Abstract
Heat shock protein 70 is an antiapoptotic chaperone protein highly expressed in human breast tumors and tumor cell lines. Here, we demonstrate that the mere inhibition of its synthesis by adenoviral transfer or classical transfection of antisense Hsp70 cDNA (asHsp70) results in massive death of human breast cancer cells (MDA-MB-468, MCF-7, BT-549, and SK-BR-3), whereas the survival of nontumorigenic breast epithelial cells (HBL-100) or fibroblasts (WI-38) is not affected. Despite the apoptotic morphology as judged by electron microscopy, the asHsp70-induced death was independent of known caspases and the p53 tumor suppressor protein. Furthermore, Bcl-2 and Bcl-XL, which protect tumor cells from most forms of apoptosis, failed to rescue breast cancer cells from asHsp70-induced death. These results show that tumorigenic breast cancer cells depend on the constitutive high expression of Hsp70 to suppress a transformation-associated death program. Neutralization of Hsp70 may open new possibilities for treatment of cancers that have acquired resistance to therapies activating the classical apoptosis pathway.
Impaired apoptosis signaling is common in cancer cells and may play an important role in tumor initiation and progression (1–4). Resistance of cancer cells to apoptosis is especially deleterious because it not only enhances the spontaneous growth of tumors but also renders them resistant to host defense mechanisms as well as various forms of therapy. Apoptosis is controlled by the expression of evolutionarily conserved genes, which either mediate or suppress the process of cell death. The best-studied mediators of apoptosis form a growing family of cysteine proteases, caspases, which function as ultimate effectors in the “classical” apoptotic process (5). Bcl-2 was the first oncogene recognized to function by inhibiting apoptosis (6). Intensive apoptosis research during the last decade has resulted in the identification of several other proteins, which may similarly promote tumorigenesis by suppressing apoptosis (2, 4). Indirect experimental evidence suggests that the major stress-inducible Hsp70 (also known as Hsp72 or Hsp70i) may be such a cancer-relevant antiapoptotic protein (2).
Hsp70 is abundantly expressed in malignant human tumors of various origins, whereas in normal cells, its expression is mainly stress inducible. All cells constitutively express a 70-kDa heat shock cognate protein (Hsc70, also known as Hsp73), which is highly homologous to Hsp70 (7, 8). Under normal conditions, Hsp70 proteins function as ATP-dependent molecular chaperones by assisting the folding of newly synthesized polypeptides, the assembly of multiprotein complexes, and the transport of proteins across cellular membranes (9–12). Under various stress conditions, the accumulation of the inducible Hsp70 enhances the ability of stressed cells to cope with increased concentrations of unfolded/denatured proteins (13, 14). Moreover, Hsp70 effectively inhibits apoptosis induced by a wide range of stimuli (13, 15–19). Interestingly, Hsp70 rescues cells from apoptosis induced by, e.g., tumor necrosis factor (TNF), without inhibiting the activation of effector caspases (19). These data suggest that Hsp70 functions either later in the apoptosis signaling cascade than any known survival-enhancing protein/drug or that Hsp70 suppresses another as yet unknown caspase-independent death pathway (19). In addition to its preferential expression in tumors and its ability to inhibit apoptosis, the role of Hsp70 in tumorigenesis is supported by data showing that its expression enhances the tumorigenic potential of rodent cells in vivo (20–22). Furthermore, the expression of Hsp70 correlates with increased cell proliferation, poor differentiation, lymph node metastases, and poor therapeutic outcome in human breast cancer (23–26).
Earlier results of a preliminary nature have suggested that the inhibition of Hsp70 synthesis in tumor cells may either sensitize them to chemotherapy or commit them to apoptosis (19, 27, 28). This study was designed to explore the possible usefulness of Hsp70 depletion in cancer therapy. For this purpose, we generated an adenoviral (Ad) vector carrying a fragment of human Hsp70 cDNA in antisense (as) orientation (Ad.asHsp70) capable of inhibiting the expression of Hsp70 without depleting the essential Hsc70. With this tool, we were then able to address the question of whether the specific Hsp70 depletion selectively affects cancer cells. Because this was the case, we next studied whether tumor cells could protect themselves from the antisense Hsp70 cDNA (asHsp70)-induced death by up-regulating known antiapoptotic proteins. Because we did not observe protection by Bcl-2 family members or caspase inhibitors, we next asked whether the death pathway silenced by Hsp70 depletion differed fundamentally from the apoptosis induced by standard chemotherapy. Although electron microscopy confirmed the induction of classical apoptosis on the morphological level, the biochemistry appeared to be different. Based on these results, the inhibition of Hsp70 synthesis now appears as a novel promising approach for treatment of human cancers resistant to standard therapies.
