Abstract
The cap-binding eukaryotic initiation factor eIF4E is phosphorylated by the mitogen-activated protein (MAP) kinase-interacting kinases (Mnk's). Three forms of the Mnk's exist in human cells: Mnk1, Mnk2a, and Mnk2b. These last two are derived from the same gene by alternative splicing and differ only at their C termini. While Mnk2a contains a MAP kinase-binding site in this region, Mnk2b lacks such a sequence and is much less readily activated by MAP kinases in vitro. Expression of Mnk2b in mammalian cells leads to increased phosphorylation of eIF4E, showing that it acts as an eIF4E kinase in vivo. While Mnk2a is cytoplasmic, a substantial amount of Mnk2b is found in the nucleus. Both enzymes contain a stretch of basic residues in their N termini that plays a role in binding to eIF4G and functions as a nuclear localization signal. Binding of eIF4G or nuclear import appears to be regulated by the C terminus of Mnk2a. Furthermore, the MAP kinase-binding site of Mnk2a regulates nuclear entry. Within the nucleus, Mnk2b and certain variants of Mnk2a that are present in the nucleus colocalize with the promyelocytic leukemia protein PML, which also binds to eIF4E.
Eukaryotic initiation factor (eIF) 4E binds to the cap structure at the 5′ end of eukaryotic mRNAs (41). eIF4E also binds the scaffold protein eIF4G, which in turn interacts with a number of other components of the translational machinery (10). These include the helicases eIF4AI and eIF4AII, the poly(A)-binding protein PABP, and the multisubunit factor eIF3, which binds to the 40S subunit of the ribosome and thus recruits it to the 5′ end of the mRNA. Control of the assembly of the resulting complex is important in regulating translation initiation. Overexpression of eIF4E can lead to cell transformation (4, 19), and microinjection of eIF4E drives progression of cells into the S phase of the cell cycle (40). Human tumors also frequently show high levels of expression of eIF4E (21, 27). These data imply that eIF4E plays an important role in cell proliferation.
eIF4E is a phosphoprotein whose phosphorylation is regulated in response to a range of stimuli. These include growth factors and mitogens (26, 47), cytokines (46, 47), and stressful stimuli (26, 47). Phosphorylation occurs at Ser209, near the C terminus of eIF4E (7, 15). Early data indicated that phosphorylation of eIF4E increased its affinity for capped mRNA (25), whereas a more recent study showed that phosphorylation of eIF4E decreased its affinity for the cap or a capped oligoribonucleotide (34). The possible importance of the phosphorylation of eIF4E in the translation initiation process has recently been reviewed (33).
Phosphorylation of eIF4E in response to insulin or phorbol esters requires the classical mitogen-activated protein (MAP) kinase (Erk) pathway (8, 47). In contrast, cytokines induce phosphorylation of eIF4E via the p38 MAP kinase pathway (46, 47). Recent work has shown that the MAP kinase-interacting kinases Mnk1 and Mnk2 can both phosphorylate eIF4E. Each interacts with eIF4G (29, 35, 49), probably via a region in their N termini, thus recruiting the kinase to the initiation factor complex that also contains eIF4E. Mnk1 and Mnk2 are each activated by Erk or p38 MAP kinase α/β, and each contains, near its C terminus, a region that binds Erk and/or p38 MAP kinase. Mnk1 shows low basal activity that is increased by stimuli that activate these upstream kinases (9, 48), while Mnk2a shows relatively high basal activity (35).
In addition to its key regulatory role in cytoplasmic translation, eIF4E is also found in the nuclear compartment, where it shows a speckled distribution in immunocytochemical analyses (2, 6, 20). Nuclear eIF4E may play a role in the export of certain mRNAs from the nucleus to the cytoplasm. One such mRNA is that for cyclin D1, which is required for S-phase progression (2). This additional role of eIF4E may be linked to its effects on the cell cycle and proliferation. Nuclear eIF4E has recently been shown to interact with the promyelocytic leukemia protein PML in so-called PML or eIF4E bodies (2, 18). The interaction of eIF4E with PML decreases the affinity of eIF4E for mRNA and may help explain the antiproliferative effects of PML (2, 43).
An important recent report that prompts a major reassessment of our understanding of translation is the observation that this process may occur in the nuclear compartment as well as in the cytoplasm (12) (but see also references 3 and 28). Nuclear translation may have a role in “proofreading” of newly synthesized mRNAs (e.g., as part of the process of nonsense-mediated decay). Nuclear eIF4E is likely to be involved in nuclear translation, and several other translation factors, including its binding partner eIF4G, are also found in the nucleus (23).
A novel splice variant of Mnk2 was recently described (37). This arises from use of two alternative 3′ exons, which gives rise to mRNAs encoding Mnk2 polypeptides (termed Mnk2a and Mnk2b) that are identical throughout their N-terminal and catalytic regions but differ at their extreme C termini. Mnk2b lacks the MAP kinase binding site found in Mnk2a. Here we show that these two proteins are differentially localized in mammalian cells and that localization is determined by features in their N and C termini. Mnk2a is cytoplasmic, while Mnk2b is partially nuclear and colocalizes with the nuclear eIF4E bodies that contain eIF4E and PML. The extreme C terminus of Mnk2a appears to modulate the subcellular localization of Mnk2a and its binding to eIF4G. The finding that the nucleus contains a kinase that acts on eIF4E adds to the growing range of components of the translational machinery now known to be present in this organelle (23). Furthermore, nuclear Mnk2b colocalizes with the PML/eIF4E bodies, suggesting that it plays a role in phosphorylating eIF4E within these nuclear bodies. This study also reveals how differential splicing can contribute additional functional diversity to the repertoire of protein kinases encoded by the human genome.
MATERIALS AND METHODS
Plasmids.
pCS3MT, pEBG-Mnk1, and pEBG-Mnk2 have been described previously (32). ID Image clones 774097 (EMBL accession number HSAA42139) and 2369160 (EMBL accession number AI762433) were obtained from Research Genetics. pBlΔNMnk2b contains the human Mnk2b sequence from position 44 of the human Mnk2 coding region (position 66 in EMBL accession number AF125532) to position 1370 cloned into pBluescript between the EcoRI and XhoI restriction sites. The complete 5′ end was generated by PCR (with primers 5′-GGAATTCGATATCCATGGTGCAGAAGAAACC-3′ and 5′-CCCAGCACATCTTCCTG-3′) with clone 774097 as the template and was subsequently cloned into pBlΔNMnk2b as an EcoRI/AatII fragment, generating pBlMnk2b. The sequence encoding the C terminus of human Mnk2a was generated by PCR (with primers 5′-CCACTCCATGGTCCTGCAG-3′ and 5′-GGCTCGAGAATTCTCAGGCGTGGTCTCCCACC-3′) with clone 2369160 as the template and was cloned into pBlMnk2b as an NcoI/XhoI fragment to produce pBlMnk2a. The complete Mnk2a and Mnk2b sequences were then cloned into pCS3MT in two steps as NcoI/XhoI (3′-part) and NcoI (5′-part) fragments. Constructs for expression of green fluorescent protein (GFP) fusions were made by cloning the full-length sequences of Mnk2a and Mnk2b as HindIII/BamHI-digested PCR fragments into pEGFP-C1 (Clontech). The C-terminal deletions were created in a similar fashion. pEGFP constructs with the N terminus of human Mnk2 up to residue 60 or 70 were created by PCR amplification of these regions and cloning as EcoRI/BamHI fragments into pEGFP-C2 (Clontech). Constructs for expression of yellow fluorescent protein (YFP)-tagged eIF4E were made by PCR amplification of human eIF4E with EcoRI and BamHI sites and cloning into pEYFP-C1 (Clontech) or pEYFP-NLS (a kind gift from A. Fox, University of Dundee) digested with EcoRI and BamHI. This pEYFP-NLS construct codes for YFP with the nuclear localization signal (NLS) from the simian virus 40 large-T antigen (PKKKRKV). To generate variants lacking the basic region (ΔBR constructs), the two parts were generated by PCR and cloned in two steps as EcoRI/HindIII and HindIII/BamHI fragments. All PCRs were performed with proofreading polymerases (HiFi Expand; Roche). Site-directed mutagenesis to create mutations in the NLS of Mnk2b or in the ERK-binding region in Mnk2a, or to create theTrp73-to-Ala mutation in eIF4E, was carried out by using the QuikChange kit (Stratagene).
Cell culture and transfections.
Human embryonic kidney 293 cells were grown in 10-cm plates in Dulbecco's modified Eagle medium (Gibco BRL) supplemented with 10% fetal bovine serum (Gibco BRL). Transient transfections were carried out by calcium phosphate precipitation of the DNA in N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES)-buffered saline with cells at a density of approximately 6 × 105 to 8 × 105 per 6-cm plate or 1.5 × 106 to 2 × 106 per 10-cm plate. PD98059 and SB203580 (both from Calbiochem) were used at final concentrations of 50 and 10 μM, respectively.
Cell harvesting.
After treatments, cells were washed once with phosphate-buffered saline (PBS) and harvested in 400 μl of harvesting buffer (20 mM HEPES·KOH [pH 7.5], 50 mM β-glycerophosphate, 0.2 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM dithiothreitol, 0.5 mM sodium orthovanadate, 1 mM phenylmethanesulfonyl fluoride [PMSF], 1 mM benzamidine, 1 μg of leupeptin/ml, 1 μg of antipain/ml, and 1 μg of pepstatin/ml). Cell debris and nuclei were spun down for 1 min at 12,000 × g, and the supernatant was transferred to new tubes.
Cell fractionation.
Cells were harvested in 400 μl of EZ lysis buffer (Sigma) with the addition of 1 mM dithiothreitol, 0.5 mM sodium orthovanadate, 1 mM PMSF, 1 mM benzamidine, 1 μg of leupeptin/ml, 1 μg of antipain/ml, and 1 μg of pepstatin/ml. After centrifugation for 5 min at 1,000 rpm in an Eppendorf 5415D tabletop centrifuge at 4°C, the cytoplasmic fraction was transferred to a new tube. The nuclear pellet was washed with 500 μl of EZ lysis buffer after centrifugation and resuspended in water, and nuclei were lysed by addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer.
Antibodies.
