Abstract
Strong evidence indicates that endosome-localized epidermal growth factor receptor (EGFR) plays an important role in cell signaling. However, elimination of endosomal signaling does not attenuate EGF-induced physiological outcomes, arguing against physiological relevance. Recently we established a system to specifically activate endosome-associated EGFR in the absence of any plasma membrane activation of EGFR and showed that endosomal EGFR signaling is sufficient to support cell survival. However, this pure endosomal signaling of EGFR does not stimulate cell proliferation, because EGFR only remained activated for less than 2 h following its stimulation at endosomes, while DNA synthesis generally requires growth factor exposure for 8 h or more. Here we report that the prolonged requirement for EGF to stimulate epithelial cell proliferation can be substituted for with two short pulses of EGF. By combining the two short pulses of EGF stimulation with our previously established method to generate endosomal EGFR signaling, we are able to generate two pulses of endosomal EGFR signaling. In this way, we demonstrated that two pulses of endosomal EGFR signaling are sufficient to stimulate cell proliferation. The first pulse of EGFR signaling induces exit from quiescence into G1 phase and appears to render cells responsive to subsequent mitogenic stimulus. This second pulse, required several hours later, drives cells through the restriction point of late G1 and into S phase. We further showed that the two pulses of endosomal EGFR signaling engaged cell cycle machinery the same way as the two pulses of standard EGFR signaling. Moreover, two pulses of endosomal EGFR signaling stimulated downstream signaling cascades in a similar way to the two pulses of standard EGFR activation. The data therefore demonstrate that signals transduced from internalized EGFR, with or without a contribution from the plasma membrane, fully satisfy the physiological requirements for S-phase entry.
The growth-stimulatory signal of epidermal growth factor (EGF) is mediated by the transmembrane EGF receptor (EGFR). Following binding of EGF, the receptor dimerizes, autophosphorylates, and then nucleates signaling complexes that include many signaling proteins, such as Grb2, SHC, phospholipase C-γ1 (PLC-γ1), the p85α subunit of phosphatidylinositol-3-kinase (PI3K), p120 Ras GAP, and Cbl. Formation of the receptor-signaling protein complexes then initiates the activation of various signaling pathways leading to cell proliferation (5, 32, 33, 37, 49). Concomitantly, these ligand-receptor complexes cluster into clathrin-coated pits, internalize into early endosomes, and eventually traffic to lysosomes for degradation (5, 8, 18, 22, 27, 36). Classically, endocytosis of mitogen-activated receptor tyrosine kinases (RTKs) has been considered a means of signal downregulation and may even be conceived of as a tumor suppressor pathway (4, 7, 9, 33, 41, 43). By clearing activated receptors from the plasma membrane and routing them through the endosomal system and on to lysosomes for degradation, the cell can safely and quickly abrogate mitogenic signals that would otherwise prove hyperproliferative and ultimately deleterious. However, accumulated evidence suggests that the internalized EGF-EGFR complex may maintain its ability to generate cell signaling from endosomes. At the endosomal location, EGF-EGFR complexes remain associated with signaling effectors, such as Grb2, SHC, p85, p120 Ras GAP, and PLC-γ (4, 11, 14, 21, 28, 40, 44, 46); are also capable of nucleating new complexes (44); and continue to signal downstream through their respective pathways (4, 14, 44).
More evidence supporting endosomal signaling comes from endocytosis-blocking experiments. Inhibition of EGFR endocytosis modulates EGF-stimulated activation of signaling proteins, especially inhibition of ERK activation (17, 20, 23, 44). Moreover, ligand-activated EGFR spends more of its lifetime internally than on the cell surface, which further suggests the importance of endosomal signaling of EGFR. However, in the few cases in which biological end points were measured, inhibition of endocytosis did not result in attenuation of biological effects (9, 20), which argues against the physiological relevance of endosomal EGFR signaling. Recently we established a system to specifically activate endosome-associated EGFR in the absence of any plasma membrane activation (44). By using this system, we examined the effects of endosomal EGFR signaling on one of the major physiological outcomes of EGFR activation, cell survival. We showed that endosomal EGFR signaling is sufficient to elicit cell survival through generation of antiapoptotic signals in response to serum withdrawal (44). This demonstrated that endosomal EGFR signaling is sufficient to generate a physiological outcome. However, it is still not clear whether endosomal EGFR signaling is sufficient to stimulate cell proliferation.
It is well established that in order for a quiescent cell to proliferate, it must be continually exposed to mitogen until a few hours prior to S phase (15, 30). In serum-arrested epithelial cells, as well as in many other cell types, this is typically a span of 7 to 9 h, entailing the initial entry into early G1 phase from the quiescent state (or G0), and on through late G1 and past the restriction point (R point) (2, 15, 30, 31, 35). The difficulty in demonstrating mitogenic outcome from endosomal EGFR signals was the inability to maintain the receptor in endosomes for a prolonged period. After 2 h, most of the activated, internalized receptor has been degraded. While it may sustain endosomal EGFR signaling, inhibition of lysosomal trafficking will alter the normal trafficking route of EGFR and thus compromise the physiological relevance of the outcome. Recent insights into growth factor-dependent mitogenesis, however, may provide an opportunity for us to determine the role of endosomal EGFR signaling in cell proliferation. It is becoming better understood that growth factor-induced signal transduction is well integrated with the cell cycle machinery (16, 19). In platelet-derived growth factor (PDGF)-induced fibroblasts, serum-arrested cells can be driven into S phase by using two discontinuous pulses of ligand spaced 8 h apart in place of continuously exposing cells to ligand for 8 h with equal proliferative kinetics (15). The initial pulse is responsible for driving resting cells into G1 and is followed by a second pulse 7 to 9 h later, which stabilizes components of the cell cycle machinery responsible for surpassing the R point and driving cells into S phase.
Since the EGFR system is very similar to the PDGF receptor (PDGFR) system, both in signaling pathways and biological outcomes, we hypothesized that an analogous EGF-induced two-pulse proliferation system exists for EGFR. Our results showed this to be the case. In MDCK and BT20 epithelial cell lines, EGF-induced mitogenesis can be achieved through two short pulses of receptor signaling spaced 8 h apart. More importantly, by combining the two short pulses of EGF stimulation with our previously established method to generate endosomal EGFR signaling, we are able to generate two pulses of endosomal EGFR signaling. In this way, we demonstrated that two pulses of endosomal EGFR signaling are sufficient to stimulate cell proliferation. Analysis of events during the G0- to S-phase transition revealed that two pulses of signal, transduced from standard EGFR signaling or endosomal EGFR signaling, engage the G1 cell cycle machinery with the same efficacy as EGF administered continually over G1. Moreover, the signaling events immediately following these two pulses are different in the induction of downstream effectors. The data, therefore, demonstrate that signals transduced from internalized EGFR, with or without contribution from the plasma membrane, fully satisfy the physiological requirements for S-phase entry.
MATERIALS AND METHODS
Antibodies and chemicals.
Goat anti-pTyr (p1086), anti-phospho-retinoblastoma (pRb), mouse anti-Erk1/2, anti-cyclin D1, rabbit anti-EGFR, anti-Erk, anti-cyclin E, anti-c-Myc, anti-PLC-γ1, anti-phospho-PLC-γ1, anti-Akt, anti-phospho-Akt, anti-SHC, and anti-Grb2 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Mouse antitubulin antibody and EGF were obtained from Upstate Biotechnology, Inc., (Lake Placid, N.Y.). The mouse anti-bromodeoxyuridine (BrdU) antibody used in mitogenesis assays was purchased from Sigma (St. Louis, Mo.) The mouse anti-EGFR antibody (Ab-1) used for immunoprecipitation was obtained from Oncogene (Boston, Mass.). Protein A-Sepharose 6MB was obtained from Pharmacia BioProcess (Uppsala, Sweden). AG-1478 and monensin were obtained from Calbiochem (La Jolla, Calif.). Unless otherwise specified, all chemicals were purchased from Sigma.
