Abstract
TP73, despite significant homology to TP53, is not a classic tumor suppressor gene, since it exhibits upregulation of nonmutated products in human tumors and lacks a tumor phenotype in p73-deficient mice. We recently reported that an N-terminally truncated isoform, ΔNp73, is upregulated in breast and gynecological cancers. We further showed that ΔNp73 is a potent transdominant inhibitor of wild-type p53 and TAp73 in cultured human tumor cells by efficiently counteracting their target gene transactivations, apoptosis, and growth suppression functions (A. I. Zaika et al., J. Exp. Med. 6:765-780, 2002). Although these data strongly suggest oncogenic properties of ΔNp73, this can only be directly shown in primary cells. We report here that ΔNp73 confers resistance to spontaneous replicative senescence of primary mouse embryo fibroblasts (MEFs) and immortalizes MEFs at a 1,000-fold-higher frequency than occurs spontaneously. ΔNp73 cooperates with cMyc and E1A in promoting primary cell proliferation and colony formation and compromises p53-dependent MEF apoptosis. Importantly, ΔNp73 rescues Ras-induced senescence. Moreover, ΔNp73 cooperates with oncogenic Ras in transforming primary fibroblasts in vitro and in inducing MEF-derived fibrosarcomas in vivo in nude mice. Wild-type p53 is likely a major target of ΔNp73 inhibition in primary fibroblasts since deletion of p53 or its requisite upstream activator ARF abrogates the growth-promoting effect of ΔNp73. Taken together, ΔNp73 behaves as an oncogene that targets p53 that might explain why ΔNp73 upregulation may be selected for during tumorigenesis of human cancers.
TP53 is the prototype tumor suppressor gene in human cancers due to its potent proapoptotic and antiproliferative function in response to cellular stress. The strongest support of this paradigm derives from the fact that the p53 pathway is inactivated either intragenically or extragenically in more than half of all human tumors. The TP73 gene product TAp73 has significant structural and functional homology to the p53 tumor suppressor (6, 11, 16, 17, 23, 44, 47). However, genetic data from human tumors and TP73-deficient mice argue against a classical Knudson-type tumor suppressor role for the TP73 gene in most tumor types. TP73-deficient mice lack a spontaneous tumor phenotype (42), and inactivating mutations in human tumors are extremely rare (more that 900 tumors covering a broad tissue spectrum have been analyzed to date [for a review, see reference 25]). Moreover, although normal human tissues express very low levels of p73, multiple primary tumor types and tumor cell lines overexpress wild-type p73, including cancers of the breast, lung, esophagus, stomach, colon, bladder, ovary, liver, bile ducts, ependymal lining, myelogenous leukemia, and neuroblastoma (25). Of note, most studies to date identifying p73 overexpression in primary human tumors have examined the total levels of p73; only a few studies have specifically measured TAp73 (21, 45).
Importantly, we and others recently reported that the human TP73 gene generates various N-terminally truncated isoform products collectively called ΔTAp73 that lack the transactivation domain (10, 12, 26, 37, 47). The most prominent truncated protein is ΔNp73, which derives either from the TA promoter via alternate splicing of exon 3 to exon 3′ or from an alternative promoter in intron 3. ΔNp73 acts as a potent transdominant inihibitor of wild-type p53 and TAp73 (10, 12, 26, 37, 47). In wild-type p53 harboring human tumor cell lines, ΔNp73 is an efficient transdominant inhibitor of transcriptional activation, apoptosis, and growth suppression and confers drug resistance mediated by wild-type p53 and TAp73 (10, 12, 26, 37, 47). Conversely, downregulation of endogenous ΔNp73 levels by antisense methods alleviates its suppressive action and enhances p53 and TAp73-mediated apoptosis in tumor cells (45). In the mouse, ΔNp73 plays an essential antiapoptotic role during developmental p53-driven death of sympathetic neurons in vivo by acting as a dominant-negative inhibitor of p53 (30). Moreover, in the mouse central nervous system, cortical neurons express the ΔNp73 protein. ΔNp73 isoforms are survival proteins in cortical neurons because their deletion causes a gradual loss of cortical neurons during the months after birth (31). Of particular interest, in a variety of human cancers, but not in normal tissues, ΔNp73 is frequently overexpressed. This includes cancers of the breast, ovary, endometrium, cervix, vagina, vulva (45), neuroblastoma (3, 7), and liver (37). Also, when measured side by side in matched female cancers, 61% of tumors exhibited preferential upregulation of ΔNp73 compared to TAp73 (in one case 2,700-fold) and 45% of tumors exhibited exclusive upregulation of ΔNp73 with no upregulation of TAp73 (45). Of note, expression of ΔNp73 is an independent molecular marker for adverse survival outcome in children with neuroblastoma (3). However, although all of these data strongly suggest an oncogenic function of ΔNp73 when deregulated, formal experimental proof had been missing. We address here this topic and directly test the putative oncogenic ability of ΔNp73 in primary cells. We found that ΔNp73 has immortalizing properties and fulfills the classic criteria for a cooperating oncogene. Taken together, our data suggest that deregulated expression of ΔNp73 bestows oncogenic activity upon the TP73 gene by functionally inactivating the suppressor actions of p53. This trait may be selected for in human cancers.
MATERIALS AND METHODS
Retroviral constructs.
Two different replication-defective retroviral expression vectors were used. The retroviral parent vector murine stem cell virus-internal ribosome entry site-green fluorescent protein (MSCV/IRES/GFP) coexpresses GFP from an IRES (gifts from I. Lemischka, Princeton University and S. Lowe, Cold Spring Harbor Laboratory). Retroviral parent vector Rpuro is derived from REBNA and contains a puromycin resistance cassette driven by a minimal cytomegalovirus promoter (29). The following cDNAs were cloned into these vectors: ΔNp73α and ΔNp73 L322Pα (a tetramerization-incompetent but DNA-binding competent mutant of ΔNp73α, corresponding to L371P in TAp73α) (45), the dominant-negative human p53 R175H mutant; mouse cMyc (a gift from M. Cole, Princeton University); E1A12S (a gift from S. Lowe); and human H-RasV12, K-RasV12, and N-RasD12 mutants (gifts from Dafna Bar-Sagi, Stony Brook University). Retroviral stocks were produced as previously described (29) by using PhoenixE packaging cells. For viral infections, 105 MEFs plated onto a 6-cm dish were incubated overnight with 3 ml of the corresponding retroviral stock. Multiple infections were performed sequentially, with a 12- to 24-h interval between each infection. The typical efficiency of infection of MEFs for the various retroviral constructs ranged from 75 to 85% as determined by their coexpressed GFP marker in fluorescence-activated cell sorting (FACS) analysis. Where required, infected fibroblasts were selected for 2 days in medium containing 2 μg of puromycin/ml. Cells were analyzed for the corresponding protein levels by immunoblots.
Cells and tissue culture.
MEFs were prepared from day 14.5 embryos of 129/Sv (129) or mixed 129/Sv × C57BL/6 (129×B6) genetic backgrounds. At least three different pools of embryos of each genotype were analyzed, sometimes after they were pooled. p53+/− and p53−/− MEFs were derived from the Trp53tm1Tyj mutant strain (14). ARF−/− MEFs and p73−/− MEFs were kindly provided by Charles J. Sherr (St. Jude Children's Research Hospital, Memphis, Tenn.) and Tyler Jacks and Elsa Flores (Massachusetts Institute of Technology, Boston), respectively. All cells were maintained in DME medium supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Gibco-BRL).
Short-term fibroblast proliferation was measured by plating equal cell numbers into six-well dishes in duplicate, followed by daily counting of trypsinized cells with a Coulter counter. Long-term proliferation assays were performed by plating 5 × 104 cells into 6-cm dishes in triplicate and counting them every 3 days with a Coulter counter. Each growth curve represents at least two independent experiments. Alternatively, cell proliferation was assessed by [3H]thymidine incorporation into DNA as described previously (13). Briefly, 2 × 104 cells per well were plated into 96-well plates in Dulbecco modified Eagle medium containing 10% FBS and 0.4 μCi of [3H]thymidine (NEN)/well. After 30 min of incubation, the cells were harvested on Unifilter-96 GF/C filter plates (Whatman) and radioactive incorporation was determined by β-scintillation counting (Pharmacia). For colony formation assays, 104 MEFs were seeded into a 6-cm dish and infected with the indicated retroviruses. Cells were selected with puromycin for 4 days, and 4 weeks later plates were fixed and stained with Giemsa. The two-sample Student t test was used to determine statistical significance.
Transformation assays.
For focus formation assays, 3 × 103 virus-infected fibroblasts were mixed with 105 uninfected MEFs and plated onto 6-cm dishes in triplicate. Cells were maintained in Dulbecco modified Eagle medium supplemented with 5% FBS and antibiotic-antimycotic (Gibco-BRL). Growth medium was changed every 3 days. After 14 to 16 days, transformation efficiency was evaluated by counting individual foci. All transformation assays were repeated at least three times. Representative plates were stained with Giemsa and photographed. Apoptosis assays were performed as previously described (45).
