Abstract
Tumor necrosis factor receptor (TNFR)-associated factor 2 (TRAF2) is one of the key factors that mediate TNF signaling. The deletion of TRAF2 renders cells more sensitive to TNF-induced apoptosis. Although TRAF2 is known to be required for TNF-induced JNK and NF-κB activation, the underlying mechanism of the increased sensitivity of TRAF2 null cells (TRAF2−/−) to TNF-induced apoptosis is not fully understood. To study the underlying mechanism, we examined the difference in gene expression between TRAF2−/− and wild-type fibroblast cells by using microarray technology. We found that one of the genes whose expression was dramatically decreased in TRAF2−/− cells was the lung Krüppel-like factor (LKLF). Our results indicate that the expression of LKLF requires TRAF2 but is independent of TNF signaling. Although it appears that TRAF2 regulates the expression of the LKLF gene at the transcription level, TRAF2 does not function as a transcription factor itself. Our results suggest that TRAF2 regulates LKLF expression through the mitogen-activated protein kinase p38 pathway. More importantly, ectopic expression of LKLF in TRAF2−/− cells protected cells against TNF-induced apoptosis. These results reveal a novel aspect of TRAF2 function: by regulating the expression of genes, such as LKLF, TRAF2 controls cell sensitivity to apoptosis.
Tumor necrosis factor receptor (TNFR)-associated factor 2 (TRAF2) is one of the key effector molecules that mediate TNF-induced cellular responses (2, 7, 15, 37). TRAF2 was first cloned by biochemical characterization of intracellular factors that associated with TNFRII (29) but was also subsequently found in the receptor complex of a number of other TNFR family members, such as CD40, CD30, and TNFR1 (2, 37). In the case of TNFR1, TRAF2 is recruited to the TNFR1 complex by the TRADD adapter protein (16). As a member of the TRAF protein family, TRAF2 shares a conserved carboxyl-terminal domain, otherwise known as the TRAF domain, which mediates receptor binding, as well as homo- and hetero-oligomerization with other TRAF proteins (2, 37). There are six TRAF proteins, of which TRAF2, -3, -4, -5, and -6 have an amino-terminal ring finger domain followed by a string of four or five zinc finger motifs (2). TRAF1 has the zinc finger domain only (2). Because TRAF proteins do not carry a motif characteristic of any enzymatic activity, they appear to function solely as adapter molecules. The ring and zinc finger domains are thought to be essential for interacting with downstream targets (2). At least eight different intracellular molecules, including TRAF1, c-IAP1, c-IAP2, I-TRAF/TANK, A20, TRIP, RIP, and NIK, have been shown to directly interact with TRAF2 (2, 7, 15, 37). The interaction between TRAF2 and these proteins has been proposed to be important in modulating the function of TRAF2 as a signal transducer.
For TNF-induced activation of the transcription factor NF-κB, both the ring finger and the first two zinc finger domains are required (2, 4, 29, 32). Initially, it was proposed that TRAF2 mediates NF-κB activation via the recruitment of the serine/threonine kinase NIK (42), which can in turn activate IKK, an IκB-specific kinase that triggers IκB degradation (3, 11, 24). However, the possible involvement of NIK in this process has been ruled out by studying the effect of genetic deletion of NIK (44). More recently, Devin et al. found that the role of TRAF2 in TNF-induced NF-κB activation is to recruit IKK to the TNFR1 complex through direct interaction with IKKα and IKKβ, the two catalytic subunits of the kinase (9, 10). When IKK is activated, it phosphorylates IκBs. The phosphorylated IκBs will then be polyubiquitinated and rapidly degraded by the proteasome (3, 11). The degradation of IκBs leads to the release of NF-κB, allowing NF-κB to translocate into the nucleus and to activate its target genes, some of which may play a role in mediating the antiapoptotic effect of NF-κΒ (5, 22, 36, 38).
In addition to mediating NF-κB activation, TRAF2 also plays a critical role in TNF-induced c-Jun N-terminal kinase (JNK)/SAPK activation (8, 22, 26, 28). TRAF2 knockout mice appeared normal at birth, but they exhibited growth impairment and died prematurely. Examination of TRAF2−/− cells revealed a severe reduction in TNF-mediated JNK/SAPK activation (43). Despite some recent studies suggesting that JNK activation plays a role in TNF-induced apoptosis (34), TRAF2 null cells, including thymocytes, other hematopoietic progenitors, and fibroblasts, are highly sensitive to TNF-induced cell death (43). Impaired NF-κB activation may partially account for the supersensitivity of these cells to TNF (43); however, the underlying mechanism of this phenomenon is still elusive.
To shed new light on the role of TRAF2 in the regulation of TNF-induced apoptosis, we used microarray technology to search for a gene(s) whose expression had been altered in TRAF2 null fibroblasts. Among the several identified genes whose expression was affected by the absence of TRAF2, expression of the lung Krüppel-like factor (LKLF) (1) was drastically diminished in TRAF2−/− cells. When TRAF2 was reintroduced into TRAF2−/− fibroblast cells, the expression of LKLF was restored. Our data indicated that the regulation of LKLF expression by TRAF2 appears to be indirect and is through the mitogen-activated protein (MAP) kinase p38 pathway. More importantly, we found that the introduction of ectopic LKLF into the TRAF2−/− cells eliminated the supersensitivity of the TRAF2−/− cells to TNF-induced apoptosis and that this protective effect of LKLF is partially explained by the fact that it up-regulates the expression of insulin-like growth factor IGF-IR1. Taken together, our study suggests that LKLF is a critical effector of TRAF2 in modulating TNF-induced apoptosis.