The antibodies used to detect marker proteins in the different fractions were obtained from Santa Cruz Biotechnology (anti-α-tubulin [TU-02] and anti-lamin B [C-20]) and from Roche (anti-GFP, which also recognizes YFP). Phosphospecific antibodies for Mnk and ERK were obtained from Cell Signaling Technology, and the anti-Myc monoclonal antibody (9E10) was from Sigma. A monoclonal antibody was raised against recombinant eIF4E (in collaboration with B. Vojtesek, Academy of Sciences of the Czech Republic, Brno, Czech Republic), and the tissue culture supernatant was used for Western blotting following isoelectric focusing. C. Lyon (University of Dundee, Dundee, United Kingdom), L. de Jong (University of Rotterdam, Rotterdam, The Netherlands), T. Schulz (University of Utrecht, Utrecht, The Netherlands), and S. Morley (University of Sussex, Brighton, United Kingdom) kindly provided the following antibodies: mouse anti-p80 coilin monoclonal serum, mouse anti-PML monoclonal serum (monoclonal antibody 5E10) (44), mouse anti-glutathione S-transferase (anti-GST) monoclonal serum, and anti-phospho-eIF4E, respectively.
Horseradish peroxidase-conjugated secondary antibodies were from Diagnostics Scotland, and fluorescein isothiocyanate (FITC)- or tetramethyl rhodamine isothiocyanate (TRITC)-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories.
Immunoprecipitation.
For anti-Myc immunoprecipitations, 2 μl of antibody (9E10 from Sigma) was added to cell extracts and incubated for 1 h at 4°C. Subsequently, protein G beads (20 μl of packed beads per immunoprecipitation) in wash buffer (harvesting buffer without Triton X-100) were added and allowed to bind the antibody for an additional hour at 4°C. The beads were then washed three times with 500 μl of wash buffer and finally resuspended in SDS-PAGE loading buffer. For detection of Mnk-bound eIF4G, cells were harvested in 10 mM HEPES·KOH (pH 7.4)-50 mM sodium fluoride-2 mM EDTA-1% Triton X-100-14 mM β-mercaptoethanol-0.5 mM sodium orthovanadate-1 mM PMSF-1 mM benzamidine-1 μg of leupeptin/ml-1 μg of antipain/ml-1 μg of pepstatin/ml. The incubations with the antibody and protein and the subsequent wash steps were carried out with the same buffer.
Expression and purification of recombinant proteins.
Human eIF4E was expressed from a pET11d plasmid in Escherichia coli BL21(DE3) and purified as described previously (42). GST-Mnk2a and GST-Mnk2b were expressed from pGEX-HA plasmids in E. coli DH5α and purified as described previously for GST-Mnk1 and GST-Mnk2 (48).
Kinase assays.
Five-microliter aliquots of the beads with bound Myc fusion proteins, obtained as described above, were incubated in a total volume of 30 μl in 20 mM HEPES-KOH (pH 7.5)-50 mM KCl-2 mM MgCl2-200 μM ATP-1 μCi of [γ-32P]ATP-200 ng of recombinant eIF4E for 1 h at 30°C. The reaction was stopped by adding SDS-PAGE sample buffer and heating the sample for 5 min at 95°C. Samples were analyzed by SDS-PAGE and autoradiography.
Isoelectric focusing.
Endogenous eIF4E was purified by m7GTP-Sepharose chromatography, separated by isoelectric focusing, and detected by Western blotting as described previously (17, 32).
Immunocytochemistry.
All fixation, permeabilization, and immunostaining steps were performed at room temperature. HEK293 cells were grown on glass coverslips (no. 1), transfected as described above, and, after the indicated times, washed in PBS and fixed for 10 min with 2% paraformaldehyde in PBS. Permeabilization was performed with 0.2% Triton X-100 in PBS for 10 min. Cells were subsequently washed with PBS, incubated with 3% bovine serum albumin in PBS for 1 h, and then incubated overnight with the primary antibody at 4°C. Three washes with PBS were carried out before incubation with the secondary antibody for 1 h. Cells were washed in PBS, and nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). Cells grown on coverslips were mounted on microscope slides with Citifluor (agar), sealed, and left to dry before examination. Bars indicate 10 μm.
Microscopy and image analysis.
Immunostained specimens were examined by using a 100× NA 1.4 Plan-Apochromat objective. Three-dimensional images were recorded on a Nikon DeltaVision Restoration microscope (Applied Precision, Inc.). For each nucleus, 20 to 24 optical sections separated by 0.2 μm were recorded. Exposures were chosen such that images remained well above the camera dark current but below the 4,096-U maximum. Alternatively, images were obtained using a Zeiss Axiovert 2000M fluorescence microscope.
RESULTS
Both isoforms of Mnk2 phosphorylate eIF4E.
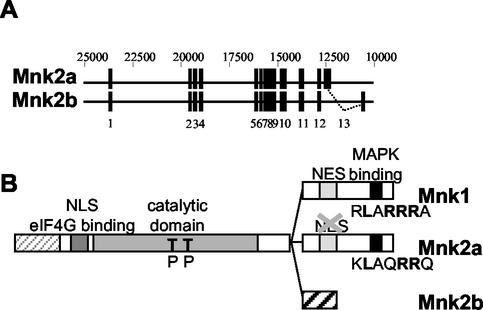
Earlier work has focused on Mnk1 and the previously identified splice variant of Mnk2 that contains a C-terminal region similar to that of Mnk1, including a binding site for MAP kinases (9, 38, 48). It is now clear that human cells possess a second splice variant in which the final exon of the previously identified Mnk2 mRNA is replaced by an alternative exon, giving rise to a completely different amino acid sequence at the extreme C terminus (Fig. 1A) (37). This splice variant (termed Mnk2b) lacks the MAP kinase-binding sites found in Mnk1 and in the other form of Mnk2, Mnk2a. However, its N-terminal and catalytic domains, and the first section of the C-terminal region, are identical to the corresponding parts of Mnk2a (Fig. 1B).
FIG. 1.
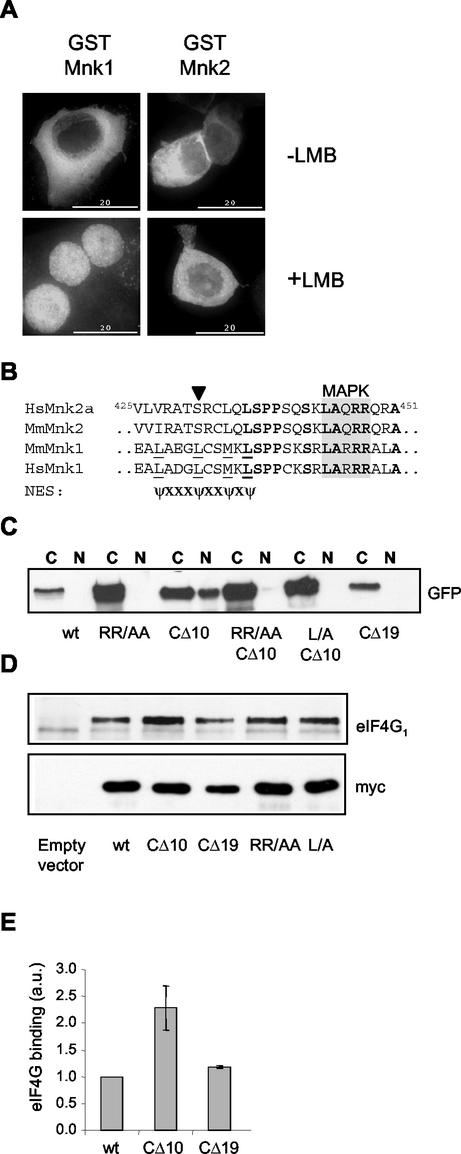
Schematic representation of the Mnk's. (A) Derivation of the mRNAs for Mnk2a and Mnk2b. Human Mnk2b is a splice variant that differs from human Mnk2b only in its C-terminal exon. The 12 exons that are shared by Mnk2a and Mnk2b, and the 13th exon, which is unique in each isoform, are indicated by solid rectangles. Numbering above the diagram refers to EMBL sequence AC007136. (B) Domains in Mnk1, Mnk2a, and Mnk2b. The different domains are discussed in the text. The human forms of Mnk2 contain an N-terminal extension (light grey striped area) of unknown function. All three Mnk's contain a basic region that has been proposed to be important in eIF4G binding (49), but this region could also contain an NLS. All variants contain a catalytic domain with conserved threonyl residues (T) in the activation domain that need to be phosphorylated (P) for activity. Mnk1 contains a consensus sequence for CRM-1-mediated nuclear export (NES). As shown in Fig. 6, one of the critical residues is not present in Mnk2a. Approximately 10 to 20 residues from the C termini of Mnk1 and Mnk2a contain a binding site for MAP kinases; the sequences for this binding site are given, with residues that are conserved among several MAP kinase substrates shown in boldface.
Mnk1 and Mnk2a have each been shown to phosphorylate eIF4E in vitro and to promote its phosphorylation in vivo (35, 49). Since Mnk2b contains the basic region in the N-terminal part of the protein that is thought to interact with eIF4G (49) and the same catalytic domain as Mnk2a, it was anticipated that Mnk2b would also phosphorylate eIF4E.
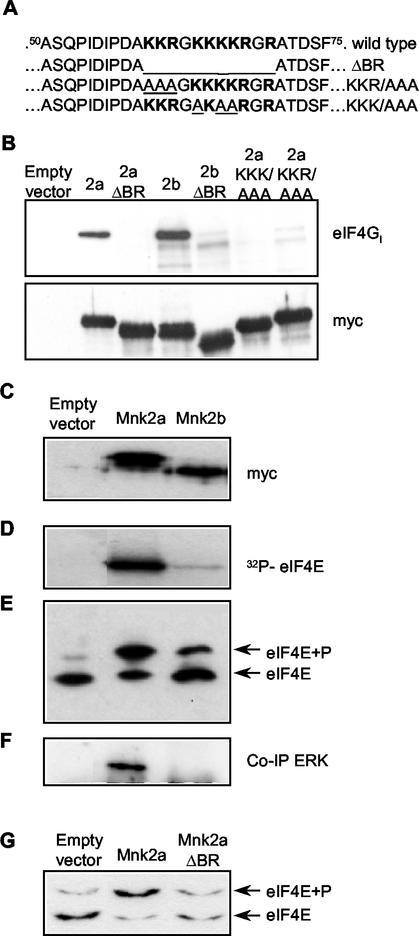
We first verified that Mnk2b could bind to eIF4G by expressing Mnk2b and, for comparison, Mnk2a in HEK293 cells as fusion proteins containing the Myc epitope tag. A region with basic residues (amino acids 60 to 70) is thought to be important for this interaction. This sequence is given in Fig. 2A. Three variants were created, one with this region completely deleted and two triple alanine mutants (Fig. 2A). All proteins were expressed at roughly similar levels, as judged by Western blotting of cell extracts with the anti-Myc antibody 9E10 (Fig. 2B, lower panel). The band observed for Mnk2b migrated slightly faster than that for Mnk2a, consistent with the smaller predicted size of the Mnk2b polypeptide. eIF4G was coimmunoprecipitated with Myc-tagged Mnk2a or Mnk2b, indicating that both isoforms bind this scaffold protein (Fig. 2B, upper panel). We consistently observed that relatively more eIF4G bound to Mnk2b than to Mnk2a, an issue which is discussed further below. Coimmunoprecipitation of endogenous eIF4G could not be detected with the empty vector or with constructs in which the basic region was deleted (Fig. 2A, ΔBR). Replacement of basic residues in either the first half (KKR/AAA) or the second half (KKK/AAA) of the basic region also abolished eIF4G binding, indicating that both sections of this region are required for stable eIF4G binding.
