Abstract
Heat shock response, which is characterized by the induction of a set of heat shock proteins, is essential for induced thermotolerance and is regulated by heat shock transcription factors (HSFs). Curiously, HSF1 is essential for heat shock response in mammals, whereas in avian HSF3, an avian-specific factor is required for the burst activation of heat shock genes. Amino acid sequences of chicken HSF1 are highly conserved with human HSF1, but those of HSF3 diverge significantly. Here, we demonstrated that chicken HSF1 lost the ability to activate heat shock genes through the amino-terminal domain containing an alanine-rich sequence and a DNA-binding domain. Surprisingly, chicken and human HSF1 but not HSF3 possess a novel function that protects against a single exposure to mild heat shock, which is not mediated through the activation of heat shock genes. Overexpression of HSF1 mutants that could not bind to DNA did not restore the susceptibility to cell death in HSF1-null cells, suggesting that the new protective role of HSF1 is mediated through regulation of unknown target genes other than heat shock genes. These results uncover a novel role of vertebrate HSF1, which has been masked underthe roles of heat shock proteins.
All living organisms respond to elevated temperatures by inducing a set of highly conserved proteins, heat shock proteins (Hsps). This response is called the heat shock response and is believed to be a universal and fundamental mechanism for cell protection against stresses such as heat shock. The heat shock response is regulated mainly at the level of transcription by heat shock transcription factors (HSFs) in eukaryotes, which bind to heat shock elements on upstream sequences of heat shock genes (45). It is well known that cells can survive an exposure to lethal temperatures when cells are preincubated at sublethal high temperatures. This phenomenon is now called induced thermotolerance. Numerous studies suggest that Hsp induction is crucial to the acquisition of the induced thermotolerance (19). Finally, heat shock response regulated by HSF is shown to be necessary for acquisition of the induced thermotolerance in the fruit fly (15), mouse embryo fibroblast cells (21), and chicken B lymphocyte DT40 cells (42).
HSFs do more than activate heat shock genes in response to elevated temperatures. It was shown that in Drosophila HSF is required under normal growth conditions for oogenesis and early development (15). Mice deficient in HSF1 show abnormal placental development, growth retardation, and female infertility (7, 46). Furthermore, mice deficient in HSF2 exhibit abnormalities in brain development and defects in spermatogenesis and oogenesis (16). In all of these cases, developmental functions of HSFs are not mediated through the induction of Hsps, suggesting that HSFs regulate unknown genes related to development. Recently, it was found that HSFs can regulate only a specific heat shock gene under normal growth conditions. In chicken DT40 cells, HSF1 and HSF3 regulate only Hsp90α expression in a cell cycle-dependent manner (25). This observation suggests the possibility that HSFs can regulate the expression of development-related genes. Another unique function of HSF1 in spermatogenesis is also proposed (28). Expression of an active HSF1 in spermatocytes blocks spermatogenesis, suggesting that HSF1 activated by elevated temperatures may induce cell death of spermatocytes. It would be necessary for injured germ cells to be actively eliminated by HSF1.
The HSF gene was originally isolated in Saccharomyces cerevisiae as a single gene that is essential for survival (40, 44). Subsequently, three mammalian HSF genes (HSF1, HSF2, and HSF4) (29, 33, 37, 38) and three chicken HSF genes (HSF1, HSF2, and HSF3) (27) were identified (for a review, see references 23 and 24). Identification of multiple members of the HSF gene family in vertebrates first left us with the question of which member mediates heat shock response. Biochemical analysis with mouse and human cells shows that HSF1 is the only factor that binds to DNA when cells are exposed to high temperatures (4, 36). Furthermore, analysis of HSF1-null mouse embryo fibroblast cells showed that HSF1 is essential and also sufficient for heat shock response (21). In contrast, in chicken cells we previously found that HSF3 as well as HSF1 binds to DNA when cells are exposed to heat shock (26), and HSF3 is necessary for burst activation of heat shock genes in chicken B lymphocyte DT40 cells (42). As HSF3 is ubiquitously expressed in most developing tissues at high levels, HSF3 may be a dominant factor for heat shock response in chickens (18).
To identify the differences in the molecular mechanisms of heat shock response between mammals and avians, we first examined the ability of chicken HSF1 (cHSF1) to activate heat shock genes in response to heat shock. We found that cHSF1 does not mediate heat shock response in either chicken and mouse cells by acquiring the amino-terminal domain containing an alanine-rich sequence. We expected that cHSF1 must have some functions other than the induced activation of heat shock genes, because the amino acid sequences of vertebrate HSF1 are highly conserved. We found that cHSF1 protects against a single exposure to moderately high temperatures independently of the expression of heat shock genes. Furthermore, we found that mammalian HSF1 also has this novel function and cHSF3 does not. Based on these results, we propose the functional diversification of vertebrate HSFs during evolution.
MATERIALS AND METHODS
Construction of chimeric HSFs.
Chicken and human HSF1 were swapped at the PflMI site (h20/c and c25/h) and the MroI site (h78/c). The cDNAs for chimeric HSF1 were subcloned into the expression vector pcDNA3.1/Zeo (Invitrogen). Plasmids encoding other chimeric HSFs were constructed by a PCR-mediated method (14). Briefly, in the case of h370/c, the primary PCR was performed with standard conditions to make a human HSF1 fragment (primers h1-ATG and h1-370) and a chicken HSF1 fragment (primers c1-333 and c1-TAA). The primers used were h1-ATG, 5′-GCG GAA TTC ACC ATG GAT CTG CCC GTG GGC-3′; h1-370, 5′-AAC GCT GAG GCA CTT TTC AGG GGT GGA GGT GGG CGG-3′; c1-333, 5′-CCG CCC ACC TCC ACC CCT GAA AAG TGC CTC AGC GTT-3′; and c1-TAA, 5′-GCG GAA TTC TTA GGA CAC GGT GGG GTC-3′ (EcoRI restriction sites are in italic, and underlining indicates a start codon and a termination codon).
After the first PCR, the inside primers were removed by selective filtration on SUPREC-02 (Takara, Japan). The secondary PCR was carried out in a total volume of 100 μl, with appropriate amounts of two PCR products produced in the primary PCR, containing 50 mM KCl, 10 mM Tris-HCl (pH 8.8), 1.5 mM MgCl2, 0.1% Triton X-100, 200 μM each deoxynucleoside triphosphate, and 2 U of Vent DNA polymerase (New England Biolabs) for 15 cycles of denaturation at 94°C (1 min), annealing at 25°C (2 min), and extension at 72°C (3 min) in an automated thermal cycler. Primers h1-ATG and c1-TAA and Vent DNA polymerase were added, and PCR was carried out for 25 cycles of 94°C denaturation (1 min), 55°C annealing (2 min), and 72°C extension (3 min). The final PCR product was digested with EcoRI at 37°C for 16 h, purified, and subcloned into the pcDNA3.1/Zeo vector.
Human HSF1 (hHSF1) or cHSF1 with deletions and point mutations were created with the QuickChange site-directed mutagenesis kit (Stratagene) as per the manufacturer's instructions. To confirm the mutations, sequencing reactions were performed with an ALFexpress AutoRead sequencing kit (Amersham Pharmacia), ALFexpress dATP labeling mix, and each synthetic oligonucleotide. Sequences were analyzed with an ALFexpress sequencer (Amersham Pharmacia).
