Abstract
To test the hypothesis that the phosphatidylinositol 3-kinase (PI3 kinase)/protein kinase Akt signaling pathway is involved in nitric oxide (NO)-induced endothelial cell migration and angiogenesis, we treated human and bovine endothelial cells with NO donors, S-nitroso-l-glutathione (GSNO) and S-nitroso-N-penicillamine (SNAP). Both GSNO and SNAP increased Akt phosphorylation and activity, which were blocked by cotreatment with the PI3 kinase inhibitor wortmannin. The mechanism was due to the activation of soluble guanylyl cyclase because 8-bromo-cyclic GMP activated PI3 kinase and the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) blocked NO-induced PI3 kinase activity. Indeed, transfection with adenovirus containing endothelial cell NO synthase (eNOS) or protein kinase G (PKG) increased endothelial cell migration, which was inhibited by cotransfection with a dominant-negative mutant of PI3 kinase (dnPI3 kinase). In a rat model of hind limb ischemia, adenovirus-mediated delivery of human eNOS cDNA in adductor muscles resulted in time-dependent expression of recombinant eNOS, which was accompanied by significant increases in regional blood perfusion and capillary density. Coinjection of adenovirus carrying dnPI3 kinase abolished neovascularization in ischemic hind limb induced by eNOS gene transfer. These findings indicate that NO promotes endothelial cell migration and neovascularization via cGMP-dependent activation of PI3 kinase and suggest that this pathway is important in mediating NO-induced angiogenesis.
Endothelial cells play critical roles in angiogenesis, a physiological or pathological neovascularization process in response to tissue ischemia and tumor growth or metastasis (6, 14). Nitric oxide (NO) has diverse biological functions, has been shown to regulate endothelial cell growth (17, 42), apoptosis (13), migration (10, 20, 26), and is essential for angiogenesis (25, 41). NO is a physiological metabolite of l-arginine to citrulline conversion by the three NO synthases (NOS) neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) (34). Many biological functions of NO, including vasodilatation, are mediated by the activation of the soluble guanylyl cyclase and the subsequent production of cyclic GMP (cGMP). This leads to the activation of cGMP-dependent protein kinase, cGMP-regulated phosphodiesterase, and cGMP-gated ion channels (9). In addition, depending on local concentration and microenvironment, NO assumes distinct chemical forms that can be either the immediate NO synthase reaction products (NO, NO−, and NO+) or NO adducts and conversion products (peroxynitrite, S-nitrosothiol, NO2−, NO3−, etc.) (34). These different forms of NO can directly modify cellular proteins, which results in cGMP-independent signaling events and the regulation of gene expression (3, 9).
Endothelial NO synthase (eNOS) produces NO constitutively at low levels but can be transiently stimulated to produce high levels of NO by hormones or environmental stimuli (19, 31). Many growth factors and hormones have been shown to exert their cellular functions, including the activation of eNOS activity, via the phosphatidylinositol 3-kinase (PI3 kinase)-protein kinase B/Akt signaling pathway (8). Akt is a serine/threonine protein kinase that is recruited to the membrane by its binding to PI3 kinase-produced phosphoinositides. At the membrane, Akt is phosphorylated and activated by phosphoinositide-dependent kinases (8, 38). Akt subsequently phosphorylates and activates eNOS, leading to the production of NO (11, 16).
The angiogenic response to vascular endothelial growth factor (VEGF) is, in part, mediated by eNOS, since VEGF-induced angiogenesis is defective in eNOS−/− mice (15). However, the precise mechanism by which NO induces the angiogenic response is not known. Because NO and PI3 kinase/Akt have certain functional similarities such as regulation of endothelial cell survival, we hypothesized that NO may exert some of its angiogenic effects via the PI3 kinase/Akt signaling pathway. The purpose of this study, therefore, was to determine the mechanisms by which NO induces endothelial cell migration and neovascularization, an important and necessary part of angiogenesis.
MATERIALS AND METHODS
Cell culture and reagents.
Human saphenous vein endothelial cells (HSVEC) were prepared as previously described (21) and used between passages 3 and 4. Human aortic endothelial cells (HAEC) were obtained from Cambrex and used at passage 4. Bovine aortic endothelial cells (BAEC) were freshly prepared as previously described and used at passage 2. Antibodies were from the following companies: Akt (H-136), p110α (H-201), and VEGF, Santa Cruz Biotech; phospho-eNOS (Ser1177) and phospho-Akt (Ser473 and Thr308), Cell Signaling Tech; p85α antiserum, Upstate Biotech; phosphotyrosine (4G10), Transduction Labs; α-tubulin (DM1A), Sigma. NO donors S-nitroso-l-glutathione (GSNO) and S-nitroso-N-acetylpenicillamine (SNAP), and 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) were from Calbiochem. LY294002 was obtained from Alexis Co., and wortmannin was from Sigma. Shingosine 1-phosphate was from BioMol. The replication-deficient adenovirus vector harboring the human endothelial nitric oxide synthase DNA (Ad.CMV-NO synthase) under the control of the cytomegalovirus (CMV) enhancer-promoter and adenovirus vector alone (Ad.CMV-null) were constructed and prepared as previously described (32). Adenovirus carrying the cDNA for the dominant-negative regulatory subunit of PI3 kinase, p85α (Ad.CMV-dnPI3K), was kindly provided by Masato Kasuga (Kobe University, Kobe, Japan).
PI3 kinase activity assay.
PI3 kinase activity assays were performed as previously described (31). Briefly, HSVEC were treated and collected in lysis buffer (20 mM Tris [pH 7.4], 10 mM EDTA, 100 mM NaCl, 1% (octylphenoxy)polyethoxyethanol, 1 mM Na3VO4, 50 mM NaF, and complete protease inhibitor [Roche]). Protein concentration was determined by a micro-BCA assay kit (Pierce) and 300 μg of cell lysate was used in the PI3 kinase reaction with substrates L-α-phosphatidylinositol-4,5-bisphosphate (Biomol) and γ-[32P]ATP. The generation of 32P-labeled l-α-phosphatidylinositol-3,4,5-trisphosphate was determined by thin-layer chromatography and autoradiography.
Akt kinase activity assay.
Akt activity was measured with an immunoprecipitation-kinase assay kit according to the manufacturer's protocol (Cell Signaling Tech). Briefly, HSVEC were treated and collected in lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mg of leupeptin per liter, and 1 mM phenylmethylsulfonyl fluoride). The protein concentration was determined, and 200 μg of protein lysate was incubated with an immobilized Akt antibody overnight. The immunoprecipitated Akt was incubated in reaction buffer (25 mM Tris [pH 7.5], 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2, 200 μΜ ATP) and 1 μg of glycogen synthase kinase 3 fusion protein for 30 min at 30°C. Akt kinase activity was determined by the amount of phosphorylated glycogen synthase kinase 3 fusion protein as detected by Western blot analysis with an antibody to phospho-glycogen synthase kinase 3 α/β.
Immunoprecipitation and Western blot analysis.
Total cell lysates were prepared as described for the PI3 kinase assay. Lysates (500 μg of protein) were incubated with antibodies to p85α, p110α, or phosphotyrosine overnight at 4°C and then with 60 μl of 50% protein A-agarose slurry for 2 h. The beads were washed four times with lysis buffer containing 150 mM NaCl and then boiled for 5 min in 30 μl of sample loading buffer. Immunoprecipitated proteins were separated by electrophoresis in sodium dodecyl sulfate-10% polyacrylamide gels and transferred onto nitrocellulose membranes (Osmonics). After blocking with phosphate-buffered saline containing 0.1% Tween 20 and 5% milk, the membranes were incubated with the antibodies indicated in the figures and detected by enhanced chemiluminescence reagent (NEN). Quantifications were performed by densitometric analysis with the NIH Image program (National Institutes of Health, Bethesda, Md.).
