Abstract
The deposition of protein aggregates in neurons is a hallmark of neurodegenerative diseases caused by polyglutamine (polyQ) proteins. We analyzed the effects of the heat shock protein (Hsp) 70 chaperone system on the aggregation of fragments of huntingtin (htt) with expanded polyQ tracts. In vitro, Hsp70 and its cochaperone Hsp40 suppressed the assembly of htt into detergent-insoluble amyloid-like fibrils in an ATP-dependent manner and caused the formation of amorphous, detergent-soluble aggregates. The chaperones were most active in preventing fibrillization when added during the lag phase of the polymerization reaction. Similarly, coexpression of Hsp70 or Hsp40 with htt in yeast inhibited the formation of large, detergent-insoluble polyQ aggregates, resulting in the accumulation of detergent-soluble inclusions. Thus, the recently established potency of Hsp70 and Hsp40 to repress polyQ-induced neurodegeneration may be based on the ability of these chaperones to shield toxic forms of polyQ proteins and to direct them into nontoxic aggregates.
Protein aggregation within neurons is a common denominator of many neurodegenerative diseases, including those caused by proteins with polyglutamine (polyQ) tract expansions (1–3). It occurs despite the presence in cells of several classes of molecular chaperones, which function in preventing misfolding and aggregation of newly synthesized and stress-denatured proteins (4, 5). Understanding whether and how molecular chaperones influence the self-association properties of the proteins involved in neurodegeneration may help to clarify the relationship of aggregates to disease (6).
Neuronal cell death in Huntington's disease (HD) occurs primarily in the cortex and striatum (7). HD is caused by dominant mutation of a gene encoding the protein huntingtin (htt) (8), a 350-kDa protein of unknown, but essential, function (9). The first exon of the HD gene contains a highly polymorphic stretch of CAG trinucleotide repeats that encode a polyQ tract. In unaffected individuals the polyQ tract typically contains between six and 39 repeats compared with 36–180 repeats in HD patients (1). It has been hypothesized that polyQ tract expansion causes a toxic gain of function, giving rise to abnormal protein–protein interactions (10, 11). Mutant forms of htt with expanded glutamine repeats form nuclear and cytoplasmic aggregates, both in transgenic mouse models and in human brain tissue (11–14). Other polyQ containing proteins that cause neurodegenerative disorders, including atrophin-1, androgen receptor, and the ataxins, form similar aggregates (1).
Aggregation of htt could be reproduced in vitro with the mutant exon 1 fragment of the protein that contains the polyQ tract (13, 15). The aggregates reveal a fibrillar morphology and are thought to result from the ability of polyQ sequences to act as “polar zippers” (3), i.e., pleated sheets of β-strands held together by hydrogen bonds between main-chain and side-chain amides.
Several lines of evidence implicate the molecular chaperone machinery and the ubiquitin/proteasome system in polyQ diseases. The nuclear inclusions containing htt are immunoreactive with antibodies to ubiquitin (11, 12) but are not readily degraded by the proteasome. In Escherichia coli and Saccharomyces cerevisiae members of the heat shock protein (Hsp)70 and Hsp40 chaperone families are essential for the rapid degradation of normal and misfolded proteins (16, 17). Affected neurons from transgenic mice expressing polyQ expanded ataxin-1 (the cause of spinocerebellar ataxia type 1) have ubiquitin-positive nuclear inclusions that contain the proteasome and the Hsp40 homolog Hdj-2/Hsdj (18). Overexpression of Hdj-2 in cultured cells suppressed the formation of nuclear inclusions by ataxin-1 (18). On the other hand, recent studies showed that increased expression of Hsp70 and Hsp40 could suppress polyQ-induced neurodegeneration in a Drosophila model without preventing the aggregation of the polyQ-expanded protein (19, 20). The mechanism by which the chaperones mitigate neurodegeneration in this experimental system remained unclear.
We analyzed the effects of members of the Hsp70 and Hsp40 families of chaperones on the aggregation behavior of htt exon 1 segments with expanded polyQ tracts. Hsp70 and Hsp40 cooperate to bind and release hydrophobic segments of unfolded polypeptides in an ATP-dependent manner (4, 21). In addition to their roles as stress proteins, they function in several important normal cellular processes, including protein folding, translocation, and degradation. Here we show that Hsp70 and Hsp40 can interact with polyQ expanded exon 1 constructs of htt and suppress or retard the formation of detergent-insoluble, fibrillar aggregates.
Materials and Methods
Plasmid Construction and Protein Purification.
E. coli constructs.
Glutathione S-transferase (GST)-HD constructs pGEX-HD20Q and pGEX-HD53Q were derived from the yeast vectors YEp105-HD20Q and YEp105-HD53Q (see below) and resulted in the expression of fusion proteins consisting of GST followed by the PreScission protease cleavage site (LEVLFQGP), nine vector-derived residues (LGSPEFIMC), a c-myc epitope (EQKLISEEDL), and exon 1 of the human HD gene containing 20 or 53 glutamines, respectively. The vector pGEX-HD53QΔP has been described (22). For the construct pGEX-MJD35Q a cDNA fragment encoding the polyQ segment was amplified by PCR from the plasmid pCMX-HAQ35 (a kind gift of A. Kakizuka, Osaka Bioscience Institute, Osaka).
Yeast constructs.
