Abstract
One of the fundamental events in metamorphosis in insects is the replacement of larval tissues by imaginal tissues. Shortly after pupariation the imaginal discs evaginate to assume their positions at the surface of the prepupal animal. This is a very precise process that is only beginning to be understood. In Drosophila, during embryonic dorsal closure, the epithelial cells push the amnioserosa cells, which contract and eventually invaginate in the body cavity. In contrast, we find that during pupariation the imaginal cells crawl over the passive larval tissue following a very accurate temporal and spatial pattern. Spreading is driven by filopodia and actin bridges that, protruding from the leading edge, mediate the stretching of the imaginal epithelia. Although interfering with JNK (Jun N-terminal kinase) and dpp (decapentaplegic) produces similar phenotypic effects suppressing closure, their effects at the cellular level are different. The loss of JNK activity alters the adhesion properties of larval cells and leads to the detachment of the imaginal and larval tissues. The absence of dpp signaling affects the actin cytoskeleton, blocks the emission of filopodia, and promotes the collapse of the leading edge of the imaginal tissues. Interestingly, these effects are very similar to those observed after interfering with JNK and dpp signaling during embryonic dorsal closure.
An important morphogenetic process, in both vertebrate and invertebrate systems, is the movement and fusion of epithelial sheets such as amphibian and avian epiboly, neural crest closure, ventral closure in Caenorhabditis elegans, and embryonic dorsal closure in Drosophila (reviewed in ref. 1). In these processes, the mechanisms responsible for the spreading of epithelial sheets are poorly understood. Further, the parameters mediating cell recognition when epithelia are brought together and fuse in register are practically unknown.
Imaginal discs of holometabolous insects are small epithelial sacs that initiate during embryogenesis in connection with the larval epidermis. They proliferate up to several thousand cells within the larval body as they remain attached by a stalk to the larval ectoderm (2). Upon metamorphosis, discs evert and differentiate to form the external structures of the adult. Shortly after puparium formation, the stalks connecting the imaginal discs to the larval epidermis open up and shorten in preparation for the evagination of the discs (3). Immediately afterward, appendages form as a result of the unfolding and extension (eversion) of the disc. Imaginal disc eversion is cytochalasin-sensitive and, hence, likely to derive from microfilament contraction. About 6 h after pupariation, the wings, halteres, and legs are fully everted (4). At that time, the proximal disc regions release their connection with the larval epidermis and expand upon it, and the peripheral disc cells grow into the neighboring larval epidermis, replacing it gradually. The contralateral discs adjoin each other and finally meet and fuse with one another and with ipsilateral adjacent discs to form the imaginal thorax as a continuum epithelium (5).
It has been reported recently that some of the signaling mechanisms involved in thorax closure [JNK and decapentaplegic (dpp) signaling] (6, 7) partly resemble those involved in the process of embryonic epithelial sealing (embryonic dorsal closure) in Drosophila (8–13).
The JNK signaling cascade is an intracellular relay pathway. The core of this cascade is the stress-activated kinases JNKK and JNK (Jun N-terminal kinase) (reviewed in ref. 14). In Drosophila, JNKK and JNK homologues are encoded by the genes hemipterous (hep) and basket (bsk). Mutants on both of these genes show an embryonic dorsal-open phenotype consequence of the lack of elongation of the cells of the lateral epidermis (15–17). During closure, JNK signaling activity is modulated by the product of the gene puckered (puc), which encodes a dual-specificity phosphatase. puc is expressed in the dorsal-most cells of the epidermis (“leading-edge” cells), and in its absence, cell recognition at the dorsal midline is impaired (10).
dpp encodes a member of the transforming growth factor β superfamily that mediates inductive interactions at early stages of embryogenesis. During embryonic dorsal closure, dpp is expressed in the row of cells making up the leading edge of the epithelia, and the loss of dpp function [in mutant alleles of its receptor thick veins (tkv)] leads to dorsal open phenotypes (18). The JNK cascade controls dpp expression in the leading-edge cells (9, 11, 12).
