Abstract
LDL receptor-related protein 6 (LRP6) is a Wnt coreceptor in the canonical signaling pathway, which plays essential roles in embryonic development. We demonstrate here that wild-type LRP6 forms an inactive dimer through interactions mediated by epidermal growth factor repeat regions within the extracellular domain. A truncated LRP6 comprising its transmembrane and cytoplasmic domains is expressed as a constitutively active monomer whose signaling ability is inhibited by forced dimerization. Conversely, Wnts are shown to activate canonical signaling through LRP6 by inducing an intracellular conformational switch which relieves allosteric inhibition imposed on the intracellular domains. Thus, Wnt canonical signaling through LRP6 establishes a novel mechanism for receptor activation which is opposite to the general paradigm of ligand-induced receptor oligomerization.
The Wnt family of secreted signaling molecules is essential in embryonic induction, cell polarity generation, and cell fate specification (46). Deregulation of Wnt signaling results in defects in development and growth control. The canonical Wnt pathway involves activation of β-catenin-dependent transcription and is evolutionarily conserved from Caenorhabditis elegans to humans. Mutations in components, which constitutively activate canonical signaling, have been identified in several tumor types, including colorectal cancer (34). Wnts bind to two coreceptors, the Frizzled-type seven-transmembrane-domain receptor (5, 17, 19, 49) and the low-density receptor-related protein (LRP) 5/6 (35, 41) or the Drosophila melanogaster ortholog Arrow (44). These interactions cause β-catenin stabilization through inhibition of its phosphorylation by glycogen synthase kinase 3β (GSK3β), which is assembled in a large cytoplasmic complex that includes Dishevelled, casein kinase I, Axin, APC, and Frat (36). As a consequence, stabilized cytoplasmic β-catenin is translocated to the nucleus and forms a complex with a family of high-mobility group-like transcription factors, including leukocyte enhancer factor-1 (LEF-1) and T-cell factors (TCF), activating transcription of target genes (4).
Frizzled family members have been shown to possess various affinities for different Wnt ligands. For example, Drosophila frizzled, Dfz1 and Dfz2, bind to Wingless (Wg), the Drosophila orthologue of Wnt, to activate the canonical pathway. However, Dfz2 has a 10-fold higher affinity for Wg than Dfz1 and plays a predominant role in transducing the Wg canonical signal in vivo (37). Frizzled family members also signal through the planar polarity pathway, which similarly involves Dsh (25) but is mediated through JNK and RhoA rather than β-catenin stabilization (7). In Drosophila, Dfz1 but not Dfz2 appears to regulate planar polarity signaling (8, 37).
LRP5, LRP6, and arrow receptors specifically function in the canonical pathway. Inactivation of arrow in Drosophila results in a phenotype similar to that of the wingless mutant (41), and mice deficient in LRP6 exhibit developmental defects resembling those caused by loss of various Wnt proteins (35). Injections of LRP6 expression RNA in Xenopus embryos enhanced Wnt-induced developmental effects (41). Thus, genetic evidence from Drosophila and the mouse indicate that both frizzled and LRP6 receptors are essential for canonical Wnt signal transduction in embryonic development. Biochemical interaction studies (5, 41) support a dual-receptor model in which independent binding to both frizzled and LRP6 by Wnts recruits these two types of receptors into a complex and elicits signaling to downstream components (32).
LRP5 loss-of-function mutations cause the human autosomal recessive disorder osteoporosis-pseudoglioma syndrome (18). In contrast, a point mutation substituting valine for glycine at codon 171 in LRP5 results in the autosomal-dominant high-bone-mass trait in humans (9, 26). This mutation appears to impair the ability of Dkk-1, an LRP binding antagonist of Wnt signaling, to inhibit LRP5 function (9). These studies strongly suggest that Wnt canonical signaling also controls important aspects of skeletal biology.
Recent studies have provided biochemical insights into the mechanism involved in Wnt signaling through LRP. Axin, a key scaffolding protein, which tethers β-catenin to GSK3β for phosphorylation and subsequent degradation, was shown to bind to the LRP5 cytoplasmic domain both in vitro and in vivo (28). A truncation mutant lacking the LRP5 extracellular domain was found to be constitutively active, implying that the extracellular domain exerts an inhibitory effect on signaling through this receptor (28). However, the mechanism by which Wnt triggers canonical signaling through its interactions with the LRP receptor has not been elucidated. In the present study we demonstrate that the LRP6 receptor forms an inactive oligomer, and the extracellular YWTD-EGF repeats are shown to be responsible for oligomerization. Deletion of the YWTD-EGF repeats converts LRP6 to an active monomer, whereas forced dimerization of such a constitutively active mutant inhibits its signaling activity and binding to Axin. Finally, we demonstrate that Wnts activate LRP6 by inducing an intracellular conformational switch from the inactive oligomeric to the active monomeric state. These findings establish a novel mechanism for ligand activation of a single-pass transmembrane receptor.
MATERIALS AND METHODS
cDNA constructs.
To generate a Flag-tagged full-length LRP6 construct, the 5′ half of the human LRP6 coding region immediately following the signal peptide sequence (residues 20 to 701) was amplified by PCR with a 3′ primer from a unique internal EcoRI site. It was then ligated to the 3′ coding region through this EcoRI site and was cloned into the pFlag-CMV1 vector (Sigma) with an NH2-terminal signal peptide and Flag tag sequences. The myc-tagged full-length LRP6 was previously described (3). To construct LRP6 deletion mutants, primers were selected to amplify the corresponding regions as indicated in Fig. 1A and 2A. The mutant cDNAs were then inserted downstream of the signal peptide and tag sequences in the vector. The 24-kDa NH2-terminal fragment of DNA gyrase B subunit (GyrB) (16) was amplified from DH5α genomic DNA and was inserted into the pFlag-CMV1 vector upstream of either the human fibroblast growth factor receptor (FGFR) 2 transmembrane and cytoplasmic (TMC) domain or the LRP6 TMC domain, each of which was amplified by PCR, to obtain the GyrB-FGFR or GyrB-LRP6 construct, respectively. For the chimeric LRP6-FGFR receptor construct, primers were used to amplify the LRP6 coding region between the internal EcoRI site and the transmembrane domain sequence (residues 702 to 1370) and were inserted into the pFlag-CMV1 vector containing the LRP6 coding region upstream of the EcoRI site (residues 20 to 701) to reconstitute the entire LRP6 extracellular domain and then were ligated to the FGFR2 TMC domain. All constructs were confirmed by DNA sequencing. Detailed cloning information is available upon request. Hemagglutinin (HA)-tagged mouse Wnt-1 and -3a cDNA clones in the pLNCX vector under the control of the cytomegalovirus promoter have previously been reported (40).
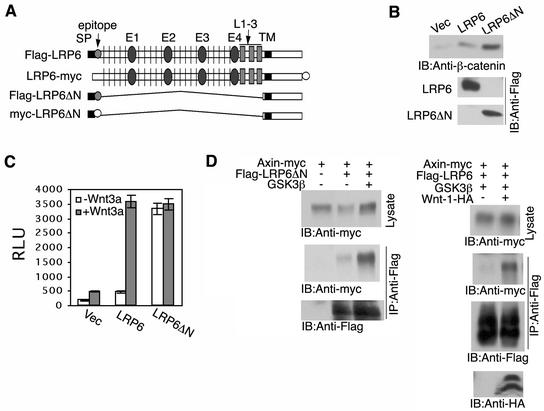
FIG. 1.