MATERIALS AND METHODS
Reagents and plasmids.
Atlas mouse arrays were purchased from Clontech. Anti-LKLF antibody was a gift from Jeffrey M. Leiden (19). Anti-RIP and anti-JNK1 antibodies were purchased from Pharmingen. Anti-IGF-IRβ, IKKβ, and antihemagglutinin antibodies were from Santa Cruz. Anti-FLAG (M2) and anti-β-actin antibodies were from Sigma. The mammalian expression plasmids for TRAF2, TRAF2(87-501), TRAF1, TRAF3, TRAF4, TRAF5, and TRAF6 have been previously described (2, 22). TRAF2DZ was constructed by PCR to delete the zinc finger domain (amino acids 98 to 271). pBPV-hIGFIR and MKK6AA were kind gifts from D. LeRoith and J. Han, respectively (18, 39). LKLF(−2047)-Luc was described previously (31). p38 inhibitor SB203580, JNK inhibitor SP600125, and MEK inhibitor U0126 were purchased from Calbiochem.
Microarrays.
Microarray assay was conducted according to the manufacturer's instructions. Briefly, total RNA was prepared from TRAF2−/− and wild-type (WT) mouse fibroblast cells and used to make probes. Two microarray membranes were hybridized with the TRAF2−/− or WT probe and exposed to X-ray films. The differentially expressed genes were identified by comparison of the results of the hybridization with the two probes.
Cell culture and transfection.
HeLa, HEK 293, and mouse fibroblast cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Cells were transfected with Lipofectamine (Gibco) as described previously (21). Stably transfected LKLF or IGF-IR cell lines were selected with hygromycin from the TRAF2−/− cells that were transfected with LKLF or IGF-IR and a hygromycin-resistant gene plasmid.
Western blot analysis.
After treatment with different reagents as described in the figure legends, cells were collected and lysed in M2 lysis buffer (20 mM Tris [pH 7], 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, 1 μg of leupeptin per ml). Fifty micrograms of cell lysate from each sample was fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blotted. The proteins were visualized by using an enhanced chemiluminescence (ECL) kit (Amersham), according to the manufacturer's instructions (21). To isolate cell extract fractions, cells were homogenized with a Dounce homogenizer, and the cytosolic and nuclear fractions were prepared as described previously (12).
Northern blot analysis.
An LKLF cDNA probe was prepared with [α-32P]dCTP by using a random primer kit (Stratagene). Equal amounts of total RNAs from WT and TRAF2−/− cells were subjected to Northern blotting. The blots were hybridized with 32P-labeled probes for 18 h with ExpressHyb hybridization solution (Clontech), washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS at 68°C, and visualized by autoradiography. To control the relative amount of RNA in each lane, after hybridization with LKLF, blots were stripped by incubation in 0.5% SDS at 95°C and reprobed with a glyceraldehyde-3-phosphate dehydrogenase cDNA probe.
Luciferase assay.
Cells were cotransfected with pGL3-LKLF(−2047)-Luc, LacZ, and different TRAF expression constructs as indicated in the figure legends. Cells were collected, and luciferase assay was conducted as described previously (21).
Apoptosis assay.
Cells were plated in 12-well plates and treated with 20 ng of TNF per ml and 100 ng of cycloheximide (CHX) per ml for the indicated periods of time. Dead cells were determined by trypan blue staining (21). The results shown are averages of three independent experiments.
Kinase assays.
Mouse fibroblast cells (5 × 105) were treated with TNF as described in the figure legends. Cells were collected in 300 μl of M2 lysis buffer. IKK complex and JNK1 were immunoprecipitated with anti-IKKα and anti-JNK1 antibodies, respectively. IKK and JNK kinase activities were determined by using 2 μg of glutathione S-transferase (GST)-IκBα(1-54) or GST-c-Jun(1-79) as a substrate, respectively (20).
RESULTS
Expression of LKLF is impaired in TRAF2−/− cells.
TRAF2−/− fibroblast cells are much more sensitive to TNF-induced apoptosis than WT fibroblasts (43). Because NF-κB activation by TNF in TRAF2−/− cells is only partially decreased, the underlying mechanism for the increased sensitivity of TRAF2−/− cells to TNF is still largely unknown. However, since blocking gene expression by either RNA or protein synthesis inhibitor sensitizes cells to TNF, it is likely that the supersensitivity of TRAF2−/− cells to TNF is due to altered gene expression. To address this possibility, we decided to study the change of gene expression in TRAF2−/− cells by utilizing cDNA microarray technology. To accelerate our study, Atlas mouse arrays (Clontech) were used, since they contain only known genes. Total RNAs from WT and TRAF2−/− mouse fibroblast cells were used to prepare cDNA probes, which were then used to hybridize microarray membranes. By comparing the results of TRAF2−/− cells with those of WT cells, the change of gene expression in TRAF2−/− cells was determined. For the DNA microarray analysis, only genes induced or repressed more than threefold in at least two of three independent experiments were counted.
