Abstract
Loss of ovarian function following menopause results in a substantial increase in bone turnover and a critical imbalance between bone formation and resorption. This imbalance leads to a progressive loss of trabecular bone mass and eventually osteoporosis, in part the result of increased osteoclastogenesis. Enhanced formation of functional osteoclasts appears to be the result of increased elaboration by support cells of osteoclastogenic cytokines such as IL-1, tumor necrosis factor, and IL-6, all of which are negatively regulated by estrogens. We show here that estrogen can suppress receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)-induced differentiation of myelomonocytic precursors into multinucleated tartrate-resistant acid phosphatase-positive osteoclasts through an estrogen receptor-dependent mechanism that does not require mediation by stromal cells. This suppression is dose-dependent, isomer-specific, and reversed by ICI 182780. Furthermore, the bone-sparing analogues tamoxifen and raloxifene mimic estrogen's effects. Estrogen blocks RANKL/M-CSF-induced activator protein-1-dependent transcription, likely through direct regulation of c-Jun activity. This effect is the result of a classical nuclear activity by estrogen receptor to regulate both c-Jun expression and its phosphorylation by c-Jun N-terminal kinase. Our results suggest that estrogen modulates osteoclast formation both by down-regulating the expression of osteoclastogenic cytokines from supportive cells and by directly suppressing RANKL-induced osteoclast differentiation.
Estrogen withdrawal following menopause leads to an increase in the production of hematopoietic growth factors such as granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor (M-CSF) and the proinflammatory cytokines IL-1, IL-6, and tumor necrosis factor (TNF) from stroma, monocytes, and lymphoid cells (1–3). An elevation in these factors is believed to stimulate the differentiation of myeloid precursor cells into osteoclasts, cells that are responsible for bone resorption (4). Estrogen replacement therapy suppresses expression of these osteoclastogenic cytokines and reduces osteoclast formation (5–8). This reduction in cytokine expression and a concomitant reduction in osteoclast formation have been proposed to account for at least one component of estrogen's beneficial effects in preventing postmenopausal osteoporosis (1–3).
Studies also suggest that estrogen acts directly on both osteoclast precursors and fully differentiated osteoclasts. In the latter case, these actions regulate osteoclast bone resorbing activity and osteoclast life span (9, 10). Indeed, loss of estrogen in vivo increases the life span of the osteoclast by decreasing apoptosis and likely contributes directly to the increased bone resorption observed following menopause (10). Estrogens also negatively regulate the production of bona fide osteoclast precursors, namely granulocyte-macrophage colony-forming unit (11–13). Taken together, these observations raise the possibility that estrogen might influence osteoclast differentiation through a mechanism independent of supportive cells.
Receptor activator of NF-κB ligand (RANKL/TRANCE/ODF) is a TNF-like factor expressed by stromal cells that is capable of stimulating osteoclast differentiation (14–18). Importantly, soluble RANKL stimulates osteoclastogenesis both in vivo and in the absence of stromal cells in vitro (19). We show here that estrogens exert direct inhibitory effects on RANKL-induced osteoclast differentiation both in primary murine bone marrow cells and in the murine macrophagic cell line RAW264.7. These effects are mediated, at least in part, through a repression in the level and functional activity of c-Jun. Collectively, our results suggest that increased osteoclast formation that follows menopause results from both overexpression of osteoclastogenic cytokines and an increase in the differentiation potential of osteoclast precursors.
Materials and Methods
Cell Culture.
Bone marrow cells from the tibiae and femurs of sham and ovariectomized mice were cultured for 24 h in α-MEM with 10% FBS to isolate nonadherent cell populations. Nonadherent cells were enriched by using Ficoll density gradient centrifugation, and then cultured in phenol red-free α-MEM supplemented with 10% charcoal-stripped FBS. The murine monocytic cell line RAW264.7 was cultured in phenol red-free α-MEM supplemented with 10% charcoal-stripped FBS. Cells were incubated with murine M-CSF, soluble human RANKL, or both in the absence or presence of 17β-estradiol, 17α-estradiol, 17β-estradiol and ICI 182780, tamoxifen, or raloxifene for the times indicated. MCF-7 cells were cultured as previously described (12). Murine M-CSF was obtained from R & D Systems. A human RANKL (residues 137–316) cDNA was expressed as a glutathione S-transferase (GST) fusion protein in BL21 cells and purified on glutathione affinity columns. 17β-Estradiol and 4-hydroxytamoxifen were obtained from Sigma, and ICI 182780 was obtained from Tocris (Ballwin, MO).
Characterization and Quantitation of Osteoclast-Like Cells.