Materials and Methods
Recombinant Adenoviruses.
The method for production of adenoviruses was a modification of the procedure described earlier (29). The adenoviral plasmids and strains were provided by HepaVec AG für Gentherapie (Berlin; www.hepavec.com). To create the Ad.asHsp70 virus, the expression unit from pcDNA-ashsp1 (19) containing cytomegalovirus immediate early promoter bases 475–796 of the published human Hsp70 sequence (30) in antisense orientation and SV40 polyadenylation signal were inserted into the adenoviral shuttle vector pHVAd2. The virus genome was generated by homologous recombination of the shuttle plasmid with the plasmid pHVAd1 containing the complete adenovirus in the strain BJ 5183 RecBC-sbcB and retransformed in the recA- strain HB101. The viral DNA was transfected into 293 cells and adenoviral plaques were propagated according to standard procedures including some modifications as described before (31). The adenovirus harboring the β-galactosidase gene under the Rous sarcoma virus promoter (Ad.β-gal) (32) was kindly provided by L. D. Stratford-Perricaudet (Centre National de la Recherche Scientifique, Villejuif, France).
Cell Culture and Treatments.
MCF-7, MDA-MB-468, SK-BR-3, and BT-549 are tumorigenic breast carcinoma cell lines, and WI-38 cells are lung-derived nontransformed fibroblasts available from the American Type Culture Collection (Manassas, VA). HBL-100 is an immortalized breast epithelial cell line kindly provided by Per Briand (Danish Cancer Society, Copenhagen, Denmark). A TNF-sensitive subclone of MCF-7 cells (MCF-7S1) is used throughout the study (33). MCF-V, MCF-Bcl-2, and MCF-Bcl-XL cells were obtained by transfecting MCF-7S1 cells with pEBS-7 vector or the same vector containing the cDNAs encoding for human Bcl-2 or Bcl-XL, respectively (33). MCF-CrmA, and MCF-CrmA-M are single-cell clones of MCF-7S1 cells overexpressing wild-type CrmA or its inactive mutant, respectively (34). All cells were propagated in RPMI medium 1640 with glutamax (GIBCO) supplemented with 10% FCS (Biological Industries, Beit Haemek, Israel), penicillin and streptomycin, and/or the required selection antibiotics, which were omitted during experiments.
The human TNF was a generous gift from Anthony Cerami (Kenneth Warren Laboratories, Tarrytown, NY). Z-Val-Ala-dl-Asp-fluoromethylketone (ZVAD) and acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD) were from Bachem.
Virus Infections and Transfections.
Subconfluent cells plated 24 h earlier were infected with adenoviruses for 15 min at 25°C with continuous rocking followed by 60 min at 37°C in RPMI medium 1640. The concentrations of virus (250–1,000 multiplicity of infection) used were always equal to the minimal concentrations of Ad.β-gal, thereby causing 100% infection in each cell line without visible toxic effects as determined by staining with 5-bromo-4-chloro-3-indolyl β-d-galactoside as described (19, 35).
Transient expression of various antisense Hsp70 constructs was obtained by transfecting subconfluent cells with pcDNA-ashsp1, -ashsp2, and -ashsp3 plasmids (19) using Lipofectamine 2000 reagents (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions. The successfully transfected cells were visualized by cotransfection (1:10) of pEGFP-N1 (CLONTECH) encoding for the green fluorescence protein. Plasmids pcDNA-Casp.8 and pcDNA-Casp.8-mut encoding for wild-type caspase 8 (FLICE) and dominant-negative mutant caspase-8, respectively, were kindly provided by Vishva Dixit (University of Michigan, Ann Arbor, MI) and served as controls for the transfection experiments. The percentage of dead green cells was determined by using an inverted Olympus IX70 fluorescence microscope and by counting a minimum of five randomly chosen fields of 100 or more green cells. The rescue experiments with Hsp70 or CrmA were performed in a similar manner by cotransfecting pEGFP-N1 (1:10) with pSV-hsp70-tag (15) or pcDNA3-CrmA (36), respectively, 24 h after the infection with adenovirus.
Apoptosis and Survival Assays.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (19) or cell counting of a minimum of eight randomly chosen fields were used to measure the viability of cells. Apoptotic nuclear changes were detected by a terminal deoxynucleotidyltransferase-mediated fluorescein-dUTP nick end-labeling kit (Boehringer Mannheim) as described (19). DEVDase activity in cell lysates was measured by using a colorimetric probe Ac-DEVD-pNA (Biomol, Plymouth Meeting, PA) as described (19).