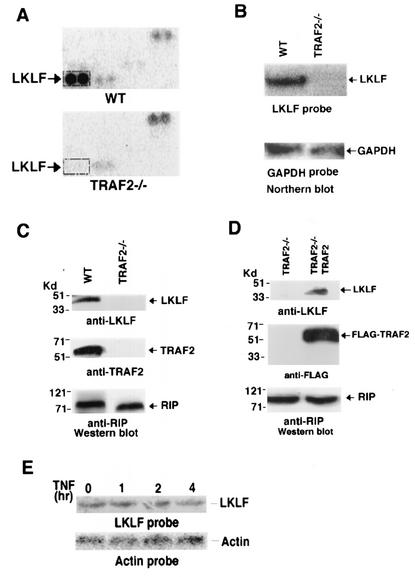
As shown in Fig. 1A, the lung Krüppel-like factor, LKLF (1), is a gene whose expression is dramatically decreased in TRAF2−/− cells. To confirm this finding, Northern blot analysis was performed with LKLF as a probe. Indeed, the results indicated that the mRNA level of LKLF was greatly decreased in TRAF2−/− cells (Fig. 1B). Furthermore, as shown in Fig. 1C, the LKLF protein in TRAF2−/− cells was barely detected by Western blotting with a specific anti-LKLF antibody (19). As a control, similar levels of RIP protein were detected in TRAF2−/− and WT cells (Fig. 1C). These results suggested that the expression of LKLF correlated with the status of the expression of TRAF2. To rule out the possibility that some other factor besides the absence of TRAF2 was responsible for the decrease in LKLF expression in TRAF2−/− cells, we tested whether LKLF expression could be reconstituted in the cells by ectopically expressing TRAF2. To do this, FLAG-TRAF2 was stably transfected into the TRAF2−/− cells (Fig. 1D). As shown in Fig. 1D, the expression of LKLF protein was restored in TRAF2−/− cells by ectopically expressing TRAF2. We also examined whether LKLF expression is regulated by TNF. TNF treatment had no detectable effect on LKLF mRNA level in WT cells (Fig. 1E), suggesting that the effect of TRAF2 on LKLF expression is independent of TNF signaling. Taken together, these results supported the notion that the expression of LKLF requires TRAF2.
FIG. 1.
Impaired LKLF expression in TRAF2−/− cells. (A) Two microarray filters were hybridized with the cDNA probes generated from RNAs from WT and TRAF2−/− mouse fibroblast cells. The results of a portion of the microarrays are shown. The spots of LKLF cDNA are indicated. (B) Equal amounts of total RNA from WT and TRAF2−/− cells were Northern blotted for LKLF with a LKLF cDNA probe. The equal input of RNA from each sample was verified with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. (C) Equal amounts of cell extracts from WT and TRAF2−/− cells were Western blotted for LKLF, TRAF2, and RIP proteins. RIP was used as a protein input control. (D) Equal amounts of cell extracts from TRAF2−/− cells and TRAF2−/− cells in which LKLF expression was reconstituted by ectopically expressing TRAF2 were Western blotted for LKLF, FLAG-TRAF2, and RIP proteins. RIP was used as a protein input control. In panels C and D, the positions of molecular mass markers (in kilodaltons) are shown to the left of the blots. (E) Equal amounts of total RNA from WT fibroblasts treated with TNF (30 ng/ml) for the indicated time points were Northern blotted with LKLF and actin probes.
TRAF2 indirectly regulates transcription of the LKLF gene.
To test the possibility that TRAF2 modulates LKLF promoter activity, we conducted reporter assays with an LKLF promoter-driven luciferase plasmid. The reporter construct, which contains the 2-kb sequence upstream of the LKLF gene and possesses full LKLF promoter activity (31), was cotransfected with different TRAF2 expression vectors into TRAF2−/− cells. Luciferase activity of each sample was measured as an indication of the LKLF promoter activity. As shown in Fig. 2A, cotransfection of the full-length TRAF2 augmented the LKLF promoter activity by 2.5-fold. Interestingly, the mutant TRAF2, TRAF2(87-501), which does not have the ring finger domain and functions as a dominant-negative mutant in TNF-induced NF-κB and JNK activation, activated the reporter as efficiently as the WT TRAF2 (Fig. 2A). In contrast, two other mutants of TRAF2, TRAF2DZ, in which the zinc finger domain was deleted, and TRAF2(272-501), in which both the ring and zinc finger domains were deleted, had little effect on the activity of the LKLF promoter. These results indicated that the zinc fingers, but not the ring finger, are required for TRAF2 to activate LKLF expression and also implied that the regulation of LKLF expression by TRAF2 is not linked to TNF signaling. The latter conclusion was further supported by the results that TNF treatment had no detectable effect on the activity of the LKLF promoter and that the expression of p65, a major NF-κB component, also had no effect on the LKLF promoter (Fig. 2B). Because six TRAF proteins have been identified (2), it is important to know whether other TRAF proteins also have the same effect on the LKLF promoter. To address this question, we performed reporter assays as described above with expression vectors of TRAF1, -2, -3, -4, -5, and -6. As shown in Fig. 2C, all TRAF proteins except TRAF2 failed to activate the LKLF promoter.
FIG. 2.