FIG. 2.
Mnk2a and Mnk2b can both phosphorylate eIF4E. (A) Amino acid sequences of residues 50 to 75 of human Mnk2a and Mnk2b. The basic region is boldfaced. The deletion of the whole region (in ΔBR) and the triple Ala mutations made in this study (KKR/AAA and KKK/AAA) are underlined. (B) The basic region is involved in eIF4G binding. HEK293 cells were transfected with the indicated constructs (the empty vector is pCS3MT), and after harvesting of the cells, Myc-tagged proteins were immunoprecipitated. Aliquots of the purified proteins were analyzed directly by Western blotting to determine expression levels (lower panel), and coimmunoprecipitated eIF4G was detected with antibodies directed against the C terminus of eIF4G (22). 2a, Mnk2a; 2b, Mnk2b. Both ΔBR constructs lack the sequence KKRGKKKKR (residues 60 to 68) (upper panel). (C) Expression of Mnk2a and Mnk2b. HEK293 cells were transiently transfected with the indicated pCS3MT constructs, and the cells were harvested as described in Materials and Methods. The Myc-tagged proteins were immunoprecipitated with anti-Myc antibodies, and the levels of expression were analyzed by Western blotting. (D) The immunoprecipitated kinases, as shown in panel B, were tested in vitro in the presence of [γ-32P]ATP for their abilities to phosphorylate recombinant eIF4E as described in Materials and Methods. The part of the autoradiogram showing labeled eIF4E is shown. (E) In vivo activitiesof human Mnk2a and human Mnk2b were assessed by purification of the endogenous eIF4E from extracts of the transfected cells and separation of the phosphorylated (eIF4E+P) and nonphosphorylated (eIF4E) forms by one-dimensional isoelectric focusing. (F) Coimmunoprecipitation of ERK was detected by probing the blot used in panel A with ERK-specific antibodies. (G) Binding of Mnk2a to eIF4G enhances eIF4E phosphorylation. HEK293 cells were transfected with an empty vector (pCS3MT) or vectors encoding Myc-tagged wild-type Mnk2a or Mnk2a with the basic region deleted. After harvesting of the cells, the phosphorylation state of endogenous eIF4E was analyzed by isoelectric focusing as described in Materials and Methods. Annotation is as for panel E.
To assess the abilities of Mnk2a and Mnk2b to phosphorylate eIF4E, each isoform was immunoprecipitated from extracts of HEK293 cells by using anti-Myc (Fig. 2C) and assayed for activity against recombinant eIF4E in vitro. The mouse ortholog of Mnk2a has high basal activity when expressed in HEK293 cells (35), and accordingly, Myc-tagged human Mnk2a also readily phosphorylated eIF4E (Fig. 2D). Myc-tagged Mnk2b expressed in HEK293 cells also phosphorylated eIF4E in vitro but displayed much lower activity than Mnk2a (Fig. 2D). The specificity of Mnk2b for the physiological site in eIF4E (Ser209) was confirmed by using purified recombinant proteins (see Fig. 3).
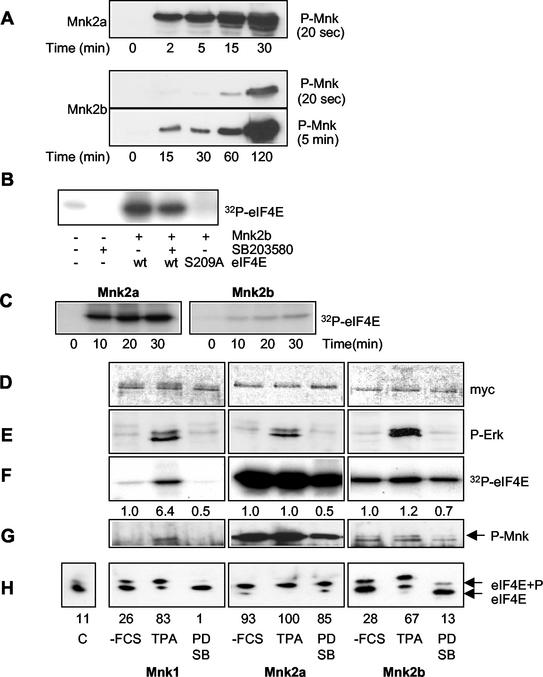
FIG. 3.
Regulation of Mnk2 activity. (A) Mnk2a is a much better substrate for MAP kinase in vitro than Mnk2b. Equal amounts (2 μg) of E. coli-expressed recombinant GST-Mnk2a (top panel) and GST-Mnk2b (bottom panels) were incubated with 6 mU of recombinant active p38 MAP kinase (kindly provided by the Division of Signal Transduction Therapy, University of Dundee) in a volume of 20 μl for the indicated times. The kinase reactions were then stopped by addition of SDS-PAGE sample buffer and heating of the sample at 95°C for 5 min. Phosphorylation of the threonines in the activation domain of the Mnk's was analyzed by Western blotting with phosphospecific antibodies (from Cell Signaling Technology). Two exposures are shown for Mnk2b, one of 20 s to allow direct comparison to the upper panel showing phosphorylation of Mnk2a, and one of 5 min to visualize phosphorylation of Mnk2b at earlier time points. The figure is an immunoblot. (B) In vitro-phosphorylated recombinant Mnk2b phosphorylates Ser209. Recombinant wild-type eIF4E or eIF4E(S209A) was incubated with recombinant Mnk2b that was activated as described above in the presence or absence of SB203580 as indicated below the gel. As a control the same amount of p38 MAPKα that was used to activate Mnk2b was also incubated with recombinant eIF4E. (C) Analysis of Mnk2a and Mnk2b activities. Recombinant GST-Mnk2a and GST-Mnk2b were phosphorylated as described above for 1 h; then the activity of p38 MAPKα was inhibited by addition of SB203580, and the activated Mnk's were incubated with recombinant eIF4E and [γ-32P]ATP for the indicated times. Phosphorylation of eIF4E was visualized by SDS-PAGE and autoradiography. (D) Expression of Myc-tagged Mnk1, Mnk2a, and Mnk2b. pCS3MT constructs encoding fusion proteins of Mnk1, Mnk2a, or Mnk2b were expressed in transfected HEK293 cells, and after the cells were harvested, the proteins were immunoprecipitated with antibodies against the Myc tag. Western blotting showed very similar expression levels of these proteins under three different conditions: −FCS, TPA, and PD SB. (i) −FCS (without fetal calf serum), after 26 h of transfection the growth medium was replaced byserum-free medium and cells were grown for an additional 16 h. (ii) TPA, cells were treated as described above, but 30 min before harvesting, they were stimulated with 500 μM TPA for 30 min. (iii) PD SB, cells were treated as described for the starved cells followed by incubation with 10 μM PD98059 and 50 μM SB203580 for 4 h before harvesting. These three conditions are indicated under panel H and apply to panels D through H. (E) Stimulation of the ERK pathway by TPA. The phosphorylation of Erk (P-Erk) was analyzed by Western blotting of the cell extracts with phosphospecific anti-Erk antibodies. (F) eIF4E kinase activity. Kinases were incubated with recombinant eIF4E and [γ-32P]ATP, and phosphorylation of eIF4E was visualized by SDS-PAGE and autoradiography. Numbers below the lanes are relative activities (as determined by using a phosphorimager [Fuji]) compared to that under the control condition (−FCS) for each separate kinase. (G) Phosphorylation of the Mnk's. The phosphorylation states of the three Mnk's under the different conditions were analyzed by Western blotting with antibodies directed against the phosphorylated threonines in the activation domain. (H) Assessment of in vivo kinase activity. The activities of the kinases were assessed by purification of endogenous eIF4E from the cell extracts by m7GTP-Sepharose chromatography, isoelectric focusing. and Western blotting with antibodies against eIF4E. The leftmost lane shows the level of eIF4E phosphorylation in untransfected control (C) cells. Numbers below the gel represent the percentage of eIF4E in its phosphorylated form, as determined by quantification of the data using ImageJ software (http://rsb.info.nih.gov/ij/).
To assess the abilities of Mnk2a and Mnk2b to phosphorylate eIF4E in vivo, extracts from HEK293 cells transfected with a vector for Mnk2a or Mnk2b (or, as a negative control, with the empty vector) were subjected to affinity chromatography on m7GTP-Sepharose followed by isoelectric focusing analysis and Western blotting. Levels of eIF4E phosphorylation were very low (<10%) in cells transfected with the empty vector. Expression of Mnk2a greatly increased eIF4E phosphorylation (to around 60 to 70%) (Fig. 2E), in line with earlier findings (32). Expression of Mnk2b also increased eIF4E phosphorylation to levels above those with the control, but to a lower extent than expression of Mnk2a (about 25 to 30%), consistent with its lower activity when assayed in vitro. These data indicate that Mnk2b phosphorylates eIF4E in vivo.
