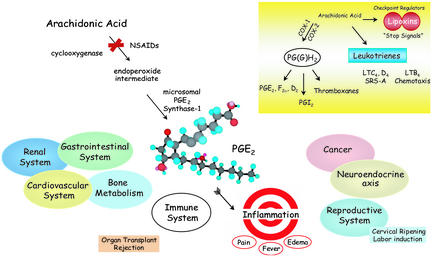
Prostaglandin (PG) E2 is almost ubiquitous in humans and evokes potent diverse actions. Utility is the price of its perfection. PGE2 is a founding member of the PGs, a class of mediators that belongs to the still growing family of bioactive autacoids known as the eicosanoids (1–3). The main classes include enzymatically generated products such as thromboxanes, leukotrienes, lipoxins, and EETs, as well as others that are produced via nonenzymatic mechanisms, e.g., isoprostanes and cyclopentaeone PGs that are increasing in number and appreciation (4, 5). PGE2 regulates key responses in the major human systems including reproductive, gastrointestinal, neuroendocrine, and immune (Fig. 1). Formed by conversion of arachidonic acid via cyclooxygenase (COX) and specific synthases, PGE2 stereospecifically exerts potent (nano- to micromolar range) tissue- and cell type-selective actions (1–6). The importance of PGs in inflammation was brought into view by the discovery of J. Vane and colleagues (7) that nonsteroidal antiinflammatory drugs (NSAIDs) like aspirin, that at the time were used clinically without a known mechanism, act by inhibiting COX production of PGs and thromboxanes. Although highly effective, the COX inhibitors (both COX-1 and COX-2) of today's clinics are still rather blunt therapies at the molecular level, because they not only block the formation of individual PGs but also knock out other “bystander” eicosanoids that may be needed to maintain homeostasis. This can lead to unwanted side effects in multiple systems, i.e., ulcers and constipation. Hence there remains considerable room for improvement in this clinical area. In this issue of PNAS, Trebino et al. (8) studied the pathogenesis of collagen-induced arthritis by using microsomal PGE synthase-1 (mPGES1) deficient mice and demonstrate the contributions of this enzyme and PGE2 in chronic inflammation and pain. Their results provide in vivo validation of this new target, taking a significant step toward the promise of more selective therapeutics. Precision and selectivity are the mantra of today's molecular medicine. In this context, the range of PGE2 actions, from protecting gastrointestinal mucosa to regulating smooth muscle and fever, set a steep challenge for designer drug hunters to achieve, namely selectivity without unwanted side effects. The findings of Trebino et al. raise hope that new classes of drugs targeting mPGES1 can be developed for the treatment of painful chronic inflammatory diseases such as rheumatoid arthritis.
Fig. 1.
Diverse actions of PGE2 and selective targeted biosynthesis in inflammation. (Inset) Eicosanoid family major enzymatic classes of cyclooxygenases and lipoxygenase pathways (see text for details).
PGE2 has a long history of celebrity and many eminent scientists devoted to unlocking its secrets, as it was among the first eicosanoids elucidated. U. von Euler and Sune Bergström each noted that in 1933 a British pharmacologist, M. W. Goldblatt, reported that human semen contained a factor that reduced blood pressure and regulated smooth muscle (1, 3). von Euler trained with H. H. Dale in England and had, just a few years earlier, isolated the bioactive peptide compound P. During systematic studies, von Euler found that extracts of prostate and vesicular glands contained potent blood-pressure-lowering factors from sheep and man that stimulated smooth muscles and were different from compound P. Importantly, von Euler discovered that the activities were of lipidic character and coined the term PG (3). Theorell demonstrated by using electrophoresis that this lipid-soluble activity behaved as an acid.
