Abstract
Epidemiologic studies demonstrate that long-term use of NSAIDs is associated with a reduced risk for the development of Alzheimer disease (AD). In this study, 20 commonly used NSAIDs, dapsone, and enantiomers of flurbiprofen were analyzed for their ability to lower the level of the 42-amino-acid form of amyloid β protein (Aβ42) in a human H4 cell line. Thirteen of the NSAIDs and the enantiomers of flurbiprofen were then tested in acute dosing studies in amyloid β protein precursor (APP) transgenic mice, and plasma and brain levels of Aβ and the drug were evaluated. These studies show that (a) eight FDA-approved NSAIDs lower Aβ42 in vivo, (b) the ability of an NSAID to lower Aβ42 levels in cell culture is highly predicative of its in vivo activity, (c) in vivo Aβ42 lowering in mice occurs at drug levels achievable in humans, and (d) there is a significant correlation between Aβ42 lowering and levels of ibuprofen. Importantly, flurbiprofen and its enantiomers selectively lower Aβ42 levels in broken cell γ-secretase assays, indicating that these compounds directly target the γ-secretase complex that generates Aβ from APP. Of the compounds tested, meclofenamic acid, racemic flurbiprofen, and the purified R and S enantiomers of flurbiprofen lowered Aβ42 levels to the greatest extent. Because R-flurbiprofen reduces Aβ42 levels by targeting γ-secretase and has reduced side effects related to inhibition of cyclooxygenase (COX), it is an excellent candidate for clinical testing as an Aβ42 lowering agent.
Introduction
Over the last two decades, a great deal of evidence has accumulated that supports the hypothesis that accumulation of the approximately 4-kDa amyloid β protein (Aβ) plays a causal role in the development of Alzheimer’s disease (AD). In the brains of patients with AD, Aβ accumulates as amyloid in senile plaques and in the walls of cerebral blood vessels as well as in more diffuse immunoreactive deposits. This accumulation is thought to result in a pathological cascade that ultimately results in neuronal dysfunction and death (1, 2).
Multiple Aβ species with varying amino and carboxyl termini are generated from the amyloid β protein precursor (APP) through sequential proteolytic cleavages by the β- and γ-secretases (3). The 40-amino-acid form (Aβ40) is the most abundantly produced Aβ peptide, whereas a slightly longer and less abundant 42-amino-acid form (Aβ42) has been implicated as the more pathogenic species (4). Under in vitro conditions, Aβ42 forms aggregates much more readily than Aβ40 and other shorter Aβ peptides, and these aggregates are toxic to a variety of cells in culture. Despite being a minor Aβ species, Aβ42 is deposited earlier and more consistently than Aβ40 in the AD brain. Studies of families with genetic mutations in presenilin 1 (PS1), presenilin 2 (PS2), and APP genes that give rise to early-onset autosomal-dominant forms of AD are consistently associated with perturbations in Aβ peptide levels and, with rare exception, these mutations selectively increase the relative levels of Aβ42 peptides (1). Study of patients with early-onset AD who have mutations in APP or presenilins have shown that Aβ42 levels are elevated by as little as 30% (5). Studies of these same mutations in transgenic mice indicate that these small increases in Aβ42 levels markedly accelerate Aβ deposition (6, 7). Collectively, these observations provide a strong rationale for selective targeting of Aβ42 and indicate that reducing Aβ42 levels by as little as 20–30% might retard the development of AD.
In addition to Aβ deposition, neurofibrillary tangle accumulation, and neuronal loss, the end-stage pathology of AD is also notable for the presence of numerous cellular and molecular markers of an inflammatory response that are often associated with the Aβ deposits (8). The cellular inflammatory response consists of widespread astrogliosis and microgliosis. A large number of molecular markers of inflammation are also increased, including multiple cytokines, interleukins, other acute-phase proteins, and complement components. Aβ aggregates appear capable of inciting an inflammatory response, and there is evidence that inflammation can promote increased Aβ production and also enhance Aβ deposition (8). Thus, an Aβ-induced inflammatory response could promote further Aβ accumulation and increased inflammation. Alternatively, it is possible that under certain circumstances the inflammatory response is beneficial and may actually promote Aβ clearance (9).
On the basis of the notion that the inflammatory response to Aβ is harmful, anti-inflammatory drugs have been suggested as beneficial agents in AD therapy (10, 11). This idea is supported by epidemiologic data, which consistently show that long-term use of nonaspirin NSAIDs is associated with protection from the development of AD (11–14). Indeed, this evidence has been used as the rationale for previous and ongoing trials of select NSAIDs in AD.
We recently found that three NSAIDs, ibuprofen, sulindac sulfide, and indomethacin, were capable of selectively lowering levels of the Aβ42 peptide. This effect was independent of their inhibition of the COX enzymes and is a novel mechanism of action for these compounds (15). On the basis of the proposed critical pathogenic role of Aβ42 in fostering Aβ deposition, this action could contribute to the apparent efficacy of NSAIDs in conferring protection from AD. In the present study, we have extended this observation by examining the effects of most commonly used NSAIDs on Aβ42 levels in cultured H4 human glioma cells and in acute dosing studies in APP transgenic Tg2576 mice. These data confirm what we initially observed in only a handful of NSAIDs; some, but not all NSAIDs lower Aβ42 levels both in cultured human neuroglioma cells and in the brains of APP Tg2576 mice. Of the NSAIDs tested, meclofenamic acid and flurbiprofen decrease Aβ42 production to the greatest extent. In our initial study, we speculated that NSAID-like compounds that lack COX inhibitory activity but retain the Aβ42 lowering activity could be identified. To test this idea, we examined racemic (R and S) as well as the R and S enantiomers of flurbiprofen and found that all lowered Aβ42 to a nearly equivalent extent. Flurbiprofen and its enantiomers also selectively lowered Aβ42 in a broken cell γ-secretase assay, a finding that is consistent with direct targeting of the γ-secretase activity that generates the Aβ peptide from APP. R-flurbiprofen lacks COX activity, and, in humans, undergoes very limited chiral inversion to the S-enantiomer that is active against COX (16). R-flurbiprofen appears to be well tolerated in humans and is currently in a phase II clinical trial for prostate cancer, on the basis of its antitumor and antimetastatic properties exhibited in mouse models of prostate cancer and colon cancer (17, 18). These data confirm our hypothesis that Aβ42 lowering compounds lacking COX activity exist.