Cell proliferation assay.
DNA synthesis was assayed by BrdU incorporation. Cells (BT20 or MDCK) were plated at 10,000 cells per glass coverslip and serum starved by incubation in serum-free medium for 36 h. Cells were then treated as necessary in the presence of 25 μM BrdU. For discontinuous treatment, BrdU was added back after each subsequent pulse or chase. After 16 to 18 h, cells were washed and fixed. Following denaturing of the DNA with 2 N HCl for 30 min at room temperature, cells were incubated with mouse anti-BrdU antibody for 1 h before addition of fluorescein isothiocyanate (FITC)-conjugated antimouse immunoglobulin G (for detection of BrdU) and 50 μg of propidium iodide per ml (to stain for total DNA). Cell nuclei were visualized in the red and green channels, and digital images were quantitated for BrdU incorporation. Percent DNA synthesis was calculated as the (number of BrdU-positive cells/total number of cells analyzed) × 100. For each experimental treatment, a minimum of 300 cells was counted.
Cell culture and treatment methods.
MDCK and BT20 cells were grown at 37°C in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and were maintained in a 5% CO2 atmosphere. Prior to treatment, cells were starved of serum for 36 h (1).
Standard EGF treatment.
Cells were treated with 100 ng of EGF per ml for the indicated pulse times. To terminate pulse and remove unbound ligand, cells were washed five times in phosphate-buffered saline (PBS) and then chased with starvation medium (2).
Endosomal EGF treatment (inactive EGF-EGFR internalization method).
The inactive EGF-EGFR internalization method has been described in detail previously (45).
(i) Treatment with monensin.
Quiescent cells were pretreated with 0.5 μM AG1478, and then monensin and EGF were added to final concentrations of 100 μM and 100 ng/ml, respectively. After a total treatment time of 1 h, the cells were washed with PBS five times to activate internalized receptors and chased with starvation medium.
(ii) Treatment without monensin.
Cells were pretreated with 0.5 μM AG1478 for 15 min, and then EGF was added to a final concentration of 100 ng/ml. After a 30-min treatment, cells were cooled down to 4°C and washed with acidic stripping buffer (100 mM acetic acid, 150 mM NaCl [pH 2.7]) for 1 min (47). The cells were then washed with PBS four times and chased with starvation medium.
To inhibit PI3K activation, wortmannin was added at 100 nM, 30 min prior to treatment. U1026, the MEK activation inhibitor, was added at 10 μM 1 h prior to treatment. For experiments testing for recovery of activation after the first pulse treatment, cells were washed with PBS 2 h after the first pulse and generally assayed at the 8-h time point (6 h later).
Immunoprecipitation.
Immunoprecipitation experiments were carried out as described previously (1). BT20 cells were lysed with immunoprecipitation buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 0.1% sodium deoxycholate, 100 mM NaF, 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM 4-[2-aminoethyl]-benzenesulfonyl fluoride, 10 μg of aprotinin per ml, 1 μM pepstatin A) overnight at 4°C. Cell lysates were then centrifuged at 21,000 × g for 30 min to remove debris. The supernatants, containing 1 mg of total protein, were incubated with 1 μg of mouse anti-EGFR antibody Ab-1.
Immunoblotting.
Immunoblotting was performed as described previously (1). Protein content of cell lysates was determined by Bradford analysis, and 30 μg of total protein was used for each sample. For the detection of EGFR, SHC, and Grb2 in the anti-EGFR immunoprecipitates, one-fifth of the immunoprecipitate from each sample was used. Protein samples were separated by electrophoresis through sodium dodecyl sulfate-10% polyacrylamide-containing gels and electrophoretically transferred onto nitrocellulose filter paper. Filters were then probed with the respective primary antibody. The primary antibodies were detected with their corresponding horseradish peroxidase-conjugated secondary antibodies followed by enhanced chemiluminescence development (Pierce Chemical, Rockford, Ill.) and light detection with Fuji (Tokyo, Japan) Super RX film.
RESULTS
The effects of one-pulse endosomal EGFR signaling on cell proliferation.
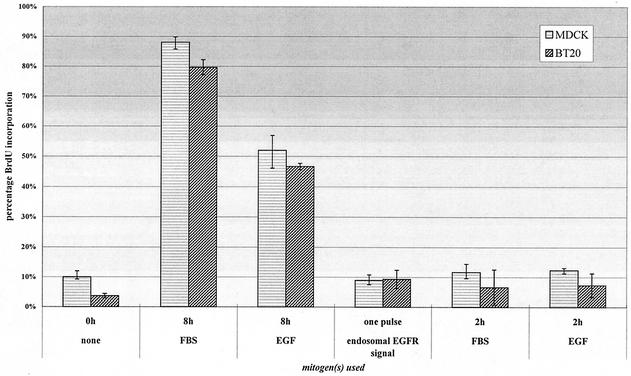
We first determined whether endosomal EGFR signaling is sufficient to stimulate cell proliferation. Cell proliferation (DNA synthesis) was determined by BrdU incorporation. As shown in Fig. 1, serum starvation for 24 h arrested MDCK and BT20 cells in G1 phase, and addition of 10% FBS or EGF (100 ng/ml) for 12 h stimulated BrdU incorporation. However, specific activation of endosome-associated EGFR did not stimulate BrdU incorporation (Fig. 1). The specific activation of endosome-associated EGFR was achieved by treating the cells with AG1478, EGF, and monensin for 30 min followed by washing and incubation with serum-free medium for 8 h. This result is not surprising, since EGFR only remained activated for less than 2 h following its activation at endosomes, and it is well established that EGF needs to be in the medium for more than 8 h to stimulate DNA synthesis (15, 30). Indeed, in control experiments, stimulation of cells with EGF or FBS alone for 2 h did not stimulate BrdU incorporation either (Fig. 1). Thus, a new system needs to be established to determine whether the pure endosomal signaling of EGFR is sufficient to stimulate cell proliferation, if allowed sufficient time.
FIG. 1.
A short pulse of either standard or endosomal EGFR signaling is insufficient to stimulate cell proliferation. For standard treatment, MDCK and BT20 cells were plated at 10,000 per coverslip and serum starved for 36 h. Cells were then stimulated for various times with either FBS (10%), EGF (100 ng/ml), or endosomal EGF treatment (see Materials and Methods) in the presence of 25 μM BrdU, after which unbound ligand was removed, and cells were incubated in starvation medium. At 18 h, cells were fixed and assayed for BrdU incorporation.
Two pulses of EGF, spaced 8 h apart, are sufficient to drive quiescent cells into S phase with kinetics similar to continuous 8-h stimulation of growth factor.
Since it is impossible to achieve continued endosomal EGFR signaling without altering EGFR trafficking, we decided to determine whether multiple pulses of endosomal EGFR signaling are sufficient to stimulate cell proliferation. In PDGF-induced fibroblasts, a recent study demonstrated that serum-arrested cells could be driven into S phase by two 30-min pulses of ligand spaced 8 h apart (15). Moreover, the kinetics of proliferation was equivalent to the case in which cells were continually exposed to mitogen. Due to the high similarity between the PDGFR and EGFR signaling systems, it is likely that a biphasic requirement for EGF may also exist in epithelial cells whose major RTK regimen included EGFR. We therefore examined whether two pulses of standard EGF treatment are sufficient to stimulate cell proliferation in MDCK and BT20 cells.