Bergström's main research was in bile acids and steroids. Because of his expertise in lipids, von Euler approached him to tackle the PG isolation and structural problem (3). Surprisingly, Bergström first carried out fatty acid oxidation studies in the mid-1930s and then returned to the area to focus on the structural elucidation of these bioactive substances. Along with his formidable team of students that included Bengt Borgström, Jan Sjövall, and Bengt Samuelsson, they elucidated in 1957 the structure of PGE1 and F1α. It took several years to recognize that the PGs were generated from arachidonic acid. Mass spectrometry was key to these discoveries. It wasn't until the early 1960s that it was possible to radiolabel arachidonic acid, and its complete biosynthetic scheme quickly followed (2). The PGE structure is conserved in mammalian systems and also is produced by marine organisms. Large quantities were found in coral, which proved serendipitous because only small amounts could be isolated from mammalian systems, limiting availability for pursuit of biological roles. PG-related compounds from coral became useful in the preparation of PG needed to explore their actions. E. J. Corey achieved total organic synthesis, in the late 1960s and early 1970s, and was then able to supply defined compounds that opened the area and established the many actions (1) we know now in target systems responsive to PGE2 as well as other prostanoids (Fig. 1).
It is in the area of inflammation that PGE2's actions are most diverse because of the many specialized cell types, complex and sometimes seemingly opposing actions that make PGE2 one of the more generally “misunderstood” eicosanoids. This confusion likely reflects differences between endogenous formation and action versus the pharmacology of adding PGE2 in vitro and in vivo. The findings of Trebino et al. (8) not only bring to the forefront notions of selective new therapeutics but also clear some of the lines of thinking on the function of PGE2 biosynthesis in vivo. PGE2 functions in the reproductive system and yet can produce bronchoconstriction as well as hypertension. It also promotes labor and dysmenorrhea attributed to its increased endometrial biosynthesis. The actions of PGE also extend to the cardiovascular and immune systems, where PGE2's ability to enhance inflammation caused by leukotrienes as well as inhibit release of mediators and regulate monocyte-macrophages and dendritic cells are current research topics (9). It is in this multicellular milieu of host defense that the mechanisms of PGE and the ability to specifically regulate its local production can have an important impact in chronic inflammatory diseases.
Acute inflammation is a protective host response to pathogens or injury that evoke its cardinal signs, namely heat, redness, swelling, pain, and loss of function (4, 5, 10), that are regulated by eicosanoids (1, 2). Regulators of inflammatory responses are of wide interest, because many of the most prevalent human illnesses, such as arthritis, asthma, and atherosclerosis, involve inflammation. Arthritis alone affects >40 million Americans, is projected to increase to ≈60 million by 2020, and is the leading cause of work-related disability among those over age 65 (11). For centuries, antiinflammatory treatments have been among the most common therapies used by healers (10). Today's worldwide sales of NSAIDs are >$6 billion and represent 3–9% of all prescriptions, and, in the U.S., >90 million prescriptions (>$2 billion) are written annually for these drugs (11). NSAIDs inhibit COX(s), which convert arachidonic acid into endoperoxide PGH2 that serves as substrate (Fig. 1 Inset) for multiple synthases (1–3). The quest for “better” NSAIDs with improved therapeutic benefits and fewer side effects remains of great interest. Indeed, identification of a second COX isoform and its rise as a clinical target have certainly given new generations of inhibitors with improved features over traditional COX inhibitors (12), but they are not without their own shortcomings.
Initiated by the innate immune system, local inflammation increases microvascular dilatation and permeability to enhance phagocyte entry into sites of microbial invasion or tissue injury. Local warmth, erythema, and swelling result from these vascular changes as well as from release of chemical substances by activated leukocytes that can inadvertently injure peripheral nerves as well as sensitize them, giving “inflammatory” pain. PGE2 is generated at sites of inflammation in substantial amounts and can mediate many of the pathologic features of inflammation (5). PGE2 is a potent vasodilator (13) and can act synergistically with other mediators and chemotaxins to increase microvascular permeability (14). In conjunction with cytokines, PGE2 is a central mediator of febrile responses (15, 16), and intradermal PGE2 is hyperalgesic in the peripheral nervous system. To affect this wide terrain of cellular responses, PGE2 interacts with specific receptors, termed EP1–4, that have a restricted patterns of expression and receptor-specific actions (reviewed in ref. 17).
Seemingly opposing modes of action make prostaglandin E2 one of the more misunderstood eicosanoids.
Despite extensive investigation establishing important roles for PGE2 in inflammation, several questions remain. Of the many enzymes, which PGE synthase is responsible for PGE2 formation during inflammation? What is its cell type distribution? How are specific synthases coupled and regulated with COX-1 and -2? Is mPGES1 a more selective target for development of new antiinflammatory drugs than COX-2? Can disease- and organ-selective drugs be developed? Trebino et al. (8) addressed some of these with mice deficient in mPGES1.
