Abstract
In fibrotic renal disease, elevated TGF-β and angiotensin II lead to increased plasminogen activator inhibitor type 1 (PAI-1). PAI-1 appears to reduce glomerular mesangial matrix turnover by inhibiting plasminogen activators, thereby decreasing plasmin generation and plasmin-mediated matrix degradation. We hypothesized that therapy with a mutant human PAI-1 (PAI-1R) that binds to matrix vitronectin but does not inhibit plasminogen activators, would enhance plasmin generation, increase matrix turnover, and decrease matrix accumulation in experimental glomerulonephritis. Three experimental groups included normal, untreated disease control, and PAI-1R–treated nephritic rats. Plasmin generation by isolated day 3 glomeruli was dramatically decreased by 69%, a decrease that was reversed 43% (P < 0.02) by in vivo PAI-1R treatment. At day 6, animals treated with PAI-1R showed significant reductions in proteinuria (48%, P < 0.02), glomerular staining for periodic acid–Schiff positive material (33%, P < 0.02), collagen I (28%, P < 0.01), collagen III (34%, P < 0.01), fibronectin (48%, P < 0.01), and laminin (41%, P < 0.01), and in collagen I (P < 0.01) and fibronectin mRNA levels (P < 0.02). Treatment did not alter overexpression of TGF-β1 and PAI-1 mRNAs, although TGF-β1 protein was significantly reduced. These observations strongly support our hypothesis that PAI-1R reduces glomerulosclerosis by competing with endogenous PAI-1, restoring plasmin generation, inhibiting inflammatory cell infiltration, decreasing local TGF-β1 concentration, and reducing matrix accumulation.
Introduction
Fibrotic renal disease is characterized by accumulation of ECM (1, 2). The amount and composition of matrix depends on a delicate balance between synthetic and degradative pathways. When matrix synthesis and deposition exceed matrix degradation, the pathological accumulation of matrix leads to fibrotic renal disease (3, 4). While our understanding of the mechanisms underlying this process has dramatically increased in recent years, research has emphasized processes that increase matrix production and therapeutic strategies to block these processes (5, 6). It is now clear that inhibition of the normal turnover of matrix also plays an important role in matrix accumulation.
Evidence supports the notion that the plasmin/protease system plays an important role in matrix degradation. Plasmin is generated by cleavage of plasminogen by either tissue-type plasminogen activator (t-PA) or urokinase-type plasminogen activator (u-PA). Plasminogen activator inhibitor type 1 (PAI-1) is the primary in vivo inhibitor of both t-PA and u-PA and acts by forming 1:1 protease-inhibitor complexes that are enzymatically inactive (7). Plasmin generation is therefore the net result of the activity of the plasminogen activators and inhibitors and can be dramatically reduced by increasing PAI-1 levels.
In vitro studies have suggested that, in addition to degrading fibrin, plasmin plays an important role in matrix degradation (4, 8, 9). Plasmin degrades the matrix proteins fibronectin (10), laminin (10), proteoglycan (11), and type IV collagen (12) as well as fibrin and also activates MMPs (13–15), the enzymes that degrade collagenous proteins.
The importance of this system in normal glomerular mesangial matrix turnover was clearly shown when matrix degradation increased fourfold after an mAb to PAI-1 was added to human mesangial cells cultured on radioactive Matrigel (16).
Data supporting the importance of the plasmin/protease system in disease has increased dramatically in recent years. Plasminogen knockout mice exhibit markedly impaired wound healing (17) and increased fibrosis after lung injury (18). In contrast, PAI-1 knockout mice show decreased lung fibrosis after bleomycin administration, although the cellular response to bleomycin is similar to that in wild-type mice (19). That PAI-1 deficiency reduces fibrosis primarily by enhancing plasmin generation was suggested by experiments where treatment of PAI-1 null mice with tranexamic acid, an inhibitor of plasmin formation, reversed the protective effect of PAI-1 deficiency (19, 20).
While PAI-1 is essentially undetectable in normal kidney, its mRNA expression and/or protein are increased in numerous models of glomerulosclerosis and in many human glomerular diseases (21–27), implicating it in the fibrotic process. PAI-1 strongly binds to the vitronectin (Vn) that is laid down at the site of tissue injury (28), concentrating PAI-1 in the fibrotic matrix where it can initially inhibit degradation of the provisional fibrin clot and later inhibit matrix degradation.
Several key modulators of renal fibrosis induce PAI-1. TGF-β increases PAI-1 production by cultured glomeruli, and overexpression of TGF-β in disease is associated with increased PAI-1 expression (refs. 29, 30; reviewed in ref. 31). Angiotensin II upregulates PAI-1 expression by mechanisms both independent of and dependent on TGF-β (32–36). Therapeutic strategies aimed at reduction of angiotensin II or TGF-β also reduce PAI-1 overexpression (3, 23, 30, 37–39).
It is now clear that increases in the trio TGF-β, angiotensin II, and PAI-1 characterize fibrotic renal disease. Currently the best available therapies involve angiotensin blockade with either an angiotensin II–converting enzyme inhibitor or an angiotensin receptor antagonist. On the horizon are therapies (such as Ab’s) that target TGF-β (40–42). Maximizing doses of these therapies or combining therapies to enhance efficacy are likely to significantly improve current regimens. Another useful approach, particularly in diseases where matrix accumulation occurs rapidly, may be to specifically target matrix degradation. We have previously shown that t-PA administration reduces matrix accumulation in anti–thy-1 nephritis (43). The data support the notion that t-PA increases plasmin generation, which in turn enhances matrix degradation.
The goal of the present study was to determine the therapeutic efficacy of an agent that was expected to manipulate the action of endogenous PAI-1 and enhance plasmin generation. A mutant human PAI-1 (PAI-1R) is a dominant-negative mutant (Thr 333 to Arg, Ala 355 to Arg) that has been shown by in vitro studies to bind Vn normally but to have no inhibitory activity on any protease (44). We hypothesized that the mutant PAI-1R, injected into nephritic rats, would compete with endogenous PAI-1 for Vn binding sites at the site of injury but would not inhibit PAs, and therefore would enhance plasmin generation and increase matrix turnover.
Methods
Animal protocols, study 1: Time course of Vn and endogenous PAI-1 staining in anti–thy-1 nephritis.
Three rats were sacrificed at each of eight timepoints from 0 to 28 days after OX-7 injection. Cortical tissue was stained for Vn and endogenous rat PAI-1.
Animal protocols, study 2: Time course of disappearance of PAI-1R from nephritic glomeruli.
Colocalization with Vn. Nine groups of two nephritic rats received intravenous PAI-1R injection (1 mg/kg body weight) 24 hours after disease induction. Groups were sacrificed at each of nine timepoints from 10 minutes to 24 hours after administration. Cortical tissue was used for dual immunostaining of Vn and PAI-1R.
Animal protocols, study 3: Therapeutic efficacy of PAI-1R.