TRAF2 transactivates the LKLF promoter in TRAF2−/− cells. (A) TRAF2−/− mouse fibroblast cells were cotransfected with 0.5 μg of LKLF(−2047)-Luc, 0.2 μg of pRSV-LacZ, and 1 μg of an empty vector plasmid or the expression vector of TRAF2, TRAF2(87-501), TRAF2(272-501), or TRAF2DZ. Twenty-four hours after transfection, the cells were collected, and luciferase activity was detected and normalized to β-galactosidase activity. Data shown are the average values from three independent experiments. (B) TRAF2−/− cells were cotransfected with 0.5 μg of LKLF(−2047)-Luc, 0.2 μg of pRSV-LacZ, and 1 μg of an empty vector plasmid or the expression vector of TRAF2 or p65. Fourteen hours after transfection, one plate of cells transfected with the empty vector was treated with TNF (30 ng/ml) for 10 h. Cells were then collected for the luciferase assay, which was performed as described above for panel A. (C) As described above for panels A and B, TRAF2−/− cells were cotransfected with LKLF(12047)-Luc, pRSV-LacZ, and an empty vector or the expression vector of TRAF1, -2, -3, -4, -5, or -6. Luciferase activity of each transfection was determined as described above for panels A and B.
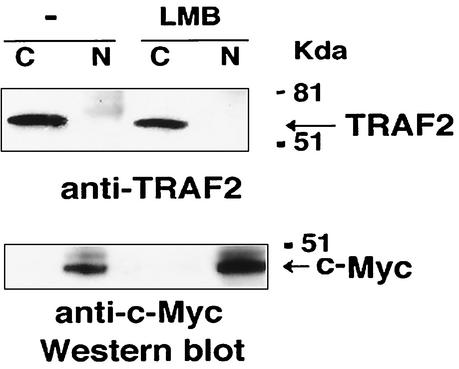
Since TRAF2 is a zinc finger protein and its zinc fingers are required for it to regulate transcription of the LKLF gene, we investigated the possibility that TRAF2 functions as a transcription factor by examining its cellular localization. As a critical effector protein of TNF signaling, TRAF2 is thought to be a cytoplasmic factor (16). To determine the cellular localization of TRAF2, we fractionated HeLa cell extracts into cytoplasmic and nuclear fractions and detected TRAF2 protein by Western blot analysis. To rule out the possibility that TRAF2 is a protein that shuttles between the nucleus and cytoplasm, we treated HeLa cells with the nuclear export inhibitor leptomycin B (LMB). Treating cells with LMB will result in the accumulation of nucleocytoplasmic shuttling proteins in the nucleus and easier detection of the trace nuclear proteins (13). As shown in Fig. 3, TRAF2 protein was detected only in cytoplasmic fractions. Detection of the nuclear protein Myc served as a control for fractionation. Also, we constructed a GFP-TRAF2 fusion protein and found it localized in the cytoplasm solely when it was ectopically expressed in the cells (data not shown). These results suggested that it is unlikely that TRAF2 acts as a transcription factor. Therefore, TRAF2 regulates transcription of the LKLF gene through other proteins.
FIG. 3.
TRAF2 localizes in the cytoplasm of the cells. HeLa cells were treated with LMB (10 μg/ml) for 15 h or were not treated (−). Nuclear (N) and cytoplasmic (C) fractions of the cells were isolated and Western blotted for TRAF2 or c-Myc, which was used as a control for nuclear proteins. The positions of molecular mass markers (in kilodaltons) and of TRAF2 and c-Myc proteins are indicated to the right of the blots.
Regulation of LKLF expression by TRAF2 involves p38 MAP kinase.
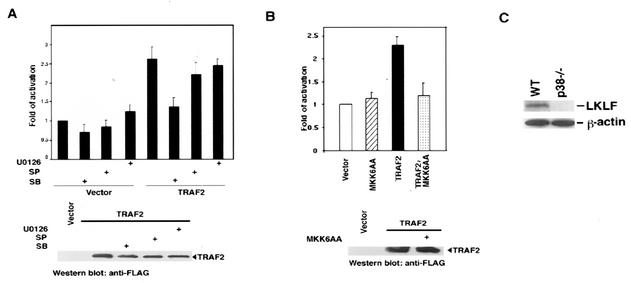
Since TRAF2 indirectly regulates transcription of LKLF, it is important to know the pathway that leads to transcriptional activation. Because TRAF2(87-501), a mutant TRAF2, activated the LKLF promoter and because, according to a recent report, it also activates p38 MAP kinase (17), we tested whether p38 MAP kinase is required for the expression of LKLF. When the p38 MAP kinase inhibitor SB203580 was used to treat the cells, as shown in Fig. 4A, induction of the LKLF promoter by TRAF2 was blocked. The expression levels of TRAF2 were not affected by SB203580 (Fig. 4A, bottom panel). In contrast, the JNK inhibitor SP600125 and MEK inhibitor U0126 had little effect on the induction of the LKLF promoter by TRAF2 (Fig. 4A). The requirement of p38 in LKLF expression by TRAF2 was further confirmed by using a dominant-negative mutant of MKK6, MKK6AA (39). As shown in Fig. 4B, MKK6AA also blocked TRAF2-induced activation of the LKLF promoter. Finally, we also checked the expression level of LKLF protein in p38 null (p38−/−) cells (33). In the p38−/− cells, the LKLF protein could not be detected (Fig. 4C). Taken together, these results indicated that TRAF2 regulates LKLF expression through the p38 MAP kinase pathway.
FIG. 4.