Ectopic expression of HSFs, chimeras, and deletion mutants.
HSF3−/− 21 cells (42) were cotransfected with an expression vector for the Gpt gene (a gift from S. Takeda) and a pUHD15-1 expression vector for a tetracycline-controlled transactivator (tTA) (11) and incubated in the presence of 20 μg of mycophenolic acid per ml (Calbiochem). A clone, tA8, in which the transactivation potential was high, was selected by performing a reporter assay with a luciferase gene under the control of a tetracycline resistance gene operator (Tet operator) sequence (11). To generate cHSF1-overexpressing cells, plasmid pUHG/cHSF1, in which a Tet operator sequence is located upstream of cHSF1 cDNA (27), was cotransfected into clone tA8 with the pZeoSV2 vector (Invitrogen) and incubated in the presence of 300 μg of zeocine per ml. Western blot analysis was performed to select cHSF1-overexpressing clones.
The hHSF1 expression vector pZeo-hHSF1 was constructed by inserting the full-length hHSF1 cDNA (29) into the pZeoSV2 vector (Invitrogen). The full-length Drosophila melanogaster HSF cDNA (a gift from C. Wu) (8) was modified by PCR mutagenesis to introduce a hemagglutinin (HA) tag flanked by BamHI sites at the 3′ terminus and NheI site at the 5′ terminus and ligated into the pcDNA3.1/Zeo vector (Invitrogen) to create pcDNA/Zeo/DmHSF-HA. The pZeo-hHSF1 and pcDNA/Zeo/DmHSF-HA vectors were transfected into HSF3−/− 21 cells and incubated in the presence of 300 μg of zeocine per ml. Western blots were performed with antiserum for HSF1 (αHSF1β) (26) or antiserum for HA (MBL Co. Ltd., Nagoya, Japan).
To express HSF in double null cells, each expression vector was cotransfected with a vector containing the hisD gene (a gift from S. Takeda, Kyoto University). The human HSF1 expression vector pCMV/hHSF1 was constructed by inserting the full-length hHSF1 cDNA into the pCMV/Blue vector (Pharmingen). Protein extracted from the cells grown in the presence of 0.5 mg of l-histidinol per ml (Sigma) was subjected to Western blot analysis with antiserum for HSF1 or HSF3 (anti-HSF3γ) (26).
To express chicken and human HSF1 in ES cells, cHSF1 and hHSF1 cDNAs were inserted into the pcDNA3.1/hygro vector (Invitrogen) by blunt-end ligation to create expression vectors pcHSF1-Hyg and phHSF1-Hyg, respectively. Each expression vector was transfected into HSF1−/− ES cells by electroporation and incubated in the presence of 100 μg of hygromycin per ml.
Generation of HSF1-null ES cells and MEF cells.
A 1.6-kb EcoRI/HindIII fragment containing the PGK-neo gene from pKJ-1 (1) was ligated into SmaI-digested pBluescript II SK(+) vector (Stratagene) by blunt-end ligation. A 3.1-kb XmnI fragment from plasmid PGKtk was ligated into that plasmid at the KpnI site by blunt-end ligation (pKO vector). A DNA fragment containing the mouse HSF1 gene was isolated from a mouse 129 lambda genomic library (Stratagene) and partially sequenced. A 5.3-kb and a 1.1-kb fragment of HSF1 gene were amplified by PCR with DNA from ES cells and inserted into the pKO vector, and a targeting vector, mHSF1-neo, was generated. Primers used were mHSF1PCR1(5′-ACA GAT GTG CAG CTG ATG AAG GGG AAA CAG GAG TG-3′) and mHSF1PCR2 (5′-CTG CCT GCA CAC TCC TCT TAG TCT GGG AGG CTC TC-3′) for the 5.3-kb fragment and mHSF1g4 (5′-CGC TCT AGA GTT AAC GGA ATT ACT AGC AGT TGT GG-3′) and mHSF1g1 (5′-CGC TCT AGA GCT AGC CAT GTT GTT GTG C-3′) for the 1.1-kb fragment.
The linearized mHSF1-neo vector was transfected into TT2 ES cells (C57BL/6 × CBA F1 origin) (39, 47), and the targeted clones were enriched with 300 μg of G418 per ml (Nacalai Tesque) and 1.5 μM ganciclovir (InvivoGen). The drug-resistant clones were screened by PCR with the primers mHSF1g3 (5′-CGC TCT AGA GTT AAC ACG GCC CCA GTA TTT AAC AG-3′) and NeoPCR1 (5′-CGC ATG CTC CAG ACT GCC-3′) (see Fig. 1D) and were confirmed by Southern blot analysis with a 3′ internal probe and neomycin resistance gene (neo) probe (see Fig. 1D and E).
FIG. 1.
Chicken HSF1 has little potential to activate heat shock genes in response to heat shock. (A) The phylogenetic tree generated in Clustal W (43) for members of the HSF family. The molecular tree was constructed by the neighbor-joining method. Gaps were excluded from all phylogenetic analyses. The numerals show bootstrap values (1,000 bootstrap replicates were performed). The unrooted tree was drawn with the program TreeView (31). The bar represents 0.1 substitutions per site. The amino acid sequences used in the tree construction are human HSF1 (hHSF1, SP accession no. Q00613), mouse HSF1 (mHSF1, SP accession no. P38532), chicken HSF1 (cHSF1, SP accession no. P38529), zebrafish HSF1 (Zebra HSF1, DDBJ accession no. AB062117), and Xenopus HSF1 (Xenopus HSF1, SP accession no. P41154), human HSF2 (hHSF2, SP accession no. Q03933), mouse HSF2 (mHSF2, SP accession no. P38533), and chicken HSF2 (cHSF2, SP accession no. P38530), chicken HSF3 (cHSF3, SP accession no. P38531), human HSF4b (hHSF4, SP accession no. Q9ULV5) and mouse HSF4b (mHSF4, SP accession no. Q9R0L1), and HSF from Saccharomyces cerevisiae (ScHSF, SP accession no. P10961), D. melanogaster (DmHSF, SP accession no. P22813), and Caenorhabditis elegans (CeHSF, PIR accession no. T27125). (B) HSF3-null cells were transfected with an expression vector for cHSF1, hHSF1, D. melanogaster HSF, or cHSF3. Clones grown in the presence of zeocine were selected for Western blot analysis with HSF1-specific antiserum αHSF1β and antiserum for HA or HSF3-specific antiserum αHSF3γ (26). Representative clones are shown. Arrows in the upper and lower columns indicate endogenous cHSF1 and cHSF3, respectively. An asterisk shows an unmodified form of HSF3 (42). Arrowheads indicate ectopically expressed HSFs. (C) Heat shock response in HSF3-null cells expressing the indicated HSFs. RNAs were extracted from cells untreated or treated with heat shock at 45°C for 60 min, and Northern blot analysis was performed with cDNA for Hsp70 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (D) Schematic representation of wild-type and mutant HSF1 loci together with the targeting vector, mHSF1-neo. Exons are represented by solid boxes, and the DNA-binding domain is encoded by exons 2 and 3. The 3′ probe for Southern blot analysis (solid bar) and primer sites (arrowheads) are indicated. Restriction enzyme sites are: Bg, BglII; Hp, HpaI; S, SalI. (E) Southern blot analysis of DNA prepared from wild-type, HSF1+/−, and HSF1−/− ES cells. DNA was digested with BglII and hybridized with the 32P-labeled 3′ probe or neo probe. (F) HSF1−/− ES cells were transfected with expression vector pcHSF1-Hyg or phHSF1-Hyg, and clones which expressed a high level of cHSF1 or hHSF1 were selected. HSF1 protein levels in each representative clone were shown by Western blot analysis with αHSF1β antiserum. The lower column represents a longer exposure. (G) Examination of heat shock response by Northern blot analysis. HSF1−/− ES cells expressing cHSF1 or hHSF1 as well as wild-type and HSF1−/− ES cells were heat shocked at 43°C for 30 min. RNAs were extracted from these cells, and Northern blot analysis was performed with 32P-labeled cDNAs for mouse Hsp70-1 and human β-actin.
