Abstract
Glucocorticoids and estrogens regulate a number of vital physiological processes. We developed a model breast cancer cell line, MCF-7 M, to examine potential mechanisms by which the ligand-bound estrogen receptor (ER) regulates glucocorticoid receptor (GR)-mediated transcription. MCF-7 cells, which endogenously express ERα, were stably transfected with mouse mammary tumor virus promoter-luciferase (MMTV-LUC) reporter and GR expression constructs. Our results demonstrate that treatment with estrogen agonists (17β-estradiol [E2], diethylstilbestrol, genistein), but not antagonists (tamoxifen or raloxifene), for 48 h inhibits GR-mediated MMTV-LUC transcription and chromatin remodeling. Furthermore, estrogen agonists inhibit glucocorticoid induction of p21 mRNA and protein levels, suggesting that the repressive effect applies to other GR-regulated genes and proteins in MCF-7 cells. Importantly, GR transcriptional activity is compromised because treatment with estrogen agonists down regulates GR protein levels. The protein synthesis inhibitor cycloheximide and the proteasome inhibitor MG132 block E2-mediated decrease in GR protein levels, suggesting that estrogen agonists down regulate the GR via the proteasomal degradation pathway. In support of this, we demonstrate that E2-mediated GR degradation is coupled to an increase in p53 and its key regulator protein Mdm2 (murine double minute 2), an E3 ubiquitin ligase shown to target the GR for degradation. Using the chromatin immunoprecipitation assay, we demonstrate an E2-dependent recruitment of ERα to the Mdm2 promoter, suggesting a role of ER in the regulation of Mdm2 protein expression and hence the enhanced GR degradation in the presence of estrogen agonists. Our study shows that cross talk between the GR and ER involves multiple signaling pathways, indicative of the mechanistic diversity within steroid receptor-regulated transcription.
Physiological and therapeutic activities of glucocorticoids and estrogens are mediated by the glucocorticoid receptor (GR) and estrogen receptor (ER), respectively. As mediators of glucocorticoid and estrogenic hormones, the GR and ER play a critical role in a diverse array of physiological processes, including metabolism, immunity, cell growth and proliferation, reproduction, and development (12, 58). Both GR and ER exert important actions in tissues other than their primary target tissues. In tissues that express both receptors, glucocorticoids often oppose the actions of estrogens. For example, in the mammary gland, glucocorticoids exert antiproliferative effects, whereas estrogens promote cell growth and proliferation (69, 79). In contrast, in bone, glucocorticoids induce bone resorption (57), whereas estrogens inhibit this action (23). Although glucocorticoids and estrogens act within the same cellular context in these biological processes, little is known about the cross talk between the GR and ER signaling pathways.
The GR and ER are both members of the steroid hormone receptor superfamily of nuclear receptors that includes the receptors for androgens, mineralocorticoids, and progestins (44). The classical mode of action of steroid hormone receptors involves binding of the ligand/receptor complex to specific DNA response elements within promoters of target genes (3). Subsequently, transcription is activated or repressed as a result of DNA-bound receptors recruiting chromatin remodeling complexes, coactivator and corepressor proteins, and the transcription initiation machinery (28, 45).
Recent studies have demonstrated a role of cross talk between receptor signaling pathways in the repression of steroid hormone receptor regulation of transcription (21, 32, 33, 67, 68). Previous studies in our laboratory have focused on the cross talk between the GR and the progesterone receptor (PR). The PR, when bound with type II antiprogestins, inhibits GR-mediated chromatin remodeling and transcription from the mouse mammary tumor virus (MMTV) promoter in T47D/A1-2 breast cancer cells (21). The inhibitory effect of PR on GR-mediated transcription of the MMTV promoter can be envisioned, because both receptors use the same DNA response elements on the MMTV promoter (44). However, our studies showed that instead of competing for DNA binding, the PR inhibits GR activity by disrupting protein-protein interactions. The antiprogestin/PR complex prevents the GR from remodeling organized chromatin by blocking GR from interacting with the chromatin remodeling complex, BRG1 (19). These experiments suggested that the MMTV promoter organized as chromatin provides a good model to unravel indirect mechanisms other than DNA binding that control gene regulation through steroid receptor cross talk.
Estrogens may affect GR regulation of MMTV transcription at a number of levels. In particular, estrogen regulation of the cell cycle can impact on GR-mediated MMTV transactivation. For example, estrogens change the expression of cyclin-dependent protein kinase inhibitor p21 and subsequently alter the activities of cyclin E/CDK2 and cyclin D1/CDK4/CDK6 complexes (55). These multiprotein kinase complexes have high levels of H1 kinase activity (62). Changes in H1 phosphorylation are a hallmark of MMTV activation by glucocorticoids in mouse mammary tumor cells (6, 38). Accordingly, a change in H1 phosphorylation status by estrogens could impact on GR-mediated activation through this mechanism. GR-mediated activation of the MMTV promoter also requires the chromatin-remodeling complex that includes BRG1 (19, 50). Chromatin remodeling proteins play a vital role in cell cycle progression, and their protein levels change at different stages of cell cycle (49, 59). The potential effects of estrogens on cell cycle regulation of these or other protein complexes involved in GR regulation of transcription may impact on GR-mediated transcriptional activity.
In the present study, we have focused on cross talk between the GR and ER by using an MCF-7 breast cancer cell line, stably expressing MMTV-luciferase (MMTV-LUC) reporter and exogenous GR. We report that estrogen agonists down regulate GR protein levels primarily through protein degradation. Our studies show that ER-directed down regulation of the GR occurs via the proteasome degradation pathway. The ER-dependent decrease in GR levels is coupled to an increase in Mdm2 protein, an E3 ubiquitin ligase recently shown to target the GR to the proteasome.
MATERIALS AND METHODS
Reagents.
Dexamethasone (DEX), 17β-estradiol (E2), diethylstilbestrol (DES), genistein, tamoxifen, cycloheximide (CHX), leptomycin B (LMB), and hydroxyurea were purchased from Sigma-Aldrich (St. Louis, Mo.). Hydrogen peroxide (H2O2) was purchased from Cumberland Swan (Smyrna, Tenn.). Raloxifene was a gift from Eli Lilly Research Laboratories (Indianapolis, Ind.), ICI 182780 (ICI) was a gift from AstraZeneca Pharmaceuticals (Macclesfield, Cheshire, United Kingdom), and MG132 was purchased from Calbiochem (La Jolla, Calif.).
Cell culture.
To generate MCF-7 cells stably expressing the GR and MMTV-long terminal repeat (LTR) promoter fused to the luciferase gene reporter, parental MCF-7 cells (American Type Culture Collection, Manassas, Va.) were cotransfected with pMMTV-LTR-LUC, pGR-NEO, and a neomycin resistance plasmid, pRSV-NEO (21), by using the calcium phosphate precipitation method (GIBCO-BRL Life Technologies, Grand Island, N.Y.). Stable clones were selected in modified Eagle medium (MEM) supplemented with 10% fetal bovine serum and 600 μg of G418/ml. Surviving clones were maintained under selection and were sequentially grown from six-well plates until confluent. Clones were then screened for the MMTV-LTR-luciferase gene by PCR and for luciferase activity in response to DEX. In subsequent passages, all cells were grown in a humidified incubator at 37°C with 5% CO2 in MEM supplemented with 2 mM glutamine, 100 μg of penicillin-streptomycin/ml, 10 mM HEPES, 10% fetal bovine serum and 300 μg of G418/ml. For all experiments, MCF-7 cells were cultured overnight in phenol red-free MEM supplemented with 5% charcoal-stripped calf serum and 2 mM glutamine. Fresh medium containing hormones was added for the length of time specified in the figure legends.
Luciferase assays.
Cells (0.2 × 106) were seeded in six-well plates in triplicate and treated with specific hormones. After hormone treatment, cells were washed twice with phosphate-buffered saline (PBS) and lysed with 500 μl of passive lysis buffer (Promega, Madison, Wis.). Luciferase activity was determined by using luciferase kits and reagents (Promega). Luciferase activity was expressed as relative light units (RLU) normalized for total protein in each sample.
RNA isolation, RNA primer extension, and RT-PCR.
Cells were left untreated or were treated with hormones for the times indicated. Total cellular RNA was prepared using Trizol reagent (GIBCO-BRL, Rockville, Md.). Primer extension analysis of total RNA was performed with single-stranded 32P-end-labeled specific oligonucleotide primers for either MMTV-LTR mRNA (MMTV-22; 5′-TCT GGA AAG TGA AGG ATA AAG TGA CGA-3′) or 18S rRNA (5′-ACC AAA GGA ACC ATA ACT G-3′) (31). For reverse transcriptase PCR (RT-PCR) analysis, cDNA was synthesized as described previously (21), and PCR was performed with the following pairs of primers. For MMTV-LUC, primers 5′-TCT GGA AAG TGA AGG ATA AAG TGA CGA-3′ and 5′-CCT CTT CTG TGT TTG TGT CTG CTG TTC-3′ were used. Human β2-microglobulin was amplified with primer sequences 5′-ACC CCC ACT GAA AAA GAT GA-3′ and 5′-ATC TTC AAA CCT CCA TGA TG-3′. Human p21 was amplified with primer sequences 5′-GCG ACT GTG ATG CGC TAA TGG-3′ and 5′-TCC CAA CTC ATC CCG GCC TC-3′. Rat GR was amplified with primer sequences 5′-GCT CAC ATT AAT ATT TGC CAA TGG-3′ and 5′-GCT GCT GCT GCT GCT GCT GC-3′. Levels of labeled PCR transcripts were analyzed on 8% polyacrylamide denaturing gels and quantified with a Molecular Dynamics PhosphorImager and ImageQuant software analysis (Molecular Dynamics, Sunnyvale, Calif.).
In vivo chromatin analysis.
Nuclei were isolated as previously described (37) and subjected to limited digestion using SstI (10 U/100 μl). After in vivo digestion, DNA was purified by phenol-chloroform extraction and ethanol precipitation. DNA samples were digested to completion using HaeIII (100 U/100 μl) to provide an internal standard for the in vivo cutting and to confirm that equivalent amounts of DNA were used for reiterative primer extension analysis. Purified DNA (10 μg) was amplified by using reiterative primer extension, Taq DNA polymerase, and 32P-labeled specific oligonucleotide complementary to MMTV sequences. Extended products were purified by phenol-chloroform extraction and ethanol precipitation. Samples were analyzed on 8% polyacrylamide gels as described previously (37).
ChIP assay.