Tumorigenicity assays.
All animal maintenance and experimentation was performed according to National Institutes of Health (NIH)-approved institutional animal care guidelines. Nude athymic mice were injected subcutaneously into the back (two sites/mouse) with 5 × 105 retrovirally transduced MEFs expressing the indicated cDNAs. Tumor growth was monitored every 3 days by palpation at the sites of injection. Animals were allowed to form tumors of up to 1.5 cm, at which point they were sacrificed. Each tumor was dissected, weighed, fixed in formalin, and processed for histopathologic examination of hematoxylin-eosin-stained sections or immunohistochemistry to verify ΔNp73 expression. The longest observation time was 13 weeks.
Protein expression analysis.
Whole-cell lysates (50 μg of protein) were separated in sodium dodecyl sulfate-10% acrylamide gels, blotted onto a Protran BA83 nitrocellulose membrane (Schleicher & Schuell), and then incubated with antibodies specific for p73 (ER15; Oncogene Sciences); p53 (CM-5; Vector Laboratories); p21 (F5), p16 (M156), pRb (IF8), cMyc (N-262), and E1A (135-5) (all from Santa Cruz Biochemicals); Ras (R02120; Transduction Laboratories); p19ARF (Ab80; Novus Biologicals); and Erk (3A7; Cell Signaling) to detect the respective proteins. Secondary antibodies were horseradish peroxidase conjugated (Amersham). The ECL protein detection system (NEN) was used.
Coimmunoprecipitation.
A spontaneously immortalized ΔNp73-expressing MEF clone (see Fig. 2E, lane 3 [bottom of panel]) was infected with a control retroviral vector or a human wtp5-expressing vector. After 48 h, coimmunoprecipitation was performed as we previously described (45), except that immunoprecipitation was done with a 1:1 mixture of DO-1 and polyclonal antibody 1801-agarose beads, followed by immunoblotting with ER-15 antibody.
FIG. 2.
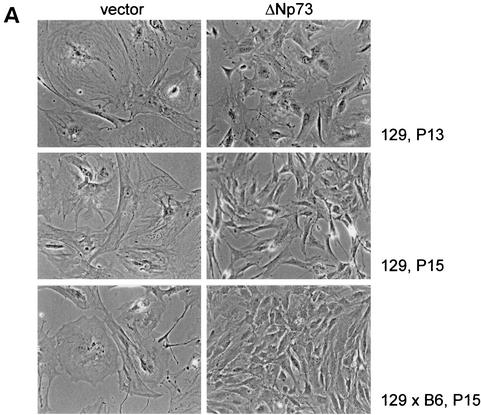
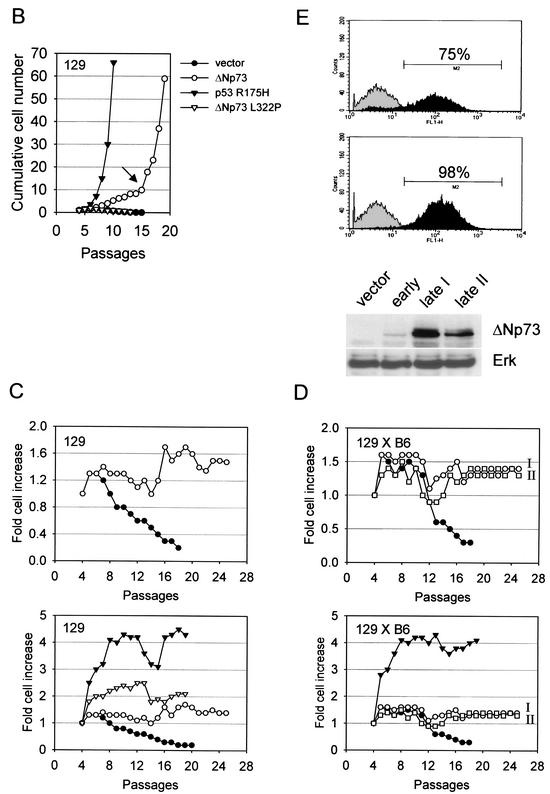
(A) ΔNp73 expression rescues primary MEFs from spontaneous cellular senescence. Morphology of MEFs of the indicated genetic background and infected with retroviruses expressing either vector GFP alone (left column) or ΔNp73α (right column) after 13 and 15 passages in culture. Control MEFs are senescent, whereas ΔNp73-expressing cells are not senescent. (Passage 8 is close to the end of their normal life span. After 12 to 15 passages, ΔNp73-expressing MEFs reproducibly produce numerous immortalized colonies that can be maintained in culture indefinitely.) (B to E) ΔNp73 expression promotes cellular immortalization. (B) Long-term growth curves of 129 MEFs infected with MSCV/IRES/GFP retroviruses expressing GFP only (•), tetramerization-defective ΔNp73 L322P (▵), ΔNp73 (○), or dominant-negative p53 R175H (▴). Cells were passaged in a 3T3 protocol. Total cell numbers per 60-mm culture dish were determined every 3 days prior to redilution for the next passage. The arrow marks the time point when numerous colonies appeared on the plates, each of which could be readily established as immortal cell lines. (C and D) Experiments similar to those in panel B with 129 MEFs (C) and 129×B6 MEFs (D) after infection with the indicated retroviruses and passage in a 3T3 protocol. Growth kinetics are plotted as the fold increase of total cell numbers after each passage. The top of the panel compares GFP vector (•) and ΔNp73 (○ and □ in panel D); the bottom of the panel compares GFP vector (•), ΔNp73 (○ and □ in panel D), p53 R175H (▵), and p53−/− (▴ in panel C). I and II refer to different populations of immortal ΔNp73 MEFs assayed in parallel. (E) A GFP-FACS analysis to determine spontaneous self-selection for high ΔNp73 expression over time is shown in the top part of the panel. Freshly MSCV/IRES/GFP-transduced 129 MEFs (passage 4) showed a ΔNp73 infectivity of 75% as assessed by GFP, whereas the same culture 11 passages later showed a ΔNp73 infectivity close to 100%. Curves: gray, parental MEFs used as a reference; black, ΔNp73-infected MEFs. At the bottom of the panel is a Western blot analysis with anti-p73 antibody after ΔNp73 expression in pooled MEFs over time. The lanes show GFP control MEFs (lane 1) and ΔNp73-infected MEFs 3 days after initial transduction (lane 2) and after spontaneous immortalization at passage 15 (lanes 3 and 4; two independent clones are shown). Erk was used as a loading control.
RESULTS
ΔNp73 accelerates growth of primary MEFs.
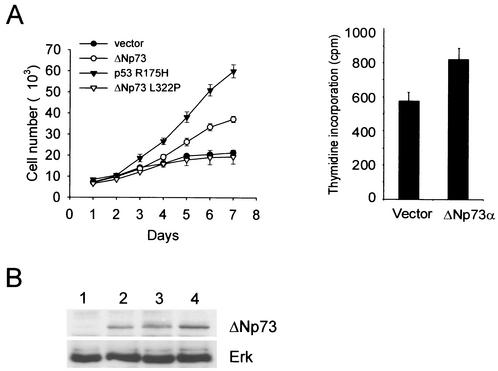
In human tumor cells, ΔNp73 interferes with p53/TAp73 function. ΔNp73 efficiently counteracts the transcriptional activation function, apoptosis, and growth suppression functions that are mediated by wild-type p53 and TAp73 and confers drug resistance to wild-type p53-harboring tumor cells (10, 12, 26, 37, 47). Moreover, we and others recently demonstrated that ΔNp73 is frequently overexpressed in human cancers (37, 47). These data suggest that ΔNp73 might also promote survival and cellular proliferation of primary cells. To examine this possibility, we generated recombinant retroviruses coexpressing wild-type ΔNp73α in conjunction with GFP generated from an IRES. As negative control, “empty” retroviruses that only express GFP or retroviruses that express mutant ΔNp73 L322Pα and GFP were used. ΔNp73 L322P (corresponding to L371P in TAp73α) is a DNA-binding-competent but tetramerization-incompetent mutant of ΔNp73α, which was previously shown to significantly reverse the dominant-negative inhibition of p53 transactivation by ΔNp73α (45). A retrovirus coexpressing the dominant-negative p53 R175H mutant and GFP served as positive control. Three days after MEFs of two different genetic backgrounds (pure 129 and mixed 129×B6) were transduced, immunoblot analysis confirmed the expression of ΔNp73 in infected MEFs (Fig. 1B). In short-term proliferation assays, wild-type ΔNp73-expressing primary fibroblasts exhibited more rapid growth rates compared to GFP-only or mutant ΔNp73-expressing control MEFs (Fig. 1A, left). The accelerated ΔNp73-induced growth rate was about half the rate induced by dominant-negative mutant p53 R175H. The increased ΔNp73-induced growth rate is also reflected in increased thymidine incorporation by 29% compared to GFP-only control MEFs (Fig. 1A, right).
