Abstract
Initiation of meiotic recombination in the yeast Saccharomyces cerevisiae requires at least 10 gene products. The initiation event creates double-strand breaks, which are then processed by other recombination enzymes. A variety of classical observations, such as the existence of recombination nodules, have suggested that the proteins catalyzing recombination form a complex. A variety of lines of evidence indicate that Rad50p, Mre11p, and Xrs2p interact, and genetic data suggesting interactions between Rec102p and Rec104p have been reported. It has recently been shown that Spo11p coimmunoprecipitates with Rec102p in meiosis as well. In this paper, we provide genetic and biochemical evidence that the meiosis-specific proteins Rec102p, Rec104p, and Spo11p all interact with each other in meiosis. Furthermore, we demonstrate that the interaction between Rec102p and Spo11p does not require Rec104p. Likewise, the interaction between Rec104p and Rec102p does not require Spo11p, although Spo11p may stabilize that association. The interactions suggest that Spo11p, Rec102p, and Rec104p may form a trimeric complex during the initiation of recombination.
For most sexually reproducing organisms, a high level of meiotic recombination is required for the proper segregation of homologous chromosomes to produce viable gametes. The single-celled eucaryote Saccharomyces cerevisiae has served as a model system with which to study meiosis and meiotic recombination (15, 37); its chromosomes undergo the typical sequence of premeiotic DNA synthesis, meiotic recombination, reductional division, equational division, and packaging (into spores). Accumulated evidence indicates that most, if not all, of the meiotic recombination events in S. cerevisiae are initiated with double-strand breaks (DSBs) (e.g., see references 15 and 30). At least 12 genes (see Table 1), which have been called early recombination genes (26), are required for the initiation of meiotic recombination (e.g., see references 18, 20, and 28). Five of the early recombination genes (RAD50, XRS2, MRE11, MRE2, and REC103) are expressed during mitosis and meiosis. Two genes (MER1 and MRE2) are meiosis-specific splicing factors required for the proper splicing of MER2 mRNA (34, 35) and seem unlikely to play a direct role in recombination initiation. The gene products of six early recombination genes (SPO11, MEI4, MER2, REC102, REC104, and REC114) are present only in meiotic cells and may therefore be considered meiosis specific. Except for MER1 and MRE2, null mutations of early recombination genes confer similar meiotic phenotypes, including a reduced sporulation rate, very low spore viability, complete elimination of intrachromosomal and interchromosomal meiotic recombination, and a failure to create meiotic DSBs (e.g., see references 17, 20, and 38).
TABLE 1.
Summary of known interactions among early exchange genes
| Gene | Interaction(s) | Method (reference) | Expression |
|---|---|---|---|
| MRE11 | Mre11p-Mre11p, Mre11p-Rad50p, Mre11p-Xrs2p | Two-hybrid analysis (22), coimmunoprecipitation (47) | Mitotic/meiotic |
| RAD50 | Rad50p-Mre11p | Two-hybrid analysis (22), coimmunoprecipitation (47) | Mitotic/meiotic |
| XRS2 | Xrs2p-Mre11p, Xrs2p-Rad50p | Two-hybrid analysis (22), coimmunoprecipitation (47) | Mitotic/meiotic |
| REC103 | Rec103p-Spo11p | Two-hybrid analysis (48) | Mitotic/meiotic |
| MER2/REC107 | Unknown | Meiotic/meiotic | |
| MER1 | Unknown | Meiotic | |
| MRE2/NAM8 | Unknown | Mitotic/meiotic | |
| REC102 | Spo11p-Rec102p, REC104-REC102 | Coimmunoprecipitation (23), high-copy-number-suppression (42) | Meiotic |
| ME14 | Unknown | Meiotic | |
| REC104 | REC104-REC102 | High-copy-number suppression (42) | Meiotic |
| REC114 | Unknown | Meiotic | |
| SPO11 | Spo11p-Rec102p, Spo11p-Rec103p | Coimmunoprecipitation (23), two-hybrid analysis (48) | Meiotic |
In other DNA metabolic processes, protein complexes carry out many of the reactions involved. For example, the DNA replication initiation complex is highly conserved in eucaryotes ranging from yeast to metazoans (7, 46). Likewise, complexes of proteins are involved in the recognition and repair of damaged DNA (such as pyrimidine dimers) (3, 39). Large structures, called recombination nodules, are associated with meiotic recombination and have been visualized along meiotic chromosomes by electron microscopy studies of Drosophila melanogaster and many other organisms (8, 10, 19, 50). It has been hypothesized that recombination nodules contain multienzyme complexes catalyzing meiotic recombination (2, 9, 10, 19). Since there is a clear precedent for protein complexes acting in DNA metabolism, since at least 10 S. cerevisiae genes are required to create DSBs to initiate meiotic recombination, and since null mutations in all early recombination genes confer similar meiotic phenotypes, it is not surprising that physical and genetic interactions have been found among some of the early recombination gene products (Table 1).
Mre11p, Rad50p, and Xrs2p can form a complex, with Mre11p serving as the binding core (22, 49). These three genes are also expressed during mitosis, and they are required for mitotic DNA recombination-repair (13). Mre11p and Rad50p have amino acid sequences that have homology to the regions of the SbcC and SbcD proteins of Escherichia coli (44). Mre11p has been reported to have DNA nuclease, DNA binding, strand dissociation, and strand-annealing activities (16, 47, 49). Rad50p has ATP binding motifs at its amino- and carboxy-terminal ends, and purified Rad50p exhibits ATP-dependent double-strand DNA binding activity (40). Special mutant alleles of RAD50 and MRE11 (e.g., rad50S) (1, 16, 49) indicate that Rad50p and Mre11p are required for both the formation and subsequent processing of meiotic DSBs.
During the preparation of this report, Kee and Keeney (23) reported that a diploid cell containing an epitope-tagged version of both SPO11 and REC102 displays a synthetic cold-sensitive meiotic Rec− phenotype, indicating a genetic interaction between the two genes. They also reported that immunoprecipitation of Rec102p coprecipitated Spo11p (23). SPO11 is a central meiosis-specific early recombination gene whose product shows moderate homology to the topoisomerase type VIA subunit of an archaebacterium (5). Spo11p is covalently linked with the 5′ ends of meiotic DSBs in rad50S cells (24). Thus, it has been proposed that SPO11 encodes the catalytic subunit for the formation of meiotic DSBs (5, 24). SPO11 homologs have been identified in complex eucaryotes, including D. melanogaster, Caenorhabditis elegans, and mice (14, 25, 31, 41), suggesting that the basic process for the initiation of meiotic recombination is evolutionarily conserved.
REC102 is one of the meiosis-specific early recombination genes in S. cerevisiae (6, 12, 27). Sequence analysis has not revealed any significant homologs in species other than S. cerevisiae (21) or functional motifs except for a potential leucine zipper domain (12). The first reported interaction between the REC102 gene and other meiotic recombination functions was the observation that overexpression of REC102 suppresses specific conditional alleles of REC104 (42). This genetic interaction observed between REC102 and REC104 led us to propose that the products of these two meiosis-specific early recombination genes would physically associate with each other, as well as with other recombination initiation functions (42). In this report, we describe genetic and physical interactions among three meiosis-specific genes, REC102, SPO11, and REC104. Our studies provide support for the hypothesis (42) that the products of these genes function in a multiprotein complex whose roles include catalysis of the initiation of meiotic recombination by the formation of DSBs.
MATERIALS AND METHODS
Yeast strains and genetic techniques.
The yeast strains used in this study are listed in Table 2. Haploids used to generate the diploids JK9-1 and LS7-1 were transformed with a 5.9-kb rec102Δ::LYS2 NsiI-BglI fragment from pJK27. Transformants were examined by both PCR and Southern analysis to confirm the rec102Δ::LYS2 deletion. The JK9-4 diploid was made by transforming the haploid parents with pJK55 digested with KpnI, thus integrating the rec102-18 allele by two-step gene replacement. Strains JK5-1-5D (rec102Δ::LYS2/rec102Δ::LYS2), JK12-4 (spo11Δ::kanr/spo11Δ::kanr rec102Δ::LYS2/rec102Δ::LYS2), and LS8-1-8B (spo11Δ::kanr/spo11Δ::kanr rec102Δ::LYS2/rec102Δ::LYS2 rec104Δ1::URA/rec104Δ1::URA3) are derivatives of K65-3D (17) and were used for coimmunoprecipitation assays.
TABLE 2.
