Abstract
Different amounts of Suppressor of Hairless (SuH)-dependent Notch (N) signaling is often used during animal development to produce two different tissues from a population of equipotent cells. During Drosophila melanogaster embryogenesis, cells with high amounts of this signaling differentiate the larval epidermis whereas cells with low amounts, or none, differentiate the central nervous system (CNS). The mechanism by which SuH-dependent N signaling is increased or decreased in these different cells is obscure. The developing epidermis is known to get enriched for the full-length N (NFull) and the developing CNS for the carboxyl terminus-truncated N (NΔCterm). Results described here indicate that this differential accumulation of N receptors is part of a mechanism that would promote SuH-dependent N signaling in the developing epidermis but suppress it in the developing CNS. This mechanism involves SuH-dependent stability of NFull, NFull-dependent accumulation of SuH, stage specific stability of SuH, and NΔCterm-dependent loss of SuH and NFull.
Drosophila melanogaster larval epidermis (cuticle) and the central nervous system (CNS) are produced from clusters of embryonic cells that have acquired the potential to become the CNS cells. These cells, called the proneural cells, express N and its ligand Delta (Dl) on their surfaces. When Delta expressed on a cell binds N expressed on the neighboring cell, a protein complex containing SuH and the full N intracellular domain (Nintra) become active in the nucleus. SuH targets the complex to the promoter regions of target genes (e.g., Enhancer of split complex) and Nintra activates transcription of these genes. This SuH- and Nintra-dependent signaling is activated in the majority of proneural cells of each cluster. These cells lose expression of neuronal genes (e.g., Achaete Scute complex) and differentiate into the epidermis. At the same time, SuH- and Nintra-dependent N signaling is suppressed in the remaining cells of the proneural clusters. These cells (called segregating neuroblasts at this stage) increase expression of the neuronal genes and differentiate into the CNS (1, 9, 11, 13, 14, 19, 21, 23, 26, 41, 42, 44, 48). The choice between becoming the epidermis cell or the CNS cell is dependent on the relative amounts of SuH- and Nintra-dependent N signaling: proneural cells with the least amount of SuH- and Nintra-dependent N signaling gradually lose all of it and become the CNS cells; the remaining cells augment SuH- and Nintra-dependent N signaling and become the epidermis cells (19).
Production of SuH- and Nintra-dependent N signaling at any time during differentiation of the CNS from the segregating neuroblasts results in loss of the CNS cells (27, 43). Nevertheless, N and Dl are expressed during, and required for, the differentiation of the CNS from the segregating neuroblasts (12, 16, 17, 22, 24, 47). Consistent with these observations, (i) N− embryos produce neither the ventral cuticle nor the CNS but become filled with proneural cells that soon stop differentiating and (ii) the hypomorphic Nts1 allele embryos produce N protein and the epidermis only in patches (9, 19, 27, 47). What is the mechanism that activates production of the epidermis (SuH- and Nintra-dependent N) signals in the developing epidermis but suppresses it in the developing CNS? How does N function in both the developing epidermis and the CNS but produce SuH- and Nintra-dependent N signaling only in the developing epidermis? The results described below suggest answers to these questions.
The predominant form of N expressed in the developing embryonic epidermis is the full-length form, NFull, while the predominant form of N expressed in the developing CNS (including the segregating neuroblasts) lacks the sequence carboxyl terminus of the CDC10/ankyrin repeats, NΔCterm (47). Results of experiments reported here indicate that accumulation of NFull in the developing epidermis would promote accumulation of SuH, which in turn would promote accumulation of NFull. This would generate a positive feed back cycle promoting SuH- and Nintra-dependent N signaling. Accumulation of NΔCterm in the developing CNS would promote degradation of SuH that in turn promotes degradation of NFull. This would generate a negative feed back cycle that suppresses SuH- and Nintra-dependent N signaling. Thus, it appears that in the course of tissue differentiation, a secondary N receptor (NΔCterm) is produced which in conjunction with the primary N receptor (NFull) would generate opposing cycles of SuH- and Nintra-dependent N signaling that is utilized to produce two different tissues from a population of equipotent cells.
MATERIALS AND METHODS
Immunostaining.
Procedures described by Lieber et al. (27) were followed and the signals were detected using horseradish peroxidase.
Western blotting and immunoprecipitation.
Procedures described by Wesley and Saez (47) were followed. Signals were detected by chemiluminescence. The amounts of proteins in extracts were quantitated using absorbance values at 280 nm and the Bio-Rad DC protein assay kit. Sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis (SDS-8% PAGE) or SDS-4% PAGE was used for Western blots. An antibody made against the heat shock 70 protein (αhsp70) was used to determine the amounts of total proteins in each lane of some blots.
Embryo production.
Embryos were collected and aged to different stages at room temperature (22°C), unless otherwise indicated. When reared at other temperatures, appropriate corrections were made for the difference in developmental rates. Embryos lacking the maternal contribution of su(H) were produced in hs-FLP1; Su(H)del47 FRT 40A P[l (2)35Bg+]/P[ovoD1] FRT 40 females following the procedure described by Morel and Schweisguth (31). These females were mated with Su(H)del47/CyO males. One hundred percent of the resultant embryos lack the maternal contribution and 50% lack the zygotic contribution as well. Uniform overexpression of different proteins was obtained by crossing Da-Gal 4 flies with UAS-SuH, -H, or -different N proteins following the procedures described by Brand and Perrimon (4). These embryos were reared at 18°C.
Somatic clones production.
wa N60g11 FRT 101 w+/FM7 flies were crossed with hs Flp; ovoD1 FRT 101/Y flies. Third-instar larvae from this cross were heat shocked for 1 h, incubated at room temperature (∼22°C) or higher for 3 h, heat shocked again for 1 h, and incubated at room temperature or higher until adult emergence.
RNA analyses.
RNAi procedures described by Clemens et al. (10) was followed. Double-stranded RNA was prepared using the DNA sequence corresponding to the first 200 amino acids of SuH. Northern blotting, cell culture, cell handling, and cell aggregation assays procedures followed are described by Wesley and Saez (47). The intracellular domain of Dl was removed by introducing a stop codon after the trans-membrane domain. A stable S2 cell line was established from this construct. Western blotting analyses showed that the resultant protein lacks the intracellular domain. All other cell lines used have been previously described (47). All cell transfections were done using the calcium phosphate procedure.
Heat shock treatment.
Embryos were heat shocked for 1 h at 37°C and allowed to synthesize proteins at room temperature for various periods of time as indicated. S2 cells were heat shocked for 30 min at 37°C and allowed to synthesize proteins for 2 h or more (as indicated) at room temperature. Clone 8 cells were incubated at 27°C for 24 h or more before protein extraction, or were heat shocked at 37°C for 30 min and incubated at room temperature for 4 to 7 h before protein extraction.
Protease inhibitor treatment.
Cells were treated for 24 h with 50 μM Lactacystin and 200 μM MG 115, or 100 μM chloroquine before heat shock induction of proteins.
Antibodies.
αN203 is described by Wesley and Saez (47), αNPCR and αNI in Lieber et al. (27), αSuH(r) in Gho et al. (15), αSuH (m) in Kidd et al. (23), and αH in Maier et al. (29). αhsp70 was purchased from Sigma and αUbiquitin was purchased from Calbiochem. αN2341 was made in hamsters against the amino acid region 2341 to 2537 in the carboxyl terminus of the Notch protein. αSuH(r1) was made in rats against His-tagged full-length SuH.
RESULTS
In the following description, NFull would refer to the largest N molecule with the full intracellular domain on Western blots and NΔCterm to the largest N molecule lacking the sequence carboxyl terminus of the CDC10/ankyrin repeats. The smaller forms derived from NFull and NΔCterm are not dealt with separately as they covary with the larger forms. N would refer to N protein in general, inclusive of all forms.
Embryonic tissues and stages not enriched for NFull are also not enriched for SuH.