Thorax closure in Drosophila represents an excellent model system for the analysis of the mechanisms involved in coordinating epithelial sheet spreading and cell recognition during development. In this paper, we present a study of thorax closure at the cellular level. We find that imaginal cells are brought together by spreading over the larval epidermis in a process mediated by extending microfilaments (filopodia), which seem to pull the contralateral epithelial sheets toward the midline. Further, we find that JNK and dpp signaling appear to have distinct roles during imaginal fusion, with JNK involved in the maintenance of the adhesiveness and integrity of the underlying larval epidermis and Dpp participating in the regulation of the cytoskeleton of the imaginal leading edge.
Materials and Methods
Drosophila Strains and Genetics.
The pucE69 allele is a P LacZ enhancer-trap insertion in the puc gene (10). The hep1 allele is an insertion of a P element in the 5′ untranslated region of the hep gene (15). hepr75 is a lethal allele generated by imprecise excision from the hep1 allele (12).
Targeted expression of UAS-driven transgenes was induced by using the following GAL4 lines. Pnr-GAL4 is expressed in wing discs in a broad domain corresponding to the central presumptive notum (19); MZ980-GAL4 (kindly provided by J. Urban) is expressed in the leading-edge cells and the presumptive scutellum of the wing disc (see Results); and Arm-GAL4 is expressed ubiquitously in imaginal and larval cells (20). The UAS lines used in this study are: UAS-TkvDN, which drives the expression of a kinase-defective form of Tkv, a type I receptor of Dpp (21); UAS-Puc, which drives the expression of Puc (10); and UAS-GFPn, which expresses a nuclear-targeted green fluorescent protein (GFP) (22).
Dissection of Larvae and Pupae.
Imaginal discs were fixed for 20 min in 4% paraformaldehyde in PBT (PBS with 0.3% Triton X-100) and antibody stained according to standard protocols.
Staged pupae were cut out at their posterior ends, and gut, fat body, salivary glands, and eye-antenna imaginal discs were removed from the anterior half of the pupal case. Open pupae were fixed overnight in 4% paraformaldehyde in PBT and subsequently dissected by cutting them in two halves, which yield a dorsal part with the wing discs attached and a ventral part carrying the leg discs. Pupae were stained with different reagents as described below.
Immunocytochemistry.
Larvae and pupae were stained with anti-β-galactosidase antibody [rabbit anti-β-Gal (Cappel), 1:10,000] to detect puc enhancer-driven expression by using standard procedures. Phalloidin staining was performed with tetramethylrhodamine B isothiocyanate-coupled phalloidin (Sigma) at a 1:100 dilution in PBT for 1 h followed by three washes with PBT. TO-PRO3 staining was performed for 5 min at a 1:50 dilution in PBT followed by several rinses with PBT. GFP was observed directly under confocal microscopy.
Results
Time Course of Wing Imaginal Discs Fusion.
In the Drosophila embryo, the dorsal side is covered by an extraembryonic cell layer, the amnioserosa, continuous with the two edges of the open epidermal sheet. After proliferation stops, the epidermal cells differentiate and elongate in the dorsoventral axis as the epidermal sheet spreads dorsally, replacing the amnioserosa and sealing the embryo. The movement is initiated in epidermal cells at the dorsal edge (“leading-edge” cells) and is later transmitted to more ventral cells (for review, see refs. 23 and 24). Mutations in components of the JNK pathway lead to a “dorsal-open” phenotype, reflecting a failure of the lateral ectoderm to expand dorsally. JNK signaling is necessary in the leading-edge cells to activate puc expression and for the appropriate organization of the cytoskeleton and membrane-associated proteins. puc negatively regulates JNK and modulates its activity at the boundary between the epidermis and the amnioserosa (10).
During larval development, the expression of puc becomes detectable in larval tissues and some imaginal cells (25) and the stalk and the region peripheral to the peripodial membrane of the wing, haltere, and leg discs (refs. 6 and 7 and data not shown). We have found that puc (monitored in the LacZ insertion pucE69 heterozygous flies) is expressed in the leading edges of all thoracic discs (wing, halteres, and legs) and its expression is maintained during the spreading and fusion of the imaginal epithelium (data not shown). Thus, puc colocalizes with the cells, leading the process of spreading and fusion in a manner that is comparable to dorsal closure in the embryo.