Yeast strains and plasmids used in this study
| Name | Description or relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| LS1-9 | LS1-4-26BMATαrec102Δ1::ura3-52ade5 CYH2smet13d trp5d leu1-12CAN1sura3-52 ade2-1 | This study |
| LS1-4-74C MATα rec102Δ1::ura3-52ADE5cyh2rmet13c trp5c leu1-ccan1rura3-52 ade2-1 | ||
| LS2-8 | LS2-3-23DMATαlys2-1 tyr1-1 his7-1ADE5 met13c cyh2rtrp5c leu1-cCAN1sura3-13ade2-1 | 42 |
| LS2-3-37A MATα lys2-1 tyr1-1 his7-2ade5 met13dCYH2strp5d leu 1-12can1rura3-13 ade2-1 | ||
| LS7-1 | LS2-3-4B1-1MATαrec104Δ-1rec102Δ2::LYS2lys2-1 tyr1-1 his7-1 ADE5 met13d cyh2rtrp5d leu1-12can1rura3-13ade2-1 | This study |
| LS2-3-20D1-1 MATα rec104Δ-1rec102Δ2::LYS2lys2-1 tyr1-1 his7-2 ade5 met13cCYH2strp5c leu1-cCAN1sura3-13ade2-1 | ||
| JK9-1 | Isogenic to LS2-8; homozygous rec102Δ2::LYS2 | This study |
| JK9-4 | Isogenic to LS2-8; homozygous rec102-18 | This study |
| LS5-1 | Isogenic to LS2-8; hemizygous rec104-8/rec104Δ-1 | 42 |
| K65-3D | HOMATαlys2-1 tyr1-1 his7-2can1rura3-13ade5 met13-d trp5-2 leu1-12ade2 | 18 |
| HOMATα lys2-1 tyr1-1 his7-2can1rura3-13ade5 met13-d trp5-2 leu1-12 ade2 | ||
| JK5-1-5D | Same as K65-3D except rec102-Δ2::LYS2/rec102-Δ2::LYS2 | This study |
| JK12-4 | Same as JK5-1-5D except spo11Δ::kanr/spo11Δ::kanr | This study |
| LS8-1-8B | Same as JK12-4 except rec104Δ1::URA/rec104Δ1::URA3 | This study |
| LS8-1-3B | Same as K65-3D except spo11::kanr/spo11::kanrrec104Δ1::URA3/rec104Δ1::URA3 | This study |
| Plasmids | ||
| pCM212 | REC102 in pRS316 | 12 |
| pEG(KG) | GST fusion protein expression vector; 2μm origin URA3 | 32 |
| pESC-URA | FLAG and MYC expression vector | Stratagene |
| pJK21 | ClaI site in polylinker region of pCM212 disrupted | This study |
| pJK22 | REC102 in pRS426 | This study |
| pJK27 | rec102Δ2::LYS2 in pRS426 | This study |
| pJK46 | rec102-18 allele in pRS316 | This study |
| pJK55 | rec102-18 allele in pRS306 | This study |
| pJK61 | REC102 in modified pRS316 with NsiI site disrupted | This study |
| pJK63 | SPO11 from pJK72 cloned in pRS316 | This study |
| pJK64 | SPO11 from pJK72 cloned in pRS426 | This study |
| pJK72 | Original clone of high-copy-number suppressor of rec102-18 | This study |
| pJK73 | Another original clone of high-copy-number suppressor of rec102-18 | This study |
| pJK74 | 3-HA tag fused with REC102 in pRS316 | This study |
| pJK75 | 3-HA tag fused with REC102 in pRS426 | This study |
| pJK81 | 2μm origin TYR1 | This study |
| pJK108 | REC102-3-HA tag in pJK81 | This study |
| pJK113 | GST-SPO11 fusion construct in pEG(KG) | This study |
| pLS37 | REC104 cloned into pESC-URA | This study |
| pLS741 | pJK74 with REC102-48 mutation | This study |
| pMH1 | REC104 cloned into pJK81 | This study |
| pMPY-3xHA | Yeast 3-HA epitope tagging vector | 42 |
| pRS306 | Integration vector; URA3 | 45 |
| pRS316 | CEN6 ARSH4 URA3 | 45 |
| pRS426 | 2μm origin URA3 | 11 |
| YDp-K | Integration vector; LYS2 | 4 |
Plasmid construction.
A description of the plasmids used in this research is given in Table 2. The REC102 gene from pCM212 (12) was cloned into pRS426 (11), a high-copy-number vector, to obtain pJK22. pJK21 was a derivative of pCM212 in which the ClaI site within the polylinker region was disrupted by partial digestion, blunting, and self-ligation. The ClaI site in the coding region of REC102 (bp +9 relative to the start codon) is thus unique. The 1.2-kb BspEI/BbuI fragment (blunted by Klenow fragment) of pJK22 containing REC102 was then removed and replaced with the 4.8-kb EcoRI/PstI fragment containing the LYS2 gene from the YDp-K plasmid (4) to obtain pJK27. The deletion starts at bp −605 and ends at bp +609 of REC102 relative to the start codon. pJK55 contains a 3.7-kb EcoRI/SpeI fragment of yeast DNA (from bp −591 to bp +2.5 kb relative to the start codon of REC102) containing the rec102-18 mutation in the integration vector pRS306 (45) and was used to integrate the rec102-18 allele into the yeast genome. To obtain pJK74, an in vivo subcloning approach was used. The three-hemagglutinin (HA) tag from pMPY-3xHA (43) was PCR amplified with the primers HA up (5′-CAATTCAGAATTTTCAAATTTTTTCCTTACCGGCTGTAACGTACAATAAGGGTGGATACCCATACGATGTTCCT-3′) and HA down (5′-TTACTAAAAAATTTTAATTGCCCAAAAGTATTTGATTAAATTATATTTTATCTATCACTGAGCAGCGTAATCTGG-3′). The first 50 nucleotides of the HA up primer correspond to the last 50 bp of the REC102 coding region, followed by two glycine codons. The last 18 nucleotides of HA up and HA down correspond to the HA epitope sequence. The first 50 bp of the HA down primer correspond to the 50 bp immediately downstream of REC102, followed by two stop codons. The PCR product generated from these primers was cotransformed with a gapped vector (pJK61 digested with NsiI), generating plasmid pJK74. The construct containing REC102, the two-glycine linker region, and the three HA repeats will be referred to as REC102-HA in this paper. The REC102-HA fusion from pJK74 was cloned into pRS426 (high-copy-number vector with URA3 as the selective marker) or pJK81 (high-copy-number vector with TYR1 as the selective marker) to obtain pJK75 or pJK108, respectively. pJK72 is one of the original clones that suppress rec102-18. The 1.9-kb BglII/SpeI fragment containing SPO11 as the only intact open reading frame from pJK72 was cloned into pRS316 or pRS426 to obtain pJK63 or pJK64, respectively. pJK113 was generated by PCR amplification of the SPO11 gene with the primers SPO11g1 (5′-AATTCTAGACATGGCTTTGGAGGGATTG-3′) and SPO11g2 (5′-TCATTATCAAAGCTTTCAATTTATA-3′). The SPO11g1 primer contains an XbaI site at its end, and the SPO11g2 primer generates a HindIII site, facilitating subcloning into the XbaI-HindIII sites of pEG(KG). pLS37 was constructed by PCR of REC104 with primer 294 (5′-CTTACCAGTAGATCTTGGGTGGTGGAATGTCCATCGAGGAGG-3′) and primer 295 (5′-CATCAGGGACTCTAAATCAAACCTCGAGGGTTCGGAGG-3′) and cloned into the BglII and SstI sites used for subcloning into pESC-URA (Stratagene). Plasmids pLS37, pJK74, and pJK113 encode fully functional FLAG-Rec104p, Rec102-HAp, and GST-Spo11p fusion proteins, as assayed by sporulation efficiency, meiotic recombination, and spore viability.
PCR mutagenesis of REC102.
Random PCR mutagenesis was performed as described previously (33, 42). Plasmid pJK21 was constructed for use as a gapped vector by digestion with ClaI (+9 of the REC102 coding region) and BseRI (440 bp downstream of REC102). The PCR-mutagenized REC102 fragment was amplified with primers REC102A (5′-CCCATGCTAGAACACAGC-3′; located at bp 536) and REC102B (5′-TTGGAGGGTACAAGCGAG-3′; located at bp +1220 relative to +1 of REC102). The PCR conditions used included 0.1 ng of pCM212 template DNA, 0.5 mM deoxynucleoside triphosphates, 2.5 U of Taq DNA polymerase (BRL), 2.5 μM primers, and 1× PCR buffer. The MnCl2 concentrations were 0.1, 0.3, and 0.5 mM in three independent reaction mixtures, which were combined with MgCl2 concentrations of 5.9, 5.7, and 5.5 mM, respectively, to generate a total concentration (Mn plus Mg) of 6 mM.
Screen for REC102 mutants that suppress rec104-8.
PCR mutagenesis was performed on REC102 as described above. PCR-mutagenized REC102 DNA and a gapped vector were transformed into LS7-1 (rec104-8/rec104-Δ1 rec102Δ::LYS2/rec102Δ::LYS2). One thousand four hundred twenty-five transformants were analyzed for meiotic recombination at 35°C by sporulating cells for 5 days and replica plating to media diagnostic for recombination. Yeast DNAs of 11 candidates were made and electroporated into E. coli to recover the REC102 mutant. Both REC102-35 and REC102-48 were recovered in this screen.
Screen for high-copy-number suppressors of rec102-18.
The screen for high-copy-number suppressors of REC102-18 was performed as described by Salem et al. (42). Five thousand three hundred fifty-five transformants were tested for meiotic recombination. Five plasmids that met the criteria of the screen were retransformed into JK9-4 and tested for sporulation frequency and meiotic recombination. Of these five candidates, four were further analyzed by restriction enzyme digestion, PCR, and sequencing.
