Abstract
We find that giant surface-enhanced Raman scattering for adsorbates on silver surfaces is present only on surfaces that exhibit self-similar fractal topology as inferred from atomic force microscopy. The fractal character results in localizing the energy of incident photons to volumes of a few nanometers on a side, millions of times smaller than the diffraction limit. Consistent with this finding, we have found an enhancement in spontaneous Raman cross section of >13 orders of magnitude for adsorbates on silver surfaces demonstrated to be fractal. The location of “hot spots” on the fractal surfaces is found to be hypersensitive to incident wavelength and polarization even though the observed Raman scattering is strictly linear in incident intensity. These observations are consistent with localization of the photon energy facilitated by the disordered nature of fractal organization through interference between the incident wave and scattered radiation from silver nanoparticle surface plasmons. We also present a surface preparation method that consistently produces fractal topologies that support single-molecule Raman scattering.
Ultra-sensitive spectroscopy to detect single molecules is of great interest in chemistry, biochemistry, and biophysics (1–3). The advent of single-molecule fluorescence studies was an important development, but requires that the molecules under investigation must be intrinsically fluorescent or chemically modified. The discovery that Raman spectroscopy could also be extended to single molecules (4) is an enormous advance because vibrational spectra contain significantly more structural information and can be used to specifically identify molecules. It is widely acknowledged that electromagnetic enhancement near metallic surfaces is a prerequisite to acquire single-molecule Raman spectra, but the detailed circumstances under which surfaces exhibit adequate enhancement remain poorly understood, and methods to prepare surfaces that will reproducibly exhibit the enhancement are cumbersome (5). Additional uncertainty exists over whether molecular resonance or charge transfer interactions with the metal are necessary to make single-molecule Raman detection viable (6).
Cross sections for Raman scattering depend on the square of the incident and scattered fields and, if these are both enhanced, Raman intensities scale as the fourth power of the field (7). Surface plasmon resonance where the local field at metal nanoparticle surfaces experiences large enhancement is thought to be part of the mechanism by which Raman cross sections are increased. However, experimental studies (8) showed only increases of 106 in surface-enhanced Raman scattering intensities using isolated silver nanoparticles at the plasmon resonance, far too low for single-molecule detection. Experiment and theory appear to confirm that single-molecule Raman scattering is observed only in metal nanoparticle aggregates (9, 10) but there is not universal agreement (4).
Experimental Procedures
Preparation of Raman Substrate Silver Films. The substrate preparation is described below. Silver films are fabricated in different ways as described below.
Silver colloid preparation and glass substrate silanization. Ninety milligrams of silver nitrate in 500 ml of solution was heated to reflux and 10 ml of 1% citrate was added immediately, followed by boiling for 2 h (11). UV-visible transmission spectra showed the plasmon absorption of the colloids was ≈400 nm. The Ag particle size was ≈10 nm as observed by scanning electron microscopy. Glass substrates were cleaned by using detergent, rinsed with deionized (DI) water, and then sonicated in acetone and methanol for 2 min each. The substrates were then placed in piranha solution (vol/vol, 3:1 H2SO4/H2O2) for 1 h at 80°C, rinsed by using large amounts of DI water, and dried under flowing dry nitrogen. The cleaned glass substrates were treated by 3% (3-mercaptopropyl)trimethoxysilane acetone solution for 5 h, rinsed with acetone, and dried with high-purity flowing nitrogen. These treated substrates were cured in a drying oven at 100°C for 1 h.