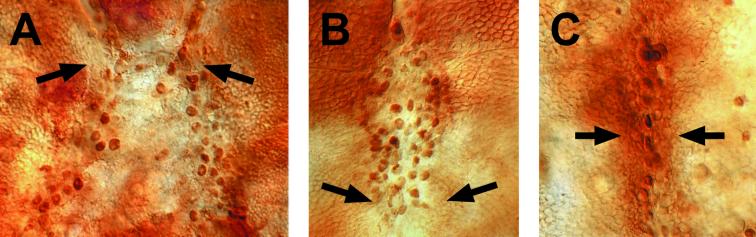
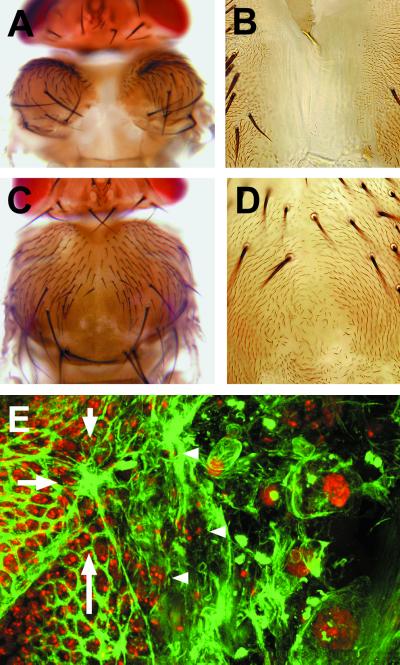
Disc stalk opening and notum and wing blade eversion initiate about 3.5 h after puparium formation (APF). Once eversion is completed, it is possible to distinguish three phases leading up to the fusion of the wing imaginal discs. (i) The disc epithelium initiates its spreading toward the dorsal midline led by the most anterior puc-expressing cells. These cells will be the first to meet their contralateral counterparts at 5.5 h APF (Fig. 1A). (ii) Immediately afterward, the most posterior imaginal cells spread, reaching the midline and fusing around 6 h APF (Fig. 1B). (iii) Finally, centrally located cells close the gap at 6.5 h APF (Fig. 1C). At about that time, notum cells start to secrete their adult cuticle.
Figure 1.
Time course of thorax closure. (A) Dorsal view of a 5.5-h APF pucE69/+ pupa stained with anti-β-galactosidase and anti-spectrin (to visualize cell perimeters) antibodies. The most anterior cells of the wing imaginal disc are the first to meet at the midline. (B) The most posterior imaginal cells move forward and initiate fusion at 6 h APF. (C) Middle cells fuse at 6.5 h APF.
Cell Behavior in Thorax Closure.
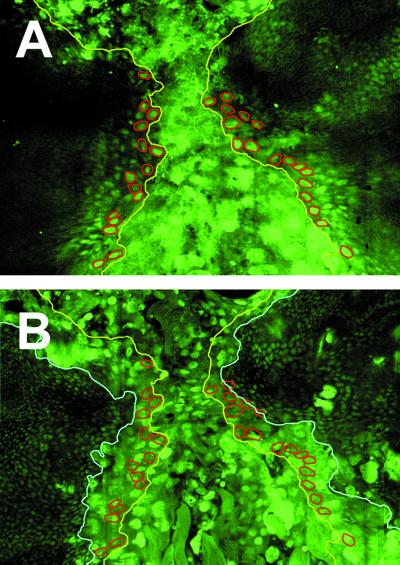
Embryonic dorsal closure proceeds through the planar stretching of epidermal cells. Meanwhile, amnioserosa cells elongate in the apical–basal axis, invaginating only at the end of closure. In contrast, in pupae, imaginal cells roll over the larval tissue on their way to the dorsal midline, leaving behind several rows of larval cells (Fig. 2). These larval cells delaminate from the edges of the larval epidermal sheet as imaginal cells proceed and undergo apoptosis.
Figure 2.