Methods
Chemicals and reagents.
All the NSAIDs and dapsone were obtained from Sigma-Aldrich (St. Louis, Missouri, USA), except for diclofenac, indomethacin, and meloxicam (Calbiochem, San Diego, California, USA) and R-and S-flurbiprofen (a gift from Encore Pharmaceuticals Inc., Riverside, California, USA).
Cell culture.
H4 neuroglioma cells expressing APP695NL (“Swedish” mutation) were used for live cell screens. Generation and culture of these cells has been described (19). H4 cells were incubated for 6 hours in the presence of the various NSAIDs in DMEM containing 1% FBS. Lactate dehydrogenase (LDH) (Promega Corp., Madison, Wisconsin, USA) assays were conducted to determine toxicity. No significant toxicity was noted at the concentrations tested.
Mass spectrometry of Aβ.
For matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS) analyses of Aβ peptides, CHO cells were treated with the indicated compounds as described previously (15). Secreted Aβ peptides were analyzed by immunoprecipitation/mass spectrometry (IP/MS) assay with minor modifications of the technique described by Wang et al. (20). Aβ was immunoprecipitated from conditioned medium with 26D6, with synthetic Aβ1–22 added as an immunoprecipitation control and mass standard. Proteins were eluted into formic acid/isopropanol/water (1:4:4) and mixed with α-cyano-4-hydroxycinnamic acid. Samples were beamed on a Perspective Voyager-DE STR Biospectrometry Workstation (PE Biosystems, Foster City, California, USA).
Broken cell γ-secretase assays.
These assays were performed essentially as previously described (21, 22). Briefly, buoyant cholesterol- rich fractions exhibiting enriched γ-secretase activity were isolated from CHO cells stably overexpressing APP695NL,I-his, an amino-terminal polyhistidine-tagged 695-amino-acid isoform of APP containing both the FAD-linked “Swedish” mutation (K595N, M596L) and the “London” mutation (V642I), by flotation through a sucrose density gradient after lysis in carbonate buffer. These buoyant fractions contain over 80% of the γ-secretase activity present in the total cell lysate. After flotation, the buoyant membranes were pelleted, washed, and resuspended in 150 mM sodium citrate (pH 7.0) with complete protease inhibitor. γ-Secretase activity was assayed in the resuspended membranes by incubation at 37°C for 2 hours. Aβ levels were determined by sandwich ELISA.
Animal studies.
Female Tg2576 mice overexpressing APP695NL were treated at 3 months of age, at which point they show high levels of soluble Aβ in brain but no signs of Aβ deposition (23, 24). NSAIDs were mixed with Kool-Aid and fed orally to the animals for 3 days unless otherwise noted. Controls were administered Kool-Aid only. The daily dose was divided into four equal doses and administered every 4–6 hours, with an 8 hour interval between the last dose at night and the first dose the next day. On the day of sacrifice, two doses were given 4 hours apart, and precisely 2 hours after the final dose animals were sacrificed and formic acid–soluble Aβ40 and Aβ42 were analyzed by ELISA as described previously (24).
Aβ ELISA.
Aβ species were analyzed by sandwich ELISA as described previously (15, 25). Mouse hemibrains were extracted with 70% formic acid and diluted 1:20 in 1 M Tris base before ELISA analysis (24). All measurements were performed in duplicate. ELISAs were performed in a blinded manner.
Analysis of compound levels in plasma and brain of Tg2576 mice.
Samples were analyzed in a blinded fashion with respect to the effect on Aβ42. After addition of naproxen as an external standard, flurbiprofen and its enantiomers were extracted from plasma using 1/10 vol of 5 N hydrochloric acid and 2 vol of ethyl acetate. For brain samples, mouse hemibrains were mixed with two equivalents of water and homogenized. The resulting homogenate was extracted in a manner identical to the plasma method. For other NSAIDs, plasma samples were mixed with 2 vol of acetonitrile (containing internal standard), and after initial homogenization in water the brain homogenate was extracted with the same procedure as used for plasma samples. Quantitation of the compounds after extraction was carried out by HPLC and tandem mass spectrometric (MS/MS) detection.
Statistical analysis.
Results were analyzed using either GraphPad Version 3.03 (Prizm; GraphPad Software Inc., San Diego, California, USA) or StatView statistical software (Jandel Scientific, Chicago, Illinois, USA). The majority of analyses used ANOVA with Dunnet’s post hoc correction for comparison of multiple samples to a control group.
Results
Multiple NSAIDs lower Aβ42 selectively in cell culture.
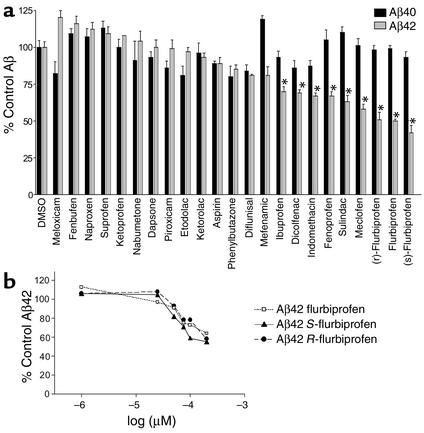
In this study, we compared the effects of 20 commonly used NSAIDs, dapsone (an antibiotic with atypical anti-inflammatory properties reported to confer protection from AD), and the two pure enantiomers of the racemic NSAID flurbiprofen on Aβ secretion in H4 human neuroglioma cells overexpressing APP695NL. This cell line secretes sufficient quantities of Aβ42 to permit reliable measurements by ELISA after 6 hours. Figure 1a shows the effects on Aβ40 and Aβ42 secretion when H4 cells were incubated in media containing 100 μM of the indicated compound for 6 hours.