Founder mice generated by injection of HSF1+/− ES clones into eight-cell embryos of ICR mice were mated with ICR mice, and their germ line transmission was confirmed. The HSF1+/− female mice were crossed with the HSF1+/− male mice to generate mouse embryonic fibroblasts. To generate HSF1−/− ES clones, HSF1+/− ES cells were incubated in the presence of 2 mg of G418 per ml and all of the drug-resistant clones were HSF1−/− ES clones. To examine heat shock response, ES cells were cultured on gelatin-coated dishes without feeder cells.
Northern blot analysis.
Total RNA was extracted from cells with Trizol reagent (Invitrogen) according to the manufacturer's instructions. GeneScreen Plus membranes (NEN) containing 10 μg of total RNA were prepared and hybridized in hybridization solution containing 50% formamide, 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% sodium dodecyl sulfate, 3× Denhardt's reagent, and 400 μg of salmon sperm DNA per ml plus 32P-labeled cDNA probes at 42°C overnight. The membranes were washed in 0.1× SSC-0.1% sodium dodecyl sulfate at 65°C. The cDNA probes used were described previously (25, 42) except for Hsp25. We isolated cDNA for chicken Hsp25 (Katoh and Nakai, unpublished observation), which belongs to an Hsp30 subfamily among the α-crystallin/small heat shock protein superfamily (17, 30). To perform Northern blot analysis of ES cells, mouse Hsp70-1 cDNA (28) and cDNA for human β-actin were used as probes.
Gel mobility shift assay, subcellular fractionation, and gel filtration analysis.
Gel mobility shift assay, subcellular fractionation, and gel filtration analysis were performed essentially as described previously (26). To detect DNA-binding activity of hHSF1, the activity of hHSF2 was wiped out by adding HSF2 antiserum to the binding reaction (25).
Cell cycle analysis.
The cell cycle was analyzed essentially as described previously (25). The cells were labeled with 20 μM bromodeoxyuridine (Amersham Pharmacia) for 10 min, washed with phosphate-buffered saline, and fixed in 70% ethanol at 4°C for 30 min. The cells were then soaked in 2 N HCl containing 0.5% Triton X-100 at room temperature for 30 min. After washing, the cells were incubated with mouse antibromodeoxyuridine antibody (1:100 dilution; PharMingen) in phosphate-buffered saline-1% bovine serum albumin at room temperature for 30 min. After washing with phosphate-buffered saline-1% bovine serum albumin, the cells were then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G antibody (1:200 dilution, Cappel) in phosphate-buffered saline-1% bovine serum albumin at room temperature for 30 min. After washing, the cells were suspended in phosphate-buffered saline containing 25 μg of propidium iodide per ml and analyzed with a flow cytometer Epics XL (Coulter). Fluorescence data were displayed as dot plots. To determine the proportion of sub-G1 cells, the fixed cells were simply stained with propidium iodide and analyzed with the flow cytometer.
Cell culture and soft agar colony formation assay.
DT40 cells were cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10−5 M β-mercaptoethanol, 10% fetal bovine serum, and 1% chicken serum at 37°C with 5% CO2. For the colony formation assay, the cells were treated with a sublethal 43°C exposure for 30 min, allowed to recover at 37°C for 2 h, and then treated with a lethal 46°C exposure for 20, 40, or 60 min. These cells were serially diluted and plated in complete medium containing 1.5% methylcellulose (1,500 cps; Aldrich, Milwaukee, Wis.). Colonies were counted at 14 days after plating. Percent survival was determined relative to the numbers of colonies from untreated cells.
Generation of adenovirus and infection.
A 1.8-kb cHSF1 cDNA fragment (27) was ligated into Adeno-X viral DNA per the manufacturer's instructions (Clontech). Infectious adenovirus was generated by transfecting HEK 293 cells, and virus titers were determined as PFU. Mouse embryonic fibroblast cells were incubated at 37°C in medium containing adenovirus (106 PFU/ml) for 24 h and then maintained at higher temperature for various periods. The number of adherent cells was counted.
RESULTS
cHSF1 has little potential to activate heat shock genes in response to heat shock.
We previously showed that a lack of cHSF3 severely reduces heat shock response in chicken B lymphocyte DT40 cells (42). This observation left us with a question of whether there is any functional difference between chicken and human HSF1. Before resolving this question, we first tried to isolate an HSF3 gene orthologue in human cells, but we failed to do so. We detected no DNA binding activity other than that of HSF1, HSF2, and HSF4 in human and mouse tissues (data not shown). Furthermore, we could not identify HSF3-related sequences from genome and expressed sequence tag databases. We reached the conclusion that HSF3 is an avian-specific factor. We created a phylogenetic tree of HSFs based on amino acid sequences from various species (Fig. 1A). The tree shows that cHSF1 belongs to the HSF1 family and chicken HSF3 (cHSF3) is far away. The predicted amino acid sequence of cHSF1 is highly identical (79%) to that of human HSF1 (hHSF1) (27).
Next, we examined heat shock response in DT40 cells deficient in cHSF1 generated previously (25). Irrespective of the high sequence identity between cHSF1 and hHSF1, Hsp70, Hsp40, and Hsp25 mRNAs were highly induced in both wild-type cells and cells deficient in cHSF1 when the cells were heat shocked at 45°C (data not shown). The expression of other major heat shock genes (Hsc70, Hsp90α, Hsp90β, and Hsp110), which are constitutively expressed, was also similar in both cell types (data not shown).
Because the molecular ratio of HSF1 and HSF3 is 1.0:3.0 in DT40 cells (19), the slight effect of cHSF1 loss on heat shock response could be due to a low level of cHSF1 and the marked reduction in heat shock gene activation in HSF3-null cells could be due to a high level of cHSF3. To rule out this possibility, we overexpressed cHSF1 in HSF3-null cells. We found that the induced level of Hsp70 mRNA in cHSF1-overexpressed HSF3-null cells was similar to that in HSF3-null cells (Fig. 1B and C). We concluded that cHSF1 have little potential to activate heat shock genes when the cells are heat shocked in DT40 cells. Next, we overexpressed HSFs from various species in HSF3-null cells (Fig. 1B). We found that the induction of Hsp70 mRNA was fully restored in HSF3-null cells ectopically expressed with hHSF1 and D. melanogaster HSF as well as cHSF3 (Fig. 1C). These results suggest that HSF1 from other species than chicken activates heat shock genes in DT40 cells.
