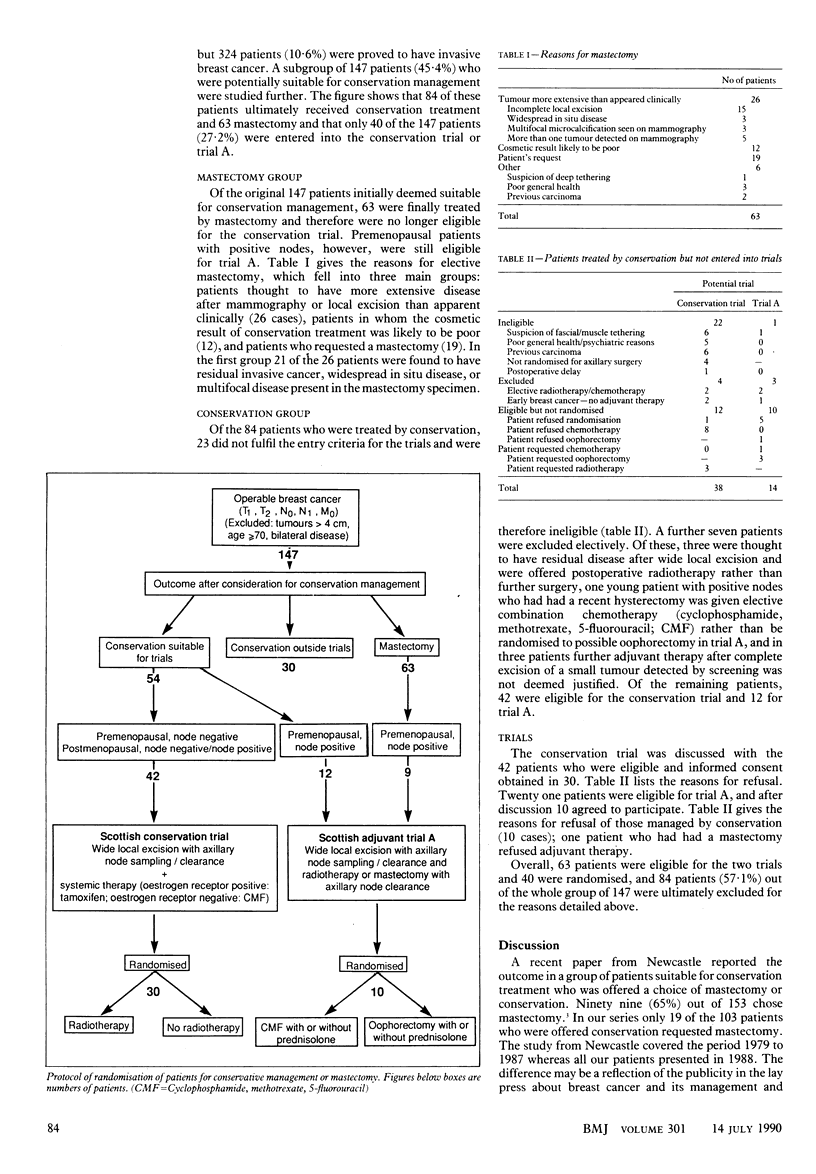
Abstract
OBJECTIVE--To investigate the rate of recruitment to early breast cancer trials and elucidate the reasons for ineligibility and refusal to participate among patients otherwise suitable for these trials. DESIGN--Prospective study of one year's cohort of patients referred to a breast unit with special reference to the subgroup suitable for conservation management and to the proportion eligible for and (after informed consent) ultimately randomised within the Scottish early breast cancer trials. SETTING--The breast unit, Longmore Hospital, Edinburgh, during 1988. PATIENTS--All 3054 patients referred to the breast unit during the year. 324 Found to have invasive breast cancer and 147 initially thought suitable for conservation management. RESULTS--63 Patients were treated by mastectomy, 19 of whom requested mastectomy rather than conservation management. 84 Patients were excluded from trials, and of the 63 eligible patients, 40 gave informed consent. Most of the 23 patients who refused the trials requested a specific adjuvant treatment after discussion of their management and the trials. CONCLUSIONS--Recruitment to prospective trials in which informed consent is required before randomisation may be slower than predicted because of a high proportion of exclusions and also refusal by patients. Trials may therefore take longer to complete and give distorted results by virtue of the unpredictable nature of the selection of patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum M., Zilkha K., Houghton J. Ethics of clinical research: lessons for the future. BMJ. 1989 Jul 22;299(6693):251–253. doi: 10.1136/bmj.299.6693.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahams D. Randomised trials and informed consent. Lancet. 1988 Oct 29;2(8618):1033–1034. doi: 10.1016/s0140-6736(88)90805-7. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Bradley C. Patient preferences and randomised clinical trials. BMJ. 1989 Jul 29;299(6694):313–315. doi: 10.1136/bmj.299.6694.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita V. T., Jr Breast cancer therapy: exercising all our options. N Engl J Med. 1989 Feb 23;320(8):527–529. doi: 10.1056/NEJM198902233200812. [DOI] [PubMed] [Google Scholar]
- Stewart H. J., Prescott R. J., Forrest P. A. Conservation therapy of breast cancer. Lancet. 1989 Jul 15;2(8655):168–169. doi: 10.1016/s0140-6736(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. G., Hart A., Dawes P. J. Mastectomy or conservation: the patient's choice. BMJ. 1988 Nov 5;297(6657):1167–1169. doi: 10.1136/bmj.297.6657.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]