Figure 1.
Effects of NSAIDs on Aβ42 production in human neuroglioma line. (a) H4 cells were treated for 6 hours with 100 μM of the compounds dissolved in DMSO vehicle, and Aβ levels in the media were determined by Aβ ELISA. Results are the average of two experiments, each performed in duplicate. Compounds are ordered by effect on Aβ42. Experimental values are compared to six control samples. *P < 0.05 by ANOVA with Dunnet’s post hoc correction. Aβ40 production was not significantly affected by any of the compounds when tested at 100 μM. (b) Dose response of racemic, R- and S-flurbiprofen. H4 cells were treated with various concentrations of racemic flurbiprofen or its purified enantiomers. All three forms of flurbiprofen selectively lower Aβ42 to approximately identical extents. At concentrations above 300 μM, moderate reductions in Aβ40 were seen, but LDH assays indicated cells treated at these doses exhibited signs of toxicity. In these experiments, control values for secreted Aβ40 and Aβ42 were greater than 500 pM and 40 pM, respectively.
In previous work, we had shown that ibuprofen, indomethacin, and sulindac sulfide lower Aβ42 levels selectively in cultured cells (15). Additional NSAIDs that selectively lowered Aβ42 levels were flurbiprofen, meclofenamic acid, fenoprofen, and diclofenac. The most effective Aβ42 lowering agents were meclofenamic acid and racemic flurbiprofen, both marketed NSAIDs. The S form of flurbiprofen is active against COX, whereas the R form is virtually inactive. In the current studies, both R- and S-flurbiprofen lowered Aβ42 levels to an extent equal to the racemate. Further dose-response studies demonstrated that the Aβ42 lowering capacity of flurbiprofen and its enantiomers was nearly identical (Figure 1b). As we had previously reported, a number of FDA-approved NSAIDs did not lower Aβ42 levels selectively in H4 cells. Some compounds, such as meloxicam, showed slight increases in Aβ42 levels that were of borderline statistical significance. Others such as phenylbutazone and diflunisal lowered Aβ40 and Aβ42 levels nonselectively. These later changes were again of borderline statistical significance. Dapsone had no significant effect on Aβ levels. When similar studies are performed in H4 cells with either 1 or 10 μM of each compound, no lowering of Aβ42 levels is seen (data not shown).
Flurbiprofen and its enantiomers shift cleavage from Aβ42 to shorter Aβ derivatives.
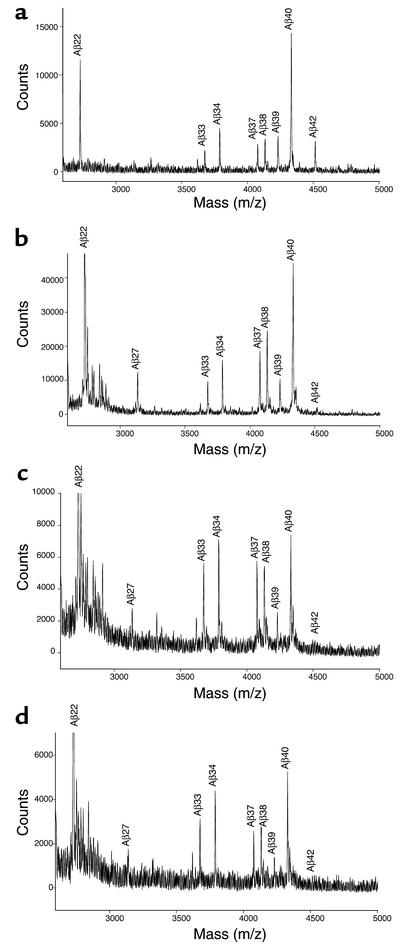
We previously reported that sulindac sulfide selectively decreased Aβ42 levels and increased levels of Aβ38, the 38-amino-acid form of Aβ (15). This finding suggested that the effect of NSAIDs was not to inhibit Aβ generation but to shift production from one Aβ species to another. To determine if flurbiprofen and its enantiomers also shift Aβ production to shorter Aβ species, IP/MS of conditioned media from CHO cells overexpressing APP751 and PS1ML was performed after treatment with flurbiprofen or its purified enantiomers. In a similar fashion to what we have observed with sulindac sulfide, flurbiprofen and its enantiomers consistently decreased the relative peak height for Aβ42 and increased the peak height of Aβ38 (Figure 2). In some experiments, levels of shorter Aβ peptides (1–33, 1–34, 1–37) were also increased. These data suggest that flurbiprofen and its enantiomers alter Aβ production through a similar mechanism as previously studied NSAIDs.
Figure 2.
IP/MS analysis of Aβ in the media of racemic, R- and S-flurbiprofen–treated cells. Plots shown are representative of two experiments, each performed in duplicate. CHO cells overexpressing APP751 and PS1ML were pretreated overnight with ethanol (a), 250 μM racemic flurbiprofen (b), 250 μM S-flurbiprofen (c), or 250 μM R-flurbiprofen (d). Conditioned medium was collected and analyzed after an additional 24 hours of treatment with the same concentration of the drug. Aβ peptides identified by their masses are indicated above the peaks. All forms of flurbiprofen reduce the Aβ42 peak and increase the levels of shorter Aβ derivatives to approximately the same extent. This effect is best seen by comparing the peak heights of the various peptides to the peak height of Aβ1–40, which is largely unchanged.
Flurbiprofen and its enantiomers directly target γ-secretase.