Exon 1 of the human HD gene containing 20 glutamines was excised from pCAG20 (13) and ligated into YEp105, resulting in YEp105-HD20Q. YEp105 is a derivative of YEp96 (2 μ TRP1) (23) under the control of the CUP1 promoter/CYC1 terminator that contains a 15-aa linker, which includes a c-myc epitope. Exon 1 of the human htt gene containing 39 and 53 glutamines was cloned into YEp105, generating YEp105-HD39Q and YEp105-HD53Q, respectively.
Protein Purification.
Constructs were transformed into E. coli SURE cells. Expression was induced with 1 mM isopropyl β-d-thiogalactoside (3.5 h at 28°C). Soluble fusion proteins were purified as described (22). Purification of bacterial DnaJ and DnaK (24), Hdj-1, and bovine Hsc70 (25) was performed as described.
Antibodies.
The following polyclonal antisera were used: HD730 (anti-GST-HD20QΔP; ref. 22), anti-DnaJ (Stressgen Biotechnologies, Victoria, Canada, Spa410), anti-DnaK (this study), anti-Hdj-1 (25), and anti-Hsp70 (Santa Cruz Biotechnology). Anti-Ssa, anti-Ssb, and anti-Ydj1 antibodies were kindly provided by E. Craig, University of Wisconsin, Madison.
HD Exon 1 Aggregation in Vitro.
GST-HD fusion protein (3 μM) was incubated at 30°C with 2.5 units of PreScission protease (Amersham Pharmacia) in buffer A (50 mM Tris⋅HCl, pH 7.0/150 mM NaCl/1 mM DTT/1 mM PMSF/0.5 μM leupeptin/0.5 μM pepstatinA) for up to 8 h. Cleavage was complete after 30 min. Chaperones were added together with the protease, unless stated otherwise. Reactions in the presence of ATP contained in addition 2 mM ATP, 50 mM KCl, 5 mM MgCl2 and ATP regenerating system (50 μg/ml creatine kinase and 8 mM creatine phosphate). Reactions were stopped by addition of an equal volume of 4% SDS/50 mM DTT, followed by heating at 95°C for 5 min. HD aggregates were filtered through cellulose-acetate filters (0.2 μm pore size) and detected on the filter with antiserum HD730 and the ECL system (Amersham Pharmacia).
GST-HD-chaperone complexes were isolated by adsorption to glutathione-Sepharose in buffer B (50 mM Tris⋅HCl, pH 7.0/150 mM NaCl) and extensive washes with buffer B/50 mM K-acetate. Complexes were eluted with buffer B/50 mM K-acetate containing either 15 mM reduced glutathione or 2 mM ATP/5 mM Mg-acetate.
HD Exon 1 Aggregation in Yeast Cells.
YEp105-HD constructs were transformed into yeast strain JN54 (MAT a, his3–11, 3–15 leu2–3, 2–112 lys2 trp1-D1 ura3–52) (26). Cells were grown in synthetic complete media (SC) at 30°C. High-level expression of HD exon 1 was achieved by growing the cells in the presence of 400 μM CuSO4. No significant genetic instability of CAG repeat segments was observed under the experimental conditions. For chaperone overexpression experiments, JN54 was cotransformed with YEp105–53Q and one of the following vectors under the control of the GAL1 inducible promoter: YcpGAL1-SSA1 (CEN Ura3), YcpGAL1-SSB1 (CEN Ura3) and YcpGAL1-YDJ1 (also named pYW5 and YDJ1-PRS316) (CEN Ura3) (27). Yeast were grown in SC media containing 2% raffinose until early log-phase (OD600 0.5) when galactose was added to 2%. Preparation of yeast cell lysates with glass beads, SDS/PAGE, immunoblotting, and immunoprecipitations were performed as described (28, 29). Filter-trap assays were either performed with SDS (see above) or buffer B. Aggregates were detected on the filter with anti-myc antibody.
Light and Electron Microscopy (EM).
Immunofluorescence was performed by using anti-myc antibody with goat anti-mouse CY3 antibody for detection (30). Nuclei were stained with 4′,6-diamidino-2-phenylindole.
For EM of HD aggregates GST-HD fusion proteins were cleaved with PreScission as described above. After 5 h reactions were diluted 5-fold in buffer B, adsorbed to copper grids (100 × 400 mesh; Plano, Wetzlar, Germany), stained with 2% uranyl acetate, and viewed in a Philips EM420 electron microscope.
Results
Hsp70 and Hsp40 Suppress Fibril Formation of HD Exon 1 in Vitro.
An established model system was used to characterize the effects of purified molecular chaperones on htt aggregation in vitro (13, 22, 31). Fusion proteins composed of GST and exon 1 of htt with normal (HD20Q) and expanded (HD53Q) polyQ repeats (see Fig. 3A) were expressed in E. coli and purified as soluble proteins. Cleavage of purified GST-HD53Q between the GST and HD parts by PreScission protease or factor Xa led to the formation of large aggregates that were insoluble in 4% SDS at 95°C (under reducing conditions) and were retained on a cellulose acetate filter with 0.2 μm cut-off (Fig. 1A). Proteolytic cleavage was complete after 30 min at 30°C (data not shown), but rapid aggregation was observed only after a 1.5- to 2-h lag phase (Fig. 1C), consistent with a nucleation-dependent mechanism of oligomerization (31, 32). In contrast, HD20Q remained soluble after its cleavage from GST and was not retained on the filter (Fig. 1A).