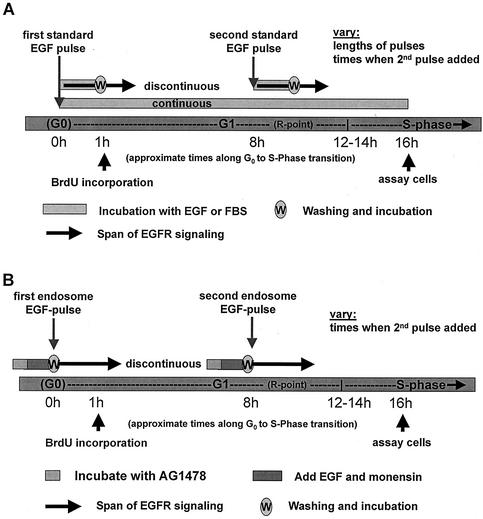
We adopted a strategy similar to that used by Jones et al. (15) (Fig. 2). Over a time course of 16 h, which includes the G0- to S-phase transition, we stimulated serum-arrested cells with mitogen either continuously (Fig. 3A) or in two temporally separate pulses. In the later case, the second pulse was administered 4, 8, or 12 h following the first (Fig. 3B). To terminate the pulse, cells were washed several times with PBS (to remove excess unbound ligand), and starvation medium was added. Using immunofluorescence, proliferation was measured by quantitating the percentage of cellular nuclei positive for BrdU incorporation, indicative of newly synthesized DNA.
FIG. 2.
Schematics of the two treatment assays employed. (A) Schematic of the continuous and discontinuous EGF stimulation assays employed. For discontinuous treatment, “w” indicates washout of unbound ligand, and solid arrows imply a continuation of signaling (from internalized receptors) after growth factor is removed from the medium. (B) Schematic of the (discontinuous) endosome-associated EGFR stimulation assay employed. For each endosome EGF pulse, the gray bar represents preincubation with AG1478, the red bar indicates incubation with EGF with or without monensin, and “w” indicates washout of AG1478 and thus the onset of endosome EGFR activation. Solid arrows imply the extent of actively signaling EGFR following this wash step.
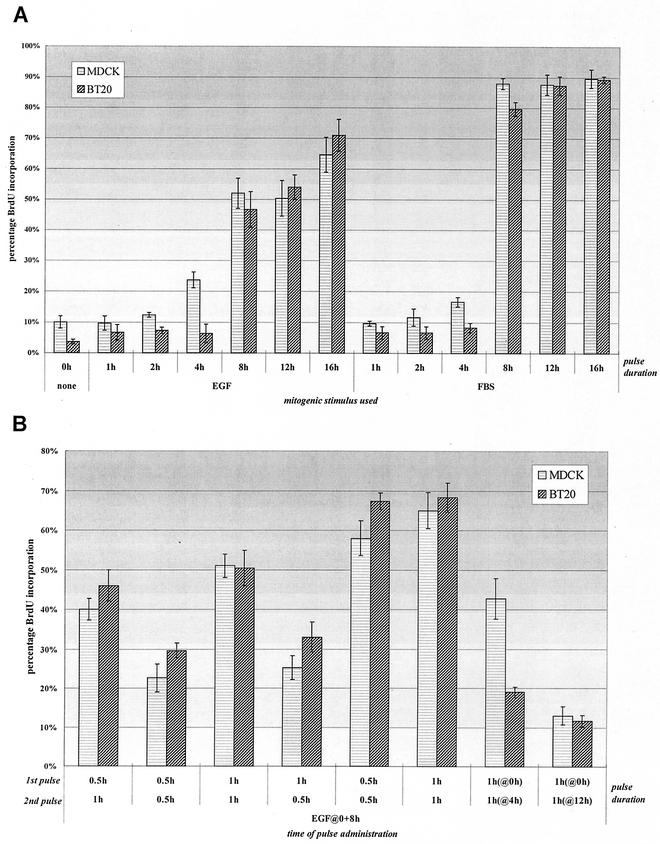
FIG. 3.
Two pulses of standard EGFR signaling are sufficient to stimulate cell proliferation. (A) Proliferation induced from a continuous mitogenic pulse. MDCK and BT20 cells were plated at 10,000 per coverslip and serum starved for 36 h. Cells were then stimulated for various times with either FBS (10%) or EGF (100 ng/ml) in the presence of 25 μM BrdU, after which unbound ligand was removed and cells were incubated in starvation medium. At 18 h, cells were fixed and assayed for BrdU incorporation. (B) Proliferation induced from two temporally separate EGF pulses. Cells were plated and starved as described above and then stimulated with EGF (or FBS) for the times indicated before removal of free ligand and culture once again in starvation media. A second pulse of growth factor was administered again at 4, 8, or 12 h and washed out after the times indicated. Cells were then fixed and assayed as indicated in panel A. Sample fields of MDCK and BT20 nuclei were quantitated by immunofluorescence of BrdU incorporation. A total of 300 cells per sample were counted, and data were obtained from triplicate experiments.
As can be seen in Fig. 3A, continual exposure to either FBS or EGF for 8 h or longer led to a marked increase in proliferating cells over the level of unstimulated controls. Although induction using FBS appeared to elicit a ∼30% greater response than when EGF was used alone, this is likely a result of compounded mitogenic signals elicited from the plethora of factors present in serum.
We next determined whether the same response could be induced if EGF was added in two temporally separate pulses. From Fig. 3B, two 1-h pulses of EGF, spaced 8 h apart, can drive cells into S phase with similar kinetics to continuous treatment (compare lane 5 of Fig. 3A with lane 3 of Fig. 3B). Interestingly, using an initial 30-min pulse of EGF favored proliferation only if the second pulse was 1 h (lanes 1 and 2). Although for PDGF-induced fibroblasts, two 30-min pulses are sufficient for mitogenesis, the same treatment in our cells gave only a weak response, lending to either physiological variation between cell types or various mitogenic potencies between growth factors. To distinguish between these two possibilities, we pulsed cells with serum 8 h apart for either 30 min or 1 h (lanes 5 and 6). As seen in the graph, either pair of FBS pulse lengths elicited proliferation in our cells, suggesting that factors present in serum, in addition to EGF, may compound the potency and/or multiplicity of mitogenic signals, therefore shortening the time required to engage downstream events necessary for mitogenesis.
We also varied the timing of the second EGF pulse (Fig. 3B). Although both cell types were mitogenically compromised if the second pulse was extended to 12 h (lane 8), there appeared to be disparity between the two cell types if the second pulse was given at 4 h (lane 7). While MDCK cells showed a strong proliferative response with this timing scheme, BT20 cells showed a more subdued response. This likely reflects the difference in cell division times (and cell cycle length) between MDCK and BT20, the former of which divides significantly faster under serum growth conditions. This was not a problem, since MDCK cells remained just as mitogenically responsive when the second pulse was given at 8 h, and thus both cell types could still be treated in parallel.
Stimulation of cell proliferation by two pulses of endosomal EGFR signaling.
The results presented above indicate that two short pulses of standard EGF treatment are sufficient to stimulate cell proliferation. We next determined whether two pulses of endosomal EGFR signaling are also sufficient to stimulate cell proliferation. We previously established a system to specifically activate EGFR at endosomes (see Materials and Methods), by first adding the reversible kinase inhibitor AG1478, followed by addition of EGF and monensin for 30 min. The EGF induces the thorough internalization of kinase-blocked receptor, while monensin prevents its recycling to the cell surface. Upon washing out the inhibitor, endosomal EGFR becomes activated and serves as a nucleation site for novel signaling complexes. By repeating this procedure twice within an 8-h interval, we would generate two pulses of endosomal EGFR signaling.