Endothelial cell migration and adhesion assays.
Endothelial cell migration was estimated in a Transwell with 24 wells (Costar) or a modified Boyden chamber with 48 wells (Neuro Probe). HAEC at passage 4 were grown to confluence in EGM-2 medium (Cambrex), and BAEC at passage 2 were grown to 90% confluence and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 0.4% fetal bovine serum (FBS) for 18 h before the experiment. Inhibitors were added 30 min prior to assays. Polyvinylpyrrolidone-free polycarbonate filters with a pore size of 8 μΜ were coated with 0.1% gelatin. Cells were briefly incubated with trypsin to obtain a single-cell suspension at 2 × 105 cells/ml in EGM-2 medium (for HAEC) or 0.4% FBS-DMEM (for BAEC). Then 2 × 104 cells (for Transwell) or 1 × 104 cells (for Boyden chamber) in suspension were added to the upper chamber with or without the inhibitors indicated in the figure legends. The bottom chamber was filled with 500 μl (for Transwell) or 26 μl (for Boyden chamber) of medium containing SNAP, 8-bromo-cyclic GMP (cGMP), or VEGF and inhibitors when indicated. The assembly was then incubated at 37°C to allow cell migration. After 4 h the cells were fixed and stained by Diff-Quik solutions (VWR).
Cells that did not migrate through the membrane were gently removed from the upper surface. Cell migration was scored in three (for Transwell) or four (for Boyden chamber) representative fields (magnification, 400×), and each group was carried out in triplicate (for Transwell) or quadruplicate (for Boyden chamber). For the cell adhesion assay, 100 μl of BAEC (2 × 104 cells) were seeded into 0.1% gelatin-coated 96-well dishes and incubated at 37°C. After 1 h, cells were washed with phosphate-buffered saline to remove unattached cells and stained. Cell attachment was quantified by measurement of absorbance at 590 nm with a microplate reader (Tecan). For the adenovirus-infected HAEC migration assay, cells at 90% confluency were incubated with the indicated adenovirus for 2 h before changing to fresh medium and incubated for another 18 h before the migration assay.
Endothelial cell proliferation assays.
BAEC (100 μl, 5,000 cells) were seeded into gelatin-coated 96-well plates and incubated in 0.4% FBS-DMEM at 37°C. After 24 or 72 h of incubation, cells were fixed and stained. The number of cells was quantified by measurement of absorbance at 590 nm with a microplate reader (Tecan).
Surgical induction of hind limb ischemia and virus administration.
Male Wistar rats (Harlan, Indianapolis, Ind.; approximately 200 g) were used for this study. Animals were anesthetized intraperitoneally with 90 mg of ketamine and 10 mg of xylazine per kg in phosphate-buffered saline. An incision was made along the inner left hind limb along the line of the femoral artery and vein. The proximal end of the femoral artery was tied with 4-0 silk suture (Ethicon) and electrocauterized. The femoral artery was dissected free from the limb and its peripheral branches. The distal end was severed, the artery was removed, and the skin was sutured closed. One week after surgery, rats were randomly divided into four groups. Group 1 was injected with 160 μl of control adenovirus (Ad.CMV-null), group 2 was injected with 160 μl of an equal mixture of adenovirus carrying eNOS cDNA (Ad.CMV-eNOS) and Ad.CMV-null, group 3 was injected with 160 μl of an equal mixture of adenovirus encoding dominant-negative PI3 kinase cDNA (Ad.CMV-dnPI3K) and Ad.CMV-eNOS, and group 4 was injected with 160 μl of an equal mixture of Ad.CMV-dnPI3K and Ad.CMV-null (virus stocks were 1011 PFU/ml in 10 mM Tris-Cl [pH 8.0]). Adenovirus injections were divided among four to five injection sites in the adductor and surrounding muscles.
Laser Doppler imaging.
A laser Doppler blood flow meter (Laser Doppler Perfusion Imager System; Lisca) was used to evaluate perfusion of both left (ischemic) and right (nonischemic) rat hind limb. The perfusion signal was subdivided into six different intervals, each displayed as a separate color. Low or no perfusion is displayed as dark blue, whereas the highest perfusion interval is displayed as red. The stored perfusion values behind the color-coded pixels remain available for data analysis. Excess hair was removed from the hind limb with a shaver. Animals were placed on a heating plate at 37°C to minimize variations in temperature. After recording the laser Doppler images, the average perfusion values of the ischemic and nonischemic limbs were calculated on the basis of colored histogram pixels. To minimize variables, including ambient light and temperature, calculated perfusion was expressed as the ratio of left (ischemic) to right (nonischemic) hind limb perfusion in each animal. Perfusion analyses were performed immediately after surgery and for 4 consecutive weeks thereafter.
Immunostaining of capillaries.
Rat hind limb skeletal muscle was excised 3 weeks after virus injection, fixed in 4% phosphate-buffered saline-buffered formaldehyde, and embedded in paraffin. Sections (4 μM) were cut and immunostained (Vectastain ABC kit; Vector Labs) with antibody to platelet endothelial cell adhesion molecule (PECAM/CD31) (PharMingen). Capillaries were counted in 10 random fields of 0.25 mm2 each per rat.
Human eNOS immunostaining.
One rat per group was killed at each time point after gene transfer (1, 2, and 3 weeks), and rat adductor skeletal muscle was perfused with phosphate-buffered saline followed by 10% buffered formaldehyde via cardiac injection. Paraffin-embedded sections were stained with a monoclonal antibody specific for human eNOS (Transduction Laboratories) with the Dako Envision immunostaining kit according to the manufacturer's instructions.
Determination of phospho-Akt (S473) and VEGF protein levels in ischemic hind limb.
Tissue samples (skeletal muscle from ischemic hind limb), obtained at day 4 after adenovirus infection, were homogenized in lysis buffer containing protease inhibitors. Phospho-Akt (S473) and VEGF levels in the lysates were determined by Western blot. As a protein loading control, membranes were reprobed with antibodies against Akt (for phospho-Akt) or α-tubulin (for VEGF). Quantifications were performed by densitometric analysis with the NIH Image program.
RESULTS
Activation of Akt by NO.
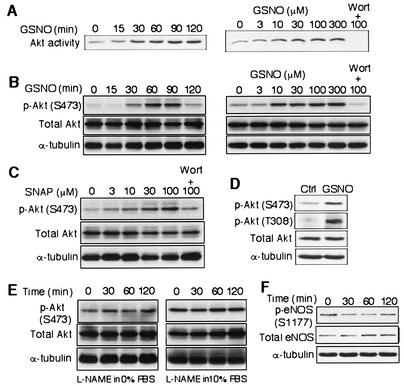
To test whether NO increases Akt kinase activity in endothelial cells (endothelial cell), human saphenous vein endothelial cells (HSVEC) were stimulated with the NO donor GSNO for various durations (Fig. 1A). Treatment with GSNO increased Akt kinase activity in a time-dependent manner (109%, 148%, 156%, 177%, and 180% increase for 15, 30, 60, 90, and 120 min, respectively, compared to time zero). Similarly, in a concentration-dependent manner, GSNO (treatment for 1 h) increased Akt kinase activity by 110%, 144%, 152%, 159%, and 165% at a GSNO concentration of 3, 10, 30, 100, and 300 μΜ, respectively, compared to no treatment (Fig. 1A). Cotreatment with the PI3 kinase inhibitor wortmannin abolished the induction of Akt kinase activity by GSNO (Fig. 1A).