Compacted nanoparticle films and cluster–cluster aggregated films. For the covalently attached silver nanoparticle films we will describe (“compacted nanoparticle films”), we drop cast colloidal solution on the modified substrates described above and allowed them to dry overnight (11, 12). The substrates were then rinsed with DI water, dried under flowing nitrogen, and kept overnight in vacuum. The atomic force microscopy (AFM) images of the substrates were taken. The substrates were treated with saturated solution of 4-mercaptopyridine for 1 h, rinsed with DI water, and dried with high-purity flowing nitrogen before Raman studies. For cluster-aggregated films, we added 5 ml of 0.2 mM fumaric acid solution to 5 ml of the silver colloidal solution. The solution color changed from dark orange to colorless while the colloidal precipitates were formed. Finally these colloidal precipitates were deposited on the (3-mercaptopropyl)trimethoxysilane-modified glass substrates. The AFM images of the substrates were taken after rinsing with DI water and drying with high-purity flowing nitrogen and overnight in vacuum. The substrates were treated with saturated solution of 4-mercaptopyridine for 1 h, rinsed with DI water, and dried with high-purity flowing nitrogen before Raman studies.
Silver “mirror films” by Tollens reaction. We added 15 M concentrated ammonium hydroxide to a beaker with 150 ml of 0.1 M silver nitrate AgNO3 while stirring until the initially formed brown precipitate dissolved (13). Seventy-five milliliters of 0.8 M KOH was added into the clear silver complex solution, and concentrated ammonium hydroxide was added dropwise to redissolve the precipitate. A second solution containing 100 ml of 0.5 M dextrose in water was made, and 0.5 ml of each solution was mixed together and dropped onto cleaned glass substrates. The AFM images of the substrates were taken after rinsing with DI water and drying overnight in vacuum. The substrates were treated with saturated solution of 4-mercaptopyridine for 1 h, rinsed with DI water, and dried with high-purity flowing nitrogen before Raman studies.
Silver nanorod substrates. Porous aluminum oxide templates with 100-nm pores were purchased from Hampton Research (Niguel, CA) (Whatman Anodisc) (14). One side of the membrane was coated with a 0.1-μm silver layer by using vacuum evaporation (10–6 Torr base pressure) to form a conductive electrode. Electrodeposition of silver rods into the pores was carried out by using a BAS 100 B/W (Bioanalytical Systems, West Lafayette, IN) electrochemical setup with a platinum wire as counter electrode and Ag/AgCl as reference electrode. Silver nanorods were grown into the oxide pores by using Technic Silver 1025 plating solution with the silver layer as working electrode. A 1.5-V potential was applied to the working electrode for the reduction of silver cation. Under these conditions, 500-s depositions were adequate to form 10-μm templated silver nanorods. After cleaning the fabricated template in an ultrasonic bath of DI water for 15 min, the template was dried under high-purity flowing nitrogen. The conductive silver layer was then attached to a cover glass slip by using quick-drying epoxy resin and dried for 15 min. The substrate was treated with 1 M NaOH for 5 h, dissolving the aluminum oxide template and leaving Ag rods attached to the Ag film on the cover glass. After rinsing, the sample surface was modified by 4-mercaptopyridine as described above.
Silver films by evaporation in vacuum. We made silver films by thermally evaporating silver from powder onto the prepared cover glass in vacuum of 10–6 Torr. In the Raman studies on these silver films, we used microscope immersion oil rather than 4-mercaptopyridine because the silver films were thick enough to be opaque, so we could not collect Raman spectra through the substrates. Therefore we recorded the Raman spectra of the oil in proximity to the substrates where the light is incident on the top of the silver films rather than through the glass substrate.
Films for single-molecule Raman spectra of 4-mercaptopyridine. We spin-cast 4-mercaptopyridine molecules on silver mirror-reaction films or on nonfractal surfaces (silver nanorod arrays and compacted nanoparticle films) by using 10–9 to 10–10 M solutions. These treated substrates were rinsed many times with DI water to remove weakly adsorbed molecules and dried with pure flowing nitrogen before Raman image studies. We estimate there is much less than one molecule per focal region at these concentrations.