Primary bone marrow monocytes (BM) or RAW264.7 cells were cultured in 48-well dishes at a density of 1 × 105 cells/well or 2 × 103 cells/well, and treated with the indicated factors at the beginning of the culture and during a medium change on day 3. Osteoclast formation was assessed by counting the total number of multinucleated (>3 nuclei), tartrate-resistant acid phosphatase (TRAP)-positive cells present per well between day 7 and 10 (BM) or on day 5 (RAW264.7) (4, 12). Osteoclast-like cells generated from RAW264.7 cells were characterized for vitronectin receptor expression as described previously (20). The ability of RAW264.7 cell-derived osteoclasts to resorb bone was assessed by first stimulating osteoclast formation on synthetic bone discs (Millenium Biologix, Kingston, Ontario, Canada) with M-CSF and RANKL for 10 days, removing the adherent cells with bleach and examining the discs for the presence of resorption lacunae by using dark field microscopy.
Analysis of Estrogen Receptor (ER).
The presence of ERα and ERβ mRNA transcripts was assessed by using RT-PCR with primer sets corresponding to nt 1210–1228 and 1655–1672 of ERα and nt 248–271 and 581–603 of ERβ. The presence of ERα protein was assessed in RAW264.7 cells by using immunocytochemistry as previously described (12). Saturation analysis was carried out by using a previously reported intact cell assay (21).
Detection of Osteoprotegerin (OPG), RANKL, RANK, and c-Fms mRNA Transcripts.
Oligonucleotide primers corresponding to murine RANKL and OPG (22) were used to amplify DNA fragments of 749 and 635 nt, respectively, from RAW264.7 cell RNA. Oligonucleotide primers corresponding to nt 533–554 and 1186–1207 of murine RANK and to nt 2653–2677 and 3381–3405 of murine c-Fms were used to amplify fragments of 674 nt and 752 nt, respectively. Cycle-dependent appearance of PCR products was assessed via UV visualization on agarose gels.
c-Jun N-Terminal Kinase (JNK1) Kinase Assays.
The activity of JNK1 was assessed by immunoprecipitating JNK1 followed by incubation of the precipitate with GST-cJun substrate (residues 1–79) (23). Reaction products were resolved on 4–20% SDS/PAGE gradient gels, dried, and autoradiographed overnight. Gel or autoradiographic signals were quantified by using a STORM PhosphorImager or a Bio-Rad Fluor-S MultiImager.
Western Blot Analysis of JNK1, c-Jun, and Phospho-cJun.
Western blot analysis was used to assess the levels of JNK1 protein in total cell lysates using anti-JNK1 Abs (Santa Cruz Biotechnologies). c-Jun was detected in nuclear extracts by similar methods by using anti-c-Jun and anti-phospho-cJun Abs from New England Biolabs. Blots were developed by using horseradish peroxidase-conjugated second Abs and visualized by using ECL (Amersham).
Transfections.
RAW264.7 cells were plated at a density of 1 × 106/well and transfected with a total of 2 μg of DNA by using Fugene reagent. The luciferase reporter plasmids pAP1-luc and pFR-luc as well as the expression vectors pFA-cJun, pFA-ATF2, pFA-cFos, and pFC-dbd were obtained from Stratagene. pCMV-βGal was used to normalize for transfection efficiencies. Cells were harvested 22–24 h following transfection, and the activities of luciferase and β-galactosidase assessed by using standard methods. Luciferase activities were normalized by using protein or β-galactosidase levels with similar results.
Results
Estrogen Suppresses Monocytic Osteoclast Formation in the Absence of Stroma.
Murine M-CSF (10 ng/ml) and soluble human RANKL (30 ng/ml) together induced the formation of significant numbers of multinucleated (>3 nuclei), TRAP-positive osteoclast-like cells from both sham and ovariectomized (OVX) bone marrow when cultured in the absence of stromal cells (Fig. 1). These multinucleated, TRAP-positive cells, which were not inducible with M-CSF or RANKL individually or with 1,25(OH)2D3, displayed features consistent with those of bona fide osteoclasts including expression of the vitronectin receptor (αVβ3) and the capacity to resorb bone (data not shown). Interestingly, estrogen deficiency produced a 2-fold increase in the number of osteoclasts formed in vitro in response to RANKL/M-CSF. Perhaps most importantly, treatment with 17β-estradiol statistically suppressed RANKL/M-CSF-induced osteoclast formation in primary cells from both sham and OVX mice 22% and 47%, respectively. As anticipated, 17α-estradiol was inactive while the general estrogen antagonist ICI 182780 reversed the effects of 17β-estradiol (24). Although reversal was incomplete in this experiment, additional studies demonstrated complete reversal in the presence of ICI 182780. These results demonstrate that estrogen can directly suppress RANKL/M-CSF-induced osteoclast formation in the absence of stromal cell mediation. 4-Hydroxytamoxifen and raloxifene also suppressed RANKL/M-CSF-induced osteoclast formation in cells derived from estrogen-deficient animals, although the compounds were less effective in cells from normal animals. These studies suggest that tamoxifen and raloxifene, prototypic selective ER modulators that are known to mimic the bone protective effects of estrogen in vivo (8, 25–27), also suppress primary monocytic cell differentiation into osteoclasts.