Assays for recombination.
Replica plating assays were done by analyzing at least two, and often three, patches of each diploid. Wild-type and (where appropriate) nonsuppressed controls were also on the plate. After 5 days on sporulation medium, the plates were replica plated to at least three and usually four media diagnostic for recombination. Quantitative plating assays were done as described by Salem et al. (42).
Coimmunoprecipitation assays.
Meiotic protein extracts were prepared from cells at 6 h in sporulation medium. Protein was extracted from 25 ml of cells by standard yeast procedures. Cells were lysed with 0.5 ml of lysis buffer (0.01 M Tris [pH 7.5], 0.1 M NaCl, 2 mM EDTA, 0.5% Triton X-100) rather than the traditional sorbitol breaking buffer. A 0.1-ml volume of 100 mM phenylmethylsulfonyl fluoride and one tablet of protease inhibitor cocktail (Boehringer Mannheim) were added per 10 ml of lysis buffer. One milligram of protein in a total of 100 μl of dilution buffer (lysis buffer plus bovine serum albumin to a final concentration of 0.05%) was used in each immunoprecipitation. Protein A beads (Amersham Pharmacia Biotech) and protein G beads (Novagen) were prepared by adding 10 mg of beads (per precipitation) to 100 μl of incubation buffer (lysis buffer plus bovine serum albumin to a final concentration of 2%). Five milligrams of beads (resuspended in incubation buffer) was added to each protein extract, and the mixture was incubated for 1 to 3 h. The beads were removed, and the appropriate antibody for immunoprecipitation was added to the precleared protein extract, and the mixture was incubated at 4°C for 1 h. Five milligrams of protein A or protein G beads was added, and samples were incubated at 4°C for 2 h. Precipitates binding to the beads were collected by centrifugation. Potential complexes were eluted from the beads by placement at 100°C for 5 min. Anti-HA (HA.11; Babco), goat anti-glutathione S-transferase (GST; Molecular Probes), and anti-FLAG (Stratagene) antibodies were used for coimmunoprecipitation and Western analysis.
RESULTS
High-copy-number REC104 suppresses specific mutations in REC102.
Our previous work (42) demonstrated that a high-copy-number (hc) plasmid containing REC102 suppressed several rec104 alleles, leading us to propose that the two meiotic early recombination genes produce interacting proteins. To further examine interactions between these two genes, we performed the reciprocal experiment to determine if hcREC104 can suppress specific alleles of REC102. Random PCR mutagenesis of REC102 generated nine temperature-sensitive (ts) alleles, although no cold-sensitive alleles were discovered in 1,425 independent transformants (Table 3). Suppression of several mutant alleles was first assayed by replica plating and then by a quantitative plating assay. The data in Fig. 1 and Table 4 indicate that hcREC104 can suppress the meiotic recombination defect caused by the ts mutant alleles rec102-10 and rec102-17. Since hcREC104 could not suppress mutant allele rec102-5, rec102-8, rec102-18, or rec102Δ, it is not bypass suppression (Table 4). The suppression of specific rec102 mutations by hcREC104 supports the hypothesis proposed by Salem et al. (42) that Rec102p and Rec104p interact.
TABLE 3.
Mutant rec102 alleles and their locations
| Allele no. | Phenotype | Altered amino acid(s)a |
|---|---|---|
| rec102-5 | Tsb | R4Q, E29D, F62V, E95K, Q166L |
| rec102-6 | Ts | D56N, K65E, F112S |
| rec102-7 | Ts | S48G, R67K, F122L |
| rec102-8 | Ts | F122S, S129T |
| rec102-10 | Ts | N109L |
| rec102-14 | Ts | Q36H, S118F |
| rec102-17 | Ts | E110G, Q119L |
| rec102-18 | Ts | Q175L |
| rec102-19 | Ts | Y111C |
| rec102-21 | Partial | D167G |
| rec102-27 | Null | I24F, R180G |
| rec102-29 | Null | V117M, W138R, A149S |
| rec102-39 | Null | S114F |
| rec102-45 | Null | E11G, H72R, S113F |
PCR mutagenesis of REC102 was carried out as described in Materials and Methods, and mutants were isolated as described in the text. Mutations in bold occur in amino acids conserved among S. cerevisiae, S. paradoxus, and S. pastorianus (21).
Ts, temperature sensitivity.
FIG. 1.
Replica plating assay for suppression of various rec102 alleles by high-copy-number REC104. The assay detected TRP5 recombinants after sporulation at a nonpermissive temperature. V, high-copy-number vector; hc104, high-copy-number REC104.
TABLE 4.
Quantitative assay for suppression of various rec102 alleles by high-copy-number REC104a
| Plasmid 1 (genotype)b | Plasmid 2 (genotype)b | Sporulation % | No. of surviving asci/totalc | RFd (103)
|
Avg fold increasee | ||
|---|---|---|---|---|---|---|---|
| Canr | Leu+ | Trp+ | |||||
| pJK33 (rec102-5) | pJK81 (vector) | 21 | 0/30 | 4.0 | 0.010 | 0.006 | 1 |
| pJK33 (rec102-5) | pMH1 (hcREC104) | 19 | 0/30 | 6.4 (1.6) | 0.007 (0.7) | 0.004 (0.7) | 0.92 |
| pJK36 (rec102-8) | pJK81 (vector) | 28 | 5/30 | 26 | 0.031 | 0.021 | 1 |
| pJK36 (rec102-8) | pMH1 (hcREC104) | 25 | 4/30 | 28 (1.1) | 0.042 (1.3) | 0.014 (0.7) | 1.1 |
| pJK38 (rec102-10) | pJK81 (vector) | 25 | 3/30 | 22 | 0.034 | 0.05 | 1 |
| pJK38 (rec102-10) | pMH1 (hcREC104) | 51 | 21/30 | 111 (5.0) | 0.41 (12) | 0.48 (9.5) | 8.3 |
| pJK45 (rec102-17) | pJK81 (vector) | 28 | 8/30 | 17 | 0.05 | 0.05 | 1 |
| pJK45 (rec102-17) | pMH1 (hcREC104) | 37 | 18/30 | 58 (3.4) | 0.11 (2.2) | 0.41 (8.2) | 4.6 |
| pJK46 (rec102-18) | pJK81 (vector) | 28 | 1/30 | 9.0 | 0.011 | 0.009 | 1 |
| pJK46 (rec102-18) | pMH1 (hcREC104) | 27 | 1/30 | 12 (1.3) | 0.008 (0.7) | 0.011 (1.2) | 1.1 |
All measurements were made in the JK9-1 (rec102Δ) diploid. JK9-1 cells were cotransformed with two plasmids. The first contained different alleles of REC102 on a CEN plasmid with URA3 as the selective marker. The second plasmid was either the control high-copy-number vector (pJK81) or pMH1 (REC104 in pJK81); both of these have TYR1 as a selective marker. Results of a plating assay for recombination are shown. Three independent transformants were examined at a nonpermissive temperature. The values shown are averages of the three cultures.
Relevant genotypes of genes on plasmids are shown in parentheses.
The viability of meiotic products was monitored by examining 30 asci to determine the fraction that could make a viable colony. At least one spore must be alive for an ascus to make a colony.
Recombination frequency (RF) is the number of recombinants divided by the total number of cells plated. The data shown are averages of three independent cultures. The values in parentheses are increases relative to the vector pJK81. Canr colonies are an indirect measure of crossing over, since recombination is required for haploidization and viability.
The average increase is the average, over all three loci, of the relative amount of recombination compared to the cells containing the vector for the same rec102 allele.
Specific alleles of REC102 suppress the rec104-8 ts allele.
To further test the Rec102p-Rec104p interaction hypothesis, we looked for evidence of allele-specific suppression. The pool of random mutations generated in REC102 were transformed into the LS7-1 diploid containing the rec104-8 ts allele. Almost 1,500 transformants were tested for the level of meiotic recombination at several loci, and two REC102 mutants with alterations in the coding region that could suppress rec104-8 were identified (Fig. 2 and Table 5). The plasmids containing these mutations were transformed into a rec102Δ strain (JK9-1) and were capable of fully complementing the Rec− phenotype (data not shown). We named these two alleles REC102-35 and REC102-48; sequence analysis revealed that the REC102-35 allele contained the amino acid changes F32L and S39R, while the REC102-48 allele contained the single amino acid change E123K. Further analysis revealed that REC102-35 could suppress both rec104-8 and rec104-12, while the suppression by the REC102-48 allele was specific to rec104-8 (data not shown). The allele-specific suppression of rec104-8 by REC102-48 is consistent with a protein-protein interaction model.
FIG. 2.
Replica plating assay for suppression of rec104-8 by specific alleles of REC102. Meiotic recombination was measured by replica plating the LS5-1 diploid containing various forms of REC102 on Trp dropout medium. All other diagnostic media showed similar responses.
TABLE 5.