Mnk2b lacks the MAP kinase-binding motif present in Mnk2a (and Mnk1). Thus, one possible explanation for its lower activity compared to that of Mnk2a could be that it fails to bind stably to MAP kinases and consequently is less readily activated by them. To assess whether Mnk2b can bind stably to Erk in vivo, Myc-tagged Mnk2a and Mnk2b were immunoprecipitated from extracts of HEK293 cells, and immunoblots were prepared and probed with anti-Erk. While a strong signal for associated Erk was observed with Mnk2a, no detectable signal was observed for Mnk2b (Fig. 2F). Thus, Mnk2b appears not to bind stably to Erk, consistent with the absence of the C-terminal Erk-binding site found in Mnk1 and Mnk2a. Given that the same region also binds p38 MAP kinase (38), it is likely that Mnk2b also fails to bind this enzyme.
The importance of the binding of Mnk's to eIF4G for the phosphorylation of eIF4E in vivo was confirmed by expressing the ΔBR variant (Fig. 2A) of Mnk2a in transfected cells. The wild-type kinase phosphorylated the endogenous eIF4E to very high levels, but this ability was markedly impaired by removal of the basic region which contains the eIF4G-binding site (Fig. 2G).
Mnk2b is a poor substrate for MAP kinase in vitro.
The absence of stable binding between Mnk2b and Erk offered a potential explanation for the lower activity of Mnk2b but left open the question whether Mnk2b could indeed be activated by Erk or p38 MAP kinase. In vitro, Mnk2a, expressed as a GST fusion protein in E. coli, is more rapidly and extensively phosphorylated by p38 MAP kinase α (p38 MAPKα) at the threonines in the activation domain than is Mnk2b (Fig. 3A). Although longer incubation with activating kinases does further increase the phosphorylation of Mnk2b, we have never been able to phosphorylate this form to anywhere near the same extent as Mnk2a (data not shown). These results presumably explain why Mnk2a exhibits higher activity in vivo (Fig. 2). However, Mnk2b was clearly activated under these conditions, as it could phosphorylate recombinant eIF4E in vitro after activation with p38 MAPKα (Fig. 3B). Similar results were obtained with active ERK2 (data not shown). Using p38 MAPKα in these assays has the advantage that it can be inactivated with SB203580. As shown in Fig. 3B, p38 MAPKα can phosphorylate eIF4E to a very small extent, and this could confuse the interpretation of experiments designed to compare the activities of Mnk2a and Mnk2b. Figure 3B also shows that Mnk2b does not phosphorylate a variant of eIF4E in which Ser209 has been replaced by an alanyl residue, confirming that Mnk2b phosphorylates eIF4E solely at the physiologically relevant site.
Comparison of the activities of Mnk2a and Mnk2b that were activated by p38 MAPKα in vitro confirmed the results shown in Fig. 2 and 3A, i.e., that the activity of Mnk2b is significantly lower than that of Mnk2a (Fig. 3C). The ratio between the signal from eIF4E phosphorylated by Mnk2a and that for Mnk2b decreases slightly upon longer incubation (e.g., a ratio of approximately eightfold at 30 min and sixfold at 2 h).
To assess the regulation of the different Mnk's by the MAP kinase-signaling pathways in vivo, we transfected HEK293 cells with a vector for Mnk1, Mnk2a, or Mnk2b, each in Myc-tagged form. Immunoblotting with anti-Myc was used to assess the relative levels of expression, which were similar (Fig. 3D). After 24 h, the cells were serum starved for an additional 16 h and then harvested directly or after treatment either with the phorbol ester tetradecanoyl phorbol acetate (TPA) (to activate Erk, as visualized by using anti-phospho-Erk antibodies [Fig. 3E]) or with one of the MAP kinase signaling inhibitors PD98059 and SB203580 (which block signaling through the ERK and p38 MAPKα/β pathways, respectively).
The activities of Mnk1, Mnk2a, and Mnk2b were assayed in anti-Myc immunoprecipitates from cells subjected to these different conditions (Fig. 3F). Mnk1 had low basal activity against recombinant eIF4E, which was increased by treatment of cells with TPA. As reported for the mouse ortholog (35), human Mnk2a had a very high basal activity that was not increased further by TPA, and this was decreased somewhat by the signaling inhibitors (Fig. 3F). Mnk2b showed much lower activity than Mnk2a, but the pattern was similar to that observed for Mnk2a, in that activity was only marginally increased by TPA and was only slightly reduced by the signaling inhibitors. A similar pattern was observed for the phosphorylation of the threonyl residues in the activation loop (T loop), as studied using a phosphospecific antibody (Fig. 3G). This antibody was designed to recognize the sequence containing the T-loop phosphothreonines in Mnk1, but it also cross-reacts with the corresponding region in the very similar sequence of Mnk2 (as can be seen in Fig. 3A). TPA increased the phosphorylation of these key regulatory sites in Mnk1, but they were already highly phosphorylated in Mnk2a, even in serum-starved cells. Treatment with SB203580 or PD98059 diminished, but did not abolish, phosphorylation of the T-loop sites in Mnk2a. Mnk2b showed a considerably lower basal level of phosphorylation at these sites, but the pattern was similar to that of Mnk2a, in that phosphorylation was not increased by TPA and was only slightly reduced by the signaling inhibitors (Fig. 3G).
The phosphorylation state of the endogenous eIF4E in the transfected and treated cells was studied to assess changes in the activities of the Mnk's in vivo. The resulting data broadly reflected the picture seen for the in vitro activity and in vivo phosphorylation of Mnk1, -2a, and -2b (Fig. 3H). However, the effects of the signaling inhibitors and TPA in cells expressing Mnk2a or Mnk2b appeared more marked than those assessed in in vitro kinase assays, especially for Mnk2b. One possible complication here is interference from the endogenous Mnk activity in the cells, which, based on its regulatory characteristics, appears to be due to Mnk1 (eIF4E phosphorylation is substantially enhanced by TPA in HEK293 cells [47]). This enzyme probably contributes substantially to the observed changes in eIF4E phosphorylation in cells expressing Mnk2b, on account of Mnk2b's relatively low intrinsic kinase activity.
Effects of mutations or deletion of the MAP kinase-binding site on the activity of Mnk2a.
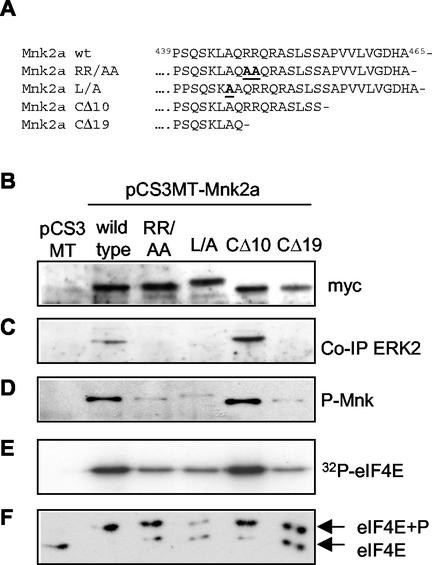
To assess whether the difference between the basal activities of the two forms of Mnk2 was due to MAP kinase binding or whether other sequences in the C termini also played a role, several variants of Mnk2a with changes in the C-terminal region were created. By site-directed mutagenesis, two variants were made with alanine substitutions for residues that are fully conserved in the MAP kinase-binding sites of several kinases that interact with MAP kinase (38, 39). Furthermore, two truncation mutants were made in which either the final 10 or the final 19 amino acids were removed. The CΔ10 mutation removes the extreme C terminus of Mnk2a but leaves the putative MAP kinase-binding site intact, while the CΔ19 truncation removes part of the MAP kinase-binding region. The amino acid sequences of the C termini of wild-type Mnk2a and these variants are depicted in Fig. 4A.
FIG. 4.
C-terminal mutations affect Mnk2a activity. (A) C-terminal sequence of human Mnk2a (residues 429 to 465) and the sequences of four variants used in these studies. In the MAP kinase-binding mutants RR/AA and L/A, the changed residues are underlined and boldfaced. (B) Loss of Erk binding reduces activity. The indicated variants were expressed from pCS3MT constructs (with pCS3MT as the empty vector control) in HEK293 cells, and Myc-tagged proteins were immunoprecipitated with anti-Myc antibodies. Western blotting with anti-Myc antibodies shows very similar expression levels for all variants. The L/A variant migrates somewhat more slowly, as it was cloned from GFP-Mnk2a(RR/AA) into the EcoRI site of pCS3MT, instead of the NcoI site, adding 15 amino acids between the Myc tag of pCS3MT and the first methionine of Mnk1. (C) Bound Erk was analyzed by probing the same blot with anti-Erk. (D) Aliquots identical to those used for panels B and C were run on a different gel, and samples were analyzed by Western blotting with phosphospecific anti-Mnk antibodies. (E) The Myc immunoprecipitates were incubated with recombinant eIF4E and [γ-32P]ATP, and phosphorylation of eIF4E was visualized by SDS-PAGE and autoradiography. (F) Endogenous eIF4E from the cell extracts used in panels B through E was purified by m7GTP-Sepharose chromatography. Phosphorylated and nonphosphorylated forms of eIF4E (as indicated on the right) were separated by isoelectric focusing and visualized by Western blotting with antibodies against eIF4E.
Upon expression in transfected cells (Fig. 4B), the RR/AA, L/A, and CΔ19 mutations completely abolished the ability of Mnk2a to bind stably to Erk, while the CΔ10 variant could still bind (Fig. 4C). This shows that even a single residue change in this binding site is sufficient to abolish MAP kinase binding. In the variants lacking a MAP kinase-binding site, phosphorylation at the threonines in the catalytic domain was markedly reduced (Fig. 4D). These variants still had detectable activity, but it was markedly lower than that of wild-type Mnk2a (Fig. 4E). This was also reflected in their ability to phosphorylate endogenous eIF4E in transfected cells (Fig. 4F). The mutated kinases that did not bind Erk thus behaved similarly to Mnk2b. The data indicate that the Erk binding site, although not absolutely essential, is very important for the activation of Mnk2a. MAP kinases can presumably still phosphorylate Mnk2b and the Mnk2a species lacking the MAP kinase-binding site by direct interaction with the T-loop region. In addition to enhancing the activation of Mnk2a, the MAP kinase-binding motif appears to play other roles in the function of Mnk2a (see below).
The isoform-specific C terminus of Mnk2b contains a consensus binding site for SH3 domains (PXXPXR). However, we were unable to find any evidence for a role for this region in binding to selected proteins that contain SH3 domains or in the regulation of Mnk2b activity in vivo (data not shown). It was also possible that the activity of Mnk2b might be modulated under specific cellular conditions. However, we failed to observe any changes in Mnk2b activity under a variety of different conditions tested, e.g., stresses (treatment of cells with arsenite or hydrogen peroxide), hyperosmolarity (treatment of cells with NaCl), or elevated cytoplasmic calcium concentrations (induced by the calcium ionophore A23187 or by carbachol), or during the cell cycle.