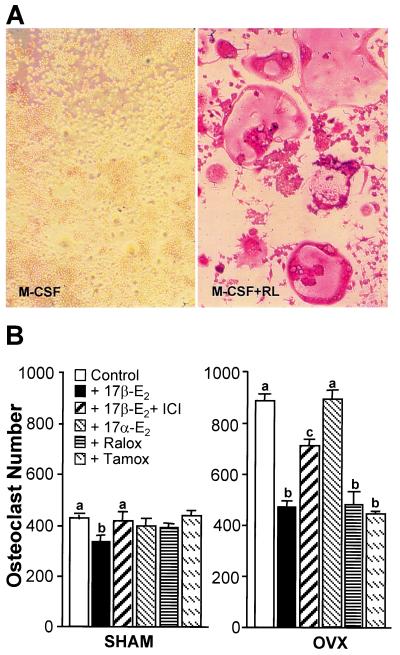
Figure 1.
Estrogens suppress M-CSF/RANKL-induced osteoclast formation in primary murine myeloid cells. Nonadherent myeloid progenitors and monocytes were isolated from sham and ovariectomized mice and plated in triplicate at 105 cells/well. (A) Murine monocytes were treated with M-CSF (10 ng/ml) or M-CSF plus RANKL (30 ng/ml) for 10 days, stained for TRAP, and photographed at ×20. (B) Cells from sham (Left) or OVX (Right) mice were treated with M-CSF/RANKL in the presence of vehicle, 17β-estradiol (10−8 M), 17β-estradiol (10−8 M) plus ICI 182780 (10−6 M), 17α-estradiol (10−8 M), 4-hydroxytamoxifen (10−7 M), or raloxifene (10−7 M). Multinucleated (>3 nuclei), TRAP-positive cells were quantitated 10 days later. Numbers represent the mean ± SE, n = 3 (a is significant vs. b and c, and b is significant vs. c at P ≤ 0.05).
Estrogen Suppresses the Formation of Osteoclasts from RAW264.7 Cells.
Monocyte/macrophagic RAW264.7 cells have been used previously as a model for osteoclast formation (17). In view of their potential utility for elucidating both essential elements of osteoclast differentiation as well as the mechanism through which estrogens might inhibit osteoclast formation, we determined whether RANKL/M-CSF-induced osteoclast formation could be inhibited by 17β-estradiol. Treatment of RAW264.7 cells with murine M-CSF (10 ng/ml) and soluble human RANKL (30 ng/ml) for 5 days led to a profound differentiation of this monocyte-macrophagic cell line into multinucleated TRAP-positive, αVβ3 integrin-positive osteoclast-like cells when compared with cells treated with M-CSF alone (Fig. 2A). These cells also formed extensive resorption lacunae when plated on synthetic bone discs, confirming the functionality of these osteoclasts (Fig. 2A). Thus, we treated RAW264.7 cells with RANKL and M-CSF in the absence or presence of 17β-estradiol, 17α-estradiol, or several additional steroidal analogues and quantitated the number of multinucleated, TRAP-positive cells present on day 5. RANKL/M-CSF-induced osteoclast formation was suppressed ≈40% by 17β-estradiol (Fig. 2B). This response was isomer-specific (17α-estradiol was inactive) (Fig. 2B, Left) and dose-dependent (Fig. 2B, Right). ICI 182780 at 10−6 M was ineffective in blocking osteoclast formation on its own (data not shown), but completely reversed the effects of 17β-estradiol (Fig. 2B) (24). In contrast, both 4-hydroxytamoxifen (10−7 M) and raloxifene (10−7 M) mimicked the effect of 17β-estradiol in reducing osteoclast formation. This estrogen-like inhibitory activity is similar to that observed in primary cells (Fig. 1) (12) and is consistent with the bone protective actions of both tamoxifen and raloxifene in vivo (25–27).
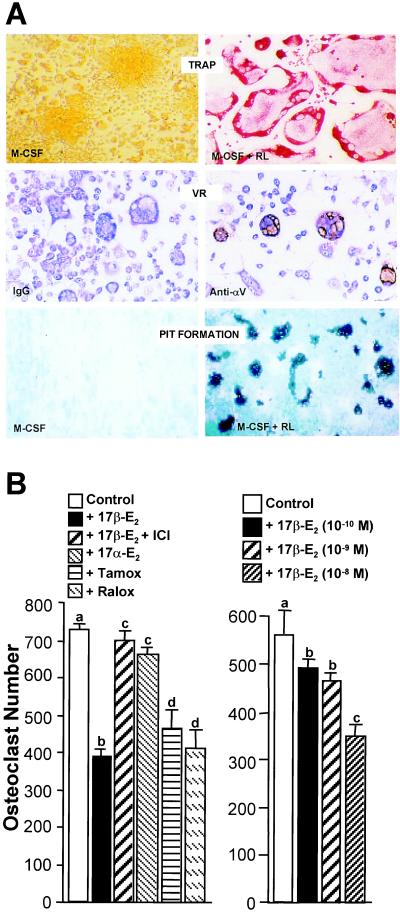
Figure 2.