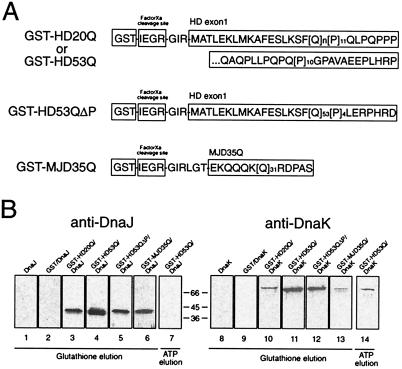
Figure 3.
Association of chaperone proteins with HD exon 1 in vitro. (A) Schematic representation of the structures of the polyQ-containing GST fusion proteins analyzed. (B) DnaJ or DnaK (6 μM each) were incubated with uncleaved GST-HD20Q, GST-HD53Q, GST-HD53QΔP, or GST-MJD35Q (3 μM each) and then precipitated with glutathione-Sepharose. GST-HD fusion proteins and associated proteins were eluted with reduced glutathione (lanes 1–6 and 8–13) or Mg-ATP (lanes 7 and 14), followed by SDS/PAGE and immunoblotting with anti-DnaJ (lanes 1–7) or anti-DnaK antibodies (lanes 8–14). Chaperones were added to Sepharose beads alone (lanes 1 and 8) or to beads containing GST (lanes 2 and 9), GST-HD20Q (lanes 3 and 10), GST-HD53Q (lanes 4, 7, 11, and 14), GST-HD53QΔP (lanes 5 and 12), or GST-MJD35Q (lanes 6 and 13).
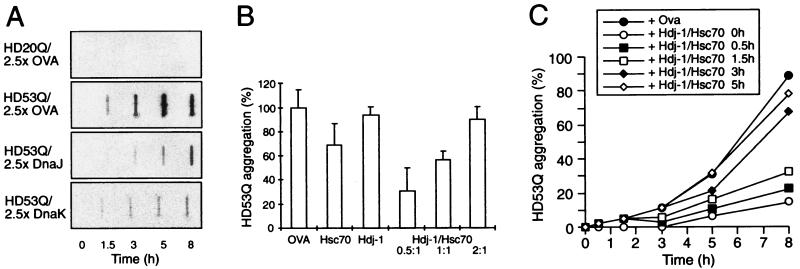
Figure 1.
Modulation of aggregation of HD exon 1 by Hsp70 and Hsp40 in vitro. (A) Time-dependent formation of SDS-insoluble aggregates of HD20Q and HD53Q (3 μM) in the presence and absence of ovalbumin (OVA) and DnaJ (Hsp40) or DnaK (Hsp70) of E. coli (7.5 μM each) in the absence of ATP as detected in filter-trap assays. Chaperones were added when aggregation was initiated by proteolytic cleavage of GST-HD fusion proteins. OVA served as a nonchaperone control protein. (B) Cooperation of mammalian Hdj-1 (Hsp40) and Hsc70 proteins in retarding SDS-insoluble HD53Q aggregation in the presence of ATP (2 mM). The ratio of Hdj-1 to Hsc70 was varied with Hsc70 and HD53Q at 3 μM. Amounts of aggregates after 8 h of incubation were quantified by Phosphorimager densitometry. Means and standard deviations of at least three independent experiments with similar results are shown. (C) Time course of formation of SDS-insoluble HD53Q aggregates with addition of Hsc70/Hdj-1/ATP (6 μM/3 μM/2 mM) occurring at different times after initiating aggregation by proteolytic cleavage of GST-HD53Q (3 μM).
Among several classes of molecular chaperones tested, including E. coli GroEL, human Hsp90, and the small Hsp αB crystallin (data not shown), only members of the Hsp70 and Hsp40 families were able to modulate the aggregation properties of HD exon 1 constructs in vitro. The chaperones did not contain cleavage sites for PreScission protease and were without effect on the cleavage of GST-HD fusion proteins (data not shown). We first tested the effects of the well-studied E. coli Hsp70 system consisting of DnaK (Hsp70) and its Hsp40 partner DnaJ. At a 2.5-fold molar excess over HD protein, both chaperones were 80–90% efficient in suppressing the formation of SDS-insoluble aggregates of HD53Q as compared with the control protein ovalbumin (Fig. 1A). Very similar results were obtained with Hsc70, the constitutively expressed Hsp70 in the mammalian cytosol (data not shown). Although DnaJ (and all other Hsp40s analyzed) acted independently of ATP, the effect of DnaK was strongly reduced by ATP, which causes the rapid release of polypeptide substrate from DnaK (21) (see Fig. 3B).