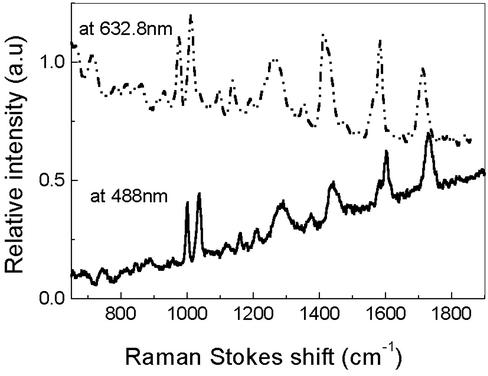
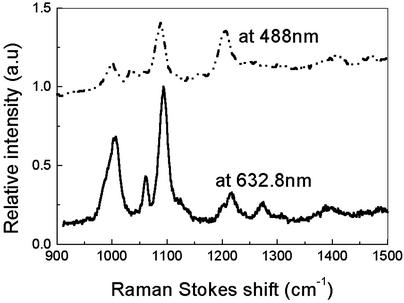
Scanning Confocal Raman Microscopy Apparatus. The sample was imaged with a 1.4 numerical aperture oil-immersion objective by using confocal microscopy (15). This setup allows us to take Raman images by using both 488-nm Ar+ and 632.8-nm HeNe laser lines. The laser powers are attenuated to 0.5–10 μW, and the spot diameter is ≈500 nm. The emission was collected with the same objective and passed through a dichroic filter (Chroma Technology, Brattleboro, VT) and a holographic notch filter (Kaiser Optical Systems, Ann Arbor, MI) to block scattered laser light inside the Nikon TE-300 inverted microscope. The Raman signal was then split, sending one part into a spectrometer with a liquid-nitrogen cooled charge-coupled device (CCD) detector and another to a single photon counting photomultiplier (Hamamatsu, Middlesex, NJ) with a 100-μm pinhole to image samples. Raman images were acquired by scanning the sample with a 2D piezoelectrically driven scanning stage (Mad City Labs, Madison, WI) controlled by a computer. Single molecules were selected for study by positioning the excitation beam over bright spots in the image. Raman spectra of hot spots in the Raman images are shown in Figs. 1 and 2.
Fig. 1.
Typical single-spot spectrum of microscope immersion oil on silver films shown in Figs. 3, 5, and 6 when excited at 632.8 or 488 nm. The CCD integration time is 5 s. Spectra are offset for clarity.
Fig. 2.
Typical single-spot spectrum of 4-mercaptopyridine on silver films shown in Figs. 3, 5, and 6 when excited at 488 or 632.8 nm. The CCD integration time is 5 s. Spectra are offset for clarity.
Results and Discussion
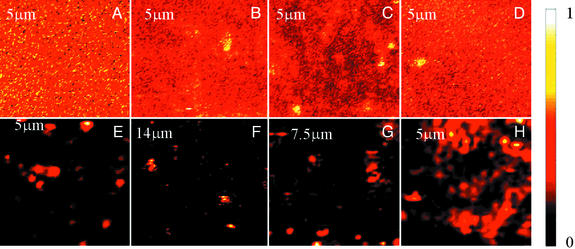
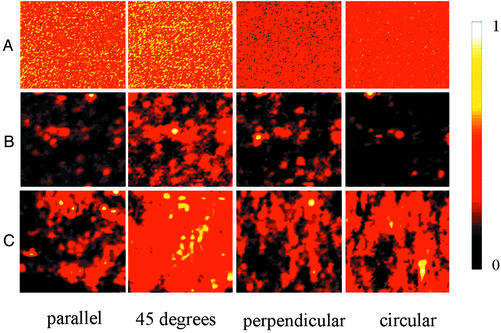
Here we report Raman spectra of molecules on the prepared silver surfaces described above. We find that the surface's ability to support giant Raman enhancement sufficient to observe single adsorbed molecule spectra can be predicted by topological measurements. Fig. 3 shows normalized Raman images of adsorbates on a variety of different silver surfaces recorded in the scanning confocal microscope. In particular, we have studied thick continuous (Fig. 3A) and discontinuous (Fig. 3B) silver films formed by thermal evaporation, compacted nanoparticle films formed by covalent attachment of silver nanoparticles to a glass substrate (Fig. 3C), films of silver nanorods grown in aluminum oxide templates (Fig. 3D), cluster–cluster aggregated films formed by induced precipitation from suspensions of colloidal silver particles (Fig. 3E), and films formed by the Tollens silver mirror reaction (Fig. 3 F–H). It is clear that some of these surfaces exhibit dramatic intensity variations (“hot spots”) whereas others do not. In Fig. 3 C–G, we have treated the surfaces with 4-mercaptopyridine and the light collected is characteristic of the adsorbate Raman spectra. In Fig. 3A, the silver films are thick enough to be opaque, so we cannot collect Raman spectra through the substrate. We therefore recorded the Raman spectra of microscope immersion oil in proximity to the substrate where the light is incident on the top of the silver film rather than through the glass substrate. To check that the appearance of hot spots does not specifically depend on mercaptopyridine or chemisorption, in Fig. 3 B and H we recorded the Raman signal of microscope immersion oil by using the same collection geometry as in Fig. 3A.