Quantitative plating assay for suppression of rec104-8 by specific alleles of REC102a
| Strain | Relevant genotype | Plasmidb | Sporulation % | No. of surviving asci/totalc | RFd (103)
|
Avg fold increasee | ||
|---|---|---|---|---|---|---|---|---|
| Met+ | Leu+ | Trp+ | ||||||
| LS5-1 | rec104-8 | pRS426 (vector) | 27 | 2/30 | 0.22 | 0.040 | 0.009 | |
| rec104-Δ1 | ||||||||
| LS5-1 | rec104-8 | pJK22 (hcREC102) | 28 | 11/30 | 2.3 (10) | 0.21 (5.3) | 0.17 (18) | 11.5 |
| rec104-Δ1 | ||||||||
| LS5-1 | rec104-8 | pLS27 (REC102-35) | 41 | 13/30 | 2.9 (13) | 0.30 (7.5) | 0.20 (22) | 14.2 |
| rec104-Δ1 | ||||||||
| LS5-1 | rec104-8 | pLS30 (REC102-48) | 78 | 22/30 | 8.9 (40) | 4.1 (102) | 1.2 (133) | 92.1 |
| rec104-Δ1 | ||||||||
Quantitative plating assay for recombination. The LS5-1 diploid was transformed with the indicated plasmid, and transformants were sporulated at the nonpermissive temperature (35°C). Sporulated cells were plated on media diagnostic for recombination, as well as complete media, to determine the frequency of recombination. All experiments were done on the same day. The results shown are averages of three independent cultures.
The relevant gene contained in the plasmid is in parentheses. The plasmids containing REC102-35 and REC102-48 were CEN plasmids.
The viability of meiotic products was monitored by examining 30 asci to determine how many could make a viable colony. At least one spore must be alive for an ascus to make a colony.
Recombination frequency (RF) is calculated as the number of recombinants divided by the total number of cells plated. The values in parentheses are fold increases relative to the vector control (pRS426). Canr colonies are an indirect measure of crossing over, since recombination is required for haploidization and viability.
The average increase is the average, over all three loci, of the relative amount of recombination compared to the cells containing the vector.
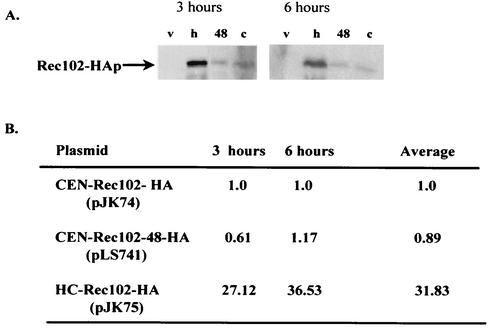
One less interesting explanation for the specific suppression of rec104-8 by REC102-48 is that the change created by the REC102-48 mutation results in a higher level of Rec102p in meiosis. This phenotype would mimic the high-copy-number suppression of rec104-8 previously reported (42). We compared the protein levels of the REC102-48 allele and the wild-type REC102 allele after tagging both with the HA epitope (43). These constructs fully complemented a rec102Δ strain for sporulation, spore viability, and recombination frequency. The data in Fig. 3 demonstrate that no more protein was detected in meiosis in REC102-48 mutant cells than in REC102 mutant cells. As expected, when REC102 was present on a high-copy-number plasmid, about 30 times as much protein was present as when it was located on a low-copy-number CEN plasmid. The data suggest that Rec102-48p interacts more effectively than wild-type Rec102p with mutant Rec104-8p.
FIG. 3.
Specific point mutations in REC102 suppress the rec104-8 mutation. (A) Western blot assay measuring the amount of Rec102-HA (v, vector alone (pRS316); h, high-copy-number Rec102-HA; 48, Rec102-48-HA [in the CEN vector pRS316]; c, CEN Rec102-HA [in the vector pRS316]). (B) Amounts of CEN-Rec102-48-HA relative to CEN-Rec102-HA. Quantitation was done with ImageQuant software on a Molecular Dynamics Phosphorimager.
Rec102p is coprecipitated with Rec104p in meiotic cells.
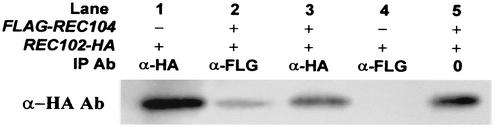
To further test the possibility that Rec102p and Rec104p associate in meiosis, we asked whether they are coimmunoprecipitated in meiotic extracts. We used the REC102-HA construct (pJK108) and a REC104 gene tagged with the FLAG epitope (pLS37). Both the REC102-HA and FLAG-REC104 constructs complement null mutations (data not shown). When antibodies to the FLAG epitope were added to protein extracts made from meiotic cells, the REC102-HA protein was also precipitated (Fig. 4). This result is consistent with the hypothesis that Rec102p and Rec104p interact. Experiments attempting to precipitate Rec102-HA and detect FLAG-REC104 were unsuccessful (see Discussion).
FIG. 4.
Coimmunoprecipitation (IP) of Rec102-HAp and FLAG-Rec104p. All proteins were isolated from an LS8-1 (rec104-Δ1/rec104-Δ1 rec102Δ/rec102Δ) diploid containing pLS37 (FLAG-Rec104p) and/or pJK108 (REC102-HA) after 6 h in sporulation medium. The antibody (Ab) used in the Western blot assay was anti-HA (α-HA). Lane 5 indicates the position of Rec102-HAp in a total protein (0) extract (100 μg loaded). Lane 2 demonstrates that Rec102-HAp can be coprecipitated by antibody to FLAG-Rec104p (α-FLG).
Genetic interactions between SPO11 and REC102.
Having shown that hcREC104 can suppress specific defects in Rec102p, we investigated whether any other genes could suppress rec102 conditional mutants. We screened a high-copy-number genomic library for suppressors of the rec102-18 ts allele. The rec102-18 allele is not suppressible by hcREC104, and high-copy-number suppressors of this allele should define recombination functions other than REC104. We used 33°C; since some recombination occurs at this temperature, we infer that some functional Rec102 protein is present. This enhanced the isolation of interacting proteins, rather than bypass suppressors.
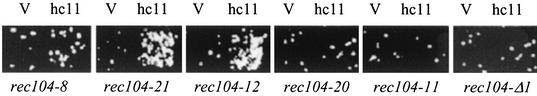
A rec102-18/rec102-18 diploid (JK9-4) was transformed with a high-copy-number genomic library (42), and 2,500 transformants were screened for meiotic recombination at several loci at 33°C. Two of the suppressor plasmids contained REC102 (data not shown). Sequence analysis demonstrated that the other two plasmids (pJK72 and pJK73) contained a region of chromosome VIII encompassing the SPO11 gene. The SPO11 gene in pJK72 was subcloned into a low-copy-number CEN plasmid and a high-copy-number 2μm plasmid to form pJK63 and pJK64, respectively (see Materials and Methods). All four plasmids (pJK72, pJK73, pJK63, and pJK64) fully complemented spo11Δ (data not shown). The pJK64 plasmid was used to examine the suppression of several rec102 alleles by replica plating (Fig. 5); weak suppression was observed for rec102-10 and rec102-17. A quantitative plating assay confirmed suppression of rec102-18 (12-fold), rec102-17 (5-fold), and rec102-10 (2-fold) (Table 6). No suppression was detected by either assay for the rec102-5, rec102-8, or rec102-19 allele. Finally, we asked if one extra copy of SPO11 could suppress rec102-18. The pJK63 (CEN) plasmid showed 5-fold suppression, compared to the 13-fold increase in recombination conferred by the JK64 (2μm) plasmid (Table 7). In comparison, hcREC102 increased meiotic recombination 53-fold in this experiment. This suggests that the suppression is quite sensitive to the amount of Spo11p present. hcSPO11 does not suppress a deletion of REC102 (data not shown), indicating that some Rec102p must be present for suppression to occur.
FIG. 5.
Replica plating assay for suppression of rec102 alleles by SPO11. Meiotic recombination was measured by replica plating on Trp dropout medium in JK9-1 diploids (rec102Δ) containing a plasmid with various mutant alleles of REC102 (shown below the replica) and either a vector (V) (pRS426) or a high-copy-number plasmid containing SPO11 (hc11) (pJK64). Tests were cone at the nonpermissive temperature. Four other media diagnostic for recombination showed similar responses.
TABLE 6.