Transactivation of the LKLF promoter by TRAF2 requires p38 MAP kinase. (A) TRAF2−/− mouse fibroblast cells were cotransfected with 0.5 μg of LKLF(−2047)-Luc, 0.2 μg of pRSV-LacZ, and 1 μg of an empty vector or the expression vector of TRAF2. As indicated, some cells were treated (+) with SB203580 (SB) (20 μM), SP600125 (SP) (20 μM), or U0126 (5 μM). Cells were collected 24 h after transfection, and the luciferase assay was performed as described in the legend to Fig. 2. The expression of TRAF2 in TRAF2-transfected cells was detected with an anti-FLAG antibody. (B) TRAF2−/− mouse fibroblast cells were cotransfected with 0.5 μg of LKLF(−2047)-Luc, 0.2 μg of pRSV-LacZ, and 1 μg of the indicated plasmids. The luciferase assay was performed as described in the legend to Fig. 2. The expression of TRAF2 was detected with an anti-FLAG antibody. (C) The protein levels of LKLF in WT and p38−/− mouse fibroblast cells were detected by Western blotting. β-Actin was used as a protein input control.
Ectopic expression of LKLF in TRAF2−/− cells renders cells more resistant to TNF-induced cell death.
The mechanism of supersensitivity of TRAF2−/− cells to TNF-induced apoptosis is still unclear, although reduced NF-κB activation may be involved (43). Since our study suggested that LKLF expression is impaired in TRAF2−/− cells and LKLF exerts antiapoptotic properties in some types of cells (30), next we investigated whether reconstitution of LKLF expression in TRAF2−/− cells affected TNF-induced apoptosis. To do this, we established LKLF stably transfected cell lines, which express substantial levels of LKLF compared to WT fibroblasts. The expression of LKLF in two of these cell lines, LKLF clone 3 and clone 7, was shown in Fig. 5A. Normally, TRAF2−/− fibroblast cells are sensitive to TNF in the presence of a low concentration of CHX. As shown in Fig. 5B, LKLF clones 3 and 7 became more resistant to TNF in the presence of 100 or 500 ng of CHX per ml compared to the pHygromycin-transfected control TRAF2−/− cells. Similarly, the LKLF-transfected TRAF2−/− cells were also more resistant to higher TNF concentration than the control TRAF2−/− cells (Fig. 5C). These results suggested that LKLF mediates the resistance of cells to TNF-induced apoptosis.
FIG. 5.
LKLF protected TRAF2−/− cells against TNF-induced apoptosis. (A) Cell extracts from WT, TRAF2−/−, and LKLF stably transfected TRAF2−/− mouse fibroblast cells (LKLF clones 3 and 7) were used to detect protein expression of LKLF and TRAF2. (B) LKLF stably transfected TRAF2−/− cells, TRAF2−/− LKLF clones 3 and 7 (LKLF #3 and LKLF #7, respectively), and pHygromycin (Hygro)-transfected TRAF2−/− cells were treated with TNF (20 ng/ml) with the indicated concentration of CHX for 8 h, and dead cells were determined by trypan blue staining as described previously (21). The percentages shown are the average values from three independent experiments. (C) The cells described above for panel B were treated with CHX (200 ng/ml) and the indicated concentration of TNF for 8 h, and dead cells were determined by trypan blue staining.
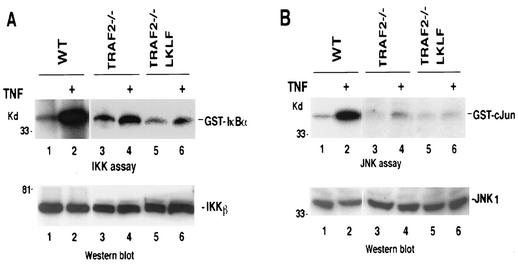
To ensure that the effect of LKLF on the sensitivity of cells to TNF-induced apoptosis was not due to its effect on TNF signaling, we investigated whether the ectopic expression of LKLF altered the status of TNF signaling in TRAF2−/− cells. Since both IKK and JNK activation by TNF are impaired in TRAF2−/− cells, we then examined IKK and JNK activation in LKLF-transfected TRAF2−/− cells. As shown in Fig. 6, the presence of LKLF did not have any effect on these two pathways in response to TNF. Therefore, these results suggested that LKLF does not affect TNF-induced apoptosis by affecting TNF-induced NF-κB and JNK activation.
FIG. 6.
The expression of LKLF in TRAF2−/− cells has no effect on TNF-induced IKK and JNK activation. (A) WT, TRAF2−/−, or TRAF2−/− LKLF clone 3 cells were treated with mouse TNF (20 ng/ml) for 10 min. Equal cell extracts were used for in vitro kinase assay to measure IKK activity and were Western blotted for the protein levels of IKKβ. (B) WT, TRAF2−/−, and TRAF2−/− LKLF clone 3 cells were treated with mouse TNF (20 ng/ml) for 15 min. JNK1 protein expression or activity from each sample was measured by Western blotting or JNK kinase assay. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the blots.
IGF-IR is a potential target of LKLF to mediate its antiapoptotic effect.
To obtain a better understanding of the antiapoptotic effect of LKLF, we reexamined the results of microarray experiments to search for genes whose expression is down-regulated in TRAF2−/− cells and whose protein products are known to have an antiapoptotic effect. Of the genes whose expression is decreased in TRAF2−/− cells according to our array results, IGF-IR is one of the genes that have previously been shown to protect cells against apoptosis (14, 45). We then confirmed IGF-IR expression in TRAF2−/− cells by Western blotting.