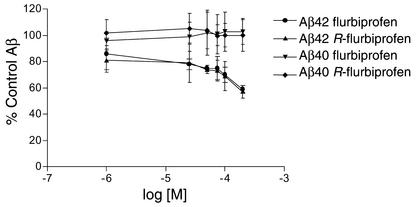
γ-Secretase refers to the enzymatic activity that carries out the final cleavage to release Aβ from APP carboxy-terminal fragments (CTFs) (1). γ-Secretase activity resides in a high-molecular-weight complex that consists of four proteins, either PS1 or PS2 coupled with nicastrin, APH-1, and PEN-2 (26, 27). Although the precise role of each member of the complex is not definitively proven, it appears that the PSs are the catalytic subunits (28). Because NSAIDs appear to shift γ-secretase cleavage independently of known cellular targets, we used a well-characterized broken cell γ-secretase assay to determine whether flurbiprofen and its enantiomers directly target γ-secretase (21, 22). This assay uses partially purified carbonate-stripped membranes that are enriched for γ-secretase activity and contain endogenous APP CTFs as substrate. Importantly, no detergents are used in this assay, since we find that solublization of γ-secretase with detergents can alter the response to various inhibitors and Aβ42 lowering NSAIDs (data not shown). Flurbiprofen and its enantiomers lower Aβ42 levels in the broken cell γ-secretase assay with a dose response similar to what was observed for cell culture without significantly affecting either Aβ40 production (Figure 3) or CTFγ production (data not shown).
Figure 3.
Flurbiprofen and R-flurbiprofen selectively lower Aβ42 in broken cell γ-secretase assays. Dose-response studies of Aβ40 and Aβ42 production in a broken cell assay shows that Aβ42 production is selectively inhibited by flurbiprofen and its enantiomers in vitro. Error bars indicate the SEM. Similar results are seen with purified S-flurbiprofen (data not shown). Data are averaged from two independent experiments with duplicate samples at each dose. Absolute control values are 1143 ± 51 pM for Aβ40 production and 91 ± 9 pM for Aβ42 production.
Multiple NSAIDs lower Aβ42 levels in the brain.
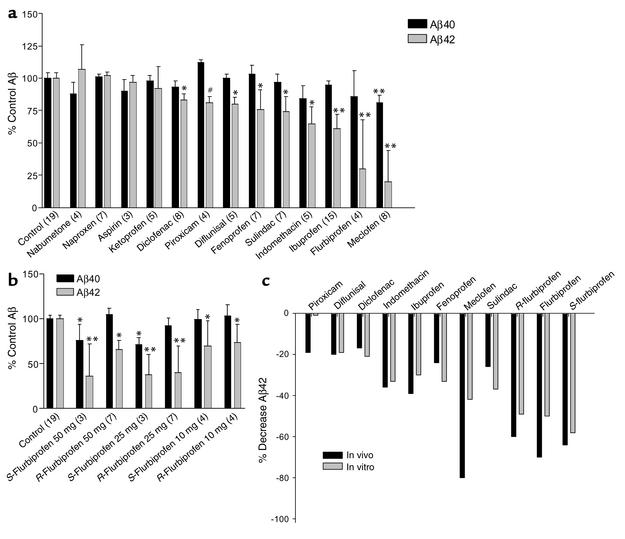
NSAIDs were orally administered to 3-month-old female Tg2576 mice for 3 days at 50 mg/kg per day except for indomethacin, which was administered at 10 mg/kg per day. The 50 mg/kg per day dose was chosen based on (a) studies showing that chronic treatment of Tg2576 mice with this dose of ibuprofen reduced plaque burden and Aβ load in the brain and (b) our previous work showing that this dose of ibuprofen significantly lowered Aβ42 levels by 39% (P < 0.001) in 3-day dosing (15, 29). Because 50 mg/kg per day of indomethacin is above its reported LD50, we used 10 mg/kg per day; this dose did not result in death or weight loss in any of the treated mice. An analysis of brain Aβ levels after the 3-day treatment shows that a number of FDA-approved NSAIDs selectively reduce Aβ42 levels (Figure 4a). In these studies, significant reductions in Aβ42 levels were noted in mice treated with flurbiprofen (70% decrease, P < 0.001), indomethacin (35% decrease, P < 0.001), fenoprofen (24% decrease, P < 0.001), meclofenamic acid (80% decrease, P < 0.001), sulindac (26% decrease, P < 0.01), diclofenac (17% decrease, P < 0.03), and diflunisal (20% decrease, P < 0.03). Small but statistically significant decreases in Aβ40 levels were noted in the S-flurbiprofen, meclofenamic acid, and indomethacin treatment groups. In addition to naproxen (that we have previously reported as having no effect on Aβ42), ketoprofen, nabumetone, and aspirin did not reduce Aβ42 levels in vivo. Piroxicam did show a trend toward lowering Aβ42 levels (20% decrease, P = 0.051).
Figure 4.
Effects of NSAIDs on Aβ42 in Tg2576 brain. The graphs illustrate the percent of control values seen in each experimental group ± SEM, and the number in parentheses indicates mice per group. Treated groups were compared with controls using ANOVA with Dunnet’s post hoc correction. (a) Survey of FDA-approved NSAIDs. Brain levels of Aβ were determined after 3 days of oral dosing and compared with controls treated with vehicle alone. All animals were treated with 50 mg/kg per day except for indomethacin, which was dosed at 10 mg/kg per day (#P = 0.051, *P < 0.03, **P < 0.01). A statistically significant 17% reduction in Aβ42 levels is seen in the diclofenac treatment group (n = 8 animals), whereas a nonsignificant trend is noted in the piroxicam treatment group (n = 4), despite the fact that the average decrease in Aβ42 levels is larger (19%). Control values for the untreated Tg2576 mice are 18.5 ± 0.7 pM/gm for Aβ40 and 7.7 ± 0.3 pM/gm for Aβ42. (b) Dose-response studies with R- and S-flurbiprofen. Brain levels of Aβ were determined after 3 days of oral dosing and compared with controls treated with vehicle alone. All of these treatments significantly lowered Aβ42 levels. Treatment with 25 and 50 mg/kg per day of S-flurbiprofen also lowered Aβ40 levels, an effect possibly attributable to toxicity; no effect was seen on Aβ40 with R-flurbiprofen treatment (*P < 0.01, **P < 0.01). (c) Comparison between Aβ42 levels in cell culture and in transgenic mice. Mean inhibition of Aβ42 production is shown in the H4 cell line (in vitro, gray bars) and in TG2576 mice (in vivo, black bars).