Immunoblot Analysis.
Equal amounts of protein (20 μg per lane) from cell lysates prepared after indicated treatments were separated by 8% SDS/PAGE. Immunodetection of proteins transferred to nitrocellulose was performed by using enhanced chemiluminescence Western blotting reagents (Amersham International). Mouse mAb against Hsp70 (H29), Hsc70 (N69 (both kindly provided by Boris Margulis, Russian Academy of Sciences, St. Petersburg, Russia), and glyceraldehyde-3-phosphate dehydrogenase were used as primary antibodies followed by peroxidase-conjugated anti-mouse IgG (Dako).
Electron Microscopy.
The infected cells were scraped off, mixed with one volume of 100% Karnovsky, incubated for 10 min, and centrifuged at 1,500 × g for 5 min. After removal of the supernatant, the pellet was stored in 70% Karnovsky's solution. After two 10-min washings in 0.1 M cacodylate buffer and centrifugation as above, the pellet was embedded in agar, cut into smaller cube and fixed in osmium, and embedded in the epoxy-embedding material Epon according to standard procedures. Sections (1 μm thick) were stained with toluidine blue and examined in a Leica DM RB microscope. Ultrathin sections were collected on Formvar-coated copper grids and stained according to standard procedures for ultrastructural examination in a JEOL 1210 or Philips 201 electron microscope.
Results
Inhibition of Hsp70 Synthesis Induces Massive Death of Breast Cancer Cells.
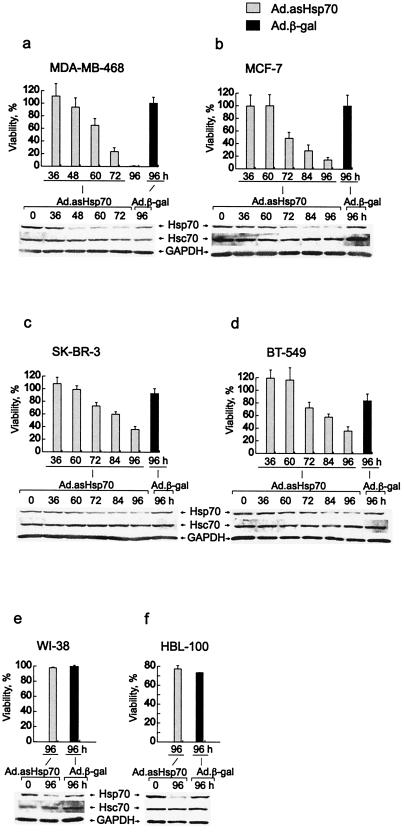
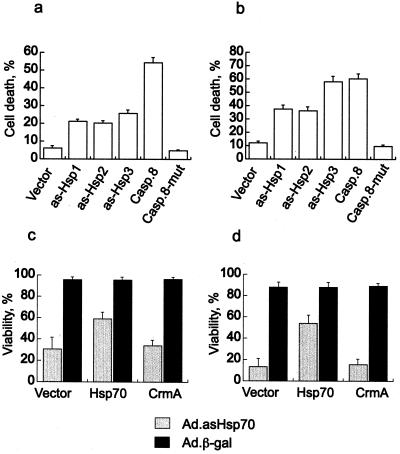
Human breast cancer cell lines with different genetic changes associated with tumorigenesis and/or resistance to apoptosis (MDA-MB-468, MCF-7, BT-549, and SK-BR-3), nontumorigenic breast-derived epithelial cells (HBL-100), and normal lung fibroblasts (WI-38) were infected with Ad.asHsp70 at a multiplicity of infection needed to achieve a 100% infection. Ad.asHsp70 specifically reduced the level of Hsp70 in all infected cell lines with no significant effects on the levels of the closely related Hsc70 (Fig. 1). Surprisingly, the majority of Ad.asHsp70-infected tumor cells died concomitant with decrease in Hsp70 protein levels ≈3–5 days after the infection (Fig. 1 a–d). Ad.asHsp70-infected cultures of MDA-MB-468 and MCF-7 cells were followed for up to 4 weeks with no appearance of any surviving colonies. Interestingly, the Ad.asHsp70-induced reduction in Hsp70 expression had no effect on the survival of nontumorigenic WI-38 and HBL-100 cells (Fig. 1 e and f). Similar infections with a control virus, Ad.β-gal, did not induce detectable cell death. The death induced by asHsp70 did not require the viral components, because eukaryotic expression plasmids (pcDNA-ashsp1, -ashsp2, and -ashsp3) encoding for three different antisense Hsp70 sequences (19) also induced significant cell death in MCF-7 and MDA-MB-468 cells (Fig. 2 a and b). That enforced expression of ectopic Hsp70 rescued Ad.asHsp70-infected breast cancer cells suggests that Ad.asHsp70-induced death is, indeed, a result of reduced Hsp70 expression in dying cells (Fig. 2 c and d).