Fig. 3.
Raman images of adsorbates (immersion oil or 4-mercaptopyridine). We fabricated 100-nm-thick (A) and 5-nm-thick (B) silver films by thermal evaporation of silver. Silver nanoparticle films (C) (called compacted nanoparticle films) were formed by covalent attachment of silver nanoparticles from colloidal suspensions to glass substrates modified by (3-mercaptopropyl)trimethoxysilane (MPS). Nanorod arrays (D) were synthesized by electrodeposition from silver chloride solution into porous alumina templates. Cluster–cluster aggregated films (E) were deposited by inducing precipitation from colloidal suspensions of citrate ion stabilized silver nanoparticles by adding fumaric acid to the suspension on the MPS-modified substrates. (F–H) Mirror reaction films were formed by using the Tollens reaction. In F, the Tollens reaction time was 5 min, whereas in G and H the time was 2 min. (C–G) Surfaces were treated by saturated 4-mercaptopyridine solutions. (A, B, and H) Surfaces were untreated and data were recorded with excitation from the exterior for the reasons explained in the text. All images were taken by scanning confocal Raman microscopy with excitation at 632.8 nm. The scan size is shown on the images. Note that each image is internally normalized to its maximum so that relative intensities between images cannot be compared. In particular, the hot spots on fractal surfaces have orders of magnitude more Raman scattering than counterparts of similar color on nonfractal surfaces.
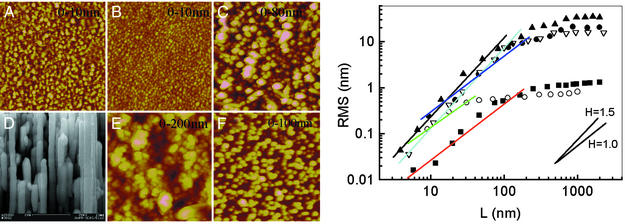
Fig. 4 Left presents topological images of these silver surfaces. All but the silver nanorod surfaces are height images recorded by using AFM. The nanorod surface is imaged by using a scanning electron microscope. Fig. 4 Right presents an analysis of these images to determine whether the surfaces exhibit self-similar scaling. The procedure we have used is to the plot rms surface roughness over areas of dimension L × L versus L, where L is length. This is analogous to the analysis of scanning tunneling micrographs (STM) used by Krim et al. (16) for 100-nm-thick gold surfaces. Scaling of rms roughness σ with image size L (L <1 μm) is observed to obey a power law, σ∝ LH, where the scaling exponent H is related to the fractal dimension D = 3-H (17). We analyzed AFM images of a 100-nm-thick gold film evaporated on a glass substrate and obtained a scaling exponent H of 1.01 ± 0.05, similar to that measured by using STM (16), indicating an ordinary 2D surface. By using the same analysis procedure, we find all of the films exhibiting Raman hot spots have fractional dimensionality D ≈1.5, whereas those that do not have D ≈2.0. Although it is not possible to apply the AFM analysis to the templated silver nanorod surface, the regularity of the pore structure (14) should lead to nonfractal character.