MCF-7 cells (0.5 × 106) were seeded in 10-cm-diameter tissue culture plates. On the next day, cells were pretreated with estrogen agonists or antagonists for 48 h at doses specified in the figure legends. For MMTV promoter, 48 h posttreatment, 1 nM DEX was added for 1 h. Following DEX treatment, cells were fixed with 1% formaldehyde at 37°C for 20 min. Cells were collected by centrifugation in PBS containing protease inhibitors. The chromatin immunoprecipitation (ChIP) assay was performed according to the Upstate Biotechnology protocol with minor modifications. Samples were diluted with ChIP dilution buffer and precleared with 80 μl of salmon sperm DNA-protein A agarose slurry for 30 min with agitation at 4°C. Immunoprecipitation was performed overnight (8 to 12 h) at 4°C with antibodies against BRG1 (H-88), transactivation/transformation-domain-associated protein (TRRAP), p53 (DO-1), normal serum immunoglobulin G (IgG) (Santa Cruz Biotech), or ERα (Upstate Biotech) as indicated on figure legends. After immunoprecipitation, 60 μl of salmon sperm DNA-protein A agarose was added for 1 h at 4°C to capture the immune complexes. Immunoprecipitates were washed five times, with one wash each with low-salt, high-salt, and LiCl buffers and two washes with TE buffer. Immune complexes were eluted twice for 15 min with 1% sodium dodecyl sulfate (SDS) in 0.1 M NaHCO3 at room temperature. DNA/protein complexes were heated at 65°C for 4 h to reverse the formaldehyde cross-linking, after which proteinase K was used to digest protein for 1 h at 45°C. DNA was purified by phenol-chloroform extraction and ethanol precipitation and amplified by PCR. Primers used for PCR were as follows: MMTV promoter, 5′-TTA AGT AAG TTT TTG GTT ACA AAC and 3′-TCT GGA AAG TGA AGG ATA AAG TGA CGA; Mdm2 promoter, 5′-TGG GCA GGT TGA CTC AGC TTT TCC TC and 3′-TGG CGT GCG TCC GTG CCC AC; p21 promoter, 5′-CCA GCC CTT TGG ATG GTT T and 3′-GCC TCC TTT CTG TGC CTG A; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promoter, 5′-AAA AGC GGG GAG AAA GTA GG and 3′-CTA GCC TCC CGG GTT TCT CT.
Western analysis.
After being washed twice with PBS, cells were pelleted by centrifugation. For whole-cell extracts, cells were lysed as previously described (19) with a minor modification of buffer X (100 mM Tris-HCl [pH 8.5], 250 mM NaCl, 1% [vol/vol] NP-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml). Cytoplasmic and nuclear extracts were prepared as previously described (31). Pelleted nuclei were resuspended in buffer X (100 mM Tris-HCl [pH 8.5], 250 mM NaCl, 1% [vol/vol] NP-40, 1 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 0.5 μg of aprotinin/ml, 0.15 mM spermine, and 0.75 mM spermidine). Nuclear pellet was lysed by a 15-min incubation with agitation at 4°C. The supernatant was recovered by centrifugation at 12,500 rpm for 10 min on a bench top refrigerated microfuge. Ten to 100 μg of protein was resolved by 6 to 14% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Amersham Biosciences Corp., Piscataway, N.J.).
Antibodies.
Immunoblotting was carried out with the following antibodies: BRG1 (Robert Kingston, Massachusetts General Hospital, Boston, Mass.); SRC1 and SRC3 (Joe Torchia, University of Western Ontario, London, Ontario, Canada); BUGR2 (B. Gametchu, Medical College of Wisconsin, Milwaukee, Wis.); E6-AP (Carolyn Smith, Baylor College of Medicine, Houston, Tex.); C terminus of Hsc70-interacting protein (CHIP) (Cam Patterson, University of North Carolina, Chapel Hill, N.C.); brm (BD Biosciences, Transduction Laboratories, San Diego, Calif.); ERα (Upstate Biotech, Lake Placid, N.Y.); p21 (BD Biosciences, Pharmingen, San Diego, Calif.), p27, cyclin D1, Hsp90, β-tubulin, PR-AB-52, and Mdm2 (Santa Cruz Biotech, Santa Cruz, Calif.); p53 (Calbiochem, Boston, Mass.); and GAPDH (Research Diagnostics Inc., Flanders, N.J.).
RESULTS
Characterization of MCF-7-MMTV-GR cells.
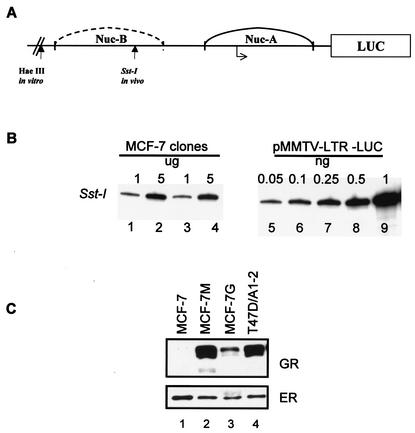
Estrogen-responsive MCF-7 cells express endogenous ER but express very low levels of GR (54). To create a system for studying the effect of estrogens on GR-mediated transcriptional activity, MCF-7 cells were stably cotransfected with an MMTV reporter plasmid, a rat GR expression plasmid, and a neomycin resistance gene construct (20, 21). From the transfected cells, several clones were selected, and cell lines were established. Of these, two G418-resistant clones were selected for further characterization. To determine the number of copies of MMTV-LUC integrated in the genome of MCF-7 cells, genomic DNA purified from clones M and G was digested to completion with SstI (Fig. 1 A). Using primers specific for MMTV sequences, DNA was analyzed by linear PCR for the number of MMTV-LUC reporter copies integrated into the genome of MCF-7 cells (31). The PCR analysis indicated that there are 20 copies of the MMTV-LUC reporter per genome in these cells (Fig. 1B). Next we determined GR protein expression by immunoblotting using a murine GR-specific monoclonal antibody. The rat GR was detected in the MCF-7 clones but not in the parental MCF-7 cells (Fig. 1C, compare lane 1 with lanes 2 and 3). The MCF-7 clone M was chosen for further experiments. Based on immunoblotting, clone M expresses approximately 100,000 receptors per cell compared to T47D/A1-2 cells, which express 80,000 receptors per cell (21).
FIG. 1.
Characterization of the MCF-7-GR clones. (A) Schematic of the proximal MMTV promoter representing the hormone-sensitive nucleosome B region and restriction enzyme sites. (B) Number of MMTV-LUC copies in MCF-7-GR clones. MCF-7 cells were cotransfected with an MMTV-LUC reporter and GR expression vector. To confirm the integration of the MMTV-reporter, genomic DNA purified from two MCF-7 clones, clones M (lanes 1 and 2) and G (lanes 3 and 4), was digested with Sst-I. Genomic DNA (1 or 5 μg) together with 0.05 to 1 ng of MMTV reporter plasmid DNA (2.5 to 50 copies per genome equivalents) (lanes 5 to 9) was analyzed by PCR. PCR products were analyzed with 8% polyacrylamide denaturing gels and exposed to a phosphorimager screen for quantitation and to determine the number of copies of the MMTV reporter integrated in the genome. (C) GR and ER expression in MCF-7-GR clones. Whole-cell extracts were prepared from parental MCF-7 cells (lane 1), MCF-7 clones M and G (lanes 2 and 3), and T47D/A1-2 (lane 4) cells. Proteins were separated on 6% SDS-PAGE, and receptor expression was monitored by Western blotting using antibodies specific for the rat GR and ER. After autoradiography, films were scanned using a densitometer, and bands were quantified.
Estrogen agonists, but not antagonists, inhibit GR transcriptional activity.
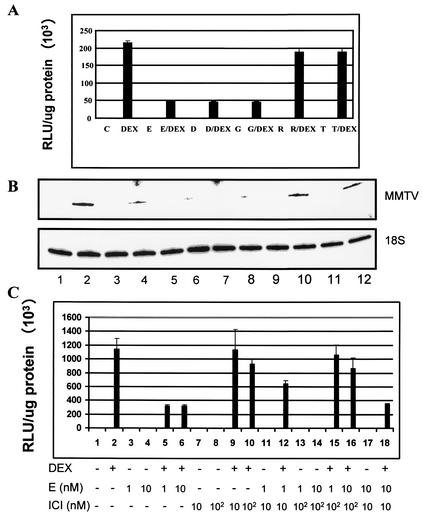
Studies with progestin antagonists suggest that the MMTV promoter provides a good model to study cross talk between steroid hormone receptors in the context of chromatin (19, 21). The present study focused on the cross talk between the GR and the ER. The GR and ER each exist as two isoforms, GRα and GRβ (77) and ERα and ERβ (35). In the present study, GR and ER refer to GRα and ERα, respectively. In preliminary experiments, we treated MCF-7 cells at various time points to determine the optimal time required to demonstrate an effect of the activated ER on GR-mediated transcriptional activity (data not shown). We found that in MCF-7 cells, pretreatment with 10 nM E2 for 48 h followed by 1 nM DEX for 16 h inhibited DEX induction of the MMTV promoter by 75% (Fig. 2A). The 48-h time point was used for all experiments.
FIG. 2.
Estrogen agonists, but not antagonists, inhibit GR-mediated transactivation. (A) MCF-7 cells were pretreated for 48 h with estrogen agonists 10 nM E2 (E), 10 nM DES (D), and 100 nM genistein (G), or antagonist 100 nM raloxifene (R) or tamoxifen (T), followed by treatment with 1 nM DEX or ER ligands plus DEX for 16 h. Lysates were harvested and analyzed for luciferase activity in triplicate samples for each treatment condition. Data are reported as RLU normalized for total protein. Results are expressed as means ± standard errors for three determinations per treatment condition. (B) Estrogen agonists inhibit glucocorticoid-induced MMTV-LUC mRNA. MCF-7 cells were pretreated for 48 h with estrogen agonists E2 and DES (10 nM) (lanes 3 to 6) or genistein (100 nM) (lanes 7 and 8) or antagonists raloxifene or tamoxifen (100 nM) (lanes 9 to 12), followed by treatment with 1 nM DEX (lane 2) or ER ligands plus DEX (lanes 4, 6, 8, 10, and 12) for 16 h. Total RNA was harvested and analyzed by primer extension with primers specific for MMTV or 18S rRNA. Levels of labeled PCR transcripts were analyzed on 8% polyacrylamide denaturing gels and exposed to phosphorimager screens for quantification. (C) The estrogen antagonist ICI attenuates E2-mediated inhibition of the MMTV promoter activity. Cells were pretreated for 48 h with 1 or 10 nM E2 (E) (lanes 3, 4, 13, 14, and 17) or 10 or 100 nM ICI (lanes 7 and 8) or ICI and E2 (lanes 11 to 18), followed by treatment with 1 nM DEX (lanes 2, 5, 6, 9, 10, 12, 15, 16, and 18) for 16 h. Lysates were harvested and analyzed for luciferase activity as described above. Data are reported as RLU normalized for total protein. The procedure was done in triplicate for each treatment condition, and data are expressed as means ± standard errors for three determinations per treatment condition.