Mnk2a and Mnk2b localize differently.
Expression of Mnk1, Mnk2a, and Mnk2b as fusions with GFP revealed striking differences in their localization. Mnk1 was entirely cytoplasmic (see also Fig. 6), and Mnk2a also was mainly cytoplasmic (although in some cells an apparent nuclear staining could be observed as well). Mnk1 has previously been shown to be mainly cytoplasmic in HEK293 cells, with a shift to the perinuclear region upon stimulation of the cells with TPA (49).
FIG. 6.
The C terminus of Mnk2a is involved in localization. (A) The localization of Mnk2 is not sensitive to leptomycin B. Mouse Mnk1 and Mnk2 were expressed as GST fusion proteins in HEK293 cells grown on coverslips. After 24 h, cells were either left untreated or incubated in the presence of 5 ng of leptomycin B (LMB)/ml for 4 h. Cells were then fixed with paraformaldehyde and permeabilized, and GST fusion proteins were visualized by using anti-GST monoclonal antibodies and FITC-conjugated anti-mouse secondary antibodies. (B) Alignment of residues 425 to 451 of human Mnk2a with corresponding regions of other Mnk's. In mouse and human Mnk1, a consensus sequence (underlined residues) can be found that could func-tion as an NES (bottom sequence, in which ψ represents a hydrophobic residue and X represents any amino acid). The solid arrowhead indicates a serine in the Mnk2 forms that would likely abolish NES function. Fully conserved amino acids are boldfaced, and the MAP kinase-binding site is shaded. (C) GFP fusion proteins of the variants indicated (see also Fig. 4) were expressed in transfected HEK293 cells. After being harvested in EZ lysis buffer, cell extracts were fractionated into cytoplasmic (C) and nuclear (N) fractions. The presence of the various forms of Mnk2a was revealed by Western blotting with antibodies against GFP. (D) C-terminal deletions affect binding of eIF4G to Mnk2a. HEK293 cells were transfected with an empty vector (pCS3MT) or vectors encoding Myc-tagged wild-type Mnk2a or Mnk2a variants as indicated. After harvesting of the cells, the expressed proteins were immunoprecipitated with antibodies against the Myc tag. Coimmunoprecipitated eIF4G was visualized by Western blotting. (E) Quantification of eIF4G binding. The signals for eIF4G and Mnk2 obtained in three similar experiments, as shown in panel D, were quantified with ImageJ software, and the relative binding of eIF4G to wild-type Mnk2a or the indicated variants was determined. Means and standard deviations are shown. The binding of eIF4G to wild-type Mnk2a was arbitrarily set at 1.
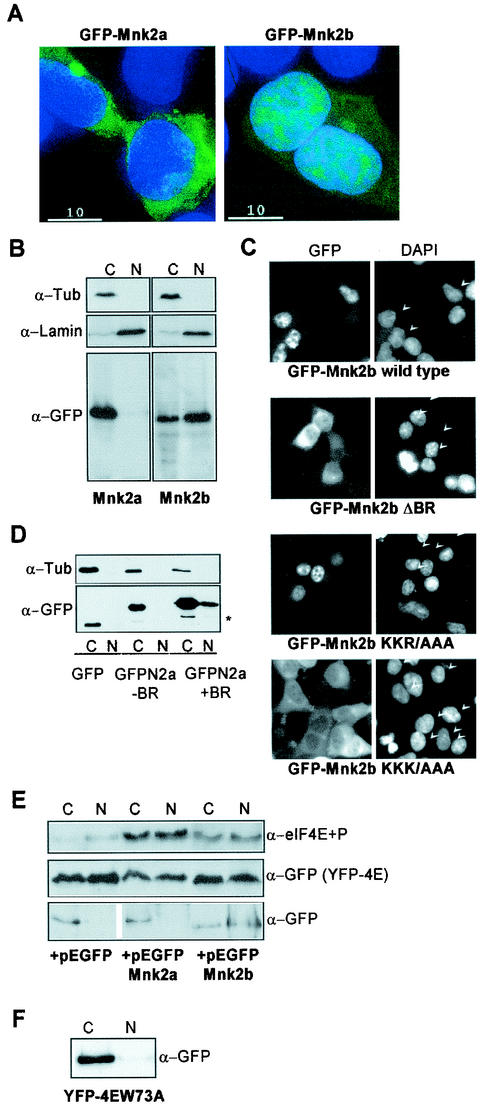
In contrast, Mnk2b was prominently visible in the nuclear compartment, where it gave a speckled or punctate pattern (Fig. 5A). Mnk2b was also evident in the cytoplasm. To investigate further the distribution of Mnk2a and Mnk2b by an alternative approach, transfected cells were fractionated into nuclear and cytoplasmic samples, and these were analyzed by immunoblotting. Use of anti-tubulin antibodies revealed that there was no contamination from cytoplasmic material in the nucleus, and conversely, anti-lamin B showed that there was little contamination by nuclear material in the cytoplasm (Fig. 5B).
FIG. 5.
Differential localization of Mnk2a and Mnk2b. (A) Mnk2b is found in the nucleus. HEK293 cells were grown on coverslips and transfected with constructs encoding GFP-tagged versions of Mnk2a or Mnk2b. After fixation of the cells with paraformaldehyde and treatment with DAPI, the presence of fluorescent proteins in the cytoplasm and nucleus was visualized as described in Materials and Methods. An overlay of immunofluorescence (green) and DNA staining in the nucleus (blue) is shown. (B) Extracts from transfected cells were fractionated to give cytoplasmic (C) and nuclear (N) fractions as described in Materials and Methods. Aliquots representing 10% of each fraction were analyzed by Western blotting with antibodies directed againstGFP, lamin B, and α-tubulin. (C) The first part of the basic region is not required for nuclear localization of Mnk2b. GFP-tagged versions of wild-type Mnk2b or the indicated variants were expressed in HEK293 cells grown on coverslips. Cells were treated as described above. Separate images for immunofluorescence (GFP) and nuclear staining (DAPI) are shown. Arrowheads in the righthand panels indicate nuclei of transfected cells. (D) The N terminus of human Mnk2 functions as an NLS. HEK293 cells were transfected with constructs expressing GFP or GFP fusions with the N terminus of human Mnk2 up to residue 60 (−BR) or up to residue 70 (+BR). Cells were harvested in EZ lysis buffer, and cytoplasmic and nuclear extracts were prepared as described in Materials and Methods. The distribution of the GFP-tagged proteins was visualized by Western blotting with anti-GFP. The asterisk indicates a product which is most likely derived from an N-terminal truncation, as similar bands are found for GFP alone or GFP fused to the N terminus of human Mnk2a up to residue 60. (E) Expression of Mnk2a or Mnk2b results in phosphorylation of eIF4E in the cytoplasm and nucleus. HEK293 cells were transfected with 5 μg of pEYFP-eIF4E in combination with 5 μg of pEGFP-C1, pEGFP-Mnk2a, or pEGFP-Mnk2b as indicated. After 24 h of transfection, cells were harvested in EZ lysis buffer, and extracts were fractionated into cytoplasmic (C) and nuclear (N) fractions. Aliquots of these fractions representing, respectively, 8 and 15% of the total were analyzed for the presence of phosphorylated eIF4E by Western blotting with a phosphospecific anti-eIF4E antibody (a generous gift from Simon Morley, University of Sussex) (top panel). Equal samples were analyzed by using anti-GFP as a control for the loading of the total amount of YFP-tagged eIF4E (center panel) and for the presence of the GFP-tagged proteins in the cytoplasmic and nuclear fractions (bottom panel). It should be noted that the part of the figure showing GFP was aligned with the GFP-Mnk's. This lefthand part was derived from the same film as the righthand part. (F) HEK293 cells were transfected with a construct encoding YFP-tagged eIF4E in which Trp73 was replaced by alanine (W73A). Cytoplasmic and nuclear fractions were made as described above, and the presence of the YFP-tagged protein was analyzed by Western blotting with antibodies against GFP.
Immunoblotting with anti-GFP to detect the GFP-Mnk fusion proteins confirmed the results of fluorescence microscopy, i.e., that much of Mnk2b is nuclear (Fig. 5B). Immunoblotting also showed that Mnk2a was exclusively cytoplasmic. The apparent nuclear staining under the microscope is most likely a result of Mnk2a localized to the perinuclear region. Thus, these two splice variants exhibit markedly different subcellular localizations.
An N-terminal polybasic region is required for nuclear localization of Mnk2b.
Many NLSs are rich in basic residues (13), and Fukunaga and Hunter suggested in their original description of human Mnk1 that the stretch of basic residues in the N terminus (see Fig. 2A) might function as an NLS (9). Indeed, Mnk1, but not a mutant lacking the first 23 residues that include this potential NLS, can bind to importin α (49). In fact, all three proteins, Mnk1, Mnk2a, and Mnk2b, contain a basic region near the N terminus. This feature is important for eIF4G binding (see Fig. 2). To assess the role of this region in the localization of Mnk2a and -2b, the same variants that were used in Fig. 2 were introduced into the GFP vector. Strikingly, Mnk2bΔBR was exclusively cytoplasmic. Thus, removal of the basic region prevented the nuclear localization of Mnk2b (Fig. 5C). It therefore seems likely that this polybasic region, or part of it, does indeed function as an NLS. The KKR/AAA variant of GFP-Mnk2b, in which the first three basic residues were replaced by alanines, still resided in the nucleus. This shows that the first part of the basic region is dispensable for nuclear localization. In contrast, changing three basic residues in the second part of the basic stretch (in KKK/AAA) rendered the fusion protein completely cytoplasmic, indicating that the integrity of the second part is essential for NLS function. In this last variant only one stretch of three basic residues remains.
Finally, we tested whether the basic residues are sufficient for nuclear localization by expressing GFP fusion proteins containing the N terminus of human Mnk2 up to residue 60 (which does not include these basic residues) or up to residue 70 (which does). GFP itself or a fusion protein of GFP with the N-terminal 60 amino acids of Mnk2a was found exclusively in the cytoplasmic fraction. The variant containing the N terminus of Mnk2 including the basic region was detected in the nucleus, showing that this basic region is capable of localizing a protein in the nucleus (Fig. 5D).