We next generated mouse embryonic stem cells (ES cells) deficient in mouse HSF1 (Fig. 1D and E) and isolated cells stably overexpressing cHSF1 or hHSF1 (Fig. 1F). Hsp70 mRNA was markedly induced in wild-type ES cells when the cells were incubated at 43°C for 30 min and not induced in HSF1-null ES cells at all (Fig. 1G). The ectopic expression of hHSF1 restored the induction of Hsp70 mRNA, whereas the ectopic expression of cHSF1 did not restore the induction. These results demonstrate that cHSF1 is unique among HSFs from various species in terms of a slight ability to activate heat shock genes when the cells are heat shocked.
N-terminal region of cHSF1 inhibits burst activation of heat shock genes.
To identify the region that causes the functional difference, we generated expression vectors for fusion proteins of chicken and human HSF1. Each expression vector was transfected, and cell lines expressing substantial levels of the HSF1 chimeras were selected for analysis of the heat shock response. We found that the substitution of a short amino-terminal sequence of cHSF1 (amino acids 1 to 25) with hHSF1 (amino acids 1 to 20) was sufficient for cHSF1 to restore heat shock response (h20/c in Fig. 2A, B). However, the short amino-terminal sequence of cHSF1 (amino acids 1 to 25) was not sufficient to suppress the ability of hHSF1 to induce the heat shock response (c25/h in Fig. 2A). We identified the amino-terminal region containing the DNA-binding domain of cHSF1 (amino acid 1 to 126) as sufficient to completely suppress the ability of hHSF1 to induce the heat shock response (c126/h in Fig. 2A). These results suggest that the amino-terminal region containing the alanine-rich sequence (Fig. 2C) and DNA binding domain in cHSF1 diverged from those in hHSF1 during evolution. These changes in amino acid sequences caused the distinct functional difference between cHSF1 and hHSF1.
FIG. 2.
Amino-terminal sequence is responsible for inability of cHSF1 to induce heat shock gene activation. (A) Schematic representation of chimeras between chicken and human HSF1 and induction of Hsp70 expression. The expression vector for each chimeric HSF1 was stably transfected into HSF3-null DT40 cells. Total RNA was extracted from cells treated with and without heat shock at 45°C for 60 min, and Hsp70 and GAPDH expression was examined by Northern blot analysis. Relative positions of functional domains are indicated: DBD, DNA binding domain; HR, hydrophobic heptad repeat; RD, regulatory domain; AD, activation domain. The h370/c chimera represents a fusion protein of human HSF1 (amino acids 1 to 370) and chicken HSF1 (amino acids 333 to 491). (B) Extracts were prepared from cell lines expressing each chimeric HSF1. Western blot analysis was performed with the HSF1 antiserum αHSF1β. (C) Alignment of chicken and human HSF1s. There are unique sequences rich in alanine residues in chicken HSF1 (underlined). The position of the h20/c substitution is indicated. DBD, DNA binding domain.
The activation of HSF1 is associated with the acquisition of DNA binding activity, nuclear translocation, and hyperphosphorylation. We examined these properties in chimeric HSFs h20/c and c126/h. hHSF1 activated heat shock genes, whereas c126/h could activate heat shock genes only a little (Fig. 3A). However, like hHSF1, c126/h bound DNA and was phosphorylated and translocated to the nucleus under heat shock conditions (Fig. 3B, C, and D). Likewise, the DNA binding activity, subcellular localization, and status of phosphorylation of h20/c were the same as those of cHSF1.
FIG. 3.
Substitution of amino-terminal sequence of cHSF1 with that of hHSF1 does not alter the DNA binding activity, heat-induced phosphorylation, and nuclear translocation of cHSF1. (A) Wild-type DT40 cells, HSF3−/− cells, and HSF3−/− cells having cHSF1, hHSF1, and chimeric h20/c or c126/h were heat shocked at 45°C for 60 min. Northern blot analysis was performed. (B) The cells shown in panel A were heat shocked at 45°C for 60 min, and whole-cell extracts were prepared. The gel shift assay was performed with the 32P-labeled heat shock element probe. (C) Western blot analysis of the whole-cell extracts isolated in panel B was performed with HSF1 antibody. (D) Cells were treated as described for panel B. Cytoplasmic (c) and nuclear (n) extracts were prepared, and Western blot analysis of the extracts was performed with HSF1 antibody.
cHSF1 is necessary for cell survival at moderately higher temperatures independently of Hsp induction.
Although cHSF1 lost the ability to activate heat shock genes under heat shock conditions, its amino acid sequences are highly conserved with those of human HSF1 (Fig. 1A). The profile of cHSF1 expression in various tissues is similar to that of mouse HSF1 (18). More importantly, cHSF1, which stays as a monomer under normal growth conditions, is converted to a trimer when cells are exposed to high temperatures, like hHSF1 (26). These observations suggest that cHSF1 has some roles under stress conditions. Therefore, we carefully analyzed the status of the cell cycle of HSF1-null cells and HSF3-null cells at high temperatures.
When cells were incubated at 43°C for 24 h, a substantial proportion of HSF1-null cells were observed in the sub-G1 fraction, whereas the proportion of the sub-G1 fraction was small in HSF3-null cells that lack heat shock response (Fig. 4A, also see Fig. 7). Double-null cells could not survive at 43°C at all, mainly because of the reduced level of Hsp90α (25).
FIG. 4.
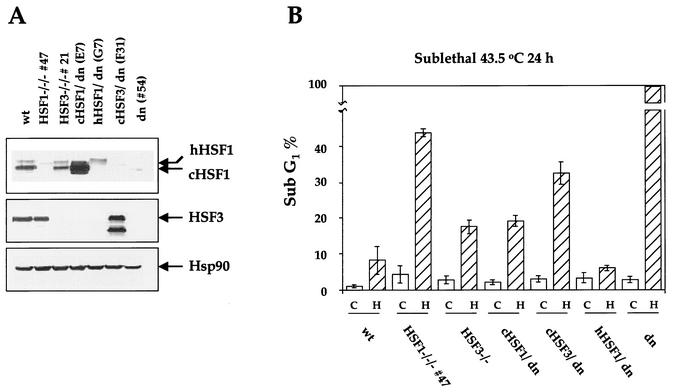
cHSF1-deficient cells are highly sensitive to high temperatures. (A) Wild-type DT40 (wt), double-null (54 cells), HSF1-null (59 cells), and HSF3-null (21 cells) cells were incubated at 43°C for 24 h. Representative cell cycle distributions of the indicated cell cultures are shown. Cells were pulse labeled for 10 min and stained with fluorescein isothiocyanate-conjugated antibromodeoxyuridine to detect bromodeoxyuridine incorporation (vertical axis) and propidium iodide to detect total DNA (horizontal axis). (B) Western blot analysis of HSF1 protein in cells of each indicated genotype as well as HSF1-null 59 cells reexpressing cHSF1 (D2). (C) The cells shown in panel B were incubated at 43.5°C for 24 h, and representative cell cycle distributions are shown. (D) Wild-type (wt) and HSF1-null 59 (HSF1−/−/−) cells were incubated for 24 h at the indicated temperatures. Proportions of sub-G1 fractions are shown. Means and standard deviations of three independent experiments are shown. (E) Cells were incubated at 43.5°C for the indicated periods, and proportions of sub-G1 fractions are shown as in panel D.
FIG. 7.