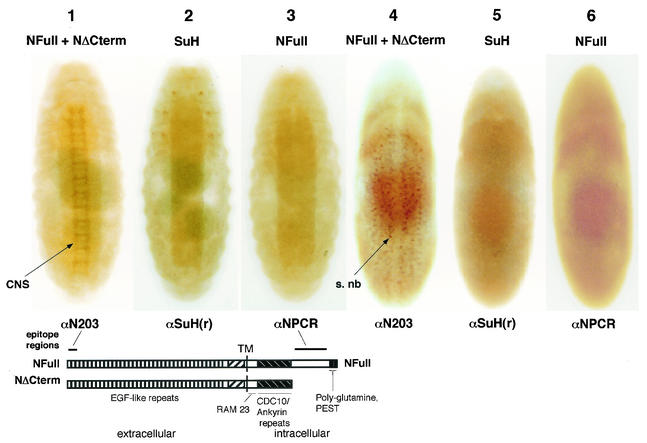
Canton S embryos were immunostained with antibodies made against SuH, the amino-terminus of N, and the carboxyl terminus of N. These embryos showed that SuH protein was present primarily in the epidermal layers of the embryo and was not enriched in the segregating neuroblasts and the developing CNS (Fig. 1, embryos 2 and 5). Thus, SuH distribution was similar to that of NFull rather than that of NΔCterm, the latter being enriched in the segregating neuroblasts and the developing CNS (compare Fig. 1, embryos 2 and 5 with embryos 1, 3, 4, and 6; the epitopes of the two N antibodies are shown beneath embryos 1 and 3). The staining observed in all the embryos was due to the primary antibody used and similar to previously published patterns (15, 47). The three pairs of SuH enriched spots on the thoracic segments (Fig. 1, embryo 2) appear to be the cells in which the autoregulatory enhancer of the Su(H) gene is active independent of Notch (3). The expression of SuH and NFull at embryonic stages 8 to 9 (embryos 5 and 6 in Fig. 1) is very general and devoid of any obvious patterns (see also references 12 and 22). In the absence of the target protein (as in SuH and N null embryos), or the primary antibody, the embryos were unstained and white (data for SuH not shown; data for N is shown 47).
FIG. 1.
The developing CNS in wild-type embryos is not enriched for SuH, like NFull but unlike NΔCterm. The wild-type strain used was yw (yellow− white−). Antibodies used for immunostaining are shown below the embryos; the proteins detected are shown above them. The staining pattern of αN203 is identical to that of antibodies made against the CDC10/ankyrin repeats (13, 22) (αNI used on Western blots in this study was made against the CDC10/ankyrin repeats but it does not work in immunostaining of embryos). Features of the N protein and the epitope regions of αN203 and αNPCR are shown below embryos 1 to 3. s. nb, segregating neuroblasts. Cells enriched for NΔCterm in embryo 4 were determined to be segregating neuroblasts by morphology and achaete expression (see also reference 47). 1 to 3 are stage 15 or 16 embryos; 4 to 6 are stage 8 or 9 embryos.
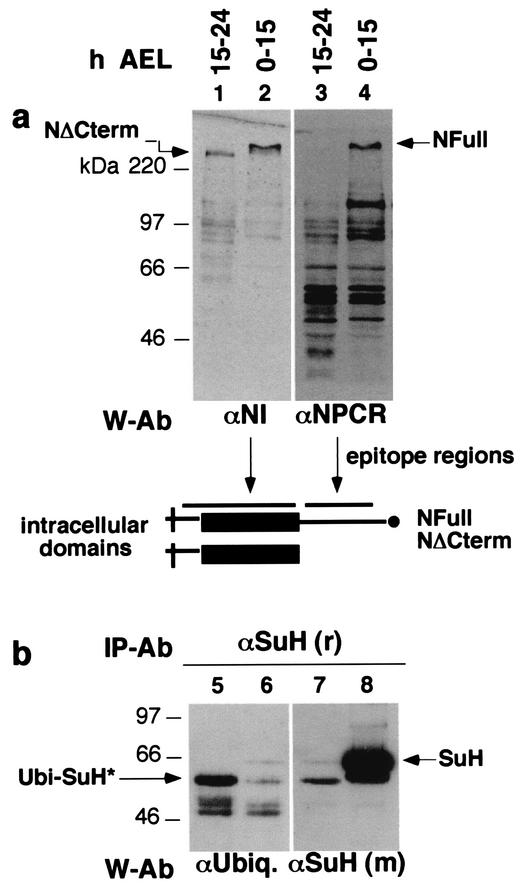
Western blotting and immunoprecipitation experiments (n = 5) also revealed a correspondence between NFull and SuH amounts. Early stages of embryos contained a high amount of NFull (Fig. 2a, lanes 2, 4) and SuH (Fig. 2b, lane 8). Late stages of embryos contained a low level of NFull (Fig. 2a, lanes 1 and 3) and SuH (Fig. 2b, lane 7). The level of NΔCterm in these embryos is higher than the amount of NFull, the opposite of the situation in early stage embryos (Fig. 2a, lanes 1 and 2). The amount of total proteins in lanes 1 and 3 is four times that in lanes 2 and 4; the amount of total proteins used for lanes 5 to 8 was the same. All the bands in lane 3 appear to be degraded or processed NFull fragments as they vary with the amount of NFull in S2 cells and embryos (data not shown). A higher level of ubiquitinated SuH (Ubi-SuH*) was detected in late stage embryos suggesting that the low level of SuH is at least partially due to targeted degradation (Fig. 2b, lanes 5 and 6). Ubi-SuH* appears to be a fragment of SuH, possibly the more stable degradation intermediate product, as it migrates faster than the nonubiquitinated form (compare lanes 5 and 8 in Fig. 2b). Straight Western blotting shows a number of slower migrating forms of SuH in old embryos (some are seen in Fig. 3b) or larvae (see Fig. 6c), but their ubiquitin status is unknown.
FIG. 2.
Embryonic stages with high levels of NFull also contain high levels of SuH. (a) The level of NFull is high in embryos from 0 to 15 h after egg laying (h AEL) and low in embryos from 15 to 24 h AEL. Total protein extracts from yw embryos were used directly for Western blotting. Antibodies used for Western blotting (W-Ab) and their epitope regions are shown below the blots. Lanes 1 and 2 and lanes 3 and 4 are the same blots. The level of total proteins in lane 1 is four times the amount in lane 2. (b) The level of SuH is high in embryos from 0 to 15 h AEL and low in those 15 to 24 h AEL. SuH, recovered from yw embryonic total protein extracts by using αSuH (r) as the immunoprecipitation antibody (IP-Ab), was used for Western blotting. Equal aliquots of extracts and antibodies were used for immunoprecipitations. Lanes 7 and 8 and lanes 5 and 6 are the same blots. αUbiq, antiubiquitin; *, possibly a fragment of SuH.
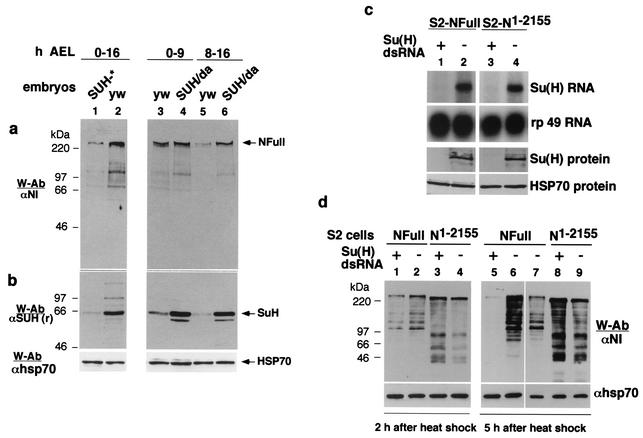
FIG. 3.
NFull is unstable in the absence of SuH. (a and b) Embryos with a low level of SuH (SUH*) contain a low amount of NFull, whereas embryos with high levels of SuH (SUH/Da) contain a high level of NFull. yw embryos served as controls. SUH* = 50% of embryos without maternal and zygotic contribution + 50% without maternal contribution but with zygotic contribution. SUH/Da = embryos produced by crossing UAS-SuH flies with daughterless Gal 4 (Da-Gal 4) flies. Total proteins from these and yw embryos were used for Western blots. yw, daGal4, and hsGal4 stocks behaved identically in all assays used in this report. yw is used as the common wild-type control. Panels a and b and HSP 70 are the same blots. (c) SuH dsRNA treatment reduces the amount of SuH RNA (top two rows) and protein (bottom two rows) in S2 cells expressing N receptors NFull and NΔCterm. Symbols: +, treated cells; −, untreated cells. rp 49 shows total RNA and HSP 70 total proteins. αSuH (m) was used to detect SuH. S2 cells not expressing any transgenes gave similar results (data not shown). (d) NFull is unstable and NΔCterm stable in SuH dsRNA S2-NFull and S2-NΔCterm cells, respectively. Total proteins from treated cells (+) and untreated cells (−) were extracted 2 and 5 h after induction of expression of N receptors and used for the Western blots. NFull is shown in lane 7 for blot alignment purposes.
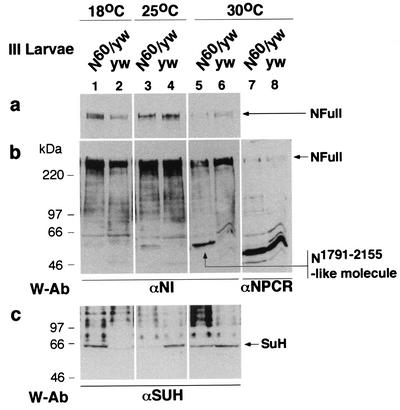
FIG. 6.