Fig. 4.
(Left) Topology of the various silver surfaces by AFM or scanning electron microscopy. (A and B) The silver films of 100 and 5 nm thick made by thermal evaporation. (C) Compacted nanoparticle films. (D) Nanorod arrays. (E) Cluster–cluster aggregated films. (F) Mirror reaction films. The size of each image is 2 μm × 2 μm. The height scales are shown in the images. (Right) Self-similarity analysis for the different silver surfaces shown in A: 100-nm-thick evaporated silver films (▪); 5.2-nm-thick evaporated silver films (○); compacted nanoparticle films (•); cluster–cluster aggregated films (▴); and mirror reaction films (▿).
The fractal character of the surfaces appears to be primarily determined by the aggregation kinetics involved in forming the films. Those that are formed via aggregation in colloidal suspensions possess self-similar fractal properties (18). In our experiments, the fractal dimensions of cluster–cluster aggregate films formed by aggregation of colloidal silver nanoparticles are ≈1.5. Under experimental conditions similar to ours, fractal dimensions of the deposited colloidal cluster–cluster aggregates of silver nanoparticles on the glass surfaces were found to be 1.45–1.65 by using the area-perimeter method (12). For silver films fabricated by the Tollens reaction, we also obtain fractal dimensions near 1.5, probably reflecting similar kinetics in the aggregation process where large clusters are formed in solution and are immobile after precipitation. In contrast, surfaces prepared by covalent attachment of the silver nanoparticles from stable colloidal solutions where the order is prescribed by uniform surface functionalization are found to be densely packed with D = 2. Similarly, evaporation onto substrates at 300 K leaves silver atoms with sufficient mobility to form relatively well-ordered surfaces with D = 2 (Fig. 4 Right). Evaporation onto cold substrates, however, can lead to fractally organized substrates (19).
Stockman (20) and Shalaev and coworkers (21) have modeled the optical properties for fractal structures formed from aggregates of silver nanoparticles, distinguishing these from nonfractal structures such as compact or periodic geometries. For cluster–cluster aggregates of silver nanoparticles, the plasmon normal modes (collective surface plasmons) are so localized that the enhancement resulting from the interaction of all of the particles through dipole–dipole coupling is concentrated in very small regions of the clusters (22). The model predicts local fields in fractal structures that can be many orders of magnitude higher than the incident fields. The locations of these hot spots are predicted to be very sensitive to the incident field polarizations and frequencies, depending critically on electromagnetic interactions between silver nanoparticles. Giant Raman signals are possible when molecules reside near these hot spots. In contrast, for compact or periodic structures, the normal modes of the surface plasmons are delocalized over larger regions determined by the long-range dipole–dipole interactions of the nanoparticle surface plasmons. Raman images from these nonfractal surfaces are expected to be approximately independent of the polarization and wavelength of the incident field.