Estrogens suppress M-CSF/RANKL-induced osteoclast formation in murine RAW264.7 cells. (A) Properties of osteoclast-like cells generated from RAW264.7 cells. Cells were induced with M-CSF or M-CSF/RANKL for 5 days and then TRAP-stained and photographed at ×20. Cells were incubated with either control IgG or Ab to vitronectin receptor (VR) αV subunit, stained with immunoperoxidase and methylene blue, and then photographed at ×10. Cells were plated on synthetic bone discs and induced with either M-CSF or M-CSF/RANKL for 10 days. Cells were removed and resorption lacunae visualized and photographed under dark-field microscopy at ×10. (B) Cells (2 × 103/well) were plated in triplicate and multinucleated (>3 nuclei), TRAP-positive osteoclasts quantitated following induction for 5 days with M-CSF/RANKL in the presence of vehicle, 17β-estradiol (10−8 M), 17β-estradiol (10−8 M) plus ICI 182780 (10−6 M), 17α-estradiol (10−8 M), 4-hydroxytamoxifen (10−7 M) or raloxifene (10−7 M) (Left) or increasing concentrations of 17β-estradiol as indicated (Right). Numbers represent the mean ± SE, n = 3 (a is significant vs. b and d; c is significant vs. d at P ≤ 0.05).
RAW264.7 Cells Express ERα.
The capacity of estrogen to suppress RANKL/M-CSF-induced osteoclastogenesis at nanomolar concentrations suggests mediation by ERs (ERα or ERβ) (28). Our own experiments suggest that primary bone marrow cells express ERα (data not shown). RT-PCR analysis of RAW264.7 cell RNA revealed the presence of both ERα and ERβ transcripts (Fig. 3A). Immunocytochemical staining selective for ERα revealed immunofluorescence localized to RAW264.7 cell nuclei and is reminiscent of that observed in MCF-7 cells (Fig. 3B). Although ERβ immunofluorescence was not detected, it is possible that the level of expression of this protein is below our limits of sensitivity. We estimated ≈500 molecules of ER/RAW264.7 cell nucleus which saturated at 1.5 × 10−9 M 17β-estradiol by using an intact whole cell ligand binding assay (data not shown). These studies suggest that the action of estrogen to block differentiation in both primary monocytes as well as in RAW264.7 cells is mediated by the ER, although the exact contribution of each isoform remains to be determined.
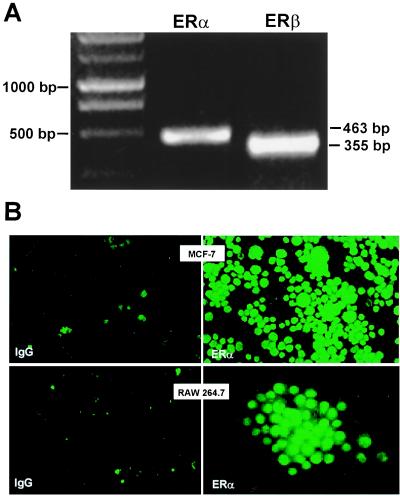
Figure 3.
Detection of ER in RAW264.7 cells. (A) Expression of ERα and ERβ mRNA transcripts using RT-PCR analysis. (B) Immunocytochemical detection of ERα in human MCF-7 breast cancer cells and in RAW264.7 cells. Cells were fixed and probed with either nonspecific IgG or anti-ERα Ab as indicated. MCF-7 and RAW264.7 cells were photographed at ×20 and ×40, respectively.
Estrogens Down-Regulate c-Jun Activity.
Osteoclast differentiation involves a sequence of events that includes differentiation, migration, fusion, activation, and survival (4, 10, 19). Despite this, a single dose of estrogen at the beginning of culture was as effective as multiple doses in inhibiting RANKL/M-CSF-induced osteoclast formation (data not shown). This finding suggests an early action of the hormone to block RANKL signaling. RANKL targets two key transcription factors, c-Jun and NF-κB, via activation of JNK1 and Iκβ, respectively (14, 16–18). We focused on c-Jun in view of its central role in both cellular proliferation and differentiation (29). Stimulation of RAW264.7 cells with RANKL led to a rapid and dose-dependent increase in JNK1 activity (Fig. 4A) and a time-dependent increase in both the phosphorylation state and levels of expression of c-Jun (Fig. 4B). These inductions were unaffected by the addition of M-CSF (data not shown). Importantly, c-Jun activation was associated with an increase in RAW264.7 cell transcription, as measured by a 4-fold enhancement of luciferase reporter gene activity under AP-1 control (Fig. 4C). AP-1 binding sites can be found in a variety of osteoclast-specific genes, including TRAP (30). Importantly, RANKL-induced transcriptional activation via AP-1 was significantly suppressed with 17β-estradiol. These results suggest both a relationship between RANKL-induced osteoclast formation and activation of AP-1-mediated gene expression, as well as a correlation between the estrogen's ability to suppress osteoclast formation and its ability to inhibit AP-1-mediated transcription.