Under cellular conditions, i.e., in the presence of ATP, Hsp70 must functionally cooperate with Hsp40 to prevent the aggregation of unfolded proteins. Hsp40s accelerate the Hsp70 ATPase, thereby generating the ADP form of Hsp70 that binds substrate tightly (21, 25). To determine whether Hsp40 and Hsp70 cooperate in a similar manner with HD53Q as the substrate, human Hdj-1 was used as the Hsp40 homolog in conjunction with Hsc70. Hdj-1 was chosen in these experiments because it has only a weak chaperone activity on its own (25), due to the absence of a zinc-finger domain that is present in other Hsp40s, including E. coli DnaJ and yeast Ydj1 (see ref. 24). Indeed, Hdj-1 alone was unable to suppress the formation of SDS-insoluble HD53Q aggregates (Fig. 1B). Similarly, in the presence of ATP, Hsc70 was inefficient in this reaction (Fig. 1B). However, when Hdj-1 was added to Hsc70/ATP at a molar ratio of 0.5:1, significantly enhanced suppression of the formation of SDS insoluble HD53Q by Hsc70 was observed (Fig. 1B). This effect of Hdj-1 was seen only in the presence of ATP (data not shown). Increasing the ratio of Hdj-1/Hsc70 abolished the ability of Hsc70/ATP to suppress the formation of SDS-insoluble HD aggregates (Fig. 1B). The mechanism underlying this unexpected effect remains to be established.
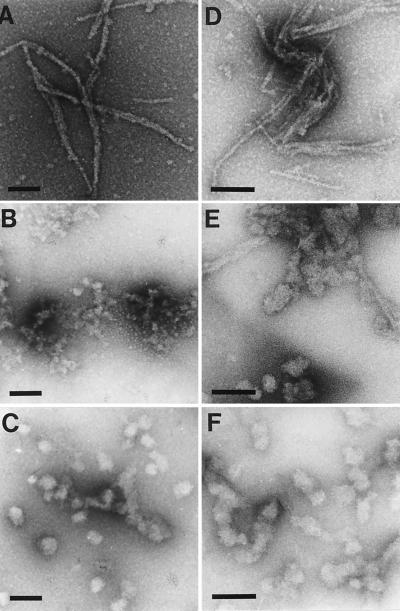
Interestingly, under the experimental conditions the chaperones prevented the formation of pelletable aggregates of HD53Q only partially (data not shown) but rather changed the physical properties of the HD aggregates from SDS insoluble to SDS soluble. EM was used to analyze the nature of the aggregates. Proteolytic cleavage of GST-HD53Q in the absence of chaperones resulted in the formation of SDS- insoluble, ordered fibrils (Fig. 2A) (13, 31). Fibrils were not observed with uncleaved GST-HD53Q (data not shown). DnaJ and DnaK (in the absence of ATP) completely prevented fibril formation and resulted in the production of small, amorphous aggregates (Fig. 2 B and C) that were SDS soluble and were not detected in the filter-trap assay (Fig. 1A). As expected, Hdj-1 alone was unable to prevent fibril formation upon cleavage of GST-HD53Q (Fig. 2D). Incubation with Hsc70/ATP resulted in the formation of a mixture of rod-shaped structures (presumably containing fibrillar material) and large clumps of HD53Q (Fig. 2E) in which the HD protein was mostly SDS insoluble (Fig. 1B). When Hdj-1 was present in addition to Hsc70/ATP, these rod-shaped aggregates were no more observed. Instead, mostly SDS-soluble, amorphous aggregates were detected (Fig. 2E). Thus, in the presence of ATP, mammalian Hsc70 and Hdj-1 effectively prevent fibrillization of polyQ expanded HD exon 1.
Figure 2.
Suppression of HD exon 1 fibril formation by Hsp40 and Hsp70 in vitro. GST-HD fusion protein (3 μM) was incubated with PreScission protease for 5 h as in Fig. 1 in the absence (A) or presence (B–F) of chaperones (6 μM). (B) DnaK; (C) DnaJ; (D) Hdj-1; (E) Hsc70/ATP; (F) Hsc70/Hdj-1/ATP (Hsc70/Hdj-1 = 2:1). Samples then were analyzed by EM. (Bar = 100 nm.)
The chaperones were effective in suppressing the formation of SDS-insoluble HD fibrils only when added during the lag phase of the aggregation reaction (Fig. 1C). Addition of chaperones after the lag phase was essentially without effect on aggregation kinetics (Fig. 1C), consistent with the view that the chaperones act preferentially on prefibrillar states of the polyQ protein during the nucleation phase of fibrillization.
Direct Association of Hsp70 and Hsp40 with HD Exon 1.
Purified DnaJ (Hsp40) and DnaK (Hsp70) was incubated with noncleaved GST-HD fusion proteins followed by precipitation with glutathione-Sepharose beads (Fig. 3A). The chaperones were specifically eluted with reduced glutathione from beads containing GST-HD20Q or GST-HD53Q (Fig. 3B, lanes 3, 4, 10, and 11), but did not interact with GST alone (Fig. 3B, lanes 2 and 9). DnaK also was eluted from GST-HD53Q upon addition of ATP (Fig. 3B, lane 14).
DnaJ and DnaK also associated with GST-HD53QΔP (Fig. 3A) lacking the C-terminal proline-rich region that follows the polyQ tract of HD exon 1 (Fig. 3B, lanes 5 and 12). Furthermore, DnaJ bound strongly to GST-MJD35Q, a fusion protein consisting of GST and a sequence derived from the expanded polyQ domain of ataxin-3 (Machado-Joseph Disease protein) (Fig. 3B, lane 6). This sequence was almost exclusively composed of a stretch of 35 glutamines flanked on either side by a few, mainly charged vector-derived amino acids (Fig. 3A) (21). The interaction of DnaK with MJD35Q was weaker but clearly detectable (Fig. 3B, lane 13). These results suggest that DnaK and DnaJ can interact directly with the regions of htt and related proteins containing the polyQ tract. In contrast to DnaK, an association of mammalian Hsc70 with uncleaved GST-HD fusion proteins could not be detected. Apparently, Hsc70 recognizes the HD protein only in a conformational state that is formed upon removal of GST. The chaperones were however recruited into SDS-insoluble HD53Q aggregates when present at low stoichiometry relative to polyQ protein (data not shown).