Extracellular domain deletion converts an inactive LRP6 to a constitutively active receptor. (A) Schematic representation of LRP6 and of extracellular domain-deleted LRP6 constructs (Flag or myc epitope tagged). Signal peptide (SP), EGF repeats (E1 to E4) separated by YWTD (vertical line) containing spacers, LDLR repeats 1 to 3 (L1-3), and transmembrane domain (TM) preceding the intracellular domain. (B) Comparison of β-catenin stabilization by LRP6ΔN and wild-type LRP6 receptors. At 48 h following transfection of 293T cells with 0.5 μg of either vector, LRP6, or LRP6ΔN, cell lysates were subjected to uncomplexed β-catenin analysis as described in Materials and Methods. IB, immunoblot. (C) Comparison of TCF signaling by LRP6ΔN and wild-type LRP6 receptors. 293T cells were transfected with 0.5 μg of vector (Vec), Flag-LRP6, or Flag-LRP6ΔN in the presence or absence of Wnt3a (0.1 μg) in 6-well dishes. In addition, the TCF reporter construct pGL3-OT was transfected at 1 μg, and the control luciferase construct, pRL-CMV, was transfected at 5 ng unless otherwise indicated. Relative luciferase units (RLU) were normalized against Renilla luciferase activity at 48 h after transfection. Results are expressed as the means ± standard deviations of two independent experiments performed in duplicate. (D) Ligand-dependent recruitment of Axin to the wild-type LRP6 receptor. Immunoprecipitation (IP) and immunoblot analyses were performed following transfection of 293T cells with 1 μg of each plasmid as indicated.
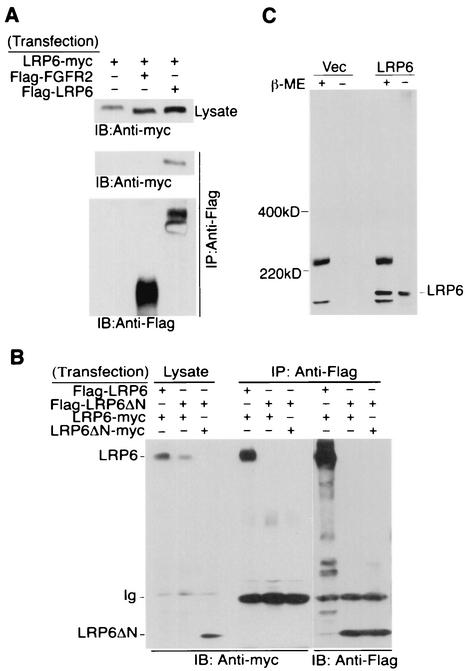
FIG. 2.
Oligomerization of the wild-type LRP6 receptors but not the mutant receptor lacking the extracellular domain. (A) Oligomerization of the wild-type LRP6 receptor. Forty-eight hours after transfection of 293T cells with 1 μg of each plasmid as indicated, immunoprecipitation (IP) and immunoblot (IB) analyses were performed from 1 mg of cell lysate. (B) Comparison of receptor complex formation by LRP6ΔN and LRP6. At 48 h following transfection of 293T cells with 2 μg each of Flag- or myc-tagged LRP6 constructs as indicated, immunoprecipitation with anti-Flag antibody was performed by using 1 mg of total cell lysate and was followed by immunoblotting with anti-myc antibody. (C) Comparison of LRP6 receptor migration under reducing and nonreducing conditions. Equal amounts of either vector or Flag-LRP6 expressing lysates were subjected to SDS-PAGE in the presence (+) or absence (−) of reducing agent β-mercaptoethanol (β-ME). Immunoblotting was performed with anti-Flag antibody. Ig, immunoglobulin.
Cell culture and transfection.
The 293T human embryonic kidney cell line was maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Transient transfection was performed by using Fugene (Roche) according to the manufacturer's instructions.
Immunoprecipitation and immunoblotting.
At 48 h posttransfection cells were washed once with phosphate-buffered saline (PBS) and were lysed on ice in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 25 mM NaF, 2 mM sodium orthovanadate, supplemented with the following proteinase inhibitors: 2 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, and 5 μg of leupeptin/ml). Lysates were cleared by centrifugation at 14,000 × g for 20 min at 4°C. Protein concentrations were then determined by using the bicinchoninic acid protein assay kit (Pierce). One or 2 mg of total cellular protein per sample was subjected to immunoprecipitation by using anti-Flag M2 agarose beads (Sigma). Beads were washed three times with lysis buffer containing 1 M NaCl and were resuspended in sodium dodecyl sulfate (SDS) loading buffer followed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were detected with corresponding anti-tag antibody, i.e., anti-Flag M2 monoclonal antibody (Sigma), anti-myc 9E10 monoclonal antibody (Santa Cruz), or anti-HA monoclonal antibody (Santa Cruz), followed by using the ECL detection system (Amersham). For detection of receptor tyrosine phosphorylation, anti-p-Tyr polyclonal antibody (Upstate) was used. Protein bands were semiquantitated by densitometry (GS-800; Bio-Rad).
Uncomplexed β-catenin analysis.
The glutathione S-transferase (GST)-E-cadherin binding assay was performed as described previously (1). Briefly, bacterially expressed GST-E-cadherin was purified with glutathione-Sepharose beads and was incubated with 1 mg of each cell lysate. GST-E-cadherin/β-catenin complexes bound to the beads were recovered by centrifugation and were analyzed by SDS-PAGE followed by immunoblotting with anti-β-catenin antibody (Transduction Laboratories).
Luciferase reporter assay.
293T cells were plated into 6-well dishes and were transiently transfected with combinations of Wnt, Hfz1, and LRP6 plasmid constructs in addition to the TOPFLASH TCF luciferase construct (generously provided by B. Vogelstein, Johns Hopkins Oncology Center, Baltimore, Md.) and the Renilla luciferase pRL-CMV construct. Relative luciferase units (RLU) were normalized against Renilla luciferase activity at 48 h after transfection. Results are expressed as means ± standard deviations of two independent experiments performed in duplicate.
Analysis of surface expression of wild-type and mutant LRP6 receptors by flow cytometry.
At 48 h following transfection of 293T cells with Flag-tagged wild-type or mutant LRP6 constructs, cells were washed twice with PBS and were suspended in PBS-0.1% EDTA. Around 5 × 105 cells were incubated in suspension with a 10-μg/ml concentration of either anti-myc antibody (negative control) or anti-Flag antibody for 1 h in blocking buffer containing 1% fetal bovine serum in PBS. Cells were then washed twice with blocking buffer and were incubated in fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G antibody (Sigma) at a 1:100 dilution in blocking buffer for 1 h. Cells were washed twice with blocking buffer and were fixed in 1% paraformaldehyde (pH 7.5). All procedures were performed at 4°C. A FACScan instrument (Becton Dickinson Immunocytometry Systems) was used for analysis.
Cell surface chemical cross-linking.
The entire procedure of cell surface chemical cross-linking was performed on intact cells as previously described (21). At 48 h following transfection of 293T cells with 2 μg of each LRP6 construct the cells were washed twice with PBS and were incubated for 2 h at 4°C with freshly prepared membrane-impermeable cross-linker solution, bis(sulfosuccinimidyl) suberate (BS3; Pierce), at 3 mg/ml in PBS without Ca2+ and Mg2+. The cells were then washed three times with PBS and were incubated for 20 min in PBS solution containing 50 mM Tris-HCl (pH 7.5) to quench residual BS3 followed by incubation in lysis buffer. Cells lysates were cleared by centrifugation and were immunoprecipitated with anti-Flag M2 agarose beads. Samples were then subjected to SDS-PAGE and immunoblotting with anti-Flag antibody.
RESULTS
Extracellular domain deletion converts an inactive LRP6 to a constitutively active receptor.