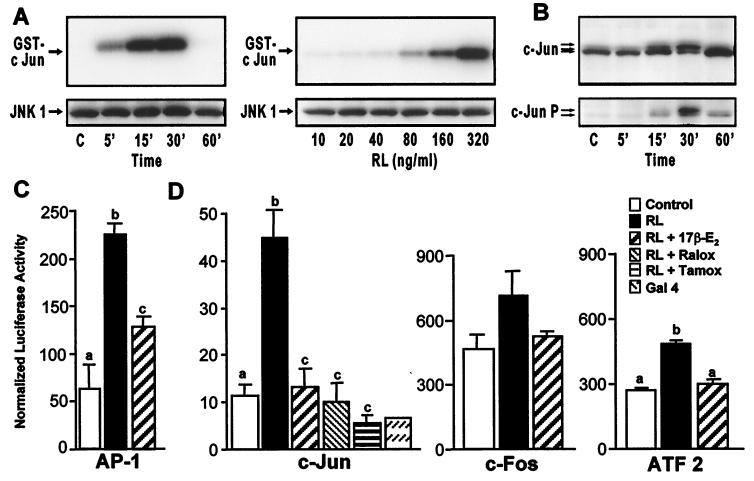
Figure 4.
Estrogen suppresses RANKL-induced activation of c-Jun and AP-1-mediated transcription in RAW264.7 cells. (A) Cells were treated for the indicated times with RANKL (80 ng/ml) or with the indicated concentrations of RANKL (RL) for 15 min and lysates examined for both JNK1 protein by Western blot and kinase activity (using GST-cJun as substrate). (B) Cells were treated for the indicated times with RANKL (80 ng/ml) and then subjected to Western blot analysis by using antibodies to c-Jun or phospho-c-Jun. Arrows indicate c-Jun and phospho-c-Jun forms. (C) Cells were transfected with a luciferase reporter plasmid (p36) containing three copies of an AP-1 response element. Cells were treated with vehicle or M-CSF and RANKL in the absence or presence of 17β-estradiol (10−8 M). Cells were harvested 24 h later and lysates assessed for luciferase and β-galactosidase activities and protein. Numbers represent the mean ± SE, n = 3 (a is significant vs. b and c; and b is significant vs. c at P ≤ 0.05). The results are representative of three independent experiments. (D) RAW264.7 cells were cotransfected with pFC2-luc and one of the following plasmids: pFA2-cJun (c-Jun), pFA-cFos (c-Fos), pFA-ATF2 (ATF2), or pFC-dbd (Gal4). Following transfection, cells were treated with RANKL in the presence of vehicle, 17β-estradiol (10−8 M), 4-hydroxytamoxifen (10−7 M), or raloxifene (10−7 M). Cells were harvested 24 h later and lysates assessed for luciferase activity, β-galactosidase activity, and protein. Numbers represent the mean ± SE, n = 3 (b is significant vs a and c at P ≤ 0.05). The results are representative of at least three independent experiments.
To explore RANKL-induced c-Jun activation and to assess related effects on the potential c-Jun partners c-Fos and ATF2 (31, 32), we examined the ability of RANKL to stimulate the transcriptional activity of transfected chimeric genes composed of the DNA binding domain of Gal4 (pFC2-dbd) fused to the activation domains of c-Jun, c-Fos, or ATF2. RANKL increased the transcriptional activity of pFA-cJun ≈4-fold (Fig. 4D). Surprisingly, although RANKL significantly increased c-Jun activity, it only weakly stimulated the activities of pFA-cFos and pFA-ATF2 (Fig. 4D). These results suggest that c-Jun is a clear activation target for RANKL, whereas c-Fos and ATF2 may not be as highly regulated. Interestingly, 17β-estradiol almost completely blocked induction of each of the transcription factor chimeras by RANKL (Fig. 4D). This finding, together with the observation that tamoxifen and raloxifene also blocked c-Jun activity (Fig. 4D), strengthens the relationship between estrogen's ability to repress transcription and its ability to suppress osteoclast differentiation. The precise role of c-Jun and the gene targets of its action during osteoclast differentiation remain to be elucidated.
Estrogen Suppresses JNK1 Activity.