Ten rats were assigned to each of the following three groups: normal controls, disease controls, and diseased animals treated with PAI-1R. PAI-1R was administered intravenously by tail vein injection twice a day from day 1 (d1) to d5 at a dose of 1 mg/kg body weight. Control rats received an equal volume of PBS. Animals were placed in metabolic cages for 24-hour urine collection from d5 to d6 and were sacrificed at d6.
Animal protocols, study 4: Effect of PAI-1R on normal rats.
Six rats were assigned to either a normal control group or a normal control group injected with PAI-1R. Dosing and sacrifice were as for Study 3 above.
Materials.
Unless otherwise indicated, materials, chemicals, and culture media were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). PAI-1R has been previously described (44) and was purified as described (45).
Animals.
The studies were performed in male Sprague Dawley rats (160–180 g) obtained from Charles River (Portage, Michigan, USA). Animal housing and care were in accordance with the NIH Guide for Care and Use of Laboratory Animals. Animals were fed a normal protein diet (22% protein, Teklad, catalog no. 86 550; Harlan Teklad, Madison, Wisconsin, USA).
Disease induction.
Glomerulonephritis was induced by tail vein injection of the monoclonal anti–thy-1 Ab OX-7 (1.75 mg/kg body weight) on d0. The OX-7 mAb was produced by cultured OX-7 cells as described previously (38). OX-7 binds to a thy-1 epitope on the surface of mesangial cells and causes complement-dependent cell lysis followed by exuberant matrix synthesis and deposition. Normal control animals were injected with the same volume of PBS.
Sacrifice.
Animals were anesthetized with isoflurane. After blood was drawn from the lower abdominal aorta, the kidney was perfused with 30 ml of cold PBS and harvested. For histological examination, cortical tissue was snap-frozen and fixed in 10% neutral-buffered formalin. Glomeruli from individual rats were isolated by graded sieving with 150-, 125-, 106-, and 75-μm mesh metal sieves as described previously (46).
Plasma levels of active rat PAI-1.
Active rat PAI-1 was measured using a commercially available kit (Molecular Innovations Inc., Southfield, Michigan, USA).
Urinary protein excretion.
Rats were housed in metabolic cages on d5, and 24-hour urinary protein excretion was measured by the Bradford method (Bio-Rad Protein Assay; Bio-Rad Laboratories Inc., Hercules, California, USA).
Light microscopy.
All microscopic examinations were performed in a blinded fashion on 3-μm sections of paraffin-embedded tissues stained with periodic acid–Schiff (PAS). Glomerular matrix expansion was evaluated as previously described (47). Briefly, in 30 glomeruli from each rat, the amount of mesangial matrix occupying each glomerulus was scored as 0 (0%), 1 (25%), 2 (50%), 3 (75%) or 4 (100%).
Immunofluorescent staining for Vn, endogenous PAI-1, and PAI-1R.
Indirect immunofluorescence was performed on 3-μm cryostat sections. Polyclonal rabbit anti-mouse Vn Ab (1:300 dilution, kindly provided by Emile de Heer, Department of Nephrology and Pathology, Leiden University Medical Center, Leiden, The Netherlands) and rabbit anti–rat PAI-1 Ab (400 μg/ml dilution; American Diagnostica Inc., Greenwich, Connecticut, USA) were used as the primary Ab’s. FITC-conjugated swine anti-rabbit IgG (DAKO Corp., Carpinteria, California, USA) was used as the secondary Ab. Intraglomerular deposition of Vn and endogenous PAI-1 in time-course study tissue was semiquantitated by scoring 20 randomly selected glomeruli per section on a 0–4 scale as described above.
For dual immunostaining, a rabbit anti–mouse Vn Ab (1:300) and a goat anti–human PAI-1 Ab (1:100; American Diagnostica Inc.) were applied at the same time and kept at 4°C overnight. TRITC-conjugated monkey anti-rabbit IgG and FITC-conjugated monkey anti-goat IgG (each at 1:200 dilution; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) were applied as secondary Ab’s at room temperature for 2 hours. Dual-immunostained sections were analyzed using a confocal microscope. Control slides treated with PBS instead of primary Ab’s showed no staining.
Immunofluorescent staining for matrix proteins and macrophages.
Monoclonal mouse anti-cellular fibronectin extra domain positive (FN-EDA+) (Harlan Sera-Lab Ltd., Loughborough, United Kingdom), rabbit anti–mouse laminin (ICN Immunobiologicals, Aurora, Ohio, USA), goat anti–human type I collagen, and goat anti–human type III collagen (Southern Biotechnology Associates, Birmingham, Alabama, USA) were used as the primary Ab’s for detection of matrix components in glomeruli at d6. FITC-conjugated rat F(ab′)2 anti-mouse IgG (H+l) (Jackson ImmunoResearch Laboratories Inc.), FITC-conjugated swine anti-rabbit IgG (DAKO Corp.), and FITC-conjugated rabbit anti-goat IgG (DAKO Corp.) were used as the secondary Ab’s. For immunostaining of fibrinogen/fibrin, FITC-conjugated rabbit anti–human fibrinogen/fibrin (DAKO Corp.) was used directly. For the determination of monocyte/macrophage infiltration into glomeruli, FITC-conjugated mouse anti–rat ED-1 Ab (Serotec Ltd., Oxford, United Kingdom) was used. Intraglomerular deposition of these ECM components was quantified by scoring 20 randomly selected glomeruli per section on a 0–4 scale as described above. The number of monocytes/macrophages per glomerulus was counted in 20 glomeruli selected randomly per section.
TGF-β1 and fibronectin content in glomeruli.
Glomeruli from individual rats were isolated and resuspended at 2 × 104 glomeruli/ml in RIPA buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, and 1 tablet/5 ml protease inhibitor mix [Complete, mini; Roche Diagnostics Corp., Indianapolis, Indiana, USA]). Glomeruli were homogenized two times on ice by sonication. Each 15-second sonication was followed by a 15-second cool down. After centrifugation at 400 g for 10 minutes at 4°C, the supernatant was stored at –70°C until analysis of glomerular TGF-β1 and fibronectin content. TGF-β1 content was measured after acid activation using a commercially available DuoSet ELISA development system (Quantikine; R&D Systems Inc., Minneapolis, Minnesota, USA) according to the manufacturer’s instructions. Fibronectin content was measured with modified inhibitory ELISA according to published methods (48).
RNA preparation and Northern hybridization.