As outlined in Fig. 2B, we modified the discontinuous treatment assay for use with our previously established endosomal EGFR system, treating the cells identically at 0 and 8 h. After preincubation for sufficient time with AG1478, EGF, and monensin in order to internalize inactive EGF-EGFR complexes, we washed out the kinase inhibitor. Since receptor activation commences following washout of the kinase inhibitor, we define this point as the onset of a “pulse.” In order to standardize the endosome EGF pulse to a standard EGF pulse of 1 h, we simply adjusted the preincubation time of our endosomal EGFR assay treatment to 1 h, which equates to 1 h of ligand binding. Although the kinetics of internalization between surface-activated and kinase-blocked (inactive) receptor may differ, 30 min of EGF addition at saturating concentrations (100 ng/ml) is ample time to internalize all EGFR in either case (44).
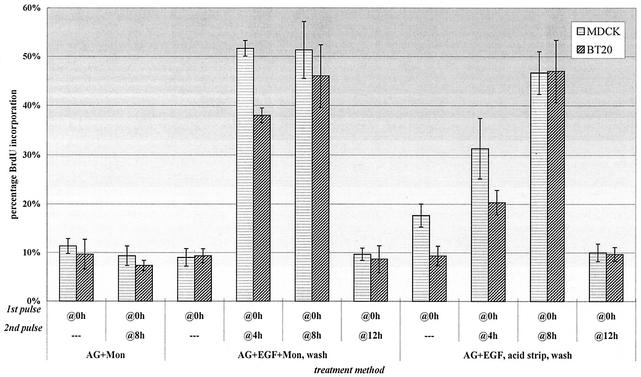
Two endosome EGF pulses, separated by 8 h, caused a proliferation rate almost identical to that with two equivalently timed standard EGF pulses (compare Fig. 3B, lane 3, with Fig. 4, lane 5). To test whether AG1478 and monensin were themselves mitogenic, we pulsed these factors without EGF at the various times indicated: as can be seen in the first two lanes, neither induced proliferation. When the timing of the second endosome EGF pulse was modulated, neither cell type led to significant proliferation if the pulses were 12 h apart (lane 6). When the second pulse was administered at 4 h (lane 4), both cell type populations underwent significant S-phase entry, with the level of BT20 entry slightly lower. Due to concern about possible nonspecific effects on cells during prolonged exposure to monensin (12, 42), we employed a monensin-free strategy for endosomal EGF treatment (see Materials and Methods). Since a fraction of inactive EGF-EGFR complexes could now recycle back to the surface in the absence of monensin, prior to washing out AG1478, we stripped off surface ligand with a mild acidic buffer. In this manner, spacing the endosome EGF pulses 8 h apart led to nearly equal proliferative rates compared to pulsing with monensin (lane 9), indicating that monensin did not nonspecifically alter the cell proliferation in our assay. Together, these results indicate that two pulses of endosomal EGFR signaling are sufficient to stimulate cell proliferation.
FIG. 4.
Two pulses of endosomal EGFR signaling are sufficient to stimulate cell proliferation. Proliferation was induced from two temporally separate endosomal EGF pulses. MDCK and BT20 cells were plated at 10,000 cells per coverslip, starved for 36 h, and then treated with AG1478 (AG) and EGF (100 ng/ml), with or without monensin (Mon), followed by washing and incubation in starvation medium. Some cells were left until the end of the assay, while others were treated as described above a second time for 4, 8, or 12 h. Cells treated without monensin were instead stripped of plasma membrane-recycled ligand prior to EGFR activation. At 18 h, cells were fixed and assayed for BrdU incorporation. Cells were counted at 300 per sample, and data were plotted as the mean of triplicate experiments.
Both standard and endosomal EGFR signaling engages the G1 cell cycle machinery.
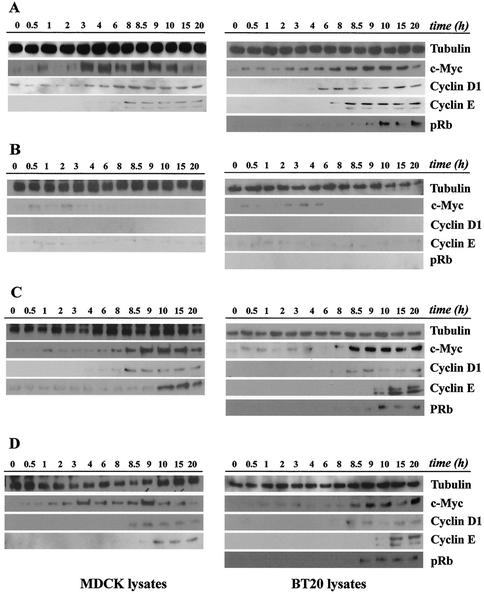
Having demonstrated the mitogenic competence of either two pulses of standard EGFR signaling or two pulses of endosomal EGFR signaling, we were next interested in seeing how these two systems engage the cell cycle machinery. In order to assess the engagement of the cell cycle under our various EGF treatments, we investigated the induction profiles of proteins in the G0- to S-phase transition over a 20-h span starting from the first mitogenic pulse (Fig. 5). c-Myc, a major downstream target of mitogenic signaling (20, 29), has been shown to affect the cell cycle at multiple points, both early and late in G1, including its role in regulating cyclin D-cdk2/4 complexes (1, 24, 29). To assess the activation of the G1 machinery itself, we directly investigated the induction of cyclins D1 and E and the phosphorylation status of pRb.
FIG. 5.
Induction of cell cycle proteins by continuous EGF exposure or by two pulses of standard and endosomal EGFR signaling. Subconfluent cultures of MDCK and BT20 cells were serum starved for 48 h and treated as indicated below. For each EGF treatment, cells were collected at the indicated times, and equal amounts of cell lysates were subjected to immunoblot analysis using antibodies to c-Myc, cyclin D1, cyclin E, and pRb. Tubulin antibody was used to assess protein loading. (A) Cells were treated continuously with EGF (100 ng/ml) until assayed. (B) Cells were treated for 1 h with EGF, after which unbound ligand was removed and cells were cultured in starvation medium. (C) Cells were treated with two 1-h pulses of EGF administered at 0 and 8 h, and pulses were terminated by washing as described above. (D) Cells were treated with AG1478 and EGF and then acid stripped of recycled ligand and washed free of AG1478 to activate internalized EGF-EGFR complexes. At 8 h, the same treatment was repeated. MDCK lysates were not analyzed for pRb phosphorylation, because the antibody didn't detect the canine protein.
As can be seen in Fig. 5, the time points are broken down into smaller intervals that correspond with when each pulse of standard or endosomal EGFR signaling (Fig. 5C and D) was initiated. As controls, we analyzed proteins over the same time course, but using continuous EGF exposure for 8 h (Fig. 5A) or a single 1-h pulse (Fig. 5B). Following initial EGF stimulation in both cell types and under all treatments, c-Myc was rapidly induced within 1 h, although for cells given only an initial EGF treatment of 1 h, c-Myc protein levels gradually declined by 8 h.
For continually treated cells, the levels of c-Myc rose steadily throughout the time course, peaking at 9 to 10 h. In MDCK cells, this rise in expression was more pronounced initially (compare 4-h data for MDCK and BT20, Fig. 5A), but was as gradual as that in BT20 cells.