PGE synthase activity is present in both cytosolic and membrane-associated fractions of cells, and optimal catalytic activity requires glutathione (18, 19). Cytosolic PGE synthases are functionally coupled to COX-1 and ubiquitously expressed (20). PGE synthase activity can also be induced by proinflammatory stimuli in leukocytes (21). Increased microsomal membrane-associated PGE synthase activity appears in lipopolysaccharide (LPS)-stimulated leukocytes with a delayed increase in PGE2 attributable to coordinate expression and functional coupling with COX-2 (22). To date, two distinct isoforms of microsomal PGES are characterized, mPGES1 and -2. mPGES-1 has high catalytic activity relative to mPGES2, and is more ubiquitously expressed (19, 23, 24). Cells from mice deficient in mPGES1 display impaired LPS-initiated PGE2 formation, but basal PGE2 generation is preserved (25). These findings indicate discrete roles for the PGE synthases and their isoforms in inflammation.
The fate of endoperoxide intermediates in the absence of a functional synthase is of interest. A reciprocal relationship between PGD2 and PGE2 formation in tumor necrosis factor (TNF)-exposed peritoneal macrophages (21) suggests a close relationship between COX and PG synthases with regulated coupling for generation of specific prostanoids. Trebino et al. (8) did not observe increases in PGI2 on disruption of mPGES-1; however, alterations in other prostanoid levels were not reported. Conversion of PGH2 to an alternate PG, nonenzymatic conversion to cyclopentaenone PGs, or the generation of novel antiinflammatory products remains unexplored in mPGES1-deficient animals.
In addition to PGE2 phlogistic properties, immunomodulatory roles are also reported (26). Distinct from its proinflammatory actions, PGE2 promotes resolution of inflammation (27). In addition, lung fibroblasts from individuals with pulmonary fibrosis display diminished COX-2 expression and PGE2 (28). Acute inflammation has four potential outcomes: complete resolution, abscess formation, healing by fibrosis, or progression to chronic inflammation (10). Unlike the early COX-2-derived PGE2 that has a central role in acute inflammation, COX-2-derived products can promote resolution via the generation of antiinflammatory lipid mediators, including prostanoids (other than PGE2), 15-epi-lipoxins, and resolvins (29–31). In the absence of mPGES-1, paw edema formation and leukocyte accumulation are reduced in delayed-type hypersensitivity. Of interest, saline injection into the contralateral paws of immunized, mPGES1 –/– mice led to increased leukocyte accumulation without increases in edema (8). These observations hint at potential additional counterregulatory roles for the enzyme and PGE2 that may become evident with further investigations of the kinetics of complex inflammatory responses, such as delayed-type hypersensitivity, which require prominent contributions from the adaptive immune system for resolution (27). In several other models of inflammation, pharmacologic inhibition of COX-2 decreases PGE2, but not leukocyte infiltration (29, 32). Moreover, eicosanoid generation at sites of inflammation is temporally regulated, and the early formation of COX-2-derived PGE2 can serve as an inducer of subsequent antiinflammatory circuits, in part, via its roles in amplifying 15-lipoxygenase expression and lipoxin generation (30). Notably, extensive clinical experience with COX inhibition by NSAIDs has provided patients with analgesia and antiinflammatory actions, but not disease-remitting properties in rheumatoid arthritis.
By targeting mPGES-1-generated PGE2, Trebino et al. (8) uncovered a striking protection from collagen-induced arthritis, with diminished pannus formation, preservation of proteoglycan at articular surfaces, and less bony erosions. Antibodies against type II collagen were detected in both control and the mPGES1-deficient mice, but the titers were decreased in mPGES1-deficient animals. Although profound immunosuppression was not present, PGE2 displays pivotal regulatory actions on innate and adaptive immune effector cells (9, 30). Toll 4 activation regulates PGE2 formation by mPGES1 (25), and, hence, further study is needed to determine whether decrements in its formation in mPGES1deficient mice may ultimately lead to adverse effects in the host's capacity to resolve acute inflammation or respond to infectious agents.