FIG. 1.
(A) Short-term growth kinetics of MEFs (129 genetic background) transduced with recombinant MSCV/IRES/GFP retroviruses expressing GFP only (•) or coexpressing dominant-negative p53 R175H (▴), ΔNp73 (○), or tetramerization-defective ΔNp73 L322P (▵) are shown on the left. ΔNp73-expressing cells have a proliferative advantage over control cells. Cells were counted daily using a Coulter counter. Initial cultures showed an infectivity of 75 to 85% as demonstrated by GFP expression. After 7 days, GFP expression had increased to >95%, indicating self-selection, a finding consistent with the observed growth advantage. Identical results were seen with MEFs from a mixed 129×B6 genetic background. Results showing that ΔNp73 increases the fraction of cells in S phase are given on the right. Thymidine incorporation of passage 5 MEFs 3 days after infection with retroviruses was determined as indicated. The results are the average counts per minute ± the standard deviation (n = 8 wells/condition) of three independent experiments. (B) Expression of retrovirally transduced ΔNp73 in 129 MEFs. GFP-only infected MEFs (lane 1) or MEFs infected with retroviral constructs coexpressing ΔNp73 (MSCV based [lane 2] and Rpuro based [lane 3]) or ΔNp73 L322P (MSCV based [lane 4]) were harvested after 3 days. Expression was determined on total cell lysates by immunoblotting 50 μg of protein per lane with p73-specific ER15 antibody.
ΔNp73 confers resistance to spontaneous replicative senescence in primary MEFs and promotes cellular immortalization.
Explanted primary rodent fibroblasts have a limited life span in culture that is attributed to the stresses imposed by tissue culture. Through a series of elegant genetic studies, it was clearly established that the p53 pathway plays a preeminent role in this spontaneous senescence phenomenon (18, 19, 34, 35). To test whether ΔNp73 has an inhibitory effect on spontaneous replicative senescence of primary fibroblasts, MEFs were subjected to long-term passaging in culture. As expected, after ca. 8 to 10 passages many cells in the GFP-only control population took on the morphology of large, flat, nondividing, and nonrefractile giant cells (Fig. 2A, left column), indicating complete senescence. In contrast, ΔNp73-expressing fibroblasts remained small, more refractile and completely lacked the characteristic giant cell formation over a period of 6 months (Fig. 2A, right column). Likewise, as expected, after 8 to 10 passages control GFP- or mutant ΔNp73-expressing fibroblasts ceased to proliferate and entered senescence, with cell numbers slowly declining thereafter (Fig. 2B to D). In contrast, ΔNp73 expression bestowed a proliferative advantage onto MEFs with a steady increase in cell numbers (Fig. 2B to D). Compared to the brisque proliferative rate of MEFs expressing the dominant-negative p53 R175H mutant, ΔNp73-induced proliferation was moderate (Fig. 2B to D). Importantly, however, ΔNp73-expressing MEFs bypassed the onset of cellular senescence and, after 12 to 15 passages, produced numerous immortalized colonies which could be readily established as cell lines and maintained in culture indefinitely (Fig. 2B to D). This result was highly reproducible and seen with independent MEF isolates from different embryos and of different genetic backgrounds (129 and 129×B6). We determined the frequency of ΔNp73-expressing immortalized clones to be 10−3, which exceeds the frequency of spontaneous immortalization of primary rodent fibroblasts by 1000-fold (10−6) (39). We have now been culturing eight immortal lines of ΔNp73-expressing MEFs for more than 6 months each, without any signs of either a decrease in their prolfierative rate or an onset of senescence. The effect of ΔNp73 expression on cellular immortalization was independent of the retroviral vehicle used since it was reproducible with both the Rpuro vector after puromycin selection and the MSCV/IRES/GFP vector. Of note, immortalization correlated with spontaneous selection for high ΔNp73 expression in unselected MSCV-transduced MEFs (Fig. 2E). This was seen as an increase in the cell number and intensity of GFP-positive cells by FACS (Fig. 2E, top; compare the passage 4 populations with 75% positive cells in the upper panel to the passage 15 populations with 98% positive cells in the lower panel; the latter are also brighter on average) and directly by the level of ΔNp73 expression (Fig. 2E, bottom; compare the early transduced population in lane 2 with two different immortalized pooled populations in lanes 3 and 4). Taken together, we conclude that ΔNp73 expression extends the life span of MEFs and allows them to escape replicative senescence.
ΔNp73 has no transforming capacity of its own.
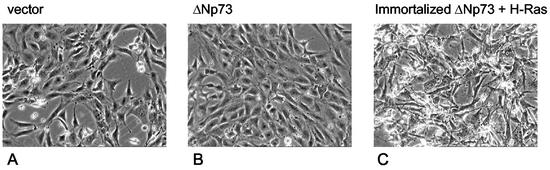
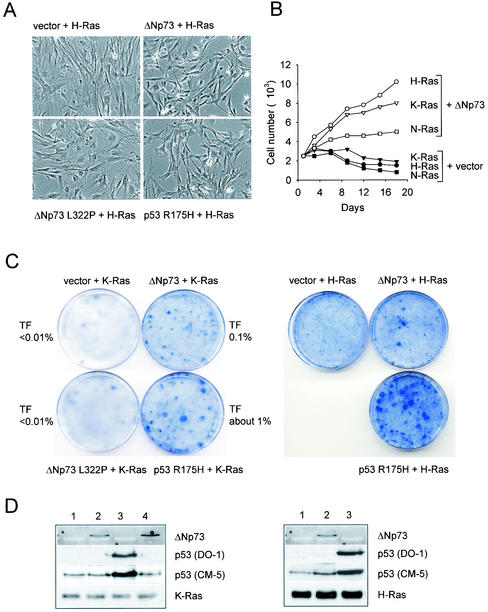
A recent study reported that forced overexpression of an unspecified form of N-terminally truncated p73β (collectively called ΔTA-p73β) in immortalized NIH 3T3 cells led to their complete oncogenic transformation (37). This result is interesting since it suggests that ΔNp73α could also be a bona fide transforming oncogene. However, in our experiments with ΔNp73α and three different sources of immortalized NIH 3T3 fibroblasts, cells failed to undergo morphological transformation and remained contact inhibited after retroviral transduction with ΔNp73α and puromycin selection (Fig. 3A and B). Nevertheless, since NIH 3T3 cells are deficient for ARF, we reexamined the possibility that ΔNp73α might be transforming in conjunction with several defined loss-of-function mutations in the p53 and retinoblastoma tumor suppressor pathways. These two central suppressor pathways have been shown to promote oncogenic transformation when combined with an oncogene from the signal transduction category. We therefore used MEFs derived from ARF-deficient or p53-deficient mice so as to model NIH 3T3 cells, but in a genetically defined way. In addition, we established immortalized fibroblast lines constitutively expressing the adenovirus E1A or the cMyc oncoproteins. However, in all cases after transduction with ΔNp73-expressing retroviruses, each of these cell types remained contact inhibited and showed no evidence of morphological transformation, whereas activated Ras transformed these cells immediately (data not shown). Moreover, in our experiments with MEFs, spontaneously immortalized MEFs that overexpressed ΔNp73 also failed to exhibit properties of fully transformed cells, such as characteristic morphological and proliferative phenotypes (see Fig. 1A and 2A). In contrast, introduction of activated H-RasV12 into spontaneously immortalized ΔNp73-expressing MEFs resulted in their clear transformation in vitro (Fig. 3C) and in vivo (see Fig. 7 and Table 1.) We conclude that ΔNp73α has properties of an immortalizing gene but not those of a transforming oncogene.
FIG. 3.
ΔNp73 lacks in vitro transforming capacity in NIH 3T3 cells. NIH 3T3 fibroblasts were infected with a control retroviral vector (A) or a ΔNp73-expressing vector (B). After infection, cells were selected in puromycin for 3 days. ΔNp73-expressing NIH 3T3 cells fail to undergo morphological transformation and remained contact inhibited. Identical results were seen with three different lines of NIH 3T3 cells. (C) For comparison, spontaneously immortalized ΔNp73-expressing MEFs, subsequently transduced with H-RasV12 retroviruses, showed strong morphological transformation in vitro and were highly tumorigenic in vivo (see Table 1).
FIG. 7.