To study the effects of ER ligands on GR-mediated transcriptional activity of the MMTV promoter, we used the synthetic estrogen (DES), the phytoestrogen genistein, and the ER antagonists raloxifene and tamoxifen. MCF-7 cells were pretreated with each compound for 48 h followed by treatment with 1 nM DEX for 16 h. Pretreatment with 10 nM E2, 10 nM DES, or 100 nM genistein inhibits MMTV-LUC reporter activity by ≈75% (Fig. 2A). In contrast to the ER agonists, the antagonists raloxifene (100 nM) and tamoxifen (100 nM) do not significantly affect GR-mediated MMTV-LUC activity (Fig. 2A). The effect of ER ligands on MMTV-LUC reporter activity reflected changes in transcription from the MMTV promoter. MCF-7 cells were treated as above, and total RNA was collected. MMTV transcription was monitored by primer extension analysis. In correlation with the observed DEX-dependent increase in reporter activity (Fig. 2A), DEX induces MMTV-LUC transcription (Fig. 2B. lane 2). Pretreatment with estrogen agonists (E2, DES, genistein) or antagonists (raloxifene, tamoxifen) alone has no significant effect on MMTV-LUC transcript (Fig. 2B, compare lane 1 with lanes 3, 5, 7, 9, and 11). In contrast, pretreatment with estrogen agonists followed by DEX inhibits glucocorticoid-induced MMTV-LUC mRNA (Fig. 2B, compare lane 2 with lanes 4, 6, and 8). Estrogen antagonists had no significant effect on DEX-induced MMTV-LUC transcript (Fig. 2B, compare lane 2 with lanes 10 and 12).
To demonstrate that the ER mediates the effects of estrogens on MMTV transcription, we tested GR transcriptional activity in the presence of the pure estrogen antagonist ICI. MCF-7 cells were pretreated with either E2 or ICI for 48 h. Both E2 and ICI were used at various dose combinations to test maximum ICI inhibition. Compared to E2, pretreatment with ICI alone followed by DEX had no effect on MMTV-LUC reporter activity (Fig. 2C, compare lane 2 with lanes 5 and 6 and 9 and 10). Pretreatment with ICI and E2 followed by DEX attenuated E2-mediated repression on GR transcriptional activity (Fig. 2C, compare lanes 5 and 6 with lanes 12, 15, and 16). The results show that ICI blocks E2 more efficiently when the two hormones are used at a 100:1 molar ratio (Fig. 2C, compare lanes 12, 15, and 16). At equal molar ratios, ICI does not attenuate E2-mediated repression of GR transcriptional activity (Fig. 2C, lane 18), consistent with the fact that E2 has higher affinity for the ER. Together, these experiments indicate that the ER mediates the repressive effect of estrogen agonists on GR-mediated transactivation.
Estrogen agonists, but not antagonists, inhibit GR's ability to remodel chromatin.
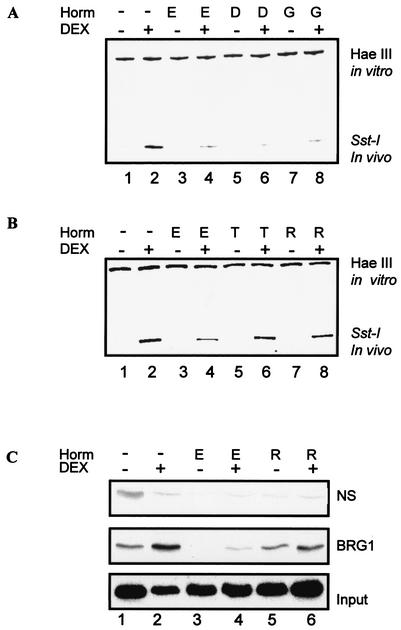
We have previously established that GR-mediated transcription from the MMTV promoter involves two distinct steps, GR recruitment of the BRG1 complex and chromatin remodeling, followed by the assembly of the transcription preinitiation complex (19, 37). Glucocorticoid induction of MMTV transcription is closely linked with the presence of a hypersensitive region within nucleosome B (nuc B) (37). In the next set of experiments, we examined whether treatment with estrogen agonists or antagonists had any effect on GR-mediated hypersensitivity, monitored by SstI cutting within nuc B (Fig. 1A). In the absence of DEX, there is limited cutting by SstI (Fig. 3A, lane 1). Treatment with DEX significantly induces SstI cutting in vivo (Fig. 3A, compare lanes 1 and 2). Pretreatment with estrogen agonists E2, DES, and genistein alone did not significantly change SstI cutting (Fig. 3A, compare lanes 1, 3, 5, and 7). However, pretreatment with estrogen agonists inhibited DEX-induced SstI hypersensitivity (Fig. 3A, compare lane 2 with lanes 4, 6, and 8). In contrast to estrogen agonists, the efficiency of SstI cutting was not affected by treatment with the estrogen antagonist tamoxifen or raloxifene (Fig. 3B, compare lane 2 with lanes 6 and 8). In vitro HaeIII cutting indicates that the amounts of DNA loaded for each treatment were equal. The results observed with SstI were also observed with AflII, another restriction enzyme that cuts in vivo within nuc B (data not shown).
FIG. 3.
Estrogen agonists inhibit glucocorticoid-induced chromatin remodeling. (A) Estrogen agonists inhibit glucocorticoid-induced SstI hypersensitivity. Nuclei were harvested from cells pretreated with 10 nM E2 and DES or 100 nM genistein (lanes 3 to 8) followed by treatment with 1 nM DEX (lane 2) or ER ligands (Horm) and DEX (lanes 4, 6, and 8) for 1 h. Nuclei were digested with Sst-I in vivo and with HaeIII in vitro to provide an internal control. DNA was amplified by reiterative primer extension using Taq DNA polymerase. PCR products were analyzed using 8% polyacrylamide denaturing gels and exposed to phosphorimager screens. (B) Estrogen antagonists do not inhibit glucocorticoid-induced SstI hypersensitivity. Cells were pretreated as described in the legend for panel A, but with 100 nM tamoxifen (lanes 5 and 6) or raloxifene (lanes 7 to 8). Cells treated with E2 (lanes 3 to 4) were included as a control. (C) BRG1 association with the MMTV promoter is inhibited by E2. Cells were untreated (lane 1) or pretreated with 10 nM E2 (lanes 3 and 4) or 100 nM raloxifene (lanes 5 and 6) followed by treatment with 1 nM DEX (lane 2) or ER ligands and DEX (lanes 4 and 6) for 1 h. Cells were harvested, and chromatin was prepared by sonication. The fragmented chromatin was immunoprecipitated with antibodies against BRG1 or nonspecific antibody control (normal serum IgG [NS]). DNA from the input and immunoprecipitates was analyzed by PCR with primers specific for the MMTV promoter. PCR products were analyzed with 6% nondenaturing gels and exposed to phosphorimager screens for further analysis. E, E2; D, DES; G, genistein; R, raloxifene; T, tamoxifen.
The ability of the GR to remodel chromatin is facilitated by the BRG1 chromatin-remodeling complex. Next, we investigated whether estrogens affected GR recruitment of the BRG1 to the MMTV promoter by using a ChIP assay (Fig. 3C). In DEX-treated cells, antibodies against BRG1, but not normal serum IgG, specifically immunoprecipitated MMTV promoter DNA (Fig. 3C, compare lanes 1 and 2 of the nonspecific panel with lanes 1 and 2 of the BRG1 panel). Treatment with E2, but not raloxifene, inhibited DEX-induced recruitment of BRG1 to the MMTV promoter (Fig. 3C, compare lane 2 with lanes 4 and 6). Together, these results show that estrogen agonists inhibit GR-mediated chromatin remodeling of the MMTV promoter, and as a result, transcription from the promoter is inhibited.
ER ligands do not alter the levels of chromatin remodeling or coactivator proteins.
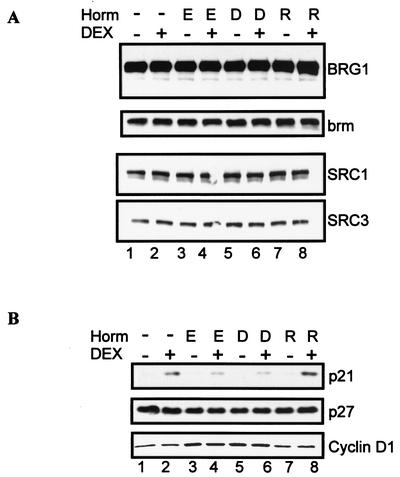
Since we show that in the presence of E2, less BRG1 is associated with the promoter by ChIP (Fig. 3C), we next analyzed BRG1 cellular protein levels to ensure that BRG1 was not limiting and to establish that the observed decrease in BRG1 bound on MMTV promoter was not due to differences in protein expression. Comparison of nontreated cells and cells treated with DEX showed that treatment with estrogen agonists and antagonists did not alter the protein levels of chromatin remodeling protein BRG1 or brm (Fig. 4A, compare lanes 1 and 2 with lanes 3 to 8). Cellular response to steroid receptors, including the GR, is tightly regulated by a number of coactivators that modify GR transcriptional activity (45). Changes in expression of coregulator proteins could contribute to estrogen-directed repression of GR activity. Therefore, we tested protein expression of SRC1 and SRC3, a subset of the SRC/p160 family of coactivator proteins. Treatment with ER ligands did not alter the expression of these proteins (Fig. 4A, compare lanes 1 and 2 with lanes 3 to 8).
FIG. 4.
Effect of ER ligands on expression of chromatin remodeling, coactivator, and cell cycle proteins. (A) Treatment with estrogen agonists and antagonists does not alter chromatin remodeling or coactivator protein levels. MCF-7 cells were pretreated with 10 nM E2 (lanes 3 and 4), 10 nM DES (lanes 5 and 6), or 100 nM raloxifene (lanes 7 and 8). Forty-eight hours posttreatment, cells were untreated (lane 1) or treated with 1 nM DEX (lane 2) or ER ligands and DEX (lanes 4, 6, and 8) for 12 h. Western blotting analysis was done with antibodies specific for BRG1, brm, SRC1, and SRC3. (B) Estrogen agonists inhibit glucocorticoid induction of p21 protein. Cells were treated as described in the legend for panel A. Western blotting analysis was performed with antibodies specific for p21, p27, and cyclin D1. E, E2; D, DES; R, raloxifene.
Chromatin remodeling proteins play a role in cell cycle progression (49, 59), and estrogen regulation of the cell cycle could impact on regulation of glucocorticoid-induced proteins. In particular, estrogens promote growth, while glucocorticoids exert antiproliferative effects on mammary epithelial cells (69, 79). To gain more insight on the physiological outcome of the cross talk between the GR and the ER, we examined whether estrogen agonists had a repressive effect on GR-regulated cell cycle proteins. For this purpose, we monitored the glucocorticoid-inducible cell cycle inhibitor, cyclin-dependent kinase inhibitor p21. Previous studies have shown that glucocorticoids induce p21 protein levels in a number of cell lines (6, 61). Cyclin D1 is a well-defined target of E2 in the MCF-7 breast epithelial cell line (1, 42) and was used as a control. In agreement with the known antiproliferative effects of glucocorticoids in breast epithelial cells, DEX induced an increase in the cyclin-dependent kinase inhibitor p21 (Fig. 4B, compare lanes 1 and 2). ER ligands alone (E2, DES, raloxifene) did not induce p21 protein expression. In contrast, pretreatment with E2 or DES, but not raloxifene, inhibits DEX-induced p21 protein expression (Fig. 4B, compare lane 2 and lanes 4, 6 and 8). Estrogens had no significant effect on the cyclin-dependent kinase inhibitor p27. However, consistent with the proliferative role of estrogen agonists on breast epithelial cells, E2 and DES, but not raloxifene, induced an increase in cyclin D1 protein levels (Fig. 4B, compare lanes 3 to 6 with 7 to 8).