Figure 1.
Inhibition of Hsp70 synthesis results in decreased survival of breast cancer cells. The indicated human breast carcinoma cell lines (a–d), lung-derived fibroblasts (e), and breast-derived nontumorigenic cells (f) were infected with Ad.asHsp70 or Ad.β-gal. The survival of cells was analyzed at indicated times after infection by counting the cells (a–d and f) or the MTT assay (e). The survival of Ad.β-gal-infected cells is expressed as a percentage compared with noninfected cells and that of Ad.asHsp70-infected cells as a percentage compared with Ad.β-gal-infected cells. The columns represent means of cell counts from a minimum of eight fields (a–d and f) or triplicate MTT assay measurements (e) ± SD. Proteins (20 μg per lane) from similarly infected cell lysates were analyzed for Hsp70, Hsc70, and glyceraldehyde-3-phosphate dehydrogenase by immunoblotting. All experiments were repeated one to three times with essentially the same results.
Figure 2.
Antisense Hsp70 DNA induces cell death in breast cancer cells and Ad.asHsp70-infected cells can be rescued by overexpression of human Hsp70. Subconfluent MCF-7 (a) or MDA-MB-468 (b) cells were transfected with pcDNA-3 vector or the same vector with three different antisense Hsp70 sequences (as-Hsp1, -2, or -3) or the entire coding sequence for caspase 8 (Casp.8) or mutated caspase 8 (Casp.8-mut). The successfully transfected cells were visualized by cotransfection (1:10) of pEGFP-N1, and the percentage of green dead cells of all green cells was counted 96 h after transfection. Rescue experiments were performed in a similar manner by cotransfecting pEGFP-N1 with an empty vector, pSV-hsp70-tag or pcDNA3-CrmA into MCF-7 (c) or MDA-MB-468 (d) cells 24 h after the infection with indicated viruses. The percentage of green-surviving cells of all green cells was counted 96 h after the infection. The values represent means of five fields each with a minimum of 100 cells ± SD. The experiments were repeated twice with essentially the same results.
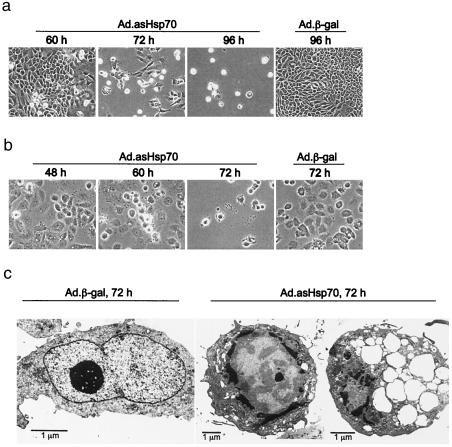
Ad.asHsp70-Infected Breast Cancer Cells Show Apoptosis-Like Morphology.
Typical apoptotic morphology characterized by loss of adherence, condensed cytoplasm, and formation of apoptotic bodies was evident in dying cells observed in the light microscopy (Fig. 3 a and b). Electron microscopy of Ad.asHsp70-infected MCF-7 (Fig. 3c) and MDA-MB-468 (not shown) revealed chromatin condensed in lumps at the inner side of the nuclear envelope and dilation of the endoplasmic reticulum to large vesicles with or without membranous material in the lumen. The dying cells had intact plasma membranes and appeared rounded often without microvilli or protrusions on the surface. Some mitochondria appeared swollen whereas others were condensed. Furthermore, DNA degradation visualized by staining with terminal deoxynucleotidyltransferase-mediated UTP end-labeling occurred in over 95% of MCF-7 and MDA-MB-468 cells 4 and 3 days after the Ad.asHsp70 infection, respectively (data not shown). Cells infected with Ad.β-gal showed no signs of apoptosis. Ultrastructurally, they had abundant mitochondria, sparse vacuoles, and chromatin appeared mostly as euchromatin (Fig. 3c). Under 5% of MCF-7 cells infected with Ad.β-gal were positive in terminal deoxynucleotidyltransferase-mediated UTP end-labeling (TUNEL) staining (data not shown).