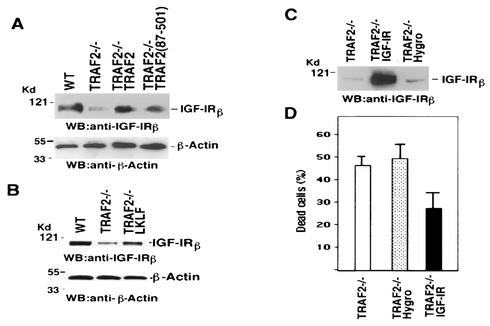
As shown in Fig. 7A, the expression of IGF-IR protein level was dramatically decreased in TRAF2−/− cells than in WT cells. Moreover, in WT TRAF2 or the TRAF2(87-501) mutant stably transfected cells, IGF-IR expression was recovered (Fig. 7A). These results indicated that the expression of IGF-IR requires TRAF2. Because expression of IGF-IR, like LKLF expression, could also be restored by the TRAF2(87-501) mutant, it is possible that LKLF and IGF-IR are regulated by TRAF2 by the same mechanism. However, since LKLF is a SP1 transcription factor and it has been suggested that the expression of IGF-IR is regulated by some SP1 transcription factors (6, 27, 35), it is also feasible that the expression of IGF-IR is regulated by TRAF2 through LKLF. To test this possibility, we then examined the expression level of IGF-IR in LKLF stably transfected TRAF2−/− cells. As shown in Fig. 7B, the expression of IGF-IR was dramatically recovered in LKLF stably transfected TRAF2−/− cells compared to the parental TRAF2−/− cells. Therefore, this result implied that IGF-IR was a potential target of LKLF. Next we investigated whether ectopic expression of IGF-IR in TRAF2−/− cells would decrease the sensitivity of cells to TNF-induced apoptosis. To do so, we generated IGF-IR stably transfected TRAF2−/− cells (Fig. 7C) and then treated those cells with TNF and CHX. As shown in Fig. 7D, the ectopic expression of IGF-IR in TRAF2−/− cells partially protected cells from TNF-induced apoptosis, compared to parental or pHygromycin-transfected TRAF2−/− cells. These results suggested that IGF-IR may account for a certain level of the antiapoptotic effect of LKLF.
FIG. 7.
Impact of IGF-IR expression on TNF-induced cell death in TRAF2−/− cells. (A) Equal amounts of cell extracts from WT, TRAF2−/−, and TRAF2-transfected TRAF2−/− cells were Western blotted (WB) for the protein levels of IGF-IRβ or β-actin. (B) Equal amounts of cell extracts from WT, TRAF2−/−, and TRAF2−/− LKLF clone 3 cells were Western blotted for the protein levels of IGF-IRβ or β-actin. (C) Equal amounts of cell extracts from TRAF2−/− and TRAF2−/− mouse fibroblast cells stably transfected with IGF-IR or pHygromycin were Western blotted for the protein level of IGF-IRβ. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the blots. (D) TRAF2−/− and TRAF2−/− mouse fibroblast cells stably transfected with IGF-IR or pHygromycin (Hygro) were treated with TNF (20 μg/ml) and CHX (200 ng/ml) for 8 h. Dead cells were determined by trypan blue staining. The results shown are average values for three independent experiments.
DISCUSSION
TRAF2 is a critical component in TNF signaling to NF-κB and JNK activation (7, 15, 37). The genetic deletion of TRAF2 resulted in the supersensitivity of cells to TNF-induced apoptosis (43). It is noted that the removal of TRAF2 from cells caused only a partial decrease of NF-κB activity induced by TNF (43). However, the impaired NF-κB activation alone could not explain the supersensitivity of TRAF2−/− cells to TNF-induced apoptosis. Therefore, it has been proposed that TRAF2 may mediate an antiapoptotic effect independent of NF-κB activation (15). In this study, we tried to address this issue by using cDNA microarray technology. We attempted to identify genes whose expression is down-regulated in TRAF2−/− cells. Of many down-regulated genes in TRAF2−/− cells, LKLF and IGF-IR are two genes known to have antiapoptotic effects (14, 30, 45). We demonstrated that TRAF2 regulates the expression of LKLF through the MAP kinase p38 pathway. More importantly, ectopic expression of LKLF in TRAF2−/− cells protects cells against TNF-induced apoptosis, partially by regulating IGF-IR expression. Therefore, our study provides a potential mechanism of TRAF2-mediated antiapoptotic effect.
TRAF2 is one of six known TRAF proteins (2). Of the six TRAF proteins, ectopic expression of TRAF2, -5, and -6, but not TRAF1, -3, and -4, leads to the activation of NF-κB and JNK (7, 15, 37). TRAF2 and -5 are required for TNF signaling, while TRAF6 is essential for interleukin-1 (IL-1) signaling. In this study, we found that only TRAF2 is capable of activating the LKLF promoter. All of the other five TRAF proteins failed to potentiate the activity of LKLF promoter when they were expressed in TRAF2−/− cells. Therefore, there is no redundant function among TRAF proteins on LKLF expression. To shed light on why only TRAF2 is capable of activating the LKLF promoter, we examined the activation of p38 by different TRAFs and found that only overexpression of TRAF2 effectively activates p38 (data not shown). In addition, the regulation of LKLF expression by TRAF2 is independent of TNF signaling, since TNF treatment has no effect on LKLF expression. The finding that the TRAF2(87-501) mutant is fully capable of activating the LKLF promoter further distinguished the regulatory function of TRAF2 on LKLF expression from TNF signaling. Hence, our study provided the first evidence that TRAF2 has additional functions other than as an effector of TNF signaling.