Estrogen agonists down regulate GR protein.
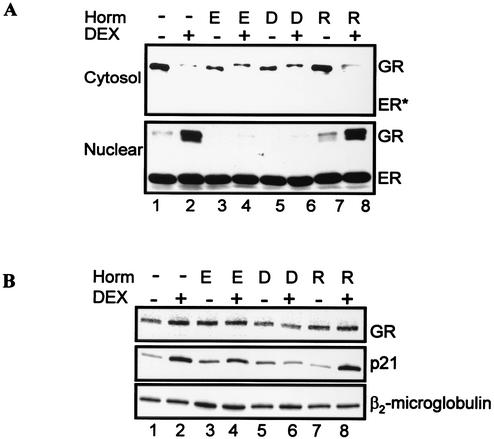
The results showing that estrogen agonists inhibit DEX induction of p21 raised the possibility that the repressive effect of estrogen agonists on GR activity is not specific for MMTV but could be a general effect on GR-regulated genes and proteins. We next asked whether estrogens had an effect on GR protein levels. It is generally accepted that the unliganded GR resides in the cytoplasm and that hormone activation leads to nuclear localization and a transcriptionally active GR (22, 29). Therefore, we monitored GR levels in the cytosol and nuclear fractions by immunoblotting. As expected, the GR is in the cytosol in the absence of DEX (Fig. 5A, cytosol results in lanes 1, 3, 5, and 7). Treatment with estrogen agonists (E2, DES), but not raloxifene, resulted in a decrease in GR levels compared to untreated cells (Fig. 5A, cytosol blot, compare lane 1 with lanes 3, 5, and 7). There is some GR present in the cytosol fraction in the cells treated with DEX in the presence of E2 and DES, raising two possibilities: the cellular fractionation was not complete because of methodology, or the GR is retained more in the cytosol in the presence of estrogen agonists (Fig. 5A, cytosol blot, compare lane 2 with lanes 4, 6, and 8). This was observed in multiple experiments. As a control, the membrane was immunoblotted with ER antibody. In contrast to the GR, the ER is not detected in the cytosol. Coincident with hormone-induced translocation, treatment with DEX localizes the GR to the nucleus (Fig. 5A, nuclear blot, lane 2). When cells were treated with DEX after pretreatment with E2 or DES, GR levels decreased and were almost undetectable with our experimental conditions (Fig. 5A, nuclear blot, compare lane 2 with lanes 4 and 6). In contrast to cells pretreated with estrogen agonists, GR levels in cells treated with raloxifene were similar to those in cells treated with DEX alone (Fig. 5A, nuclear blot, compare lane 2 with lane 8). ER levels did not significantly change with hormone treatment, and in particular, DEX treatment does not have a significant effect on ER protein levels. In a similar experiment, treatment with genistein, but not tamoxifen, decreased GR protein levels (data not shown). These results indicate that treatment with estrogen agonists, but not antagonists, results in a decrease in GR protein levels in MCF-7 cells.
FIG. 5.
Activated ER decreases GR protein levels. (A) Effect of ER ligands on GR protein levels. MCF-7 cells were untreated (lane 1) or pretreated with 10 nM E2 (lanes 3 and 4), 10 nM DES (lanes 5 and 6), or 100 nM raloxifene (lanes 7 and 8). Forty-eight hours posttreatment, cells were treated with 1 nM DEX (lane 2) or ER ligands and DEX (lanes 4, 6, and 8) for 12 h. Cytoplasmic and nuclear extracts were analyzed by Western blotting with antibodies specific for GR and ER. (B) Effect of ER ligands on GR and p21 mRNA levels. Cells were treated as described above, and total RNA was prepared as described in Materials and Methods. GR, p21, and β2-microglobulin mRNA transcripts were analyzed by RT-PCR. E, E2; D, DES; R, raloxifene.
To ascertain that the decrease in GR protein expression was not associated with altered transcription of the GR gene, we monitored GR mRNA expression. It was unlikely that we would detect any differences in GR mRNA, since we constructed a cell line that would constitutively express the rat GR under control of a simian virus 40 promoter. Rat GR mRNA expression was monitored by RT-PCR with primers specific for the rat GR cDNA sequence after pretreatment with estrogen agonists and antagonists. Treatment with ER agonists or antagonists does not significantly change GR mRNA expression (Fig. 5B, compare lanes 1 and 2 with lanes 3 to 8). In contrast to the GR transcript, E2 and DES, but not raloxifene, inhibit DEX induction of p21 mRNA. Together, these results show that in MCF-7 cells pretreated with estrogen agonists, GR protein levels are decreased. Furthermore, this decrease occurs at a step downstream of transcription of the GR gene, suggesting that estrogen agonists may affect GR protein stability.
ER-dependent down regulation of the GR requires de novo protein synthesis and is mediated by the proteasome degradation pathway.
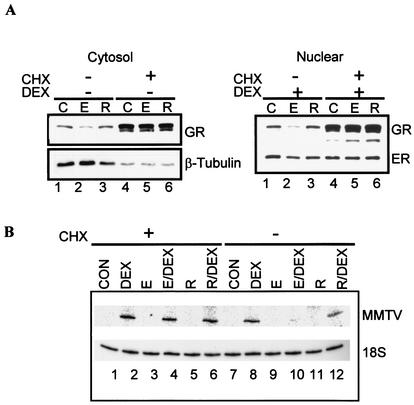
We next investigated potential mechanisms by which estrogen agonists decrease GR protein levels. The finding that estrogen agonists do not significantly affect GR mRNA levels suggests that the decrease in GR protein is not at the transcription level but rather downstream, perhaps at a translation or posttranslational step. To test whether the E2-mediated decrease in GR protein levels required de novo protein synthesis, we asked whether inhibiting protein synthesis would block the E2-mediated decrease in GR protein levels. Cells were pretreated with E2 or raloxifene alone or with ER ligands in the presence of 1 μg of CHX/ml for 48 h. Cell lysates were prepared from the cytosol and nuclear fractions, and the GR was monitored by Western blotting. Pretreatment with E2, but not raloxifene, decreases the GR in the cytosol and in the nucleus (Fig. 6A, compare lane 2 with lanes 1 and 3). Pretreatment with E2 in the presence of CHX blocks E2-directed down regulation of the GR (Fig. 6A, compare lanes 2 and 5). As a control in the cytosol, the same membrane was stripped and immunoblotted with β-tubulin antibody. In contrast to the GR, β-tubulin protein levels do not significantly change with E2 treatment (Fig. 6A, compare lanes 1 and 2). However, unlike the GR, β-tubulin protein levels decrease in the presence of CHX. Similarly, the nuclear proteins were immunoblotted with ER antibody as control. In the presence of CHX, ER protein levels do not change significantly. Notably, treatment with CHX not only blocks E2-mediated decrease in GR protein levels but also increases basal levels of the GR (Fig. 6A, compare lanes 1 and 2 with lanes 4 and 5). Together, these results indicate that the mechanism by which estrogen agonists down regulate GR protein levels requires de novo protein synthesis but does not involve the synthesis of the GR protein itself. Instead, the results suggest that E2 decreases the GR by a mechanism that involves GR protein turnover or stability. Interestingly, upon CHX treatment and GR accumulation, we detect additional GR antibody reactive material that could represent proteolytic fragments and/or splice variants. A resolution of this issue is currently under active investigation.
FIG. 6.
ER-dependent down regulation of the GR requires de novo protein synthesis and proteasome degradation. (A) CHX blocks ER mediated decrease in GR. MCF-7 cells were untreated (cytosol results, lane 1) or pretreated for 48 h with 10 nM E2 (lane 2) or 100 nM raloxifene (lane 3) in the presence or absence of 1 μg of CHX/ml (lanes 4 to 6). After 48 h, cells were untreated or treated with 1 nM DEX (nuclear results, lane 1) or ER ligands and DEX (lanes 2 and 3) for 12 h. Western blotting analysis was done with antibodies specific for GR, ER, and β-tubulin. (B) CHX blocks the inhibitory effect of E2 on GR transactivation. MCF-7 cells were untreated (lanes 1 and 7) or pretreated for 48 h with 10 nM E2 (lanes 3, 4, 9, and 10) or 100 nM raloxifene (lanes 5, 6, 11, and 12) in the presence or absence of 1 μg of CHX/ml (lanes 1 to 6). After 48 h, cells were treated with 1 nM DEX (lane 2 and 8) or ER ligands and DEX (lanes 4, 6, 10 and 12) for 12 h. Total RNA was harvested and analyzed by primer extension with specific primers for MMTV and 18S rRNA. PCR products were analyzed with 8% polyacrylamide denaturing gels and exposed to phosphorimager screens for further analysis. (C) The proteasome inhibitor MG132 blocks ER-mediated degradation of the GR. MCF-7 cells were untreated (lane 1) or pretreated for 48 h with 10 nM E2 (lane 2) or 100 nM raloxifene (lane 3) in the presence or absence of 0.5 μM MG132 (lanes 4 to 6). After 48 h, cells were treated with 1 nM DEX or ER ligands and DEX for 12 h. Western blotting analysis was done with antibodies specific for GR and ER. (D) The proteasome inhibitor MG132 blocks GR degradation, but not PR degradation. MCF-7 cells were untreated (lane 1) or pretreated for 48 h with 10 nM E2 (lane 2) or 100 nM raloxifene (lane 3) in the presence or absence of 0.5 μM MG132 (lanes 4 to 6). Nuclear extracts were prepared, and Western blotting analysis was done with antibodies specific for PR. (E) MG132 reverses the inhibitory effect of E2 on GR transactivation. MCF-7 cells were untreated (lanes 1 and 7) or pretreated for 48 h with 10 nM E2 (lanes 3, 4, 9, and 10) or 100 nM raloxifene (lanes 5, 6, 11, and 12) in the presence or absence of 0.5 μM MG132 (lanes 7 to 12). After 48 h, cells were treated with 1 nM DEX (lanes 2 and 8) or ER ligands and DEX (lanes 4, 6, 10, and 12) for 12 h. Total RNA was analyzed by RT-PCR with specific primers for MMTV and β2-microglobulin. PCR products were analyzed with 8% polyacrylamide denaturing gels and exposed to phosphorimager screens. E, E2; R, raloxifene.
We further investigated whether treatment with CHX restores GR-mediated transcriptional activation in the presence of E2. Cells were treated as in previous experiments, and total RNA was collected. MMTV-LUC mRNA was monitored by primer extension analysis. Pretreatment with E2, but not raloxifene, alone or in the presence of CHX did not induce MMTV-LUC mRNA (Fig. 6B, compare lanes 1 and 7 with lanes 3, 5, 9, and 11). Treatment with DEX alone or in the presence of CHX induces MMTV-LUC transcript (Fig. 6B, compare lanes 1 and 7 with 2 and 8). In contrast, pretreatment with E2, but not raloxifene, inhibits DEX induction of MMTV-LUC transcript (Fig. 6B, compare lane 10 with lanes 8 and 12), and this effect is blocked by CHX (Fig. 6B, compare lanes 4 and 10). These experiments suggest that inhibiting protein synthesis blocks E2-mediated decrease in GR protein levels and restores GR-mediated transcriptional activity, as shown by an increase in MMTV-LUC transcript.