N60g11/yw third-instar larvae produce a higher level of an N1791-2155-like molecule at higher temperatures (25 and 30°C), when loss of SuH-dependent N signaling phenotype is observed, and contain lower levels of NFull (a and b) and SuH (c) (lanes 3 to 6). At 18°C, when gain of SuH-dependent N signaling phenotype is observed, N60g11/yw larvae contain a low level of N1791-2155-like molecule and high levels of NFull and SuH (lanes 1 and 2). The presence of more SuH in lane 5 than in lane 3 is possibly due to the effect of high temperature on SuH (Fig. 4b). The N1791-2155-like molecule is considered to be the intracellular domain of N60g11 as it is not recognized by the αNPCR (lanes 7 and 8; reprobing of the blot in lanes 5 and 6) and is of the size expected of the intracellular domain of N60g11 (including the transmembrane domain). A molecule of similar size produced by N1-2155 in S2 cells (N1-2155 is 20 amino acids longer than N60g11) gets biotinylated in cell surface biotinylation experiments (data not shown).
The amount of NFull, NΔCterm, SuH, and ubiquitinated SuH was also determined in 0 to 9 and 8 to 16 h after egg laying embryos. The level of NFull and SuH were always high, and the level of NΔCterm and ubiquitinated SuH always low, in 0 to 9 h embryos. The levels of the same proteins in 8 to 16 h embryos were variable, with the level of NFull and SuH always being lower and the level of NΔCterm and ubiquitinated SuH higher than in 0 to 9 h embryos (data not shown).
Reduction in the amount of SuH in embryos reduces the amount of NFull.
SuH amount in embryos was reduced following the procedure described by Morel and Schweisguth (31) for generating SuH− germ line clonal embryos. Fifty percent of these embryos would lack both maternal and zygotic contribution; the other 50% would lack the maternal contribution but contain the zygotic contribution. The amount of SuH in embryos was increased using the binary UAS/Gal4 system (4). UAS-SuH flies were crossed with flies carrying daughterless promoter driven Gal 4 (Da-Gal 4). The resulting Da-Gal 4/UAS-SuH would overexpress SuH more or less uniformly throughout the embryo. Protein extracts from these embryos were analyzed by Western blotting.
Embryos in which SuH amount was reduced had a lower level of NFull (Fig. 3a and b, lanes 1 and 2). Overexpression of SuH resulted in accumulation of NFull (Fig. 3a and b, lanes 3 to 6). The effect of SuH on accumulation of NFull was stronger in 8 to 16 h embryos compared with 0 to 9 h embryos (compare lanes 5 and 6 with lanes 3 and 4 in Fig. 3a and b). The same results were obtained in three independent repetitions of the experiments. As could be expected from the general pattern of reduction or increase in SuH expression, immunostaining of these embryos did not show any interesting pattern of loss or accumulation of SuH or NFull.
The effect of loss of SuH on NΔCterm could not be examined in embryos as NΔCterm is derived from NFull (47): lower levels of NFull would mean lower levels of NΔCterm. Therefore, the effect of SuH on NΔCterm was examined in cultured S2 cells treated with dsRNA to specifically eliminate SuH RNA. Figure 3c shows that S2 cells with heat shock-inducible constructs of NFull (S2-NFull) and an NΔCterm-like protein N1-2155 (S2-N1-155) (47) treated with SuH dsRNA contained low levels of SuH RNA and protein. Following induction of expression, NFull was found to be unstable in cells with low levels of SuH (Fig. 3d, lanes 1 and 2 and lanes 5 and 6). On the other hand, the stability of N1-2155 was unaffected (Fig. 3d, lanes 3 and 4 and lanes 8 and 9). The same results were obtained in five repetitions of the experiments.
NΔCterm-like proteins reduce the amount of NFull in middle stage embryos.
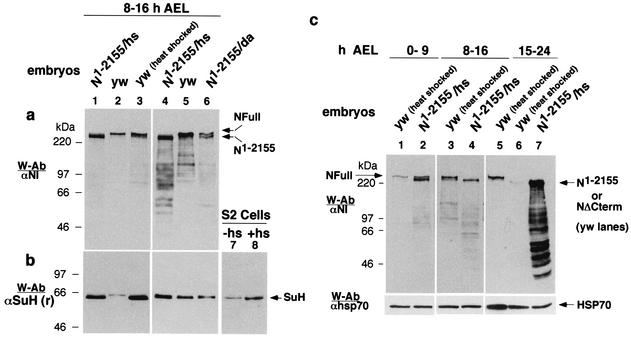
The amount of NΔCterm-like proteins in embryos was increased by expression of N1-2155 proteins using the UAS/Gal4 system. High levels of N1-2155, obtained using the heat shock-Gal4 driver (hsGal4), resulted in almost a complete loss of NFull (Fig. 4a, lanes 1 and 2). Relatively moderate amounts of N1-2155, obtained using the daGal4 driver, resulted in moderate loss of NFull (Fig. 4a, lanes 4 to 6).
FIG. 4.
Expression of NΔCterm reduces the amount of NFull in middle-aged embryos. (a) Expression of N1-2155 at high levels (N1-2155/hs) results in almost complete loss of NFull (lanes 1 to 3) and at intermediate level (N1-2155/Da) results in partial loss of NFull (lanes 4 to 6). Non-heat-shocked (yw) and heat-shocked (yw [heat shocked]) wild-type embryos were used as controls. Total protein extracts were used for the Western blots. The same amount of total proteins was loaded in lanes 1 to 3 and in lanes 4 to 6.UAS-N1-2155 flies were crossed to Da- or hs-Gal4 flies to express N1-2155 at different levels. (b) The effect of N1-2155 on SuH amount was modest with Da-Gal 4 induction (lanes 5 and 6) and obscured with heat shock induction (lanes 1 to 5 and lanes 7 and 8). Blots in a were reprobed with αSuH (r) for lanes 1 to 6. Total protein extracts from S2 cells that were heat shocked (+hs) or not (−hs) was used for lanes 7 and 8. (c) N1-2155 affects NFull differently at different stages of embryogenesis. Extracts of total proteins from different stages of embryos that were wild type and heat shocked (yw [heat shocked]) or containing heat shock induced N1-2155 (N1-2155/hs) were used for the Western blots. NFull in lane 5 was used to align lanes 6 and 7 with others. Lanes 5 to 7 were exposed to film for a longer time to show NΔCterm in lane 6.
The effect of N1-2155 on NFull amount and the stability of N1-2155 expression in embryos showed a dependence on the developmental stages of the embryos. In early-stage embryos, 0 to 9 h after egg laying, N1-2155 increased the amount of NFull (Fig. 4c, lanes 1 and 2). The increase was modest but consistent (n = 3). N1-2155 was very unstable in these embryos, becoming undetectable in less than 40 min after heat shock. In middle-stage embryos, 8 to 16 h after egg laying, N1-2155 almost completely suppressed expression of NFull (Fig. 4c, lanes 3 and 4). N1-2155 was relatively more stable in these embryos, being detectable for more than an hour following heat shock. In late-stage embryos (15 to 24 h after egg laying)—when NFull and SuH levels are low, and NΔCterm is the predominant N receptor (Fig. 2a and b)—N1-2155 was expressed at very high levels and was very stable, being detectable for hours following heat shock induction (Fig. 4c, lanes 5 to 7). Loss of NFull appeared to be from cells normally expressing NFull as immunostaining of embryos showed that N1-2155 accumulated everywhere NFull is normally expressed (data not shown).
In the above experiments, the effect of N1-2155 on SuH could not be determined. One reason was that the heat shock itself increased the amount of SuH, both in embryos and cultured S2 cells (Fig. 4b, lanes 2 and 3 and lanes 7 and 8). Another possible reason is that N1-2155 required further modifications, like conversion into the heterodimeric receptor or Delta binding, to affect the amount of SuH. These could have been the reasons for the very modest effect of Da-Gal 4 driven expression of N1-2155 on SuH amount (Fig. 4a and b, lanes 5 and 6; the reduction was observed in four repetitions of the experiment). In order to overcome these limitations, the effect of NΔCterm on SuH amount was examined with molecules corresponding to the intracellular domains of NFull and NΔCterm, expressed through the daGal4 promoter.