Because TRAF2 has five zinc finger domains, it has been speculated that it may function as a transcription factor (23). A previous study suggested that TRAF2 may localize in the nucleus and has some transcriptional activity when overexpressed (25). We examined the localization of the endogenous TRAF2 in WT fibroblasts but failed to detect TRAF2 in the nucleus. We also used the green fluorescent protein (GFP)-TRAF2 fusion protein to examine TRAF2 localization in HeLa cells and mouse fibroblasts. No GFP-TRAF2 was detected in the cell nucleus. We realized that the cell system used by the previous study (human umbilical vein endothelial cells) is different from the ones we used (HeLa cells and mouse fibroblasts). Therefore, it is possible that the discrepancy on TRAF2 localization between the previous study and ours may be due to the specificity of different cell types. We conclude that TRAF2 does not function as a transcription factor in the regulation of LKLF expression in mouse fibroblasts. Our finding that the dominant-negative mutant of MKK6 blocked TRAF2-induced LKLF expression further supports this conclusion.
LKLF belongs to the SP1 transcription factor family (35). All members of this family have three Krüppel-like zinc fingers in their DNA-binding domains. LKLF is highly expressed in the lung, and it is also found in the heart, spleen, skeletal muscle, and testis (1). Mice lacking LKLF by gene targeting die at midgestation around day 12.5 due to severe hemorrhage. LKLF is required for blood vessel assembly and homeostasis during mammalian embryogenesis (19, 40, 41). Despite the pivotal role of LKLF in development, regulation of expression of LKLF as well as the target genes downstream of LKLF is not understood well. Recently, it was reported that LKLF is expressed in T cells and that expression of LKLF is regulated by cytokines, such as IL-2 and IL-7 (30). The expression of LKLF has been correlated with long-term survival of memory T cells both in vitro and in vivo (30). In this study, we found that the maintenance of LKLF expression in mouse fibroblasts requires TRAF2 and that the regulatory effect of TRAF2 on LKLF expression is achieved through MAP kinase p38. These findings are the first to shed light on the regulation of LKLF expression, although the mechanism of p38 function in this process needs further investigation. More importantly, when LKLF was ectopically expressed in TRAF2−/− cells, it protected cells against TNF-induced apoptosis. However, because LKLF is a transcription factor, it must protect cells by regulating the expression of other proteins. We identified IGF-IR as a potential target gene of LKLF. But because the expression of IGF-IR in TRAF2−/− cells produces less of a protective effect than LKLF does, it is possible that LKLF regulates the expression of multiple genes like IGF-IR to protect cells against TNF-induced cell death.
NF-κB activity has been found to protect cells against apoptosis (5, 22, 36, 38). In TRAF2−/− cells, NF-κB activation by TNF treatment is only partially decreased (43). However, the TRAF2−/− cells became supersensitive to TNF-induced apoptosis (43). Therefore, it has been speculated that TRAF2 may provide the survival signal through a NF-κB-independent pathway (15, 43). Identification of LKLF as a transcription factor that is dependent on TRAF2 but independent of TNF signaling indicates the existence of such a pathway. More importantly, our study suggests that, most likely, the supersensitivity of TRAF2−/− cells to TNF-induced apoptosis is the result of down-regulation of expression of certain proteins, such as LKLF. Therefore, TRAF2 serves a larger role than as just an effector of TNF family signaling.
Acknowledgments
We thank W. C. Yeh and T. W. Mak for the TRAF2−/− fibroblasts, M. Karin for the p38−/− fibroblasts, J. M. Leiden and C.-N Ting for the LKLF antibody, J. Han for the MKK6AA construct, and D. LeRoith for the pBPV-hIGFIR plasmid.
REFERENCES
- 1.Anderson, K. P., C. B. Kern, S. C. Crable, and J. B. Lingrel. 1995. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol. Cell. Biol. 15:5957-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arch, R. H., R. W. Gedrich, and C. B. Thompson. 1998. Tumor necrosis factor receptor-associated factors (TRAFs): a family of adapter proteins that regulates life and death. Genes Dev. 12:2821-2830. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and D. Baltimore. 1996. NF-κB: ten years after. Cell 87:13-20. [DOI] [PubMed] [Google Scholar]
- 4.Baud, V., Z. G. Liu, B. Bennett, N. Suzuki, Y. Xia, and M. Karin. 1999. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 13:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNFα-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 6.Beitner-Johnson, D., H. Werner, C. T. Roberts, Jr., and D. LeRoith. 1995. Regulation of insulin-like growth factor I receptor gene expression by Sp1: physical and functional interactions of Sp1 at GC boxes and at a CT element. Mol. Endocrinol. 9:1147-1156. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, J. R., and J. S. Pober. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482-6491. [DOI] [PubMed] [Google Scholar]
- 8.Brenner, D. A., M. O'Hara, P. Angel, M. Chojkier, and M. Karin. 1989. Prolonged activation of jun and collagenase genes by tumor necrosis factor-alpha. Nature 337:661-663. [DOI] [PubMed] [Google Scholar]