Mnk2 expression increases the amount of phosphorylated nuclear eIF4E.
What could be the function of the nuclear Mnk2b? Although eIF4E has been found in the nuclear compartments of various cell types, we have consistently found that only a very small amount of eIF4E, if any, is present in the nucleus in HEK293 cells (16). Similarly, eIF4G is almost completely cytoplasmic in HEK293 cells (data not shown). However, when eIF4E is expressed as a YFP fusion protein in HEK293 cells, a significant amount of YFP-eIF4E is found in the nucleus (Fig. 5E, center panel). Coexpression of Mnk2b, which was present in both the cytoplasm and the nucleus (Fig. 5E, bottom panel), resulted in increased phosphorylation of the nuclear YFP-eIF4E (top panel). Surprisingly, similar data were obtained with the cytoplasmic variant, Mnk2a. These results, therefore, do not allow us to distinguish whether eIF4E is phosphorylated in the cytoplasm and subsequently transported to the nucleus or directly phosphorylated in the nucleus, e.g., by Mnk2b. It is possible that nucleocytoplasmic shuttling of eIF4E is sufficiently rapid that the phosphorylated eIF4E equilibrates between the cytoplasm and the nucleus so that similar proportions of the protein become phosphorylated in each compartment irrespective of whether the initial phosphorylation event occurred in the cytoplasm or in the nucleus. This would require mechanisms for the swift transport of eIF4E into or out of the nucleus. Indeed, a nucleocytoplasmic shuttling protein for eIF4E, 4E-T, has been described recently (5), and eIF4E contains a nuclear export signal (M. Ferraiuolo and N. Sonenberg, personal communication). 4E-T binds to the dorsal site of eIF4E (5), a region also involved in binding eIF4G and 4E-BP1. In agreement with this observation, we found that YFP-tagged eIF4E with Trp73 mutated to Ala, a variant that does not bind 4E-T (5), is almost completely cytoplasmic (Fig. 5F).
The N terminus of Mnk2a is involved in localization and eIF4G binding.
The common N terminus of Mnk2a and Mnk2b contains a functional NLS, and Mnk1 contains a similar sequence motif, prompting the question why Mnk1 and Mnk2a are not found within the nucleus. As indicated in Fig. 1B, Mnk1 contains a canonical leucine-rich nuclear export sequence (NES), and Mnk1 export has been shown to be sensitive to leptomycin B (Fig. 6A) (23), indicating that export of Mnk1 is mediated by CRM1 and likely explaining why Mnk1 is primarily cytoplasmic. The unique C terminus of Mnk2a also contains a sequence similar to that of a CRM1-type NES. However, one of the key leucine residues in this sequence is replaced by serine (Fig. 6B). Therefore, one would expect Mnk2a to be nuclear, since it contains an NLS but no apparent NES. However, it is actually found in the cytoplasm. Possible explanations for this discrepancy are (i) that some feature of the unique C terminus of Mnk2a prevents nuclear import of Mnk2a or (ii) that this region of Mnk2a contains an alternative non-CRM1 NES that results in its export to the cytoplasm. To test these possibilities, we made truncation mutants in which the last 10 (CΔ10) or 19 (CΔ19) amino acids of Mnk2a were removed. Strikingly, whereas Mnk2a is normally cytoplasmic, the CΔ10 mutant is partially nuclear (Fig. 6C). This result does not tell us which of the two possibilities is true. However, removal of a further 9 amino acids to give Mnk2aCΔ19 yielded a protein whose localization was again cytoplasmic. This strongly argues against an NES in the extreme C terminus of Mnk2a (option ii), as such an NES would be removed in CΔ19 as well. One would expect such a variant to be found in the nucleus, like the Mnk2a CΔ10 mutant, rather than in the cytoplasm.
The idea that the C terminus of Mnk2a interferes with the NLS function of the basic region implies that this region would also interfere with eIF4G binding. Therefore, we also compared eIF4G binding to wild-type Mnk2a with that to the CΔ10 and CΔ19 variants (Fig. 6D and E). Removal of the last 10 amino acids of Mnk2a reproducibly increased its binding to eIF4G twofold, supporting the idea that this region reduces the accessibility of the basic region in the N terminus. A variant in which a further 9 residues were deleted (Mnk2aCΔ19), as well as the forms that cannot bind MAP kinase (i.e., Mnk2a RRA and Mnk2a LA), showed similar levels of eIF4G binding to the wild-type protein.
An intact MAP kinase-binding motif is required for nuclear localization of Mnk2aCΔ10.
The nine residues removed to produce Mnk2aCΔ19 contain part of the MAP kinase-binding site and are required for the stable interaction of Mnk2a with Erk (Fig. 4C). To assess whether this feature was also needed for the nuclear localization of Mnk2aCΔ10, we prepared two new variants of this protein in which the remaining MAP kinase docking site was subjected to point mutations. The mutations introduced are the same as those in the variants whose sequences are given in Fig. 4A, i.e., RR/AA and L/A. These mutant proteins, Mnk2a-RR/AACΔ10 and Mnk2a-L/ACΔ10, were completely cytoplasmic, unlike Mnk2aCΔ10 itself, which is partially nuclear (Fig. 6C). Mutation of these residues within wild-type Mnk2a had no effect on its localization; it remained cytoplasmic (as shown for the RR/AA mutant in Fig. 6C). Thus, it seems likely that the residues required for binding to Erk are also required for the efficient entry of Mnk2aCΔ10 into the nucleus.
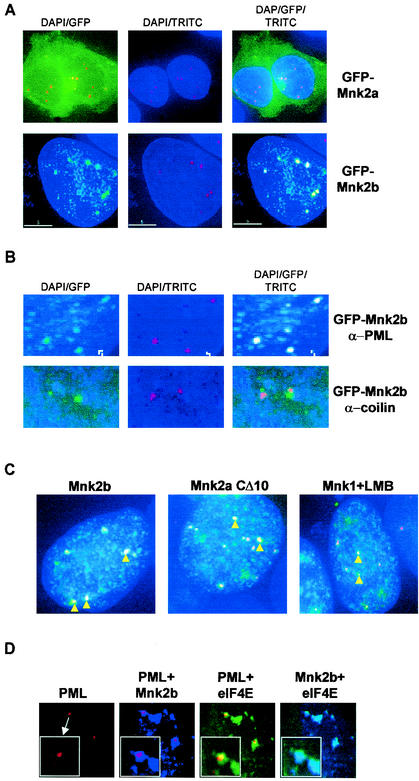
Mnk2b colocalizes with nuclear PML.
The finding that Mnk2b is partially nuclear was of particular interest, given that a fraction of the cellular eIF4E is also known to be nuclear (2, 6, 20) and given the recent observation that nuclear eIF4E associates with PML (2). PML and eIF4E are found within nuclear PML bodies (2), which are now also termed eIF4E bodies (43). To study the localization of Mnk2b within the nucleus, we made use of antibodies to PML in conjunction with GFP-Mnk2b in immunofluorescence experiments. As expected, a significant proportion of the Mnk2b was nuclear. This material was found to colocalize with PML, as judged by the coincidence of the GFP and TRITC (anti-PML) signals (Fig. 7A). This indicates that Mnk2b is a component of the PML/eIF4E bodies.
FIG. 7.
Mnk2b colocalizes with PML. (A) HEK293 cells grown on coverslips were transfected with the indicated constructs. After incubation with antibodies against PML, microscopic images were recorded as described in Materials and Methods. DAPI indicates DAPI staining of the nuclei, GFP indicates fluorescence microscopy to detect GFP-tagged proteins, and TRITC indicates the use of TRITC-conjugated secondary antibodies to detect PML. (B) Colocalization of GFP-Mnk2b with PML and not with coilin. Coverslips with GFP-Mnk2b-transfected cells were incubated with an anti-PML or anti-coilin antibody and with TRITC-conjugated secondary antibodies as indicated. An enlargement of the nucleus showing Mnk2b-PML speckles is shown. (C) HEK293 cells grown on coverslips were transfected with GFP-Mnk2b, GFP-Mnk2aCΔ10, or GFP-Mnk1. In the last case, the transfected cells were treated with leptomycin B (LMB) (5 ng/ml) for 4 h before fixation. Colocalization with PMLwas determined as described in Materials and Methods. Nuclei of the transfected cells were visualized by staining with DAPI (blue). Yellow arrowheads indicate some of the bodies in which PML and the GFP-tagged kinases colocalize. (D) Colocalization of Mnk2b, eIF4E, and PML. HEK293 cells were transfected with constructs expressing CFP-tagged Mnk2b and YFP-tagged eIF4E. The latter construct contains an additional sequence corresponding to the simian virus 40 T-antigen NLS in order to increase the levels of eIF4E in the nucleus. CFP and YFP intensities were very similar in these experiments. After fixation and permeabilization, PML was visualized by immunofluorescence as described in Materials and Methods. DeltaVision microscopy images of nuclei were recorded using the filters for TRITC (PML), TRITC and CFP (PML + Mnk2b), TRITC and YFP (PML + eIF4E), and CFP and YFP (Mnk2b + eIF4E). These four images were taken from the same Z-plane. Insets in each panel show a part of the image in greater detail.
To study the specificity of the colocalization of Mnk2b with PML, we tested whether Mnk2b would also colocalize with other nuclear bodies. One other type of nuclear body is the so-called Cajal bodies, of which coilin is a component. However, Mnk2b did not colocalize with coilin (Fig. 7B), and Mnk2b therefore appears not to be associated with such structures.
During our studies on the localization of the Mnk's, we noticed that Mnk2aCΔ10 and, in leptomycin B-treated cells, Mnk1 gave a punctate appearance in the nucleus. In both cases (Fig. 7C), we observed clear colocalization of these proteins with PML. This strongly suggests that the colocalization of Mnk2b with PML is not specifically conferred by its different C terminus but involves features that are shared with Mnk1 and Mnk2a.
To confirm that Mnk2b and PML colocalize with eIF4E in the nucleus, a cyan fluorescent protein-tagged version of Mnk2b was coexpressed in transfected cells with YFP-tagged eIF4E (Fig. 7D). To increase the amount of eIF4E in the nucleus, YFP-eIF4E was expressed from a construct that codes for an NLS at the N-terminal end of eIF4E. As shown in Fig. 7A to C, Mnk2b colocalized with PML in speckles; in Fig. 7D, the overlay is evident from the purple PML bodies. eIF4E was also found in these PML bodies (yellow overlap), although the overlap was not as complete as that seen for Mnk2b and PML. In general, Mnk2b and eIF4E were found to colocalize throughout the nucleus (Fig. 7D, rightmost panel, light blue overlap).