HD Exon 1 Aggregation in S. cerevisiae.
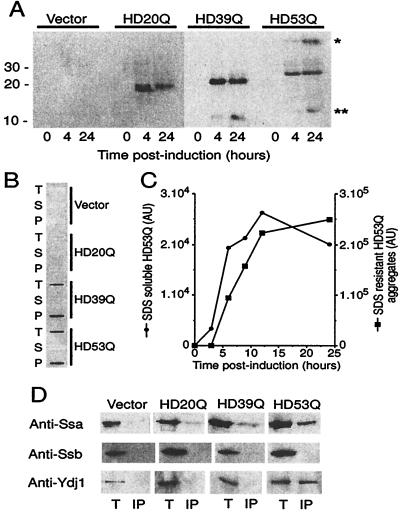
The budding yeast S. cerevisiae has been useful in the analysis of prion-like aggregation phenomena (33). To determine whether chaperones can modulate the aggregation of polyQ-expanded proteins in vivo, the exon 1 segment of htt with normal (HD20Q) and expanded (HD39Q and HD53Q) polyQ tracts was expressed in yeast under control of the Cup1 promoter. The constructs were myc-tagged at the amino terminus. In cells transformed with HD20Q, HD39Q, or HD53Q and induced with copper, protein bands of ≈18, 23 and 28 kDa, respectively were detected in total lysates by immunoblot analysis (Fig. 4A). As observed previously, the polyQ proteins migrated at an apparent molecular mass 5–8 kDa greater than predicted from their sequences (13). In lysates from cells expressing HD53Q, high-molecular weight HD exon 1 protein also was detected (Fig. 4A, *). This form of HD represented either SDS-insoluble aggregation intermediates or fragments of large SDS-insoluble deposits of HD53Q, described below, which did not migrate into the gel. In addition, lower molecular weight degradation products of the HD constructs were detected in cells expressing HD39Q and HD53Q (Fig. 4A, **). The expression of HD exon 1 fragments had no measurable effect on cell growth at 23°-37°C (data not shown).
Figure 4.
Expression and aggregation of mutant HD exon 1 in yeast. (A) Expression of myc-tagged HD20Q, HD39Q, and HD53Q was analyzed over 24 h by SDS/PAGE and immunoblotting of cell lysates with anti-myc antibodies. (B) PolyQ length-dependent formation of SDS-insoluble HD aggregates. Equal protein amounts of cell lysates were analyzed in filter-trap assays after incubation in SDS/DTT at 95°C. T, total cell lysate; S and P, supernatant and pellet fractions after centrifugation of total cell lysate (20,000 × g, 10 min), respectively. (C) Kinetics of HD expression and aggregation. Expression of SDS-soluble HD53Q and formation of SDS-insoluble HD53Q aggregates were monitored by SDS/PAGE and filter-trap assay of total cell lysates as in A. Data are plotted in arbitrary densitometer units for equal amounts of cell protein analyzed. Densitometric units for SDS-soluble and -insoluble HD53Q are only approximately comparable. (D) Association of Hsp70 and Hsp40 with HD exon 1 in yeast. Immunoprecipitation of myc-tagged HD20Q, HD39Q, and HD53Q with anti-myc antibody from total cell lysates after 24 h of expression followed by immunoblotting of precipitates with anti-Ssa (yeast Hsp70), anti-Ssb (yeast Hsp70), and anti-Ydj1 (yeast Hsp40) antibodies. Immunoprecipitations were performed in the absence of ATP. T, total lysate; IP, immunoprecipitate. Protein amounts loaded in T correspond to approximately 10% of those loaded in IP. Anti-myc immunoprecipitation was ≈50% effective.
In addition to the high molecular weight material on SDS/PAGE, lysates from cells expressing HD39Q and HD53Q, but not from cells expressing HD20Q, contained large, SDS-insoluble HD exon 1 aggregates that were detected in filter-trap assays (Fig. 4B). After 24 h of expression the majority of HD53Q was in these large aggregates (Fig. 4C). Synthesis of HD53Q as a SDS-soluble protein preceded the appearance of SDS-insoluble aggregates that formed with a lag phase of 2–3 h (Fig. 4C). The maximal concentration of the expressed HD constructs in the yeast cytosol was estimated at 5–10 μM (for soluble HD20Q) (data not shown). SDS-insoluble aggregates of HD53Q were already detectable at a concentration of approximately 0.5 μM of soluble HD53Q. For comparison, the concentration of Hsp70 proteins in the yeast and mammalian cytosol is in the range of 30 to 50 μM (ref. 34 and unpublished data). These results indicate that the aggregation properties of HD fragments with normal and expanded polyQ tracts can be faithfully reproduced in S. cerevisiae, consistent with recent observations (35).
Association of Hsp70 and Hsp40 with HD Exon 1 in Vivo.