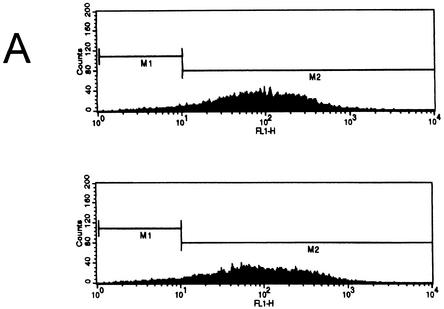
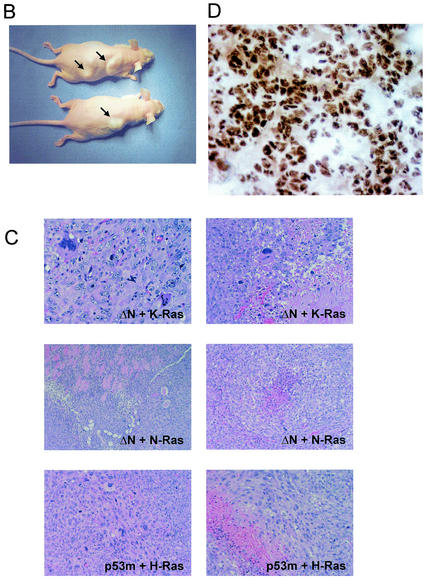
ΔNp73 cooperates with oncogenic Ras in promoting cellular transformation in vivo. (A) Characterization of MEFs for nude mouse assays used in Table 1. The percentage of ΔNp73 expressing cells as measured by GFP. FACS analysis of 129 MEFs cotransduced with ΔNp73 and K-RasV12 (top) and with ΔNp73 and N-RasD12 (bottom) immediately prior to subcutaneous injection into nude mice. (B) Photographs of nude mice 7 weeks after injections of ΔNp73+H-RasV12-infected 129 MEFs. (C) Corresponding sections of tumors generated by 129 MEFs cotransduced with the indicated retroviruses. Formalin-fixed and paraffin-embedded hematoxylin-eosin-stained sections show a typical sarcomatous morphology with tumor necrosis, anaplasia, and invasion into fat and muscle tissue. p53m indicates p53 R175H. Magnification, ×200. (D) ΔNp73 expression is maintained in fibrosarcomas. Immunohistochemical detection of ΔNp73 on a section from a ΔNp73+K-RasV12-generated tumor is shown. Detection was done by using biotin-streptavidin-horseradish peroxidase. Magnification, ×600.
TABLE 1.
ΔNp73 cooperates with oncogenic Ras in promoting tumorigenicity in vivo
| MEF type | No. of mice with tumors/ total no. of mice | No. of tumors/mouse | Avg wt of tumor (g) | Mean latency period (wk) ± SD |
|---|---|---|---|---|
| 129 MEFsa | ||||
| GFP+K-RasV12 | 0/4 | NAe | NA | NA |
| ΔNp73+K-RasV12 | 3/5 | 1, 1, 2 | 1.75 | 10 ± 3 |
| ΔNp73+N-RasD12 | 2/2 | 1, 2 | 0.62 | 10 ± 3 |
| ΔNp73+H-RasV12 | 2/4 | 1, 2 | 0.53 | 4 ± 1 |
| p53 H175+K-RasV12 | 5/5 | 2, 2, 2, 2, 2 | 1.37 | 2.5 |
| p53 H175+N-RasD12 | 1/1 | 2 | 1.5 | 3 |
| B6 MEFsb | ||||
| GFP+H-RasV12 | 0/4 | NA | NA | NA |
| ΔNp73+H-RasV12 | 2/4 | 1, 1 | 0.25 | 9 ± 2 |
| ΔNp73+K-RasV12 | 0/4 | NA | NA | NA |
| p53−/−+H-RasV12 | 2/2 | 2, 2 | 0.6 | 2.5 |
| p53 H175+H-RasV12 | 2/2 | 2, 2 | 0.6 | 2.5 |
| Spontaneously immortalized ΔNp73 129 MEFsc | ||||
| +H-RasV12 (expt 1) | 2/2 | 2, 2 | 0.45 | 1.4d |
| +H-RasV12 (expt 2) | 2/2 | 2, 2 | 0.52 | 1.4d |
| Passaged and in vitro-transformed 129 MEFs (ΔNp73+H-RasV12) | 2/2 | 1, 1 | 0.7 | 4.5 ± 1 |
| p73−/− MEFs (GFP+H-RasV12) | 0/2 | NA | NA | NA |
MSCV based.
Percent Rpuro based, after selection.
Expt 1, two populations pooled, MSCV based; expt 2, three clones pooled, Rpuro based after selection.
That is, 10 days.
NA, not applicable.
ΔNp73 rescues Ras-induced senescence in primary MEFs and cooperates with oncogenic Ras in cellular transformation in vitro.
Introduction of activated oncogenes into primary cells provokes a dramatic p53-dependent growth arrest reminiscent of premature senescence, whereas ablation of p53 function in this context allows cellular transformation of primary cells (34). Specifically, it is well established that in MEFs the ARF/p53 pathway is essential for the antioncogenic response elicited by activated Ras alleles. p53+/+ or p53+/− MEFs are resistant to the formation of neoplastic foci upon Ras transfection, whereas p53−/− MEFs readily form foci (27, 34). Given the finding that ΔNp73 allows cells to bypass spontaneous replicative senescence, we next sought to determine whether ΔNp73 also confers resistance to Ras-induced premature senescence. We infected MEFs with retroviruses expressing ΔNp73, followed by infection with retroviruses expressing constitutively active H-RasV12, K-RasV12, and N-RasD12 2 days later (Fig. 4). Consistent with previous observations, expression of all three Ras alleles in control-infected primary fibroblasts resulted in large, flat cells and cessation of growth at passage 5 to 6 (Fig. 4A and B). In contrast, introduction of all three Ras alleles into ΔNp73-expressing cells reproducibly led to their moderate but clear morphological transformation with small, refractile spindle-shaped cells that resembled cells which coexpressed mutant p53 and Ras. In contrast, cells coexpressing the mutant ΔNp73 L322P and Ras resembled control cells (Fig. 4A). Also, fibroblasts coexpressing ΔNp73 and Ras isoforms continued to proliferate slowly but steadily for over 10 passages, whereas GFP+Ras fibroblasts slowly declined in numbers (Fig. 4B). Since the inability to undergo Ras-mediated senescence often signifies susceptibility to Ras-mediated transformation, we next sought to determine whether ΔNp73 also acts as a facilitator in this process. Indeed, after 10 ± 2 passages (30 to 40 days), ΔNp73+Ras-expressing MEF populations proliferated more rapidly and produced fully transformed colonies. In repeat experiments, the frequency of such secondary colonies varied between ∼5 × 10−5 and ∼10−4. Moreover, MEFs coexpressing ΔNp73 and either H-RasV12 or K-Ras V12 lost contact inhibition and produced foci in focus formation assays, albeit to a lesser extent than cells with mutant p53 (Fig. 4C). The efficiency of focus formation of ΔNp73+Ras-coexpressing fibroblasts was 0.1 to 0.2% compared to 1% in cells coexpressing dominant-negative p53 R175H+Ras. This phenotypic manifestation of transformation was specific for ΔNp73, since cells coexpressing mutant ΔNp73 L322P and Ras failed to do so (Fig. 4C). On the other hand, ΔNp73+Ras-expressing MEFs failed to grow in soft agar. It is important to note that these ΔNp73+Ras-expressing foci showed poor growth properties and were difficult to establish as long-term cell lines. However, the frequency with which they arose in multiple repeat experiments by different investigators (O. Petrenko and A. Zaika) indicated that these foci did not arise by spontaneous clonal expansion, in particular since the GFP+Ras control plates produced no foci. In general, these foci had a limited proliferative potential and, after a small and variable number of population doublings, developed the phenotypic characteristic of senescence. Of the 15 to 20 ΔNp73+H-RasV12 or ΔNp73+K-RasV12 colonies picked, eventually all of them stopped growing, and no secondary cultures could be derived from these foci. To confirm that we do not simply select for clones that lose p53 in the retrovirally infected MEF series from Fig. 4C, which theoretically could be an artifact of growing MEFs in stressful conditions, we verified continued p53 expression by immunoblots with an antibody that recognizes mouse p53 (Fig. 4D). More importantly, we sequenced nine independently derived spontaneously immortalized ΔNp73 clones for their p53 gene status and found wild-type status in all nine clones.
FIG. 4.