Mammalian HSF1 as well as chicken HSF1 is a survival factor in DT40 cells. (A) Double-null cells were stably transfected with an expression vector for cHSF1, hHSF1, or cHSF3. Protein levels of HSF1, HSF3, and Hsp90 were determined by Western blot analysis in wild-type cells (wt), HSF1-null cells (line 47), HSF3-null cells (line 21), double null cells (line 54), and null cells reexpressing cHSF1(E7), hHSF1 (G7), or cHSF3 (F31). (B) Proportions of sub-G1 fractions in the cells described in A are shown before (open bar) and after (hatched bar) incubation at 43.5°C for 24 h. All of the double-null cells died after the treatment. Note that the reexpression of hHSF1 completely restored cell survival after heat treatment.
Furthermore, we examined the dosage effect of HSF1. We found that the proportions of the sub-G1 fraction were 6, 10, 19, and 52% in HSF1+/+/+, HSF1+/+/−, HSF1+/−/−, and HSF1−/−/− cells at 43.5°C, respectively (Fig. 4B, C). Restoration of cHSF1 into HSF1-null cells reduced the proportion of sub-G1 fraction (21%). The nuclei of these cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI), and fragmented nuclei were evident in HSF1-null cells (data not shown). HSF1-null cells were more susceptible to death even at a mild temperature, such as 41°C, than wild-type cells (Fig. 4D). When HSF1-null cells were incubated at 43.5°C, cell death began to be observed at 12 h after the incubation (Fig. 4E). These results demonstrated that cHSF1 is necessary for survival of DT40 cells after a mild heat shock.
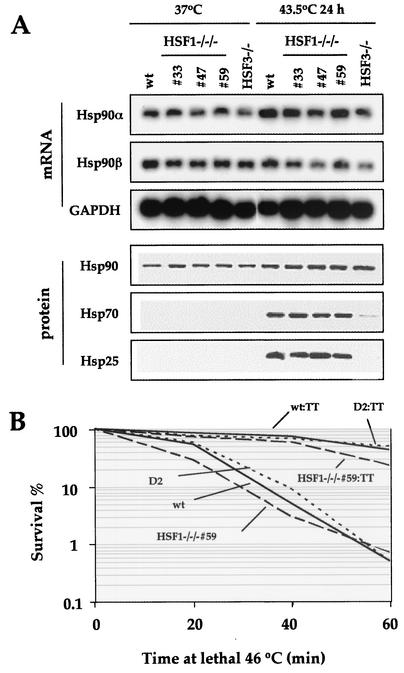
We previously showed that cHSF1 or cHSF3 is required for Hsp90α expression, and this expression is crucial for progression in the cell cycle (25). Therefore, we examined Hsp90α and Hsp90β expressions before and after heat shock. The levels of constitutive and induced expression of Hsp90s in HSF1-null cells were similar to those in wild-type cells (Fig. 5A). This result demonstrates that HSF3 is sufficient for the regulation of Hsp90 expression, and the high sensitivity of HSF1-null cells to heat is not related to Hsp90 expression.
FIG. 5.
Expression of Hsp90 and induced thermotolerance in HSF1-null cells. (A) Expression of heat shock proteins, including Hsp90, in three independently generated cHSF1-null clones (33, 47, and 59). Wild-type, HSF1-null, and HSF3-null cells were incubated at 43.5°C for 24 h. Accumulations of Hsp90α and Hsp90β mRNAs were examined by Northern blot analysis. Accumulations of Hsp90, Hsp70, and Hsp25 proteins were determined by Western blot analysis. (B) cHSF1-null cells acquire induced thermotolerance. cHSF1-null cells (HSF1−/−/− 59) and cHSF1-restored cells (D2) as well as wild-type cells (wt) grown at 37°C were incubated at a lethal 46°C temperature for the indicated periods. The numbers of surviving cells were counted by a colony formation assay, and percent survival is shown. Some cells were treated at a sublethal 43°C for 30 min and then allowed to recover for 2 h before lethal heat shock (wt:TT, HSF1−/−/− 59:TT, and D2:TT). Means of three independent experiments are shown.
We next examined the cell survival when cells were exposed to extremely high temperatures, such as 46°C, for a short period. The numbers of colonies were counted at 14 days after heat shock. We found that the survival of HSF1-null cells was similar to that of wild-type cells and cHSF1-reexpressing HSF1-null cells (Fig. 5B). As was expected from the fact that the heat shock response is intact in HSF1-null cells, HSF1-null cells as well as wild-type cells could survive a lethal 46°C exposure when these cells were preconditioned with a sublethal 43°C exposure for 30 min and allowed to recover at 37°C for 2 h (Fig. 5B). We can distinguish the toxic effect of the mild heat shock to cells from that of the extreme heat shock. Induction of Hsps, which is mediated by cHSF3, is necessary for resistance to extremely high temperatures in chicken cells, whereas cHSF1 is required for resistance to moderately high temperatures.
To test whether HSF1-null cells were more sensitive to other stresses than wild-type cells, cells were exposed to ionizing radiation and UV. Twenty-four hours after the treatments, the numbers of sub-G1 cells were examined (Fig. 6). The ratios were about twofold higher in HSF1-null cells than in wild-type cells and were decreased by restoring cHSF1 expression (D2 in Fig. 6). These results suggest that HSF1 suppresses cell death induced by various stresses.
FIG. 6.
HSF1-null cells are sensitive to radiation and UV. (A) Wild-type (wt) cells, HSF1-null 59 cells, and cHSF1-restored 59 cells (D2) were exposed to 600 rads of irradiation and then incubated for 24 h. Proportions of sub-G1 fractions in control and irradiated cells are shown. (B) Cells were exposed to UV at 10 J/m2 and then incubated for 24 h. Means and standard deviations of three independent experiments are shown.
Mammalian HSF1 has a novel function.
Does cHSF3 have the same function as cHSF1? To answer this question, the proportion of sub-G1 cells was examined in HSF3-null cells. It was found that the sub-G1 proportion in HSF3-null cells was ≈18% after incubation at 43.5°C for 24 h (Fig. 7A and B). Considering that the proportion in wild-type cells was ≈8% and that in HSF1-null cells was ≈44%, we concluded that only a few cHSF3-null cells died. A slight increase in dead cells due to a lack of cHSF3 may be explained by the loss of Hsp accumulation. On the contrary, we reexpressed cHSF3 in double-null cells. All of the double-null cells died after incubation at 43.5°C for 24 h (Fig. 7B) (25). Reexpression of cHSF3 partially suppressed cell death, probably due to the increase in Hsp90 expression (Fig. 7A, B) (25). However, many of these cells (≈33%) still died. Furthermore, the proportion of the sub-G1 fraction in double null cells reexpressing cHSF1 (≈19%) was similar to that in HSF3-null cells (≈18%) when heat shocked. These results indicate that cHSF3 cannot complement the cHSF1 defect and suggest that the heat shock response does not play a significant role in cell survival at moderately high temperatures.
Next, we wanted to answer the question whether human HSF1 has the same role as chicken HSF1. We clearly showed that reexpression of hHSF1 completely rescued cell death (≈6%) induced by a moderate heat shock (Fig. 7A, B). The complete complementation of the cHSF1 and cHSF3 defect by hHSF1 suggests that hHSF1 has two distinct roles, those of cHSF1 and cHSF3. To solidify this hypothesis, we generated mouse embryonic fibroblast cells lacking HSF1. Levels of the constitutive expression of major heat shock genes in HSF1-null mouse embryonic fibroblast cells were similar to those in wild-type cells, although heat shock response was lacking in HSF1-null cells (Fig. 8A). When mouse embryonic fibroblast cells were incubated at moderately high temperatures, we observed that cells detached from culture dishes and then died (data not shown). We calculated the numbers of attached cells to estimate the viability of cells because all of the detached cells were going to die. Nine hours after being incubated at 41.5°C or 42°C, the number of attached HSF1-null cells was about half of that of wild-type cells (Fig. 8B, C, and D). This result indicates that mouse HSF1 is required for resistance to moderately high temperatures, like cHSF1.