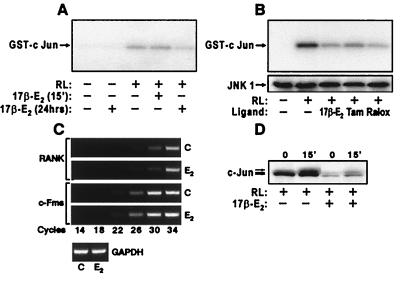
From a mechanistic perspective, several nonexclusive possibilities exist: (i) estrogen intervenes at the transcriptional level (33) to suppress RANKL-induced JNK1 activation, possibly by decreasing RANK or c-Fms expression, (ii) estrogen inhibits c-Jun gene expression, and (iii) estrogen alters the transcriptional capacity of c-Jun through a transrepression mechanism involving direct or indirect interaction between ER and c-Jun (or its heterodimer partners, e.g., c-Fos) (32). We tested the first possibility by treating RAW264.7 cells for either 24 h or 15 min with 17β-estradiol (10−8 M), stimulating the cells with RANKL, and then measuring the activity of JNK1. RANKL treatment for 5 min stimulated JNK1 activity over 12-fold (Fig. 5A), an increase that was unchanged following treatment with 17β-estradiol for 15 min. A 24-h pretreatment with the hormone, however, reduced JNK1 activity by 30% (Fig. 5A). 4-Hydroxytamoxifen and raloxifene were also effective suppressors, reducing RANKL-induced JNK1 activity by 45% and 62%, respectively, relative to 52% with 17β-estradiol (Fig. 5B). These findings appear to rule out direct protein–protein interactions and suggest that estrogen acts to block c-Jun activation.
Figure 5.
Estrogen suppresses JNK1 activity but does not alter RANK and c-Fms expression in RAW 264.7 cells. (A) Cells were pretreated with vehicle or 17β-estradiol (10−8 M) (15 min or 24 h) and then stimulated for 5 min with RANKL (80 ng/ml). Cell lysates were examined for JNK1 activity. RANKL-induced JNK1 activity was reduced 0 and 30% following a 15-min and 24-h treatment with estrogen, respectively. (B) Cells were treated for 24 h with vehicle, 17β-estradiol (10−8 M), 4-hydroxytamoxifen (10−7 M), or raloxifene (10−7 M) and then stimulated for 5 min with RANKL (80 ng/ml). Extracts were examined for JNK1 activity and by Western blot analysis. (C) RNA was isolated from cells treated for 24 h with either vehicle or 17β-estradiol (10−8 M) and subjected to RT-PCR by using oligonucleotide primer pairs for murine RANK, c-Fms, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The cycle-dependent appearance of specific DNA products was assessed by agarose gel electrophoresis. d, Cells were treated for 24 h with either vehicle or 17β-estradiol (10−8 M), and stimulated for 15 min with RANKL and nuclear extracts subjected to Western blot analysis by using Abs to c-Jun.
Estrogen Does Not Alter Expression of RANK or c-Fms but Down-Regulates c-Jun.
Upstream gene targets for down-regulation of RANKL/M-CSF signaling by estrogen include the growth factors' receptors RANK and c-Fms. Based on a transcriptional mechanism of estrogen action, we evaluated levels of RANK and c-Fms transcripts in RAW264.7 cells by using an RT-PCR approach. Neither RANK nor c-Fms mRNA levels were suppressed after a 24-h treatment with 17β-estradiol (Fig. 5C). This observation was supported by Western blot analysis of RANK protein (data not shown). A 24-h pretreatment with estrogen did, however, lead to a dramatic decrease (70%) in total c-Jun (Fig. 5D). These data indicate that estrogen inhibits RANKL-induced osteoclast differentiation through a partial blockade of c-Jun expression as well as repression of a select, but apparently nonreceptor, component(s) integral to JNK activation and downstream signaling.
Discussion
Our investigation has revealed that osteoclast precursors are direct targets of estrogen's action. Thus, 17β-estradiol and other bone protective compounds such as tamoxifen and raloxifene (25–27) oppose the differentiating effects of M-CSF and RANKL and reduce the formation of osteoclast-like cells from undifferentiated precursors. Although estrogen inhibits the activity of mature osteoclasts (9) and shortens their life span via apoptosis (10), our observation that a single dose of estrogen reduces osteoclast formation suggests an early action to limit the differentiation of precursors into mononuclear osteoclasts. These actions are consistent with earlier studies which suggest that estrogen also functions to retard myeloid cell lineage progression, thereby negatively influencing the pool of myeloid progenitor cells such as granulocyte-macrophage colony-forming unit with the capacity to differentiate into osteoclasts (11, 12, 34). These observations clearly demonstrate that estrogen can oppose RANKL/M-CSF-induced osteoclast formation in both monocytes and RAW264.7 cells, and does so in the absence of supportive cells.