ΔNp73 rescues Ras-induced senescence in primary MEFs. (A) Primary MEFs coexpressing ΔNp73 and oncogenic Ras exhibit a transformed phenotype in vitro. 129 MEFs coexpressing GFP and H-RasV12, ΔNp73 and H-RasV12, ΔNp73 L322P and H-RasV12, or p53 R175H and RasV12 were evaluated. Morphologically, the ΔNp73+H-RasV12 cells were highly refractile, were no longer contact inhibited, and resembled cells transformed by mutant p53+RasV12. This effect was not seen with tetramerization-defective ΔNp73 L322P+H-RasV12. (B) ΔNp73 rescues Ras-induced senescence in primary MEFs. The growth properties of 129 primary MEFs that were infected with retroviruses expressing GFP or ΔNp73, followed by infection with H-RasV12-, K-RasV12-, or N-RasD12-expressing retroviruses 2 days later, were evaluated. Cells were plated at equal densities and then counted every 3 days. Whereas Ras-expressing MEFs ceased to grow after 12 days and slowly lost cells due to concomitant cell death, ΔNp73+Ras-expressing MEFs continue to proliferate. MEFs were at passage 5 at the time of infection; 20 days later corresponds to six additional passages. After further passage in culture, fully transformed cells arose in the ΔNp73 cultures. Two such pooled populations were injected into nude mice, leading to the development of high-penetrance, short-latency tumors (see Table 1). (C) ΔNp73 and Ras cooperate in promoting focus formation. 129 MEFs were transduced with retroviral vectors expressing GFP+Ras, ΔNp73+Ras, mutant ΔNp73+Ras, and p53 R175H+Ras. Infected cells were selected with puromycin for 48 h. A total of 3 × 103 cells were mixed with 105 uninfected MEFs and plated onto 6-cm dishes in triplicate. After 14 days, plates were stained with Giemsa and photographed. K-RasV12 was used on the left, and H-RasV12 was used on the right. Loss of contact inhibition was also seen with N-RasD12 (not shown). (D) Expression analysis of MEFs from the focus formation assays in panel C 4 days after transduction with retroviruses encoding RasV12+GFP (lane 1), RasV12+ΔNp73 (lane 2), RasV12+p53 R175H (lane 3), and RasV12+ΔNp73 L322P (lane 4). Immunoblot analyses of 50-μg portions of total cell extracts with the indicated antibodies were done. DO-1 was specific for human p53, whereas CM-5 was raised against mouse p53, although it cross-reacts with human p53. (E) Expression analysis of MEFs from focus formation assays in panel C 4 days after transduction with retroviruses encoding RasV12+GFP (lane 1), RasV12+ΔNp73 (lane 2), RasV12+p53 R175H (lane 3), and RasV12+ΔNp73 L322P (lane 4). Immunoblot analyses of 50-μg portions of the total cell extracts with the indicated antibodies were carried out. For panels D and E, K-Ras was used on the left and H-Ras was used on the right.
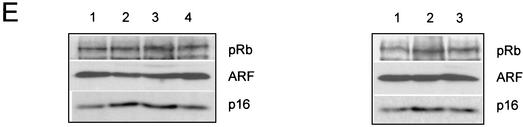
Although our results thus far implicate p53 to be a major target of ΔNp73, it might not be the only one, since disrupting the p53 pathway in MEFs has a strong immortalizing effect that could easily obscure any other effect of ΔNp73 on cell cycle regulators. Based on a recent report that “ΔTAp73” (i.e., Ex2/3Del p73) also induces pRB hyperphosphorylation in human tumor cells and human diploid fibroblasts after high adenovirally driven ΔNp73 expression (38), we examined whether ΔNp73-induced changes in the pRB tumor suppressor pathway are detectable in our MEF series from Fig. 4D. However, we failed to see consistent changes in the migration pattern of pRB (indicative of hyperphosphorylation) or changes in the levels of p16INK4a and p19ARF (Fig. 4E). This negative result could have several explanations, among them that the moderate expression levels achieved by retroviruses might not reveal this effect, that the effect depends on a particular isoform (Ex2/3Del p73), or that a species difference exists. Taken together, these results suggest that constitutive ΔNp73 expression rescues a proportion of primary cells from Ras and p53-mediated growth arrest by inhibiting p53 function, thus extending their life span and promoting cellular transformation.
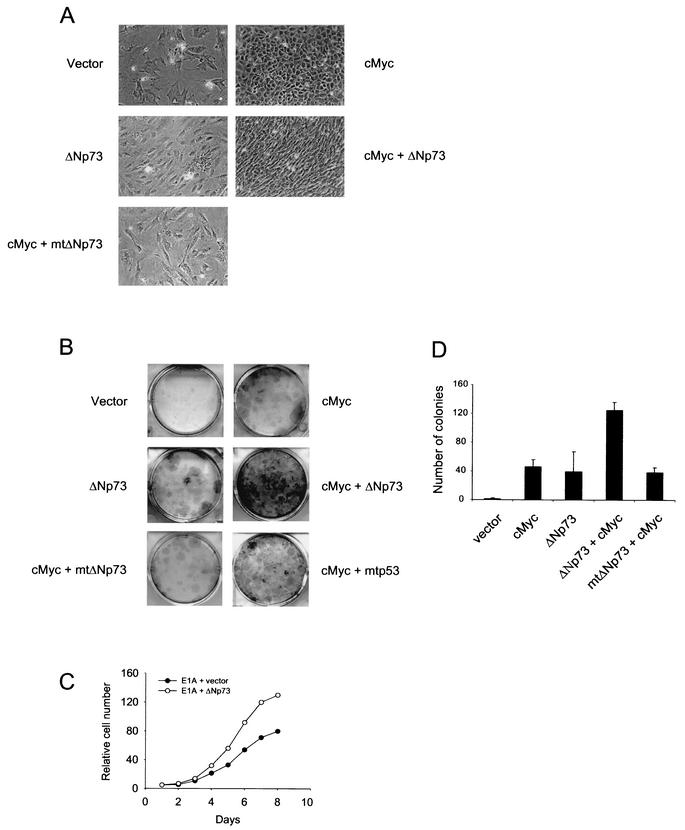
ΔNp73 cooperates with cMyc and E1A in cell proliferation and colony formation.
Ectopic expression of the cMyc proto-oncogene in primary fibroblasts results in their immortalization (1, 20, 46). cMyc-mediated immortalization of fibroblasts is frequently associated with p53 mutations or ARF loss (46) and cMyc strongly collaborates with loss of p53 in causing mouse lymphomagenesis (2, 8). Conversely, under serum-free conditions, high levels of cMyc can reveal a built-in apoptogenic function of cMyc that is signaled via the ARF pathway and depends on an intact p53 function (1, 9, 46). Since our previous results in MEFs suggested that ΔNp73 exerts its action by functionally inhibiting wild-type p53, we sought to determine whether ΔNp73 also accelerates the proliferative potential of cMyc-expressing wild-type MEFs. Indeed, ΔNp73 dramatically increased the profilerative capacity of cMyc-expressing primary fibroblasts, as shown by proliferative morphology and by colony formation assays (Fig. 5A and B). In both assays, this effect was abolished when ΔNp73 was replaced by mutant ΔNp73 L322P (Fig. 5A and B).
FIG. 5.
ΔNp73 cooperates with cMyc and E1A in cell proliferation and colony formation. (A) Morphology of 129 MEFs infected with retroviruses expressing GFP only, ΔNp73 only, cMyc only, or cMyc together with wild-type or mutant ΔNp73. mtΔNp73 stands for ΔNp73 L322P. (B) Colony formation assay of MEFs transduced with the indicated plasmids. The bar graph shows the quantitation of three independent experiments plus the standard deviation. (C) Short-term growth curve of MEFs infected with retroviruses coexpressing either E1A and GFP or E1A and ΔNp73.
Moreover, the viral E1A oncoprotein and cMyc share many functional properties. Both are immortalizing oncogenes in primary rodent fibroblasts (22, 32) and, like cMyc, E1A also paradoxically induces concomitant apoptosis in wild-type MEFs in an ARF-p53-dependent manner (5). Among its many effects, E1A releases the E2F transcription factors from pRB inhibition, and E2F1 then selectively induces ARF expression (4), which then in turn stabilizes p53. Consistent with this notion, coexpression of E1A with ΔNp73 enhanced the proliferative rate of MEFs compared to cells infected with E1A+GFP vector (Fig. 5C). Together, these data support the idea that ΔNp73 promotes the full proliferative potential of cMyc and E1A in primary fibroblasts by inhibiting the concomitant p53-mediated apoptotic failsafe response.
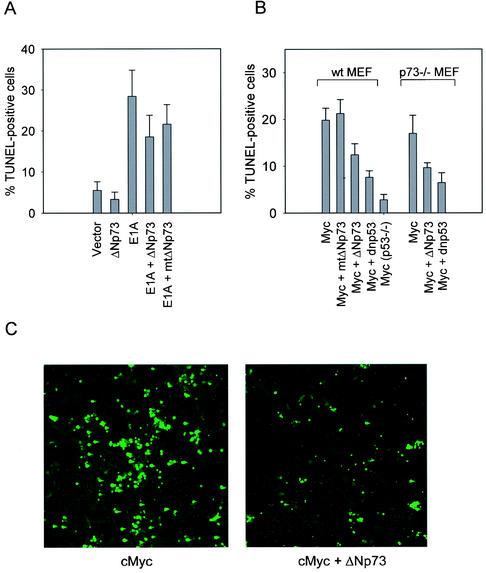
ΔNp73 compromises p53-dependent apoptosis in MEFs.