FIG. 8.
Hypersensitivity of HSF1-null mouse embryonic fibroblast cells to high temperatures is restored by ectopic expression of chicken HSF1. (A) Northern blot analysis of heat shock genes in HSF1+/+, HSF1+/−, and HSF1−/− mouse embryonic fibroblast cells before and after heat shock at 43°C for 30 min. (B) Sixteen hours after the HSF1+/+ (open bars) and HSF1−/− (gray bars) mouse embryonic fibroblast cells were plated on culture dishes, the dishes were maintained in an incubator at each indicated temperature. Numbers of attached cells were counted at 9 h after the incubation. Means and standard deviations of three independent experiments are shown. (C) HSF1+/+ (diamonds) and HSF1−/− (squares) mouse embryonic fibroblast cells were incubated at 41.5°C for the indicated periods, and numbers of attached cells were counted. (D) Morphology of MEF cells incubated at 41.5°C under a Axiovert 200 microscope (Zeiss). (E) Mouse embryonic fibroblast cells were incubated in the presence of adenovirus expressing LacZ (Ad-LacZ) or chicken HSF1 (Ad-cHSF1). Western blot analysis of HSF1 was performed with these extracts. The level of endogenous HSF1 in HSF1+/+ mouse embryonic fibroblast cells was too low to be detected. (F) Twenty-four hours after the mouse embryonic fibroblast cells were plated, cells were maintained in the presence or absence of adenovirus (106/ml) for 24 h. Then the cells were incubated at 42°C for the indicated periods. Protein levels of Hsp90, Hsp70, Hsp27, and actin were examined by Western blot analysis with extracts from these cells. (G) Numbers of attached cells were counted at the indicated times after incubation at 42°C. Means and standard deviations of three independent experiments are shown.
The protective effect of mouse HSF1 in mouse embryonic fibroblast cells would be due to the upregulation of heat shock genes. To exclude this possibility, we expressed cHSF1 into HSF1-null mouse embryonic fibroblast cells. When HSF1-null mouse embryonic fibroblast cells were infected with Ad-cHSF1, cHSF1 was expressed, but heat shock response was not restored (Fig. 8E, F). Nevertheless, the numbers of attached HSF1-null cells at 41.5°C were restored by being infected with Ad-cHSF1 (Fig. 8G). These results clearly demonstrate that mammalian HSF1 as well as chicken HSF1 has a new protective function.
New protective role of HSF1 is mediated through regulation of unknown target genes.
To examine whether the new protective role of HSF1 is mediated through regulation of target genes, we generated double-null DT40 cells stably expressing wild-type hHSF1 or hHSF1 mutants in which the DNA-contacting arginine at position 71 (20) was substituted with glycine (hHSF1R71G) or alanine (hHSF1R71A) (Fig. 9A, B). hHSF1R71G and hHSF1R71A were converted to trimers but failed to bind to DNA (Fig. 9C, D). As a result, ectopic expression of hHSF1R71G or hHSF1R71A in double-null cells restored neither the constitutive expression of Hsp90α nor heat shock-induced activation of heat shock genes (Fig. 9E). Therefore, double-null cells expressing hHSF1R71G or hHSF1R71A could not survive at 43°C (Fig. 9F). In contrast, the level of constitutive Hsp90α expression and the heat-induced levels of heat shock proteins in HSF1-null cells were the same as in wild-type cells (Fig. 5A). We generated HSF1-null DT40 cells expressing hHSF1 or hHSF1R71G (Fig. 9G) and found that the proportion of the sub-G1 fraction of HSF1-null cells expressing hHSF1R71 at 43.5°C was similar to that of HSF1-null cells (Fig. 9H). These results suggest that the new protective role of HSF1 is also mediated through regulation of target genes other than heat shock genes.
FIG. 9.
DNA binding activity is necessary for human HSF1 to restore resistance to high temperatures. (A) Schematic representation of mutant human HSF1. The arginine at amino acid position 71 in human HSF1 is shown to contact DNA directly in S. cerevisiae HSF (20) and was mutated to alanine (hHSF1R71A) or glycine (hHSF1R71G). The conserved domains in other vertebrate HSFs are indicated: DBD, DNA binding domain; HR-A/B, amino-terminal hydrophobic repeat; HR-C, carboxyl-terminal hydrophobic repeat. (B) HSF1 protein levels were determined by Western blotting with extracts from wild-type DT40, double null cells (dn), and double null cells expressing hHSF1, hHSF1R71G, or hHSF1R71A. (C) Gel filtration analysis of extracts isolated from double null cells expressing hHSF1 (G7) and hHSF1R71G (R71G) before and after heat shock at 45°C for 30 min. The predicted elution positions of monomeric and trimeric forms of HSF1 are indicated at the bottom (26). (D) The cell extracts prepared in panel C were subjected to the gel mobility shift assay with the 32P-labeled ideal HSE oligonucleotide. Binding reaction mixes were incubated in the presence of anti-HSF2 antiserum to wipe out HSF2 binding activity. (E) Northern blot analysis of cells before and after heat shock at 45°C for 60 min. (F) Proportions of sub-G1 fractions in cells are shown before (open bar) and after (hatched bar) incubation at 43.5°C for 24 h. (G) HSF1 protein levels were determined by Western blotting with extracts from wild-type DT40 cells, HSF1-null cells (line 59), and HSF1-null cells expressing hHSF1 (hHSF1/line 59) and hHSF1R71G (R71G/line 59). (H) Proportions of sub-G1 fractions in cells are shown before (open bar) and after (hatched bar) incubation at 43.5°C for 24 h. Means and standard deviations of three independent experiments are shown.
DISCUSSION
Thermotolerance regulated by HSFs.
It is unclear how cells are induced to die when exposed to high temperatures, as the physiological effects of heat are complex. Cells in different states of metabolism and in different stages of differentiation may be induced to die by different mechanisms. There must be many targets of extreme high temperatures that induce cell death. Therefore, heat shock proteins are not the only proteins that protect cells from death. Nevertheless, heat shock proteins are recognized as major players in the protection of cells from stresses such as heat shock. As the expression of a set of heat shock proteins is regulated by HSFs, HSFs should be involved in protection of cells against heat shock.
Two HSF-mediated mechanisms by which cells protect themselves from death due to exposure to high temperatures are known. First, it is well known that cells pretreated with a sublethal heat shock can survive lethal heat shock. This phenomenon is classically called induced thermotolerance and is regulated by HSFs (HSF1 in mammals and HSF3 in avians) through the activation of heat shock genes (21, 42). Heat shock proteins protect against denaturation and aggregate formation of cellular proteins under heat shock conditions and support their renaturation when the cells recover to grow at normal temperatures (10). At the same time, heat shock proteins inhibit several molecules, such as Apaf-1 and cytochrome c, which are involved in mitochondrion-mediated death pathways (5, 6, 32, 35).