FIG. 1.
NO increases Akt kinase activity and phosphorylation in endothelial cells. (A) Akt kinase activity was measured after HSVEC were stimulated with GSNO (100 μΜ) for the indicated times (left) and concentrations for 1 h (right). As a control experiment, HSVEC was also treated for 30 min with wortmannin (Wort, 25 nm) before GSNO stimulation (right). (B) Akt phosphorylation at the Ser473 position was measured after BAEC were stimulated with GSNO at the indicated times (left) and concentrations for 1 h (right). BAEC was also treated for 30 min with wortmannin (Wort, 25 nm) before GSNO stimulation (right). (C) Akt phosphorylation at the Ser473 position was measured after BAEC were stimulated with SNAP at the indicated concentration for 1 h. BAEC was also treated for 30 min with wortmannin (Wort, 25 nm) before 100 μΜ SNAP stimulation. (D) Phosphorylation at the Ser473 (S473) and Thr308 (T308) positions of Akt was measured after stimulating BAEC with GSNO (300 μΜ). (E) BAEC were treated with l-nitroarginine methylester (L-NAME, 1 mM) in DMEM with 0% (left) or 10% (right) FBS for the indicated times and analyzed for Akt phosphorylation at the Ser473 position. The same blots were stripped and reprobed with antibodies to Akt (total Akt) and to α-tubulin to demonstrate equal protein loading. (F) BAEC were stimulated by GSNO (300 μΜ) and analyzed for the phosphorylation of eNOS at the Ser1177 position. The same blots were stripped and reprobed with antibodies to eNOS (total eNOS) and to α-tubulin to confirm protein loading.
Since activation of Akt kinase requires Ser/Thr phosphorylation (1), we examined the effects of NO on Akt serine phosphorylation. Treatment of bovine aortic endothelial cells (BAEC) with GSNO increased Akt phosphorylation (phospho-Akt; S473) in a time-dependent manner, producing 89%, 194%, 347%, 417%, and 151% increases after 15, 30, 60, 90, and 120 min, respectively. Similarly, in a concentration-dependent manner, GSNO increased phospho-Akt (S473) by 93%, 160%, 165%, 199%, and 208% at 3, 10, 30, 100, and 300 μΜ, respectively, which was inhibited by cotreatment with wortmannin (Fig. 1B).
BAEC treated with another NO donor, SNAP, also showed increases in Akt phosphorylation in a concentration-dependent manner (131% at 3 μΜ, 159% at 10 μΜ, 229% at 30 μΜ, and 275% at 100 μΜ), which was blocked by pretreatment with wortmannin (Fig. 1C). In addition, similar effects of NO donors and wortmannin on Akt phosphorylation were observed in endothelial cells from other vascular beds (i.e., HSVEC and HAEC) (data not shown). GSNO (100 μΜ, 1 h) also increased the phosphorylation of Akt on Thr308 by 266% compared to control or untreated cells (Fig. 1D).
Interestingly, we did not find that inhibiting endogenous eNOS activity by the NO synthase inhibitor l-nitroarginine methylester affected Akt kinase phosphorylation in either the absence (120% at 30 min, 87% at 60 min, and 109% at 120 min, not significant) or presence (92% at 30 min, 97% at 60 min, and 80% at 120 min, not significant) of serum (Fig. 1E). Although GSNO activated PI3 kinase and Akt, GSNO did not increase eNOS phosphorylation (Ser1177) (Fig. 1F).
Activation of PI3 kinase by NO.
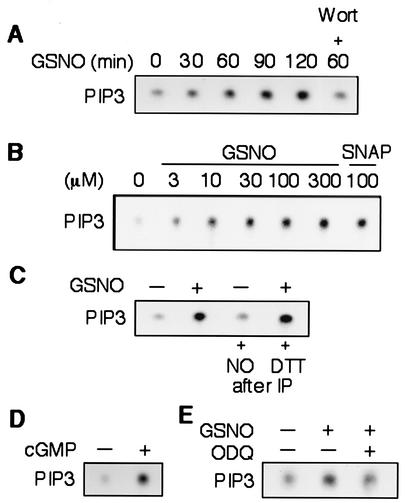
Since the activation of Akt is dependent upon PI3 kinase, we next examined the effect of NO on PI3 kinase activity. Treatment of HSVEC with GSNO (300 μΜ) increased PI3 kinase activity within 30 min, and the effect was sustained for at least 2 h (Fig. 2A). Cotreatment with wortmannin prevented GSNO-induced PI3 kinase activity. In a concentration-dependent manner, GSNO (3 to 300 μΜ) increased PI3 kinase activity, with maximal activity occurring at a GSNO concentration of 100 μΜ. The induction of PI3 kinase by NO was not limited to that released by GSNO, since the other NO donor, SNAP, also produced a similar effect (Fig. 2B). Furthermore, the induction of PI3 kinase activity by NO was not due to a direct effect such as nitrosylation of NO on PI3 kinase because incubating immunoprecipitated PI3 kinase with GSNO did not increase its activity and the addition of dithiothreitol to the immunoprecipitated PI3 kinase from NO-stimulated cells did not decrease the induced PI3 kinase activity (Fig. 2C).
FIG. 2.
NO increases PI3 kinase activity. (A) HSVEC were stimulated by GSNO (300 μΜ), and increases in phosphatidylinositol-3,4,5-trisphosphate (pip3) production were measured with and without wortmannin (Wort, 25 nm). (B) HSVEC were stimulated z increasing concentrations of GSNO (3 to 300 μΜ) or SNAP (100 μΜ) for 1 h, and cell lysates were subjected to the PI3 kinase activity assay. (C) HSVEC protein lysates after immunoprecipitating (IP) by an antibody to phosphotyrosine were treated with GSNO (300 μΜ) or with dithiothreitol (DTT, 1 mM) for 30 min before the PI3 kinase activity assay. Lysates from HSVEC stimulated by GSNO (300 μΜ) were used as a positive control. (D) PI3 kinase activity in HSVEC stimulated or not 8-bromo-cGMP (300 μΜ) for 1 h. (E) PI3 kinase activity in endothelial cells stimulated by GSNO in the presence or absence of the soluble guanylyl inhibitor ODQ (1 μΜ) for 1 h. Untreated cells were used as a negative control.
Since a major target of NO is the soluble guanylyl cyclase, we tested whether NO induced PI3 kinase activity via the cGMP-dependent pathway. Indeed, treatment of HSVEC with 8-bromo-cGMP (300 μΜ), a stable cGMP analogue, increased PI3 kinase activity (Fig. 2D). Moreover, we showed that the specific soluble guanylyl cyclase inhibitor ODQ (1 μΜ) was able to suppress the induction of PI3 kinase activity by NO (Fig. 2E).
NO increased endothelial cell migration via PI3 kinase.