Total RNA was extracted immediately from freshly isolated glomeruli by a guanidinium isothiocyanate method using Trizol reagent (Invitrogen, Gaithersburg, Maryland, USA) according to the manufacturer’s instructions. Isolated glomeruli from ten rats were pooled for subsequent RNA extraction. For Northern analysis, RNA was denatured and fractionated by electrophoresis through 1.0% agarose gel (30 μg/lane) and transferred to a BrightStar-plus nylon membrane (Ambion Inc., Austin, Texas, USA). Nucleic acids were immobilized by UV irradiation. Membranes were prehybridized with ULTRAhyb solution (Ambion Inc.) and hybridized with DNA probes (1 × 106 cpm/ml) labeled with 32P-dATP using the Random Primed StripAble DNA Probe Synthesis and Removal Kit (Ambion Inc.). The blots were washed in 2× SSC with 0.1% SDS at 42°C for 10 minutes and in 0.1× SSC with 0.1% SDS at 42°C for 20 minutes. DNA probes used were: GAPDH cDNA, a gift from P. Kondaiah and M.B. Sporn, Dartmouth University, Hanover, New Hampshire, USA (49); TGF-β1 cDNA, kindly provided by H.L. Moses, Vanderbilt University Cancer Center, Nashville, Tennessee, USA (50); PAI-1 cDNA, kindly provided by T.D. Gelehrter, University of Michigan, Ann Arbor, Michigan, USA (51); FN-EDA+ cDNA, a generous gift from R.O. Hynes, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA (52); and type I collagen, kindly provided by Phillip Gray, University of Utah, Salt Lake City, Utah, USA (53). Three blots per probe were performed. Autoradiographic films were scanned on a laser densitometer (Ultrascan XL; Pharmacia LKB Biotechnology Inc., Pleasant Hill, California, USA). For quantitative densitometric measurements of Northern blots, all the signals were normalized to GAPDH levels used for equal loading.
Plasmin activity of isolated glomeruli.
Normal and d3 nephritic rats were sacrificed 10 minutes after PAI-1R (n = 4) or PBS injection (n = 4). Intact glomeruli from individual rats were isolated as described above. Total glomerular plasmin activity was measured using a plasmin-specific chromogenic substrate, Chromozym PL (Roche Molecular Biochemicals, Indianapolis, Indiana, USA). This substance is specifically cleaved by plasmin into a residual peptide and 4-nitroaniline, which can be detected spectrophotometrically. Intact glomeruli were suspended at 2 × 104 glomeruli per ml of 50 mM Tris (pH 8.2) with 0.1% Triton X-100 and sonicated for 15 seconds on ice. After centrifugation at 200 g for 10 minutes at 4°C, 80 μl of glomerular supernatant and 20 μl of 3 mM Chromozym PL and 0.2 μM plasminogen (DiaPharma Group Inc., West Chester, Ohio, USA) were added per well. Absorbance was measured at 405 nm three times over a 2-hour interval. The increase in absorbance, corresponding to plasmin activity, was calculated. The standard linear curve was generated with serial dilutions of porcine plasmin. Results were expressed as U/108 glomeruli.
Statistics and calculation of percentage reduction in disease severity.
Data are expressed as mean ± SEM. The significance of differences in the measured values between groups was analyzed by the Student t test or Welch’s t test. P < 0.05 was considered statistically significant. The disease-induced increase in a variable was defined as the mean value for the disease control group minus the mean value of the normal control group. The percent reduction in disease severity in a PAI-1R-treated group was calculated as follows:
[1 – (PAI-1R–treated group mean – normal control group mean)/(disease control group mean – normal control group mean)] × 100.
Results
Study 1: Time course of Vn and endogenous PAI-1 staining in anti–thy-1 nephritis
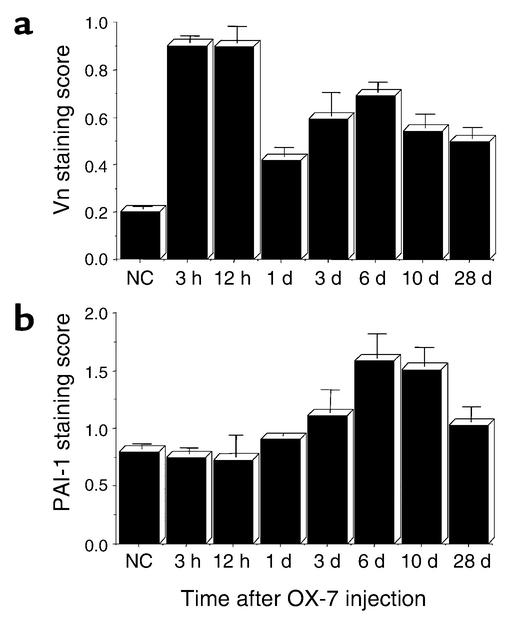
PAI-1 binds to Vn in both plasma and ECM. To determine when Vn and endogenous PAI-1 would be present in glomeruli from nephritic rats we did a time-course study. The results are shown in Figure 1. Vn staining in glomeruli increases dramatically as early as 3 hours after anti–thy-1 Ab is injected and remains elevated throughout the course of disease (Figure 1a). Endogenously produced PAI-1 deposition into glomerular ECM increases less rapidly, showing a small increase at d1 and peaking at d6 of disease (Figure 1b). We therefore decided to begin PAI-1R therapy on d1, when Vn is present to which PAI-1R can bind.
Figure 1.
Time course of glomerular Vn (a) and endogenous PAI-1 (b) staining in OX-7–induced nephritis. Data are from three rats at each timepoint. NC, normal control.
Study 2: Time course of disappearance of PAI-1R from nephritic glomeruli
Colocalization with Vn.
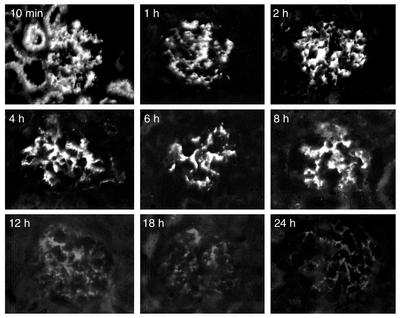
To determine whether injected PAI-1R was present in nephritic glomeruli, whether it bound to Vn, and how long it remained, staining was evaluated on kidney sections from two rats at nine timepoints after PAI-1R injection (Figure 2). The staining Ab was specific for human PAI-1 and did not stain rat PAI-1. No staining was seen in normal control or nephritic kidney. PAI-1R shows strong glomerular and tubular staining at 10 minutes after injection. The tubular staining disappears by 1 hour after injection, suggesting that at 10 minutes some PAI-1R is being filtered, while PAI-1R present at 1 hour is likely bound in the glomeruli. This PAI-1R remains in nephritic glomeruli for at least 8 hours and disappears by 12 hours.
Figure 2.
Time course of disappearance of PAI-1R from nephritic glomeruli, shown by injected PAI-1R staining in OX-7–induced nephritic glomeruli at d1. Representative photomicrograph of glomeruli from two rats at each timepoint injected with PAI-1R at 1 mg/kg body weight.