Figure 3.
Breast cancer cells infected with Ad.asHsp70 display an apoptotic morphology. MCF-7 (a and c) and MDA-MB-468 (b) breast cancer cells were infected with indicated adenoviruses and analyzed for morphological changes by phase contrast microscopy (a and b) or electron microscopy (c) at indicated times after the infection.
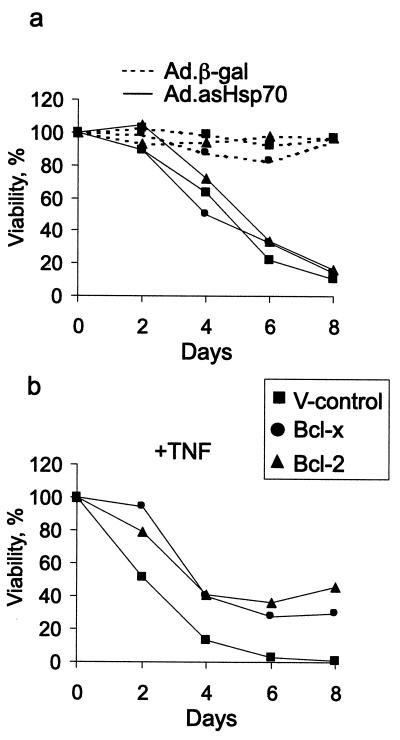
Bcl-2 and Bcl-XL Fail to Rescue Breast Cancer Cells from Ad.asHsp70-Induced Cell Death.
To study the ability of potent antiapoptotic proteins Bcl-2 and Bcl-XL to rescue breast cancer cells from cell death induced by reduced expression of Hsp70, we infected MCF-7 cells overexpressing these proteins (33) with Ad.asHsp70. Neither Bcl-2 nor Bcl-XL had an effect on Ad.asHsp70-induced cell death in MCF-7 cells. The ectopic Bcl-2 and Bcl-XL proteins were functionally active because they conferred almost 50% protection from apoptosis induced by a long-term treatment with otherwise lethal concentrations of TNF (Fig. 4).
Figure 4.
Bcl-2 and Bcl-XL fail to protect breast cancer cells from Ad.asHsp70-induced cell death. Subconfluent MCF-7 cells transfected with an empty vector (V-control) or those overexpressing Bcl-2 or Bcl-XL were infected with indicated viruses (a) or treated with 10 ng/ml of TNF (b). The survival of the cells as compared with noninfected cells was analyzed by MTT assay at indicated times after the infection or TNF treatment. The values represent means of triplicate measurements. The experiments were repeated twice with essentially the same results.
Ad.asHsp70-Induced Apoptosis-Like Cell Death Is Caspase Independent.
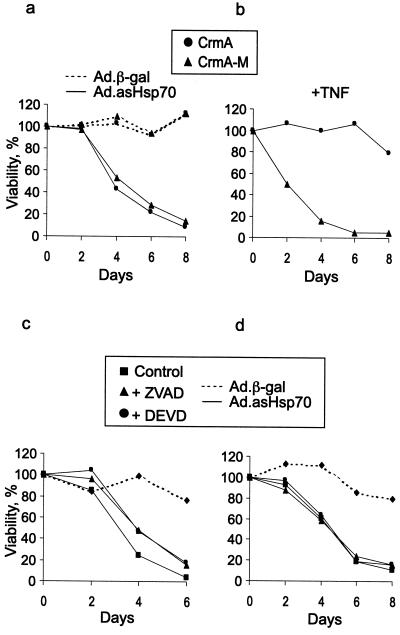
Next we tested whether caspases, the major mediators of classical apoptosis pathways, are involved in death induced by reduced Hsp70 expression. Surprisingly, the overexpression of a cowpox virus-derived caspase inhibitor CrmA (34) as well as treatments with either a potent broad-spectrum caspase inhibitor, ZVAD, or an inhibitor of caspase-3-like caspases, DEVD, had no effect on Ad.asHsp70-induced death of MCF-7 cells (Figs. 2c and 5 a, b, and d). Similarly, both caspase inhibitors as well as forced expression of CrmA failed to rescue MDA-MB-468 cells from asHsp70 (Figs. 2d and 5c). Caspase inhibitors resulted, however, in a slight delay in the death process of MDA-MB-468 cells. To ensure effective concentrations of caspase inhibitors in a long-term assay, fresh media with fresh inhibitors was added after a 3-day incubation. Furthermore, ZVAD and DEVD were used at 100- and 2.5-times higher concentrations, respectively, than required for a complete inhibition of TNF-induced cell death of MCF-7 cells when TNF was added 3 days after the inhibitors (data not shown).