To further evaluate the effects of the flurbiprofen enantiomers in vivo, animals were dosed with 10, 25, or 50 mg/kg per day of purified R- or S-flurbiprofen. All treatment regimens resulted in significant reductions in Aβ42 levels (Figure 4b). At 50 mg/kg per day, S-flurbiprofen decreased Aβ42 levels by 64% (P < 0.001), and R-flurbiprofen decreased Aβ42 levels by 34% (P < 0.001). At 25 mg/kg per day, S-flurbiprofen decreased Aβ42 levels by 62% (P < 0.001), and R-flurbiprofen decreased Aβ42 levels by 60% (P < 0.001). At 10 mg/kg per day, S-flurbiprofen decreased Aβ42 by 30% (P < 0.01), and R-flurbiprofen decreased Aβ42 levels by 26% (P < 0.01). Treatment with 25 and 50 mg/kg per day of S-flurbiprofen also decreased Aβ40 levels significantly (19% decrease at 25 mg/kg per day, P < 0.01; 24% decrease at 50 mg/kg per day, P < 0.03).
As shown in Figure 4c, the ability of a given NSAID to selectively lower Aβ42 levels in H4 glioma cells was a good but not perfect predictor of the in vivo response. Nine of the eleven compounds, which selectively lowered Aβ42 levels in H4 cells, lowered Aβ42 levels in APP transgenic mice. Piroxicam, which did not alter Aβ42 levels in cell culture, selectively lowered Aβ42 levels in vivo, and diflunisal, which lowered both Aβ42 and Aβ40 levels in cultured cells, showed a small but significant selective decrease in Aβ42 levels in APP transgenic mice.
Plasma Aβ levels were assessed by Aβ ELISA in a subgroup of mice treated with various NSAIDs. In 8 out of 12 mice, flurbiprofen and its enantiomers nonselectively decreased plasma Aβ40 and Aβ42 levels by 30–50%, but there was no correlation with brain Aβ levels. Similar results were seen with ibuprofen, for which three of six animals analyzed showed decreased levels of both Aβ40 and Aβ42, but again there was no correlation with brain Aβ levels. No change in plasma Aβ levels was seen in the meclofenamic acid–treated animals, even though there was on average an 80% decrease in brain Aβ42 levels. Despite the lack of correlation between brain Aβ42 and plasma Aβ42 levels in these 3-day studies, in a limited longer-term study that we have conducted with ibuprofen (1–2 weeks), we have seen selective lowering of plasma Aβ42 levels in seven of nine animals. A modest but nonsignificant correlation (r2 = 0.27) between plasma Aβ42 levels and brain Aβ42 levels was also observed.
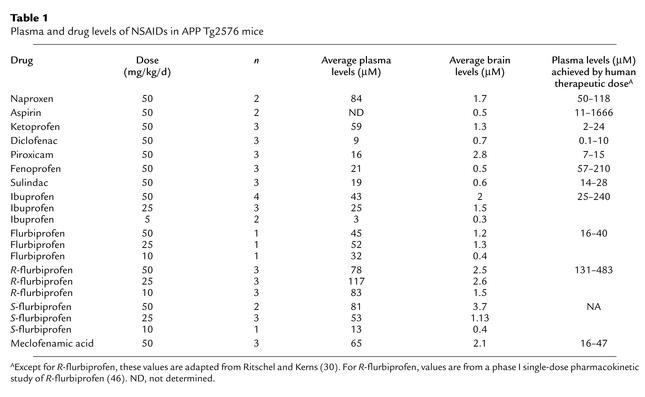
Drug levels in the brain.
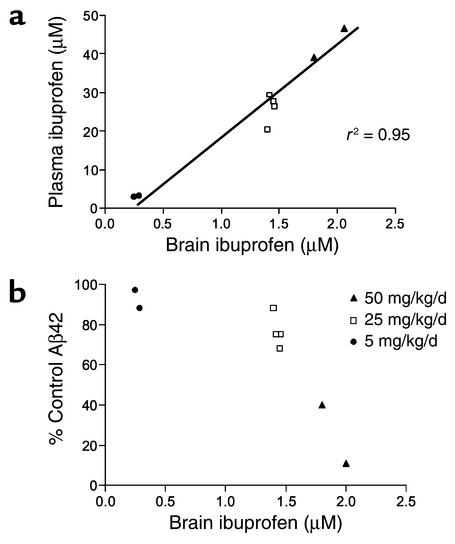
Two hours after the last drug dose, mice were sacrificed and plasma and brain samples analyzed by LC/MS/MS techniques for the level of the drug administered. The results are listed in Table 1. These data show that despite a high dose (in milligrams per kilogram per day), the plasma levels of many of these drugs are comparable to plasma levels achieved during recommended therapeutic dosing in humans (30). Plasma levels of naproxen, diclofenac, fenoprofen, sulindac, flurbiprofen, and ibuprofen are within the therapeutic range for humans. Levels of ketoprofen and meclofenamic acid are slightly above the human therapeutic range. For mice treated with purified enantiomers of flurbiprofen, both R and S enantiomers were measured. As expected from previous rodent studies, there was racemization (31). For S-flurbiprofen, 6–7% was converted to the R form, and for R-flurbiprofen, 22–30% was converted to the S form. As noted in previous pharmacokinetic studies, the brain penetration of the NSAIDs is low (32). In addition to these single-dose studies, a more extensive study of dose response to ibuprofen was conducted. In this case, ibuprofen was dosed as described at 50 mg/kg per day, 25 mg/kg per day, or 5 mg/kg per day, and the levels of brain Aβ42 and plasma and brain ibuprofen levels were determined. As shown in Figure 5a, there was an excellent correlation between plasma and brain levels of ibuprofen. There was also a strong correlation between Aβ42 lowering and the drug levels in the brain (Figure 5b) and plasma (data not shown).