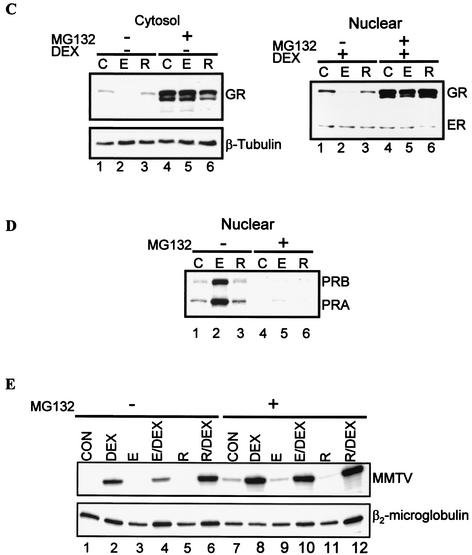
The experiments using CHX suggest that the E2-mediated decrease in GR involves protein turnover. Regulated proteolysis by the proteasome accounts for turnover of most short- and long-lived proteins, including some nuclear receptors (13, 36, 43, 51). Germane to our current studies, the ubiquitin-proteasome pathway has been shown to degrade the GR (16, 65, 71, 73). To examine if E2 down regulates the GR through a proteasome-dependent pathway, we asked whether the proteasome inhibitor MG132 would block E2-mediated down regulation of the GR. Cells were cultured overnight and cotreated with hormones plus 0.5 μM MG132 for 48 h. Cytosol and nuclear fractions were prepared and analyzed by Western blotting. In both the cytosol and the nucleus, pretreatment with E2, but not raloxifene, decreases GR protein levels (Fig. 6C, compare lane 2 with lanes 1 and 3). Pretreatment with MG132 blocks E2-directed down regulation of the GR (Fig. 6C, compare lanes 2 and 5). For a control, the same membrane was stripped and immunoblotted with β-tubulin (cytosol) or ER antibody (nuclear). In contrast to the GR, β-tubulin protein levels do not significantly change with E2 treatment (Fig. 6C, compare lanes 1 and 2). Treatment with MG132 does not increase β-tubulin protein from basal levels.
To ascertain that the effects inhibiting the proteasome were specific for the GR, we determined PR levels under similar experimental conditions. As expected, treatment with E2, but not raloxifene, increases PR protein levels (Fig. 6D, compare lane 2 with lanes 1 and 3). Treatment with MG132 inhibits ER-mediated increase of the PR and in fact inhibits basal PR protein expression. These results indicate that E2-mediated proteasomal degradation is specific for the GR (Fig. 6D, lane 5). To establish whether the GR accumulated after proteasome inhibition was transcriptionally competent, GR-mediated changes in MMTV-LUC transcript levels were analyzed. Pretreatment with E2 or with raloxifene alone or in the presence of MG132 did not induce MMTV-LUC mRNA (Fig. 6E, compare lanes 1, 3, and 5 with lanes 7, 9, and 11), although MG132 increases basal activity of the promoter (Fig. 6E, compare lanes 1 and 7). Treatment with DEX alone or in the presence of MG132 induces MMTV-LUC mRNA (Fig. 6E, compare lanes 1 and 7 with lanes 2 and 8). Pretreatment with E2, but not raloxifene, inhibits DEX induction of MMTV-LUC mRNA (Fig. 6E, compare lane 4 with lanes 2 and 6), and MG132 blocks this effect (Fig. 6E, compare lanes 4 and 10). Taken together, these results indicate that E2-mediated down regulation of the GR is blocked by a proteasome inhibitor, suggesting that E2 enhances proteasomal degradation of the GR.
ER-dependent GR degradation is coupled to an increase in Mdm2 protein.
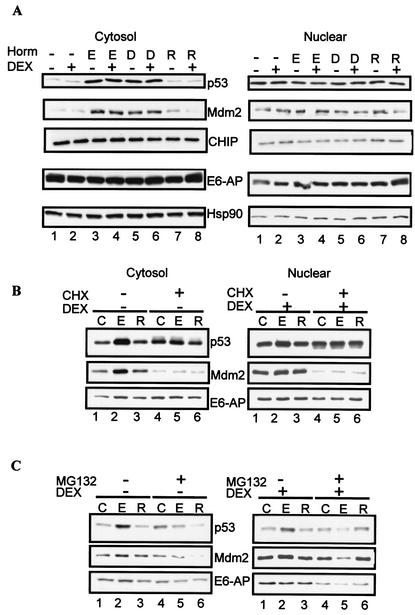
Our experiments in which we inhibit protein synthesis with CHX led us to hypothesize that treatment with estrogen agonists results in the synthesis of ER-regulated protein(s) that targets the GR to the proteasome. In the next set of experiments, we examined the changes in protein levels of the tumor suppressor p53 and its inhibitor Mdm2. Recent experiments have shown Mdm2 to be regulated by estrogens as well as to target the GR for ubiquitylation (27, 65). As a control, membranes were immunoblotted with antibodies for hsp90, a chaperone protein that stabilizes the GR (73); E6-AP, a E3 ubiquitin ligase that has been shown to function as a coactivator for the ER (52) and to target p53 for proteasome degradation (63); and CHIP, a chaperone-dependent E3 ligase that induces ubiquitylation of the GR (10). In the cytosol, estrogen agonists (E2, DES), but not raloxifene, increase p53 protein levels two- to threefold compared to levels in untreated or DEX-treated cells (Fig. 7A, compare lanes 3 to 6 with lanes 1, 2, 6, and 8). Concomitantly with the increase in p53, Mdm2 increases in the cytosol after treatment with E2 and DES. In contrast to p53 and Mdm2, neither E6-AP nor hsp90 or CHIP changes significantly after treatment with ER ligands.
FIG. 7.
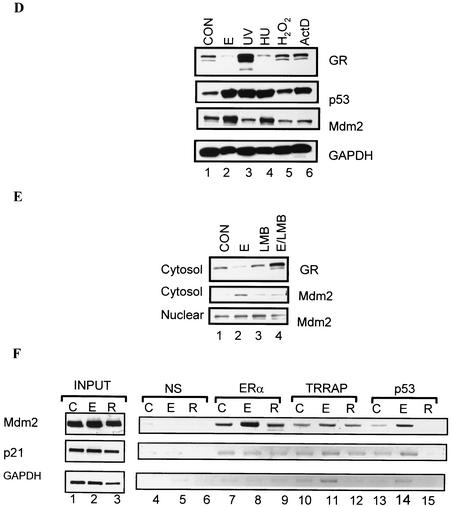
ER-dependent GR degradation is coupled to an increase in Mdm2 protein expression. (A) Estrogen agonists increase p53 and Mdm2 protein levels in the cytosol. MCF-7 cells were pretreated for 48 h with 10 nM E2 (lanes 3 and 4), 10 nM DES (lanes 5 and 6), or 100 nM raloxifene (lanes 7 and 8). After 48 h, cells were left untreated (lane 1) or treated with 1 nM DEX (lane 2) or ER ligands and DEX (lanes 4, 6, and 8) for 12 h. Western blotting analysis was done with antibodies specific for p53, Mdm2, CHIP, E6-AP, and hsp90. (B) CHX blocks Mdm2 protein expression. MCF-7 cells were untreated (lane 1) or pretreated for 48 h with 10 nM E2 (lane 2) or 100 nM raloxifene (lane 3) in the presence or absence of 1 μg of CHX/ml (lanes 4 to 6). (C) MG132 blocks E2-mediated increase in Mdm2 protein levels. Cells were treated as described in the legend for panel B in the presence or absence of 0.5 μM MG132, and Western blotting analysis was performed as described in the legend for panel B. (D) An increase in Mdm2 is correlated with GR degradation. MCF-7 cells were treated with various agents that induce p53, namely, 10 nM E2 (48 h; lane 2), 100 J of UV/m2 (16 h) (lane 3), 1.5 mM hydroxyurea (24 h) (lane 4), 1 mM H2O2 (3 h) (lane 5), or 1 μg of actinomycin D/ml (3 h) (lane 6). Whole-cell extracts were prepared and subjected to SDS-PAGE, and proteins were analyzed by Western blotting with antibodies specific for GR, p53, Mdm2, and GAPDH as a control. (E) E2-mediated GR degradation is coupled to an increase in cytosolic Mdm2. MCF-7 cells were untreated (lane 1) or treated with 10 nM E2 (lane 2), 5 nM LMB (lane 3), or E2 and LMB (lane 4) for 48 h. Cytosol and nuclear extracts were prepared and subjected to SDS-PAGE and Western blotting. (F) E2 induces ERα and p53 association with the Mdm2 promoter. Cells were untreated (lane 1) or treated with 10 nM E2 (lane 2) or 100 nM raloxifene (lane 3) for 48 h. Cells were harvested, and chromatin was prepared by sonication. The fragmented chromatin was immunoprecipitated with antibodies against nonspecific antibody control (normal serum IgG [NS]) (lanes 4 to 6), ERα (lanes 7 to 9), TRRAP (lanes 10 to 12), or p53 (lanes 13 to 15). A proportion of the fragmented chromatin was saved before immunoprecipitation as input (lanes 1 to 3). DNA from the input and immunoprecipitates was analyzed by PCR with primers specific for the Mdm2, p21, or GAPDH promoters. PCR products were analyzed with a 1.5% agarose gel. E, E2; D, DES; R, raloxifene; ActD, actinomycin D; HU, hydroxyurea; C, untreated.
To gain insight on whether p53 and Mdm2 could be the candidate molecules targeting the GR for proteasome degradation, we asked whether CHX and MG132 blocked E2-mediated up regulation of these proteins. Cytoplasmic and nuclear extracts from the experiments illustrated in Fig. 6A and C were immunoblotted with antibodies for p53, Mdm2, and E6-AP. In contrast to the GR (see Fig. 6A, lane 2), pretreatment with E2 increases p53 levels compared to the control or cells treated with DEX (Fig. 7B, compare lanes 1 and 2), and the effect is predominantly in the cytosol. Raloxifene does not significantly change p53 levels compared to the control (Fig. 7B, compare lanes 1 and 3). In both cytosol and the nucleus, pretreatment with CHX blocks E2-mediated increase in p53 and maintains the protein at basal levels (Fig. 7B, compare lane 2 with lanes 4 to 6). As shown in Fig. 7A, E2 increases Mdm2 protein levels, as observed for p53 (Fig. 7B, compare lanes 1 and 2). However, in contrast to p53 or the GR (see Fig. 6A, lanes 4 to 6), pretreatment with CHX and E2 not only blocks E2-mediated increase in Mdm2 but also blocks basal Mdm2 levels in both the cytosol and the nucleus. E6-AP protein levels do not significantly change with hormone or CHX treatment. The observation that there are distinct differences in the effects of the protein synthesis inhibitor on the GR, p53, and Mdm2 protein levels suggests differences in protein turnover among these proteins. In particular, CHX inhibits Mdm2 synthesis, suggesting that this protein could play a role in E2-induced GR degradation.