DISCUSSION
Splice variants of Mnk2.
In this study we have investigated the functional properties of a distinct splice variant of the eIF4E kinase Mnk2. The human genome contains about 500 protein kinase genes. However, earlier predictions suggested that mammalian cells would possess substantially more protein kinases than this (11). The present study demonstrates how the functional diversity of that limited repertoire of kinase genes can be extended by alternative splicing to generate distinct isoforms from a single kinase gene, in this case isoforms that differ in their subcellular localization. The existence of multiple isoforms seems a common feature of many components in the MAP kinase signaling pathways (see, e.g., references 31 and 45), although Mnk2 seems so far to be the only downstream kinase in these pathways that has been reported to exist in two splice variants.
Mnk2b has previously been shown to bind estrogen receptor β (37), which is nuclear when in its active state. The nuclear localization of Mnk2b may allow it to phosphorylate active estrogen receptors, e.g., when these receptors are bound to DNA. This would be highly reminiscent of phosphorylation of estrogen receptor α by another Erk substrate, p90rsk (14). Further work will be required to identify nuclear substrates and functions for Mnk2b.
Colocalization with PML.
The finding that Mnk2b is located in the nucleus and associates with bodies containing PML is important in view of other recent work in this area. PML has recently been shown to bind eIF4E and to decrease its affinity for capped mRNA (2). It is clearly possible that the colocalization of Mnk2b and eIF4E within PML bodies allows Mnk2b to phosphorylate eIF4E, an effect similar to the facilitating effect of the scaffold protein eIF4G on the phosphorylation of eIF4E by Mnk1 in the cytoplasm (29). Whether PML itself or another component of PML/eIF4E bodies functions as the relevant scaffold remains to be elucidated. To try to address this, we mutated two prolines in the C terminus of Mnk2b to alanines, thereby destroying a possible domain for binding to RING domains, a so-called FRODO domain (1), and also a possible SH3-binding domain. However, this mutation did not affect colocalization with PML bodies (data not shown). This finding is consistent with the data in Fig. 7C, which imply that Mnk-PML colocalization is conferred by features shared by all Mnk isoforms. It is because of its nuclear localization that Mnk2b colocalizes with PML, not because its C terminus contains a specific binding site for PML.
Phosphorylation of eIF4E also weakens its affinity for capped mRNA (34), so the two effects (PML binding and phosphorylation of eIF4E) may cooperate in reducing the transport of certain transcripts, such as that for cyclin D1 (2, 18), into the cytoplasm. Mnk2b may, of course, also act to phosphorylate other components of PML bodies. A similar situation arises within eIF4F complexes, where Mnk1 phosphorylates eIF4G (30) as well as eIF4E. However, our initial data indicate that Mnk2b does not phosphorylate PML.
Regulation of activity by MAP kinase binding.
Consistent with its lack of the MAP kinase-interaction motif, Mnk2b does not bind stably to Erk, unlike Mnk1 and Mnk2a (9, 48) (Fig. 2). Mnk2b is much less readily phosphorylated by MAP kinases in vitro, and although it can phosphorylate eIF4E both in vitro and in vivo, it shows much lower activity than Mnk2a (Fig. 2 and 3). Surprisingly, Mnk2b has higher basal activity in vivo than Mnk1. It is not clear what causes this difference, but Mnk2b, in contrast to Mnk1, seems to be significantly phosphorylated under basal conditions at critical threonyl residues in the activation domain of the kinase. It is possible that this region is less accessible to phosphatases in the Mnk2 isoforms than in Mnk1. An important implication of the differences in activity between Mnk1 and the Mnk2 isoforms is that, in cells that primarily express Mnk1, eIF4E phosphorylation is likely to be determined by two factors. One is the state of activation of the upstream pathways, i.e., the Erk and p38 MAPKα/β pathways, that switch on Mnk1. The second is the degree of assembly of eIF4F complexes, which are required for efficient eIF4E phosphorylation, since they bring together Mnk1 and its substrate, eIF4E, through their common binding partner, eIF4G (29). In contrast, since Mnk2a and, to a lesser extent, Mnk2b have high basal activity, in cells mainly expressing Mnk2a and -2b one would expect the main factor affecting eIF4E phosphorylation to be the levels of eIF4F complexes.
Involvement of the C terminus in localization.
A further major difference between Mnk1 and -2a, on the one hand, and Mnk2b, on the other, lies in their subcellular localization. While Mnk1 and Mnk2a are cytoplasmic, a substantial fraction of Mnk2b is found in the nuclear compartment. All three proteins contain a polybasic sequence in the N terminus. That of Mnk1 has been shown to bind the nuclear-transport factor importin α (9). Here we show that this motif does indeed function as an NLS.
Given that Mnk1 and Mnk2a are actually cytoplasmic, it seemed likely that they also contained features that either mediated their efficient reexport to the cytoplasm or impaired the function of the NLS. Indeed, inspection of the sequence of Mnk1 revealed the presence of a feature similar to a canonical CRM1-type NES containing multiple hydrophobic residues. The C termini of Mnk2a and Mnk2b do not contain such a sequence motif, although Mnk2a does contain a similar feature, but with a serine at the position of one of the essential leucyl residues. As one would expect, leptomycin B does not affect the localization of Mnk2a (Fig. 6A).
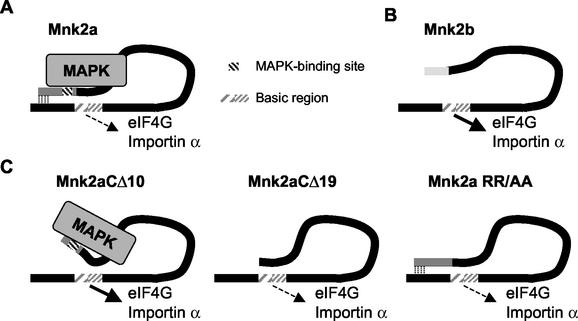
Removal of the last 10 amino acids from Mnk2a yielded a truncated protein that located to the nucleus, while removal of the C-terminal 19 amino acids or mutation of the MAP kinase-binding site resulted in complete cytoplasmic localization. How are these data to be explained? One possible model is depicted in Fig. 8. In this model, Mnk2a folds such that its C terminus reduces the accessibility of the N-terminal basic region. This might even be due to a direct interaction between the N and C termini. In this conformation, Mnk2a can bind both to eIF4G and to importin but only with low efficiency.
FIG. 8.
Involvement of the N and C termini in the regulation of Mnk2. (A) Schematic presentation of Mnk2a bound to MAP kinase (MAPK). The basic region is shown with two different patterns to indicate that the NLS and the eIF4G-binding site overlap but are not identical. Limited binding of importin α and eIF4G can occur in the wild-type Mnk2a, as indicated by the dashed arrow. (B) Schematic presentation of Mnk2b. The shorter C-terminal region of Mnk2b does not interfere with access to the N terminus, and therefore Mnk2b can bind better to importin α and eIF4G (as indicated by the solid arrow). (C) Possible explanation of the effects of changes in the C terminus of Mnk2a. In Mnk2aCΔ10 the interaction between the N and C termini is reduced, allowing a conformational change dependent on binding to MAPK. This results in increased access for importin α and eIF4G. Due to the lack of a MAPK-binding site in the CΔ19 or RR/AA (or L/A) variant, this conformational change cannot occur and the basic region is less accessible.
MAP kinase binding is clearly not required for the import of Mnk2b into the nucleus, as our data show that Mnk2b does not bind stably to Erk. It is possible that the shorter C-terminal region of Mnk2b, which also has a quite different sequence, does not block access to the polybasic region containing the NLS. Such a scenario would also be consistent with the better ability of Mnk2b (compared to Mnk2a) to bind to eIF4G, a property that also requires the polybasic region (as depicted in Fig. 8B).
We suggest that removal of the last 10 residues in Mnk2a may weaken the interaction between the N and C termini, so that MAP kinase binding now reduces the ability of the C terminus to occlude access to the basic region in the N terminus. This would enhance the binding of eIF4G and importin, leading to the increased nuclear localization of this variant (Fig. 6). Removal of a further 9 residues or mutation of residues in the MAP kinase-binding site would eliminate MAP kinase binding and result in the “closure” of the structure again, decreasing the accessibility of the N terminus. In variants lacking the MAP kinase-binding site, the conformational change induced by binding to MAP kinase would not occur, explaining the cytoplasmic localization of such variants.
The C terminus of Mnk1 is similar to that of Mnk2a and may also occlude its N-terminal NLS, but studying this question was beyond the scope of this investigation. The ability of the nuclear-export inhibitor leptomycin B to render Mnk1 nuclear demonstrates that the NLS of Mnk1 is not completely blocked (23): even if import is slow, Mnk1 can eventually accumulate in the nucleus when its export is inhibited. Other reports have indicated that the localization of two other kinases that lie downstream of MAP kinases, MAPKAPK2 and MAPKAPK5, is also determined by interplay between their NLSs, C termini, and binding to MAP kinases (24, 36).
Role of Mnk2b in the nucleus.
A splice variant of human Mnk2 that is present in the nucleus could be involved in various processes in the nucleus. If it plays a role similar to that of the other Mnk's in the cytoplasm, i.e., phosphorylation of eIF4E, this could impinge on two aspects of gene expression. (i) Mnk2b activity could be involved in nuclear translation, the process that is thought to function as a pioneer round of translation for the purpose of proofreading mRNAs (12). However, it should be noted that the existence of nuclear translation has recently been strongly disputed (3, 28). (ii) Transport of certain mRNAs could be regulated by phosphorylation of eIF4E. In this regard, the colocalization with PML might be significant, as PML, through its interaction with eIF4E, is thought to regulate the transport into the cytoplasm of a subset of mRNAs, e.g., cyclin D1 mRNA (2). Phosphorylation of eIF4E by Mnk2b could add an extra regulatory step in mRNA transport. It is also feasible that Mnk2b has additional nuclear substrates, for example, other components of the PML bodies or proteins involved in estrogen signaling.
Acknowledgments
This work was supported by a Programme Grant from the United Kingdom Medical Research Council.
We thank Carole Lyon and Paul Andrews (University of Dundee) for valuable suggestions and technical assistance.