Table 1.
Plasma and drug levels of NSAIDs in APP Tg2576 mice
Figure 5.
In vivo relationship between ibuprofen levels and brain Aβ42 levels. Tg2576 mice were dosed with 5, 25, or 50 mg/kg per day, and plasma and brain levels of ibuprofen were determined. (a) Ibuprofen levels in brain and plasma are highly correlated. Plasma ibuprofen concentrations ranged from 3 to 47 μM and brain levels from 0.3 to 2.1 μM. There was a strong correlation between brain and plasma levels (r2 = 0.95); as expected, there was a wide range in levels based on the dosage of ibuprofen. Approximately 6% of detectable plasma ibuprofen is found in the CNS. (b) A high degree of correlation (r2 = 0.96, three-order polynomial) is seen between ibuprofen concentrations in the CNS and Aβ42 production. Aβ42 levels were significantly decreased by either 25 or 50 mg/kg per day of ibuprofen; at 5 mg/kg per day of ibuprofen there was a small, nonsignificant decrease in Aβ42 levels.
Discussion
Despite considerable advances in the understanding of AD, no therapeutic interventions that halt or reverse the underlying disease process are available (33). On the basis of the amyloid cascade hypothesis, a number of therapeutic strategies targeting various steps in the production, deposition, or clearance of Aβ are being evaluated in preclinical or clinical studies. These treatments include (a) β- and γ-secretase inhibitors that target the proteases that produce Aβ, (b) anti-Aβ immunotherapies to promote Aβ clearance, and (c) agents that target Aβ aggregates. Like any novel therapeutic approach, the development of these treatments may be impeded by potential nontarget- and target-based toxicity. Given that such agents are likely to be administered over long-periods to elderly individuals, these agents must demonstrate excellent safety profiles. Because of these concerns, a great deal of recent attention has focused on potential treatment with agents, such as NSAIDs and statins, implicated as protective factors in epidemiologic studies, since these agents have well-characterized toxicity profiles (11, 34, 35).
Numerous epidemiologic studies have provided evidence that chronic intake of NSAIDs is associated with a decreased risk AD (11–14). Although the epidemiologic studies would suggest a protective role for NSAIDs in AD, several therapeutic trials of various nonaspirin NSAIDs have been conducted. Two small placebo-controlled pilot studies, one with indomethacin and another with diclofenac in combination with misoprostol, showed some trends toward reducing the cognitive decline in patients with AD (36, 37). However, the results in each trial were confounded by their small size and the large withdrawal rates among those receiving the NSAIDs. FDA-approved selective COX2 inhibitors, celecoxib (Celebrex) and rofecoxib (Vioxx), have also been evaluated in therapeutic AD clinical trials. Final results from these trials have not been reported, but interim reports have failed to report any efficacy. Also ongoing are treatment trials using ibuprofen, dapsone, naproxen, and celecoxib. Of note, the AD Anti-Inflammatory Prevention trial (ADAPT) is assessing the efficacy of naproxen and celecoxib in preventing or delaying the onset of AD in a large cohort. Thus, ADAPT is the only ongoing trial that will test whether certain NSAIDs may reduce the risk of developing AD.
Inhibition of the two isoforms of cyclooxygenase (COX1 and COX2) is the primary pharmacological action of NSAIDs that results in their anti-inflammatory properties (38). Some NSAIDs activate the peroxisome proliferator γ nuclear transcription factor; this activation may have anti-inflammatory consequences relevant to AD (39, 40). Although it has been proposed that NSAIDs exert their beneficial effects by reducing the inflammatory responses in the AD brain, the mechanism of action underlying the therapeutic effects of NSAIDs in AD remains uncertain. Our recent finding that ibuprofen, indomethacin, and sulindac lowered Aβ42 production independently of their effects on COX suggests that this property could contribute to their apparent protective efficacy in AD (15). To better evaluate this possibility, we analyzed 20 NSAIDs, which represent nearly all of those commonly used, for their effects on Aβ42 in cell culture. NSAIDs capable of lowering Aβ42 levels in cell culture, as well as several that failed to lower Aβ42 levels, were then tested in acute dosing studies in transgenic mice. These data show that diclofenac, diflunisal, ibuprofen, sulindac, fenoprofen, indomethacin, flurbiprofen, and meclofenamic acid reduce Aβ42 levels in the brains of mice. Other compounds, including naproxen, aspirin, nabumetone, and ketoprofen, did not lower Aβ42 levels in vivo.
Overall, there was a good degree of association between Aβ42 lowering effects in cell culture and in vivo. However, several discrepancies are apparent. These discrepancies were only seen in compounds, with modest effects, decreasing Aβ42 levels by 17–20%. To determine if compounds that minimally perturb Aβ42 levels in acute dosing paradigms can affect Aβ deposition, long-term dosing studies in APP transgenic mice, such as those already carried out on ibuprofen (29), will be needed.
A concern with our previous study was that the Aβ42 lowering effect of ibuprofen, sulindac sulfide, and indomethacin was only apparent in cultured cells at relatively high concentrations (>25–50 μM), and maximal lowering typically did not occur unless cells were treated with even higher concentrations of these drugs. It was also thought that the 50 mg/kg per day dosing level in animals would represent a dose likely to be nonphysiologic in humans. In this study, we directly assessed both the plasma levels and brain levels of several NSAIDs after 3 days of dosing. These drug levels likely represent steady-state or near steady-state levels of the compounds. These data demonstrate that, despite a high dose, the plasma levels of most NSAIDs measured did not exceed the levels that can be achieved with therapeutic doses in humans (30). On the basis of these studies, we conclude that recommended dosing regimens of certain NSAIDs in humans are likely to achieve drug levels in humans that could potentially lower Aβ42 levels.