In a separate experiment, we monitored the effects of inhibiting the proteasome on p53, Mdm2, and E6-AP protein levels. As shown above, pretreatment with E2, but not raloxifene, increases p53 and Mdm2 levels (Fig. 7C, compare lane 2 with lanes 1 and 3). As shown for the GR, treatment with the proteasome inhibitor MG132 attenuated the E2-mediated response by blocking the increase in p53 and Mdm2 (Fig. 7C, compare lanes 2 and 5). Interestingly, MG132 inhibits E2-mediated up regulation of Mdm2 in the nucleus (Fig. 7C, compare lanes 2 and 5, nuclear blot). These data support the hypothesis that Mdm2 is an ER target, and the result is consistent with recent reports showing that MG132 inhibits ER transcriptional activity (43).
Since we find that GR degradation is correlated with an increase in both p53 and Mdm2, we wanted to determine which protein was more directly correlated with GR degradation. We employed a variety of stress-inducing agents known to induce p53 and monitored GR protein levels by immunoblotting (Fig. 7D). Compared to control cells, treatment with E2 increases p53 and down regulates the GR (Fig. 7D, compare lanes 1 and 2). Both UV irradiation and hydroxyurea increased p53 to the same extent as E2 (Fig. 7D, compare lane 2 with lanes 3 and 4). However, p53 induced by UV is not coupled to GR degradation, while p53 induced by hydroxyurea is associated with a decrease in GR. Treatment with hydrogen peroxide or actinomycin D increased p53 above control levels but did not significantly change GR protein levels (Fig. 7D, compare lane 1 with lanes 5 and 6). Analysis of the effect of these agents on the expression of Mdm2 protein provided a more distinct answer. Our results show that p53 is associated with an increase in Mdm2 in cells treated with E2 (lane 2) and hydroxyurea (lane 4) but not with cells treated with UV irradiation (lane 3). Importantly, an increase in p53 that is associated with an increase in Mdm2 correlates with a decrease in GR, indicating that GR degradation is coupled to an increase in Mdm2 rather than p53.
Until recently, Mdm2 has been described as an E3 ubiquitin ligase that primarily interacts with p53 and promotes its ubiquitylation and proteasomal degradation (26, 34). However, Mdm2 has recently been shown to ubiquitinate and target the GR to the proteasome (65). Nuclear export of Mdm2 has been shown to be essential for its E3 ubiquitin ligase activity (70). The export from the nucleus to the cytosol is mediated by a nuclear export signal sequence in a number of proteins that interact with CRM-1 receptor. The streptomyces metabolite LMB directly interacts with the CRM-1 receptor and inhibits nuclear export of proteins possessing the nuclear export signal sequence. To determine if E2-mediated decrease in GR was associated with cytoplasmic localization of Mdm2, we used LMB to inhibit nuclear export of Mdm2. In control cells, less than 5% of Mdm2 is localized in the cytosol (Fig. 7E, lane 1, cytosol versus nuclear blot). Treatment with E2 leads to accumulation of Mdm2 in the cytosol (Fig. 7E, lane 2, cytosol versus nuclear blot). In response to LMB alone, Mdm2 is predominantly nuclear (lane 3). Furthermore, LMB blocks E2-mediated nuclear export of Mdm2 and consequently blocks GR degradation (Fig. 7E, compare lanes 2 and 4). Since the results with various stress-inducing agents indicate that Mdm2 specifically correlates with GR degradation, the result with LMB suggests that estrogen agonists sequester Mdm2 in the cytosol to facilitate GR degradation.
To examine a direct role of the ER in the increase in Mdm2 expression, we employed the ChIP technique to determine whether ER protein binds to the Mdm2 promoter. After formaldehyde cross-linking and shearing of chromatin by sonication, protein/DNA complexes were immunoprecipitated with antibodies against normal serum IgG, ERα, TRRAP, and p53. PCR was performed with Mdm2-specific primers spanning the p53 binding site. Results show that ERα is recruited to the region of the Mdm2 promoter spanning the p53 binding site when cells are treated with E2, but not raloxifene (Fig. 7F, compare lanes 8 and 9). Mdm2 is a p53 target; hence p53 is recruited to the promoter in a E2-dependent manner (Fig. 7F, compare lane 14 with lanes 13 and 15). TRRAP is an acetyltransferase multiprotein complex that is required for p53 transcriptional regulation of Mdm2 (4) and also for E2-dependent cell growth in MCF-7 cells (76). Consequently, recruitment of the TRRAP complex to the Mdm2 promoter is E2 dependent (Fig. 7F, compare lane 11 with lanes 10 and 12). No PCR product was observed with DNA immunoprecipitated with normal serum IgG (Fig. 7F, lanes 4 to 6). We also examined recruitment of these factors on p21 promoter, another p53 target gene. Neither ERα nor p53 was recruited to p21 promoter with primers spanning the p53 binding site. No PCR product with immunoprecipitated DNA was observed on the GAPDH promoter, suggesting that the recruitment of these factors is specific for Mdm2 promoter. These results indicate that ERα is recruited on the Mdm2 promoter and that the p53 induced by E2 is preferentially recruited to the Mdm2 promoter, but not to the p21 promoter, supporting our observation that E2 increases Mdm2, but not p21 protein levels. Together, these experiments suggest that Mdm2 may be the candidate protein induced by E2 to facilitate GR degradation.
DISCUSSION
Glucocorticoids and estrogens play an important role in regulating physiological processes such as immune response, reproduction, and cell growth (12, 58). Consequently in the present study, we investigated the role of GR/ER cross talk on GR-mediated transcriptional activity. Our data show that estrogen agonists, but not antagonists, inhibit GR-mediated chromatin remodeling and transcriptional activity of the MMTV promoter integrated in the MCF-7 breast cancer cell line. Similar inhibitory effects were observed when the PR was bound with type II antiprogestins such as Org 31710 and ZK112993 in the T47D/A1-2 breast cancer cell line (19, 21).
In the studies with antiprogestins, the PR-GR antagonism could be predicted. For instance, the GR and PR bind similar DNA response elements on the MMTV promoter (44), and the antagonism could result from competition for DNA binding sites. However, as our studies have shown, the PR represses GR-mediated MMTV transcription primarily by disrupting GR protein-protein interactions with the chromatin remodeling complex proteins (19). Glucocorticoid-induced chromatin remodeling is a hallmark of the GR-mediated transcriptional activation of the MMTV promoter (19, 37, 50). We show that estrogen agonists decrease chromatin remodeling within proximal promoter. Our previous studies with progestin antagonists showed that type II antiprogestins inhibit the ability of the GR to remodel chromatin by sequestering the BRG1 complex (19). In the case of ER-GR antagonism, squelching of cofactors may not be the primary mechanism by which estrogen agonists inhibit GR activity.
Using a number of experimental approaches, we systematically dissected the mode of estrogen agonist repression on GR-mediated transcriptional activity. In MCF-7 cells, treatment with estrogen agonists leads to a proteasome-dependent decrease in GR protein levels. We were surprised by this result, because a previous study (33) had shown that E2-dependent decreases in GR protein could be explained by a decrease in GR mRNA. To circumvent the effects of E2 on GR mRNA levels, we constructed an MCF-7/GR cell line that stably expresses the GR from a rat cDNA driven by a constitutive simian virus 40 early promoter. This would allow us to focus on pathways downstream of the GR gene transcription that are involved in ER-GR cross talk. In the present study, treatment with ER ligands did not significantly change GR mRNA levels compared to control samples. Nevertheless, treatment with estrogen agonists result in a significant decrease in GR protein, suggesting that other ER-dependent pathways can affect GR protein levels. Our experiments in which we inhibit protein synthesis by using CHX suggest that estrogen agonists down regulate the GR primarily by a mechanism that requires de novo protein synthesis. It is unlikely that estrogen agonists inhibit synthesis of GR protein, since we show that in the presence of CHX, E2-mediated decrease in GR protein is blocked and the GR increases above basal levels. The mechanism by which CHX increases basal GR levels is not understood, but a similar observation has been made for the ER when cells are treated with CHX (30, 72). However, our hypothesis that ER-mediated decrease in GR levels requires de novo protein synthesis is supported by a recent study (74). Using experiments similar to those reported here, Wormke et al. show that the aryl hydrocarbon receptor-dependent decrease in ERα does not require de novo protein synthesis, although it is mediated by the proteasome degradation pathway (74).
The ubiquitin proteasomal degradation multicomplex accounts for turnover of most short-lived proteins, including nuclear receptors (9, 13, 36, 43, 51). The levels of GR are tightly regulated and have been shown to be subject to proteasome degradation (16, 65, 71). By inhibiting proteasomal degradation, the inhibitor MG132 blocks ER agonist-dependent degradation of the GR. This is consistent with the hypothesis that ER agonists induce proteasomal degradation of the GR. Our experiments also show that proteasome inhibition represses ER functional activity in MCF-7 cells as MG132 inhibits ER-mediated increase in PR protein levels (43). The results observed with the PR also support recent reports that MG132 does not increase basal PR protein levels (51).
The increase in cytosolic p53 and Mdm2 in the presence of estrogen agonists provides a mechanistic basis to explain how estrogen agonists could target the GR to the proteasome. A number of studies have shown that p53 antagonizes GR transcriptional activity (24, 64, 65). Mdm2 is an E3 ubiquitin ligase that has p53 as one of its major substrates (26, 34). However, recently, a trimeric complex that includes the GR, p53, and Mdm2 was shown to enhance ligand-dependent ubiquitylation and degradation of the GR in HepG2 and human umbilical vein endothelial cells (65), suggesting that Mdm2 is involved in degradation of proteins other than p53.
A number of our experiments suggest that Mdm2 could be the candidate protein that is induced by E2 to facilitate GR proteasomal degradation in MCF-7 cells. Particularly, inhibiting de novo protein synthesis blocks E2-mediated increase in Mdm2, but not p53. Inhibiting proteasome degradation has been shown to inhibit ER-dependent transcriptional activity (43), and MG132 blocks E2-mediated increase in Mdm2 in our experiments. The experiments with agents that induce p53 but do not result in GR degradation also support the idea that Mdm2 protein expression is correlated to GR degradation. Finally, ERα is recruited to the Mdm2 promoter in an E2-dependent manner, suggesting a direct role of the ER in Mdm2 transcription. In support of our findings, GR and p53 are not the only targets of the Mdm2 E3 ubiquitin ligase activity. A recent study has shown that Mdm2 potentiates Akt phosphorylation-dependent ubiquitylation and degradation of the androgen receptor, another member of the steroid receptor superfamily (40). In addition, Mdm2 induces ubiquitylation and proteasome degradation of the histone acetyltransferase multiprotein complex, Tip60, and the β2-adrenergic receptor/β-arrestin complex (39, 66). These studies support a role of Mdm2 E3 ubiquitin ligase activity in ER-dependent degradation of the GR.