Figure 5.
Caspase inhibitors fail to protect breast cancer cells from Ad.asHsp70-induced cell death. Subconfluent MCF-7 cells overexpressing CrmA or its inactive mutant (CrmA-M) were infected with indicated viruses (a) or treated with 10 ng/ml TNF (b). Subconfluent MDA-MB-468 (c) or MCF-7 (d) cells were left untreated or treated with 10 μM ZVAD or 100 μM DEVD for 1 h before the infection with indicated adenoviruses. In all experiments, the medium was changed at day 4 with unchanged concentrations of inhibitors and TNF. The survival of the cells as compared with noninfected cells was analyzed by MTT assay at indicated times after the infection or TNF treatment. The values represent means of triplicate measurements. The experiments were repeated twice with essentially the same results.
To investigate whether caspases are at all activated by Ad.asHsp70, we then measured the activity of DEVD-specific effector caspases in cell lysates prepared from infected breast cancer cells 2–4 days after the infection. Despite the massive apoptosis induced by Ad.asHsp70, the DEVDase activity in Ad.asHsp70-infected MCF-7 and MDA-MB-468 cells was indistinguishable from that in Ad.β-gal-infected cells at days 2, 3, and 4 postinfection (data not shown).
Discussion
The data presented above clearly show that the high expression of Hsp70 is required for the survival of tumorigenic breast cancer cells. Abrogation of Hsp70 synthesis by adenoviral transfer of a fragment of Hsp70 cDNA resulted in extensive cell death of all breast cancer cell lines tested. Our data strongly suggest that the death induced by Ad.asHsp70 was caused by the mere inhibition of Hsp70 synthesis and not to virus infection or other nonspecific effects of the virus. First, transient transfection of eucaryotic expression plasmids encoding for antisense Hsp70 fragments were able to induce cell death in breast cancer cells in the absence of adenoviral sequences. Second, similar infections with a control adenovirus (Ad.β-gal) or another adenovirus identical to Ad.asHsp70 except for the asHsp70 sequence (data not shown) did not result in increased apoptosis, indicating that viral components per se cannot kill breast cancer cells. Third, ectopic expression of Hsp70 was able to rescue cells from Ad.asHsp70-induced death. Finally, nontumorigenic cells were resistant to Ad.asHsp70 infection, ruling out nonspecific toxicity of the virus as the cause of the death. Furthermore, inhibition of the synthesis of other proteins by asHsp70 is an unlikely explanation for death induced by asHsp70, because nonoverlapping antisense Hsp70 sequences (asHsp70- and asHsp70–2) were equally effective in inducing cell death in breast cancer cells, and Ad.asHsp70 specifically reduced expression of Hsp70 without affecting that of Hsc70, whose cDNA sequence has the highest homology of known human cDNAs to the antisense sequences used.
The precise nature of the death pathway silenced by Hsp70 still needs to be studied. We show, however, that despite the obvious apoptotic morphology, it is clearly distinct from the classical apoptosis pathway. The caspases have been firmly established as major mediators of the execution phase of apoptosis (5). Signaling from death receptors of the TNF-receptor family can lead to direct activation of a caspase cascade, whereas most other apoptotic stimuli lead to mitochondrial changes and a subsequent activation of caspases. Even though it is clear today that cells can die effectively in the absence of caspase activation, only in a few cases has caspase activation been shown to be absent in cells displaying an apoptotic morphology (37–39). Ad.asHsp70-induced apoptosis differs, however, also from most other caspase-independent apoptotic pathways by being insensitive to protection conferred by antiapoptotic proteins of the Bcl-2 family. Thus, it is interesting to note that Hsp70-mediated protection from apoptosis induced by, e.g., TNF or staurosporine, is accompanied by neither the inhibition of the release of cytochrome c from mitochondria nor inhibition of effector caspases (19). Our data evaluated in light of this earlier observation suggest that Hsp70 may inhibit another as yet unknown apoptotic pathway. After various apoptotic stimuli, such as TNF, this pathway would then be activated simultaneously with the classical caspase cascade but turned on after down-regulation of Hsp70 expression in tumor cells even in the absence of caspase activation. Translocation of apoptosis-inducing factor from the mitochondrion into the nucleus could, for example, explain chromatin condensation even in the absence of caspase activation. This is, however, an unlikely scenario, because Bcl-2 has been reported to effectively inhibit the release of apoptosis-inducing factor from the mitochondria (40). In addition to caspases, other proteases including cathepsins, calpains, and serine proteases have been implicated in various apoptosis models (41–44). The possible role of these proteases in the Ad.asHsp70-induced death pathway is presently under investigation.