Second, we suggested in a previous report that HSF can regulate the expression of some set of specific heat shock genes in normally growing cells. Cells may be more resistant to a single exposure to thermal stress when the constitutive expression of some heat shock proteins is elevated by HSF. In the case of chicken DT40 cells, HSF1 and HSF3 redundantly upregulate only Hsp90α expression in the absence of stress (25). Either HSF1 or HSF3 is sufficient to activate the Hsp90α gene in normal growth conditions. Similarly, Hsp90α expression is specifically decreased in S. cerevisiae when an activation domain of HSF is mutated (22). A reduction in Hsp90α causes instability of Cdc2 at high temperatures that promotes cell cycle transition from the G2 phase to the mitosis phase (25). Therefore, when cells deficient in both HSF1 and HSF3 are exposed to moderate temperatures, the cell cycle transition is blocked at the G2 phase, and these cells then die. This is a particular case where the target molecule of heat stress is clear. Probably, heat shock genes regulated by HSFs under normal growth conditions may be different in various cell types (Izu and Nakai, unpublished data).
In this report, we demonstrate another novel mechanism of cell protection from thermal stress regulated by HSF1. In chicken cells, cHSF1 has little function in the induction of heat shock genes but has a marked thermoprotective effect. This effect is mediated neither through the induction of heat shock genes nor through the regulation of constitutive expression of some heat shock genes, such as Hsp90α described above. HSF3 is sufficient for the induction of heat shock genes and upregulation of the Hsp90α gene in chicken cells. One reason that the cell cycle block at 43°C in double-null cells was not fully restored by overexpressing Hsp90α (25) should be the lack of this HSF1 function. With this third mechanism of HSF1 function, cells can protect themselves against a single exposure to moderately high temperatures. This function is distinct from the induced thermotolerance that is the acquisition of resistance against short-term exposure to extremely high temperatures (Fig. 5B).
The proportion of sub-G1 cells in HSF1-null DT40 cells was the same as that in wild-type cells at 24 h after exposure to extremely high temperatures such as 46°C (data not shown). Furthermore, chicken HSF3, a strong activator of heat shock genes upon heat shock, lacks this novel function (Fig. 7). These results clearly demonstrate that the third function of HSF1 can be completely dissociated from the function that activates heat shock genes under heat shock conditions. In the course of analysis of the molecular mechanisms of cell death of spermatocytes exposed to thermal stress (28), we recently found that heat shock genes such as Hsp110, Hsp90α, Hsp70-1, and Hsp27 are not induced in spermatogonia in response to thermal stress. However, spermatogonia in HSF1-deficient mice is highly sensitive to thermal stress (H. Izu and A. Nakai, unpublished data), suggesting that HSF1 protects spermatogonia independently of the activation of heat shock genes. This result may support the biological significance of the third role of HSF1.
How does HSF1 protect against thermal stress independently of the expression of heat shock genes? We provide evidences that the DNA binding activity of HSF1 is necessary to play the new role (Fig. 9). Some target genes other than heat shock genes should be involved in the cell cycle progression or death pathway. As the cell cycle progression is not blocked at any specific stage (Fig. 4), the machinery of cell cycle progression may not be inhibited in HSF1-null cells. To resolve this question, we need to identify the target genes regulated by HSF1.
Molecular evolution of vertebrate HSFs.
In this report, we resolve the question of why chicken HSF3 is essential for burst activation of heat shock genes although there is cHSF1 in chicken DT40 cells (42). Unexpectedly, cHSF1 has little ability to activate heat shock genes upon heat shock in chicken cells and does not activate heat shock genes at all in mouse cells. More surprisingly, only a short stretch of amino acid sequences rich in alanine in the amino terminus was revealed to suppress the transcriptional activity of cHSF1.
All members of the HSF gene family have common structural features, including a conserved DNA binding domain which exhibits a winged helix-turn-helix motif, an extended hydrophobic repeat (HR-A/B) involved in trimerization, a transactivation domain, and a regulatory domain that regulates its transcriptional activity. With some exceptions, HSFs also have a carboxyl-terminal hydrophobic repeat (HR-C), which has been suggested to function in suppression of trimer formation by interaction with the HR-A/B (23). Both chicken and human HSF1 have these common domains (27). Functionally, both factors can bind to DNA (26), and the potential to activate transcription when the carboxyl terminus downstream of the DNA binding domain of cHSF1 is fused to the GAL4 DNA binding domain is heat inducible (29). Furthermore, the regulatory domain of hHSF1 can be functionally substituted for by that of cHSF1 (12). Recently, it was reported that a loop domain within the DNA binding domain of HSF1 confers DNA binding specificity (2). However, the amino acid sequences of the loop domain in cHSF1 are exactly the same as those in hHSF1. These data suggest that the inability of cHSF1 to activate heat shock genes may not be due to only the conserved domains. We found in this report that the amino-terminal region containing an alanine-rich sequence and the DNA-binding domain is sufficient to cause the functional difference between the two orthologues. As cHSF1 can bind to DNA, the unique sequences might inhibit exposure of the activation domain to basal transcriptional machinery. Alternatively, the unique domain could recruit some corepressor that suppresses transcription because many transcriptional repressors have alanine-rich sequences (13).
We demonstrate that human HSF1 is functionally equivalent to chicken HSF1 plus HSF3. It is speculated that there is likely a common ancestral HSF gene and that gene duplication occurred during the evolution of avians. The nucleotide sequences of cHSF3 diverged significantly from that of HSF1 against selective forces during evolution (Fig. 1A). If so, is any advantage obtained with cHSF3 instead of cHSF1 in avians? It is noteworthy that there are two markedly physiological differences between mammals and avians. First, avians develop in eggs, where the temperature cannot be kept constant. The chicks are cold-blooded shortly after hatching. Therefore, developing embryos and chicks may be frequently exposed to thermal stress. Second, the physiological temperatures in avians are relatively high compared with those in mammals (3). In the absence of stress, cHSF3 remains a dimer, which is more stable against heat than a monomer form of cHSF1 (26, 41). The unique physiology of avians might be selective forces in the evolution of the heat-stable cHSF3.
There is a related example of molecular change during evolution. A transcriptional repression domain rich in alanine in homeotic gene products is conserved in insects but is absent from homeotic gene products in other species (9, 34). The evolution of this domain in insects supports the morphological diversification unique in insects. In the case of HSF1, the alanine-rich sequence is acquired only in avians. Although it could not suppress the ability to activate heat shock genes in hHSF1, the substitution of the alanine-rich domain with the corresponding domain of hHSF1 was sufficient to restore the ability (Fig. 2). We have no answer yet as to why cHSF1 has lost the ability to mediate the heat shock response.
Acknowledgments
We thank S. Takeda, C. Wu, and D. J. Thiele for reagents.
This work was supported in part by Grants-in-Aid for Scientific Research (B) on Priority Area (A)-Cell Cycle, on Priority Area (C)-Cancer, from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the Novartis Foundation for the Promotion of Science, and the UBE Foundation.
The first two authors contributed equally to this work.