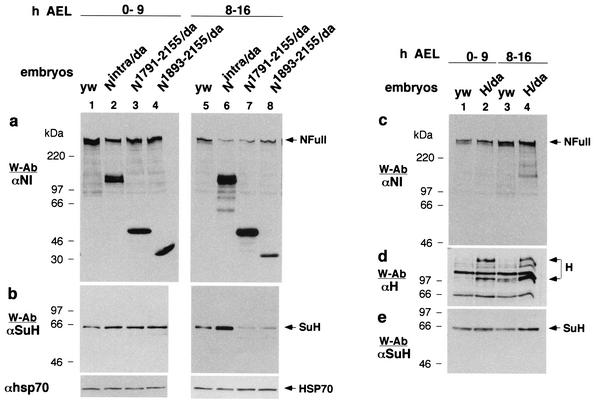
Nintra corresponds to the intracellular domain of NFull (27, 43). N1791-2155 corresponds to the intracellular domain of NΔCterm (it contains the sequence from near the end of transmembrane domain to the end of CDC10/ankyrin repeats). N1893-2155 is composed mostly of the CDC10/ankyrin repeats (7) and shows activities that are comparable to that of NΔCterm (47). The effect of expression of these molecules on NFull and SuH was examined in early- and mid-stage embryos (stages expressing relatively higher levels of NFull and SuH [Fig. 3a and b; Fig. 4a and b]). In early-stage embryos, all three molecules (Nintra, N1791-2155, and N1893-2155) increased the amount of SuH (Fig. 5a and b, lanes 1 to 4). The increase was modest but consistent (n = 5). In mid-stage embryos, Nintra increased the amount of SuH but N1791-2155 and N1893-2155 decreased it (Fig. 5a and b, lanes 5 to 8). All three molecules reduced expression of NFull in mid-stage embryos but had minimal effect in early-stage embryos (Fig. 5a, lanes 1 to 8). Hairless is known to bind both SuH and N (2, 8, 38, 46). Its overexpression did not reduce SuH amounts, as overexpression of N1791-2155 and N1893-2155 did, and did not reduce NFull amount as all N intracellular molecules did, indicating that the effects of N molecules were specific (Fig. 5c to e).
FIG. 5.
Intracellular domain molecules of NFull and NΔCterm have different effects on the amount of SuH in middle age embryos. (a and b) Da-Gal4 expression of the intracellular domain molecules of NΔCterm (N1791-2155/Da and N1893-2155/Da) reduces the amount of SuH in middle age embryos (lanes 5, 7, and 8) while that of the intracellular domain molecule of NFull (Nintra/Da) increases it (lanes 5 and 6). All molecules increased the amount of SuH in younger embryos (lanes 1 to 4) and of NFull in middle aged embryos (lanes 5 to 8). yw embryos served as controls. The same amount of total proteins was loaded in all lanes. (c to e) H does not affect the amount of SuH and NFull in the same manner. Total proteins extracted from wild-type embryos (yw) and embryos overexpressing Hairless using the Da-Gal 4 driver (H/Da) were used for the Western blots. (d) Joined arrows point to the two described forms of H (29). A separate blot generated along with those in panels c and e is shown.The same amount of total proteins was loaded in all lanes. Panels c and e are the same blots.
When N1791-2155 and N1893-2155 were grossly overexpressed using the hsGal4, daGal4, armadillo-Gal4, or actin-Gal4 drivers, the majority of embryos failed to hatch into larvae, just as those expressing Nintra from the same promoters (data not shown). Consistent with previously published results (27, 36, 43), they all produced epidermis at the expense of the CNS (data not shown). This is likely to be due to (i) the weak activities of N1791-2155 and N1893-2155 (23, 25, 30) producing higher than wild-type levels of SuH- and Nintra-dependent N signaling when grossly overexpressed, (ii) the residual effect of N1791-2155 and N1893-2155 activities that are similar to those of Nintra in early stages of embryogenesis (Fig. 5a and b, lanes 1 to 4), or (iii) repression of proneural cluster formation (prior to lateral inhibition) by constitutive activation of the deltex-dependent pathway by N1791-2155 and N1893-2155 (34).
Whether a proneural cell becomes the neuronal cell or the epidermal cell appears to be based on a 1.5- to 2-fold difference in SuH- and Nintra-dependent signaling (19). Lanes 1 and 2 in Fig. 3a give an idea of the maximum difference in levels of NFull and SuH between animals that are wild type and null for SuH- and Nintra-dependent N signaling (as SuH− embryos are). All heterologous promoters tested so far (12 in number) produce more than 10 times the amount of N produced in wild-type flies. The consequence of such general and high accumulation for processes acting on proteins (like enrichment for different forms of Notch in different cells) is also unknown. Thus, although the heterologous promoters were very useful in revealing the intrinsic differences in the activities of the intracellular domains of NFull and NΔCterm, they were inadequate for determining whether the negative effect of NΔCterm on NFull and SuH amounts translates to a reduction in SuH- and Nintra-dependent N signaling. Therefore, a Notch allele that produces an NΔCterm-like protein from the wild type Notch promoter was used to determine whether loss of SuH- and Nintra-dependent N signaling is associated with increased amount of the intracellular domain of NΔCterm-like protein and decreased amounts of NFull and SuH.
Loss of N signaling is associated with an increased amount of NΔCterm-like protein.
N60g11 is a temperature sensitive allele of the Notch gene that produces an NΔCterm-like protein due to a frame shift mutation just carboxyl terminus of the CDC 10/ankyrin repeats (28, 47). N60g11/+ flies show gain of lateral inhibition signaling (SuH- and Nintra-dependent or Deltex-dependent N signaling) at 18°C (manifest as loss of bristles) and loss at room temperature or higher (manifest as notched wings); wing development at 18°C and bristle development at higher temperatures are normal (6, 28, 34). In our experiments, wing notching was observed in 0% of N60g11//yw flies reared at 18°C (with variable loss of bristles), in 30% of flies reared at 25°C, and in 91% of the flies reared at 30°C (with no loss of bristles at 25 and 30°C). In the same experiments, yw flies did not show wing notching or loss of bristles at any temperature (300 flies were scored in each instance and the experiment was repeated twice). Based on the data presented in Fig. 4 and 5, N60g11/yw larvae were expected to accumulate higher levels of the intracellular domain of N60g11 (an N1791-2155-like molecule), and lower levels of NFull and SuH, with increasing temperature when compared with levels in yw/yw (FM7 Act-GFP/yw) larvae. Results of Western blotting experiments (n = 3) showed realization of these expectations. The amount of an N1791-2155-like molecule increased with temperature; the levels of NFull and SuH in N60g11/yw larvae were higher than those in yw/yw larvae in larvae reared at 18°C but lower in larvae reared at 25 and 30°C (Fig. 6). The results were consistent in three repetitions of the experiments. The difference in the amount of SuH in N60g11/yw and yw/yw larvae was much stronger at 25°C than at 30°C (Fig. 6c). This is possibly due to heat shock induction of SuH (Fig. 4b; data not shown). It is possible that the much stronger accumulation of N1791-2155-like molecule at 30°C more than overcame the effect of heat shock induced increase in SuH amount. The mechanism by which N60g11 promotes accumulation of NFull at 18°C is unknown (N60g11 might be affecting normal processing and trafficking of NFull).
The above experiment with N60g11 showed that the decrease in SuH- and Nintra-dependent N signaling is likely to be due to increase in amounts of an NΔCterm-like molecule. Although possible, it is unlikely to be due to temperature-dependent reduction in NFull amount (that has nothing to do with N60g11) because N-/yw flies show reduced frequency of wing notching at 30°C compared with the frequency at 25°C, as could be expected with increase in SuH amount (data not shown). It is also unlikely to be due to the Deltex-dependent N signaling (34), as N60g11/yw flies that are reared at 25°C or above do not show any microchaetae phenotypes (28). On the contrary, they show a high frequency of wing notching, a phenotype related to loss of SuH- and Nintra-dependent N signaling (Deltex-dependent N signaling is not involved in this phenotype [34]). These observations indirectly argued that NΔCterm-like molecules, expressed close to the wild-type levels and in the pattern dictated by the wild-type promoter, are null for SuH- and Nintra-dependent N signaling. They did not show whether NΔCterm-like proteins expressed in this manner are null for SuH- and Nintra-dependent N signaling in third instar larvae (this has been shown in embryos [6]). This issue was examined by generating N60g11/N60g11 somatic clones at the temperature where N60g11/+ flies do not produce wing notching, i.e., at the room temperature (∼22°C). More than 45% (174 of 386) of N60g11 FRT101/ovoD1 FRT 101 flies with the capability to produce N60g11/N60g11 somatic clones, reared at room temperature or above, showed wing notching when somatic recombination was induced by heat shock, compared with 0% (0 of 518) in the control ovoD1 FRT101/FM7 lac z flies. Wing notching was not observed when somatic recombination was not induced (in 409 and 314 flies, respectively). The frequency of wing notching was slightly higher at 25 and 30°C, and slightly lower at 18°C, as could be expected from N60g11 suppressing or promoting accumulation, respectively, of NFull in N60g11/yw mother cells (Fig. 6; data not shown). Thus, NΔCterm-like molecules, expressed close to wild-type levels and in the pattern dictated by the wild-type Notch gene promoter, are not only null for SuH- and Nintra-dependent N signaling but also have the potential to reduce this signaling by reducing the amount of NFull and SuH.