The previous results suggest that ΔNp73 should also compromise p53-dependent apoptosis in primary fibroblasts after challenge with DNA-damaging drugs. Since treatment of primary fibroblasts with chemotherapeutic drugs induces a cell cycle arrest response, we first sensitized them for apoptosis (33) by infecting wild-type MEFs with either E1A or cMyc-expressing retroviruses 2 days prior to treatment with camptothecin or etoposide (5 μM for 10 h). As shown in TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assays, coexpression of ΔNp73 in E1A- or cMyc-sensitized wild-type MEFs significantly decreased DNA damage-triggered, p53-mediated apoptosis compared to E1A- or cMyc vector-only control cells. This effect was abrogated by mutant ΔNp73 L322P, demonstrating specificity (Fig. 6). The inhibition of apoptosis by ΔNp73 is not as strong as that seen with dominant-negative mutant p53 R175H, which in turn is weaker than a p53−/− homozygous deletion. This relative ranking of inhibitor strength between ΔNp73, dominant-negative p53 and a complete lack of p53 was seen throughout our various assays. Could the effects of ΔNp73 also be dependent on the presence of TAp73? We addressed this question by comparing the etoposide-triggered apoptotic response of p73−/− MEFs to wild-type MEFs (Fig. 6B, right). The results show that the ΔNp73 effect of suppressing apoptosis in this system appears to be largely independent of the presence of TAp73. This result is further supported by our finding (see below) that nude mice injected with GFP+H-RasV12-transduced p73−/− MEFs behave like wild-type MEFs and fail to produce tumors (Table 1). Together, these findings support the notion that wtp53, rather than TAp73, might be a critical target for ΔNp73 inhibition.
FIG. 6.
ΔNp73 compromises p53-dependent apoptosis in primary fibroblasts. (A) 129 wild-type MEFs were infected with the indicated retroviruses 2 days prior to treatment with camptothecin (5 μM for 10 h). (B) 129 wild-type MEFs, p53−/− MEFs, or p73−/− MEFs were infected with the indicated retroviruses 2 days prior to treatment with etoposide (5 μM for 10 h). (A and B) Quantitation of cell death in expressing cells by TUNEL assays. ΔNp73 expression in E1A- or cMyc-sensitized MEFs decreased DNA damage-triggered, p53-mediated apoptosis. The results represent three independent experiments plus the standard deviation. mtΔNp73 stands for ΔNp73 L322P; dnp53 stands for p53 R175H. (C) Representative fields of TUNEL assays from panel B.
ΔNp73 cooperates with oncogenic Ras in promoting tumorigenicity in vivo.
To determine whether ΔNp73 cooperates with Ras and promotes tumorigenesis in vivo, early-passage MEFs (passage 4) were sequentially transduced with ΔNp73, followed by Ras 2 days later, and then injected into athymic nude mice. Because K-Ras and N-Ras mutations are more frequent than H-Ras mutations in human tumors (24), it was important to include K-RasV12 and N-RasD12 in addition to H-RasV12. We used 129 MEFs transduced with MSCV-based retroviruses without selection and C57BL/6 MEFs transduced with Rpuro-based retroviruses after 4 days of puromycin selection. ΔNp73 expression in unselected MSCV-transduced MEFs was >90%, as verified by FACS analysis with the GFP comarker (Fig. 7A). In all cases, cell populations derived from three independent infections were pooled and injected into the back of nude mice. The results of these experiments are shown in Table 1 and Fig. 7. Whereas control mice injected with cells coexpressing GFP and Ras completely failed to produce tumors, mice injected with 129 MEFs expressing ΔNp73+K-RasV12 (three of five mice), ΔNp73+N-RasD12 (two of two mice), or ΔNp73+H-RasV12 (two of four mice) produced one or two tumors each (Table 1; examples are shown in Fig. 7B and C). The latency periods until the appearance of readily visible tumors varied from 10 ± 3 weeks in the case of K-RasV12 and N-RasD12 to 4 weeks in the case of H-RasV12. Histologic examination confirmed that these tumors were highly invasive fibrosarcomas composed of pleomorphic spindle cells arranged in a herring bone pattern with multiple foci of necrosis and anaplasia. This morphology resembled tumors produced by mutant p53 R175H+H-RasV12 (Fig. 7C). The tumorigenic effect of ΔNp73 in the context of Ras was again seen with C57BL/6 MEFs, where two of four mice produced tumors in cooperation with H-RasV12 within 9 ± 2 weeks of latency. On the other hand, K-RasV12 failed to cooperate with ΔNp73 in B6 MEFs in the four tested mice, possibly suggesting some influence of genetic background. More animals will have to be tested to clarify this question. Of note, at the time of harvest fibrosarcomas exhibited maintained expression of ectopic ΔNp73 (Fig. 7D). Also, as already previously seen in our in vitro growth and transformation assays, the in vivo oncogenic cooperativity of ΔNp73 with Ras alleles was weaker than that of dominant-negative p53 R175H or complete p53 loss, which produces tumors with all Ras isoforms within a short latency period (2.5 to 3 weeks) and with a 100% frequency (Table 1). On the other hand, when MEFs that had been preimmortalized by ΔNp73 were subsequently transduced with H-RasV12, tumors formed very rapidly in four of four animals with an even shorter latency period than in the mutant p53 animals (10 days of latency). Moreover, when MEFs that had been originally cotransduced with ΔNp73 and H-RasV12 and then passaged in culture for 10 to 15 passages (30 to 40 days) until they showed brisk growth rates were injected into nude mice, they also rapidly produced tumors in two of two animals (4.5 ± 1 weeks of latency). Taken together, these results suggest that ΔNp73 expression allows primary fibroblasts that contain oncogenic Ras mutations to escape premature senescence in the animal with a certain frequency and maintain these cells in an immortalized state. Over time, such cells are then likely to acquire secondary oncogenic mutations, resulting in efficient tumorigenic conversion in vivo.
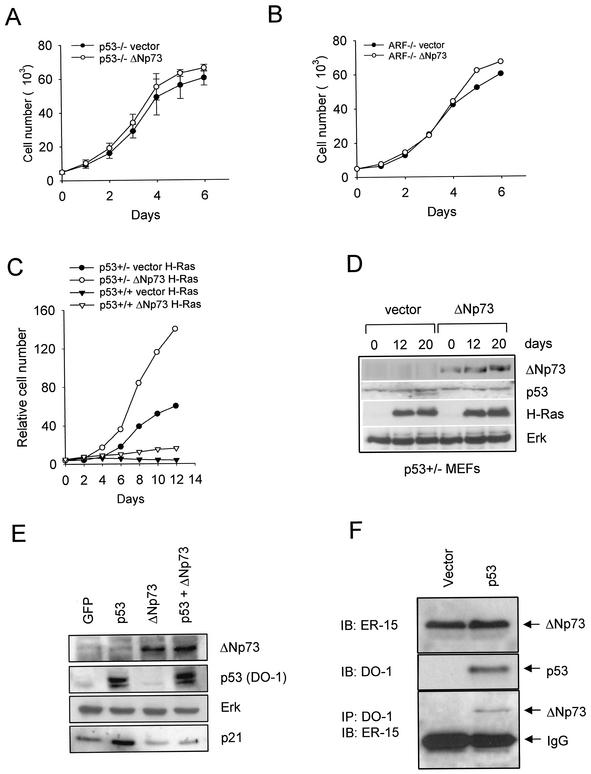
Wild-type p53 is a major target of ΔNp73-mediated effects in primary fibroblasts.
All our data thus far strongly support the idea that p53 is a major target that underlies the proliferative effects of ΔNp73 in the various biologic contexts tested here. To verify this notion, we used genetically defined p53 pathway deletion mutants of MEFs and assayed them for loss of ΔNp73 effects. Indeed, deletion of p53 or its requisite upstream activator ARF completely abrogated the growth-promoting effect of ΔNp73 compared to vector alone (Fig. 8A and B). Moreover, ΔNp73-expressing heterozygous p53+/− MEFs, which contain only half the dose of p53 protein compared to p53+/+ MEFs, exhibited enhanced rescue from Ras-induced senescence compared to wild-type MEFs (Fig. 8C, compare the distance between the filled symbols to the distance between the open symbols). This result is consistent with the notion that the cellular p53 concentrations in heterozygous cells are more efficiently inhibited by the available ectopic ΔNp73 (Fig. 8C). Figure 8D is an example of the expression controls run for Fig. 8C. Taken together, we conclude that wild-type p53 is a major target of ΔNp73 inhibition and is largely responsible for ΔNp73-mediated oncogenic effects in primary fibroblasts.
FIG. 8.