In cells induced with two pulses of EGF, there appeared to be a distinct induction of this protein following the second pulse. The later rise in c-Myc levels was most pronounced in BT20 cells 30 min following stimulation with the second pulse of EGF (Fig. 5C and D). However, there was no difference between two pulses of standard and EGFR signaling.
Whether cells were treated with one or two pulses over 8 h, the relative kinetics of cyclin induction and pRb phosphorylation appeared to be similar. An ordered pattern of induction could be observed, beginning with the later elevation of c-Myc, followed by the appearance of detectable levels of cyclin D1 and then cyclin E, and ending in phosphorylation of pRb. In a few instances where pRb is detected prior to the appearance of cyclin E, it can be seen that the pRb is only hypophosphorylated, presumably via cyclin D-cdk complexes, as indicated by slightly lower mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In continually stimulated cells, the G1 cell cycle machinery was engaged starting between 4 and 6 h, consistent with the findings comparing continuous and discontinuous PDGF treatments in fibroblasts (15). The pRb levels themselves remained constant throughout the G1-S time course (data not shown). MDCK lysates were not analyzed for pRb, since our antibody was not reactive with the canine Rb protein. These data show that the cell cycle machinery leading to S phase is engaged to the same extent whether cells are treated continuously or in two distinct pulses. Moreover, it appears that there is little difference in cell cycle engagement between mitogenic signals initiated from standard EGFR activation and those initiated from the activation of endosome-associated EGFR.
Both standard and endosomal EGFR signaling activates downstream effectors in a similar pattern.
We showed that the two pulses of endosomal EGFR signaling engaged cell cycle machinery the same way as the two pulses of standard EGFR signaling. Next, we wanted to more closely examine the signal transduction pathways downstream of EGFR under these two different situations. Time points 2 h following the first pulse (0 to 2 h) or the second pulse (8 to 10 h) were monitored for the standard and endosome EGF treatments. For each time point, cell lysates were analyzed for activity (phosphorylation) of EGFR and other key signaling proteins. Phosphotyrosine profiles of EGFR over a 20-h time course with continuously treated cells show only a slight enhancement of phosphorylation some 2 h after the initial induction, which remains relatively constant until the end of the time course (data not shown).
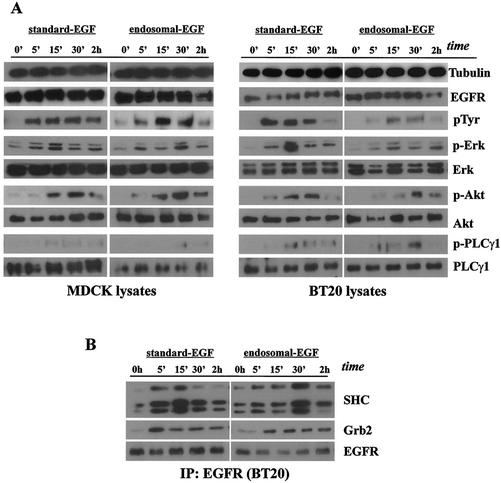
Consistent with our previous findings, the first pulse of EGFR activation stimulated mitogen-activated protein kinase (MAPK), Akt, and PLC-γ1 (Fig. 6A) (44). EGFR immunoprecipitated with Grb2 and SHC after both standard EGFR activation and activation of endosome-associated EGFR (Fig. 6B). The endosomal EGFR signaling resulted in the slower activation of downstream proteins due to the slower activation of EGFR itself.
FIG. 6.
Stimulation of EGFR and various signal transduction pathways by the first pulse of standard and endosomal EGFR signaling. Subconfluent cultures of MDCK and BT20 cells were serum starved for 48 h and treated for 1 h with EGF (100 ng/ml) to assay receptor activation and signal transduction originating at the plasma membrane (surface EGF) or incubated with AG1478 and EGF, acid stripped of recycled ligand, and washed free of AG1478 to assay receptor activation and signal transduction originating at the endosome (endosomal EGF). (A) Initial activation of EGFR and downstream signaling effectors following surface and endosome EGF treatment. Cell lysates, collected at the times indicated, were subjected to immunoblot analysis with antibodies to EGFR, phosphotyrosine 1186 of EGFR (p1186), phospho-Erk1/2, Erk1/2, phospho-Akt, Akt, phospho-PLC-γ1, and PLC-γ1. Tubulin antibody was used to assess protein loading. For the endosome EGF pulse, samples collected at 0 h preceded the acid strip step, although they had already been preincubated with AG1478 and EGF. (B) Ligand-induced association of EGFR with SHC and Grb2 following surface and endosome EGF treatment. BT20 lysates were immunoprecipitated (IP) with mouse anti-EGFR antibody and subjected to immunoblot analysis with antibodies to Grb2, SHC, and EGFR.
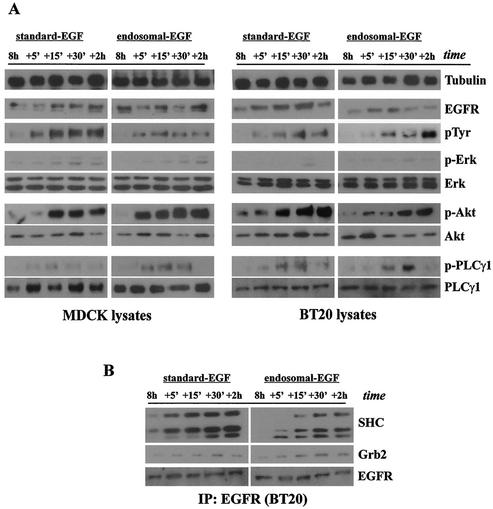
Standard EGFR activation and the activation of endosome-associated EGFR by the second pulse of EGF resulted in a very similar activation pattern of downstream signaling molecules. Akt was strongly activated, PLC-γ1 was moderately activated, and ERK was weakly activated (Fig. 7A). Moreover, both the standard EGFR activation and the activation of endosome-associated EGFR resulted in the association of EGFR with Grb2 and SHC (Fig. 7B). These results indicate that the second phase of activation of endosome-associated EGFR stimulated the downstream signaling cascade in a similar way to the second phase of standard EGFR activation.
FIG. 7.
Stimulation of EGFR and various signal transduction pathways by the second pulse of standard and endosomal EGFR signaling. Subconfluent cultures of MDCK and BT20 cells were serum starved for 48 h and then treated discontinuously at 0 h and again at 8 h with surface EGF or endosome EGF pulses. Cells were either treated for 1 h with EGF (100 ng/ml) to assay receptor activation and signal transduction originating at the plasma membrane (surface EGF) or incubated with AG1478 and EGF, acid stripped of recycled ligand, and washed free of AG1478 to assay receptor activation and signal transduction originating at the endosome (endosome EGF). (A) Later activation of EGFR and downstream signaling effectors following the second pulse of EGF. Cell lysates, collected at the times indicated, were subjected to immunoblot analysis with antibodies specific for EGFR, phosphotyrosine 1186 of EGFR (p1186), phospho-Erk1/2, Erk1/2, phospho-Akt, Akt, phospho-PLC-γ1, and PLC-γ1. Tubulin antibody was used to assess protein loading. For the endosome EGF pulse, samples collected at 8 h preceded the acid strip step, although they had already been preincubated with AG1478 and EGF. (B) Ligand-induced association of EGFR with SHC and Grb2 following the second pulse of EGF. BT20 lysates were immunoprecipitated (IP) with mouse anti-EGFR antibody and subjected to immunoblot analysis with antibodies to Grb2, SHC, and EGFR.