One of the perplexing aspects of these data is that they demonstrate a discrepancy between potency in cell culture systems and potency in APP transgenic mice. This is especially true if one presumes that these agents are working against specific targets in the CNS. If this is the case, then the low micromolar levels of these drugs present in the brain appear capable of lowering Aβ42 levels to the same extent as seen with 100 μM treatment in a human cell line. Several factors could account for this large increase in apparent potency. First, if drugs accumulate in specific compartments in the brain where the target is also localized, this colocalization could account for the discrepancy in potency. Second, the target in the brain could differ in some ways from the target in cell culture. For example, it is possible that the brain form of γ-secretase is subtly distinct from the form in cultured cells. Third, it is possible that a metabolite of the NSAIDs exhibits higher potency for the target than the parent compounds. Alternatively, it is possible that the drugs do not act centrally but instead reduce peripheral Aβ42 levels, which results in enhanced efflux of Aβ42 from the brain. This type of “peripheral sink” mechanism has been postulated to account for the Aβ lowering effect of anti-Aβ immunotherapy (41, 42). To determine if such a mechanism might contribute to the Aβ42 lowering effect we have observed with certain NSAIDs, we also examined plasma Aβ levels in a subset of treated animals. There was no correlation between Aβ42 plasma levels and Aβ42 levels in the brain after these 3-day dosing studies. However, longer-term studies with ibuprofen indicate that selective lowering of plasma Aβ42 does occur with at least one NSAID and loosely correlates with brain Aβ42 levels. Additional studies will be needed to distinguish among these possibilities.
The finding that multiple commonly used NSAIDs can lower Aβ42 provides a framework in which to evaluate both previous and future epidemiologic studies and AD clinical trials with respect to anti-inflammatory versus Aβ42 lowering mechanisms. Although none of the published epidemiologic studies on NSAIDs and AD has reported effects of individual NSAIDs, the Cache County Study, the Baltimore Longitudinal Study of Aging, and the Rotterdam Study have compared aspirin or oral salicylates with nonaspirin NSAIDs (12–14). In each of these studies, nonaspirin NSAIDs, when taken for more than 2 years, showed a greater protective effect than aspirin. In the Rotterdam Study, detailed data on NSAID use was provided; but no analysis based on individual drug use was reported (12). In our study, two of the six most commonly used NSAIDs (ibuprofen and indomethacin) significantly lowered Aβ42 levels in APP transgenic mice. Two other NSAIDs, diclofenac and piroxicam, slightly reduced Aβ42 levels in vivo. Together, these four drugs accounted for 74% of NSAID use in the Rotterdam Study, whereas naproxen and ketoprofen, which had no effect on Aβ42 levels, accounted for 21% of NSAID use. Given these observations, insights into the possible mechanisms of action of NSAIDs in AD could be generated by either reevaluating existing data or collecting new data that focus on whether or not the NSAID analyzed has the potential to lower Aβ42 levels. In a similar fashion, these data may help to evaluate results from previous and ongoing clinical trials of various NSAIDs. Only one of the current trials is using an NSAID, ibuprofen, which has the potential to lower Aβ42 levels. Neither of the selective COX2 inhibitors, celecoxib and rofecoxib, appears capable of lowering Aβ42 in vitro or in vivo (T. Golde and E. Koo, unpublished data).
A drawback to the potential clinical use of conventional NSAIDs in AD, either as Aβ42 lowering agents or as anti-inflammatory medications, is the gastrointestinal and renal toxicity thought to be mediated by their inhibition of COX. Because the Aβ42 lowering effect is independent of COX, we suggested that a rational drug candidate for the treatment or prevention of AD is an Aβ42 lowering agent either lacking or having greatly reduced COX-inhibiting activity (15). In contrast to rodents, in humans the COX-inactive R-flurbiprofen is minimally converted to the S enantiomer (16, 31). This enables administration of higher doses of R-flurbiprofen with a reduced incidence of COX-mediated side effects. Here we show that R- and S-flurbiprofen are nearly equipotent in their ability to lower Aβ42 levels. Similar data in cultured cells have recently been reported by another group (43). Both enantiomers of flurbiprofen and racemic flurbiprofen also seem to function through a mechanism similar that which we previously reported. Like sulindac sulfide, which lowers Aβ42 and selectively increases Aβ38 levels, flurbiprofen and its enantiomers lower Aβ42 and increase Aβ38 levels. Thus, these findings with R-flurbiprofen are consistent with our prediction that such Aβ42 lowering compounds exist. In this study, we further show that the γ-secretase complex is the target of flurbiprofen and its enantiomers, since these compounds selectively lower Aβ42 in a broken cell γ-secretase assay. Additional studies will be needed to determine the precise mechanism through which Aβ42 lowering NSAIDs such as flurbiprofen interact with γ-secretase. Although we have not completed long-term studies of flurbiprofen and its enantiomers in APP transgenic mice, a nitrous oxide–releasing flurbiprofen derivative, NCX-2216, has been reported to be highly effective at reducing Aβ accumulation in long-term studies in an AD mouse model (44). This derivative is converted to flurbiprofen in vivo. R-flurbiprofen is also reported to inhibit NF-κB. Inhibition of NF-κB has been postulated to reduce the inflammatory response in AD (45). However, the Aβ42 lowering effect of R-flurbiprofen and its effects on NF-κB appear to be independent of each other (43). Taken together, these data suggest that R-flurbiprofen is a compound that can be used to test our hypothesis that Aβ42 levels can be safely lowered in humans with minimal effects on COX activity or the physiological functions of γ-secretase. Although there is a strong rationale for selective targeting of Aβ42, no Aβ therapeutic has been sufficiently tested in humans to determine its possible efficacy in the treatment or prevention of AD. Such evidence will not be forthcoming for a number of years. Rigorous clinical testing of R-flurbiprofen and other NSAIDs that lower Aβ42 will be necessary to determine if they have the ability to lower Aβ42 in humans and therapeutic efficacy in AD.