Recent studies have demonstrated that an N-terminally truncated LRP5 mutant lacking the extracellular domain was constitutively active for canonical signaling (28). In order to extend these observations to LRP6, we generated full-length LRP6 and LRP6ΔN expression vectors (Fig. 1A) and measured their effects on expression levels of uncomplexed β-catenin (1). Exogenous expression of LRP6 was associated with a low basal level of stabilized β-catenin, while LRP6ΔN induced a much higher level at a lower protein expression level (Fig. 1B). The TOPFLASH luciferase reporter contains TCF-binding sites and can be directly activated by the β-catenin/TCF complex (24). When TCF signaling was measured by this approach, wild-type LRP6 exhibited only a low basal activity (Fig. 1C), which was dramatically increased in response to cotransfection with Wnt3a, a Wnt ligand known to activate the β-catenin signaling pathway in mammalian cells (37). As previously reported for LRP5ΔN (28), LRP6ΔN exhibited a constitutively high level of TCF signaling activity, which was not influenced by Wnt (Fig. 1D).
The LRP5ΔN has been reported to interact with Axin, and the efficiency of this interaction was increased in response to exogenous expression of GSK-3β (28). As shown in Fig. 1D (left panel), LRP6ΔN was specifically coimmunoprecipitated with Axin, and the efficiency was enhanced by GSK3β cotransfection. Under the same conditions, the exogenously expressed LRP6 receptor did not or only very weakly interacted with Axin, and Wnt-1 significantly enhanced this interaction (Fig. 1D, right panel). All of these results indicated that the extracellular domain of the LRP6 receptor inhibits function of its intracellular domain and that Wnt ligands act to relieve this inhibition.
Wild-type LRP6 receptor but not LRP6ΔN forms a homomeric complex.
There is extensive evidence that oligomer formation plays a mechanistic role in the activation of many receptors (27). Thus, we next investigated whether the structure of the LRP6 receptor might provide insights concerning its ability to signal by coexpressing Flag and myc-tagged wild-type receptors in 293T cells and performing coimmunoprecipitation analysis. As shown in Fig. 2A, wild-type LRP6 receptors possessing different tags were detected within the same complex, while an unrelated receptor, FGFR2, did not show any interaction with LRP6 under the same conditions. Since the signaling potential of LRP6 alone was dramatically upregulated by deleting the extracellular domain, we next compared the receptor complex formation ability of the wild-type receptor with that of the LRP6ΔN mutant by coimmunoprecipitation analysis. As shown in Fig. 2B, while the wild-type receptor was detected as a homomeric complex, the mutant receptor failed to demonstrate detectable interaction either with itself or with the wild-type LRP6. These findings indicated that wild-type LRP6 existed as an inactive homomeric oligomer, while the constitutively active LRP6ΔN mutant was monomeric.
To investigate whether intermolecular disulfide linkages were responsible for LRP6 dimer formation, we compared LRP6 receptor migration under reducing and nonreducing conditions. As shown in Fig. 2C, the majority of the LRP6 protein migrated similarly under both conditions, indicating that the LRP6 homomeric complex detected by coimmunoprecipitation shown in Fig. 2B did not involve intermolecular disulfide linkage. Studies published after submission of this manuscript indicate that chaperone proteins are required for LRP6 to achieve a cell surface location when expressed in COS cells and that aberrant disulfide bridges leads to the accumulation of predominately high-molecular-weight aggregates LRP5 or 6 under nonreducing conditions (13, 20). Since the great majority of LRP6 migrated at the size of the monomer when expressed in 293T cells and analyzed under nonreducing conditions (Fig. 2C), these chaperones do not appear limiting under the conditions of exogenous LRP6 expression in 293T cells.
Extracellular domain YWTD-EGF repeat regions are responsible for LRP6 oligomer formation.
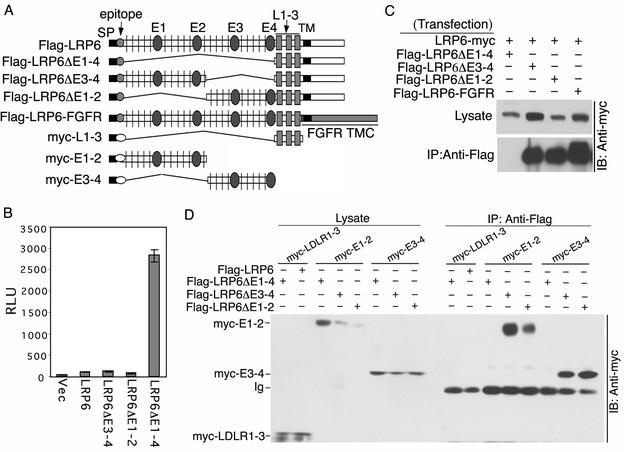
To map the region required for the LRP6 receptor oligomerization, we generated several additional LRP6 receptors with different deletions within the extracellular domain (Fig. 3A). LRP6ΔE1-4 deleted all four YWTD-EGF repeats but retained the LDL receptor (LDLR) repeats, while LRP6ΔE3-4 or LRP6ΔE1-2 lacked the first or last two YWTD-EGF repeats, respectively. Figure 3B shows that like LRP6ΔN, the LRP6ΔE1-4 mutant receptor was constitutively active, while LRP6ΔE3-4 and LRP6ΔE1-2 exhibited low basal activities comparable to that of wild-type LRP6. To investigate the ability of these mutants to form complexes, we coexpressed each Flag-tagged mutant receptor with the myc-tagged wild-type LRP6 followed by coimmunoprecipitation with anti-Flag antibody. The constitutively active LRP6ΔE1-4 mutant failed to form complexes with wild-type LRP6, while both inactive LRP6ΔE3-4 and LRP6ΔE1-2 were able to do so (Fig. 3C), further correlating constitutive signaling activity with the inability to form receptor complexes. These results also indicated that the YWTD-EGF repeat subdomains but not the LDLR repeat subdomain within the LRP6 extracellular domain were involved in receptor oligomer formation.
FIG. 3.
Extracellular domain YWTD-EGF repeat regions are responsible for LRP6 oligomer formation. (A) Schematic representation of additional LRP6 receptor deletion constructs (Flag or myc tagged) and of secreted extracellular subdomains (myc tagged). The transmembrane (TM) and cytoplasmic (C) domains of LRP6 were replaced by the TMC domains of FGFR2 to generate the LRP6-FGFR chimeric receptor. SP, signal peptide. (B) Comparison of signaling activities of different LRP6 deletion mutants in a TCF luciferase reporter assay. Analysis was performed following transfection of 293T cells with 0.25 μg of each LRP6 construct per well as described in the legend to Fig. 1C. (C and D) Comparison of receptor complex formation by different LRP6 mutants or extracellular subdomains. At 48 h following transfection of 293T cells with 2 μg of each LRP6 construct as indicated, immunoprecipitation (IP) with anti-Flag antibody and immunoblotting (IB) with anti-myc antibody were performed as described in the legend to Fig. 1D. All Flag-tagged constructs were expressed at comparable levels and immunoprecipitated efficiently with anti-Flag antibody (data not shown). Vec, vector; Ig, immunoglobulin.
To confirm that the YWTD-EGF repeat subdomains were responsible for oligomer formation, we generated secretable myc-tagged fragments, including E1-2 (YWTD-EGF repeats 1 and 2), E3-4 (YWTD-EGF repeats 3 and 4), and the LDLR repeats (Fig. 3A). Each subdomain was coexpressed with different Flag-tagged LRP6 receptor mutants followed by immunoprecipitation of the Flag-tagged receptors. As shown in Fig. 3D, the LDLR repeats failed to interact with either the LRP6ΔE1-4 mutant or the wild-type receptor. In contrast, both E1-2- and E3-4-secreted subdomains formed complexes with LRP6ΔE3-4 or LRP6ΔE1-2 but not with LRP6ΔE1-4. All of these results established that the extracellular YWTD-EGF repeat subdomains were responsible for LRP6 oligomer formation.