We do not exclude other mechanisms by which ER can mediate GR protein degradation. Disrupting chaperone/GR heterocomplexes has been shown to facilitate GR degradation (10, 14, 73). Apart from Mdm2, CHIP is the only other protein that has been shown to induce ubiquitylation and degradation of the GR (10). Particularly, although CHIP induces GR degradation and was recently demonstrated to induce androgen receptor degradation, these effects were not completely reversed by proteasome inhibitors (8). Our results show that ER-mediated decrease in GR levels is reversed by a proteasome inhibitor and that Mdm2, but not CHIP, is a direct target of the ER, since CHIP protein levels do not change after treatment with ER ligands. Other studies have shown a number of steroid receptor interacting proteins to be components of the ubiquitin proteasome degradation pathway (15). Among these proteins is the E3 ubiquitin ligase E6-AP, a coactivator for the ER (52) that also targets p53 for proteasome degradation (63). It is possible that the ER activated by estrogen agonists can enhance GR degradation by directly interacting with E6-AP, but our findings do not support a role of E6-AP in GR degradation. The lack of a role of E6-AP in GR degradation is also supported by a previous observation showing that E6-AP acts as a coactivator in GR transactivation assays (52).
The ER-mediated decrease in GR protein is reasonable, considering that exposure to estrogen agonists and environmental compounds that mimic estrogen action has been shown to decrease other types of receptors in vivo and in vitro. For example, exposure of MCF-7 cells to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) has been shown to decrease ER protein levels (72), an effect confirmed recently to be mediated by proteasomes (74). An earlier study had postulated that TCDD may promote this effect by inducing an enzyme that acts in concert with E2 to target the ER to the ubiquitin proteasome pathway (2), a model similar to what we propose with the GR. In another study, exposure to genistein was shown to down regulate androgen receptors in the rat prostate (18) and uterus (11). These studies support a role of ER signaling in regulation of steroid hormone receptor protein stability.
Together with the decrease in GR protein, we show that estrogen agonists increase both p53 and Mdm2 in the cytoplasm of MCF-7 breast cancer cells. Our results agree with previous reports showing that p53 overexpression correlates with up regulation of Mdm2 (5), and in MCF-7 cells E2 treatment increases p53 (46, 48, 56). Although colocalization of p53 and Mdm2 in cellular compartments is a well-known phenomenon (7, 17, 25, 75), simultaneous cytosolic colocalization of both proteins has not been reported to be steroid hormone-dependent in breast cancer cells. Studies have shown that wild-type p53 is functionally inactivated by abnormal cytoplasmic sequestration in breast cancer cells (46, 47), but Mdm2 expression in the cytosol was not examined. Previous studies have argued that when p53 is sequestered in the cytosol, Mdm2-dependent p53 degradation is enhanced (17). However, it is clear from our experiments that the cytoplasmic p53 induced by estrogen agonists is resistant to Mdm2-mediated degradation. Interestingly, unliganded ER was shown to protect p53 from Mdm2 inactivation in a transformed MCF-7 cell line (41). This phenomenon is similar to that described in neuroblastoma cells, where it was also shown that resistance of p53 to Mdm2 degradation could not be attributed to a failure of the E3 ubiquitin ligase activity of Mdm2 or the proteasome degradation function. It is not clear how estrogen agonists promote p53 stability in the cytoplasm. However, certain covalent modifications (78) can sequester p53 in the cytosol, impeding its transcriptional activity. More recently, a specific protein, Parc, which anchors p53 in the cytosol and perhaps protects p53 from degradation, has been described (53).
Nuclear localization is essential for p53 to function in the control of cell growth and tumorigenesis. Indeed, estrogen agonists alter p53 function. The p53 induced by estrogen agonists is recruited to the Mdm2 promoter, but not to the p21 promoter. Importantly, ERα is recruited to the Mdm2 promoter together with the acetyltransferase multiprotein complex TRRAP that has been shown to potentiate E2-dependent cell growth (76) and that is also required for Mdm2 transcriptional regulation by p53 (4). Complementary to our finding of ER regulation of Mdm2, recent studies have shown that expression of Mdm2 is correlated with the expression of ERα and is up regulated by E2 and DES (27, 41, 60). The shift in p53-dependent, ER-mediated Mdm2 regulation supports an increasing role of the ER in tumorigenesis.
In this study, we engineered an MCF-7/GR cell line to investigate GR-ER cross talk using the MMTV-LUC as a model for GR-mediated transcriptional activity. We demonstrate that estrogen agonists enhance proteasomal degradation of the GR, and as a result, estrogen agonists can impact on transcriptional regulation by the GR. Our findings suggest that in MCF-7 cells, estrogen agonists, in their role of promoting cell growth, increase p53 stability and shift its transcriptional activity from the cell cycle inhibitor p21 to Mdm2, a known potent E3 ubiquitin ligase. Thus, it is plausible that in the presence of estrogen agonists, a cascade of events leading to p53 stability can divert the Mdm2 E3 ubiquitin ligase activity towards destruction of the GR in MCF-7 cells. In contrast to ER agonists, ER antagonists play a permissive role in mediating GR activity. Thus, cross talk between GR and ER involves a converging of multiple signaling pathways, which, with the addition of the tumor suppressor p53 and the E3 ubiquitin ligase Mdm2, suggests a further layer of complexity in steroid receptor regulation of transcription.
Acknowledgments
We thank Alex Merrick, Julie Hall, Cary Weinberger, Pratibha Hebbar, John Couse, Tatsuya Sueyoshi, and Daniel Menendez (N.I.E.H.S.) and Christy Fryer (The Salk Institute for Biological Studies, La Jolla, Calif.) for critical review of the manuscript. We thank members of the Archer laboratory for helpful discussions during the course of this study. We are especially grateful to the following people for generously supplying antibodies used in this study: Robert Kingston (Massachusetts General Hospital, Boston, Mass.), Joe Torchia (University of Western Ontario, London, Canada), B. Gametchu (Medical College of Wisconsin, Milwaukee, Wis.), Carolyn Smith (Baylor College of Medicine, Houston, Texas), and Cam Patterson (University of North Carolina, Chapel Hill, North Carolina).
REFERENCES
- 1.Altucci, L., R. Addeo, L. Cicatiello, S. Dauvois, M. G. Parker, M. Truss, M. Beato, V. Sica, F. Bresciani, and A. Weisz. 1996. 17β-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cells. Oncogene 12:2315-2324. [PubMed] [Google Scholar]
- 2.Angus, W. G., M. Campaigne Larsen, and C. R. Jefcoate. 2000. TCDD elevates erbB2 expression and signaling in T47D cells by reversing serum potentiation of estrogen receptor activity, independent of estrogen levels and enhanced ER down-regulation. Mol. Cell. Endocrinol. 170:1-13. [DOI] [PubMed] [Google Scholar]
- 3.Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 81:1269-1304. [DOI] [PubMed] [Google Scholar]
- 4.Ard, P. G., C. Chatterjee, S. Kunjibettu, L. R. Adside, L. E. Gralinski, and S. B. McMahon. 2002. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol. Cell. Biol. 22:5650-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barak, Y., and M. Oren. 1992. Enhanced binding of a 95 kDa protein to p53 in cells undergoing p53-mediated growth arrest. EMBO J. 11:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee, R. N., G. C. Banks, K. W. Trotter, H. L. Lee, and T. K. Archer. 2001. Histone H1 phosphorylation by Cdk2 selectively modulates mouse mammary tumor virus transcription through chromatin remodeling. Mol. Cell. Biol. 21:5417-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd, S. D., K. Y. Tsai, and T. Jacks. 2000. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat. Cell Biol. 2:563-568. [DOI] [PubMed] [Google Scholar]
- 8.Cardozo, C. P., C. Michaud, M. C. Ost, A. E. Fliss, E. Yang, C. Patterson, S. J. Hall, and A. J. Caplan. 2003. C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Arch. Biochem. Biophys. 410:134-140. [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover, A., A. Orian, and A. L. Schwartz. 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22:442-451. [DOI] [PubMed] [Google Scholar]
- 10.Connell, P., C. A. Ballinger, J. Jiang, Y. Wu, L. J. Thompson, J. Hohfeld, and C. Patterson. 2001. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3:93-96. [DOI] [PubMed] [Google Scholar]
- 11.Cotroneo, M. S., J. Wang, I. A. Eltoum, and C. A. Lamartiniere. 2001. Sex steroid receptor regulation by genistein in the prepubertal rat uterus. Mol. Cell. Endocrinol. 173:135-145. [DOI] [PubMed] [Google Scholar]
- 12.Couse, J. F., and K. S. Korach. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20:358-417. [DOI] [PubMed] [Google Scholar]
- 13.Dace, A., L. Zhao, K. S. Park, T. Furuno, N. Takamura, M. Nakanishi, B. L. West, J. A. Hanover, and S. Cheng. 2000. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc. Natl. Acad. Sci. USA 97:8985-8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFranco, D. B. 2002. Navigating steroid hormone receptors through the nuclear compartment. Mol. Endocrinol. 16:1449-1455. [DOI] [PubMed] [Google Scholar]
- 15.Dennis, A. P., R. U. Haq, and Z. Nawaz. 2001. Importance of the regulation of nuclear receptor degradation. Front. Biosci. 6:D954-959. [DOI] [PubMed] [Google Scholar]
- 16.Deroo, B. J., C. Rentsch, S. Sampath, J. Young, D. B. DeFranco, and T. K. Archer. 2002. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol. Cell. Biol. 22:4113-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman, D. A., and A. J. Levine. 1998. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 18:7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz, W. A., J. Wang, I. E. Eltoum, and C. A. Lamartiniere. 2002. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Mol. Cell. Endocrinol. 186:89-99. [DOI] [PubMed] [Google Scholar]
- 19.Fryer, C. J., and T. K. Archer. 1998. Chromatin remodeling by the glucocorticoid receptor requires the BRG1 complex. Nature 393:88-91. [DOI] [PubMed] [Google Scholar]
- 20.Fryer, C. J., H. K. Kinyamu, I. Rogatsky, M. J. Garabedian, and T. K. Archer. 2000. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J. Biol. Chem. 275:17771-17777. [DOI] [PubMed] [Google Scholar]
- 21.Fryer, C. J., S. K. Nordeen, and T. K. Archer. 1998. Antiprogestins mediate differential effects on glucocorticoid receptor remodeling of chromatin structure. J. Biol. Chem. 273:1175-1183. [DOI] [PubMed] [Google Scholar]
- 22.Galigniana, M. D., P. R. Housley, D. B. DeFranco, and W. B. Pratt. 1999. Inhibition of glucocorticoid receptor nucleocytoplasmic shuttling by okadaic acid requires intact cytoskeleton. J. Biol. Chem. 274:16222-16227. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher, J. C., S. E. Fowler, J. R. Detter, and S. S. Sherman. 2001. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J. Clin. Endocrinol. Metab. 86:3618-3628. [DOI] [PubMed] [Google Scholar]