NFull promotes accumulation of SuH RNA more strongly than NΔCterm.
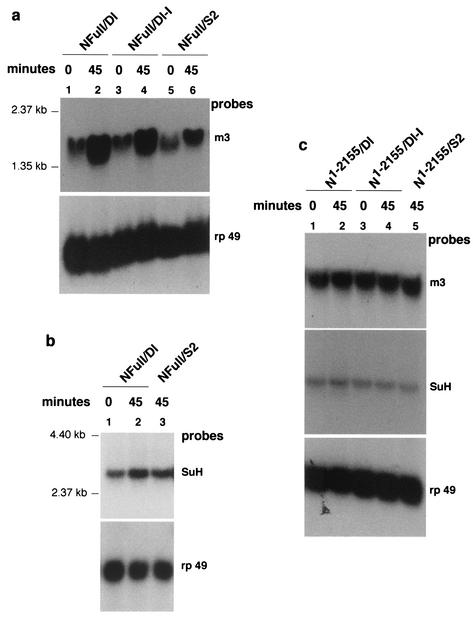
NFull is recently shown to activate the SuH- and Nintra-dependent N signaling pathway target gene Enhancer of split m3 in S2 cell aggregation assays (30a). Both S2-NFull and S2- N1-2155 used here bind Dl expressed on S2 cells and form aggregates with S2-Dl cells (47). Therefore, the effect of activation of NFull and NΔCterm (N1-2155) receptors on SuH gene [su(H)] RNA levels was examined in cell aggregation experiments. As expected, S2-NFull cells treated with S2-Dl cells promoted strong accumulation of the Enhancer of split m3 RNA (Fig. 7a, lanes 1 and 2). S2-NFull cells mixed with cells not expressing Dl (S2 cells) also showed significant response, presumably due to autoactivation of NFull at high levels of expression (Fig. 7a, lanes 5 and 6 [compare with lanes 1 and 2]). However, the response of S2-NFull cells mixed with S2 cells was always significantly lower than the response of S2-NFull cells mixed with S2-Dl cells (n = 5). This response of Enhancer of split m3 is due to the NFull intracellular domain: S2 cells expressing Dl lacking the intracellular domain (S2-Dl-I) gave a similar result (Fig. 7a, lanes 3 and 4) and S2-cells expressing NFull lacking the intracellular domain showed no response (data not shown). In these same experiments, S2-NFull cells were found to promote accumulation of su(H) RNA (Fig. 7b). The response was not as strong as the response of Enhancer of split m3 (compare Fig. 7b and a). This is not surprising, as the abundance of su(H) RNA is very low (both in S2 cells and embryos) compared with that of the Enhancer of split m3. More than 40 μg of total RNA had to be loaded per lane to obtain reasonable su(H) RNA signals, whereas Enhancer of split m3 response could be observed with just 10 μg of RNA (40 μg of RNA were loaded per lane in all these experiments to provide a measure of relative abundance and response).
FIG. 7.
NFull promotes accumulation of SuH RNA more strongly than NΔCterm. (a) NFull promotes accumulation of RNA of Enhancer of split m3, a target of SuH- and Nintra-dependent N signaling. The promotion is strong in the presence of Dl (compare lanes 2 and 4 with lane 6 in the upper panel). RNA was extracted from cells immediately after mixing of the different cells (0 min) or after 45 min. (b) NFull also promotes accumulation of SuH RNA. c. NΔCterm promotes expression of Enhancer of split m3 and su(H) RNA only weakly, if at all. The blots were reprobed with rp 49 probe to reveal relative levels of total RNA in the lanes. Forty micrograms of total RNA was loaded in all lanes. m3 and rp 49 probes were generated using the primers described by Mishra-Gorur et al. (30a). SuH probe was generated using the full coding sequence.
In parallel experiments, S2-N1-2155 treated with S2-Dl cells showed a weak response, if at all, with regard to both Enhancer of split m3 and su(H) genes (Fig. 7c). The Dl independent response (i.e., autoactivation) that was always observed with NFull was not observed with S2-N1-2155 (n = 3) even though (i) the amounts of N proteins expressed in the two cell lines are comparable (Fig. 3d) (47) and (ii) the rate and size of cell aggregation formation were similar (data not shown). These results are consistent with previous reports that N intracellular domains lacking the carboxyl terminus sequence (similar to that lacking in N1-2155) are poor activators of SuH and Nintra N signaling target genes, both in vivo and in vitro (23, 25, 30). Thus, NFull promotion of SuH accumulation could be at least in part due to promotion of su(H) RNA accumulation.
NΔCterm promotes ubiquitination of NFull and SuH derived fragments.
Mammalian Notch1 receptor is shown to be ubiquitinated and targeted for 26S proteasome or lysosome degradations (20, 32, 33). Analysis of embryonic extracts showed that an ubiquitinated form of SuH is the predominant form when SuH is low in embryos (Fig. 2b). These observations raised the possibility that increased expression of N1791-2155 would lead to ubiquitination of NFull and SuH that targets them for degradation. This possibility was explored in cultured cells.
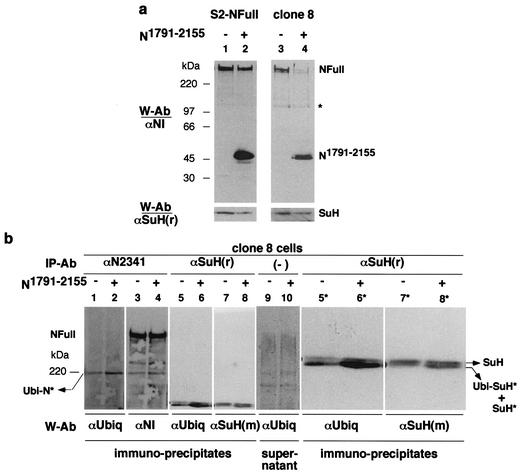
Transfection of heat shock induced N1791-2155 into S2-NFull cells resulted in reduced amounts of NFull and SuH (Fig. 8a, lanes 1 and 2). Clone 8 cells express NFull endogenously and respond to S2-Dl cells just as S2-NFull cells do (C. S. Wesley, personal observation). Transfection of N1791-2155 into clone 8 cells resulted in stronger reductions in the amounts of NFull and SuH (Fig. 8a, lanes 3 and 4). While the S2 cells are derived from embryos, the clone 8 cells are derived from imaginal disks (45). This developmental stage difference in origins, and the embryonic stage-dependent response of SuH to N1791-2155 expression (Fig. 5a and b), might be the bases for difference in the responses of S2-NFull and clone 8 cells.
FIG. 8.