Imaginal cells spread over larval cells during thorax closure. Confocal images of a 5-h APF pupa expressing a nuclear GFP (GFPn) under the control of Arm-Gal4. GFPn is expressed in all imaginal (small diploid nuclei) and larval (large polyploid nuclei) cells. (A) Dorsal surface focal plane. Leading-edge imaginal cells are highlighted in red. The edge of the spreading discs is marked in yellow. (B) A focal plane situated 6 μm below the dorsal surface. The edge of the underlying larval epidermis is marked in light blue.
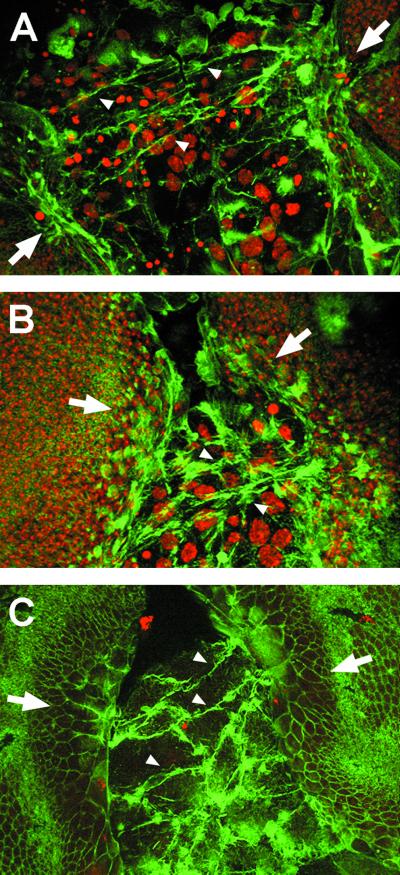
During embryonic dorsal closure, filamentous actin and nonmuscle myosin accumulate at the leading edge of the lateral epidermis and form a mechanically contiguous contractile band, or purse string (26). To evaluate the role of the cytoskeleton in thorax closure, we monitored the presence of actin. At early stages, we observed that imaginal cells contact across, over the larval epidermis, emitting filopodia (Fig. 3A). These filopodia form linear and branched structures that seem to originate in the imaginal epithelial edges and contact the imaginal cells of contralateral discs. Later, once the anterior ends of the discs have been brought together, filopodia and actin bridges are evident at posterior positions (Fig. 3 B and C). At these stages, the cells of the leading edge change shape and undergo a prominent elongation toward the midline. This contradicts previous reports, which describe only round, polygonal cells and an accumulation of actin in the central midline upon fusion (7). Changes of shape align with the filamentous bridges, suggesting a mechanical role for these actin-rich structures.
Figure 3.
Imaginal cells extend filopodia that connect contralateral discs. (A) Confocal image projection of a 5-h APF pupa stained with phalloidin to label polymerized actin and TO-PRO3 to mark nuclei. Long, thick filopodia extend from the wing imaginal disc edges, expand over the larval tissue, and eventually connect the confronting discs (arrowheads). (B) At 6 h APF, after discs contacted anteriorly, filopodia (arrowheads) extend from more posterior areas of the leading edge (confocal projection). (C) Dorsal surface confocal image of a 6-h APF pupa. Filopodia link the contralateral discs, and imaginal cells change shape extending toward the midline.
Spreading and Fusion of Imaginal Cells Depend on JNK Signaling.
The JNK signaling cascade participates in the spreading and fusion of discs during pupation. Wing discs of maternally rescued homozygous hepr75 (D-JNKK) animals remain in their initial position in the prepupa, they do not spread, and in many cases disc eversion does not take place (ref. 6; unpublished observations). Milder defects can be observed in several other conditions in which JNK signaling is impaired. Thus, in hypomorphic heteroallelic combinations of kayak (a gene coding for the Drosophila homologue of the transcription factor c-Fos) or hep, the thoracic epithelia fail to reach the midline and fuse (7). Similar abnormalities are observed after overexpressing Puc or dominant-negative Fos with a Pnr-Gal4, a line that is expressed in the larval tissue and the central notum region (“pannier domain of expression”) (19).