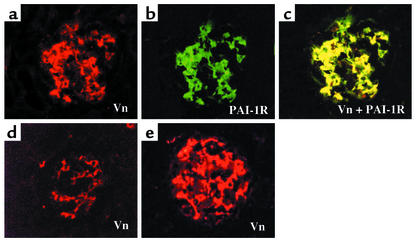
The results of dual immunostaining are shown in Figure 3. Vn stained in red (Figure 3a) and PAI-1R stained in green (Figure 3b) produced strong yellow staining (Figure 3c) when viewed with a confocal filter, indicating that injected PAI-1R was colocalized with rat Vn. Based on these results, PAI-1R was administered therapeutically twice a day at a dose of 1 mg/kg body weight.
Figure 3.
Colocalization of PAI-1R with Vn. A glomerulus from a d1 animal sacrificed 1 hour after PAI-1R injection. Staining for (a) rat Vn (red) and (b) PAI-1R (green). (c) Double staining for Vn and PAI-1R. Injected PAI-1R colocalized with endogenous rat Vn in the mesangium. Normal control (d) and disease control (e) rats showed Vn staining only in glomerulus, and no staining for human PAI-1 in kidney.
Study 3: Therapeutic efficacy of PAI-1R
Effects of PAI-1R on plasma levels of active rat PAI-1.
Normal and disease control rats had very similar plasma levels of active rat PAI-1 (3.32 ± 0.21 and 3.22 ± 0.45 ng/ml, respectively). PAI-1R–treated, nephritic rats had slightly, though not significantly, elevated PAI-1 levels (4.25 ± 0.35 ng/ml; P = 0.07). These results indicate that PAI-1R injection had little effect on endogenous PAI-1 levels in plasma.
Effects of PAI-1R on urinary protein excretion in anti–thy-1 nephritis.
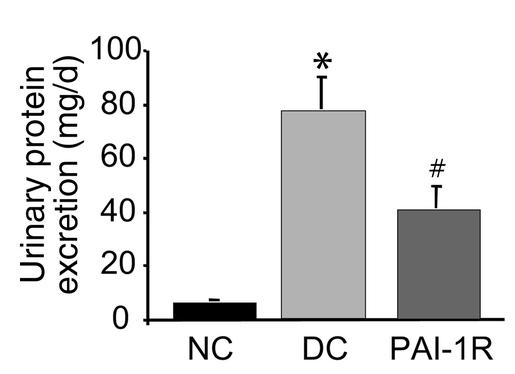
Twenty-four–hour urinary protein excretion was measured from d5 to d6 (Figure 4). Disease-induced increases in urinary protein excretion were reduced 47.7% by PAI-1R treatment compared with the disease control group (39.66 ± 8.61 mg/d vs. 76.42 ± 12.01 mg/d, respectively; P < 0.02).
Figure 4.
Effects of PAI-1R on 24-hour urinary protein excretion from d5 to d6. Urinary protein excretion was significantly lower in the PAI-1R–treated, nephritic group. *P < 0.001 vs. normal control. #P < 0.02 vs. disease control (DC).
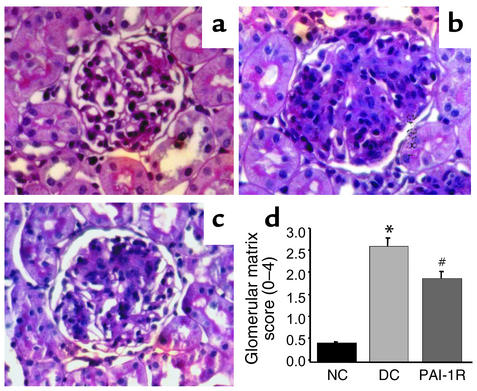
PAS staining.
Representative glomeruli stained with PAS are shown in Figure 5. The glomeruli from the disease control rats showed marked accumulation of ECM expressed as PAS-positive material at d6 (Figure 5b) compared with normal glomeruli (Figure 5a). Treatment of nephritic rats with the mutant PAI-1 resulted in much less mesangial ECM accumulation in glomeruli (Figure 5c). Figure 5d shows a graphical representation of the mean ± SEM of PAS matrix score for each group. PAS score increased from 0.45 ± 0.02 in normal control rats to 2.63 ± 0.22 in disease control rats as a result of nephritis. PAI-1R treatment decreased the matrix score significantly (P < 0.02) from 2.63 ± 0.22 in the disease control group to 1.90 ± 0.18. This is a 33% reduction in the disease-induced increase in PAS staining score.
Figure 5.
Glomerular histology. Representative photomicrographs of glomeruli from normal control rats (a), disease control rats treated with PBS (b), and PAI-1R–treated, nephritic rats (c) at d6. (d) Graphic representation of PAS staining scores of each group. PAI-1R treatment resulted in a significant reduction in PAS staining score compared with disease control rats. *P < 0.001 vs. normal control; #P < 0.02 vs. disease control.
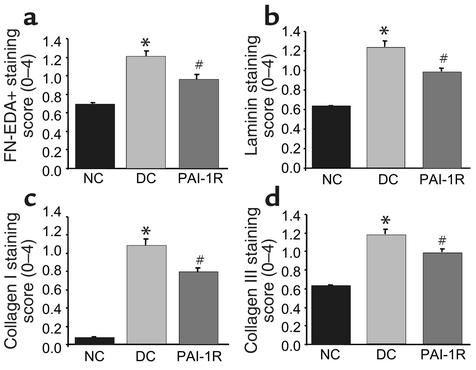
Immunofluorescent staining.
The results of the semiquantitative analysis of immunofluorescent staining for ECM proteins are shown in Figure 6. Compared with the disease control group, the staining scores were significantly lower in the PAI-1R–treated, nephritic group at d6 for FN-EDA+ (1.20 ± 0.06 vs. 0.96 ± 0.05; P < 0.01), laminin (1.23 ± 0.06 vs. 0.63 ± 0.01; P < 0.01), type I collagen (1.07 ± 0.09 vs. 0.79 ± 0.05; P < 0.01), and type III collagen (1.17 ± 0.06 vs. 0.98 ± 0.04; P < 0.01). These represent decreases in disease-induced ECM accumulation of 48% for FN-EDA+, 41% for laminin, 28% for type I collagen, and 34% for type III collagen.
Figure 6.
Immunofluorescent staining score for ECM proteins at d6. Glomerular staining for FN-EDA+ (a), laminin (b), type I collagen (c), and type III collagen (d) were lower in the PAI-1R–treated, nephritic group. *P < 0.001 compared with normal control. #P < 0.01 compared with disease control.
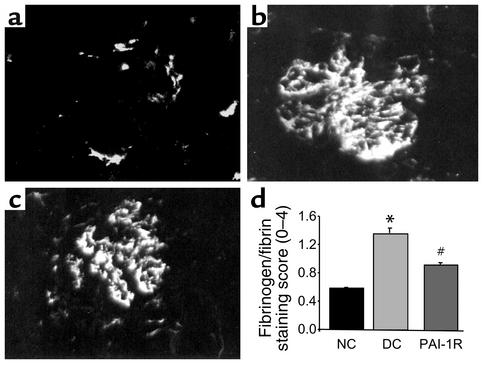
To evaluate the effect of PAI-1R on fibrinogen/fibrin deposition in nephritic glomeruli, direct immunofluorescent staining was performed on d6 tissues (Figure 7). The staining score for fibrinogen/fibrin was reduced by 56% in the PAI-1R–treated, nephritic group compared with disease control (P < 0.001).