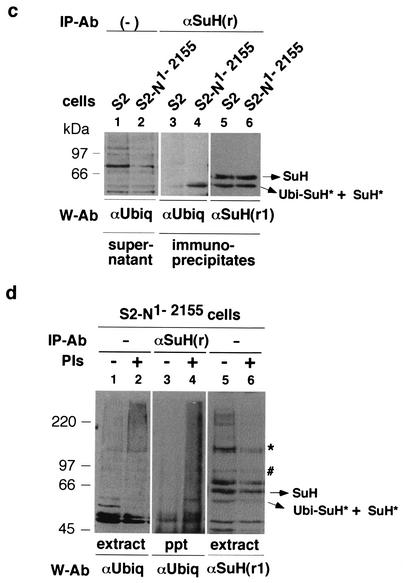
NΔCterm promotes accumulation of ubiquitinated N and SuH fragments. (a) Increased expression of N1791-2155 in clone 8 cells results in a stronger loss of NFull and SuH than in S2-NFull cells. Compare lanes 3 and 4 with lanes 1 and 2. Equal amounts of total proteins were loaded in lanes 1 and 2, and in lanes 3 and 4. The band marked with an asterisk serves as an indicator of relative loading. Proteins were electrophoresed in SDS-8% PAGE gels. (b) N1791-2155 promotes accumulation of ubiquitinated N (Ubi-N*) and a smaller SuH fragment (SuH*) that is ubiquitinated (Ubi-SuH*; see c below). Compare lanes 2 and 4 with lanes 1 and 3 for N; lanes 6 and 8 with lanes 5 and 7 for SuH. Panels 5*, 6*, 7*, and 8* are magnified (×2) images of relevant portions of panels 5 to 8. The blot in lanes 1 and 2 was reprobed for lanes 3 and 4, the blot in lanes 5 and 6 for lanes 7 and 8. Lanes 9 and 10 is the blot of supernatants of SuH immunoprecipitates indicating the levels of total proteins in extracts. The blot of supernatants of N immunoprecipitates was almost identical (not shown). Proteins were electrophoresed in SDS-4% PAGE gels. (c) N1-2155 also promotes accumulation of Ubi-SuH* (lanes 3 and 4). Only a fraction of SuH* molecules appear to be ubiquitinated as the amount of SuH* was similar in S2 and S2-N1-2155 cells (lanes 5 and 6). Lanes 1 and 2 indicate the total amount of proteins in the extracts used for lanes 3 and 4. Lanes 5 and 6 show the blot from lanes 3 and 4 reprobed with an SuH antibody. Proteins were electrophoresed in SDS-8% PAGE gels. (d) Treatment of S2-N1-2155 cells with 26 S proteasome inhibitors (M115 + lactacystin) promotes accumulation of ubiquitinated proteins as expected (lanes 1 and 2) but loss of SuH proteins (lanes 3 and 4). SuH is recognized by polyclonal rat αSuH (r) from F. Schweisguth, by polyclonal mouse αSuH (m) from S. Kidd and T. Lieber, by polyclonal rat αSuH (r1) generated by us (three different animals), and by the monoclonal rat αSuH antibody from S. Artavanis-Tsakonas. SuH* is recognized weakly by αSuH (r) and αSuH (m); the latter more so than the former. The band marked with an asterisk is also recognized by αSuH (m) (strongly) and αSuH (r) (weakly). The band marked with # is also recognized weakly by αSuH (r). The remaining bands shown in lane 4 are associated with heat shock induced SuH. Whether or not they are SuH fragments or related proteins is unknown. PIs, protease inhibitors.
In further experiments, clone 8 cells were transfected with N1791-2155 and total proteins extracted about 7 h after induction of expression. Although longer or overnight incubations yielded the greatest reductions in the expression of NFull and SuH, shorter incubations yielded the best recovery of ubiquitinated N and SuH fragments. However, at this time period, reductions in NFull and SuH were not always obvious indicating that all forms of these proteins are rapidly degraded once a certain level of ubiquitination is attained. NFull and SuH were immunoprecipitated from the total protein extracts and analyzed by SDS-4% PAGE to detect proteins in the whole range of sizes of SuH and N proteins followed in the study. NFull and SuH were immunoprecipitated from the same extracts, and the same amounts of extracts were used for each immunoprecipitation. An antibody specific to the N carboxyl terminus was used to avoid recovery of N1791-2155 molecules. The resulting Western blots were probed first with a ubiquitin antibody and subsequently reprobed with an N or SuH antibodies. Results of these experiments (n = 5) showed that immunoprecipitates from N1791-2155-treated samples contained higher levels of a ubiquitinated N fragment produced from the endogenous N, Ubi-N*, and a higher level of a smaller endogenous SuH fragment (SuH*) that is ubiquitinated, Ubi-SuH* (Fig. 8b, lanes 1 to 4 and 5 to 8, respectively; lanes 5* to 8* are magnifications [2×] of lanes 5 to 8; lanes 9 and 10 show that total proteins contents were similar in extracts used). Ubi-N* and Ubi-SuH* are considered to be partially degraded fragments as they migrate faster than NFull and full-length SuH. Note that the Ubi-SuH* fragment in embryos also migrated faster than SuH (Fig. 2b).
N1-2155 also promoted accumulation of Ubi-SuH* between three and five hours following heat shock induction (Fig. 8c). These experiments (n = 3) indicated that a fraction of SuH* is ubiquitinated because the levels of SuH* in S2 and S2-N1-2155 were comparable but the level of Ubi-SuH* was higher in S2-N1-2155 cells (Fig. 8c, lanes 3 to 6). Note that Ubi-SuH* produced in S2 cells is almost the same size as the Ubi-SuH* produced in embryos (compare Fig. 8c, lanes 3 to 6, with Fig. 2b, lanes 5 to 8). S2-N1-2155 cells were treated with protease inhibitors lactacystin and MG115 to determine if SuH is degraded by the 26 S proteasome. If it were, SuH was expected to accumulate in treated cells. Interestingly, S2-N1-2155 cells treated with protease inhibitors showed loss of SuH proteins (Fig. 8d, compare lane 6 with lane 5). There was a general increase in the level of ubiquitinated proteins in the sample indicating that the proteasome inhibitors had the expected effect in the experiments (Fig. 8d, lanes 1 and 2). Immunoprecipitates from aliquots of the same extracts showed an increase in Ubi-SuH* compared with mock-treated cells (Fig. 8d, lanes 3 and 4; the blot could not be exposed to film for the period used for lanes 3 and 4 of Fig. 8c because protease treatment increased background in immunoprecipitations). Thus, it appears that SuH is degraded by a protease whose accumulation is under the control of the 26 S proteasome, but not its activity. A reduction in the activity of the 26 S proteasome might result in accumulation of this protease leading to loss of SuH. We did not detect any significant effect with the lysosomal inhibitor chloroquine. Protease inhibitor experiments with NFull gave variable and inconclusive results possibly due to interactions between SuH-dependent and -independent processes (data not shown).
The SuH blot shown in Fig. 8d (lane 4) is the one probed with a newly made SuH antibody [αSuH(r1)] that recognizes many more fragments than any other antibody used in the study. SuH, SuH*, and Ubi-SuH* are recognized by four different antibodies (three polyclonals and one monoclonal), made in four different labs, in two different animals (rat and mouse). Therefore, these are the only bands considered to be SuH in this study. Three independent antibodies recognize the band marked with just an asterisk; two recognize the band marked with a pound sign. These and few other bands are present at very low levels in embryonic, larval, or pupal extracts and become apparent upon overexposure of the blots to films, or covary with heat shock induced SuH (data not shown). Future studies might reveal the relation, if any, of these fragments to the function and degradation of SuH and how this degradation relates to ubiquitination and degradation of NFull.
DISCUSSION
Production of SuH- and Nintra-dependent N signaling at any time during CNS differentiation results in loss of CNS cells (27, 43). But, both N and Delta are required for, and present during, differentiation of the CNS from the segregating neuroblasts (16, 17, 22, 24, 47). Furthermore, gross and nonspecific overexpression of Delta fails to perturb either the segregation of neurobalsts or the development of the CNS from the segregating neuroblasts (39). These observations indicate that some mechanism other than Delta distribution exists in embryos to (i) reduce SuH- and Nintra-dependent N signaling in the segregating neuroblasts and (ii) to maintain it at this low level, or even eliminate it, during differentiation of these cells into the CNS. The first task might be accomplished by enriching for NΔCterm. The carboxyl terminus of N is required for production of high amounts of SuH- and Nintra-dependent N signaling (Fig. 7) (23, 25, 30). NΔCterm lacks this sequence (47), shows weak SuH- and Nintra-dependent N signaling activity (Fig. 7; our clonal analysis) (6), and is enriched in the segregating neuroblasts and the developing CNS (47) (Fig. 1). The second task might be accomplished by a mechanism involving the differential stability of NΔCterm and NFull when SuH amount is low (Fig. 2 and 3) and the effect of NΔCterm on NFull and SuH ubiquitination and stability (Fig. 4, 5, and 8). These possibilities are supported by the observation that the level of NΔCterm is high and those of NFull and SuH are low when SuH- and Nintra-dependent N signaling is low during development (Fig. 6).
The observation that NFull promotes accumulation of both Enhancer of split m3 and su(H) does not necessarily mean that it regulates both these genes by the same mechanism. It is possible that NFull promotes su(H) RNA accumulation through an in-direct, hitherto unknown, mechanism. It might also be a Dl independent mechanism because NFull and NΔCterm-like N1-2155 differed the most (qualitatively) in their Dl independent effect on Su(H) RNA accumulation (Fig. 7). Interestingly, Dl expression ceases in segregating neuroblasts immediately after their specification and resumes later on during differentiation of these neuroblasts into the CNS (24).
Besides the transcriptional activation sequence, the carboxyl terminus present in NFull but absent in NΔCterm also contains: (i) one of the binding sites of Numb, an endocytic protein known to suppress N signaling (18, 37, 40) and (ii) the PEST sequence involved in protein turnover (35). These sequences might be important elements of the mechanism governing SuH-dependent stability of NFull. Thus, the N carboxyl terminus might be a target for regulation in instances where one of two developing tissues requires a high amount of SuH- and Nintra-dependent N signaling and the other requires a low amount or none. For example, retention or removal of the N carboxyl terminus might determine whether a proneural cell becomes the epidermal cell or the CNS cell, respectively.