Coimmunoprecipitation experiments were performed to determine whether the HD exon 1 segments expressed in yeast were associated with chaperones. Immunoprecipitation of myc-tagged HD53Q with anti-myc antibody from total lysates resulted in the coprecipitation of Hsp70s of the Ssa class and their Hsp40 partner protein Ydj1 (Fig. 4D), a close homolog of E. coli DnaJ and human Hdj-2. Ssa and Ydj1 did not coimmunoprecipitate with HD20Q and only Ssa associated detectably with HD39Q, although to a lower extent than with HD53Q (Fig. 4D). Thus, the association of these chaperones with HD exon 1 shows a similar dependence on the length of the polyQ tract as observed for the formation of SDS-insoluble HD aggregates.
Notably, Hsp70s of the Ssb class were not coimmunoprecipitated with any of the HD exon 1 constructs (Fig. 4D). Ssa and Ssb proteins are closely related, but functionally distinct, members of the yeast Hsp70 chaperone family. Ssb proteins interact predominantly with nascent polypeptides on ribosomes, whereas the Ssa proteins act posttranslationally (26, 34).
Hsp70 and Hsp40 Inhibit Formation of Detergent-Insoluble HD Aggregates in Vivo.
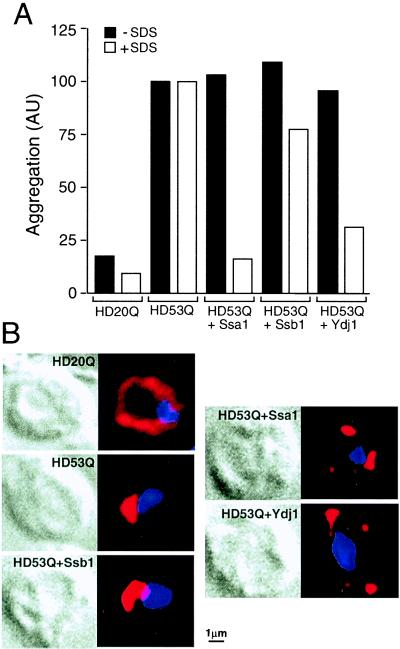
The effects of increased expression of chaperones on HD exon 1 aggregation in vivo were evaluated by coexpressing HD53Q with Hsp70 (Ssa1 or Ssb1) or Ydj1 in yeast. The chaperones were 2- to 3-fold overexpressed under control of a galactose-regulated promoter without interfering with the simultaneous induction of HD53Q expression by copper (data not shown). Strikingly, in yeast cells transformed with Ssa1 or Ydj1, but not in cells overexpressing Ssb1, highly efficient suppression of the assembly of SDS-insoluble HD53Q aggregates was observed in filter-trap assays (Fig. 5A). As in vitro, the chaperones did not suppress HD exon 1 aggregation per se but rather mediated the formation of SDS-soluble, presumably amorphous, HD53Q aggregates (Fig. 5A).
Figure 5.
Effects of overexpression of Hsp70 and Hsp40 on HD exon 1 aggregation in yeast. (A) Total yeast lysates expressing HD exon 1 constructs and chaperones for 24 h were analyzed by filter-trap assays with and without prior incubation with SDS. Cells were either transformed with YEp105HD20Q and YEp105-HD53Q alone or with YEp105-HD53Q in combination with pGAL-SSA1, pGAL-SSB1, or pGAL-YDJ1 as indicated. Amounts of aggregation are given in arbitrary units normalized to the amounts of HD aggregation detected in cells expressing HD53Q alone. Note that the small amounts of aggregates of HD20Q are close to the background levels of the assay. (B) Cells expressing either myc-tagged HD20Q or HD53Q alone or HD53Q in combination with increased levels of Ssa1, Ssb1, or Ydj1 as in A were analyzed by immunofluorescence. (Left) Differential interference contrast photographs. (Right) HD fluorescence (red). DNA was stained with 4′,6-diamidino-2-phenylindole (blue). Examples representative of the majority of yeast cells examined are shown. Upon overexpression of Ssa1 or Ydj1 cells typically contained 3–5 distinct HD53Q deposits.
Yeast cells expressing HD20Q and HD53Q for 24 h were analyzed by indirect immunofluorescence (Fig. 5B). HD20Q displayed a diffuse cytoplasmic staining. In contrast, HD53Q was typically concentrated in a single large (≈1–2 μm in diameter), mostly peri-nuclear aggregate (Fig. 5B). Coexpression of Ssa1 or Ydj-1, but not of Ssb1, resulted in a decrease in the size of the HD53Q aggregates to ≈0.3–1 μm and an increase in their number, but not in diffuse cytoplasmic staining (Fig. 5B). The fluorescence intensity and the general appearance of the aggregates remained similar, although the inclusions formed upon chaperone overexpression were SDS soluble (Fig. 5A). This change in physical properties of the deposits may reflect the inhibition by the Hsp70 system of the formation of amyloid-like, SDS-insoluble HD fibrils in yeast in vivo.
Discussion
At least eight inherited neurodegenerative disorders are caused by otherwise unrelated proteins containing polyQ expansions (1, 6). Here we have shown that the cellular machinery of molecular chaperones is able to profoundly change the aggregation properties of these proteins in vitro and in vivo. Hsp70 and certain Hsp40 family members interact with fragments of htt containing the polyQ tract and, as shown in vitro, inhibit the ordered assembly of SDS-resistant HD exon 1 fibrils. This ability to prevent fibrillization may underlie the recently demonstrated activity of the Hsp70/Hsp40 system to mitigate the neurotoxicity of polyQ expanded proteins in a Drosophila disease model (19, 20).