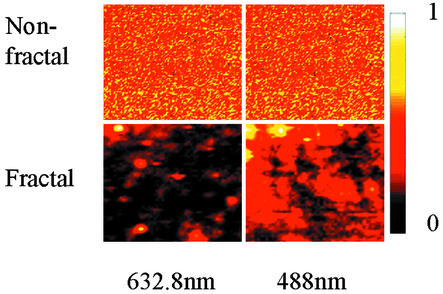
To confirm these predictions, we measured Raman images on fractal surfaces and nonfractal surfaces for different incident laser polarizations and wavelengths as shown in Figs. 5 and 6. The Raman intensity distributions for nonfractal structures are homogeneous and nearly independent of both excitation polarization and frequency. For fractal surfaces, the Raman intensity distributions are extremely inhomogeneous and hypersensitive to the excitation polarizations and wavelengths. For example, the hot spots for various polarizations are completely distinct even though these polarizations are not orthogonal. Remarkably, simple intuition about field superposition does not apply even though the Raman scattering intensity is strictly linear in incident intensity. In fact, the interference between incident and scattered waves required to generate hot spots critically depends on field direction. This qualitative agreement with the predictions of the numerical simulations on fractal surfaces (20) argues strongly that the dimensionality of the surface is the essential feature in localizing the field to produce the giant enhancements required single-molecule Raman spectroscopy.
Fig. 5.
Raman images of the same area on selected fractal and nonfractal surfaces for various excitation laser polarizations where “parallel” is chosen arbitrarily. (A) Raman images of microscope immersion oil on silver films (100 nm thick) with laser power of 9 μW. (B) Raman images of 4-mercaptopyridine adsorbed on the cluster–cluster aggregated films with 0.6-μW excitation power. (C) Raman images of immersion oil on mirror reaction films with 1.9-μW excitation power. The excitation wavelength in each case is 632.8 nm. The size of each image is 5 μm × 5 μm. Note that each image is internally normalized to its maximum so that relative intensities between images cannot be compared. In particular, the hot spots on fractal surfaces have orders of magnitude more Raman scattering than counterparts of similar color on nonfractal surfaces.
Fig. 6.
Raman images of a single area on selected fractal and nonfractal surfaces for different excitation wavelengths. (Upper) The Raman images of immersion oil on the 100-nm-thick silver film. (Lower) The Raman images of 4-mercaptopyridine adsorbed on cluster–cluster aggregated films. The size of each image is 5 μm × 5 μm. Note that each image is internally normalized to its maximum so that relative intensities between images cannot be compared. In particular, the hot spots on fractal surfaces have orders of magnitude more Raman scattering than counterparts of similar color on nonfractal surfaces.
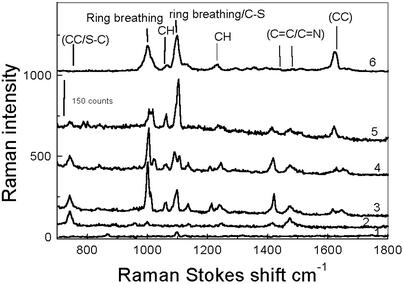
To quantify the field enhancement of the observed hot spots, we studied the single-molecule Raman scattering from 4-mercaptopyridine molecules. We observed this on our fractal silver films, whereas it was not observed under similar conditions on the nonfractal films. Fig. 7 shows single-molecule Raman spectra of 4-mercaptopyridine as a function of time. The Raman spectra of 4-mercaptopyridine were assigned as shown by using the bulk Raman and surface-enhanced Raman scattering spectra (23). The strong temporal fluctuations in intensity and spectra, along with a study of spot density versus solute concentration, are evidence for single-molecule scattering. We estimated the enhancement of the effective Raman cross section of 4-mercaptopyridine molecules by comparison with droplets of toluene solution. Raman spectra of toluene solution on the cover glass without silver films were recorded by using the same optical excitation and collection geometry as for the studies of single molecules of 4-mercaptopyridine. The focal volume was estimated to be 56 fL (24), and the concentration of neat toluene is 9.4 mol/liter, so there are ≈3.3 × 1011 toluene molecules in the focus. Judging from relative sizes of the signals in toluene and 4-mercaptopyridine (see Fig. 7), we estimated the effective cross-section increase caused by electromagnetic field enhancement in the latter to be a factor of ≈5 × 1013, assuming no effect of electronic resonance in the Raman scattering from interaction with the silver (25). If we assume equal field enhancement for the incident and scattered waves, the Raman signal should scale as the fourth power of field enhancement. This reasoning suggests apparent increases of a factor of 2,500 in incident field and 7 × 106 in incident intensity. If we think of the incident photon as a packet of energy localized to around a cubic wavelength (in this case 632 nm), this energy must be compressed to a cube 200 times smaller on each side (in this case 3 nm!) to account for the Raman enhancement. This result confirms that fractal surfaces of metal films can localize the incident field into a region of a few nanometers, in agreement with theoretical predictions.