Interestingly, the protein activity profile following the second mitogenic pulse was quite different from that following the first mitogenic pulse (Fig. 6 and 7). When samples of protein lysates, taken over 2 h after the second induction, were analyzed for their activity, we found both quantitative and qualitative differences from the first mitogenic pulse.
Receptor protein levels, analyzed over 2 h following the second EGF pulse, were moderately lower than the initial levels (compare EGFR immunoblots from Fig. 6A and 7A). One possibility for this could be that the cell, following the lysosomal degradation of EGF-EGFR complexes from the first pulse, had not yet replenished its receptor number to the level it had initially. Despite this lower receptor quantity, both EGFR and downstream effectors were still significantly activated. Curiously, the kinetic pattern for these events differed from that of the first pulse. Where the first pulse of EGF stimulation led to a rapid activation pattern of EGFR and the p85 subunit of PI3K, rising within 5 to 15 min and declining by 2 h, the second response, in our two cell types, showed a delayed pattern of activity that remained high even at 2 h. Stimulation of Akt following the second EGF pulse not only was more prolonged, but appeared significantly stronger as well. The patterns of PLC-γ stimulation appeared to be similar in both pulses, although MAPK phosphorylation, as seen in the second pulse, was significantly lower under both standard induction and endosome EGF induction. Similar levels of MAPK protein over both pulses ruled out the possibility that the markedly reduced signal was due to the presence of less MAPK protein at 8 h.
For endosomal EGFR signaling, we found that SHC association was stronger at later times than with standard EGFR signaling. This pattern was weakly parallel for Grb2 (Fig. 6B and 7B). We also showed that both Grb2 and SHC associated with EGFR to a slightly lesser extent compared with its association following the initial induction, with the reduction in Grb2 association being more pronounced. The lower level of MAPK induction and reduced association with Grb2 and SHC imply reduced activity via the classical Ras-MAPK pathway. Moreover, these patterns were parallel from both standard and endosomal EGFR signaling.
The role of Erk and PI3K activation in cell proliferation induced by two pulses of EGFR signaling.
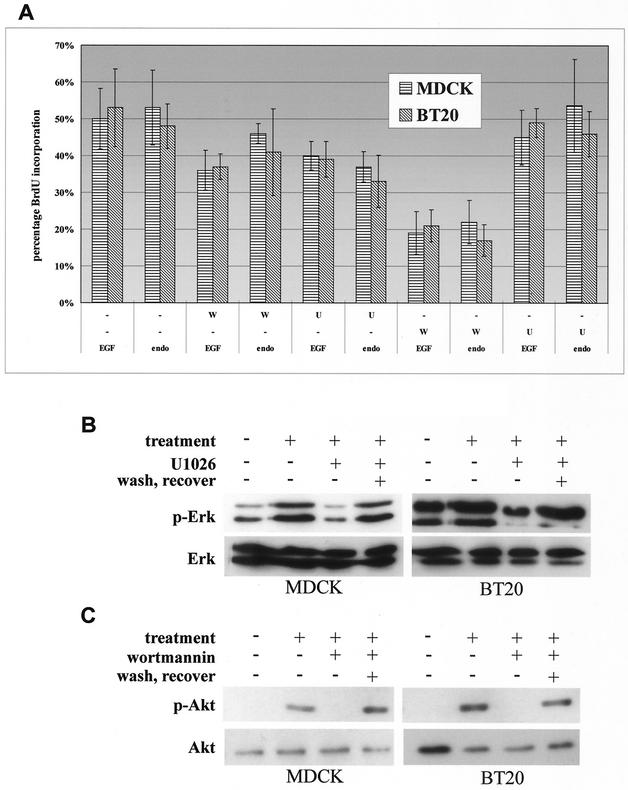
To determine the role of Erk and PI3K activation in cell proliferation induced by two pulses of EGFR signaling, we selectively inhibited Erk and PI3K activation induced by each pulse of EGFR signaling and then examined the effects on cell proliferation. As shown in Fig. 8A, inhibition of Erk activation by U1026 or inhibition of PI3K activation by wortmannin following the first pulse of EGFR signaling resulted in partial inhibition of cell proliferation. While inhibition of PI3K activation following the second pulse of EGFR signaling significantly blocked the EGF-induced cell proliferation, inhibition of Erk activation following the second pulse of EGFR signaling had no effects on EGF-induced cell proliferation. No differences were observed between the levels of EGFR signaling initiated from the cell surface of the endosomes (Fig. 8A). The efficacy of U1026 in inhibiting Erk activation and of wortmannin in inhibiting PI3K activation was determined by immunoblotting with anti-p-Erk and anti-p-Akt antibodies (Fig. 8B). Together, these results suggest that while the first phase of Erk and PI3K activation is involved in initiating the cell cycle leading up to the restriction point before S-phase entry, the second phase of PI3K activation plays a significant role in driving the cell cycle into S phase.
FIG. 8.
The role of Erk and PI3K activation in cell proliferation induced by two pulses of EGFR signaling in BT20 and MDCK cells. (A) Effect of wortmannin and U1026 on cell proliferation induced by two pulses of EGFR signaling from both standard and endosomal treatments. Cells were treated discontinuously at 0 h and again at 8 h with surface EGF (EGF) or endosome EGF (endo) pulses. The cell proliferation assay is described in Materials and Methods. W, treatment with wortmannin (100 nM); U, treatment with U1026 (10 μM). (B and C) Immunoblot analysis of the inhibition of Erk (B) and Akt (C) phosphorylation by U1026 (B) and wortmannin (C) on both MDCK and BT20 cells following an initial pulse of endosome EGFR stimulation (treatment). Wash and recovery of Erk and Akt activation were assayed in the last column of each blot. Here, cells were washed after the first pulse at 0 h, and lysates were assayed for recovery following the second endosome EGF pulse treatment at 8 h. Cell treatment and immunoblot analysis are described in Materials and Methods.
DISCUSSION
For more than a decade, investigators have debated the existence of endosomal signaling (4, 6, 7). Initially, endocytosis of ligand-activated receptors was considered a mechanism to attenuate signaling. Recent evidence suggests that internalized receptors maintain their ability to generate cellular signals after endocytosis to endosomes. The strongest evidence supporting endosomal signaling comes from endocytosis inhibition experiments (17, 20, 38, 44, 48). However, despite intensified efforts at understanding cell signaling from endosomes (15, 16, 24, 30, 39), no direct evidence existed to demonstrate the activation of signal transduction pathways from this location or to support the physiological relevance of endosomal signaling (10). Recently we established a system to specifically activate endosome-associated EGFR in the absence of any EGFR activation at the plasma membrane. We showed that endosomal EGFR signaling is sufficient to support cell survival and to activate major signaling pathways (44). However, one important but difficult question that remained unanswered was whether endosomal EGFR signaling is sufficient to stimulate cell proliferation. The difficulty in addressing this question is due to the insufficient activation time of endosome-associated EGFR. EGFR only remains activated for <2 h following its activation at endosomes. It is generally accepted that in order to elicit a proliferative response, quiescent cells typically require ligand exposure until about 2 h prior to S phase, which generally corresponds to 7 to 9 h (15, 30). Indeed, by using our previously established method, we showed that endosomal EGFR signaling is not sufficient to stimulate the proliferation of BT20 and MDCK cells. Thus, for endosomal EGFR signaling to be validated as fully mitogenic, we first had to establish a means to ensure that signals were exclusively endosomal, but exposure was prolonged enough to effect proliferation.