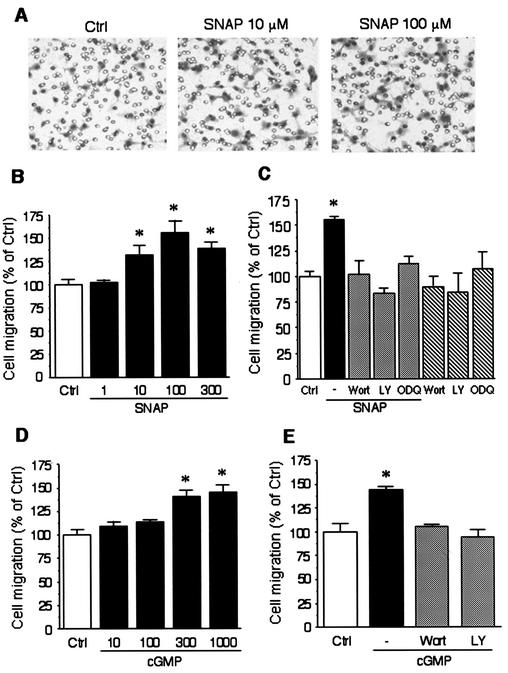
Since both PI3 kinase and NO are implicated in endothelial cell migration, we tested whether PI3 kinase mediates NO-induced endothelial cell migration. In HAEC, treatment with SNAP increased cell migration in a concentration-dependent manner (133 ± 10%, 156 ± 13%, and 138 ± 7% increase with 10, 100, and 300 μΜ SNAP, respectively, P < 0.05 compared to untreated cells) (Fig. 3A and B). When cells were pretreated with the PI3 kinase inhibitors wortmannin (25 nM) and LY294002 (10 μΜ) or a soluble guanylyl cyclase inhibitor ODQ (1 μΜ), NO-induced endothelial cell migration was greatly attenuated (Fig. 3C). Furthermore, treatment with 8-bromo-cGMP (10 to 1,000 μΜ) increased endothelial cell migration in a concentration-dependent manner (Fig. 3D), which was blocked by wortmannin (25 nM) and by LY294002 (10 μΜ) (Fig. 3E). These results suggested that the effect of NO on endothelial cell migration required cGMP-dependent activation of PI3 kinase. Because adhesion to the membrane filter may also influence endothelial cell transmigration, we investigated whether NO affects endothelial cell adhesion. Neither SNAP, 8-Br-cGMP, wortmannin, LY294002, nor ODQ affected endothelial cell adhesion compared to untreated cells.
FIG. 3.
NO promotes endothelial cell migration via PI3 kinase and cGMP-dependent protein kinase. (A) HAEC migration through the membrane filter in response to 10 μΜ and 100 μΜ SNAP was analyzed in a Boyden chamber. Original magnification, 200×. (B) Cell migration was measured in BAEC (percent of control) treated with increasing concentrations of SNAP (1 to 300 μΜ). (C) Migration was measured in BAEC treated with SNAP (100 μΜ) in the presence or absence of the PI3 kinase inhibitors wortmannin (Wort, 25 nm) and LY294002 (LY, 10 μΜ) or the soluble guanylyl cyclase inhibitor ODQ (1 μΜ). (D) Cell migration was measured in BAEC (percent of control) treated with increasing concentrations of 8-bromo-cGMP (cGMP, 10 to 1000 μΜ). (E) Migration was measured in BAEC treated with 8-bromo-cGMP (300 μΜ) in the presence or absence of wortmannin (Wort, 25 nm) or LY294002 (LY, 10 μΜ). Asterisks indicate a statistically significant difference (P < 0.05) between the treated cells and untreated cells (control, Ctrl). The migration data presented are shown as mean ± standard error of the mean. Each experiment was performed in quadruplicate.
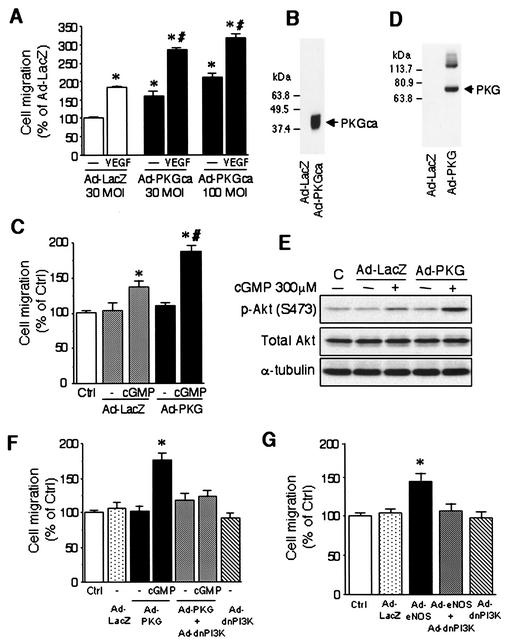
To demonstrate that cGMP-dependent protein kinase G (PKG) activity is directly involved in NO-induced endothelial cell migration, we next infected HAEC cells with an adenovirus expressing a constitutively active form of PKG (Ad-PKGca). Compared with cells infected with the control adenovirus expressing β-galactosidase (Ad-LacZ) at a multiplicity of infection (MOI) of 30, HAEC infected with Ad-PKGca at 30 and 100 MOI showed significant increases in migration (163 ± 13% and 210 ± 12%, respectively), an effect that was comparable to VEGF stimulation (184 ± 4%) (Fig. 4A). The combination of Ad-PKGca and VEGF further enhanced endothelial cell migration (286 ± 6% and 319 ± 9% at 30 and 100 MOI of Ad-PKGca, respectively), suggesting an additive effect between PKG and VEGF (Fig. 4A). Furthermore, BAEC infected with an adenovirus expressing a wild type PKG1α (Ad-PKG) also augmented 8-Br-cGMP-induced endothelial cell migration (Fig. 4C).
FIG. 4.
PI3 kinase and Akt kinase activities are required for eNOS- and cGMP-dependent protein kinase-promoted endothelial cell migration. (A) HAEC were infected with either a control adenovirus expressing β-galactosidase (Ad-LacZ) at 30 MOI or an adenovirus expressing a constitutively active form of the cGMP-dependent protein kinase (Ad-PKGca) at 30 or 100 MOI. Cell migration through the membrane filter in the presence or absence of VEGF (10 ng/ml) after 24 h is shown. Cell migration was quantified as described in Materials and Methods. The data are shown as mean ± standard error of the mean and expressed as a percent of that of the Ad-LacZ-infected cells without VEGF. Asterisks indicate a statistically significant difference (P < 0.05) from Ad-LacZ-infected cells without VEGF, and # indicates a statistically significant difference (P < 0.05) from Ad-LacZ-infected cells with VEGF. (B) PKG catalytic domain (≈42 kDa) transgene products were detected in lysates from HAEC infected with Ad-PKGca at 100 MOI. (C) BAEC were infected with Ad-LacZ or an adenovirus expressing a wild-type cGMP-dependent protein kinase 1α (Ad-PKG) at 50 MOI for 16 h before measurement of migration as described in the text. Cell migration through the membrane filter in the presence and absence of 8-bromo-cGMP was measured. The data are shown as mean ± standard error of the mean and expressed as a percentage of the control. Asterisks indicate a statistically significant difference (P < 0.05) from Ad-LacZ-infected cells without 8-bromo-cGMP, and # indicates a statistically significant difference (P < 0.05) from Ad-LacZ-infected cells with 8-bromo-cGMP. (D) PKG1α (≈76 kDa) transgene products were detected in lysates from BAEC infected with Ad-PKG. (E) BAEC were infected with Ad-LacZ or wild-type Ad-PKG at 50 MOI for 16 h. Akt phosphorylation at the Ser473 position was measured after BAEC were incubated in the presence or absence of 8-bromo-cGMP (300 μm) for 1 h. (F) BAEC were infected with Ad-LacZ (100 MOI), Ad-PKG (50 MOI) plus Ad-LacZ (50 MOI), Ad-PKG (50 MOI) plus Ad-dnPI3K (50 MOI), or Ad-dnPI3K (50 MOI) plus Ad-LacZ (50 MOI) for 16 h before measurement of migration as described in the text. Asterisks indicate a statistically significant difference (P < 0.05) from Ad-PKG-infected cells without 8-bromo-cGMP. (G) BAEC were incubated with Ad-LacZ (100 MOI), Ad-eNOS (50 MOI) plus Ad-LacZ (50 MOI), Ad-eNOS (50 MOI) plus Ad-dnPI3K (50 MOI), or Ad-dnPI3K (50 MOI) plus Ad-LacZ (50 MOI) for 16 h before measurement of migration as described in the text. Migration was quantified by counting cells which migrated through the membrane filter. The data are shown as mean ± standard error of the mean and expressed as a percentage of the control. Asterisks indicate a statistically significant difference (P < 0.05) from Ad-LacZ-infected cells.