The discovery of RANKL, an essential inducer of osteoclastogenesis that is expressed on the surface of stroma and other regulatory cells (14–19), made these studies possible. The capacity of soluble RANKL to induce differentiation in the absence of supportive cells enabled us to test whether estrogens could act directly on osteoclast precursors. Our finding that estrogen suppresses cellular responsiveness to osteoclastogenic factors such as RANKL and M-CSF clearly demonstrates a function separate from its actions in regulating the expression or activity of signaling molecules such as granulocyte-macrophage-CSF, M-CSF, and RANKL or IL-1, IL-6, and TNF from support cells (1–3). These direct effects of estrogen on osteoclast precursors do not, however, obviate the importance of the sex steroid's indirect effects via stromal cells to modulate osteoclastogenesis (1, 2, 5–7). Future studies will be necessary to assess the relative contribution of both mechanisms to estrogen deficiency bone loss.
In the present studies, estrogen appears to down-regulate RANKL-induced c-Jun gene expression and activation, and to inhibit AP-1 (c-Jun/c-Fos)-mediated gene transcription. Although these effects appear to be transient, it is likely that they precipitate downstream events essential to the differentiation and formation of functional osteoclasts. c-Jun expression and activation are indeed essential for osteoclast differentiation both in vivo and in vitro (35, 36). Additional RANKL-activated transcription factors may also play a role. For example, NF-κB is activated by RANKL both in monocytes (14, 16–18) and in RAW264.7 cells (our preliminary data) and is required in vivo for osteoclast formation (37). Interestingly, ER directly inhibits NF-κB actions on the IL-6 promoter (38). What is the mechanism through which estrogen suppresses osteoclast differentiation? Dramatic suppression of c-Jun expression likely plays a dominant role early in the process that eventually leads to differentiation. In addition, estrogen-mediated down-regulation of the activity of JNK1, a key player in RANKL-induced osteoclast formation in vivo, is probably also important. The lack of change in JNK1 protein levels, however, suggests that a more upstream signaling component(s) is the target. Given the time required for estrogen's actions, this component is likely to be transcriptionally regulated. Our experiments seem to rule out the most obvious candidate, RANK itself. This finding may be consistent with the general ability of estrogen to down-regulate monocytic response to a variety of stimulators including IL-1 (39) and lipopolysaccharide (40). Whatever the target, a similar regulatory action would be anticipated of 4-hydroxytamoxifen and raloxifene, despite the fact that they induce antagonist conformations in the C terminus of ER (41, 42).
In summary, we have shown that 17β-estradiol suppresses RANKL and M-CSF-induced osteoclast formation from both primary bone marrow monocytes and RAW264.7 cells. Suppression involves down-regulation of signaling components within the RANKL transduction pathway that are essential for c-Jun expression and activation, although neither RANK nor c-Fms appear to be specific targets. Future studies will focus on defining elements within the RANKL signaling pathway that are directly regulated by the estrogenic hormone.
Acknowledgments
We thank Glenn Doerman for help in preparing the figures. This work was supported by a grant from the National Institutes of Health (DK-56059).
Abbreviations
- M-CSF
macrophage colony stimulating factor
- RANK
receptor activator of NF-κB
- RANKL
RANK ligand
- ER
estrogen receptor
- OPG
osteoprotegerin
- JNK1
c-Jun N-terminal kinase
- TRAP
tartrate resistant acid phosphatase
- OVX
ovariectomy
- AP-1
activator protein 1
- ATF2
activating transcription factor 2
- TNF
tumor necrosis factor
- GST
glutathione S-transferase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130200197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130200197
References
- 1.Manolagas S C, Jilka R L. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici R. J Bone Miner Res. 1996;8:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 3.Turner R T, Riggs B L, Spelsberg T C. Endocr Rev. 1994;15:275–300. doi: 10.1210/edrv-15-3-275. [DOI] [PubMed] [Google Scholar]
- 4.Roodman G D. Exp Hematol. 1999;27:1229–1241. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 5.Jilka R L, Hangoc G, Girosole G, Passeri G, Williams D C, Abrams J S, Boyce B, Broxmeyer H, Manolagas S C. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 6.Kimble R B, Vannice J L, Bloedow D C, Thompson R C, Hopfer W, Kung V, Brownfield C, Pacifici R. J Clin Invest. 1994;93:1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimble R B, Bain S D, Pacifici R. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 8.Cosman F, Linsay R. Endocr Rev. 1999;20:418–434. doi: 10.1210/edrv.20.3.0371. [DOI] [PubMed] [Google Scholar]