A recent finding by Jones et al. (15) showing that the requirement for a prolonged pulse of PDGF to stimulate cell proliferation can be replaced with two short pulses of PDGF allowed us to establish a new system to determine whether endosomal EGFR signaling is sufficient to stimulate cell proliferation. In PDGF-induced fibroblasts, a full mitogenic outcome was found to be effected through two distinct phases of signaling, corresponding to the times when mitogen is required to drive cells from quiescence and then later through the R point (15). Under this discontinuous sequence, the most favorable response was elicited when mitogenic pulses were administered 7 to 9 h apart. Applying this finding to the EGFR system, we found that EGF-induced mitogenesis in epithelial cells follows a similar biphasic mechanism, corresponding to times when the cells require growth factor for cell cycle progression. Two short pulses of standard EGF-induced signaling, spaced 8 h apart, were sufficient to elicit proliferation with kinetics similar to that of 8 h of continuous EGF treatment (Fig. 3A and B). More importantly, two pulses of endosomal EGFR signaling, spaced 8 h apart, are also sufficient to stimulate cell proliferation in a similar way to two pulses of standard EGFR signaling (Fig. 4). Interestingly, for cell proliferation induced by two pulses of endosomal EGFR signaling, the response was smaller when the second pulse was given at 4 h under the monensin-free conditions versus conditions in which monensin was included. We are not completely clear what causes this discrepancy. However, it is possible that in the presence of monensin, more EGFR is accumulated in the endosome: thus, the endosomal EGFR signaling is stronger and lasts longer. Alternatively, following the acidic wash to strip EGF from the surface EGFR, the cells may need some time to recover from the wash.
Underlying support for the physiological relevance of endosomal EGFR signaling comes from the proliferation experiments following standard (discontinuous) EGF treatment (Fig. 1C). If the duration of either EGF pulse was 30 min or less, the proliferative response was compromised, while two 1-h pulses were as proliferative as continuous 8-h EGF treatment. This implicates that the minimum time required for ligand exposure (and thus the minimal signaling requirement) is at least 30 min, a span well exceeding the occupancy time of activated EGFR at the plasma membrane. It is therefore likely that the majority of EGF-induced mitogenic signals are actually endosomally derived. Furthermore, elimination of the initial plasma membrane component from the total mitogenic signal quantity does not compromise the proliferative response.
When we investigated the time course of G1 cell cycle events by using either standard or endosomal EGF treatments, we found no differences in how the G1 machinery was engaged. In both cases, the transcription factor c-Myc was induced early on (0.5 to 2 h) in response to the first pulse and more strongly following the second pulse (8.5 h) (Fig. 5C and D). Even under continuous EGF treatment, c-Myc induction appeared to follow a biphasic pattern (Fig. 5B), albeit not as pronounced as for the discontinuous systems. Likewise, an ordered sequence of cell cycle events followed the second pulse under either standard or endosomal EGF treatment: beginning with an elevation in cyclin D1 levels half an hour after the onset of the second pulse, followed by an increase in cyclin E levels, and finally followed by the hyperphosphorylation of pRb, an event defining the R point (16, 34).
We then investigated the specific signaling events effected from each mitogenic pulse following both standard and endosome EGF treatments. In addition to EGFR and its phosphorylation state, we analyzed the activation profiles of MAPK (of the Ras-MAPK pathway), Akt (of the PI3K-Akt pathway), and PLC-γ1 (Fig. 6A and 7A). Although it is well established that under standard EGF treatment all three of these proteins are activated or phosphorylated (3, 4, 13, 14, 32), we wanted to determine whether differential levels of activity existed, depending on location (standard compared to endosomal signaling) or time (first compared to second pulse). In most cases, differences between standard and endosome-stimulated systems were kinetic. Activation of proteins under endosome EGF induction was generally more delayed than standard EGFR activation. This may reflect the incomplete removal of AG1478 and delayed activation of the internalized receptor following our treatment. Although phosphorylation of these three proteins was qualitatively similar whether EGFR was activated by the standard treatment or from endosomes, we did observe differences in protein activity between the two pulses themselves. Following the second pulse, activation of Akt was higher and more prolonged than after the first pulse. Moreover, there was significantly less MAPK activity, even though the protein levels were similar to those with the first pulse. This observation may in part be explained by our immunoprecipitation experiments (Fig. 6B and 7B). Activated EGFR communicates with Ras through a signaling complex containing Grb2, SHC, and mSos, the latter of which acts as a Ras-specific guanine nucleotide exchange factor (25, 26, 32). In a comparison of the EGFR immunoprecipitates from both pulses, lower levels of Grb2 and SHC were recruited to the receptor following the second EGF induction. Additionally, EGFR levels themselves were reduced at this time, as seen by EGFR immunoblots of both cell lysates and immunoprecipitated protein. This and perhaps a lower level of available Grb2 and SHC (as a consequence of previous proteolytic degradation) could help to explain the lower level of activation of MAPK activity. These results are consistent with both epithelial cell types used. Overall, these differences lend credence to the emerging idea that distinct, although not necessarily mutually exclusive, events are needed to drive cells into S phase. Furthermore, these events are initiated equally well from EGFR either at endosomes or at the plasma membrane.
Finally, we carried out more experiments to determine which specific signaling pathways are responsible for mediating the biphasic proliferative response. We specifically inhibited PI3K activation and Erk activation induced during the first and the second pulses of EGFR activation and then examined the effects on cell proliferation (Fig. 8). Our results suggest that while the first phase of Erk and PI3K activation is involved in initiating the cell cycle leading to the S-phase entry, the second phase of PI3K activation plays a significant role in driving the cell cycle into S phase. These findings are in agreement with previous reports regarding EGFR and other RTKs (15, 20).
Although the different activity profiles between these two phases of mitogenic signaling raise many provocative questions for future study, they are not the principal issue addressed in this report. In the present investigation, we elucidated the biphasic nature of EGF-induced proliferative signaling in order to address the mitogenic role of endosomal EGFR. Linking this with our system, which allows for the specific activation of endosome-associated EGFR without initial activation at the plasma membrane, we have validated the mitogenic function of endosomal EGFR. Endosomal EGFR signaling is fully competent in all aspects of signal transduction and biological function. The plasma membrane, although necessary for ligand recruitment, is not a privileged site for EGFR signaling. These findings argue for a much more profound role for endosome signaling in general. It can be hoped that in light of the multitude of RTKs that exist, future investigations will reveal analogous receptor systems that function at the endosomal level and lend further credence to the importance of endosomal signal transduction.
Acknowledgments
This work was supported in part by grants from the Canadian Institutes of Health Research (CIHR), the Alberta Heritage Foundation for Medical Research (AHFMR), and the Natural Science and Engineering Council of Canada (NSERC). Z.W. is a CIHR Scholar and an AHFMR Scholar.