Chaperone Effects on HD Exon 1 Aggregation in Vitro and in Vivo.
Hsp70 is known to preferentially recognize extended peptide segments that are enriched in hydrophobic residues, such as leucine and isoleucine (21). Yet, Hsp70 binding to HD exon 1 apparently involves an interaction with the polyQ-containing segment of the protein (Fig. 3B) whose polar glutamines are thought to act as hydrogen donors and acceptors in forming fibrils rich in β-sheet structure (3). Although the flanking regions of the polyQ tract may participate in the chaperone interaction, both Hsp70 as well as DnaJ and Ydj1 associate with HD exon 1 in a manner dependent on the length of the polyQ segment. The ability of these chaperones to shield the polyQ tract can explain their striking capacity to prevent or retard HD fibrillization. In contrast, the Hsp40 homolog Hdj-1, which lacks the zinc finger domain present in DnaJ and Ydj1, is inefficient in suppressing HD fibrillization (Figs. 1B and 2D), but strongly enhances the ability of mammalian cytosolic Hsc70 in modulating HD exon 1 aggregation in an ATP-dependent process (Figs. 1B and 2F).
Unlike E. coli DnaK, mammalian Hsc70 does not associate detectably with nonaggregated HD exon 1 fused to GST but rather recognizes the protein in a conformational state that is generated upon cleavage from the GST domain, but is not yet committed to fibrillization. Consistent with this interpretation the chaperones are most effective in suppressing fibril formation when added during the lag phase of polymerization (Fig. 1C). However, under the experimental conditions used the interaction of Hsp70 and Hsp40 with the polyQ protein does not prevent (and perhaps even promotes) the formation of SDS-soluble, amorphous aggregates (Fig. 4).
Upon expression in yeast, polyQ expanded exon 1 constructs of htt are first soluble in the cytosol, but after a critical cellular concentration is reached, large SDS-resistant aggregates form (Figs. 4 and 5). SDS-insoluble inclusions are also present in the brain of HD patients, and amyloid-like fibrils have been detected by EM in the SDS-insoluble HD exon 1 aggregates isolated from COS-1 cells (31). Thus, it is likely that the large, SDS-resistant deposits formed by mutant exon 1 in yeast in vivo represent the cellular equivalent of the SDS-insoluble exon 1 fibrils formed in vitro.
Increased expression of Hsp70 and Hsp40 chaperones in the yeast cytosol modulates the physical properties of HD exon 1 aggregates in a manner remarkably similar to that observed in vitro. The chaperones retard or suppress the formation of SDS-insoluble HD aggregates in favor of the formation of smaller, SDS-soluble inclusions (Fig. 5). Similar results were obtained upon simultaneous overexpression of human Hsp70 and Hsp40 (Hdj-1) in COS-1 cells (A.S. and E.E.W., unpublished observations). The association of yeast Hsp70 (Ssa) and Hsp40 (Ydj1) with detergent-soluble HD exon 1 (Fig. 4D) suggests that, as in vitro, the chaperones act on early aggregation intermediates.
Pathomechanistic Implications.
Recent observations in a Drosophila disease model demonstrated the potential of Hsp70 and Hsp40 (Hdj-1) to suppress the neurotoxicity of polyQ expanded protein fragments (19, 20). Interestingly, overexpression of Hsp70 or Hdj-1 in the fly eye did not prevent the formation of polyQ containing aggregates in neurons as judged by immunofluorescence. Our findings suggest a possible explanation for these observations: Hsp70 chaperones may suppress neurodegeneration by retarding the accumulation of amyloid-like aggregates, or their precursors, and by causing the formation of nonamyloid, detergent-soluble polyQ inclusions. As seen in the yeast model, the two forms of aggregate do not necessarily differ dramatically in their appearance by light microscopy but the detergent-soluble aggregates generated at elevated chaperone levels eventually may be disposed of by the proteasome machinery. It is also possible that, by shielding amyloidogenic precursors, the chaperones prevent the recruitment of other glutamine-rich proteins into stable aggregates and, hence, their functional inactivation that may lead to neurodegeneration. The chaperones may act essentially by channeling toxic protein into nontoxic aggregates, thus preventing or delaying disease initiation and perhaps slowing disease progression.
Searching for ways to increase the levels of Hsp70/Hsp40 in neurons may open up promising avenues for a therapy of polyQ expansion diseases and perhaps other neurodegenerative disorders caused by amyloidogenic proteins.
Acknowledgments
We thank M. Knop, M. Matzer, and G. Pfeiffer for valuable help with immunofluorescence and EM experiments, and E. Craig and S. Lindquist for generously providing yeast strains and plasmids. P.J.M. is supported by a fellowship from the Hereditary Disease Foundation. E.E.W. is supported by grants from the Huntington's Disease Society of America, Deutsche Forschungsgemeinschaft, and the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie.