Consistent with these results, infection with Ad-PKG augmented Akt phosphorylation induced by 8-Br-cGMP (phospho-Akt [S473]/total Akt, 150% at 300 μΜ 8-Br-cGMP in Ad-LacZ and 226% at 300 μΜ 8-Br-cGMP in Ad-PKG compared with Ad-LacZ without 8-Br-cGMP) (Fig. 4E). The expression of PKG in infected endothelial cells with Ad-PKGca (Fig. 4B) or Ad-PKG (Fig. 4D) was confirmed by immunoblotting for PKG. The promigratory effects of Ad-PKG and Ad-eNOS on endothelial cells were completely abolished by coinfection with Ad-dnPI3K but not Ad-LacZ (Fig. 4F and 4G), indicating that the promigratory effects of NO and PKG are mediated by PI3 kinase.
NO increased endothelial cell proliferation via PI3 kinase.
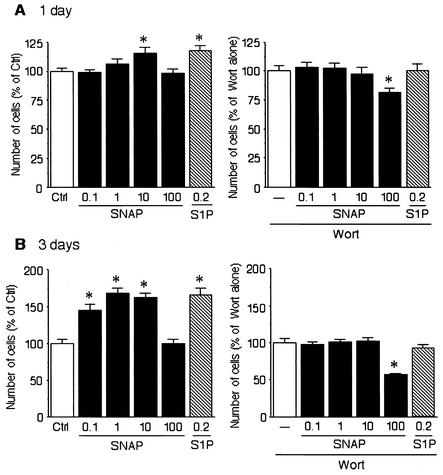
Since endothelial cell proliferation is an essential step in angiogenesis, we next investigated whether NO can enhance endothelial proliferation. BAEC were incubated with SNAP in DMEM including 0.4% FBS for 24 h (Fig. 5A) or 72 h (Fig. 5B). Sphingosine 1-phosphate was used as a positive control. Treatment of BAEC with SNAP (0.1, 1, and 10 μΜ for 72 h) enhanced endothelial cell proliferation (Fig. 5B, left), which was completely abolished by pretreatment with wortmannin (25 nM) (Fig. 5B, right). These findings suggest that both endothelial cell migration and proliferation are mediated by PI3 kinase. Interestingly, higher concentrations of SNAP (i.e., 100 μΜ) did not induce endothelial cell proliferation and, in conjunction with wortmannin, decreased endothelial cell proliferation below baseline values.
FIG. 5.
Enhancement of endothelial cell proliferation with SNAP is involved in PI3 kinase pathway. (A) BAEC were incubated with SNAP or sphingosine-1 phosphate (S1P) (left) or with wortmannin (Wort, 25 nM) (right) in DMEM with 0.4% FBS for 24 h. (B) BAEC were incubated with SNAP or shingosine 1-phosphate (left) or with wortmannin (Wort, 25 nM) (right) in DMEM with 0.4% FBS for 72 h. The data are shown as mean ± standard error of the mean and expressed as a percentage of the control (Ctrl) or the wortmannin-alone value. Asterisks indicate a statistically significant difference (P < 0.05) from control or wortmannin alone.
Adenovirus-mediated delivery of eNOS in vivo.
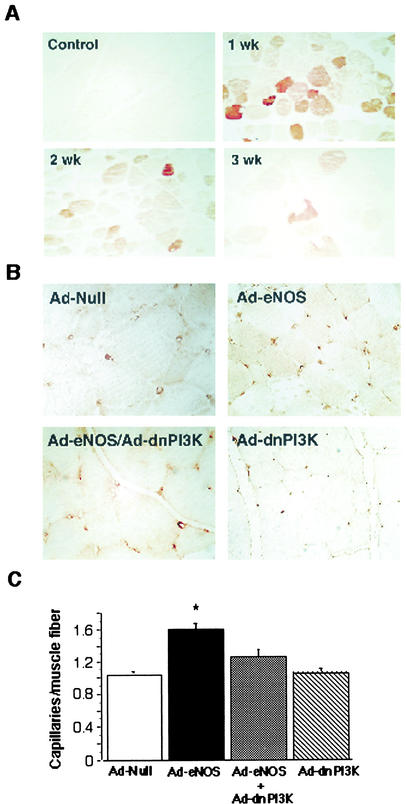
To determine the physiological relevance of NO-mediated endothelial cell migration, we performed adenoviral delivery of human eNOS in a rat hind limb ischemia model. In order to localize the expression of recombinant human eNOS followingAd.CMV-eNOS gene transfer in ischemic adductor muscles, we employed immunohistochemistry with an antibody against eNOS that specifically recognizes the human form. Rats were sacrificed (one per group) 1, 2, and 3 weeks after gene transfer. Strong reactivity was seen in adductor muscles 1 week after gene transfer, which diminished to weaker levels by week 3 (Fig. 6A). Human eNOS was not detected in control tissue from a noninjected contralateral limb.
FIG. 6.
Effect of eNOS gene transfer on capillary density in the ischemic hind limb of rats. (A) Paraffin-embedded sections were immunostained for eNOS expression as described in the text. Representative sections were taken from a control section (control) from the uninjected contralateral limb and 1, 2, and 3 weeks after eNOS gene transfer. (B) Adenovirus carrying eNOS, null, or dnPI3K was locally injected into the hind limb of rats after the femoral artery was removal. Three weeks later, capillary density was assessed by immunostaining paraffin-embedded sections with an antibody against PECAM-1 (CD31). Sections from injected rats with null, eNOS plus null, eNOS plus dnPI3K, and dnPI3K plus null viruses are shown. (C) Results were quantified, and data are expressed as capillaries/muscle fiber (mean ± standard error of the mean, n = 5 to 6 per group). *, P < 0.01 versus null or eNOS plus dnPI3K.
Increased capillary density by eNOS gene transfer is mediated by PI3 kinase.
To address the question of whether eNOS gene delivery increased the number of capillaries during ischemia, we measured the capillary density of skeletal muscle sections by immunostaining with antibody against CD-31. Representative sections are shown from rats injected with Ad.CMV-null (Ad-null), Ad.CMV-eNOS/Ad.CMV-null (Ad-eNOS), Ad.CMV-eNOS/Ad.CMV-dnPI3K (Ad-eNOS/Ad-dnPI3K), and Ad.CMV-null/Ad.CMV-dnPI3K (Ad-dnPI3K) (Fig. 6B). Quantitative analysis showed a significant increase in capillary density in rats receiving Ad-eNOS (relative to null adenovirus) that increased until week 3, which was antagonized by concomitant administration of adenovirus carrying the dominant-negative PI3 kinase gene (Ad-dnPI3K) (Fig. 6C).