Quantitative plating assay for recombinationa
| Plasmid 1 (genotype) | Plasmid 2 (genotype) | Sporulation % | No. of asci surviving/totalc | RFd (103)
|
Avg fold increasee | ||
|---|---|---|---|---|---|---|---|
| Canr | Leu+ | Trp+ | |||||
| pJK33 (rec102-5) | pJK81 (vector) | 21 | 0/30 | 4.0 | 0.010 | 0.006 | 1 |
| pJK33 (rec102-5) | pJK82 (hcSPO11) | 15 | 0/30 | 5.0 (1.3) | 0.005 (0.5) | 0.005 (0.9) | 0.84 |
| pJK36 (rec102-8) | pJK81 (vector) | 28 | 5/30 | 26 | 0.031 | 0.031 | 1 |
| pJK36 (rec102-8) | pJK82 (hcSPO11) | 32 | 5/30 | 32 (1.2) | 0.016 (0.5) | 0.025 (1.2) | 0.9 |
| pJK38 (rec102-10) | pJK81 (vector) | 25 | 3/30 | 22 | 0.034 | 0.005 | 1 |
| pJK38 (rec102-10) | pJK82 (hcSPO11) | 38 | 8/30 | 62 (2.9) | 0.047 (1.4) | 0.011 (2.2) | 2.1 |
| pJK45 (rec102-17) | pJK81 (vector) | 28 | 8/30 | 17 | 0.05 | 0.05 | 1 |
| pJK45 (rec102-17) | pJK82 (hcSPO11) | 42 | 15/30 | 66 (3.9) | 0.16 (3.2) | 0.036 (7.2) | 4.8 |
| pJK46 (rec102-18) | pJK81 (vector) | 28 | 1/30 | 9.0 | 0.011 | 0.009 | 1 |
| pJK46 (rec102-18) | pJK82 (hcSPO11) | 58 | 26/30 | 126 (13) | 0.15 (14) | 0.082 (9.1) | 12 |
Quantitative plating assay for meiotic recombination. The JK9-1 diploid was transformed with two plasmids, and three independent cotransformants were sporulated at the nonpermissive temperature. Sporulated cells were plated on media diagnostic for recombination, as well as complete media, to determine the frequency of recombination. All experiments were done on the same day. The results shown are averages of the three independent cultures. Sporulation was determined by counting at least 200 cells from each culture and was averaged from three independent cultures.
bAll measurements were made in the rec102Δ diploid JK9-1 at the nonpermissive or seminonpermissive temperature. hc, high-copy-number 2μm plasmid; CEN, low-copy-number centromere plasmid.
The viability of meiotic products was monitored by examining 30 asci to determine how many could make a viable colony. At least one spore must be alive for an ascus to make a colony.
Recombination frequency (RF) is calculated as the number of recombinants divided by the total number of cells plated. The values in parentheses are increases relative to the vector control (pRS426). Canr colonies are an indirect measure of crossing over, since recombination is required for haploidization and viability.
The average increase is the average, over all three loci, of the relative amount of recombination compared to the cells containing the vector.
TABLE 7.
Suppression analysis by quantitative plating experiment with a rec102-18 homozygous diploida
| Plasmid (relevant gene) | Sporu- lation % | Spore viability (%)b | RF (103)c
|
Avg valued | ||
|---|---|---|---|---|---|---|
| Canr | Trp+ | His+ | ||||
| pJK22 (hcREC102) | 61 | 88 (58/64) | 270 | 3.3 | 0.27 | 53 |
| pJK64 (hcSPO11) | 44 | 63 (38/60) | 120 | 0.28 | 0.103 | 13 |
| pJK63 (CENSPO11) | 39 | 21 (13/60) | 52 | 0.12 | 0.043 | 5.1 |
| pRS426 (vector) | 32 | 0.6 (1/160) | 9 | 0.023 | 0.008 | 1.0 |
Quantitative plating assay in diploid JK9-4 containing chromosomal copies of the rec102-18 allele. hc, high-copy-number 2μm plasmid; CEN, low-copy-number centromere plasmid.
Spore viability was calculated as the number of viable spores divided by the number of spores dissected. At least 15 asci were dissected for all diploids. Forty asci were examined for the pRS426-containing diploid, and only one colony grew; spore viability was calculated as 1/160.
Recombination frequency (RF) is the number of recombinants divided by the total number of cells plated. Data were averaged from three independent cultures. All cultures were grown at the semipermissive temperature of 33°C. Canr colonies are an indirect measure of crossing over, since crossing over is required for haploidization and viability.
The average value represents the average of the relative values of recombination (plasmid/pRS426) for all three of the loci examined. The values are relative to that observed in cells containing pRS426.
High-copy-number SPO11 suppresses the meiotic recombination defect conferred by some rec104 mutations.
Since Rec102p and Rec104p interact and an increased SPO11 dosage suppresses defects in REC102, we asked if increasing the dosage of SPO11 might also suppress mutant alleles of REC104. A series of isogenic diploids containing various rec104 mutations were transformed with a high-copy-number plasmid (pJK64) containing SPO11. We assayed meiotic recombination by replica plating and then by a quantitative plating assay (Fig. 6 and Table 8). The data indicate that increased amounts of SPO11 suppress meiotic defects in diploids containing either the rec104-12 or the rec104-21 allele but not rec104-20 or the rec104-11 allele. There is very weak suppression of the rec104-8 allele; this explains why SPO11 was not detected in the previous screen for high-copy-number suppressors of rec104-8 (42). Increased dosage of SPO11 cannot suppress a null deletion of REC104, indicating that the suppression is not bypass suppression and that some Rec104p must be present for the suppression to occur.
FIG. 6.
Replica plating assay for suppression of various rec104 alleles by high-copy-number SPO11. Meiotic recombination was measured by replica plating on Trp dropout medium in diploids homozygous for various mutant alleles of REC104 and either a vector (V) (pRS426) or a high-copy-number plasmid containing SPO11 (hc11) (pJK22). Four other media diagnostic for recombination showed similar responses.
TABLE 8.
Quantitative plating assay for suppression of various rec104 alleles by high-copy-number SPO11a
| Strainb | Relevant genotype | Plasmid | Sporulation % | No. of asci surviving/totalc | RFd (103)
|
Avg fold increase | ||
|---|---|---|---|---|---|---|---|---|
| Canr | Leu+ | Trp+ | ||||||
| NW5-1 | rec104-11 | pRS426 | 24 | 3/20 | 2.1 | 0.037 | 0.019 | 1.0 |
| NW5-1 | rec104-11 | pJK22 | 22 | 4/20 | 3.0 (1.4) | 0.026 (0.7) | 0.011 (0.6) | 0.89 |
| NW4-1 | rec104-20 | pRS426 | 34 | 6/20 | 3.5 | 0.042 | 0.031 | 1.0 |
| NW4-1 | rec104-20 | pJK22 | 37 | 5/20 | 4.6 (1.3) | 0.036 (0.86) | 0.045 (1.5) | 1.2 |
| LS5-1 | rec104-8 | pRS426 | 21 | 3/20 | 3.6 | 0.089 | 0.012 | 1.0 |
| rec104-Δ1 | ||||||||
| LS5-1 | rec104-8 | pJK22 | 28 | 5/20 | 4.7 (1.3) | 0.12 (1.3) | 0.029 (2.4) | 1.7 |
| rec104-Δ1 | ||||||||
| NW2-1 | rec104-21 | pRS426 | 39 | 4/20 | 4.3 | 0.033 | 0.073 | 1.0 |
| NW2-1 | rec104-21 | pJK22 | 58 | 13/20 | 47.9 (11) | 0.573 (17) | 1.10 (15) | 14.5 |
| NW3-1 | rec104-12 | pRS426 | 23 | 4/20 | 1.7 | 0.029 | 0.077 | 1.0 |
| NW3-1 | rec104-12 | pJK22 | 47 | 10/20 | 29.9 (17.6) | 0.86 (29.6) | 1.6 (20.1) | 22.4 |
Quantitative plating assay for meiotic recombination. The indicated diploid was transformed with the appropriate plasmid, and three independent transformants were sporulated at the nonpermissive temperature. Sporulated cells were plated on media diagnostic for recombination, as well as complete media, to determine the frequency of recombination. All experiments were done on the same day. The results shown are averages of the three independent cultures.
Each yeast diploid strain was homozygous for the rec104 mutant allele indicated.
The viability of meiotic products was monitored by examining 20 asci to determine the fraction that could make a viable colony. At least one spore must be alive for an ascus to make a colony.
Recombination frequency (RF) is the number of recombinants divided by the total number of cells plated. The data shown are averages of three independent cultures. The values in parentheses are increases relative to the vector control (pRS426). Canr colonies are an indirect measure of crossing over, since crosing over is required for haploidization and viability.
Coimmunoprecipitation of Spo11p and Rec102p.