The only clue to the nature of this apoptotic pathway comes from the results showing that Ad.asHsp70 preferentially kills tumorigenic cells. Although proliferation and death appear opposing and mutually contradictory, substantial evidence now indicates that several growth-inducing oncoproteins, e.g., Myc and Ras, indeed promote cell death (4, 45). The coupling of cell division to cell death can thus function as an effective barrier that must be circumvented for cancer to occur. The first example of such a dual tumorigenesis model came from studies showing that Bcl-2, when coexpressed with Myc, was able to promote tumor growth by increasing the survival of the cells and not by further inducing proliferation (46). The following studies on its mechanism of action have since established Bcl-2 as an oncogene that effectively inhibits apoptosis signaling upstream of the release of cytochrome c from the mitochondria to cytosol (2, 45). Thus, the major role of Bcl-2 in tumorigenesis seems to be able to block proapoptotic effects of oncogenes like c-myc activating the classical apoptosis pathway. Other oncogenes, however, trigger death pathways distinct from the classical apoptosis and thus may require survival proteins such as Hsp70 to be coexpressed to overcome the growth inhibition caused by the concomitant induction of death. For example, oncogenic Ras was recently shown to activate a caspase-independent cell death program that could not be inhibited by Bcl-2 in human glioblastoma cells (47). However, this death pathway differs fundamentally from that induced by Hsp70 depletion in breast cancer cells. Glioblastoma cells killed by activated Ras lacked, namely, all of the morphological features of apoptosis.
Thus, the exact character of the tumor-associated death pathway silenced by Hsp70 still needs to be studied. In addition to known caspases, functional p53 tumor suppressor protein is also dispensable for Ad.asHsp70-induced apoptosis, because MDA-MB-468, BT-549, and SK-BR-3 cell lines harboring inactivating mutations in p53 (48, 49) died effectively after Ad.asHsp70 infection. In conclusion, our findings suggest that this death pathway functions as a hindrance of neoplasia, and its activation may form bases for novel therapies aimed against numerous tumors that have acquired resistance against apoptosis-based therapies.
Acknowledgments
We dedicate this work to the memory of Michael Strauss. We thank Marcel Leist for critical comments and Birgit Poulsen and Klaus Kokholm for excellent technical assistance. This work was supported by the Danish Medical Research Council and Danish Cancer Society.
Abbreviations
- Ad
adenoviral
- as
antisense
- DEVD
acetyl-Asp-Glu-Val-Asp-aldehyde
- Hsp70
70-kDa heat shock protein
- TNF
tumor necrosis factor
- asHsp70
antisense Hsp70 cDNA
- Hsc70
heat shock cognate protein
- Ad.asHsp70
Hsp70 cDNA in antisense orientation
- ZVAD
Z-Val-Ala-dl-Asp-fluoromethyl ketone
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
References
- 1.Bold R J, Termuhlen P M, McConkey D J. Surg Oncol. 1997;6:133–142. doi: 10.1016/s0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 2.Jäättelä M. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- 3.Reed J C. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg R A. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 6.Vaux D L, Cory S, Adams J M. Nature (London) 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 7.Tavaria M, Gabriele T, Kola I, Anderson R L. Cell Stress Chaper. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiang J G, Tsokos G C. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 9.Beckmann R P, Mizzen L A, Welch W J. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Thomas J O. Mol Cell Biol. 1992;12:2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami H, Pain D, Blobel G. J Cell Biol. 1988;107:2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang H-L, Terlecky S, Plant C P, Dice J F. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 13.Kabakov A E, Gabai V L. Heat Shock Proteins and Cytoprotection: ATP-Deprived Mammalian Cells. Austin, TX: Landes; 1997. [Google Scholar]