Interestingly, the perfusion and capillary density of the group that received Ad-dnPI3K without Ad.CMV-eNOS was not significantly different from that of the group receiving Ad.null or the Ad.CMV-eNOS/Ad-dnPI3K combination. The adenovirus injections were performed 1 week after surgery; and during that week, the majority of spontaneous angiogenesis occurred. However, there was a residual amount of natural recovery from weeks 1 through 3 which was not affected by Ad-dnPI3K administration. These results indicate that delivery of the eNOS gene leads to increases in the number of capillaries formed in the ischemic hind limb, a process which is dependent upon PI3 kinase activation.
Laser Doppler perfusion imaging.
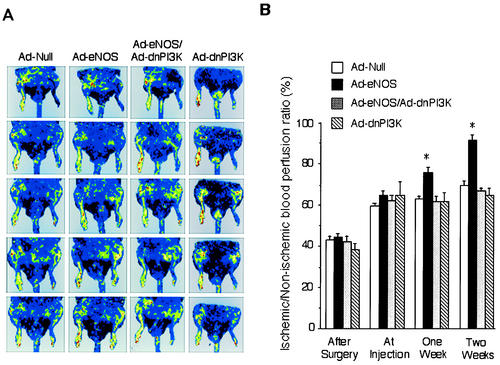
To determine the physiological consequences of increased capillary density, we measured the ability of eNOS gene transfer to augment blood perfusion in the ischemic hind limb of rats by laser Doppler imaging. This noninvasive technique allows blood flow to be assessed at multiple time points. Blood perfusion recovery was measured weekly, and body temperature was maintained by keeping animals on a heating pad. By 1 week after gene transfer, quantitative analysis showed a significant increase in blood flow in rats receiving Ad-eNOS (relative to null adenovirus) that increased until week 3 and was blocked by concomitant administration of adenovirus carrying the dominant-negative PI3 kinase gene (Ad-dnPI3K) (Fig. 7A). There was no significant difference between Ad-null and Ad-dnPI3K. Results were quantified and expressed as recovery of ischemic to normoperfused limb (eNOS/null, 94.3% ± 1.6% versus 75.1% ± 2.0% and 75.9% ± 1.7%, null and eNOS/dnPI3K, respectively, P < 0.01, n = 6 to 8, at week 3) (Fig. 7B).
FIG. 7.
Regional blood flow in the rat ischemic hind limb after gene transfer. Rats underwent unilateral femoral artery ligation and removal. One week after surgery, rats were locally injected (adductor muscle) with control adenovirus (Ad.CMV-null), an equal mixture of adenoviruses carrying the eNOS gene (Ad.CMV-eNOS) and null virus, an equal mixture of Ad.CMV-eNOS and adenovirus carrying the dominant negative PI3 kinase gene (Ad.CMV-dnPI3K), or an equal mixture of dnPI3 kinase and null virus. (A) A laser Doppler perfusion imager system (Lisca) was used to evaluate perfusion of both the left (ischemic) and right (nonischemic) rat hind limbs. The perfusion signal is subdivided into six different intervals, each displayed as a separate color. Low or no perfusion is displayed as dark blue, whereas the highest perfusion interval is displayed as red. The stored perfusion values behind the color-coded pixels remain available for data analysis. (B) After recording the laser Doppler images, average perfusion values of the ischemic and nonischemic limbs were calculated on the basis of colored histogram pixels. To minimize variables, including ambient light and temperature, calculated perfusion was expressed as the ratio of left (ischemic) to right (nonischemic) hind limb perfusion in each animal. Blood flow was monitored weekly for 3 weeks after adenovirus injection. Results were quantified, and data are expressed as the ischemic/nonischemic blood perfusion ratio (mean ± standard error of the mean, n = 6 to 8 per group). *, P < 0.01 versus null or eNOS plus dnPI3K.
Phospho-Akt and VEGF expression in ischemic hind limb of rats.
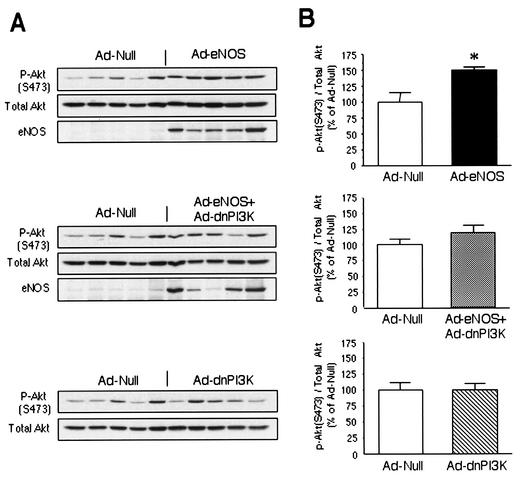
To determine whether Akt or VEGF is involved in the proangiogenic effect of NO, we measured phospho-Akt (S473) and VEGF expression in the adductor muscle at day 4 after gene transfer. Ad-eNOS gene transfer increased the phospho-Akt level (151 ± 4% compared to Ad-null, P < 0.05) (Fig. 8A and 8B, upper). In contrast, there was no significant change in animals treated with Ad-eNOS plus Ad-dnPI3K or Ad-dnPI3K alone compared to Ad-null (Fig. 8B). These results indicate that both NO- and VEGF-induced angiogenesis is mediated by PI3 kinase. However, the angiogenic effect of NO is not mediated by VEGF, since Ad-eNOS gene transfer did not affect VEGF protein levels (Ad-eNOS, 96 ± 8%; Ad-eNOS plus Ad-dnPI3K, 103 ± 15%; and Ad-dnPI3K, 100 ± 20%, P > 0.05 compared with Ad-null).
FIG. 8.
Phospho-Akt levels in rat ischemic hind limb after gene transfer. (A) Phospho-Akt (S473), Akt, and eNOS protein levels in lysates from rats infected with Ad-null, Ad-eNOS plus Ad-null, Ad-eNOS plus Ad-dnPI3K, or Ad-dnPI3K plus Ad-null. (B) Quantifications were performed by densitometric analysis with the NIH Image program. The data are shown as the phospho-Akt (S473)/Akt ratio and expressed as a percentage of that of Ad-null-infected rats (mean ± standard error of the mean, n = 5). Asterisks indicate a statistically significant difference (P < 0.05) from Ad-null-infected rats.
DISCUSSION
Mounting evidence exists to support the concept that nitric oxide mediates angiogenesis (2, 30, 32, 36). However, the molecular mechanisms that govern this action remain unclear. It is well known that Akt/protein kinase B lies downstream of PI3 kinase. Akt phosphorylates eNOS (11, 16), which leads to elevated NO formation and subsequent endothelial cell growth and migration (10, 24). In this study, we found that NO itself may directly activate PI3 kinase-Akt signaling, leading to angiogenesis in a rat model of hind limb ischemia. Consistent with a recent study (32), we show that adenovirus-mediated delivery of eNOS cDNA promotes neovascularization in ischemic hind limbs, as evidenced by increased blood perfusion recovery and capillary density. However, delivery of adenovirus carrying the dominant negative PI3 kinase cDNA construct (Ad.CMV-dnPI3K) either alone or in combination with eNOS was able to block the increased perfusion recovery and capillary density in ischemic limbs of rats. Thus, these results indicate that PI3 kinase mediates a critical pathway in NO- and VEGF-mediated angiogenesis. These in vivo results demonstrate that elevated eNOS expression or activity is sufficient to activate the PI3 kinase-Akt signaling pathway via PKG, leading to functional neovascularization in ischemic tissues.