LRP6 receptor dimers are present at the cell surface.
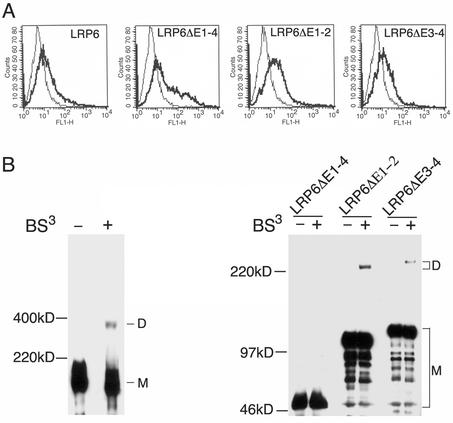
Several reports have shown that in human embryonic kidney 293 cells, exogenously expressed LRP6 reaches the cell surface and mediates Wnt signaling and Dkk-1 binding (3, 29, 30). To compare the surface expression of various LRP6 receptor constructs, we subjected 293T transfectants to fluorescence-activated cell sorter (FACS) analysis with anti-Flag antibody. As shown in Fig. 4A, N-terminally Flag-tagged wild-type LRP6, LRP6ΔE1-4, LRP6ΔE1-2, and LRP6ΔE3-4 were each readily detectable by Flag antibody staining on the surface of transfected cells.
FIG. 4.
Homodimerization of LRP6 receptors on the cell surface. (A) Analysis of cell surface expression of wild-type and mutant LRP6 receptors. Forty-eight hours following transfection of 293T cells with 2 μg of Flag-tagged wild-type LRP6, LRP6ΔE1-4, LRP6ΔE1-2, or LRP6ΔE3-4 construct, living cells were processed for immunostaining with anti-myc (control) or anti-Flag antibody and were subjected to FACS analysis as described in Materials and Methods. (B) Dimerization of wild-type and mutant LRP6 receptors on the cell surface. Forty-eight hours following transfection of 293T cells with 2 μg of Flag-tagged wild-type LRP6, LRP6ΔE1-4, LRP6ΔE1-2, or LRP6ΔE3-4 construct, cross-linking was performed on intact cells in the presence (+) or absence (−) of BS3 as described in Materials and Methods. Cell lysates were immunoprecipitated with anti-Flag M2 agarose beads and were subjected to SDS-PAGE followed by immunoblotting with anti-Flag antibody. M, monomer; D, dimer.
To establish that the LRP6 receptor is oligomerized at the cell surface, BS3-mediated chemical cross-linking was performed as previously reported (21) on intact 293T cells transiently transfected with the same Flag-tagged receptors. BS3, which reacts with free NH2 groups in proteins, is membrane impermeable due to its charged nature and therefore only cross-links surface-expressed proteins. As shown in the left panel of Fig. 4B, the full-length LRP6 receptor migrated as a monomer of ∼200 kDa in SDS-PAGE in both non-cross-linked and cross-linked samples. An additional band of ∼400 kDa was observed only in BS3-cross-linked samples, implying the presence of LRP6 receptor dimers at the cell surface. Similarly, LRP6ΔE1-2 and LRP6ΔE3-4 migrated as monomers of ∼115 and ∼130 kDa, respectively, and were detected as homodimers of ∼230 and 260 kDa, respectively, in cells exposed to the cross-linker (Fig. 4B, right panel). In striking contrast, the constitutively active mutant LRP6ΔE1-4 (∼50-kDa band as monomer) was not detectable as a homodimer at the cell surface under the same cross-linking conditions (Fig. 4B, right panel). These findings together with the results described above established that LRP6 is expressed at the cell surface as a non-disulfide-linked dimer, which forms as a result of interactions of its YWTD-EGF repeat domain.
Forced dimerization inhibits LRP6 intracellular domain functions.
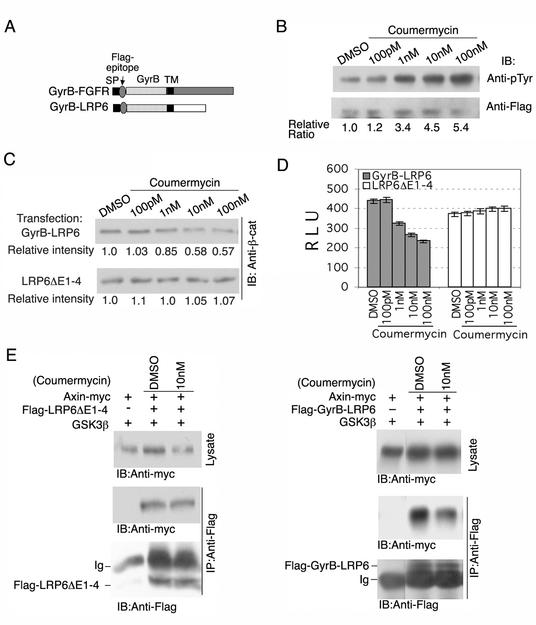
To determine whether dimerization negatively regulates LRP6 receptor functions, we generated a GyrB-LRP6 chimera by replacing the LRP6 extracellular domain with the amino-terminal 24-kDa subdomain of the B subunit of bacterial DNA gyrase (GyrB) (Fig. 5A). The same GyrB subdomain has been used to induce dimerization of intracellular fusion proteins by coumermycin, an antibiotic which binds GyrB with a stoichiometry of 1:2 (16). To verify that coumermycin was able to induce dimer formation of a transmembrane protein, a GyrB-FGFR chimera was constructed in which the 24-kDa subdomain of gyrase B replaced the extracellular domain of FGFR2 (Fig. 5A). As shown in Fig. 5B, coumermycin treatment induced tyrosine autophosphorylation of this chimeric receptor, establishing that it was able to cause dimerization and activation of the intracellular kinase domain. In striking contrast, coumermycin exposure resulted in reduced Wnt signaling by GyrB-LRP6, which was constitutively active at a level comparable to that of the LRP6ΔE1-4 mutant, as measured both by the uncomplexed β-catenin assay (Fig. 5C) and by TOPFLASH TCF reporter analysis (Fig. 5D).
FIG. 5.
Forced dimerization of the GyrB-LRP6 chimeric receptor inhibits LRP6 intracellular domain functions. (A) Schematic representation of GyrB-FGFR and GyrB-LRP6 constructs, in which the extracellular domains of FGFR2 and LRP6 were replaced by the amino-terminal 24K subdomain of the bacterial DNA gyrase B subunit (GyrB). TM, transmembrane domain; SP, signal peptide. (B) Coumermycin activates the GyrB-FGFR chimeric receptor. At 24 h following transfection of 293T cells with 0.5 μg of GyrB-FGFR, coumermycin was added at the concentrations indicated. Around 16 h later cell lysates were obtained and GyrB-FGFR was immunoprecipitated (IP) with anti-Flag antibody, following SDS-PAGE and immunoblotting with anti-p-Tyr. The same blot was stripped and reprobed with anti-Flag antibody. Normalized quantification of the immunoblots is shown at the bottom (Relative Ratio). DMSO, dimethyl sulfoxide. (C) Coumermycin inhibits constitutive β-catenin stabilization by GyrB-LRP6. Around 0.5 μg of GyrB-LRP6 or LRP6ΔE1-4 control plasmid was transfected into 293T cells. The conditions of coumermycin treatment were the same as those for panel B. Uncomplexed β-catenin analysis was performed as described in the legend to Fig. 1B. Quantified representation of the immunoblot is shown at the bottom of each blot (Relative intensity). (D) Coumermycin inhibits constitutive TCF signaling of GyrB-LRP6. At 6 h following transfection of 293T cells with 50 ng of GyrB-LRP6 or LRP6ΔE1-4 in 6-well dishes, coumermycin was added as indicated for 18 h. TCF-dependent transcription was measured as described in the legend to Fig. 1C. (E) Coumermycin inhibits the interaction between GyrB-LRP6 and Axin. At 48 h following transfection of 293T cells with 0.25 μg of Axin-myc, 1 μg of GSK3β, together with 0.5 μg of either Flag-LRP6ΔE1-4 or Flag-GyrB-FGFR, coumermycin was added at the concentration indicated for 30 min, and immunoprecipitation with anti-Flag antibody and immunoblot (IB) with anti-myc antibody were performed as described in the legend to Fig. 1D.