- 9.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2001. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 10.Devin, A., Y. Lin, S. Yamaoka, Z. Li, M. Karin, and Z. G. Liu. 2001. The alpha and beta subunits of IκB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol. Cell. Biol. 21:3986-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 12.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 14.Hadsell, D. L., and G. Abdel-Fattah. 2001. Regulation of cell apoptosis by insulin-like growth factor I. Adv. Exp. Med. Biol. 501:79-85. [DOI] [PubMed] [Google Scholar]
- 15.Heyninck, K., and R. Beyaert. 2001. Crosstalk between NF-κB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol. Cell Biol. Res. Commun. 4:259-265. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov, V. N., Ø. Fodstad, and Z. Ronai. 2001. Expression of ring finger-deleted TRAF2 sensitizes metastatic melanoma cells to apoptosis via up-regulation of p38, TNF and suppression of NF-κB activities. Oncogene 20:2243-2253. [DOI] [PubMed] [Google Scholar]
- 18.Kato, H., T. N. Faria, B. Stannard, C. T. Roberts, Jr., and D. LeRoith. 1993. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor. Characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (IR-3). J. Biol. Chem. 268:2655-2661. [PubMed] [Google Scholar]
- 19.Kuo, C. T., M. L. Veselits, K. P. Barton, M. M. Lu, C. Clendenin, and J. M. Leiden. 1997. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11:2996-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, Y., A. Devin, A. Cook, M. M. Keane, M. Kelliher, S. Lipkowitz, and Z. G. Liu. 2000. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IκB kinase and c-Jun N-terminal kinase. Mol. Cell. Biol. 20:6638-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, Y., A. Devin, Y. Rodriguez, and Z. G. Liu. 1999. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13:2514-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 23.Mackay, J. P., and M. Crossley. 1998. Zinc fingers are sticking together. Trends Biochem. Sci. 23:1-4. [DOI] [PubMed] [Google Scholar]
- 24.Malinin, N. L., M. P. Boldin, A. V. Kovalenko, and D. Wallach. 1997. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385:540-544. [DOI] [PubMed] [Google Scholar]
- 25.Min, W., J. R. Bradley, J. J. Galbraith, S. J. Jones, E. C. Ledgerwood, and J. S. Pober. 1998. The N-terminal domains target TNF receptor-associated factor-2 to the nucleus and display transcriptional regulatory activity. J. Immunol. 161:319-324. [PubMed] [Google Scholar]
- 26.Natoli, G., A. Costanzo, A. Ianni, D. J. Templeton, J. R. Woodgett, C. Balsano, and M. Levrero. 1997. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275:200-203. [DOI] [PubMed] [Google Scholar]
- 27.Ohlsson, C., N. Kley, H. Werner, and D. LeRoith. 1998. p53 regulates insulin-like growth factor-I (IGF-I) receptor expression and IGF-I-induced tyrosine phosphorylation in an osteosarcoma cell line: interaction between p53 and Sp1. Endocrinology 139:1101-1107. [DOI] [PubMed] [Google Scholar]
- 28.Reinhard, C., B. Shamoon, V. Shyamala, and L. T. Williams. 1997. Tumor necrosis factor α-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 16:1080-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothe, M., S. C. Wong, W. J. Henzel, and D. V. Goeddel. 1994. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 78:681-692. [DOI] [PubMed] [Google Scholar]
- 30.Schober, S. L., C. T. Kuo, K. S. Schluns, L. Lefrancois, J. M. Leiden, and S. C. Jameson. 1999. Expression of the transcription factor lung Kruppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J. Immunol. 163:3662-3667. [PubMed] [Google Scholar]
- 31.Schrick, J. J., M. J. Hughes, K. P. Anderson, M. L. Croyle, and J. B. Lingrel. 1999. Characterization of the lung Kruppel-like transcription factor gene and upstream regulatory elements. Gene 236:185-195. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi, M., M. Rothe, and D. V. Goeddel. 1996. Anatomy of TRAF2. Distinct domains for nuclear factor-κB activation and association with tumor necrosis factor signaling proteins. J. Biol. Chem. 271:19935-19942. [DOI] [PubMed] [Google Scholar]
- 33.Tamura, K., T. Sudo, U. Senftleben, A. M. Dadak, R. Johnson, and M. Karin. 2000. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 34.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 35.Turner, J., and M. Crossley. 1999. Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem. Sci. 24:236-240. [DOI] [PubMed] [Google Scholar]
- 36.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNF-alpha-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 37.Wajant, H., and P. Scheurich. 2001. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int. J. Biochem. Cell. Biol. 33:19-32. [DOI] [PubMed] [Google Scholar]
- 38.Wang, C. Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 39.Wang, X., C. H. McGowan, M. Zhao, L. He, J. S. Downey, C. Fearns, Y. Wang, S. Huang, and J. Han. 2000. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20:4543-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wani, M. A., R. T. Means, Jr., and J. B. Lingrel. 1998. Loss of LKLF function results in embryonic lethality in mice. Transgenic Res. 7:229-238. [DOI] [PubMed] [Google Scholar]
- 41.Wani, M. A., S. E. Wert, and J. B. Lingrel. 1999. Lung Kruppel-like factor, a zinc finger transcription factor, is essential for normal lung development. J. Biol. Chem. 274:21180-21185. [DOI] [PubMed] [Google Scholar]
- 42.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 43.Yeh, W. C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 44.Yin, L., L. Wu, H. Wesche, C. D. Arthur, J. M. White, D. V. Goeddel, and R. D. Schreiber. 2001. Defective lymphotoxin-beta receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291:2162-2165. [DOI] [PubMed] [Google Scholar]
- 45.Yu, H., and T. Rohan. 2000. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer. Inst. 92:1472-1489. [DOI] [PubMed] [Google Scholar]