Figure 7.
Immunofluorescent staining for glomerular fibrinogen/fibrin at d6. Representative photomicrograph of a normal rat (a), a disease control (b), and a PAI-1R–treated, nephritic rat (c). Graphic representation of fibrinogen/fibrin staining scores for each group (d). PAI-1R treatment resulted in a significant reduction in glomerular fibrin deposition compared with disease control rats. *P < 0.001 vs. normal control; #P < 0.001 vs. disease control.
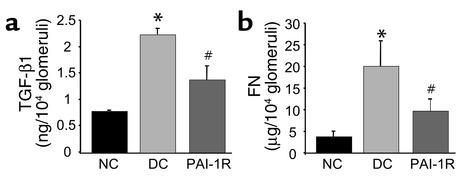
Effects of PAI-1R on TGF-β1 and fibronectin content in glomeruli.
Injection of anti–thy-1 Ab resulted in a 2.86-fold increase in TGF-β1 and a 5.4-fold increase in fibronectin content of glomeruli isolated at d6 (Figure 8). TGF-β1 and fibronectin content were reduced with PAI-1R by 58.6% (P < 0.01) and 63.3% (P < 0.01), respectively (Figure 8).
Figure 8.
Effects of PAI-1R on TGF-β1 and fibronectin (FN) content in glomeruli at d6. The glomerular content of total TGF-β1 (a) and fibronectin (b) was significantly lower in the PAI-1R–treated group. *P < 0.001 vs. normal control; #P < 0.01 vs. disease control.
Effects of PAI-1R on glomerular mRNA levels of TGF-β1, PAI-1, fibronectin, and type I collagen in anti–thy-1 nephritis.
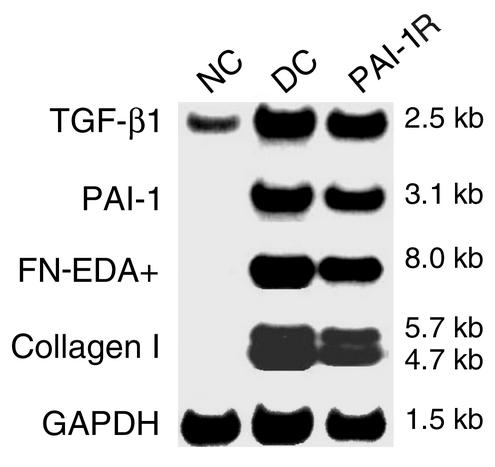
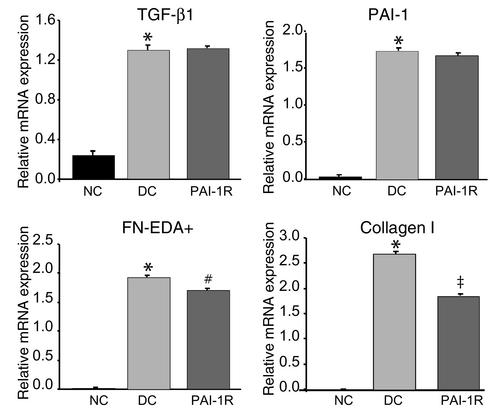
As shown in Figure 9 and Figure 10, glomerular mRNA analysis revealed a fivefold increase in TGF-β1 mRNA expression and dramatic increases in PAI-1, FN-EDA+, and type I collagen mRNA expression in disease control rats compared with normal control rats. PAI-1R administration significantly reduced the levels of FN-EDA+ and type I collagen mRNAs, by 11% (P < 0.02) and 31% (P < 0.01), respectively, but did not affect the overexpression of TGF-β1 and PAI-1 mRNAs.
Figure 9.
Effects of PAI-1R treatment on glomerular mRNA expression in anti–thy-1 nephritis at d6. Representative Northern blot is shown.
Figure 10.
Relative glomerular mRNA expression of TGF-β1, PAI-1, fibronectin, and type I collagen. Glomerular mRNA levels of fibronectin and type I collagen were significantly reduced at d6, but the overexpression of TGF-β1 and PAI-1 was not changed in the PAI-1R–treated group. *P < 0.001 vs. normal control; #P < 0.02 vs. disease control; ‡P < 0.01 vs. disease control.
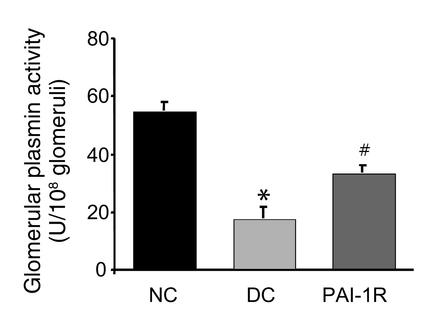
Effect of PAI-1R on plasmin activity of glomeruli in anti–thy-1 nephritis.
As shown in Figure 11, d3 nephritic glomeruli exhibited a dramatic decrease in plasmin activity compared with normal rat glomeruli (17.39 ± 3.83 vs. 54.65 ± 2.99 U/108 glomeruli, respectively; P < 0.001). The disease-induced decrease in glomerular plasmin activity was significantly reversed by PAI-1R treatment (treated, diseased, 33.36 ± 2.41 U/108 glomeruli vs. untreated, diseased, 17.39 ± 3.83 U/108 glomeruli; P < 0.02).
Figure 11.
Effects of PAI-1R on the glomerular plasmin activity of nephritic rats at d3. Kidneys were removed 10 minutes after PBS or PAI-1R injection (1 mg/kg body weight). Nephritic glomeruli had decreased plasmin activity, which was elevated by in vivo presacrifice injection of PAI-1R (n = 4 rats per group). *P < 0.01 vs. normal control; #P < 0.02 vs. disease control.
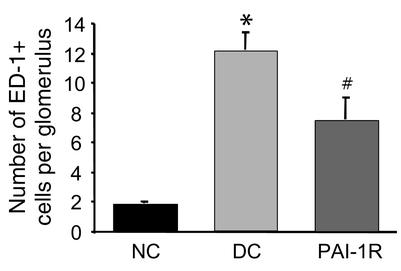
Effects of PAI-1R on monocyte/macrophage infiltration in anti–thy-1 nephritis.
The number of monocytes/macrophages was determined in kidney sections from all rats in each group (Figure 12). Nephritic glomeruli from disease control rats contained higher numbers of monocytes/macrophages than did glomeruli from normal control rats (12.1 ± 1.2 vs. 1.8 ± 0.1; P < 0.001). The average number of monocytes/macrophages per glomerular cross section in nephritic rats treated with PAI-1R was 46% lower than that in disease controls (7.3 ± 1.5 vs. 12.1 ± 1.2; P < 0.02).