NO exerts many important effects on the vascular wall in addition to its vasodilatory effect. These include suppressing the inflammatory responses induced by various cytokines (33), inhibiting apoptosis (13), and regulating cell migration and angiogenesis (25, 41). Previous studies have shown that the PI3 kinase/Akt signaling pathway activates eNOS (11, 16). It is therefore intriguing that exogenous NO increased PI3 kinase and Akt activity, since such an effect could potentially form a positive feedback mechanism for PI3 kinase, Akt, and eNOS activation in endothelial cells. Interestingly, we did not find that the activating effects of NO on PI3 kinase and Akt led to eNOS activation, as the Akt-dependent phosphorylation on eNOS (Ser1177) was not increased by NO stimulation. These results suggest that NO may interfere with Akt-dependent eNOS phosphorylation or promote “dephosphorylation” of eNOS. Indeed, previous studies have shown that NO can feedback inhibit eNOS activity (5, 18, 29). Furthermore, we found that basal Akt kinase phosphorylation was not affected by inhibiting endogenous eNOS activity, suggesting that endogenous PI3 kinase and Akt activities were not modulated by basal eNOS activity, probably due to an insufficient amount of NO produced by basal eNOS activity. It is possible that this lack of a positive feedback loop is due to a discriminating signal transduction by Akt to its many downstream effectors or to the different subcellular localization and availability of exogenous versus endogenous sources of NO.
Angiogenesis is a complex process involving endothelial cell proliferation, migration, remodeling of extracellular matrix, and formation of tubular structures (6, 26). Endothelial cell migration requires both increased cell motility and chemotaxis that guides the direction of cell movement. The endothelial cell migration assay employed in our studies detects both increased cell migration and motility. Therefore, although a rapid NO diffusion across the membrane after its release by NO donors may prevent the formation of an NO gradient to induce a directional cell migration, NO may increase endothelial cell motility and result in increased cell redistribution across the membrane. This mechanism is consistent with the finding that endothelial cells expressing the constitutive form of PKG showed an increased migration across the porous membrane in the absence of any stimulus.
Our findings showing that NO increased endothelial cell migration/motility and proliferation are consistent with the ability of NO to promote angiogenesis in a rabbit cornea model of angiogenesis and that angiogenesis is impaired in eNOS−/− mice (25, 40, 41). Furthermore, although both Akt and eNOS activities are required for VEGF-induced endothelial cell migration (23), only Akt activity is required for sphingosine-1 phosphate-induced endothelial cell migration (24). Therefore, the activation of PI3 kinase/Akt activities and promotion of endothelial cell migration by NO agree with the notion that PI3 kinase/Akt signal activation is essential for endothelial cell angiogenic response. However, depending on the external stimuli, the activation of eNOS by Akt can be dispensable for endothelial cell migration. It should be noted that exogenous NO has been reported to inhibit endothelial cell migration (20) and failed to restore spontaneous angiogenesis in the ischemic limb of eNOS−/− mice (25). It is possible that in those experiments, the older passages of endothelial cells responded differently to higher concentrations of NO donors in vitro and that the direct systemic administration of an NO donor could not elicit an optimal local pharmacological response in vivo. Indeed, recent studies suggest that NO donors increase endothelial cell migration (28, 39).
NO can directly modulate protein functions by S-nitrosylation on susceptible cysteine residues (4). This results in the inhibition of endothelial cell apoptosis and NF-κB-dependent gene transcription by inactivating caspases and the p50 subunit of NF-κB (12, 22). However, we did not find that NO directly activated PI3 kinase in vitro, nor did we find that dithiothreitol, which removes protein nitrosylation, suppressed NO-induced PI3 kinase activity. Therefore, NO does not appear to activate PI3 kinase through nitrosylation. Instead, our data support the idea that NO activates PI3 kinase via the cGMP-dependent pathway. Interestingly, we recently also found that NO increased FLICE inhibitory protein (FLIP) expression in endothelial cells and that this increase in FLIP protein expression was suppressed by wortmannin (unpublished observations). These findings are in agreement with a recent report showing that PI3 kinase/Akt signaling suppresses endothelial cell apoptosis by regulating antiapoptotic FLIP expression in endothelial cells (35).
A previous study suggests that tyrosine phosphorylation of p85α leads to the activation of PI3 kinase (7). Compared to unstimulated cells, we did find more p85α and p110α proteins immunoprecipitated by an antibody to phosphotyrosine in NO-stumulated BAEC in Western blot analysis (data not shown). However, when we performed the reverse experiment by first immunoprecipitating p110α or p85α followed by Western blot analysis with phosphotyrosine antibody, neither p110α nor p85α had detectable tyrosine phosphorylation before or after NO stimulation even though these two proteins were immunoprecipitated, as confirmed in subsequent Western blot analyses (data not shown). We speculate that NO/cGMP may act through a tyrosine-phosphorylated adaptor protein to increase the PI3 kinase activity.
Our results support an important role of NO in maintaining vascular integrity by increasing endothelial cell motility and promoting endothelial cell migration during wound healing and in response to tissue ischemia (25, 40). Furthermore, our findings have implications for certain pathophysiological conditions where high local concentrations of NO are produced by the inducible NO synthase in vascular smooth muscle cells, inflammatory macrophages and monocytes, and some tumor cells. Indeed, we have shown that activated macrophages produced NO at a level comparable to that used in the current study (27, 33). It remains to be determined whether in a paracrine or juxtacrine system, high levels of NO secreted from vascular smooth muscle cells and macrophages could actually promote angiogenesis at sites of vascular inflammation (27). It is also interesting that clinical and experimental studies support a positive relationship between tumor malignancy and NO synthase activity in certain tumors (37). Thus, it is possible that tumor-derived NO induces endothelial cell migration and contributes to the angiogenesis required for tumor cell invasiveness.
In summary, we have shown that NO increases endothelial cell migration and produces functional neovascularization via the PI3 kinase/Akt signaling pathway. Further studies aimed at elucidating the molecular mechanism(s) by which PKG activates the PI3 kinase/Akt signaling pathway may help uncover novel therapeutic strategies for regulating the angiogenic process.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL52233, HL48743, HL70274, DK62729, HL67574, and HL29397) and the American Heart Association Bugher Foundation.