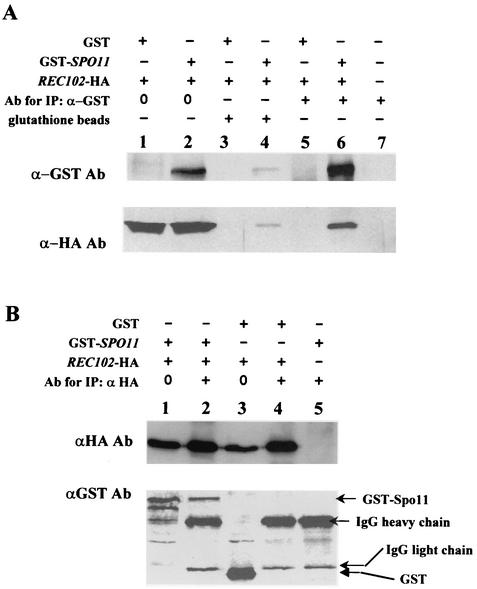
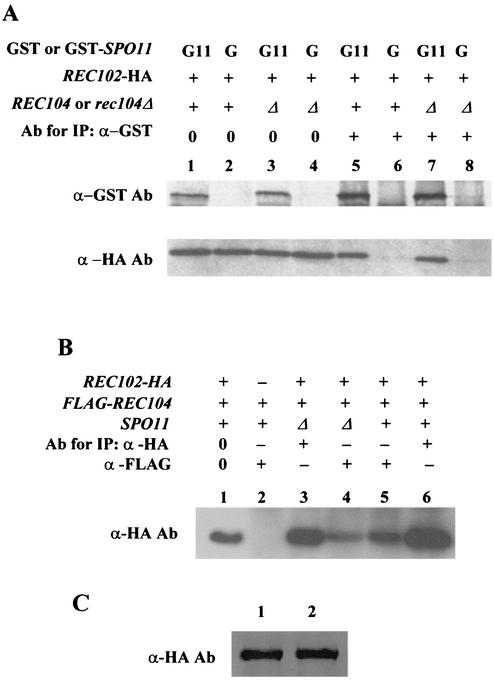
Kee and Keeney (23) recently demonstrated that Spo11p coimmunoprecipitates with epitope-tagged Rec102p from meiotic protein extracts. The high-copy-number suppression by SPO11 of specific rec102 alleles shown in this work supports their conclusion that these two proteins associate. To confirm that Spo11p and Rec102p associate in our strain background, and to also demonstrate that the Rec102 protein coimmunoprecipitates with Spo11p, we first labeled each protein with epitope tags other than those used by Kee and Keeney. We used Rec102-HAp (discussed above), and to follow Spo11p, the GST epitope was added to the amino terminus. The resulting construct (GST-SPO11) complemented an spo11Δ null mutation (data not shown). This method of tagging SPO11 also allowed us to use the GST moiety alone as a control for specific precipitation. Finally, since the GST moiety binds to a column matrix containing glutathione, it provided an alternative method by which to determine whether Rec102p was associated with Spo11p.
The data in Fig. 7A (lanes 5 and 6) demonstrate that when GST-Spo11p was precipitated by an antibody to GST, Rec102-HAp was coprecipitated. When the meiotic protein extract was applied to a glutathione column, GST-Spo11p was isolated (Fig. 7A, lane 4) and Rec102-HAp was also recovered from the column. The recovery of protein by this method was low. A coimmunoprecipitation experiment with the GST tag alone resulted in the isolation of GST (data not shown) but no Rec102-HAp (Fig. 7A, lane 3). A reciprocal immunoprecipitation experiment was done by first immunoprecipitating Rec102-HAp and then determining if GST-Spo11p was coprecipitated (Fig. 7B, lanes 2 and 4). The data indicate that GST-Spo11p was coprecipitated with antibodies to Rec102-Hap, similar to the observation of Kee and Keeney (23). The antibody to Rec102-Hap did not coprecipitate the GST moiety (Fig. 7B, lane 4).
FIG. 7.
Spo11p interacts with Rec102p during meiosis. All proteins were isolated from diploid JK12-4 (rec102Δ/rec102Δ spo11Δ/spo11Δ) after 6 h in sporulation medium. The number 0 refers to total yeast protein with no immunoprecipitation (IP). The plus and minus signs above the lanes indicate which components were present. (A) Lanes 1, 3, and 5 are protein from cells containing plasmids pJK108 (REC102-HA) and pEG-KG (a GST expression vector [31]). Lanes 2, 4, and 6 are from cells containing plasmids pJK108 and pJK113 (a GST-SPO11 expression plasmid). Lanes 5 and 6 demonstrate that Rec102-HA coimmunoprecipitates with Gst-Spo11p. Lanes 3 and 4 demonstrate that Rec102-HA copurifies with Gst-Spo11p on a glutathione column. (B) Reciprocal coimmunoprecipitation between Rec102p and Spo11p. All proteins were isolated from diploid JK12-4. Lane 1 contains total protein and shows both Rec102-HAp and GST-Spo11p. Lane 2 demonstrates that GST-Spo11p coimmunoprecipitates with Rec102-HAp. Lane 3 is total protein with only GST present. Lane 4 demonstrates that precipitation of Rec102-Hap does not coprecipitate GST. Lane 5 demonstrates that no protein is precipitated if Rec102-HA is not present. Ab, antibody; IgG, immunoglobulin G.
Rec102p-Spo11p association does not require Rec104p; Rec102p-Rec104p association does not require Spo11p.
All of the experiments described above are consistent with interactions among all three proteins (Rec102p, Rec104p, and Spo11p). To determine if it is possible for some of these pairwise interactions to occur in the absence of the third protein, we first investigated whether immunoprecipitation of GST-Spo11p can still coprecipitate Rec102-HAp in the absence of Rec104p. The experiments were done with a rec104-Δ1 mutant strain. Figure 8A demonstrates that Rec102-HAp coprecipitated with Gst-Spo11p equally well whether Rec104p was present or not.
FIG. 8.
Rec104p is not required for Rec102p-Spo11p association, and Spo11p is not required for Rec102p-Rec104p association. All protein was isolated from cells after 6 h in sporulation medium. The specific antibody (Ab) used to develop the Western blot is shown at the left of the lanes. The plus and minus signs above the lanes indicate which components were present. The number 0 refers to total yeast protein with no immunoprecipitation (IP). (A) Protein was isolated from isogenic rec102Δ spo11Δ REC104 (LS8-1-1A) and rec102Δ spo11Δ rec104-Δ1 (LS8-1-8B) diploids. The REC102-HA gene was carried on plasmid pJK108 and was present in all lanes. The GST-SPO11 construct was carried on plasmid pJK113, and the control GST expression vector was pEG-KG. Total protein (80 μg) was present in lanes 1 to 4 as controls. (B) Protein was isolated from isogenic rec102Δ rec104-Δ1 SPO11 (JK12-4) and rec102Δ rec104-Δ1 spo11Δ (LS8-1-8B) diploids. Lane 1 is total protein from JK12-4. The REC102-HA gene was carried on plasmid pJK108. The FLAG-REC104 gene was carried on pLS37. See Fig. 2 for some controls. (C) The amount of Rec102-HAp present in meiosis is the same in diploid cells that are SPO11 (LS2-8) or spo11Δ (JK12-4) mutants. Lanes: 1, LS2-8; 2, JK12-4. Samples were taken at 6 h, and equal amounts of protein were loaded.
An analogous experiment was performed to determine whether Rec102p and Rec104p can interact in the absence of Spo11p (Fig. 8B). A comparison of lanes 4 and 5 indicates that Rec102-HAp was coprecipitated with FLAG-Rec104p whether Spo11p was present or absent. However, we noted that the amount of Rec102-HAp that was precipitated in the absence of SPO11 was somewhat less than that observed in its presence (see Discussion). This was not because there is less Rec102-HA protein in a spo11Δ mutant cell (Fig. 8C).
DISCUSSION
Interactions between REC102 and REC104.
Our previous work demonstrated that overexpression of REC102 suppresses specific conditional alleles of REC104, providing the first evidence for a possible interaction between two meiosis-specific early recombination genes and supporting the idea that Rec102p and Rec104p associate in a recombination initiation complex (42). Two alternative models, that excess REC102 can bypass the need for REC104 protein or that excess REC102 increases the expression of REC104, were excluded in that work (42). In this paper, we further characterize the interaction between the REC102 and REC104 genes by both genetic and biochemical approaches. Several lines of new evidence support the hypothesis that the products of these two early recombination genes interact in meiosis. First, hcREC104 can suppress specific mutant alleles of REC102. Second, two REC102 alleles (REC102-35 and REC102-48) can suppress a ts allele of REC104 (rec104-8). The suppression of rec104-8 by the REC102-48 allele is not due to an increased concentration of Rec102-48p, which indicates that the mechanism is different from high-copy-number suppression. The fact that REC102-48 can suppress only the rec104-8 allele is also consistent with a protein-protein interaction. Third, Rec102-HAp was coimmunoprecipitated along with FLAG-Rec104p. (We could not detect the reciprocal coimmunoprecipitation. This type of asymmetry has been seen before among recombination proteins. For example, Kee and Keeney [23] reported that they could not detect the precipitation of Rec102p by Spo11p.) However, we believe that the high-copy-number suppression, the allele-specific suppression, and the coimmunoprecipitation observed all support the hypothesis that Rec104p and Rec102p interact in a meiotic recombination initiation complex. We note with interest that the interactions between these proteins was not detected in an extensive search (in mitotic and meiotic cells) for two-hybrid interactions carried out in our laboratory (29). Likewise, the genomic two-hybrid analysis for Rec102p and Rec104p described in reference 48 lists no interactions between these two proteins. If proteins are present and active only in a relatively brief period during meiosis, perhaps other approaches are more sensitive than two-hybrid assays.