Although these results showed that GyrB-FGFR fusion protein exhibited a robust tyrosine phosphorylation increase in response to coumermycin (over fivefold at 100 nM), we observed a maximum of approximately twofold inhibition by coumermycin of the GyrB-LRP6 fusion protein's signaling activity as determined by both TCF reporter and uncomplexed β-catenin assays (Fig. 5). This difference likely reflects the opposite effects of dimerization on these signaling responses. For example, one would expect only approximately 40 to 50% inhibition of β-catenin signaling with dimerization of approximately 40 to 50% of the GyrB-LRP6 fusion protein. In contrast, the same efficiency of dimer formation would result in a major increase in GyrB-FGFR activation (tyrosine phosphorylation), since the basal level of activity of this protein was relatively low. Under the same conditions, coumermycin had no effect on the level of the chimeric GyrB-LRP6 protein (data not shown) or any effect on constitutive signaling by the LRP6ΔE1-4 mutant, which cannot be dimerized by coumermycin (Fig. 5C and D). These results established that forced dimerization of the LRP6 intracellular domain inhibited canonical Wnt signaling activity by this receptor, which was directly opposite to oligomerization-induced activation of the GyrB-FGFR chimeric receptor observed under the same conditions.
We next investigated the ability of GyrB-LRP6 to interact with Axin in the absence or presence of coumermycin exposure. As shown in Fig. 5E, the LRP6ΔE1-4 mutant formed complexes with Axin detectable by coimmunoprecipitation, and coumermycin had no effect on this complex formation (left panel). However, the amount of Axin in complexes with the GyrB-LRP6 chimeric receptor was specifically and rapidly reduced by coumermycin treatment within 30 min (right panel). These findings strongly support the concept that dimerization of the LRP6 intracellular domain inhibits its signaling activity at least in part by inhibiting Axin recruitment.
Wnts induce a conformational switch in the LRP6 receptor extracellular domain that relieves allosteric inhibition imposed on the intracellular domain.
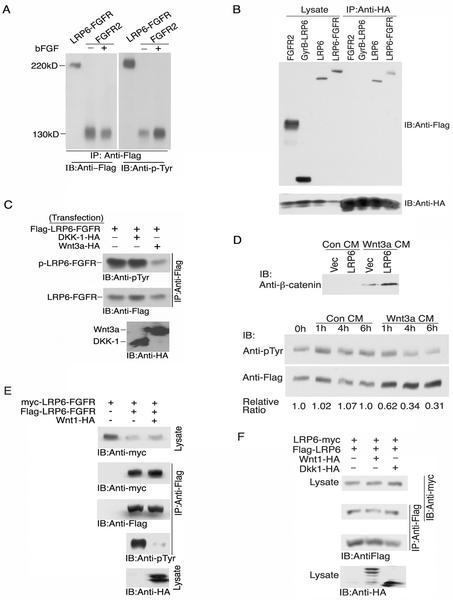
Since Wnts activate LRP6 to initiate canonical Wnt signaling (29, 41), we reasoned that the mechanism might involve a ligand-induced switch of the LRP6 receptor from an inactive dimeric or oligomeric state to an active monomeric state. To test this hypothesis, we constructed another chimeric receptor, LRP6-FGFR (Fig. 3A), in which the LRP6 extracellular domain was fused to the transmembrane and intracellular domains of FGFR2. Thus, the tyrosine kinase domain served as a reporter for intermolecular interactions of the cytoplasmic domains, since it is well known that the tyrosine kinase activation involves clustering and transphosphorylation of the induced receptor oligomers (27). As shown in Fig. 6A, expression of this chimeric receptor in 293T cells was associated with its constitutive tyrosine autophosphorylation at a level comparable to that observed with FGF stimulation of the FGFR2 and was considerably higher than that of the nonstimulated FGFR2 expressed at similar protein levels. These results strongly implied that oligomers formed by the LRP6 external domains positioned the chimeric tyrosine kinase domains in sufficiently close proximity to induce intermolecular kinase activation with an efficiency similar to that induced by FGF clustering of FGFR2 (Fig. 6A).
FIG. 6.
Wnt ligand interactions with the oligomeric LRP6 external domain relieve allosteric inhibition on the LRP6 intracellular domain. (A) Constitutive tyrosine autophosphorylation of a LRP6-FGFR chimeric receptor. Flag-LRP6-FGFR (1 μg) and Flag-FGFR2 (0.5 μg) were transfected in 293T cells. Basic FGF (bFGF) (100 ng/ml) was added at 24 h to Flag-FGFR2-transfected cells. At 48 h, lysates (2 mg) were immunoprecipitated (IP) with anti-Flag antibody and were subjected to SDS-PAGE analysis and immunoblotting (IB) with anti-p-Tyr polyclonal antibody. The same blot was stripped and reprobed with anti-Flag antibody to determine receptor protein levels. (B) Complex formation between Wnt ligand and LRP6 receptors. 293T cells were transfected with 1 μg of Wnt3a-HA together with 1 μg of Flag-FGFR2, Flag-GyrB-LRP6, Flag-LRP6, or Flag-LRP6-FGFR. At 48 h, cell lysates were immunoprecipitated with anti-HA antibody followed by SDS-PAGE and immunoblot with anti-Flag or anti-HA antibody. (C) Wnt3a but not Dkk-1 inhibits constitutive tyrosine autophosphorylation of the LRP6-FGFR chimera. 293T cells were transfected with Flag-LRP6-FGFR (1 μg) and either vector (Vec), Dkk-1-HA (3 μg), or Wnt3a-HA (3 μg). At 48 h, cell lysates were immunoprecipitated with anti-Flag antibody followed by SDS-PAGE and immunoblotting with either anti-p-Tyr, anti-Flag, or anti-HA antibody. Normalized quantification of the immunoblots is shown at the bottom. (D) Activation of LRP6 receptor and inhibition of constitutive receptor tyrosine autophosphorylation of LRP6-FGFR by exogenously added Wnt3a CM. Twenty-four hours following transfection of 293T cells with 0.5 μg of vector or LRP6, control (Con) CM (from L cells, ATCC no. CRL-2648) or Wnt3a CM (prepared from Wnt3a stably transfected L cells, ATCC no. CRL-2647) was added for 1 h, and the cell lysates were subjected to uncomplexed β-catenin analysis (upper panel). Twenty-four hours following transfection of 0.5 μg of Flag-LRP6-FGFR in 293T cells, control CM or Wnt3a CM was added for various times as indicated, and cell lysates were then immunoprecipitated with anti-Flag antibody followed by SDS-PAGE and immunoblot analysis with anti-p-Tyr. The same blot was stripped and reblotted with anti-Flag (lower panel). (E) Wnt ligand induces conformational alteration in LRP6 receptor oligomers. 293T cells were transfected with 1 μg of Flag-LRP6-FGFR and myc-LRP6-FGFR together with 4 μg of either vector or Wnt-1-HA plasmids. At 48 h, cell lysates were immunoprecipitated with anti-Flag antibody followed by SDS-PAGE and immunoblotting with anti-myc, anti-p-Tyr, anti-Flag, or anti-HA antibody as described above. (F) Effects of Wnt-1 and Dkk-1 on oligomerization of the wild-type LRP6 receptor. 293T cells were transfected with 0.5 μg of Flag-LRP6-FGFR and myc-LRP6-FGFR together with 2 μg of vector, Wnt-1-HA, or Dkk-1-HA plasmid. At 48 h cell lysates were immunoprecipitated with anti-Flag antibody followed by SDS-PAGE and immunoblotting as described above.