REFERENCES
- 1.Borden, K. L. 2000. RING domains: master builders of molecular scaffolds? J. Mol. Biol. 295:1103-1112. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, N., M. Sharma, A. Kentsis, J. M. Perez, S. Strudwick, and K. L. Borden. 2001. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J. 20:4547-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlberg, J. E., E. Lund, and E. B. Goodwin. 2003. Nuclear translation: what is the evidence? RNA 9:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Benedetti, A., and R. E. Rhoads. 1990. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc. Natl. Acad. Sci. USA 87:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dostie, J., M. Ferraiuolo, A. Pause, S. A. Adam, and N. Sonenberg. 2000. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J. 19:3142-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dostie, J., F. Lejbkowicz, and N. Sonenberg. 2000. Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. J. Cell Biol. 148:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn, A., and C. G. Proud. 1995. Serine 209, not serine 53, is the major site of phosphorylation in initiation factor eIF-4E in serum-treated Chinese hamster ovary cells. J. Biol. Chem. 270:21684-21688. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, A., R. G. Vries, and C. G. Proud. 1997. Signalling pathways which regulate eIF4E. Biochem. Soc. Trans. 25:192S. [DOI] [PubMed]
- 9.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gingras, A.-C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 11.Hunter, T. 1994. 1001 protein kinases redux—towards 2000. Semin. Cell. Biol. 5:367-376. [DOI] [PubMed] [Google Scholar]
- 12.Iborra, F. J., D. A. Jackson, and P. R. Cook. 2001. Coupled transcription and translation within nuclei of mammalian cells. Science 293:1139-1142. [DOI] [PubMed] [Google Scholar]
- 13.Jans, D. A., C. Y. Xiao, and M. H. Lam. 2000. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22:532-544. [DOI] [PubMed] [Google Scholar]
- 14.Joel, P. B., J. Smith, T. W. Sturgill, T. L. Fisher, J. Blenis, and D. A. Lannigan. 1998. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol. Cell. Biol. 18:1978-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi, B., A. L. Cai, B. D. Keiper, W. B. Minich, R. Mendez, C. M. Beach, J. Stepinski, R. Stolarski, E. Darzynkiewicz, and R. E. Rhoads. 1995. Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J. Biol. Chem. 270:14597-14603. [DOI] [PubMed] [Google Scholar]
- 16.Kleijn, M., G. C. Scheper, M. L. Wilson, A. R. Tee, and C. G. Proud. 2002. Localisation and regulation of the eIF4E-binding protein 4E-BP3. FEBS Lett. 532:319-323. [DOI] [PubMed] [Google Scholar]
- 17.Kleijn, M., H. O. Voorma, and A. A. M. Thomas. 1995. Phosphorylation of eIF-4E and initiation of protein synthesis in P19 embryonal carcinoma cells. J. Cell. Biochem. 59:443-452. [DOI] [PubMed] [Google Scholar]
- 18.Lai, H. K., and K. L. Borden. 2000. The promyelocytic leukemia (PML) protein suppresses cyclin D1 protein production by altering the nuclear cytoplasmic distribution of cyclin D1 mRNA. Oncogene 19:1623-1634. [DOI] [PubMed] [Google Scholar]
- 19.Lazaris-Karatzas, A., K. S. Montine, and N. Sonenberg. 1990. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 345:544-547. [DOI] [PubMed] [Google Scholar]
- 20.Lejbkowicz, F., C. Goyer, A. Darveau, S. Neron, R. Lemieux, and N. Sonenberg. 1992. A fraction of the mRNA 5′ cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc. Natl. Acad. Sci. USA 89:9612-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, B. D., L. Liu, M. Dawson, and A. De Benedetti. 1997. Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer 79:2385-2390. [PubMed] [Google Scholar]
- 22.Li, W., G. J. Belsham, and C. G. Proud. 2001. Eukaryotic initiation factors 4A (eIF4A) and 4G (eIF4G) mutually interact in a 1:1 ratio in vivo. J. Biol. Chem. 276:29111-29115. [DOI] [PubMed] [Google Scholar]
- 23.McKendrick, L., E. Thompson, J. Ferreira, S. J. Morley, and J. D. Lewis. 2001. Interaction of eukaryotic translation initiation factor 4G with the nuclear cap-binding complex provides a link between nuclear and cytoplasmic functions of the m7 guanosine cap. Mol. Cell. Biol. 21:3632-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng, W., L. L. Swenson, M. J. Fitzgibbon, K. Hayakawa, E. Ter Haar, A. E. Behrens, J. R. Fulghum, and J. A. Lippke. 2002. Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. J. Biol. Chem. 277:37401-37405. [DOI] [PubMed] [Google Scholar]
- 25.Minich, W. B., M. L. Balasta, D. J. Goss, and R. E. Rhoads. 1994. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc. Natl. Acad. Sci. USA 91:7668-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley, S. J., and L. McKendrick. 1997. Involvement of stress-activated protein kinase and p38/RK mitogen-activated protein kinase signaling pathways in the enhanced phosphorylation of initiation factor 4E in NIH 3T3 cells. J. Biol. Chem. 272:17887-17893. [DOI] [PubMed] [Google Scholar]
- 27.Nathan, C. A., S. Franklin, F. W. Abreo, R. Nassar, A. De Benedetti, and J. Glass. 1999. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J. Clin. Oncol. 17:2909-2914. [DOI] [PubMed] [Google Scholar]
- 28.Nathanson, L., T. Xia, and M. P. Deutscher. 2003. Nuclear protein synthesis: a re-evaluation. RNA 9:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyronnet, S., H. Imataka, A. C. Gingras, R. Fukunaga, T. Hunter, and N. Sonenberg. 1999. Human eukaryotic translation initiation factor 4G (eIF4G) recruits Mnk1 to phosphorylate eIF4E. EMBO J. 18:270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raught, B., A. C. Gingras, S. P. Gygi, H. Imataka, S. Morino, A. Gradi, R. Aebersold, and N. Sonenberg. 2000. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J. 19:434-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz, V., I. Arozarena, and P. Crespo. 2000. Distinct carboxy-termini confer divergent characteristics to the mitogen-activated protein kinase p38α and its splice isoform Mxi2. FEBS Lett. 474:169-174. [DOI] [PubMed] [Google Scholar]
- 32.Scheper, G. C., N. A. Morrice, M. Kleijn, and C. G. Proud. 2001. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol. Cell. Biol. 21:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheper, G. C., and C. G. Proud. 2002. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur. J. Biochem. 269:5350-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheper, G. C., B. van Kollenburg, J. Hu, Y. Luo, D. J. Goss, and C. G. Proud. 2002. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J. Biol. Chem. 277:3303-3309. [DOI] [PubMed] [Google Scholar]
- 35.Scheper, G. C., R. van Wijk, and A. A. M. Thomas. 2001. Regulation of the activity of eukaryotic initiation factors in stressed cells, p. 39-52. In R. E. Rhoads (ed.), Signaling pathways for translation. Springer-Verlag, Berlin, Germany. [PubMed]
- 36.Seternes, O. M., B. Johansen, B. Hegge, M. Johannessen, S. M. Keyse, and U. Moens. 2002. Both binding and activation of p38 mitogen-activated protein kinase (MAPK) play essential roles in regulation of the nucleocytoplasmic distribution of MAPK-activated protein kinase 5 by cellular stress. Mol. Cell. Biol. 22:6931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slentz-Kesler, K., J. T. Moore, M. Lombard, J. Zhang, R. Hollingsworth, and M. P. Weiner. 2000. Identification of the human mnk2 gene (MKNK2) through protein interaction with estrogen receptor beta. Genomics 69:63-71. [DOI] [PubMed] [Google Scholar]
- 38.Smith, J. A., C. E. Poteet-Smith, D. A. Lannigan, T. A. Freed, A. J. Zoltoski, and T. W. Sturgill. 2000. Creation of a stress-activated p90 ribosomal S6 kinase. The carboxyl-terminal tail of the MAPK-activated protein kinases dictates the signal transduction pathway in which they function. J. Biol. Chem. 275:31588-31593. [DOI] [PubMed] [Google Scholar]
- 39.Smith, J. A., C. E. Poteet-Smith, K. Malarkey, and T. W. Sturgill. 1999. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 274:2893-2898. [DOI] [PubMed] [Google Scholar]
- 40.Smith, M. R., M. Jaramillo, Y. L. Liu, T. E. Dever, W. C. Merrick, H. F. Kung, and N. Sonenberg. 1990. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 2:648-654. [PubMed] [Google Scholar]
- 41.Sonenberg, N., M. A. Morgan, W. C. Merrick, and A. J. Shatkin. 1978. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc. Natl. Acad. Sci. USA 75:4843-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern, B. D., M. Wilson, and R. Jagus. 1993. Use of nonreducing SDS-PAGE for monitoring renaturation of recombinant protein synthesis initiation factor, eIF-4α. Protein Expr. Purif. 4:320-327. [DOI] [PubMed] [Google Scholar]
- 43.Strudwick, S., and K. L. Borden. 2002. The emerging roles of translation factor eIF4E in the nucleus. Differentiation 70:10-22. [DOI] [PubMed] [Google Scholar]
- 44.Stuurman, N., A. de Graaf, A. Floore, A. Josso, B. Humbel, L. de Jong, and R. van Driel. 1992. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 101:773-784. [DOI] [PubMed] [Google Scholar]
- 45.Tournier, C., A. J. Whitmarsh, J. Cavanagh, T. Barrett, and R. J. Davis. 1997. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 94:7337-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tschopp, C., U. Knauf, M. Brauchle, M. Zurini, P. Ramage, D. Glueck, L. New, J. Han, and H. Gram. 2000. Phosphorylation of eIF-4E on Ser 209 in response to mitogenic and inflammatory stimuli is faithfully detected by specific antibodies. Mol. Cell. Biol. Res. Commun. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 47.Wang, X., A. Flynn, A. J. Waskiewicz, B. L. Webb, R. G. Vries, I. A. Baines, J. A. Cooper, and C. G. Proud. 1998. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J. Biol. Chem. 273:9373-9377. [DOI] [PubMed] [Google Scholar]
- 48.Waskiewicz, A. J., A. Flynn, C. G. Proud, and J. A. Cooper. 1997. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16:1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waskiewicz, A. J., J. C. Johnson, B. Penn, M. Mahalingam, S. R. Kimball, and J. A. Cooper. 1999. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol. 19:1871-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]