REFERENCES
- 1.Amati, B., K. Alevizopoulos, and J. Vlach. 1998. Myc and the cell cycle. Front. Biosci. 3:D250-D268. [DOI] [PubMed] [Google Scholar]
- 2.Balciunaite, E., S. M. Jones, A. Toker, and A. Kazlauskas. 2000. PDGF initiates two distinct phases of PKC activity that make unequal contributions to the G0 to S transition. Curr. Biol. 10:261-267. [DOI] [PubMed] [Google Scholar]
- 3.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 4.Burke, P., K. Schooler, and H. S. Wiley. 2001. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell 12:1897-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter, G. 1987. Receptors for epidermal growth factor and other polypeptide mitogens. Annu. Rev. Biochem. 56:881-914. [DOI] [PubMed] [Google Scholar]
- 6.Ceresa, B. P., and S. L. Schmid. 2000. Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol. 12:204-210. [DOI] [PubMed] [Google Scholar]
- 7.Clague, M. J., and S. Urbe. 2001. The interface of receptor trafficking and signaling. J. Cell Sci. 114:3075-3081. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, S., and R. A. Fava. 1985. Internalization of functional epidermal growth factor: receptor/kinase complexes in A-431 cells. J. Biol. Chem. 260:12351-12358. [PubMed] [Google Scholar]
- 9.Di Fiore, P. P., and G. N. Gill. 1999. Endocytosis and mitogenic signaling. Curr. Opin. Cell Biol. 11:483-488. [DOI] [PubMed] [Google Scholar]
- 10.Di Fiore, P. P., and P. De Camilli. 2001. Endocytosis and signaling: an inseparable partnership. Cell 106:1-4. [DOI] [PubMed] [Google Scholar]
- 11.Di Guglielmo, G. M., P. C. Baass, W. J. Ou, B. I. Posner, and J. J. Bergeron. 1994. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 13:4269-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doebler, J. A. 2000. Comparative effects of carboxylic ionophores on membrane potential and resistance of NG108-15 cells. Toxicol. In Vitro 14:235-243. [DOI] [PubMed] [Google Scholar]
- 13.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 14.Haugh, J. M., A. C. Huang, H. S. Wiley, A. Wells, and D. A. Lauffenburger. 1999. Internalized epidermal growth factor receptors participate in the activation of p21(ras) in fibroblasts. J. Biol. Chem. 274:34350-34360. [DOI] [PubMed] [Google Scholar]
- 15.Jones, S. M., and A. Kazlauskas. 2001. Growth-factor-dependent mitogenesis requires two distinct phases of signaling. Nat. Cell Biol. 3:165-172. [DOI] [PubMed] [Google Scholar]
- 16.Jones, S. M., and A. Kazlauskas. 2001. Growth factor-dependent signaling and cell cycle progression. Chem. Rev. 101:2413-2423. [DOI] [PubMed] [Google Scholar]
- 17.Jones, S. M., R. Klinghoffer, G. D. Prestwich, A. Toker, and A. Kazlauskas. 1999. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr. Biol. 9:512-521. [DOI] [PubMed] [Google Scholar]
- 18.Kay, D. G., W. H. Lai, M. Uchihashi, M. N. Khan, B. I. Posner, and J. J. Bergeron. 1986. Epidermal growth factor receptor kinase translocation and activation in vivo. J. Biol. Chem. 261:8473-8480. [PubMed] [Google Scholar]
- 19.Kerkhoff, E., and U. R. Rapp. 1998. Cell cycle targets of Ras/Raf signaling. Oncogene 17:1457-1462. [DOI] [PubMed] [Google Scholar]
- 20.Kong, M., C. Mounier, V. Dumas, and B. I. Posner. 2002. EGF-induced DNA synthesis: key role for Src phosphorylation of the docking protein Gab2. J. Biol. Chem. 278:5837-5844. [DOI] [PubMed]
- 21.Kuruvilla, R., H. Ye, and D. D. Ginty. 2000. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron 27:499-512. [DOI] [PubMed] [Google Scholar]
- 22.Lai, W. H., P. H. Cameron, J. J. Doherty II, B. I. Posner, and J. J. Bergeron. 1989. Ligand-mediated autophosphorylation activity of the epidermal growth factor receptor during internalization. J. Cell Biol. 109:2751-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Land, H., A. C. Chen, J. P. Morgenstern, L. F. Parada, and R. A. Weinberg. 1986. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol. Cell. Biol. 6:1917-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leone, G., J. DeGregori, R. Sears, L. Jakoi, and J. R. Nevins. 1997. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387:422-426. (Erratum, 387:932.) [DOI] [PubMed]
- 25.Marshall, C. J. 1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4:82-89. [DOI] [PubMed] [Google Scholar]
- 26.Marshall, C. J. 1996. Cell signaling. Raf gets it together. Nature 383:127-128. [DOI] [PubMed] [Google Scholar]
- 27.Merion, M., and W. S. Sly. 1983. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J. Cell Biol. 96:644-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, W. E., and R. J. Lefkowitz. 2001. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 13:139-145. [DOI] [PubMed] [Google Scholar]
- 29.Obaya, A. J., M. K. Mateyak, and J. M. Sedivy. 1999. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene 18:2934-2941. [DOI] [PubMed] [Google Scholar]
- 30.Pardee, A. B. 1974. A restriction point for control of normal animal cell proliferation. Proc. Natl. Acad. Sci. USA 71:1286-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardee, A. B. 1989. G1 events and regulation of cell proliferation. Science 246:603-608. [DOI] [PubMed] [Google Scholar]
- 32.Pawson, T. 1995. Protein modules and signaling networks. Nature 373:573-580. [DOI] [PubMed] [Google Scholar]
- 33.Pawson, T. 1997. New impressions of Src and Hck. Nature 385:582-585. [DOI] [PubMed] [Google Scholar]
- 34.Peeper, D. S., et al. 1997. Ras signaling linked to the cell-cycle machinery by the retinoblastoma protein. Nature 386:177-181. (Erratum, 386:521.) [DOI] [PubMed]
- 35.Planas-Silva, M. D., and R. A. Weinberg. 1997. The restriction point and control of cell proliferation. Curr. Opin. Cell Biol. 9:768-772. [DOI] [PubMed] [Google Scholar]
- 36.Schlessinger, J. 1986. Allosteric regulation of the epidermal growth factor receptor kinase. J. Cell Biol. 103:2067-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlessinger, J., and A. Ullrich. 1992. Growth factor signaling by receptor tyrosine kinases. Neuron 9:383-391. [DOI] [PubMed] [Google Scholar]
- 38.Shen, Y., L. Xu, and D. A. Foster. 2001. Role for phospholipase D in receptor-mediated endocytosis. Mol. Cell. Biol. 21:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79:551-555. [DOI] [PubMed] [Google Scholar]
- 40.Slepnev, V. I., and P. De Camilli. 2000. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 1:161-172. [DOI] [PubMed] [Google Scholar]
- 41.Sorkin, A., and G. Carpenter. 1993. Interaction of activated EGF receptors with coated pit adaptins. Science 261:612-615. [DOI] [PubMed] [Google Scholar]
- 42.Tartakoff, A. M. 1983. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32:1026-1028. [DOI] [PubMed] [Google Scholar]
- 43.Vieira, A. V., C. Lamaze, and S. L. Schmid. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274:2086-2089. [DOI] [PubMed] [Google Scholar]
- 44.Wang, Y., S. Pennock, X. Chen, and Z. Wang. 2002. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol. Cell. Biol. 22:7279-7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Y., S. Pennock, X. Chen, and Z. Wang. 2002. Internalization of inactive EGF receptor into endosomes and the subsequent activation of endosome-associated EGF receptors. Epidermal growth factor. Sci. STKE 161:PL17. [Online.] www.stke.org/cgi/content/full/sigtrans;2002/161/p117. [DOI] [PubMed]
- 46.Wang, Z., P. Tung, and M. Moran. 1996. Association of p120 ras GAP with endocytic components and colocalization with epidermal growth factor (EGF) receptor in response to EGF stimulation. Cell Growth Differ. 7:123-133. [PubMed] [Google Scholar]
- 47.Waterman, H., I. Alroy, S. Strano, R. Seger, and Y. Yarden. 1999. The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing. EMBO J. 18:3348-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wettey, F. R., S. F. Hawkins, A. Stewart, J. P. Luzio, J. C. Howard, and A. P. Jackson. 2002. Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science 297:1521-1525. [DOI] [PubMed] [Google Scholar]
- 49.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]