Although Wnt-1 has been reported to bind the LRP6 receptor independent of frizzled in an in vitro interaction assay (41), a recent study failed to detect this interaction between Drosophila wingless and the arrow receptor (47). To test the ability of Wnt3a to form complexes with LRP6, we performed coexpression and coimmunoprecipitation analyses. As shown in Fig. 6B, the LRP6 receptor and Wnt3a were readily detected in the same complex, while an unrelated receptor, FGFR2, as well as the GyrB-LRP6 mutant lacking the LRP6 extracellular domain which expressed at higher levels, failed to interact with Wnt3a under the same conditions (Fig. 6B). These results indicate that Wnt3a interacts with the LRP6 receptors either directly or indirectly in the same complex.
We next investigated the effects of known LRP6 ligands on the intracellular conformation of the LRP6-FGFR chimera. As shown in Fig. 6C, Wnt3a caused a striking reduction in the level of LRP6-FGFR autophosphorylation, while Dickkopf 1 (Dkk-1), an inhibitory ligand of LRP6 (3, 29, 38), had no detectable effect at comparable expression levels of both HA-tagged ligands. Since these experiments were conducted under conditions in which Wnt3a and LRP6 were coexpressed in the same cell, it was important to establish that the LRP6 conformational changes described could be triggered by exogenous Wnt addition. Wnts are generally tightly cell associated (10, 33), but several recent reports have documented Wnt producer cell lines in which biologically active Wnts are released into culture fluids (11, 39, 43). As shown in the upper panel of Fig. 6D, Wnt3a condition medium (CM) produced by L cells induced a readily detectable increase in uncomplexed β-catenin in 293T cells and synergized with exogenously expressed LRP6 to induce a much higher level of uncomplexed β-catenin. When the same CM was added to LRP6-FGFR chimera expressing 293T cells, we observed a reduction within 1 h in the level of LRP6-FGFR receptor tyrosine phosphorylation, which persisted for at least 6 h (Fig. 6D, lower panel). To exclude the possibility of other secreted factors, which might influence the tyrosine phosphorylation of LRP6-FGFR, CM from the same cells without Wnt3a expression was tested and shown to have no effect (Fig. 6D). These results firmly establish that exogenous Wnts act directly at the cell surface to activate canonical signaling by inducing a rapid conformational change in the LRP6/FGFR oligomer to induce a more open conformation of the cytoplasmic domains.
To distinguish whether the Wnt ligand completely dissociates the LRP6 oligomers leading to monomeric receptors or only induces a more open conformational of the intracellular domain, we coexpressed two different tagged LRP6-FGFR chimeric receptors in the absence or presence of Wnt-1 coexpression. As shown in Fig. 6E, under conditions in which Wnt-1 coexpression almost completely inhibited tyrosine phosphorylation of LRP6-FGFR chimeric receptor, homomeric complex formation of the Flag- and myc-tagged LRP6-FGFR receptors was not detectably affected. Similarly, expression of neither Wnt-1 nor Dkk-1 had any detectable effects on the formation of wild-type LRP6 receptor homomeric complex (Fig. 6F). These results imply that the Wnt ligand acts by altering the conformation of the LRP6 receptor oligomer rather than by completely disrupting the oligomeric receptor complex.
DISCUSSION
The identification of LDL receptor-related proteins, LRP5 and LRP6 (32, 38) and their Drosphila orthologue Arrow (44), as Wnt coreceptors has led to important biochemical insights into the role of this receptor in canonical signaling. Mao et al. (28) demonstrated that the intracellular domain of wild-type LRP5 receptor recruits Axin, a component of the β-catenin degradation complex (36), to the membrane in response to Wnt triggering. However, the lack of any recognizable catalytic motif in the LRP5 and LRP6 intracellular domain (12) and the fact that this domain interacts with Axin in both yeast and mammalian cells (28) suggested that posttranslational modifications triggered by Wnt were not required for LRP5-Axin interactions. Mutational analysis independently revealed that an LRP5 deletion mutant lacking the external domain but preserving the transmembrane and cytoplasmic domains was constitutively active, implying that the external domain negatively regulates LRP5 function (28). Our present studies have extended these observations to LRP6 and have led to the elucidation of a novel mechanism for ligand activation of a transmembrane receptor.
In striking contrast to the general mechanism of receptor activation by oligomerization (27), we demonstrated that wild-type LRP6 forms an inactive homomeric complex, whereas constitutively active LRP6 mutants were monomeric. These results suggested that the extracellular domain of the wild-type receptor might inhibit signaling by oligomerization. We mapped LRP6 oligomerization to the YWTD-EGF repeat region of the external domain through analysis of a series of LRP6 mutants. An inverse correlation between receptor oligomerization and signaling was confirmed by use of a chimeric GyrB-LRP6 receptor also lacking the LRP6 external domain, which was replaced by GyrB. This mutant exhibited constitutive signaling activity, which was inhibited by coumermycin-induced forced dimerization. Axin interaction with the cytoplasmic domain of this chimera was also inhibited by coumermycin-induced receptor dimerization. All these results support a model in which LRP6 dimerization mediated by the extracellular YWTD-EGF repeat region inhibits interaction of the LRP6 intracellular domain with Axin as well as LRP6 signaling functions. This model is consistent with the possibility that LRP5 and LRP6 heterodimers may also occur. If so, such heteromeric receptor complexes may possess different affinities for Wnt ligands or even different intracellular signaling specificity compared to those of LRP5 or LRP6 homomeric oligomers.
Two studies published after submission of this paper reported the requirement of a chaperone protein, Mesd, or of the Drosophila ortholog Boca for proper folding and cell surface location of functional LRP6 or arrow receptors (13, 20). The majority of exogenously expressed LRP6 in COS-1 cells was shown to accumulate as aggregates in the endoplasmic reticulum through aberrant intermolecular disulfide linkages, presumably due to the lack of sufficient expression of Mesd proteins in these cells (20). In human embryonic kidney 293 cells, exogenously expressed LRP6 has been reported to function at the cell surface and to mediate Wnt signaling and Dkk-1 binding (3, 29, 30). Consistent with these reports, we were able to detect the expression of wild-type and various LRP6 mutant receptors at the cell surface by FACS analysis in 293 cells. When expressed in 293 cells under nonreducing conditions, the majority of the LRP6 receptor was not observed as high-molecular-weight aggregates or LRP6 oligomers. These findings establish both that oligomer formation of the wild-type LRP6 receptor does not involve intermolecular disulfide linkages and that chaperone proteins cannot be limiting in 293 cells. By using a cell membrane-impermeable cross-linker, homodimers of wild-type LRP6, LRP6ΔE1-2, and LRP6ΔE3-4 were detected at the cell surface, while the constitutively active LRP6ΔE1-4 mutant failed to be cross-linked under the same conditions. These results strongly support the concept that the functional LRP6 receptor exists as an oligomer at the cell surface and that oligomerization is mediated by the YWTD-EGF repeats.