The stability of NFull might be dependent on the amount of SuH available to it rather than on the amount of SuH in the cell. This could explain why overexpression of N1-2155, Nintra, N1791-2155, and N1893-2155 all lead to loss of NFull, independent of the amount of SuH (Fig. 4 and 5a and b), and why N60g11/yw larvae at 30°C are associated with a stronger reduction in SuH- and Nintra-dependent N signaling (compared with larvae at 25°C) despite the heat shock related increase in SuH. N1-2155, Nintra, N1791-2155, N1893-2155, and the intracellular domain of N60g11 all contain the SuH binding sites, RAM 23 and CDC10/ankyrin repeats regions (14, 23, 44; personal observation), and they might have titrated SuH away from the endogenous NFull. Thus, enrichment for NΔCterm in the segregating neuroblasts might first reduce the amount of SuH- and Nintra-dependent N signaling compared with other cells since it is has a weaker activity than NFull. Subsequently, over time, it might produce complete loss of SuH and NFull by promoting their ubiquitination and degradation. This two-step gradual process is supported by the observation that reductions in NFull and SuH amounts are not obvious in the segregating neuroblasts even though these cells must have reduced SuH- and Nintra-dependent N signaling compared with their neighboring cells to escape the epidermal fate.
Since NFull is unstable in the absence of SuH, it is very likely that NΔCterm reduces the amount of SuH and this in turn reduces the amount of NFull. SuH is a very stable protein. The maternally contributed SuH can function until the end of embryogenesis, i.e., for 20 to 24 h (15). It is possible that the only way to reduce SuH amount on a much shorter time scale, as during differentiation of a tissue, is by developmental stage- or cell-specific degradation. The different effects of NΔCterm on SuH amount in early- and middle-stage embryos could be due to the difference in the ability of these embryos to degrade SuH. In early-stage embryos, without the ability to degrade SuH, both the weakly active NΔCterm and strongly active NFull might merely add to the stable pool of SuH. In middle-stage embryos, with the ability to degrade SuH, the difference in the effects of NΔCterm and NFull on SuH accumulation in conjunction with the difference in the effect of SuH on NΔCterm and NFull stabilities might provide a mechanism for increasing SuH- and Nintra-dependent N signaling in some cells while decreasing the same in others.
Alleles resembling NΔCterm and N60g11, the Mcd Notch alleles, have been shown to constitutively activate a deltex-dependent pathway that suppresses neural fates by suppressing formation of the proneural clusters at a stage preceding lateral inhibition (5, 6, 7, 34). The activity of NΔCterm at the embryonic proneural stage was not explored in this study as it behaved similarly to NFull at this stage (Fig. 5a). This deltex-dependent pathway is unlikely to have been involved in reduction of SuH- and Nintra-dependent N signaling in N60g11/yw flies reared at 25 or 30°C, because these flies do not show the microchaetae phenotype associated with Mcd alleles but show a high frequency of the wing margin phenotype (reference 28 and this study) that is not associated with Mcd alleles (34). Interestingly, Ramain et al. (34) report that loss of SuH enhanced the effect of Mcd alleles, indicating that these alleles too might negatively affect SuH accumulation, just as NΔCterm-like molecules did in this study. Thus, the developmental stage- or tissue-specific reduction of SuH might be an important function of NΔCterm, albeit with different consequences: promotion of neurogenesis during lateral inhibition stage but its suppression at an earlier stage.
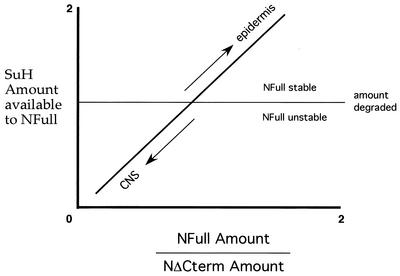
Based on (i) NFull, NΔCterm, and SuH activities described above, (ii) NΔCterm behaving as a null allele when expressed near wild-type levels, and (iii) and the indication that lateral inhibition is based on an initial 1.5- to 2-fold difference in activities, a model for the mechanism by which SuH- and Nintra-dependent N signaling is increased in the developing epidermis but decreased in the developing CNS during lateral inhibition in vivo is proposed in Fig. 9. It might serve as a useful framework for further studies. Commitment of cells to become the epidermis or the CNS takes place when SuH is being actively degraded in the embryos. Cells with a higher NFull/NΔCterm ratio are able to accumulate SuH above the amount lost to degradation. Increase in SuH amount increases the availability of SuH to NFull and thereby the NFull/NΔCterm ratio (as it would stabilize NFull), which in turn would further increase SuH amount. This positive loop would lead to stable production of the SuH- and Nintra-dependent N signals in the epidermis cells. On the other hand, cells with a lower NFull/NΔCterm ratio are not only unable to accumulate SuH above the amount lost to degradation but also increase SuH degradation. A low level of SuH would decrease the availability of SuH to NFull and thereby the NFull/NΔCterm ratio (as it would destabilize NFull), which in turn would further decrease the amount of SuH and NFull. This negative loop would lead to loss of NFull and SuH and thereby the ability to produce SuH- and Nintra-dependent signals in the CNS cells. The most interesting feature of this model is that the interactions among the various components of a single signal transduction pathway are engineered to produce a developmental toggle switch, rather than an on-off switch that sends differentiating cells along one pathway or the other. Proteolytic removal of the carboxyl terminus of N, which yields NΔCterm from NFull, appears to function as the button of this switch.
FIG. 9.
A model of the mechanism by which the activities of NFull, NΔCterm, and SuH promote SuH- and Nintra-dependent N signaling in the developing epidermis but suppress it in the developing CNS, in middle-stage embryos.
Acknowledgments
We thank T. Lieber and S. Kidd for N materials; M. Young for his support of initial parts of the study; F. Schweisguth and V. Morel for SuH materials; A. Preiss and D. Maier for H antibody; J. Posakony for the UAS-H stock; A. Martinez-Arias for the N60g11 FRT stock; M. Rand and S. Artavanis-Tsakonas for the SuH monoclonal antibody; the Drosophila Stock Center for the daGal4, hsGal4, and other fly stocks; L. Saez, and T. Lieber, S. Kidd, R. Cagan, N. Baker, and U. Wesley for comments on the manuscript.
This work was supported by the NIH (NINDS) grant NS43122-01 to C.S.W.