(A and B) In the absence of p53 or ARF, ΔNp73 has no effect on proliferation. The growth kinetics of p53−/− MEFs and p19ARF−/− MEFs transduced with a control empty vector (•) or ΔNp73-expressing vector (○) are shown. ΔNp73-expressing cells showed no proliferative advantage over vector control cells. (C) Compared to p53+/+ MEFs, p53+/− MEFs exhibit enhanced ΔNp73-mediated rescue from Ras-induced growth arrest. Growth curves of 129 MEFs of the indicated genotypes transduced with recombinant empty Rpuro vector or ΔNp73, followed by transduction with H-RasV12 4 days later, are shown. The growth advantage bestowed by ΔNp73 in p53+/+ MEFs was further increased in p53+/− MEFs. Note the compressed y-axis scale compared to Fig. 1A. (D) Expression of transduced ΔNp73 and H-RasV12 over time in p53+/− MEFs from panel C. Cells were selected in puromycin for 5 days. Lanes: 1, 2, and 3, vector+H-RasV12 0, 12, and 20 days, respectively, after Ras infection; 4, 5, and 6, ΔNp73+H-RasV12 at 0, 12, and 20 days, respectively, after Ras infection, which occurred 4 days after vector/ΔNp73 infection.Immunoblots were performed with the indicated antibodies. Endogenous Erk is used as loading control. (E) ΔNp73 inhibits the transactivation function of wild-type p53 in primary fibroblasts. 129 wild-type MEFs were infected with retroviruses encoding either GFP, human wild-type p53, ΔNp73, or both p53 and ΔNp73. After 48 h, cells were analyzed by immunoblotting for endogenous p21Waf1. Retroviral expression was confirmed as indicated. Endogenous Erk was used as loading control. (F) Physical interaction between ΔNp73α and wild-type p53 protein in primary fibroblasts. At the bottom of the panel is shown the detection of a protein complex between ΔNp73α and wild-type p53 in an immortal ΔNp73-expressing MEF clone 48 h after infection with either a control or a p53-expressing retrovirus. Immunoprecipitations of equal amounts of total protein (2 mg each) with a mixture of the anti-p53 antibodies DO-1 and 1801 covalently coupled to agarose beads, followed by immunoblotting with anti-ΔNp73 antibody ER-15, were carried out. The immunoglobulin G light chain is indicated. At the top, immunoblots (50 μg of total protein) with the indicated antibodies are shown to confirm expression.
To further demonstrate the inhibitory effect of ΔNp73 overexpression on p53 transactivation function, we examined the induction of endogenous p21Waf1 by p53 in the presence or absence of ΔNp73. We found that endogenous p21 levels in wild-type MEFs infected with GFP+ΔNp73 become markedly depressed compared to MEFs infected with GFP only (Fig. 8E, lanes 1 and 3). Moreover, this effect is even more pronounced in wild-type MEFs expressing ectopic p53 compared to MEFs that coexpress p53 and ΔNp73 (Fig. 8E, lanes 2 and 4). This result fully confirms our previously published results in human cancer cell lines (45).
Concerning a mechanism of transdominance, we had previously already demonstrated the existence of a physical interaction between ΔNp73 and wild-type p53 in human cancer cell lines and primary human tumor tissues (45). More importantly, we can also detect mixed complexes between ΔNp73 and wild-type p53 in coimmunoprecipitations from MEFs (Fig. 8F). This was seen in ΔNp73-expressing immortalized MEFs infected with p53-encoding retrovirus, thus providing a mechanism for transdominance and confirming our previous findings in human tumors.
DISCUSSION
Using classical in vitro and in vivo transformation assays, we show here that ΔNp73 exerts oncogenic functions in primary cells. ΔNp73 facilitates immortalization of MEFs, rescues them from Ras-induced senescence, and cooperates with cMyc and E1A in driving their proliferation. Most importantly, ΔNp73 cooperates with all three isoforms of oncogenic Ras in inducing MEF-derived malignant fibrosarcomas in vivo. On the other hand, ΔNp73 has no transforming capacity of its own, at least in primary fibroblasts and in the NIH 3T3 strains used in the present study. Taken together, our results show that ΔNp73 can be classified as an immortalizing protein that cooperates with a classical oncogene from the signal transduction category in completely transforming primary fibroblasts.
Primary fibroblasts respond to oncogenic Ras expression by undergoing an irreversible premature arrest that resembles replicative senescence (34). Premature senescene acts as a “failsafe” tumor suppressor mechanism in this context. Immortalization is the critical event toward complete oncogenic transformation because immortalization events disrupt the normal antioncogenic response to Ras signaling. It has been clearly established that immortalization can be achieved by loss of one of the two crucial tumor suppressor and senescence regulators p19ARF or p53 (for a review, see reference 36). Alternatively, coexpression of immortalizing proteins such as cMyc, DRIL1, c-jun, and c-fos also bypasses Ras-induced senescence (15, 22, 28, 40, 41). Deregulated ΔNp73 expression rescues a proportion of primary fibroblasts from Ras- and p53-mediated growth arrest by inhibiting p53 function, thus extending their life span and promoting cellular transformation. Mechanistically, wild-type p53 is a major target of ΔNp73 inhibition and functional p53 inhibition appear to be largely responsible for the ΔNp73-mediated oncogenic effects in primary fibroblasts. This conclusion is based on the fact that p53−/− MEFs and ARF−/− MEFs have abrogated the growth-promoting effect of ΔNp73. Also, ΔNp73 compromises p53-dependent apoptosis in oncogene-sensitized MEFs that were subject to DNA damage.
We propose the following view of the role of ΔNp73 in oncogenicity as a reasonable explanation: ΔNp73 overexpression is required to initiate transformation by immortalizing a fraction of primary fibroblasts, but deregulated ΔNp73 alone is not sufficient to fully transform these immortalized cells. ΔNp73, introduced into wild-type cells, causes functional inactivation of p53, which may be temporary or permanent but, importantly, generates a window of opportunity for the acquisition of random oncogenic mutations through the ensuing genetic instability. This epigenetic compromise of the p53 suppressor function is the driving force for facilitating definitive secondary genetic losses in growth-restraining pathways and gains in growth-promoting pathways. In a preliminary analysis of ΔNp73-immortalized clones, we found that three of nine clones had lost their p19ARF expression but retained wild-type p53 expression (unpublished data). We are currently assessing gene expression patterns and p53 pathway status in ΔNp73-expressing Ras-transformed MEF clones. At any rate, in this system the compromised activation of the ARF/p53 pathway due to its inhibition by ΔNp73 is the critical initial driving force for an increased probability that genetically compromised clones will emerge. This notion is consistent with the fact that escape from Ras-induced premature senescence is insufficient for oncogenic transformation by activated Ras (30). This is also consistent with the relatively long latency period of 4 to 10 weeks and the incomplete penetrance of tumor incidence that we observed in vivo. Conversely, spontaneously immortalized ΔNp73 MEFs had time to acquire secondary mutations while in culture and therefore are tumorigenic after Ras expression within a very short latency of 10 days and a 100% incidence (Table 1).
We show here that deregulated ΔNp73 expression in primary cells can be a biologically significant transdominant inhibitor of p53 function. However, many additional questions need to be addressed in the future. These questions include whether ΔNp73 is required to maintain the transformed phenotype of cells, what the relationship is between ΔNp73 and other TP73 gene products (TAp73 in particular), and whether ΔNp73 has immortalizing properties outside the p53/TAp73 function. Emerging evidence from the analysis of primary human tumors shows that deregulated ΔNp73 expression is rather frequent (3, 7, 37, 43, 45). Moreover, in at least one tumor type, neuroblastoma (which is almost exclusively wild type for p53), ΔNp73 can act as such a strong oncogene that it acts as a prognostic marker for poor clinical outcome, independent of the established relevant markers of age, stage, and MYCN amplification (3). This brings up the possibility that ΔNp73, rather than the opposing TAp73, is the critical product of TP73 upregulation in cancers. This idea is supported by our observation that p73−/− MEFs plus H-RasV12, unlike p53−/− MEFs plus H-RasV12, are nontumorigenic in nude mice (Table 1). A comprehensive analysis of ΔNp73 deregulation in a broad spectrum of human tumors is needed to substantiate the clinical relevance of this novel inactivation mechanism of p53.
Acknowledgments
We thank Troy Joseph for technical assistance.
This work was supported by grants from the NIH/NCI to U.M.M.