Fig. 7.
Bulk and single-molecule Raman spectra of 4-mercaptopyridine on Tollens reaction silver surfaces. The CCD exposure time is 5 s for each spectrum and the excitation is 5 μW at 632.8 nm. Spectra 2–5 are representative Raman spectra of the same spot with time. Spectrum 6 is the bulk surface-enhanced Raman scattering spectrum of 4-mercaptopyridine for comparison. Spectrum 1 is the Raman spectrum of toluene on coverglass by using the same optical geometry and excitation power and a CCD integrated time of 15 s. The assignments of several prominent modes are listed on the spectra. In particular, they are C—H in plane wag (843 cm–1), C—N double bond stretch (1,200 cm–1), etc.
Conclusions and Implications
Our experimental findings are helpful in understanding why it is possible to record Raman spectra of single molecules adsorbed on small aggregates of silver nanoparticles whereas evaporated films and organized nanocluster surfaces do not show the same behavior (26, 27). Another important aspect of our work is the discovery that Tollens reaction surfaces will organize fractally and reproducibly exhibit single-molecule Raman scattering from adsorbed species. This procedure is superior to previous methods that rely on fortuitous attachment of adsorbates to clusters of aggregates formed by precipitation from colloidal suspensions. One advantage is the absence of a coating on the colloidal silver (such as citrate ions used to stabilize the nanoparticles against aggregation) that may remain and produce spurious Raman scattering. Also, these surfaces have the advantage that it is possible to use them to obtain single-molecule Raman scattering for molecules without functional groups to attach them to the metal surfaces. Given the susceptibility of single-molecule Raman scattering to impurities caused by its extreme sensitivity, this feature is important. We hope that the improved understanding and control of substrates described here will contribute to important applications for ultrasensitive Raman spectroscopy in biochemical research and clinical medicine.
Acknowledgments
Z.W. acknowledges Prof. L. Novotny and Dr. A. Hartschuh (Institute of Optics, University of Rochester) and Dr. T. Huser (Lawrence Livermore National Laboratory, Livermore, CA) for helpful discussions and technical support. We are grateful to Prof. E. M. Conwell for reading this manuscript. This work was supported by National Science Foundation Grant CTS-9970663.
Abbreviations: DI, deionized; AFM, atomic force microscopy; CCD, charge-coupled device.
References
- 1.Ambrose, W. P., Goodwin, P. M., Jett, J. H., Orden, A. V., Werner, J. H. & Keller, R. A. (1999) Chem. Rev. 99, 2929–2956. [DOI] [PubMed] [Google Scholar]
- 2.Levene, M. J., Korlach, J., Turner, S. W., Foquet, M., Craighead, H. G. & Webb, W. W. (2003) Science 299, 682–686. [DOI] [PubMed] [Google Scholar]
- 3.Lu, H. P., Xun, L. & Xie, X. S. (1998) Science 282, 1877–1882. [DOI] [PubMed] [Google Scholar]
- 4.Nie, S. & Emory, S. S. (1997) Science 275, 1102–1106. [DOI] [PubMed] [Google Scholar]
- 5.Kerker, M., ed. (1990) Selected Papers on Surface-Enhanced Raman Scattering (SPIE Optical Engineering Press, Bellingham, WA).