The patterns of suppression and interaction suggest possible regions where the two proteins might interact. We note that the REC102-48 allele consists of the change E123K and that it specifically suppresses the rec104-8 allele, which consists of two changes (G32W and T70K) located in the amino half of the protein. Thus, the middle portion of the 200-amino-acid Rec102p protein may interact with the amino-terminal portion of Rec104p. Consistent with this is the high-copy-number suppression of rec102-17 (E110G, Q119L) and rec102-10 (N109L), both with alterations in the middle of the protein, by hcREC104 (Table 9). Likewise, hcREC102 suppresses the rec104-8 allele, which, as mentioned above, is located in the amino-terminal half of the protein. It is not possible to infer regions of interaction from the suppression of rec104 alleles by hcREC102 because the ts alleles of rec104 that were suppressed (except for rec104-8) contained at least four substitutions. We argue that these data suggest possible regions of interaction to be pursued in future studies.
TABLE 9.
Alleles showing different responses to high-copy-number suppressiona
| Mutant allele | Phenotype | Amino acid change(s) | High-copy-number suppression |
|---|---|---|---|
| rec104-12 | Tsb | T37I, F46K, G90N, I120V | +++ (hcSPO11), + (hcREC102) |
| rec104-8 | Ts | G32W, T70K | − (hcSPO11), +++ (hcREC102) |
| rec102-18 | Ts | Q175L | +++ (hcSPO11), 0 (hcREC104) |
| rec102-10 | Ts | N109L | − (hcSPO11), ++ (hcREC104) |
Comparison of the alleles that were suppressed by increased copies of one gene but not the other. hc, high copy number. The degree of suppression is depicted qualitatively as the relative increase in recombination. The following scale was used: <1.5×, 0; 1.5 to 2×, −; 2 to 4×, ±; 4 to 8×, +; 8 to 12×, ++; >12×, +++. Data for hcREC102 suppression of rec104 alleles come from Salem et al. (42).
Ts, temperature sensitivity.
Interactions between REC102 and SPO11.
To discover other recombination functions that interact with REC102, we used a missense ts allele (rec102-18) that could not be suppressed by hcREC104. The only gene (other than REC102 itself) isolated as a high-copy-number suppressor after examination of 2,500 transformants (or ∼2.1 genomes) was SPO11. hcSPO11 could not suppress a rec102 deletion mutation, indicating that it could not functionally substitute for REC102. Likewise, it could only suppress certain alleles of rec102. Our finding that untagged SPO11 can, when present at a high copy number, suppress specific alleles of untagged REC102 supports the recent work by Kee and Keeney (23) demonstrating coimmunoprecipitation of Spo11-HA3His6p by Rec102-Myc9p. These authors also demonstrated that a diploid containing both tagged alleles (Rec102-Myc9p and Spo11-HA3His6p) displayed a synthetic cold sensitivity for meiotic recombination. Our suppression data led us to the same conclusion as that of Kee and Keeney, i.e., that Rec102p and Spo11p associate with each other in meiotic recombination.
We were motivated, in part, to examine the coimmunoprecipitation between Spo11p and Rec102p because our strains derive from S288C and are different from the SK1 background used by Kee and Keeney (23). The work reported here substantiates and extends the association between Spo11p and Rec102p. First, by using proteins with epitope tags different from those used by Kee and Keeney (23), we also detected coimmunoprecipitation in our strains. Second, we were able to observe association between these two proteins in a novel way by using a glutathione column that was not dependent on the use of precipitating antibodies. Third, we note that Kee and Keeney (23) reported that they were not able to immunoprecipitate native Spo11; this precluded them from determining whether Rec102p was coprecipitated with Spo11p. (They found that Spo11p was coprecipitated with two different tagged forms of Rec102, but not the converse.) Because we were able to precipitate Spo11-GSTp, as well as isolate the tagged form on a glutathione column, we were able to show that Rec102p coimmunoprecipitates with Spo11p. We note that the amounts of Rec102p associated with Spo11 seems to differ in these two approaches. With immunoprecipitation, the ratio of Spo11p to Rec102p appears higher than with the column; this may be due to the difference in technique. Given the central importance of Spo11p as the potential source of DSBs, our genetic and biochemical data provide even more support for an interaction between Spo11p and Rec102p in meiotic recombination. In passing, we note that neither our own extensive search for two-hybrid interactions for REC102 (29) nor those currently described in the genomic two-hybrid search (48) detected interactions between Rec102p and Spo11p.
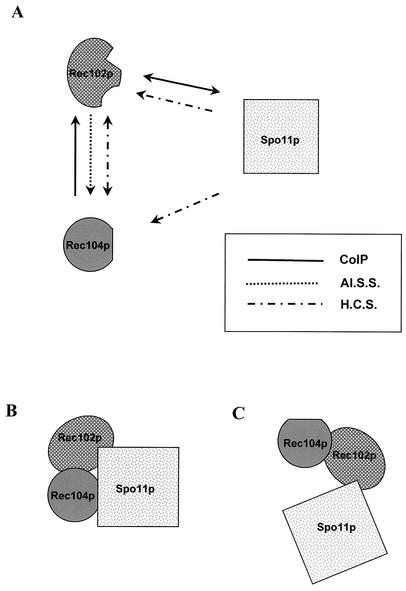
Three-way protein interactions.
The observations made in this paper and those made by Kee and Keeney (23) and Salem et al. (42) all argue that the SPO11, REC102, and REC104 gene products interact in a complex for the initiation of recombination. Figure 9 summarizes the known interactions among these three proteins and raises the question of how the three proteins interact in the recombination complex. Of the known interactions, it is obvious from Fig. 9 that the least data exist for the Spo11p-Rec104p interaction. To begin to address how these proteins interact, we examined the coimmunoprecipitation of two of the proteins in the absence of the third. The data in this paper clearly show that Rec102p and Spo11p can coimmunoprecipitate in the absence of Rec104p. Not only does this indicate that Rec104p is not required for the interaction, it demonstrates that neither genetic recombination per se nor the ability to form DSBs is required for the association. Likewise, Rec102p and Rec104p interact in a strain without any Spo11p, indicating that Spo11p is not absolutely necessary for this association. The tagged Rec104p protein, however, appeared to coprecipitate somewhat less Rec102p in the spo11 deletion strain than in the SPO11 mutant strain. If this is true, it suggests that Spo11p stabilizes the interaction between Rec104p and Rec102p.
FIG. 9.
Interactions among Rec102p, Rec104p, and Spo11p. (A) Summary of all known interactions among Rec102p, Rec104p, and Spo11p. CoIP, coimmunoprecipitation; Al.S.S, allele-specific suppressor; H.C.S., high-copy-number suppressor. The interaction for which there is the least evidence is that between Rec104p and Spo11p. The data are consistent with the notion that either all three proteins interact together (B) or Rec102p acts as a bridge between Rec104p and Spo11p (C). See the text for a discussion.
We believe that our data provide support for two types of interaction. In one, Rec102p and Spo11p interact and Rec102p and Rec104p interact but Spo11p and Rec104p do not directly interact (Fig. 9C). Another model supposes that all three proteins interact but that the interactions between any two are not dependent on the presence of the third (Fig. 9C). We feel that the fact that hcSPO11 suppresses a different set of rec104 alleles than hcREC102 (Table 9) and the difference between the amounts of Rec102p that coprecipitate in the SPO11 versus the spo11 mutant strain together suggest that Spo11p directly interacts with Rec104p (Fig. 9B). Future experiments will determine which model is correct and, of course, incorporate the other recombination initiation genes. Homologs of REC102 and REC104 have been found in other Saccharomyces species (21, 36), although no clear homologs of these genes have been discovered in other genera. Given the conservation and importance of SPO11, we hypothesize that genes do exist in other eucaryotes that carry out a function analogous to that of both Rec102p and Rec104p.
Other factors in the initiation complex.
There are at least 10 genes coding for proteins that are required for meiotic DSB formation. It is certainly conceivable that all of them are present in the initiation complex, at least at some time before the formation of DSBs. Usui et al. (49) reported that three proteins (40, 24, and 22 kDa) from a meiotic extract bind to GST fused with the C-terminal fragment of Mre11p; none of these bands was observed in the extract from mitotic cells. On the basis of size alone, these authors suggested that the two small proteins are Rec102p and Rec104p. If either Rec102p or Rec104p actually does interact with Mre11p, this indicates that at least six of the early recombination gene products (Rec102p, Rec104p, Spo11p, Rad50p, Mre11p, and Xrs2p) are present in the recombination initiation complex. Given the potential danger of making 150 DSBs in chromosomes during meiosis, it would not be surprising to find that a large complex of proteins controls and catalyzes the events.
Acknowledgments
The initial stages of this work were supported by National Institutes of Health grant R01-GM36846 to R.E.M. The final stages were supported by the University of Iowa. Laura Salem was supported during part of this work by a National Institutes of Health Genetics Predoctoral Training Grant awarded to the Program in Genetics. Both N.M. and M.H. were supported by a Howard Hughes summer undergraduate research fellowship during this work.
We thank Natalie Morey and Martin Hove, who helped characterize the high-copy-number suppressors as part of their Honors Thesis in Biology. We thank Stuart Haring for excellent suggestions on improving the paper.