- 9.Oursler M J, Pederson L, Pyfferoen J, Osdoby P, Fitzpatrick L, Spelsberg T C. Endocrinology. 1993;132:1373–1380. doi: 10.1210/endo.132.3.8440193. [DOI] [PubMed] [Google Scholar]
- 10.Hughes D E, Dai A, Tiffee J C, Mundy G R, Boyce B F. Nat Med. 1996;2:1132–1134. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 11.Girasole G, Jilka R L, Passeri G, Boswell S, Boder G, Williams D C, Manolagas S C. J Clin Invest. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shevde N K, Pike J W. Blood. 1996;87:2683–2692. [PubMed] [Google Scholar]
- 13.Erben R G, Raith S, Eberle J, Stangassinger M. Am J Physiol. 1998;274:E476–E483. doi: 10.1152/ajpendo.1998.274.3.e476. [DOI] [PubMed] [Google Scholar]
- 14.Wong B R, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett III F S, Frankel W N, et al. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki H-S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacey D L, Timms E, Tan H-L, Kelley M J, Dunstan C R, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 17.Hsu H, Lacey D L, Dunstan C R, Solovyev I, Colombero A, Timms E, Tan H-L, Elliott G, Kelley M J, Sarosi I, et al. Proc Natl Acad Sci USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa T, Nishihara T, Takahasho N, Suda T. J Immunol. 1999;163:434–442. [PubMed] [Google Scholar]
- 19.Suda T, Takahasho N, Udagawa T, Jimi E, Gillespie M T, Martin T J. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 20.Martin T J, Udagawa N. Trends Endocrinol Metab. 1998;9:6–12. doi: 10.1016/s1043-2760(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 21.Nagel S, vom Saal F S, Welsons W V. Proc Soc Exp Biol Med. 1998;217:300–309. doi: 10.3181/00379727-217-44236. [DOI] [PubMed] [Google Scholar]
- 22.Horwood N J, Elliott J, Martin T J, Gillespie M T. Endocrinology. 1998;139:4743–4746. doi: 10.1210/endo.139.11.6433. [DOI] [PubMed] [Google Scholar]
- 23.Darnay B G, Haridas V, Ni J, Moore P A, Aggarwal B B. J Biol Chem. 1998;273:20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 24.Wakeling A E, Bowler J J. Endocrinology. 1987;112:R7–R10. doi: 10.1677/joe.0.112r007. [DOI] [PubMed] [Google Scholar]
- 25.Love R R, Bardon H S, Mazess R B, Epstein S, Chapell R J. Arch Intern Med. 1994;154:2585–2588. [PubMed] [Google Scholar]
- 26.Black L J, Sato M, Rowley E R, Magee D E, Bekele A, Williams D C, Gullinam G J, Bendele R, Kauffman R R, Bensch W R, et al. J Clin Invest. 1994;93:63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans G L, Turner R T. Bone. 1995;17:181S–190S. doi: 10.1016/8756-3282(95)00291-k. [DOI] [PubMed] [Google Scholar]
- 28.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 29.Angel P, Karin M. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 30.Reddy S V, Kuzhandaivelu N, Acosta L G, Roodman G D. Bone. 1995;16:587–593. doi: 10.1016/8756-3282(95)00086-s. [DOI] [PubMed] [Google Scholar]
- 31.Foletta V C, Segal D H, Cohen D R. J Leukocyte Biol. 1998;63:139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- 32.Minden A, Karin M. Biochim Biophys Acta. 1997;1333:F85–104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 33.Katzenellenbogen J A, O'Malley B W, Katzenellenbogen B S. Mol Endocrinol. 1996;10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- 34.Zecchi-Orlandini S, Formigli L, Tani A, Benvenuti S, Fiorelli G, Papucci L, Capaccioli S, Orlandini G E, Brandi M L. Biochem Biophys Res Commun. 1999;255:680–685. doi: 10.1006/bbrc.1999.0215. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo K, Bakiri L, Yaniv M, Wagner E F. J Bone Miner Res. 1999;14(Sup. 1):S177. (Abstr.). [Google Scholar]
- 36.David J-P, Sabapathy K, Behrens A, Berger B, Hochedinger K, Hoffmann O, Wagner E F. J Bone Miner Res. 1999;14(Sup. 1):S148. (Abstr.). [Google Scholar]
- 37.Franzoso G, Carlson L, Xing L, Poljak L, Shores E W, Brown K D, Leonardi A, Tran T, Boyce B F, Siebenblist U. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein B, Yang M X. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava S, Weitzmann M N, Cenci S, Ross F P, Adlar S, Pacifici R. J Clin Invest. 1999;104:503–513. doi: 10.1172/JCI7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deshpande R, Khalili H, Pergolizzi R G, Michael S D, Chang M-D. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 41.Brzozowski A, Pike A, Dauter Z, Hubbard R, Bonn T, Engstrom O, Ohman L, Greene G, Gustafsson J, Carlquist M. Nature (London) 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 42.Shiau A K, Barstad D, Loria P M, Cheng L, Kushner P J, Agard D A, Greene G L. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]