Disruption of mPGES1 displayed a similar phenotype in collagen-induced arthritis as pharmacological inhibition or genetic disruption of COX-2. Although there is evidence for an in vivo link between COX-2 and mPGES1, it does not preclude the formation of other COX-2–derived products, such as the 15-epi-lipoxins or resolvins that display antiinflammatory and proresolving properties (31). In addition to disruption of these proresolving biosynthetic circuits for ATL (33) and resolvins (31), inhibition of COX-2 is associated with several adverse effects, including impaired renal and cardiac function as well as increased thrombogenesis (34, 35). Similar reductions in inflammation with mPGES1 and COX-2 knockouts now provide new and potentially more selective targets for new antiinflammatory drug design that could spare the COX-2-derived proresolving lipid mediators.
In summation, PGE2's biological actions are tissue- and organ-specific. The results of Trebino et al., that mice, deficient in microsomal PGE2, have improved collagen-induced arthritis, are encouraging and suggest that we are at the dawn of new classes of therapeutic drugs that regulate the eicosanoids' senior member, PGE2. Presumably, this approach should be better tolerated with fewer unwanted side effects than those drugs that block upstream of specific PG synthases. It appears that COX-2 inhibitors can lead to an increase in thrombogenesis and cardiovascular disease incidence (34, 35). Trebino et al. (8) emphasize that unwanted side effects of inhibiting PGE2 generation might be alleviated by targeting mPGE2 synthase 1. New classes of drugs with precision are likely to abound.
Acknowledgments
We thank Mary H. Small and Katie Gotlinger for expert assistance with this manuscript and the National Institutes of Health for support of the research in our laboratories.
See companion article on page 9044.
References
- 1.Bergström, S. (1982) in Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures (Almqvist and Wiksell, Stockholm), pp. 129–148.
- 2.Samuelsson, B. (1982) in Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures (Almqvist and Wiksell, Stockholm), pp. 153–174.
- 3.von Euler, U. S. (1978) in The Excitement and Fascination of Science: Reflections by Eminent Scientists, ed. Gibson, W. C. (Annual Reviews, Palo Alto, CA), Vol. 2, pp. 675–686. [Google Scholar]
- 4.Serhan, C. N. & Ward, P. A. (1999) Molecular and Cellular Basis of Inflammation (Humana, Totowa, NJ).
- 5.Lawrence, T., Willoughby, D. A. & Gilroy, D. W. (2002) Nat. Rev. Immunol. 2, 787–795. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson, B., Dahlén, S. E., Lindgren, J. Å., Rouzer, C. A. & Serhan, C. N. (1987) Science 237, 1171–1176. [DOI] [PubMed] [Google Scholar]
- 7.Vane, J. R. (1982) in Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures (Almqvist & Wiksell, Stockholm), pp. 181–206.
- 8.Trebino, C. E., Stock, J. L., Gibbons, C. P., Naiman, B. M., Wachtmann, T. S., Umland, J. P., Pandher, K., Lapointe, J.-M., Saha, S., Roach, M. L., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 9044–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocca, B. & FitzGerald, G. A. (2002) Intl. Immunopharmacol. 2, 603–630. [DOI] [PubMed] [Google Scholar]
- 10.Majno, G. (1975) The Healing Hand: Man and Wound in the Ancient World (Harvard Univ. Press, Cambridge, MA).