REFERENCES
- 1.Askew, D. S., R. A. Ashmun, B. C. Simmons, and J. L. Cleveland. 1991. Constitutive cMyc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6:1915-1922. [PubMed] [Google Scholar]
- 2.Blyth, K., A. Terry, M. O'Hara, E. W. Baxter, M. Campbell, M. Stewart, L. A. Donehower, D. E. Onions, J. C. Neil, and E. R. Cameron. 1995. Synergy between a human cMyc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene 10:1717-1723. [PubMed] [Google Scholar]
- 3.Casciano, I., K. Mazzocco, L. Boni, G. Pagnan, B. Banelli, G. Allemanni, M. Ponzoni, G. P. Tonini, and M. Romani. 2002. Expression of ΔNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 3:246-251. [DOI] [PubMed] [Google Scholar]
- 4.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 14:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Stanchina, E., M. E. McCurrach, F. Zindy, S. Y. Shieh, G. Ferbeyre, A. V. Samuelson, C. Prives, M. F. Roussel, C. J. Sherr, and S. W. Lowe. 1998. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 15:2434-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz, S., K. Rother, C. Bamberger, H. Schmale, J. Mossner, and K. Engeland. 2002. Differential regulation of transcription and induction of programmed cell death by human p53-family members p63 and p73. FEBS Lett. 1-3:93-99. [DOI] [PubMed] [Google Scholar]
- 7.Douc-Rasy, S., M. Barrois, M. Echeynne, M. Kaghad, E. Blanc, G. Raguenez, D. Goldschneider, M. J. Terrier-Lacombe, O. Hartmann, U. Moll, D. Caput, and J. Benard. 2002. ΔN-p73α accumulates in human neuroblastic tumors. Am. J. Pathol. 2:631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elson, A., C. Deng, J. Campos-Torres, L. A. Donehower, and P. Leder. 1995. The MMTV/cMyc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene 11:181-190. [PubMed] [Google Scholar]
- 9.Evan, G. I., A. H. Wyllie, C. S. Gilbert, T. D. Littlewood, H. Land, M. Brooks, C. M. Waters, L. Z. Penn, and D. C. Hancock. 1992. Induction of apoptosis in fibroblasts by cMyc protein. Cell 69:119-128. [DOI] [PubMed] [Google Scholar]
- 10.Grob, T. J., U. Novak, C. Maisse, D. Barcaroli, A. U. Luthi, F. Pirnia, B. Hugli, H. U. Graber, V. De Laurenzi, M. F. Fey, G. Melino, and A. Tobler. 2001. Human ΔNp73 regulates a dominant-negative feedback loop for TAp73 and p53. Cell Death Differ. 8:1213-1223. [DOI] [PubMed] [Google Scholar]
- 11.Irwin, M., M. C. Marin, A. C. Phillips, R. S. Seelan, D. I. Smith, W. Liu, E. R. Flores, K. Y. Tsai, T. Jacks, K. H. Vousden, and W. G. Kaelin. 2000. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407:645-648. [DOI] [PubMed] [Google Scholar]
- 12.Ishimoto, O., K. Kawahara, K. Enjo, M. Obinata, T. Nukiwa, and S. Ikawa. 2002. Possible oncogenic potential of ΔNp73: a newly identified isoform of human p73. Cancer Res. 62:636-641. [PubMed] [Google Scholar]
- 13.Jackman, J., and P. M. O'Connor. 1998. Cell cycle analysis, p. 8314. In J. S. Bonifacino (ed.), Current protocols in cell biology, vol. I. John Wiley & Sons, Inc., New York, N.Y.
- 14.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Jochum, W., E. Passegue, and E. F. Wagner. 2001. AP-1 in mouse development and tumorigenesis. Oncogene 19:2401-2412. [DOI] [PubMed] [Google Scholar]
- 16.Jost, C. A., M. C. Marin, and W. G. Kaelin, Jr. 1997. p73 is a simian p53-related protein that can induce apoptosis. Nature 389:191-194. [DOI] [PubMed] [Google Scholar]
- 17.Kaghad, M., H. Bonnet, A. Yang, L. Creancier, J. C. Biscan, A. Valent, A. Minty, P. Chalon, J. M. Lelias, X. Dumont, P. Ferrara, F. McKeon, and D. Caput. 1997. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90:809-819. [DOI] [PubMed] [Google Scholar]
- 18.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 5:649-659. [DOI] [PubMed] [Google Scholar]
- 19.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 6851:83-86. [DOI] [PubMed] [Google Scholar]
- 20.Kohl, N. E., and H. E. Ruley. 1987. Role of cMyc in the transformation of REF52 cells by viral and cellular oncogenes. Oncogene 2:41-48. [PubMed] [Google Scholar]
- 21.Kovalev, S., N. D. Marchenko, S. Swendeman, M. LaQuaglia, and U. M. Moll. 1998. Expression level, allelic origin and mutation analysis of the p73 gene in neuroblastoma tumors and cell lines. Cell Growth Differ. 9:897-903. [PubMed] [Google Scholar]
- 22.Land, H., L. F. Parada, and R. A. Weinberg. 1983. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 5927:596-602. [DOI] [PubMed] [Google Scholar]
- 23.Lissy, N. A., P. K. Davis, M. Irwin, W. G. Kaelin, and S. F. Dowdy. 2000. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 407:642-645. [DOI] [PubMed] [Google Scholar]
- 24.McCormick, F., and A. Wittinghofer. 1996. Interactions between Ras proteins and their effectors. Curr. Opin. Biotechnol. 4:449-456. [DOI] [PubMed] [Google Scholar]
- 25.Moll, U. M., S. Erster, and A. Zaika. 2001. p53, p63, and p73: solos, alliances, and feuds among family members. Biochim. Biophys. Acta 1552:47-59. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa, T., M. Takahashi, T. Ozaki, K. Watanabe, S. Todo, H. Mizuguchi, T. Hayakawa, and A. Nakagawara. 2002. Autoinhibitory regulation of p73 by ΔNp73 to modulate cell survival and death through a p73-specific target element within the ΔNp73 promoter. Mol. Cell. Biol. 22:2575-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmero, I., C. Pantoja, and M. Serrano. 1998. p19ARF links the tumour suppressor p53 to Ras. Nature 6698:125-126. [DOI] [PubMed] [Google Scholar]
- 28.Peeper, D. S., A. Shvarts, T. Brummelkamp, S. Douma, E. Y. Koh, G. Q. Daley, and R. Bernards. 2002. A functional screen identifies hDRIL1 as an oncogene that rescues RAS-induced senescence. Nat. Cell Biol. 2:148-153. [DOI] [PubMed] [Google Scholar]
- 29.Petrenko, O., A. Beavis, M. Klaine, R. Kittappa, I. Godin, and I. R. Lemischka. 1999. The molecular characterization of the fetal stem cell marker AA4. Immunity 10:691-700. [DOI] [PubMed] [Google Scholar]
- 30.Pozniak, C. D., S. Radinovic, A. Yang, F. McKeon, D. R. Kaplan, and F. D. Miller. 2000. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 289:304-306. [DOI] [PubMed] [Google Scholar]
- 31.Pozniak, C. D., F. Barnabe-Heider, V. V. Rymar, A. F. Lee, A. F. Sadikot, and F. D. Miller. 2002. p73 is required for survival and maintenance of CNS neurons. J. Neurosci. 22:9800-9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruley, H. E. 1983. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 5927:602-606. [DOI] [PubMed] [Google Scholar]
- 33.Samuelson, A. V., and S. W. Lowe. 1997. Selective induction of p53 and chemosensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc. Natl. Acad. Sci. USA 22:12094-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 5:593-602. [DOI] [PubMed] [Google Scholar]
- 35.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 6851:86-91. [DOI] [PubMed] [Google Scholar]
- 36.Sherr, C. J. 1998. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 19:2984-2991. [DOI] [PubMed] [Google Scholar]
- 37.Stiewe, T., S. Zimmermann, A. Frilling, H. Esche, and B. M. Putzer. 2002. Transactivation-deficient ΔTA-p73 acts as an oncogene. Cancer Res. 13:3598-3602. [PubMed] [Google Scholar]
- 38.Stiewe, T., J. Stanelle, C. C. Theseling, B. Pollmeier, M. Beitzinger, and B. M. Putzer. 2003. Inactivation of retinoblastoma (Rb) tumor suppressor by oncogenic isoforms of the p53 family member p73. J. Biol. Chem. 278:14230-14236. [DOI] [PubMed] [Google Scholar]
- 39.Todaro, G. J, H. Green, and M. R. Swift. 1966. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science 741:1252-1254. [DOI] [PubMed] [Google Scholar]
- 40.Vogt, P. K. 2000. Fortuitous convergences: the beginnings of JUN. Nat. Rev. Cancer 6:465-469. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg, R. A. 1989. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 14:3713-3721. [PubMed] [Google Scholar]
- 42.Yang, A., N. Walker, R. Bronson, M. Kaghad, M. Oosterwegel, J. Bonnin, C. Vagner, H. Bonnet, P. Dikkes, A. Sharpe, F. McKeon, and D. Caput. 2000. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404:99-103. [DOI] [PubMed] [Google Scholar]
- 43.Zaika, A. I., S. Kovalev, N. D. Marchenko, and U. M. Moll. 1999. Overexpression of the wild-type p73 gene in breast cancer tissues and cell lines. Cancer Res. 59:3257-3263. [PubMed] [Google Scholar]
- 44.Zaika, A., M. Irwin, C. Sansome, and U. M. Moll. 2001. Oncogenes induce and activate endogenous p73 protein. J. Biol. Chem. 276:11310-11316. [DOI] [PubMed] [Google Scholar]
- 45.Zaika, A. I., N. Slade, S. H. Erster, C. Sansome, T. W. Joseph, M. Pearl, E. Chalas, and U. M. Moll. 2002. ΔNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is upregulated in human tumors. J. Exp. Med. 6:765-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 15:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, J., J. Jiang, W. Zhou, and X. Chen. 1998. The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res. 58:5061-5065. [PubMed] [Google Scholar]