Acknowledgments
These studies were funded by the NIH/National Institute on Aging (P01 AG20206 to E. Koo and T. Golde and NS39072 to T. Golde). Additional resources from the Mayo Foundation were used to support the Tg2576 mouse colony that provided the mice used in these studies. J. Eriksen was supported by a John Douglas French Foundation fellowship grant. S. Sagi was supported by an NIH training grant (T 32 AG00216). No funding from industry was used for these studies. We thank W. Wechter for the purified enantiomers of flurbiprofen; W. Chen and X. Jiang, at Merck Research Laboratories San Diego, for their analysis of NSAID levels in the brain and plasma; R. Wang for assisting in the IP/MS analyses; and S. Younkin for the Tg2576 mice used in the study.
Footnotes
See the related Commentary beginning on page 321.
Conflict of interest: Kevin W. Jessing and Kenton H. Zavitz are employees of Myriad Pharmaceuticals. Following the submission and review of this manuscript, Myriad Pharmaceuticals began a phase II trial to determine the efficacy of R-flurbiprofen for use in Alzheimer disease.
Nonstandard abbreviations used: Alzheimer’s disease (AD); amyloid β protein (Aβ); amyloid precursor protein (APP); presenilin (PS); matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS); immunoprecipitation/mass spectrometry (IP/MS); tandem mass spectrometry (MS/MS); carboxy-terminal fragments (CTFs); AD Anti-Inflammatory Prevention trial (ADAPT).
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. . Biochim. Biophys. Acta. 2000; 1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 4.Younkin SG. The role of A beta 42 in Alzheimer’s disease. J. Physiol. (Paris). 1998;92:289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]
- 5.Scheuner D, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 6.Duff K, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 7.Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyss-Coray T, et al. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aisen PS. Inflammation and Alzheimer’s disease: mechanisms and therapeutic strategies. Gerontology. 1997;43:143–149. doi: 10.1159/000213842. [DOI] [PubMed] [Google Scholar]
- 11.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 12.in t’Veld BA, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 13.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 14.Zandi PP, et al. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- 15.Weggen S, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 16.Geisslinger G, Lotsch J, Menzel S, Kobal G, Brune K. Stereoselective disposition of flurbiprofen in healthy subjects following administration of the single enantiomers. Br. J. Clin. Pharmacol. 1994;37:392–394. doi: 10.1111/j.1365-2125.1994.tb04295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechter WJ, et al. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 2000;60:2203–2208. [PubMed] [Google Scholar]
- 18.Wechter WJ, et al. Treatment and survival study in the C57BL/6J-APC(Min)/+(Min) mouse with R-flurbiprofen. Life Sci. 2000;66:745–753. doi: 10.1016/s0024-3205(99)00645-1. [DOI] [PubMed] [Google Scholar]
- 19.Murphy MP, et al. Presenilin 1 regulates pharmacologically distinct gamma-secretase activities. Implications for the role of presenilin in gamma-secretase cleavage. J. Biol. Chem. 2000;275:26277–26284. doi: 10.1074/jbc.M002812200. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Sweeney D, Gandy SE, Sisodia SS. The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J. Biol. Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 21.McLendon C, et al. Cell-free assays for γ-secretase activity. FASEB J. 2000;14:2383–2386. doi: 10.1096/fj.00-0286fje. [DOI] [PubMed] [Google Scholar]
- 22.Wahrle S, et al. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao K, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 24.Kawarabayashi T, et al. Age-dependent changes in brain, CSF, and plasma amyloid protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki N, et al. An increased percentage of long amyloid β protein is secreted by familial amyloid β protein precursor (βAPP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 26.Takasugi N, et al. The role of presenilin cofactors in the γ-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 27.Edbauer D, et al. Reconstitution of gamma-secretase activity. Nat. Cell Biol. 2003;7:7. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe MS, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 29.Lim GP, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritschel, W.A., and Kerns, G.L. 1999. Handbook of basic pharmacokinetics... including clinical applications. J.I. Graubart, editor. American Pharmaceutical Association. Washington, DC, USA. 563 pp.
- 31.Wechter WJ, Bigornia AE, Murray ED, Jr, Jee WS. Chiral pharmacokinetics of Rac-flurbiprofen and pharmacodynamics of anabolic bone response in the normal rat. Chirality. 1994;6:457–459. doi: 10.1002/chir.530060602. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Halim MS, Sjoquist B, Anggard E. Inhibition of prostaglandin synthesis in rat brain. Acta Pharmacol. Toxicol. (Copenh.). 1978;43:266–272. doi: 10.1111/j.1600-0773.1978.tb02264.x. [DOI] [PubMed] [Google Scholar]
- 33.Golde TE. Alzheimer disease therapy: can the amyloid cascade be halted? J. Clin. Invest. 2003;111:11–18. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 35.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 36.Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology. 1999;53:197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 37.Rogers J, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 38.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol. Sci. 1999;20:465–469. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 39.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of β-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. J. Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heneka MT, et al. Peroxisome proliferator-activated receptor gamma agonists protect cerebellar granule cells from cytokine-induced apoptotic cell death by inhibition of inducible nitric oxide synthase. J. Neuroimmunol. 1999;100:156–168. doi: 10.1016/s0165-5728(99)00192-7. [DOI] [PubMed] [Google Scholar]
- 41.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 42.DeMattos RB, et al. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morihara T, Chu T, Ubeda O, Beech W, Cole GM. Selective inhibition of Aβ42 production by NSAID R-enantiomers. J. Neurochem. 2002;83:1009–1012. doi: 10.1046/j.1471-4159.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- 44.Jantzen PT, et al. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tegeder I, et al. Inhibition of NF-κB and AP-1 activation by R- and S-flurbiprofen. FASEB J. 2001;15:2–4. doi: 10.1096/fj.00-0130fje. [DOI] [PubMed] [Google Scholar]
- 46.Murray ED, Jr, et al. Phase I single-dose pharmacokinetics and lack of enantiomer bioinversion of E-7869 (R-flurbiprofen) Clin. Pharmacol. Ther. 2000;67:103. [Google Scholar]