- 6.Otto, A. (2001) Phys. Status Solidi. A 188, 1455–1470. [Google Scholar]
- 7.Garcia-Vidal, F. J. & Pendry, J. B. (1996) Phys. Rev. Lett. 77, 1163–1166. [DOI] [PubMed] [Google Scholar]
- 8.Kneipp, K., Kneipp, H., Kartha, V. B., Mamasmy, R., Deinum, G., Itzkan, I., Dasari, R. R. & Feld, M. S. (1998) Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 57, R6281–R6284. [Google Scholar]
- 9.Kneipp, K., Kneipp, H., Manoharan, R., Hanlon, E. B., Itzkan, I., Dasari, R. R. & Feld, M. S. (1998) Appl. Spectrosc. 52, 1493–1497. [Google Scholar]
- 10.Markel, V. A., Shalaev, V. M., Zhang, P., Huynh, W., Tay, L., Haslett, T. L. & Moskovits, M. (1999) Phys. Rev. B 59, 10903–10909. [Google Scholar]
- 11.Park, S., Im, J., Im, J., Chun, B. & Kim, J. (1999) Microchem. J. 63, 71–91. [Google Scholar]
- 12.Wensleers, W., Stellacci, W., Meyer-Friedrichsen, T., Mangel, T., Bauer, C. A., Pond, S. J. K., Marder, S. R. & Perry, J. W. (2002) J. Phys. Chem. B 106, 6853–6863. [Google Scholar]
- 13.Saito, Y., Wang, J. J., Smith, D. A. & Batchelder, D. N. (2002) Langmuir 18, 2959–2961. [Google Scholar]
- 14.Sauer, G., Brehm, G., Schneider, S., Nielsch, K., Wehrspohn, R. B., Choi, J. & Gosle, U. (2002) J. Appl. Phys. 91, 3243–3247. [Google Scholar]
- 15.Huser, T., Yan, M. & Rothberg, L. J. (2000) Proc. Natl. Acad. Sci. USA 97, 11187–11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krim, J., Heyvaert, I., Haesendonck, C. V. & Bruynseraede, Y. (1993) Phys. Rev. Lett. 70, 57–60. [DOI] [PubMed] [Google Scholar]
- 17.Mandelbrot, B. B. (1983) The Fractal Geometry of Nature (Freeman, New York).
- 18.Weitz, D. A. & Oliveria, M. (1984) Phys. Rev. Lett. 52, 1433–1436. [Google Scholar]
- 19.Douketis, C., Haslett, T. L., Wang, Z., Moskovits, M. & Lannotta, S. (2000) J. Chem. Phys. 113, 11315–11323. [Google Scholar]
- 20.Stockman, I. M. (1997) Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 56, 6494–6507. [Google Scholar]
- 21.Markel, A. V., Shalaev, M. V., Stechel, B. E., Kim, W. & Armstrong, L. R. (1996) Phys. Rev. B 53, 2425–2436. [DOI] [PubMed] [Google Scholar]
- 22.Moskovits, M., Tay, L., Yang, J. & Haslett, T. (2002) Top. Appl. Phys. 82, 215–226. [Google Scholar]
- 23.Hu, J., Zhao, B., Xu, W., Li, B. & Fan, Y. (2002) Spectrochim. Acta A 58, 2827–2834. [DOI] [PubMed] [Google Scholar]
- 24.Kneipp, K., Wang, Y., Kneipp, H., Perelman, L. T., Itzkar, I., Dasari, R. & Feld, M. S. (1997) Phys. Rev. Lett. 78, 1667–1670. [Google Scholar]
- 25.Krug, J. T., Wang, G. D., Emory, S. R. & Nie, S. (1999) J. Am. Chem. Soc. 121, 9208–9214. [Google Scholar]
- 26.Michaels, M. A., Jiang, J. & Brus, L. (2000) J. Phys. Chem. B 104, 11965–11971. [Google Scholar]
- 27.Xu, H., Bjerneld, E. J., Kall, M. & Borjesson, L. (1999) Phys. Rev. Lett. 83, 4357–4360. [Google Scholar]