Ligand activation of tyrosine kinase receptors involves ligand-mediated receptor oligomerization, which induces activation of the kinase domains (45). By generation of an LRP6-FGFR chimera it was possible to establish that oligomerization mediated by the LRP6 external domain caused sufficiently close juxtaposition of the intracellular tyrosine kinase domains to cause their chronic activation. We demonstrated that both coexpressed and exogenously added Wnt induced a conformational switch which separated the intracellular domains of the LRP6-FGFR chimera from the close proximity required to maintain kinase activation without dissociation of the oligomeric receptor complex. Thus, Wnt signaling through LRP6 invokes a new paradigm in which Wnts act to relieve allosteric inhibition imposed on the intracellular domain of an inactive receptor oligomer. This is in direct contrast to the general mechanism of ligand-induced receptor clustering responsible for the activation of hormone and growth factor receptors, lymphokine receptors, T-cell and B-cell receptors, and many other families of cell surface receptors (27). According to this model, the Axin-binding site in the intracellular domain of the LRP receptor would be sequestered within the LRP6 oligomer, presumably through mutual steric hindrance. Wnt ligands would alter the conformation of this receptor oligomer to relieve allosteric inhibition, allowing access to substrates such as Axin and producing an active LRP6 receptor.
Previous studies have shown that forced dimerization of CD45, a member of the receptor-like protein tyrosine phosphatase (RPTP) family involved in T-cell receptor (TCR) signaling, inhibits its activity and results in the loss of TCR signaling (15). Similarly, dimerization inhibits the activity of RPTPα (22). It has been proposed on the basis of crystal structure that dimerization negatively regulates phosphatase activity through interaction of an inhibitory wedge on one monomer with the catalytic cleft in the other to block substrate access (6, 22, 31). While inhibitory ligands for these receptors have been postulated to negatively regulate RPTPs by causing their dimerization, such ligands have not been identified (45). In fact, a recent study suggests that CD45 may not require an inhibitory ligand to form a dimer. Instead, CD45 phosphatase activity may be regulated by the differential dimerization of alternatively spliced isoforms (48).
Genetic and biochemical studies have provided strong evidence that the seven transmembrane-spanning frizzled receptor and the single transmembrane-spanning LRP5 or LRP6 receptor cooperate in Wnt canonical signaling (41). Previous studies have indicated that the frizzled receptor itself forms a dimer through interactions involving its cysteine-rich domain, and these interactions are independent of Wnt (2, 14). Specific mutations within the frizzled cytoplasmic tail have been shown to inhibit canonical signaling (42), implying that it possesses intracellular signaling functions. Tamai et al. (41) reported that Wnt binds to frizzled and LRP6 independently and recruits these two receptors into a complex. However, the interaction between Wingless and arrow was not detectable in Drosophila (47). Although we were able to detect specific Wnt3a/LRP6 complexes by overexpression and coimmunoprecipitation as has been previously reported (23, 28), this approach does not determine the affinity of the interaction or whether this interaction involves other components of the receptor complex. Our findings as well as those of others (28) indicate that the NH2-terminally truncated LRP mutants lacking the extracellular domain are capable of canonical signaling independent of either a Wnt ligand or frizzled receptor. Thus, the mechanism by which frizzled cooperates with LRP to transduce the Wnt signal remains to be elucidated. Nonetheless, it is likely that at physiological Wnt ligand and receptor levels the engagement of both frizzled and LRP coreceptors is required to achieve efficient intracellular coupling to the canonical pathway. Based on our results, it is conceivable that an LRP mutant lacking the intracellular domain might act to increase Wnt canonical signaling by forming a receptor heterodimer with one free cytoplasmic tail. However, there are reports that the LRP extracellular domain inhibits canonical signaling (29, 41). If so, such inhibition may reflect the complexity of in vivo signaling involving LRP interactions not only with Wnt ligands but also with frizzled coreceptors.
Missense mutations in the YWTD-EGF repeat region of LRP5 have been found both in the human autosomal-recessive disorder osteoporosis-pseudoglioma syndrome (18) and the autosomal-dominant high-bone-mass trait (9, 26). Our present findings suggest possible functional consequences of these mutations in disease. Such mutations could alter the association or dissociation of LRP oligomers, which might influence the conformational switch mediated by Wnt ligands or the ability of the inhibitory ligand, Dkk-1, to downregulate the receptor through interactions with LRP (3, 29) and a recently identified coreceptor, kremen (30). In fact, one report indicates that the YWTD-EGF repeat mutation at codon 171 in the high-bone-mass trait interferes with Dkk-1 function (9). Constitutive activation of canonical signaling due to mutations in certain Wnt pathway components is etiologically involved in a number of human tumors (36). Our present findings suggest that genetic lesions in the LRP receptor, which impaired its ability to form oligomers, might activate canonical signaling, making LRP a potential candidate for oncogenic activation in tumors.
Acknowledgments
We thank F. Hess for generously providing LRP6 cDNA, F. Costantini for myc-tagged Axin cDNA, and R. Hazan for Flag-tagged FGFR2 cDNA. We are grateful to A. Gazit and A. Yaniv for helpful discussion.
This work was supported by NCI grant CA71672-04 (S.A.A.).
REFERENCES
- 1.Bafico, A., A. Gazit, S. S. Wu-Morgan, A. Yaniv, and S. A. Aaronson. 1998. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene 16:2819-2825. [DOI] [PubMed] [Google Scholar]
- 2.Bafico, A., A. Gazit, T. Pramila, P. W. Finch, A. Yaniv, and S. A. Aaronson. 1999. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J. Biol. Chem. 274:16180-16187. [DOI] [PubMed] [Google Scholar]
- 3.Bafico, A., G. Liu, A. Yaniv, A. Gazit, and S. A. Aaronson. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683-686. [DOI] [PubMed] [Google Scholar]
- 4.Barker, N., P. J. Morin, and H. Clevers. 2000. The Ying-Yang of TCF/β-catenin signaling. Adv. Cancer Res. 77:1-24. [DOI] [PubMed] [Google Scholar]
- 5.Bhanot, P., M. Brink, C. H. Samos, J. C. Hsieh, Y. Wang, J. P. Macke, D. Andrew, J. Nathans, and R. Nusse. 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382:225-230. [DOI] [PubMed] [Google Scholar]
- 6.Bilwes, A. M., J. den Hertog, T. Hunter, and J. P. Noel. 1996. Structural basis for inhibition of receptor protein-tyrosine phosphatase-alpha by dimerization. Nature 382:555-559. [DOI] [PubMed] [Google Scholar]
- 7.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 8.Boutros, M., J. Mihaly, T. Bouwmeester, and M. Mlodzik, 2000. Signaling specificity by Frizzled receptors in Drosophila. Science 288:1825-1828. [DOI] [PubMed] [Google Scholar]
- 9.Boyden, L. M., J. Mao, J. Belsky, L. Mitzner, A. Farhi, M. A. Mitnick, D. Wu, K. Insogna, and R. P. Lifton. 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346:1513-1521. [DOI] [PubMed] [Google Scholar]