- 24.Ganguli, G., J. Back, S. Sengupta, and B. Wasylyk. 2002. The p53 tumour suppressor inhibits glucocorticoid-induced proliferation of erythroid progenitors. EMBO Rep. 3:569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer, R. K., Z. K. Yu, and C. G. Maki. 2000. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat. Cell Biol. 2:569-573. [DOI] [PubMed] [Google Scholar]
- 26.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 27.Hori, M., J. Shimazaki, S. Inagawa, and M. Itabashi. 2002. Overexpression of MDM2 oncoprotein correlates with possession of estrogen receptor alpha and lack of MDM2 mRNA splice variants in human breast cancer. Breast Cancer Res. Treat. 71:77-83. [DOI] [PubMed] [Google Scholar]
- 28.Hsiao, P. W., B. J. Deroo, and T. K. Archer. 2002. Chromatin remodeling and tissue-selective responses of nuclear hormone receptors. Biochem. Cell. Biol. 80:343-351. [DOI] [PubMed] [Google Scholar]
- 29.Htun, H., J. Barsony, I. Renyi, D. L. Gould, and G. L. Hager. 1996. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc. Natl. Acad. Sci. USA 93:4845-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Htun, H., L. T. Holth, D. Walker, J. R. Davie, and G. L. Hager. 1999. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol. Biol. Cell. 10:471-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinyamu, H. K., C. J. Fryer, K. B. Horwitz, and T. K. Archer. 2000. The mouse mammary tumor virus promoter adopts distinct chromatin structures in human breast cancer cells with and without glucocorticoid receptor. J. Biol. Chem. 275:20061-20068. [DOI] [PubMed] [Google Scholar]
- 32.Kraus, W. L., K. E. Weis, and B. S. Katzenellenbogen. 1995. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol. Cell Biol. 15:1847-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan, A. V., S. Swami, and D. Feldman. 2001. Estradiol inhibits glucocorticoid receptor expression and induces glucocorticoid resistance in MCF-7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 77:29-37. [DOI] [PubMed] [Google Scholar]
- 34.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 35.Kuiper, G. G., E. Enmark, M. Pelto-Huikko, S. Nilsson, and J. A. Gustafsson. 1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 93:5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, H. L., and T. K. Archer. 1994. Nucleosome-mediated disruption of transcription factor-chromatin initiation complexes at the mouse mammary tumor virus long terminal repeat in vivo. Mol. Cell. Biol. 14:32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, H. L., and T. K. Archer. 1998. Prolonged glucocorticoid exposure dephosphorylates histone H1 and inactivates the MMTV promoter. EMBO J. 17:1454-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legube, G., L. K. Linares, C. Lemercier, M. Scheffner, S. Khochbin, and D. Trouche. 2002. Tip60 is targeted to proteasome-mediated degradation by Mdm2 and accumulates after UV irradiation. EMBO J. 21:1704-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, H. K., L. Wang, Y. C. Hu, S. Altuwaijri, and C. Chang. 2002. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 21:4037-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, G., J. A. Schwartz, and S. C. Brooks. 2000. Estrogen receptor protects p53 from deactivation by human double minute-2. Cancer Res. 60:1810-1814. [PubMed] [Google Scholar]
- 42.Lobenhofer, E. K., L. Bennett, P. L. Cable, L. Li, P. R. Bushel, and C. A. Afshari. 2002. Regulation of DNA replication fork genes by 17beta-estradiol. Mol. Endocrinol. 16:1215-1229. [DOI] [PubMed] [Google Scholar]
- 43.Lonard, D. M., Z. Nawaz, C. L. Smith, and B. W. O'Malley. 2000. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol. Cell 5:939-948. [DOI] [PubMed] [Google Scholar]
- 44.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 46.Molinari, A. M., P. Bontempo, E. M. Schiavone, V. Tortora, M. A. Verdicchio, M. Napolitano, E. Nola, B. Moncharmont, N. Medici, V. Nigro, I. Armetta, C. Abbondanza, and G. A. Puca. 2000. Estradiol induces functional inactivation of p53 by intracellular redistribution. Cancer Res. 60:2594-2597. [PubMed] [Google Scholar]
- 47.Moll, U. M., G. Riou, and A. J. Levine. 1992. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc. Natl. Acad. Sci. USA 89:7262-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moudgil, V. K., S. Dinda, N. Khattree, S. Jhanwar, P. Alban, and C. Hurd. 2001. Hormonal regulation of tumor suppressor proteins in breast cancer cells. J. Steroid Biochem. Mol. Biol. 76:105-117. [DOI] [PubMed] [Google Scholar]
- 49.Muchardt, C., and M. Yaniv. 1999. The mammalian SWI/SNF complex and the control of cell growth. Semin. Cell Dev. Biol. 10:189-195. [DOI] [PubMed] [Google Scholar]
- 50.Muller, W. G., D. Walker, G. L. Hager, and J. G. McNally. 2001. Large-scale chromatin decondensation and recondensation regulated by transcription from a natural promoter. J. Cell Biol. 154:33-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nawaz, Z., D. M. Lonard, A. P. Dennis, C. L. Smith, and B. W. O'Malley. 1999. Proteasome-dependent degradation of the human estrogen receptor. Proc. Natl. Acad. Sci. USA 96:1858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nawaz, Z., D. M. Lonard, C. L. Smith, E. Lev-Lehman, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1999. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol. Cell. Biol. 19:1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikolaev, A. Y., M. Li, N. Puskas, J. Qin, and W. Gu. 2003. Parc: a cytoplasmic anchor for p53. Cell 112:29-40. [DOI] [PubMed] [Google Scholar]
- 54.Oakley, R. H., J. C. Webster, C. M. Jewell, M. Sar, and J. A. Cidlowski. 1999. Immunocytochemical analysis of the glucocorticoid receptor alpha isoform (GRalpha) using GRalpha-specific antibody. Steroids 64:742-751. [DOI] [PubMed] [Google Scholar]
- 55.Pestell, R. G., C. Albanese, A. T. Reutens, J. E. Segall, R. J. Lee, and A. Arnold. 1999. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr. Rev. 20:501-534. [DOI] [PubMed] [Google Scholar]
- 56.Qin, C., T. Nguyen, J. Stewart, I. Samudio, R. Burghardt, and S. Safe. 2002. Estrogen up-regulation of p53 gene expression in MCF-7 breast cancer cells is mediated by calmodulin kinase IV-dependent activation of a nuclear factor kappaB/CCAAT-binding transcription factor-1 complex. Mol. Endocrinol. 16:1793-1809. [DOI] [PubMed] [Google Scholar]
- 57.Rackoff, P. J., and C. J. Rosen. 1998. Pathogenesis and treatment of glucocorticoid-induced osteoporosis. Drugs Aging 12:477-484. [DOI] [PubMed] [Google Scholar]
- 58.Reichardt, H. M., F. Tronche, S. Berger, C. Kellendonk, and G. Schutz. 2000. New insights into glucocorticoid and mineralocorticoid signaling: lessons from gene targeting. Adv. Pharmacol. 47:1-21. [DOI] [PubMed] [Google Scholar]
- 59.Reyes, J. C., J. Barra, C. Muchardt, A. Camus, C. Babinet, and M. Yaniv. 1998. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha). EMBO J. 17:6979-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Risinger, J. I., L. A. Terry, and J. Boyd. 1994. Use of representational difference analysis for the identification of mdm2 oncogene amplification in diethylstilbestrol-induced murine uterine adenocarcinomas. Mol. Carcinog. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 61.Rogatsky, I., J. M. Trowbridge, and M. J. Garabedian. 1997. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol. Cell. Biol. 17:3181-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell, A., J. Hendley, and D. Germain. 1999. Inhibitory effect of p21 in MCF-7 cells is overcome by its coordinated stabilization with D-type cyclins. Oncogene 18:6454-6459. [DOI] [PubMed] [Google Scholar]
- 63.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 64.Sengupta, S., J. L. Vonesch, C. Waltzinger, H. Zheng, and B. Wasylyk. 2000. Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells. EMBO J. 19:6051-6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sengupta, S., and B. Wasylyk. 2001. Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes Dev. 15:2367-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shenoy, S. K., P. H. McDonald, T. A. Kohout, and R. J. Lefkowitz. 2001. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294:1307-1313. [DOI] [PubMed] [Google Scholar]
- 67.Shyr, C. R., Y. C. Hu, E. Kim, and C. Chang. 2002. Modulation of estrogen receptor-mediated transactivation by orphan receptor TR4 in MCF-7 cells. J. Biol. Chem. 277:14622-14628. [DOI] [PubMed] [Google Scholar]
- 68.Smith, C. L., H. Htun, R. G. Wolford, and G. L. Hager. 1997. Differential activity of progesterone and glucocorticoid receptors on mouse mammary tumor virus templates differing in chromatin structure. J. Biol. Chem. 272:14227-14235. [DOI] [PubMed] [Google Scholar]
- 69.Sutherland, R. L., O. W. Prall, C. K. Watts, and E. A. Musgrove. 1998. Estrogen and progestin regulation of cell cycle progression. J. Mammary Gland Biol. Neoplasia 3:63-72. [DOI] [PubMed] [Google Scholar]
- 70.Tao, W., and A. J. Levine. 1999. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc. Natl. Acad. Sci. USA 96:3077-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallace, A. D., and J. A. Cidlowski. 2001. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J. Biol. Chem. 276:42714-42721. [DOI] [PubMed] [Google Scholar]
- 72.Wang, X., W. Porter, V. Krishnan, T. R. Narasimhan, and S. Safe. 1993. Mechanism of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated decrease of the nuclear estrogen receptor in MCF-7 human breast cancer cells. Mol. Cell. Endocrinol. 96:159-166. [DOI] [PubMed] [Google Scholar]
- 73.Whitesell, L., and P. Cook. 1996. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol. Endocrinol. 10:705-712. [DOI] [PubMed] [Google Scholar]
- 74.Wormke, M., M. Stoner, B. Saville, K. Walker, M. Abdelrahim, R. Burghardt, and S. Safe. 2003. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell. Biol. 23:1843-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xirodimas, D. P., C. W. Stephen, and D. P. Lane. 2001. Cocompartmentalization of p53 and Mdm2 is a major determinant for Mdm2-mediated degradation of p53. Exp. Cell Res. 270:66-77. [DOI] [PubMed] [Google Scholar]
- 76.Yanagisawa, J., H. Kitagawa, M. Yanagida, O. Wada, S. Ogawa, M. Nakagomi, H. Oishi, Y. Yamamoto, H. Nagasawa, S. B. McMahon, M. D. Cole, L. Tora, N. Takahashi, and S. Kato. 2002. Nuclear receptor function requires a TFTC-type histone acetyl transferase complex. Mol. Cell 9:553-562. [DOI] [PubMed] [Google Scholar]
- 77.Yudt, M. R., and J. A. Cidlowski. 2001. Molecular identification and characterization of a and b forms of the glucocorticoid receptor. Mol. Endocrinol. 15:1093-1103. [DOI] [PubMed] [Google Scholar]
- 78.Zaika, A., N. Marchenko, and U. M. Moll. 1999. Cytoplasmically “sequestered” wild type p53 protein is resistant to Mdm2-mediated degradation. J. Biol. Chem. 274:27474-27480. [DOI] [PubMed] [Google Scholar]
- 79.Zhou, F., B. Bouillard, M. O. Pharaboz-Joly, and J. Andre. 1989. Nonclassical antiestrogenic actions of dexamethasone in variant MCF-7 human breast cancer cells in culture. Mol. Cell. Endocrinol. 66:189-197. [DOI] [PubMed] [Google Scholar]