Figure 12.
Number of monocytes/macrophages infiltrating glomeruli in anti–thy-1 nephritis at d6. PAI-1R treatment resulted in a significant reduction in monocyte/macrophage infiltration compared with disease control rats. *P < 0.001 vs. normal control; #P < 0.02 vs. disease control.
Study 4: Effect of PAI-1R on normal rats
To detect effects of PAI-1R injection in normal rats, six PAI-1R–treated and six PBS-treated normal rats were injected and sacrificed as in Study 3. Levels of active rat PAI-1 in plasma, urinary protein excretion, staining for PAS+ material and specific matrix components, ED-1+ cells in glomeruli and TGF-β1 and fibronectin content in glomeruli, and collagen I and TGF-β1 mRNA levels were very similar in untreated and PAI-1R–injected normal rats. No comparisons reached statistical significance. In contrast, PAI-1 mRNA levels were 59% higher (0.24 ± 0.02 vs. 0.15 ± 0.01) and fibronectin mRNA levels were 76% higher (0.26 ± 0.05 vs. 0.15 ± 0.01) in rats injected with PAI-1R than in control rats. To look at this more closely, Northern blots were rerun with disease control group RNA from Study 3 and RNA from Study 4. It was seen that disease caused very large increases in PAI-1 and fibronectin mRNA, while injection of PAI-1R into normal rats produced very small but significant increases that were only 5.4% and 7.4% of the disease-induced increases seen for PAI-1 and fibronectin, respectively. Although we cannot be certain, the similarity between the PAI-1R–injected and PBS-injected normal rats for most measures suggests that PAI-1R injection has little effect in normal rats.
Discussion
The protein Vn is a multifunctional glycoprotein found in plasma, platelets, and ECM of many normal tissues (54), particularly during wound healing in the vessel wall and the skin (55). In anti–thy-1 nephritis, Vn deposition occurs in the glomerular mesangium. The time course of Vn staining shown in Figure 1 indicates that Vn deposition is strongly increased as early as 3 hours after glomerular injury begins. In plasma and the ECM, PAI-1 is associated with Vn, which stabilizes PAI-1 in its active conformation and converts it to an efficient inhibitor of thrombin (56–58). It is also thought that Vn serves to localize PAI-1 to the ECM where it regulates local proteolytic activity (59). In anti–thy-1 nephritis, endogenous PAI-1 is strongly induced as well, but its deposition into glomerular ECM increases more slowly than that of Vn.
Physiologically, when native PAI-1 binds to a protease such as u-PA or t-PA, protease cleavage of the PAI-1 reactive center loop induces a rapid conformational change in PAI-1 that results in an approximately 250-fold reduction in PAI-1 affinity for Vn. The loss of affinity for Vn results in rapid repartitioning of the PAI-1/protease complex from Vn in the ECM to the clearance receptor, leading to subsequent endocytosis and degradation of the complex (60–62). As the PAI-1/protease complex is removed, the Vn molecule becomes available to bind another PAI-1 molecule. The noninhibitory PAI-1R used in these studies has the same affinity for Vn as native PAI-1 but has no inhibitory activity toward any protease (44). It also does not lose its affinity for Vn (44) following protease binding and cleavage and therefore would be expected to bind to Vn longer than native PAI-1.
Immunostaining studies showed colocalization of PAI-1R and Vn and persistence of PAI-1R in nephritic rat glomeruli, suggesting that the injected PAI-1R is targeted to Vn within the nephritic glomerulus where it should effectively compete with endogenous native PAI-1 for binding sites on Vn. While there, PAI-1R partially reverses the disease-induced decrease in glomerular plasmin activity (Figure 11). Although not proven here, it is likely that this increased plasmin leads to enhanced degradation of the pathological ECM. Indeed, among the therapeutic consequences of PAI-1R treatment were significant decreases in PAS-positive material (Figure 5) and in the specific matrix proteins FN-EDA+, laminin, type I collagen, and type III collagen (Figure 6), and decreases in fibrinogen/fibrin accumulation (Figure 7).
However, several findings suggest that PAI-1R acts by mechanisms in addition to enhancing matrix degradation. PAI-1R treatment reduced overexpression of FN-EDA+ mRNA, type I collagen mRNA, glomerular TGF-β1 content, and glomerular fibronectin content (Figure 8). These results would not be expected if the sole action of PAI-1R were to increase ECM turnover. Indeed, previous work from this laboratory showed that t-PA treatment of anti–thy-1 nephritis only reduced ECM protein accumulation with no effect on mRNA or protein levels (43).
A possible mechanism by which PAI-1R reduces the severity of anti–thy-1 nephritis may be reduction of inflammatory cell migration into the glomerulus. Inflammatory cells characterize many experimental and human renal diseases (63–65) and are thought to contribute to disease by releasing numerous factors, including TGF-β, PDGF, bFGF, and IL-6 (65). The PAI-1/Vn binding site localizes to the first 50 residues of Vn, a region that also contains the Arg-Gly-Asp (RGD) cell-attachment site used by a number of cell types for adhesion and migration (66). Therefore, independent of its antiproteolytic activity, PAI-1 may block cell adhesion and migration for the time it is bound to Vn, an idea supported by the finding that PAI-1 null mice show enhanced smooth muscle cell migration (67). The noninhibitory mutant PAI-1R does not lose its affinity for Vn and therefore would be expected to block cell migration even in the presence of proteases, a finding shown in vitro (44, 67). In the present study, monocyte/macrophage infiltration was reduced by 46% in PAI-1R–treated, nephritic rats (Figure 12). Fewer inflammatory cells should reduce TGF-β, and we found that glomerular TGF-β1 content was reduced by 59% compared with disease control glomeruli (Figure 8).
Another mechanism by which PAI-1R treatment may reduce glomerular TGF-β concentration was suggested by a recent study of Schoppet et al. in which matrix Vn was shown to have high affinity for TGF-β (68). Although TGF-β binding to Vn did not alter TGF-β receptor binding or signal transduction, the observation that Vn has high affinity for TGF-β suggests that TGF-β binding to Vn could increase the local concentration of TGF-β at the cell-ECM interface and thereby influence its functions. The peptide sequence of Vn directly involved in TGF-β binding overlaps binding sites for PAI-1 and u-PA receptor (66, 68, 69). Interestingly, in studies in vitro, PAI-1 and TGF-β competed for Vn binding sites and PAI-1 was able to release bound TGF-β from Vn in a concentration-dependent manner (68). This raises the possibility that PAI-1R binding to Vn may increase TGF-β clearance at the cell-ECM interface by effectively competing with TGF-β for Vn binding sites, decreasing glomerular TGF-β concentration. Since TGF-β induces both fibronectin and collagen, a decrease in TGF-β protein might lead to decreases in fibronectin and collagen mRNA, as we observed.