REFERENCES
- 1.Bailey, A. M., and J. W. Posakony. 1995. Suppressor of Hairless directly activates transcription of Enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 9:2609-2622. [DOI] [PubMed] [Google Scholar]
- 2.Bang, A. G., A. M. Bailey, and J. W. Posakony. 1995. Hairless promotes stable commitment to the sensory organ precursor cell fate by negatively regulating the activity of the Notch signaling pathway. Dev. Biol. 172:479-494. [DOI] [PubMed] [Google Scholar]
- 3.Barolo, S., R. J. Walker, A. D. Polyanovsky, G. Freschi, T. Keil., and J. W. Posakony. 2000. A Notch-independent activity of Suppressor of Hairless is required for normal mechanoreceptor physiology. Cell 103:957-969. [DOI] [PubMed] [Google Scholar]
- 4.Brand, A. H., and N. Perrimon. 1993. Targetted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 5.Brennan, K., M. Baylies, and A. Martinez-Arias. 1999. Repression by Notch is required before Wingless signaling during muscle progenitor cell development in Drosophila. Curr. Biol. 9:707-710. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, K. R., R. Tateson, T. Lieber, J. P. Couso, V. Zecchini, and A. Martinez-Arias. 1999. The abruptex mutations of Notch disrupt the establishment of proneural clusters in Drosophila. Dev. Biol. 216:230-242. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, K., R. Tateson, K. Lewis, and A. Martinez-Arias. 1997. A functional analysis of Notch mutation in Drosophila. Genetics 147:177-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brou, C., F. Logeat, M. Lecourtois, J. Vandekerckhove, P. Kourilsky, F. Schweisguth, and A. Israel. 1994. Inhibition of the DNA-binding activity of Drosophila Suppressor of Hairless and of its human homolog, KBF2/RBP-Jk, by direct protein-protein interaction with Drosophila Hairless. Genes Dev. 8:2491-2503. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera, C. V. 1990. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development 109:733-742. [PubMed] [Google Scholar]
- 10.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Strooper, B., W. Annaert, P. Cupers, P. Saftig, K. Craessaerts, J. S. Mumm, E. H. Schroeter, V. Schrijvers, M. S. Wolfe, W. J. Ray, A. Goate, and R. Kopan. 1999. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398:518-522. [DOI] [PubMed] [Google Scholar]
- 12.Fehon, R. G., K. Johansen, I. Rebay, and S. Artavanis-Tsakonas. 1991. Complex cellular and subcellular regulation of Notch expression during embryonic and imaginal development of Drosophila: Implications for Notch function. J. Cell Biol. 113:657-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehon, R. G., P. J. Kooh, I. Rebay, C. L. Regan, T. Xu, M. Muskavitch, and S. Artavanis-Tsakonas. 1990. Molecular interaction between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61:523-534. [DOI] [PubMed] [Google Scholar]
- 14.Fortini, M. E., and S. Artavanis-Tsakonas. 1994. The Suppressor of Hairless protein participates in Notch receptor signaling. Cell 79:273-282. [DOI] [PubMed] [Google Scholar]
- 15.Gho, M., M. Lecourtois, G. Geraud, J. W. Posakony, and F. Schweisguth. 1996. Subcellular localization of Suppressor of Hairless in Drosophila sense organ cells during Notch signaling. Development 122:1673-1682. [DOI] [PubMed] [Google Scholar]
- 16.Giniger, E., L. Y. Jan, and Y. N. Jan. 1993. Specifying the path of the intersegmental nerve of the Drosophila embryo: a role for Delta and Notch. Development 117:431-440. [DOI] [PubMed] [Google Scholar]
- 17.Giniger, E. 1998. A role for Abl in Notch signaling. Neuron 20:667-681. [DOI] [PubMed] [Google Scholar]
- 18.Guo, M., L. Y. Jan, and Y. N. Jan. 1996. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron 17:27-41. [DOI] [PubMed] [Google Scholar]
- 19.Heitzler, P., and P. Simpson. 1991. The choice of cell fate in the epidermis of Drosophila. Cell 64:1083-1092. [DOI] [PubMed] [Google Scholar]
- 20.Jehn, B. M., I. Dittert, S. Beyer, K. von der Mark, and W. Bielke. 2002. c-Cbl binding and ubiquitination-dependent lysosomal degradation of membrane-associated Notch1. J. Biol. Chem. 277:8033-8040. [DOI] [PubMed] [Google Scholar]
- 21.Jennings, B., A. Preiss, C. Delidakis, and S. Bray. 1994. The Notch signaling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development 120:3537-3548. [DOI] [PubMed] [Google Scholar]
- 22.Kidd, S., M. K. Baylies, G. P. Gasic, and M. W. Young. 1989. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 3:1113-1129. [DOI] [PubMed] [Google Scholar]
- 23.Kidd, S., T. Lieber, and M. W. Young. 1998. Ligand induced cleavage and regulation of nuclear entry of Notch in Drosophila melanogaster embryos. Genes Dev. 12:3728-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kooh, P. J., R. G. Fehon, and M. A. T. Muskavitch. 1993. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development 117:493-507. [DOI] [PubMed] [Google Scholar]
- 25.Kurooka, H., K. Kuroda, and T. Honjo. 1998. Roles of the ankyrin repeats and C-terminal region of the mouse Notch1 intracellular region. Nucleic Acids Res. 26:5448-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecourtois, M., and F. Schweisguth. 1995. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split Complex genes triggered by Notch signaling. Genes Dev. 9:2598-2608. [DOI] [PubMed] [Google Scholar]
- 27.Lieber, T., S. Kidd, E. Alcamo, V. Corbin, and M. W. Young. 1993. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 7:1949-1965. [DOI] [PubMed] [Google Scholar]
- 28.Lyman, D., and M. W. Young. 1993. Further evidence for function of the Drosophila Notch protein as a transmembrane receptor. Proc. Natl. Acad. Sci. USA 90:10395-10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier, D., J. Marquart, A. Thompson-Fontaine, I. Beck, E. Wurmbach, and A. Preiss. 1997. In vivo structure-function analysis of Drosophila HAIRLESS. Mech. Dev. 67:97-106. [DOI] [PubMed] [Google Scholar]
- 30.Matsuno, K., M. J. Go, X. Sun, D. E. Eastman, and S. Artavanis-Tsakonas. 1997. Suppressor of Hairless-independent events in Notch signaling imply novel pathway elements. Development 124:4265-4273. [DOI] [PubMed] [Google Scholar]
- 30a.Mishra-Gorur, K., M. D. Rand, B. Perez-Villamil, and S. Artavanis-Tsakonas. 2002. Down regulation of Delta by proteolytic processing. J. Cell Biol. 159:313-324. [DOI] [PMC free article] [PubMed]
- 31.Morel, V., and F. Schweisguth. 2000. Repression by Suppressor of Hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 14:377-388. [PMC free article] [PubMed] [Google Scholar]
- 32.Oberg, C., J. Li, A. Pauley, E. Wolf, M. Gurney, and U. Lendahl. 2001. The notch intracellular domain is ubiquitinated and negatively regulated by the mammalian sel-10 homolog. J. Biol. Chem. 276:35847-35853. [DOI] [PubMed] [Google Scholar]
- 33.Qiu, L., C. Joazeiro, N. Fang, H-Y. Wang, C. Elly, Y. Altman, D. Fang, T. Hunter, and Y.-C. Liu. 2000. Recognition and ubiquitination of Notch by Itch, a Hect-type E3 ubiquitin ligase. J. Biol. Chem. 275:35734-35737. [DOI] [PubMed] [Google Scholar]
- 34.Ramain, P., K. Khechumian, L. Seugnet, N. Arbogast, C. Ackermann, and P. Heitzler. 2001. Novel alleles reveal a Deltex-dependent pathway repressing neural fate. Curr. Biol. 11:1729-1738. [DOI] [PubMed] [Google Scholar]
- 35.Rechsteiner, M. 1988. Regulation of enzyme levels by proteolysis: the role of pest regions. Adv. Enzyme Regul. 27:135-151. [DOI] [PubMed] [Google Scholar]
- 36.Roehl, H., and J. Kimble. 1993. Control of cell fate in C. elegans by a Glp-1 peptide consisting primarily of ankyrin repeats. Nature 264:632-635. [DOI] [PubMed] [Google Scholar]
- 37.Santolini, E., C. Puri, A. E. Salcini, M. C. Gagliani, P. G. Pelicci, C. Tacchetti, and P. P. Di Fiore. 2000. Numb is an endocytic protein. J. Cell Biol. 151:1345-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweisguth, F., and J. W. Posakony. 1994. Antagonist activities of Suppressor of Hairless and Hairless control alternative cell fates in the Drosophila adult epidermis. Development 120:1433-1441. [DOI] [PubMed] [Google Scholar]
- 39.Seugnet, L., P. Simpson, and M. Haenlin. 1997. Transcriptional regulation of Notch and Delta: requirement for neuroblast segregation in Drosophila. Development 120:2015-2025. [DOI] [PubMed] [Google Scholar]
- 40.Spana, E., and C. Doe. 1996. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21-26. [DOI] [PubMed] [Google Scholar]
- 41.Struhl, G., and A. Adachi. 1998. Nuclear access and action of Notch in vivo. Cell 93:649-660. [DOI] [PubMed] [Google Scholar]
- 42.Struhl, G., and I. Greenwald. 1999. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398:522-525. [DOI] [PubMed] [Google Scholar]
- 43.Struhl, G., K. Fitzgerald, and I. Greenwald. 1993. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74:331-345. [DOI] [PubMed] [Google Scholar]
- 44.Tamura, K., Y. Taniguchi, S. Minoguchi, T. Sakai, T. Tun, T. Furukawa, and T. Honjo. 1995. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-jκ/Su(H). Curr. Biol. 5:1416-1423. [DOI] [PubMed] [Google Scholar]
- 45.Van Leeuwen, F., C. H. Samos, and R. Nusse. 1994. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature 368:342-344. [DOI] [PubMed] [Google Scholar]
- 46.Wang, S., S. Younger-Shepherd, L. Y. Jan, and Y. N. Jan. 1997. Only a subset of the binary cell fate decisions mediated by Numb/Notch signaling in Drosophila sensory organ lineage requires Suppressor of Hairless. Development 124:4435-4446. [DOI] [PubMed] [Google Scholar]
- 47.Wesley, C. S., and L. Saez. 2000. Analysis of Notch lacking the carboxyl terminus identified in Drosophila embryos. J. Cell Biol. 149:683-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye, Y., N. Lukinova, and M. E. Fortini. 1999. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature 328:525-552. [DOI] [PubMed] [Google Scholar]