We are grateful to M. Kasuga (Kobe University, Kobe, Japan) for providing Ad-dnPI3K. We also thank Y.-T. Tai (Dana Farber Cancer Institute, Boston, Mass.) for providing Ad-β-Gal and R. E. Pratt (Brigham and Women's Hospital, Boston, Mass.) for Ad-PKGca and Ad-PKG1α.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Babaei, S., and D. J. Stewart. 2002. Overexpression of endothelial NO synthase induces angiogenesis in a coculture model. Cardiovasc. Res. 55:190-200. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C. 2001. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 11:66-75. [DOI] [PubMed] [Google Scholar]
- 4.Broillet, M. C. 1999. S-nitrosylation of proteins. Cell. Mol. Life Sci. 55:1036-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buga, G. M., J. M. Griscavage, N. E. Rogers, and L. J. Ignarro. 1993. Negative feedback regulation of endothelial cell function by nitric oxide. Circ. Res. 73:808-812. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet, P. 2000. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6:389-395. [DOI] [PubMed] [Google Scholar]
- 7.Cuevas, B. D., Y. Lu, M. Mao, J. Zhang, R. LaPushin, K. Siminovitch, and G. B. Mills. 2001. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J. Biol. Chem. 276:27455-27461. [DOI] [PubMed] [Google Scholar]
- 8.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 9.Denninger, J. W., and M. A. Marletta. 1999. Guanylate cyclase and the ·NO/cGMP signaling pathway. Biochim. Biophys. Acta 1411:334-350. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler, S., E. Dernbach, and A. M. Zeiher. 2000. Phosphorylation of the endothelial nitric oxide synthase at Ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 477:258-262. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler, S., I. Fleming, B. Fisslthaler, C. Hermann, R. Busse, and A. M. Zeiher. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601-605. [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler, S., J. Haendeler, M. Nehls, and A. M. Zeiher. 1997. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 185:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimmeler, S., and A. M. Zeiher. 1999. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 6:964-968. [DOI] [PubMed] [Google Scholar]
- 14.Folkman, J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1:27-31. [DOI] [PubMed] [Google Scholar]
- 15.Fukumura, D., T. Gohongi, A. Kadambi, Y. Izumi, J. Ang, C. O. Yun, D. G. Buerk, P. L. Huang, and R. K. Jain. 2001. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA 98:2604-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulton, D., J. P. Gratton, T. J. McCabe, J. Fontana, Y. Fujio, K. Walsh, T. F. Franke, A. Papapetropoulos, and W. C. Sessa. 1999. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooch, K. J., C. A. Dangler, and J. A. Frangos. 1997. Exogenous, basal, and flow-induced nitric oxide production and endothelial cell proliferation. J. Cell Physiol. 171:252-258. [DOI] [PubMed] [Google Scholar]
- 18.Griscavage, J. M., A. J. Hobbs, and L. J. Ignarro. 1995. Negative modulation of nitric oxide synthase by nitric oxide and nitroso compounds. Adv. Pharmacol. 34:215-234. [DOI] [PubMed] [Google Scholar]
- 19.Janssens, S. P., A. Shimouchi, T. Quertermous, D. B. Bloch, and K. D. Bloch. 1992. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J. Biol. Chem. 267:14519-14522. [PubMed] [Google Scholar]
- 20.Lau, Y. T., and W. C. Ma. 1996. Nitric oxide inhibits migration of cultured endothelial cells. Biochem. Biophys. Res. Commun. 221:670-674. [DOI] [PubMed] [Google Scholar]
- 21.Liao, J. K., W. S. Shin, W. Y. Lee, and S. L. Clark. 1995. Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J. Biol. Chem. 270:319-324. [DOI] [PubMed] [Google Scholar]
- 22.Marshall, H. E., and J. S. Stamler. 2001. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 40:1688-1693. [DOI] [PubMed] [Google Scholar]
- 23.Morales-Ruiz, M., D. Fulton, G. Sowa, L. R. Languino, Y. Fujio, K. Walsh, and W. C. Sessa. 2000. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 86:892-896. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Ruiz, M., M. J. Lee, S. Zollner, J. P. Gratton, R. Scotland, I. Shiojima, K. Walsh, T. Hla, and W. C. Sessa. 2001. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J. Biol. Chem. 276:19672-19677. [DOI] [PubMed] [Google Scholar]
- 25.Murohara, T., T. Asahara, M. Silver, C. Bauters, H. Masuda, C. Kalka, M. Kearney, D. Chen, J. F. Symes, M. C. Fishman, P. L. Huang, and J. M. Isner. 1998. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 101:2567-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murohara, T., B. Witzenbichler, I. Spyridopoulos, T. Asahara, B. Ding, A. Sullivan, D. W. Losordo, and J. M. Isner. 1999. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 19:1156-1161. [DOI] [PubMed] [Google Scholar]
- 27.Peng, H. B., M. Spiecker, and J. K. Liao. 1998. Inducible nitric oxide: an autoregulatory feedback inhibitor of vascular inflammation. J. Immunol. 161:1970-1976. [PubMed] [Google Scholar]
- 28.Purdie, K. J., G. S. Whitley, A. P. Johnstone, and J. E. Cartwright. 2002. Hepatocyte growth factor-induced endothelial cell motility is mediated by the upregulation of inducible nitric oxide synthase expression. Cardiovasc. Res. 54:659-668. [DOI] [PubMed] [Google Scholar]
- 29.Ravichandran, L. V., R. A. John, and A. Rengasamy. 1995. Direct and reversible inhibition of endothelial nitric oxide synthase by nitric oxide. Am. J. Physiol. 268:H2216-H2223. [DOI] [PubMed] [Google Scholar]
- 30.Rikitake, Y., K. Hirata, S. Kawashima, M. Ozaki, T. Takahashi, W. Ogawa, N. Inoue, and M. Yokoyama. 2002. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 22:108-114. [DOI] [PubMed] [Google Scholar]
- 31.Simoncini, T., A. Hafezi-Moghadam, D. P. Brazil, K. Ley, W. W. Chin, and J. K. Liao. 2000. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, R. S., Jr., K. F. Lin, J. Agata, L. Chao, and J. Chao. 2002. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hind limb ischemia. Arterioscler. Thromb. Vasc. Biol. 22:1279-1285. [DOI] [PubMed] [Google Scholar]
- 33.Spiecker, M., H. B. Peng, and J. K. Liao. 1997. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J. Biol. Chem. 272:30969-30974. [DOI] [PubMed] [Google Scholar]
- 34.Stuehr, D. J. 1999. Mammalian nitric oxide synthases. Biochim. Biophys. Acta 1411:217-230. [DOI] [PubMed] [Google Scholar]
- 35.Suhara, T., T. Mano, B. E. Oliveira, and K. Walsh. 2001. Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP). Circ. Res. 89:13-19. [DOI] [PubMed] [Google Scholar]
- 36.Tamarat, R., J. S. Silvestre, N. Kubis, J. Benessiano, M. Duriez, M. deGasparo, D. Henrion, and B. I. Levy. 2002. Endothelial nitric oxide synthase lies downstream from angiotensin II-induced angiogenesis in ischemic hind limb. Hypertension 39:830-835. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen, L. L., and D. W. Miles. 1998. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev. 17:107-118. [DOI] [PubMed] [Google Scholar]
- 38.Toker, A., and A. C. Newton. 2000. Cellular signaling: pivoting around PDK-1. Cell 103:185-188. [DOI] [PubMed] [Google Scholar]
- 39.Urbich, C., A. Reissner, E. Chavakis, E. Dernbach, J. Haendeler, I. Fleming, A. M. Zeiher, M. Kaszkin, and S. Dimmeler. 2002. Dephosphorylation of endothelial nitric oxide synthase contributes to the antiangiogenic effects of endostatin. FASEB J. 16:706-708. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki, K., H. D. Edington, C. McClosky, E. Tzeng, A. Lizonova, I. Kovesdi, D. L. Steed, and T. R. Billiar. 1998. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J. Clin. Investig. 101:967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziche, M., L. Morbidelli, E. Masini, S. Amerini, H. J. Granger, C. A. Maggi, P. Geppetti, and F. Ledda. 1994. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 94:2036-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziche, M., A. Parenti, F. Ledda, P. Dell'Era, H. J. Granger, C. A. Maggi, and M. Presta. 1997. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ. Res. 80:845-852. [DOI] [PubMed] [Google Scholar]