- 14.Nollen E A, Brunsting J F, Roelofsen H, Weber L A, Kampinga H H. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jäättelä M, Wissing D, Bauer P A, Li G C. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jäättelä M, Wissing D. J Exp Med. 1993;177:231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vayssier M, Banzet N, Francois D, Bellmann K, Polla B S. Am J Physiol. 1998;275:L771–L779. doi: 10.1152/ajplung.1998.275.4.L771. [DOI] [PubMed] [Google Scholar]
- 19.Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jäättelä M. Int J Cancer. 1995;60:689–693. doi: 10.1002/ijc.2910600520. [DOI] [PubMed] [Google Scholar]
- 21.Seo J S, Park Y M, Kim J I, Shim E H, Kim C W, Jang J J, Kim S H, Lee W H. Biochem Biophys Res Comm. 1996;218:582–587. doi: 10.1006/bbrc.1996.0103. [DOI] [PubMed] [Google Scholar]
- 22.Volloch V Z, Sherman M Y. Oncogene. 1999;18:3648–3651. doi: 10.1038/sj.onc.1202525. [DOI] [PubMed] [Google Scholar]
- 23.Ciocca D R, Clark G M, Tandon A K, Fuqua S A, Welch W J, McGuire W L. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- 24.Lazaris A C, Chatzigianni E B, Panoussopoulos D, Tzimas G N, Davaris P S, Golematis B C. Breast Cancer Res Treat. 1997;43:43–51. doi: 10.1023/a:1005706110275. [DOI] [PubMed] [Google Scholar]
- 25.Vargas-Roig L M, Fanelli M A, Lopez L A, Gago F E, Tello O, Aznar J C, Ciocca D R. Cancer Detect Prev. 1997;21:441–451. [PubMed] [Google Scholar]
- 26.Vargas-Roig L M, Gago F E, Tello O, Aznar J C, Ciocca D R. Int J Cancer. 1998;73:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y, Zhao X, Kariya Y, Teshigawara K, Uchida A. Cancer Immunol Immunother. 1995;40:73–78. doi: 10.1007/BF01520287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur J, Ralhan R. Int J Cancer. 2000;85:1–5. doi: 10.1002/(sici)1097-0215(20000101)85:1<1::aid-ijc1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milarski K L, Morimoto R I. Proc Natl Acad Sci USA. 1986;83:9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand K, Arnold W, Bartels T, Lieber A, Kay M A, Strauss M, Dorken B. Cancer Gene Ther. 1997;4:9–16. [PubMed] [Google Scholar]
- 32.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jäättelä M, Benedict M, Tewari M, Shayman J A, Dixit V M. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 34.Wissing D, Mouritzen H, Egeblad M, Poirier G G, Jäättelä M. Proc Natl Acad Sci USA. 1997;94:5073–5077. doi: 10.1073/pnas.94.10.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandig V, Brand K, Herwig S, Lukas J, Bartek J, Strauss M. Nat Med. 1997;3:313–319. doi: 10.1038/nm0397-313. [DOI] [PubMed] [Google Scholar]
- 36.Tewari M, Dixit V M. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 37.Mathiasen I S, Lademann U, Jäättelä M. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- 38.Okuno S, Shimizu S, Ito T, Nomura M, Hamada E, Tsujimoto Y, Matsuda H. J Biol Chem. 1998;273:34272–34277. doi: 10.1074/jbc.273.51.34272. [DOI] [PubMed] [Google Scholar]
- 39.Borner C, Monney L. Cell Death Differ. 1999;6:497–507. doi: 10.1038/sj.cdd.4400525. [DOI] [PubMed] [Google Scholar]
- 40.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Constantini P, Loeffer M, et al. Nature (London) 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 41.Ruggiero V, Johnson S E, Baglioni C. Cell Immunol. 1987;107:317–325. doi: 10.1016/0008-8749(87)90240-1. [DOI] [PubMed] [Google Scholar]
- 42.Wright S C, Wei Q S, Zhong J, Zheng H, Kinder D H, Larrick J W. J Exp Med. 1994;180:2113–2123. doi: 10.1084/jem.180.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lotem J, Sachs L. Proc Natl Acad Sci USA. 1996;93:12507–12512. doi: 10.1073/pnas.93.22.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K K. Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 45.Evan G, Littlewood T. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 46.Strasser A, Harris A W, Bath M L, Cory S. Nature (London) 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 47.Chi S, Kitanaka C, Noguchi K, Mochizuki T, Nagashima Y, Shirouzu M, Fujita H, Yoshida M, Chen W, Asai A, et al. Oncogene. 1999;18:2281–2290. doi: 10.1038/sj.onc.1202538. [DOI] [PubMed] [Google Scholar]
- 48.Bartek J, Vojtesek B, Lane D P. Eur J Cancer. 1992;29A:101–107. doi: 10.1016/0959-8049(93)90584-3. [DOI] [PubMed] [Google Scholar]
- 49.Hedenfalk I A, Baldetorp B, Borg A, Oredsson S M. Cytometry. 1997;29:321–327. doi: 10.1002/(sici)1097-0320(19971201)29:4<321::aid-cyto9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]