REFERENCES
- 1.Alani, E., R. Padmore, and N. Kleckner. 1990. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell 61:419-436. [DOI] [PubMed]
- 2.Ashley, T., and A. Plug. 1998. Caught in the act: deducing meiotic function from protein immunolocalization. Curr. Top. Dev. Biol. 37:201-239. [DOI] [PubMed] [Google Scholar]
- 3.Batty, D. P., and R. D. Wood. 2000. Damage recognition in nucleotide excision repair of DNA. Gene 241:193-204. [DOI] [PubMed] [Google Scholar]
- 4.Berben, G., J. Dumont, V. Gilliquet, P. A. Bolleand, and F. Hilger. 1991. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7:475-477. [DOI] [PubMed] [Google Scholar]
- 5.Bergerat, A., B. de Massy, D. Gadelle, P. C. Varoutas, A. Nicolas, and P. Forterre. 1997. An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature 386:414-417. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava, J., J. Engerbreght, and G. S. Roeder. 1992. The rec102 mutant of yeast is defective in meiotic recombination and chromosome synapsis. Genetics 130:59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant, J. A., K. Moore, and S. J. Aves. 2001. Origins and complexes: the initiation of DNA replication. J. Exp. Bot. 52:193-202. [PubMed] [Google Scholar]
- 8.Carpenter, A. T. 1975. Electron microscopy of meiosis in Drosophila melanogaster females. II. The recombination nodule—a recombination-associated structure at pachytene? Proc. Natl. Acad. Sci. USA 72:3186-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter, A. T. 1979. Synaptonemal complex and recombination nodules in wild-type Drosophila melanogaster females. Genetics 92:511-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter, A. T. C. 1988. Thoughts on recombination nodules, meiotic recombination, and chiasmata, p. 529-548. In R. Kucherlapati and G. R. Smith (ed.), Genetic recombination. American Society for Microbiology, Washington, D.C.
- 11.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. [DOI] [PubMed] [Google Scholar]
- 12.Cool, M., and R. E. Malone. 1992. Molecular and genetic analysis of the yeast early meiotic recombination genes REC102 and REC107/MER2. Mol. Cell. Biol. 12:1248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Amours, D., and S. P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell. Biol. 3:317-327. [DOI] [PubMed] [Google Scholar]
- 14.Dernburg, A. F., K. McDonald, G. Moulder, R. Barstead, M. Dresser, and A. M. Villeneuve. 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94:387-398. [DOI] [PubMed] [Google Scholar]
- 15.Dresser, M. E. 2000. Meiotic chromosome behavior in Saccharomyces cerevisiae and (mostly) mammals. Mutat. Res. 451:107-127. [DOI] [PubMed] [Google Scholar]
- 16.Furuse, M., Y. Nagase, H. Tsubouchi, K. Murakami-Murofushi, T. Shibata, and K. Ohta. 1998. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 17:6412-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galbraith, A. 1995. Ph.D. thesis. University of Iowa, Ames.
- 18.Galbraith, A. M., and R. E. Malone. 1992. Characterization of REC104, a gene required for early meiotic recombination in the yeast Saccharomyces cerevisiae. Dev. Genet. 13:392-402. [DOI] [PubMed] [Google Scholar]
- 19.Heyting, C. 1996. Synaptonemal complexes: structure and function. Curr. Opin. Cell Biol. 8:389-396. [DOI] [PubMed] [Google Scholar]
- 20.Jiao, K., S. A. Bullard, L. Salem, and R. E. Malone. 1999. Coordination of the initiation of recombination and the reductional division in meiosis in Saccharomyces cerevisiae. Genetics 152:117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao, K., J. J. Nau, M. Cool, W. M. Gray, J. S. Fassler, and R. E. Malone. 2002. Phylogenetic footprinting reveals multiple regulatory elements involved in control of the meiotic recombination gene, REC102. Yeast 19:99-114. [DOI] [PubMed] [Google Scholar]
- 22.Johzuka, K., and H. Ogawa. 1995. Interaction of Mre11 and Rad50: two proteins required for DNA repair and meiosis-specific double-strand break formation in Saccharomyces cerevisiae. Genetics 139:1521-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kee, K., and S. Keeney. 2002. Functional interactions between SPO11 and REC102 during initiation of meiotic recombination in Saccharomyces cerevisiae. Genetics 160:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keeney, S., C. N. Giroux, and N. Kleckner. 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88:375-384. [DOI] [PubMed] [Google Scholar]
- 25.Mahadevaiah, S. K., J. M. Turner, F. Baudat, E. P. Rogakou, P. de Boer, J. Blanco-Rodriguez, M. Jasin, S. Keeney, W. M. Bonner, and P. S. Burgoyne. 2001. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 27:271-276. [DOI] [PubMed] [Google Scholar]
- 26.Malone, R. E. 1983. Multiple mutant analysis of recombination in yeast. Mol. Gen. Genet. 189:405-412. [DOI] [PubMed] [Google Scholar]
- 27.Malone, R. E., S. Bullard, M. Hermiston, R. Rieger, M. Cool, and A. Galbraith. 1991. Isolation of mutants defective in early steps of meiotic recombination in the yeast Saccharomyces cerevisiae. Genetics 128:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao-Draayer, Y., A. M. Galbraith, D. L. Pittman, M. Cool, and R. E. Malone. 1996. Analysis of meiotic recombination pathways in the yeast Saccharomyces cerevisiae. Genetics 144:71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao-Drayyer, Y. 1998. Ph.D. thesis. University of Iowa, Ames.
- 30.Martini, E., and S. Keeney. 2002. Sex and the single (double-strand) break. Mol. Cell 9:700-702. [DOI] [PubMed] [Google Scholar]
- 31.McKim, K. S., and A. Hayashi-Hagihara. 1998. mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev. 12:2932-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell, D. A., T. K. Marshall, and R. J. Deschenes. 1993. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast 9:715-722. [DOI] [PubMed] [Google Scholar]
- 33.Muhlrad, D., R. Hunter, and R. Parker. 1992. A rapid method for localized mutagenesis of yeast genes. Yeast 8:79-82. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa, T., and H. Ogawa. 1997. Involvement of the MRE2 gene of yeast in formation of meiosis-specific double-strand breaks and crossover recombination through RNA splicing. Genes Cells 2:65-79. [DOI] [PubMed] [Google Scholar]
- 35.Nandabalan, K., and G. S. Roeder. 1995. Binding of a cell-type-specific RNA splicing factor to its target regulatory sequence. Mol. Cell. Biol. 15:1953-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nau, J. J., K. R. Summers, A. M. Galbraith, S. A. Bullard, and R. E. Malone. 1997. Isolation of early meiotic recombination genes analogous to S. cerevisiae REC104 from the yeasts S. paradoxus and S. pastorianus. Curr. Genet. 31:7-14. [DOI] [PubMed] [Google Scholar]
- 37.Petes, T. D., R. E. Malone, and L. S. Symington. 1991. Recombination in yeast, p. 407-521. In J. R. Broach, J. R. Pringle, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces (genome dynamics, protein synthesis, and energetics). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Pittman, D. L. 1996. Ph.D. thesis. University of Iowa, Ames.
- 39.Prakash, S., and L. Prakash. 2000. Nucleotide excision repair in yeast. Mutat. Res. 451:13-24. [DOI] [PubMed] [Google Scholar]
- 40.Raymond, W. E., and N. Kleckner. 1993. RAD50 protein of S. cerevisiae exhibits ATP-dependent DNA binding. Nucleic Acids Res. 21:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romanienko, P. J., and R. D. Camerini-Otero. 2000. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 6:975-987. [DOI] [PubMed] [Google Scholar]
- 42.Salem, L., N. Walter, and R. Malone. 1999. Suppressor analysis of the Saccharomyces cerevisiae gene REC104 reveals a genetic interaction with REC102. Genetics 151:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider, B. L., W. Seufert, B. Steiner, Q. H. Yang, and A. B. Futcher. 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11:1265-1274. [DOI] [PubMed] [Google Scholar]
- 44.Sharples, G. J., and D. R. Leach. 1995. Structural and functional similarities between the SbcCD proteins of Escherichia coli and the RAD50 and MRE11 recombination and repair proteins of yeast. Mol. Microbiol. 17:1215-1217. [DOI] [PubMed] [Google Scholar]
- 45.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takisawa, H., S. Mimura, and Y. Kubota. 2000. Eukaryotic DNA replication: from pre-replication complex to initiation complex. Curr. Opin. Cell Biol. 12:690-696. [DOI] [PubMed] [Google Scholar]
- 47.Trujillo, K. M., and P. Sung. 2001. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50*Mre11 complex. J. Biol. Chem. 276:35458-35464. [DOI] [PubMed] [Google Scholar]
- 48.Uetz, P., and R. E. Hughes. 2000. Syst. and large-scale two-hybrid screens Curr. Opin. Microbiol. 3:303-308. [DOI] [PubMed] [Google Scholar]
- 49.Usui, T., T. Ohta, H. Oshiumi, J. Tomizawa, H. Ogawa, and T. Ogawa. 1998. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95:705-716. [DOI] [PubMed] [Google Scholar]
- 50.Zickler, D., and L. W. Olson. 1975. The synaptonemal complex and the spindle plaque during meiosis in yeast. Chromosoma 50:1-23. [DOI] [PubMed] [Google Scholar]