- 10.Bradley, R. S., and A. M. Brown. 1990. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 9:1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley, R. S., and A. M. Brown. 1995. A soluble form of Wnt-1 protein with mitogenic activity on mammary epithelial cells. Mol. Cell. Biol. 15:4616-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, S. D., R. C. Twells, P. J. Hey, R. D. Cox, E. R. Levy, A. R. Soderman, M. L. Metzker, C. T. Caskey, J. A. Todd, and J. F. Hess. 1998. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem. Biophys. Res. Commun. 248:879-888. [DOI] [PubMed] [Google Scholar]
- 13.Culi, J., and R. S. Mann. 2003. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell 112:343-354. [DOI] [PubMed] [Google Scholar]
- 14.Dann, C. E., J. C. Hsieh, A. Rattner, D. Sharma, J. Nathans, and D. J. Leahy. 2001. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412:86-90. [DOI] [PubMed] [Google Scholar]
- 15.Desai, D. M., J. Sap, J. Schlessinger, and A. Weiss. 1993. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell 73:541-554. [DOI] [PubMed] [Google Scholar]
- 16.Farrar, M. A., J. Alberol-Ila, and R. M. Perlmutter. 1996. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383:178-181. [DOI] [PubMed] [Google Scholar]
- 17.Gazit, A., A. Yaniv, A. Bafico, T. Pramila, M. Igarashi, J. Kitajewski, and S. A. Aaronson. 1999. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene 18:5959-5966. [DOI] [PubMed] [Google Scholar]
- 18.Gong, Y., R. B. Slee, N. Fukai, G. Rawadi, S. Roman-Roman, A. M. Reginato, H. Wang, T. Cundy, F. H. Glorieux, D. Lev, M. Zacharin, K. Oexle, J. Marcelinoi, W. S. Suwair Heeger, G. Sabatakos, S. Apte, W. N. Adkins, J. Allgrove, M. Arslan-Kirchner, J. A. Batch, P. Beighton, G. C. Black, R. G. Boles, L. M. Boon, C. Borrone, H. G. Brunner, G. F. Carle, B. Dallapiccola, A. De Paepe, B. Floege, M. L. Halfhide, B. Hall, R. C. Hennekam, T. Hirose, A. Jans, H. Juppner, C. A. Kim, K. Keppler-Noreuil, A. Kohlschuetter, D. LaCombe, M. Lambert, E. Lemyre, T. Letteboer, L. Peltonen, R. S. Ramesar, M. Romanengo, H. Somer, E. Steichen-Gersdorf, B. Steinmann, B. Sullivan, A. Superti-Furga, W. Swoboda, M. J. van den Boogaard, W. Van Hul, M. Vikkula, M. Votruba, B. Zabel, T. Garcia, R. Baron, B. R. Olsen, and M. L. Warman. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513-523. [DOI] [PubMed] [Google Scholar]
- 19.He, X., J. P. Saint-Jeannet, Y. Wang, J. Nathans, I. Dawid, and H. Varmus. 1997. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275:1652-1654. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, J. C., L. Lee, L. Zhang, S. Wefer, K. Brown, C. DeRossi, M. E. Wines, T. Rosenquist, and B. C. Holdener. 2003. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112:355-367. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, G., J. den Hertog, and T. Hunter. 2000. Receptor-like protein tyrosine phosphatase alpha homodimerizes on the cell surface. Mol. Cell. Biol. 20:5917-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, G., J. den Hertog, J. Su, J. Noel, J. Sap, and T. Hunter. 1999. Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-alpha. Nature 401:606-610. [DOI] [PubMed] [Google Scholar]
- 23.Kato, M., M. S. Patel, R. Levasseur, I. Lobov, B. H. Chang, D. A. Glass II, C. Hartmann, L. Li, T. H. Hwang, C. F. Brayton, R. A. Lang, G. Karsenty, and L. Chan. 2002. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157:303-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 25.Krasnow, R. E., L. L. Wong, and P. N. Adler. 1995. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development 121:4095-4102. [DOI] [PubMed] [Google Scholar]
- 26.Little, R. D., J. P. Carulli, R. G. Del Mastro, J. Dupuis, M. Osborne, C. Folz, S. P. Manning, P. M. Swain, S. C. Zhao, B. Eustace, M. M. Lappe, L. Spitzer, S. Zweier, K. Braunschweiger, Y. Benchekroun, X. Hu, R. Adair, L. Chee, M. G. FitzGerald, C. Tulig, A. Caruso, N. Tzellas, A. Bawa, B. Franklin, S. McGuire, X. Nogues, G. Gong, K. M. Allen, A. Anisowicz, A. J. Morales, P. T. Lomedico, S. M. Recker, P. Van Eerdewegh, R. R. Recker, and M. L. Johnson. 2002. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 70:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemmon, M. A., and J. Schlessinger. 1994. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem. Sci. 19:459-463. [DOI] [PubMed] [Google Scholar]
- 28.Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr III, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell. 7:801-809. [DOI] [PubMed] [Google Scholar]
- 29.Mao, B., W. Wu, Y. Li, D. Hoppe, P. Stannek, A. Glinka, and C. Niehrs. 2001. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411:321-325. [DOI] [PubMed] [Google Scholar]
- 30.Mao, B., W. Wu, G. Davidson, J. Marhold, M. Li, B. M. Mechler, H. Delius, D. Hoppe, P. Stannek, C. Walter, A. Glinka, and C. Niehrs. 2002. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417:664-667. [DOI] [PubMed] [Google Scholar]
- 31.Majeti, R., A. M. Bilwes, J. P. Noel, T. Hunter, and A. Weiss. 1998. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science 279:88-91. [DOI] [PubMed] [Google Scholar]
- 32.Nusse, R. 2001. Developmental biology making head or tail of Dickkopf. Nature 411:255-256. [DOI] [PubMed] [Google Scholar]
- 33.Papkoff, J., and B. Schryver. 1990. Secreted int-1 protein is associated with the cell surface. Mol. Cell. Biol. 10:2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peifer, M., and P. Polakis. 2000. Wnt signaling in oncogenesis and embryogenesis-a look outside the nucleus. Science 287:1606-1609. [DOI] [PubMed] [Google Scholar]
- 35.Pinson, K. I., J. Brennan, S. Monkley, B. J. Avery, and W. C. Skarnes. 2000. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407:535-538. [DOI] [PubMed] [Google Scholar]
- 36.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 37.Rulifson, E. J., C. H. Wu, and R. Nusse. 2000. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol. Cell. 6:117-126. [PubMed] [Google Scholar]
- 38.Semenov, M. V., K. Tamai, B. K. Brott, M. Kuhl, S. Sokol, and X. He. 2001. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11:951-961. [DOI] [PubMed] [Google Scholar]
- 39.Shibamoto, S., K. Higano, R. Takada, F. Ito, M. Takeichi, and S. Takada. 1998. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3:659-670. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu, H., M. A. Julius, M. Giarre, Z. Zheng, A. M. Brown, and J. Kitajewski. 1997. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 8:1349-1358. [PubMed] [Google Scholar]
- 41.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 42.Umbhauer, M., A. Djiane, C. Goisset, A. Penzo-Mendez, J. F. Riou, J. C. Boucaut, and D. L. Shi. 2000. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 19:4944-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Leeuwen, F., C. H. Samos, and R. Nusse. 1994. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature 368:342-344. [DOI] [PubMed] [Google Scholar]
- 44.Wehrli, M., S. T. Dougan, K. Caldwell, L. O'Keefe, S. Schwartz, D. Vaizel-Ohayon, E. Schejter, A. Tomlinson, and S. DiNardo. 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407:527-530. [DOI] [PubMed] [Google Scholar]
- 45.Weiss, A., and J. Schlessinger. 1998. Switching signals on or off by receptor dimerization. Cell 94:277-280. [DOI] [PubMed] [Google Scholar]
- 46.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell. Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 47.Wu, C. H., and R. Nusse. 2002. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem. 277:41762-41769. [DOI] [PubMed] [Google Scholar]
- 48.Xu, Z., and A. Weiss. 2002. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat. Immunol. 3:764-771. [DOI] [PubMed] [Google Scholar]
- 49.Yang-Snyder, J., J. R. Millerm, J. D. Brown, C. J. Lai, and R. T. Moon. 1996. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. 6:1302-1306. [DOI] [PubMed] [Google Scholar]