Abbreviations
- HD
Huntington's disease
- htt
huntingtin
- polyQ
polyglutamine
- Hsp
heat shock protein
- GST
glutathione S-transferase
- EM
electron microscopy
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140202897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140202897
References
- 1.Zoghbi H Y, Orr H T. Curr Opin Neurobiol. 1999;9:566–570. doi: 10.1016/S0959-4388(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 2.Martin J B. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 3.Perutz M F. Trends Biochem Sci. 1999;24:58–63. doi: 10.1016/s0968-0004(98)01350-4. [DOI] [PubMed] [Google Scholar]
- 4.Hartl F U. Nature (London) 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J L, Craig E A. Cell. 1997;90:201–204. doi: 10.1016/s0092-8674(00)80327-x. [DOI] [PubMed] [Google Scholar]
- 6.Sisodia S S. Cell. 1998;95:1–4. doi: 10.1016/s0092-8674(00)81743-2. [DOI] [PubMed] [Google Scholar]
- 7.Vonsattel J P, Myers R H, Stevens T J, Ferrante R J, Bird E D, Richardson E P., Jr J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 8.The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 9.Nasir J, Floresco S B, O'Kusky J R, Diewert V M, Richman J M, Zeisler J, Borowski A, Marth J D, Phillips A G, Hayden M R. Cell. 1995;81:811–823. doi: 10.1016/0092-8674(95)90542-1. [DOI] [PubMed] [Google Scholar]
- 10.Trottier Y, Lutz Y, Stevanin G, Imbert G, Devys D, Cancel G, Saudou F, Weber C, David G, Tora L, et al. Nature (London) 1995;378:403–406. doi: 10.1038/378403a0. [DOI] [PubMed] [Google Scholar]
- 11.Davies S W, Turmaine M, Cozens B A, DiFiglia M, Sharp A H, Ross C A, Scherzinger E, Wanker E E, Mangiarini L, Bates G P. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 12.DiFiglia M, Sapp E, Chase K O, Davies S W, Bates G P, Vonsattel J P, Aronin N. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 13.Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates G P, Davies S W, Lehrach H, Wanker E E. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 14.Becher M W, Kotzuk J A, Sharp A H, Davies S W, Bates G P, Price D L, Ross C A. Neurobiol Dis. 1998;4:387–397. doi: 10.1006/nbdi.1998.0168. [DOI] [PubMed] [Google Scholar]
- 15.Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, Bates G P, Lehrach H, Wanker E E. Proc Natl Acad Sci USA. 1999;96:4604–4609. doi: 10.1073/pnas.96.8.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes S A, Dice J F. J Cell Biol. 1996;132:255–258. doi: 10.1083/jcb.132.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottesman S, Wickner S, Maurizi M R. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 18.Cummings C J, Mancini M A, Antalffy B, DeFranco D B, Orr H T, Zoghbi H Y. Nat Genet. 1998;19:148–154. doi: 10.1038/502. [DOI] [PubMed] [Google Scholar]
- 19.Warrick J M, Chan H Y E, Gray-Board G L, Chai Y H, Paulson H L, Bonini N M. Nat Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 20.Kazemi-Esfarjani P, Benzer S. Science. 2000;287:1837–1840. doi: 10.1126/science.287.5459.1837. [DOI] [PubMed] [Google Scholar]
- 21.Bukau B, Horwich A L. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 22.Wanker E E, Scherzinger E, Heiser V, Sittler A, Eickhoff H, Lehrach H. Methods Enzymol. 1999;309:375–386. doi: 10.1016/s0076-6879(99)09026-6. [DOI] [PubMed] [Google Scholar]
- 23.Ecker D J, Khan M I, Marsh J, Butt T R, Crooke S T. J Biol Chem. 1987;262:3524–3527. [PubMed] [Google Scholar]
- 24.Szabo A, Korszun R, Hartl F U, Flanagan J. EMBO J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- 25.Minami Y, Hohfeld J, Ohtsuka K, Hartl F U. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 26.Nelson R J, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig E A. Cell. 1992;71:97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- 27.Stone D E, Craig E A. Mol Cell Biol. 1990;10:1622–1632. doi: 10.1128/mcb.10.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewalt K L, Hendrick J P, Houry W A, Hartl F U. Cell. 1997;90:491–500. doi: 10.1016/s0092-8674(00)80509-7. [DOI] [PubMed] [Google Scholar]
- 29.Siegers K, Waldmann T, Leroux M R, Grein K, Shevchenko A, Schiebel E, Hartl F U. EMBO J. 1999;18:75–84. doi: 10.1093/emboj/18.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knop M, Pereira G, Geissler S, Grein K, Schiebel E. EMBO J. 1997;16:1550–1564. doi: 10.1093/emboj/16.7.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, Bates G P, Lehrach H, Wanker E E. Proc Natl Acad Sci USA. 1999;96:4604–4609. doi: 10.1073/pnas.96.8.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochet J-C, Landsbury P T. Curr Opin Struct Biol. 2000;10:60–68. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 33.Lindquist S. Cell. 1997;89:495–498. doi: 10.1016/s0092-8674(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 34.Pfund C, Lopez-Hoyo N, Ziegelhoffer T, Schilke B A, Lopez-Buesa P, Walter W A, Wiedmann M, Craig E A. EMBO J. 1998;17:3981–3989. doi: 10.1093/emboj/17.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krobitsch S, Lindquist S. Proc Natl Acad Sci USA. 2000;97:1589–1594. doi: 10.1073/pnas.97.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]