REFERENCES
- 1. C. N. Adra, P. H. Boer, and M. W. McBurney. 1987. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene 60:65-74. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, S. G., P. C. Liu, K. Klyachko, R. I. Morimoto, and D. J. Thiele. 2001. The loop domain of heat shock transcription factor 1 dictates DNA-binding specificity and responses to heat stress. Genes Dev. 15:2134-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschoff, J., and U. von Saint Paul. 1973. Brain temperature as related to gross motor activity in the unanesthetized chicken. Physiol. Behav. 10:529-533. [DOI] [PubMed] [Google Scholar]
- 4.Baler, R., G. Dahl, and R. Voellmy. 1993. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol. Cell. Biol. 13:2486-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beere, H. M., B. B. Wolf, K. Cain, et al. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 6.Bruey, J. M., C. Ducasse, P. Bonniaud, et al. 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2:645-652. [DOI] [PubMed] [Google Scholar]
- 7.Christians, E., A. A. Davis, S. D. Thomas, and I. J. Benjamin. 2000. Maternal effect of Hsf1 on reproductive success. Nature 407:693-694. [DOI] [PubMed] [Google Scholar]
- 8.Clos, J., J. T. Westwood., P. B. Becker, S. Wilson, K. Lambert, and C. Wu. 1990. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell 63:1085-1097. [DOI] [PubMed] [Google Scholar]
- 9.Galant, R., and S. B. Carroll. 2002. Evolution of a transcriptional repression domain in an insect Hox protein. Nature 415:910-913. [DOI] [PubMed] [Google Scholar]
- 10.Glover, J. R., and S. Lindquist. 1998. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73-82. [DOI] [PubMed] [Google Scholar]
- 11.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, M., T. Schuetz, K. Sullivan, and R. E. Kingston. 1995. A heat shock-responsive domain of human HSF1 that regulates transcription activation domain function. Mol. Cell. Biol. 15:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna-Rose, W., and U. Hansen. 1996. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 12:229-234. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, Inc., Los Angeles, Calif.
- 15.Jedlicka, P., M. A. Mortin, and C. Wu. 1997. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 16:2452-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallio, M., Y. Chang, M. Manuel, et al. 2002. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J. 21:2591-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawazoe, Y., M. Tanabe, and A. Nakai. 1999. Ubiquitous and cell-specific members of the avian small heat shock protein family. FEBS Lett. 455:271-275. [DOI] [PubMed] [Google Scholar]
- 18.Kawazoe, Y., T. Tanabe, N. Sasai, K. Nagata, and A. Nakai. 1999. HSF3 is a major heat shock responsive factor during chicken embryonic development. Eur. J. Biochem. 265:688-697. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist, S. 1986. The heat-shock response. Annu. Rev. Biochem. 55:1151-1191. [DOI] [PubMed] [Google Scholar]
- 20.Littlefield, O., and H. C. Nelson. 1999. A new use for the ‘wing' of the ′winged' helix-turn-helix motif in the HSF-DNA cocrystal. Nat. Struct. Biol. 6:464-470. [DOI] [PubMed] [Google Scholar]
- 21.McMillan, D. R., X. Xiao, L. Shao, K. Graves, and I. J. Benjamin. 1998. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 273:7523-7528. [DOI] [PubMed] [Google Scholar]
- 22.Morano, K. A., N. Santoro, K. A. Koch, and D. J. Thiele. 1999. A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol. Cell. Biol. 19:402-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto, R. I. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788-3796. [DOI] [PubMed] [Google Scholar]
- 24.Nakai, A. 1999. New aspects in the vertebrate heat shock factor system: Hsf3 and Hsf4. Cell Stress Chaperones 4:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai, A., and X. Ishikawa. 2001. Cell cycle transition under stress conditions controlled by vertebrate heat shock factors. EMBO J. 20:2885-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai, A., Y. Kawazoe, M. Tanabe, K. Nagata, and R. I. Morimoto. 1995. The DNA-binding properties of two heat shock factors, HSF1 and HSF3 are induced in the avian erythroblast cell line HD6. Mol. Cell. Biol. 15:5168-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakai, A., and R. I. Morimoto. 1993. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol. Cell. Biol. 13:1983-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakai, A., M. Suzuki, and M. Tanabe. 2000. Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1. EMBO J. 19:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakai, A., M. Tanabe, Y. Kawazoe, J. Inazawa, R. I. Morimoto, and K. Nagata. 1997. HSF4, a new member of human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 17:469-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norris, C. E., M. A. Brown, E. Hickey, L. A. Weber, and L. E. Hightower. 1997. Low-molecular-weight heat shock proteins in a desert fish (Poeciliopsis lucida): homologs of human Hsp27 and Xenopus Hsp30. Mol. Biol. Evol. 14:1050-1061. [DOI] [PubMed] [Google Scholar]
- 31.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 32.Pandey, P., A. Saleh, A. Nakazawa, et al. 2000. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 19:4310-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabindran, S. K., G. Giorgi, J. Clos, and C. Wu. 1991. Molecular cloning and expression of a human heat shock factor, HSF1. Proc. Natl. Acad. Sci. USA 88:6906-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronshaugen, M., N. McGinnis, and W. McGinnis. 2002. Hox protein mutation and macroevolution of the insect body plan. Nature 415:914-917. [DOI] [PubMed] [Google Scholar]
- 35.Saleh, A., S. M. Srinivasula, L. Balkir, P. D. Robbins, and E. S. Alnemri. 2000. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2:476-483. [DOI] [PubMed] [Google Scholar]
- 36.Sarge, K. D., S. P. Murphy, and R. I. Morimoto. 1993. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 13:1392-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarge, K. D., Z. Zimarino, K. Holm, C. Wu, and R. I. Morimoto. 1991. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 5:1902-1911. [DOI] [PubMed] [Google Scholar]
- 38.Schuetz, T. J., G. J. Gallo, L. Sheldon, P. Tempst, and R. E. Kingston. 1991. Isolation of a cDNA for HSF2: evidence for two heat shock factor genes in humans. Proc. Natl. Acad. Sci. USA 88:6911-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinkai, Y., H. Satoh, N. Takeda, M. Fukuda, E. Chiba, T. Kato, T. Kuramochi, and Y. Araki. 2002. A testicular germ cell-associated serine-threonine kinase, MAK, is dispensable for sperm formation. Mol. Cell. Biol. 22:3276-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorger, P. K., and H. R. B. Pelham. 1988. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54:855-864. [DOI] [PubMed] [Google Scholar]
- 41.Tanabe, M., A. Nakai, Y. Kawazoe, and K. Nagata. 1997. Different thresholds in the response of two heat shock transcription factors, HSF1 and HSF3. J. Biol. Chem. 272:15389-15395. [DOI] [PubMed] [Google Scholar]
- 42.Tanabe, M., Y. Kawazoe, S. Takeda, R. I. Morimoto, K. Nagata, and A. Nakai. 1998. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO J. 17:1750-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiederrecht, G., D. Seto, and C. Parker. 1988. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell 54:841-853. [DOI] [PubMed] [Google Scholar]
- 45.Wu, C. 1995. Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Biol. 11:441-469. [DOI] [PubMed] [Google Scholar]
- 46.Xiao, X., X. Zuo, A. A. Davis, D. R. McMillan, B. B. Curry, J. A. Richardson, and I. J. Benjamin. 1999. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 18:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagi, T., T. Tokunaga, Y. Furuta, S. Nada, M. Yoshida, T. Tsukada, Y. Saga, N. Takeda, Y. Ikawa, and S. Aizawa. 1993. A novel ES cell line, TT2, with high germline-differentiating potency. Anal. Biochem. 214:70-76. [DOI] [PubMed] [Google Scholar]