- 11.Centers for Disease Control and Prevention (1999) Morbid. Mortal. Wkly. Rep. 48, 349. [Google Scholar]
- 12.Needleman, P. & Isakson, P. C. (1997) J. Rheumatol. 24, Suppl. 49, 6–8. [PubMed] [Google Scholar]
- 13.Williams, T. J. & Peck, M. J. (1977) Nature 270, 530–532. [DOI] [PubMed] [Google Scholar]
- 14.Raud, J., Dahlén, S.-E., Sydbom, A., Lindbom, L. & Hedqvist, P. (1988) Proc. Natl. Acad. Sci. USA 85, 2315–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello, C. A., Bernheim, H. A., Duff, G. W., Le, H. V., Nagabhushan, T. L., Hamilton, N. C. & Coceani, F. (1984) J. Clin. Invest. 74, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballou, L. R. (2002) in Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury, eds. Honn, K. V., Marnett, L. J., Nigam, S. & Serhan, C. N. (Kluwer/Plenum, New York), Vol. 5, pp. 585–591. [Google Scholar]
- 17.Narumiya, S. & FitzGerald, G. A. (2001) J. Clin. Invest. 108, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe, K., Kurihara, K., Tokunaga, Y. & Hayaishi, O. (1997) Biochem. Biophys. Res. Commun. 235, 148–152. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsson, P.-J., Thoren, S., Morgenstern, R. & Samuelsson, B. (1999) Proc. Natl. Acad. Sci. USA 96, 7220–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanioka, T., Nakatani, Y., Semmyo, N., Murakami, M. & Kudo, I. (2000) J. Biol. Chem. 275, 32775–32782. [DOI] [PubMed] [Google Scholar]
- 21.Fournier, T., Fadok, V. & Henson, P. M. (1997) J. Biol. Chem. 272, 31065–31072. [DOI] [PubMed] [Google Scholar]
- 22.Murakami, M., Naraba, H., Tanioka, T., Semmyo, N., Nakatani, Y., Kojima, F., Ikeda, T., Fueki, M., Ueno, A., Oh-ishi, S. & Kudo, I. (2000) J. Biol. Chem. 275, 32783–32792. [DOI] [PubMed] [Google Scholar]
- 23.Tanikawa, N., Ohmiya, Y., Ohkubo, H., Hashimoto, K., Kangawa, K., Kojima, M., Ito, S. & Watanabe, K. (2002) Biochem. Biophys. Res. Commun. 291, 884–889. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus, M., Kubata, B. K., Eguchi, N., Fujitani, Y., Urade, Y. & Hayaishi, O. (2002) Arch. Biochem. Biophys. 397, 336–341. [DOI] [PubMed] [Google Scholar]
- 25.Uematsu, S., Matsumoto, M., Takeda, K. & Akira, S. (2002) J. Immunol. 168, 5811–5816. [DOI] [PubMed] [Google Scholar]
- 26.Kitsis, E. A., Weissmann, G. & Abramson, S. B. (1991) J. Rheumatol. 18, 1461–1465. [PubMed] [Google Scholar]
- 27.Bandeira-Melo, C., Serra, M. F., Diaz, B. L., Cordeiro, R. S. B., Silva, P. M. R., Lenzi, H. L., Bakhle, Y. S., Serhan, C. N. & Martins, M. A. (2000) J. Immunol. 164, 1029–1036. [DOI] [PubMed] [Google Scholar]
- 28.Wilborn, J., Crofford, L. J., Burdick, M. D., Kunkel, S. L., Strieter, R. M. & Peters-Golden, M. (1995) J. Clin. Invest. 95, 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilroy, D. W., Colville-Nash, P. R., Willis, D., Chivers, J., Paul-Clark, M. J. & Willoughby, D. A. (1999) Nat. Med. 5, 698–701. [DOI] [PubMed] [Google Scholar]
- 30.Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K. & Serhan, C. N. (2001) Nature Immunol. 2, 612–619. [DOI] [PubMed] [Google Scholar]
- 31.Serhan, C. N., Hong, S., Gronert, K., Colgan, S. P., Devchand, P. R., Mirick, G. & Moussignac, R.-L. (2002) J. Exp. Med. 196, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muscará, M. N., Vergnolle, N., Lovren, F., Triggle, C. R., Elliott, S. N., Asfaha, S. & Wallace, J. L. (2000) Br. J. Pharmacol. 129, 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorucci, S., De Lima, O. M., Jr., Mencarelli, A., Palazzetti, B., Distrutti, E., McKnight, W., Dicay, M., Ma, L., Romano, M., Morelli, A. & Wallace, J. L. (2002) Gastroenterology 123, 1598–1606. [DOI] [PubMed] [Google Scholar]
- 34.FitzGerald, G. A. & Patrono, C. (2001) N. Engl. J. Med. 345, 433–442. [DOI] [PubMed] [Google Scholar]
- 35.Marcus, A. J., Broekman, M. J. & Pinsky, D. J. (2002) N. Engl. J. Med. 347, 1025–1026. [DOI] [PubMed] [Google Scholar]