TGF-β is a potent inducer of PAI-1 production, therefore PAI-1 mRNA expression should also have been reduced if TGF-β protein is reduced. This did not happen, suggesting a feedback loop in which interference with the action of native PAI-1 leads to enhanced PAI-1 mRNA production.
The present study demonstrates that PAI-1R, a mutant human protein, is targeted to Vn in nephritic glomeruli where it is likely to remain longer than native PAI-1. While there, PAI-1R significantly reduces pathological ECM accumulation by a combination of mechanisms including competing with endogenous native PAI-1 for Vn binding sites, restoring plasmin generation, inhibiting inflammatory cell infiltration, and decreasing local TGF-β1 concentration. We conclude that this human mutant PAI-1, and other therapeutic agents aimed at enhancing degradation of pathological ECM, may have important clinical application.
Acknowledgments
The authors thank Linda Hoge for her excellent technical assistance. This work was supported by NIH grants DK-60508 (to N.A. Noble), DK-49374 (to W.A. Border), and DK-43609 (to W.A. Border).
Footnotes
See the related Commentary beginning on page 326.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: tissue-type plasminogen activator (t-PA); urokinase-type plasminogen activator (u-PA); plasminogen activator inhibitor type 1 (PAI-1); vitronectin (Vn); mutant human PAI-1 (PAI-1R); day 1 (d1); periodic acid–Schiff (PAS); fibronectin extra domain positive (FN-EDA+).
References
- 1.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 2.Border WA, Okuda S, Nakamura T. Extracellular matrix and glomerular disease. Semin. Nephrol. 1989;9:307–317. [PubMed] [Google Scholar]
- 3.Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 4.Schnaper HW. Balance between matrix synthesis and degradation: a determinant of glomerulosclerosis. Pediatr. Nephrol. 1995;9:104–111. doi: 10.1007/BF00858986. [DOI] [PubMed] [Google Scholar]
- 5.Gaedeke J, Peters H, Noble NA, Border WA. Angiotensin II, TGF-β and renal fibrosis. Contrib. Nephrol. 2001;135:153–160. doi: 10.1159/000060162. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Noble N, Border W. Therapeutic strategies to halt renal fibrosis. Curr. Opin. Pharmacol. 2002;2:177–181. doi: 10.1016/s1471-4892(02)00144-3. [DOI] [PubMed] [Google Scholar]
- 7.Loskutoff DJ, Sawdey M, Mimuro J. Type 1 plasminogen activator inhibitor. Prog. Hemost. Thromb. 1989;9:87–115. [PubMed] [Google Scholar]
- 8.Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am. J. Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- 9.Mignatti P. Extracellular matrix remodeling by metalloproteinases and plasminogen activators. Kidney Int. 1995;47:S12–S14. [PubMed] [Google Scholar]
- 10.Liotta LA, et al. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41:4629–4636. [PubMed] [Google Scholar]
- 11.Mochan E, Keler T. Plasmin degradation of cartilage proteoglycan. Biochim. Biophys. Acta. 1984;800:312–315. doi: 10.1016/0304-4165(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 12.Mackay AR, Corbitt RH, Hartzler JL, Thorgeirsson UP. Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res. 1990;50:5997–6001. [PubMed] [Google Scholar]
- 13.He CS, et al. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4–aminophenyl)mercuric acetate. Biochemistry. 1990;29:5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-DeSimone N, et al. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J. Biol. Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 16.Baricos WH, Cortez SL, El-Dahr SS, Schnaper HW. ECM degradation by cultured human mesangial cells is mediated by a PA/plasmin/MMP-2 cascade. Kidney Int. 1995;47:1039–1047. doi: 10.1038/ki.1995.150. [DOI] [PubMed] [Google Scholar]
- 17.Romer J, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat. Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 18.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am. J. Pathol. 2000;157:177–187. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori N, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J. Clin. Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eitzman DT, et al. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J. Clin. Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes JL, Mitchell RJ, Torres ES. Expression of plasminogen activator-inhibitor-1 (PAI-1) during cellular remodeling in proliferative glomerulonephritis in the rat. J. Histochem. Cytochem. 1995;43:895–905. doi: 10.1177/43.9.7642963. [DOI] [PubMed] [Google Scholar]
- 22.Keeton M, Ahn C, Eguchi Y, Burlingame R, Loskutoff DJ. Expression of type 1 plasminogen activator inhibitor in renal tissue in murine lupus nephritis. Kidney Int. 1995;47:148–157. doi: 10.1038/ki.1995.17. [DOI] [PubMed] [Google Scholar]
- 23.Ma LJ, et al. Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int. 2000;58:2425–2436. doi: 10.1046/j.1523-1755.2000.00426.x. [DOI] [PubMed] [Google Scholar]
- 24.Shihab FS, et al. Transforming growth factor-beta and matrix protein expression in acute and chronic rejection of human renal allografts. J. Am. Soc. Nephrol. 1995;6:286–294. doi: 10.1681/ASN.V62286. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, et al. Increased levels of transforming growth factor-beta in HIV-associated nephropathy. Kidney Int. 1999;55:579–592. doi: 10.1046/j.1523-1755.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 27.Paueksakon P, Revelo MP, Ma LJ, Marcantoni C, Fogo AB. Microangiopathic injury and augmented PAI-1 in human diabetic nephropathy. Kidney Int. 2002;61:2142–2148. doi: 10.1046/j.1523-1755.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence DA, et al. Characterization of the binding of different conformational forms of plasminogen activator inhibitor-1 to vitronectin. Implications for the regulation of pericellular proteolysis. J. Biol. Chem. 1997;272:7676–7680. doi: 10.1074/jbc.272.12.7676. [DOI] [PubMed] [Google Scholar]
- 29.Tomooka S, Border WA, Marshall BC, Noble NA. Glomerular matrix accumulation is linked to inhibition of the plasmin protease system. Kidney Int. 1992;42:1462–1469. doi: 10.1038/ki.1992.442. [DOI] [PubMed] [Google Scholar]
- 30.Peters H, Border WA, Noble NA. Targeting TGF-beta overexpression in renal disease: maximizing the antifibrotic action of angiotensin II blockade. Kidney Int. 1998;54:1570–1580. doi: 10.1046/j.1523-1755.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 31.Eddy A. Plasminogen activator inhibitor-1 and the kidney. Am. J. Physiol. Renal Physiol. 2002;283:F209–F220. doi: 10.1152/ajprenal.00032.2002. [DOI] [PubMed] [Google Scholar]
- 32.Olson JA, Jr, Shiverick KT, Ogilvie S, Buhi WC, Raizada MK. Angiotensin II induces secretion of plasminogen activator inhibitor 1 and a tissue metalloprotease inhibitor-related protein from rat brain astrocytes. Proc. Natl. Acad. Sci. U. S. A. 1991;88:1928–1932. doi: 10.1073/pnas.88.5.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelezna B, et al. Angiotensin-II induction of plasminogen activator inhibitor-1 gene expression in astroglial cells of normotensive and spontaneously hypertensive rat brain. Mol. Endocrinol. 1992;6:2009–2017. doi: 10.1210/mend.6.12.1491687. [DOI] [PubMed] [Google Scholar]
- 34.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. J. Clin. Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feener EP, Northrup JM, Aiello LP, King GL. Angiotensin II induces plasminogen activator inhibitor-1 and-2 expression in vascular endothelial and smooth muscle cells. J. Clin. Invest. 1995;95:1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagami S, et al. Dual effects of angiotensin II on the plasminogen/plasmin system in rat mesangial cells. Kidney Int. 1997;51:664–671. doi: 10.1038/ki.1997.96. [DOI] [PubMed] [Google Scholar]
- 37.Peters H, Border WA, Noble NA. Angiotensin II blockade and low-protein diet produce additive therapeutic effects in experimental glomerulonephritis. Kidney Int. 2000;57:1493–1501. doi: 10.1046/j.1523-1755.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 38.Peters H, Border WA, Noble NA. Tandem antifibrotic actions of L-arginine supplementation and low protein diet during the repair phase of experimental glomerulonephritis. Kidney Int. 2000;57:992–1001. doi: 10.1046/j.1523-1755.2000.00927.x. [DOI] [PubMed] [Google Scholar]
- 39.Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo A. Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- 40.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta-1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 41.Ziyadeh FN, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyajima A, et al. Antibody to transforming growth factor-β ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 43.Haraguchi M, Border WA, Huang Y, Noble NA. t-PA promotes glomerular plasmin generation and matrix degradation in experimental glomerulonephritis. Kidney Int. 2001;59:2146–2155. doi: 10.1046/j.1523-1755.2001.00729.x. [DOI] [PubMed] [Google Scholar]
- 44.Stefansson S, et al. Inhibition of angiogenesis in vivo by plasminogen activator inhibitor-1. J. Biol. Chem. 2001;276:8135–8141. doi: 10.1074/jbc.M007609200. [DOI] [PubMed] [Google Scholar]
- 45.Kvassman J-O, Shore JD. Purification of human plasminogen activator inhibitor (PAI-1) from Escherichia coli and separation of its active and latent forms by hydrophobic chromography. Fibrinolysis. 1995;9:215–221. [Google Scholar]
- 46.Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor-β and proteoglycan production in experimental glomerulonephritis. J. Clin. Invest. 1990;86:453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-β1 underlies development of progressive kidney fibrosis. Kidney Int. 1994;45:916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- 48.Rennard SI, Berg R, Martin GR, Foidart JM, Robey PG. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal. Biochem. 1980;104:205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- 49.Lark MW, Saphos CA, Walakovits LA, Moore VL. In vivo activity of human recombinant tissue inhibitor of metalloproteinases (TIMP). Activity against human stromelysin in vitro and in the rat pleural cavity. Biochem. Pharmacol. 1990;39:2041–2049. doi: 10.1016/0006-2952(90)90627-w. [DOI] [PubMed] [Google Scholar]
- 50.Derynck R, Jarrett A, Chen EY, Goeddel DV. The murine transforming growth factor-β precursor. J. Biol. Chem. 1986;261:4377–4379. [PubMed] [Google Scholar]
- 51.Bruzdzinski CJ, Riordan-Johnson M, Nordby EC, Suter SM, Gelehrter TD. Isolation and characterization of the rat plasminogen activator inhibitor-1 gene. J. Biol. Chem. 1990;265:2078–2084. [PubMed] [Google Scholar]
- 52.Schwarzbauer JE, Tamkun JW, Lemischka IR, Hynes RO. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983;35:421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- 53.Genovese C, Rowe D, Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984;23:6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- 54.Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb. Res. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 55.Preissner KT. Structure and biological role of vitronectin. Annu. Rev. Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- 56.Declerck PJ, et al. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin) J. Biol. Chem. 1988;263:15454–15461. [PubMed] [Google Scholar]
- 57.Knudsen BS, Harpel PC, Nachman RL. Plasminogen activator inhibitor is associated with the extracellular matrix of cultured bovine smooth muscle cells. J. Clin. Invest. 1987;80:1082–1089. doi: 10.1172/JCI113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrence DA, Berkenpas MB, Palaniappan S, Ginsburg D. Localization of vitronectin binding domain in plasminogen activator inhibitor-1. J. Biol. Chem. 1994;269:15223–15228. [PubMed] [Google Scholar]
- 59.Mimuro J, Schleef RR, Loskutoff DJ. Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood. 1987;70:721–728. [PubMed] [Google Scholar]
- 60.Stefansson S, et al. Plasminogen activator inhibitor-1 contains a cryptic high affinity binding site for the low density lipoprotein receptor-related protein. J. Biol. Chem. 1998;273:6358–6366. doi: 10.1074/jbc.273.11.6358. [DOI] [PubMed] [Google Scholar]
- 61.Stefansson S, Lawrence DA, Argraves WS. Plasminogen activator inhibitor-1 and vitronectin promote the cellular clearance of thrombin by low density lipoprotein receptor-related proteins 1 and 2. J. Biol. Chem. 1996;271:8215–8220. doi: 10.1074/jbc.271.14.8215. [DOI] [PubMed] [Google Scholar]
- 62.Argraves KM, et al. The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J. Biol. Chem. 1995;270:26550–26557. doi: 10.1074/jbc.270.44.26550. [DOI] [PubMed] [Google Scholar]
- 63.Herbert, M.-J., and Brady, H.R. 1997. Leukocyte adhesion. In Immunologic renal diseases. E.G. Neilson and W.G. Couser, editors. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 519–545.
- 64.Johnson, R.J., Klebanoff, S.J., and Couser, W. G. 1997. Neutrophils. In Immunologic renal diseases. E.G. Neilson and W.G. Couser, editors. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 547–558.
- 65.Nikolic-Paterson, D.J., Lan, H.Y., and Atkins, R.C. 1997. Macrophages in immune renal injury. In Immunologic renal diseases. E.G. Neilson and W.G. Couser, editors. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 575–592.
- 66.Seiffert D, Ciambrone G, Wagner NV, Binder BR, Loskutoff DJ. The somatomedin B domain of vitronectin. Structural requirements for the binding and stabilization of active type 1 plasminogen activator inhibitor. J. Biol. Chem. 1994;269:2659–2666. [PubMed] [Google Scholar]
- 67.Redmond EM, et al. Endothelial cells inhibit flow-induced smooth muscle cell migration: role of plasminogen activator inhibitor-1. Circulation. 2001;103:597–603. doi: 10.1161/01.cir.103.4.597. [DOI] [PubMed] [Google Scholar]
- 68.Schoppet M, Chavakis T, Al-Fakhri N, Kanse SM, Preissner KT. Molecular interactions and functional interference between vitronectin and transforming growth factor-beta. Lab. Invest. 2002;82:37–46. doi: 10.1038/labinvest.3780393. [DOI] [PubMed] [Google Scholar]
- 69.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator-inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J. Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]