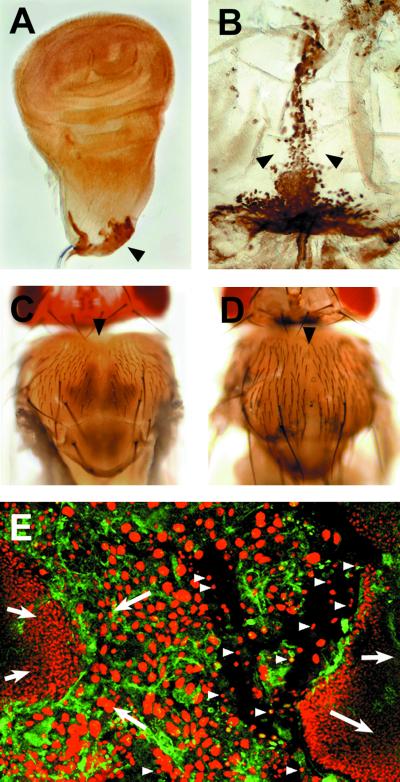
To analyze the role of JNK signaling in leading-edge cells, we used the MZ980-Gal4 line. This Gal4 line is expressed specifically in the presumptive edge cells of wing (Fig. 4 A and B) and haltere (not shown) discs and in the intervening larval cells (data not shown). Expressing Puc ectopically with MZ980-Gal4 results in adults with a mild thorax cleft phenotype (Fig. 4C) reminiscent of hep hypomorphic alleles (hep1) (Fig. 4D).
Figure 4.
JNK signaling is involved in imaginal disc spreading and fusion, and it is necessary for the integrity of the larval tissue (A and B). The pattern of expression of the MZ980-Gal4 is revealed by the targeted expression of β-galactosidase. (A) Expression in the stalk region of third instar imaginal discs. (B) Expression in the dorsal junction and the scutellum of a 10-h APF pupa. (C) Mild thorax cleft observed after Puc overexpression with the MZ980-Gal4. (D) Weak thorax cleft observed in hypomorphic hep1 animals. (E) Cellular defects associated to the strong hepr75 condition. Larval cells disassociate from the imaginal epithelia and undergo premature apoptosis (arrowheads). No filopodia are observed, and the imaginal tissue detaches and initiates retraction.
Complete failure of JNK signaling (in hepr75 animals) abolishes both spreading and fusion. In many zygotic null animals thorax closure does not proceed and wings occasionally fail to evert (6). We found that in hepr75 animals, the expression of actin is down-regulated in both the larval and the epidermal tissues. In these mutants, filopodia departing from leading-edge cells are rare or missing and imaginal cells do not change shape (Fig. 4E). In addition, the larval epidermal cells detach from each other and from the imaginal epidermis, round up, and their nuclei constrict, breaking up the integrity of the larval epithelium. As a consequence, the imaginal discs never spread and fuse.
Dpp Signaling Is Required for Thorax Closure and Affects Cytoskeleton Dynamics.
Hypomorphic mutations in dpp cause a thoracic cleft phenotype reminiscent of that observed in hep1 (27). Further, this failure in thorax closure also is observed in several mutant combinations of the dpp receptors thick veins (tkv) and punt (28, 29) and the dpp signal transducer medea (30). dpp in imaginal discs is expressed in a very complex and dynamic pattern, and it accumulates in the stalk cells that will generate the imaginal leading edge. Later, during closure, dpp is found in the anterior part of the dorsal midline, in a fraction of the cells expressing puc (6, 7). Interestingly, dpp expression is not affected in a hepr75-null background (6), in contrast to the embryo, where the expression of dpp in the most dorsal epidermis is controlled by JNK signaling.
To study the cellular activities depending on dpp during thorax closure, we interfered with dpp signaling by expressing a dominant negative form of the receptor Tkv (TkvDN). When TkvDN is expressed in the pannier domain, it leads to a strong cleft phenotype, where both heminota remain extremely contracted and isolated (Fig. 5A). In this condition, we found an intervening naked cuticle joining the heminota (Fig. 5B). A more restricted ectopic expression was generated with the MZ980-Gal4 line. In this combination, which probably represents a partial reduction in dpp activity, the thorax cleft is attenuated (Fig. 5C) and cuticular polarity defects are observed at the midline (Fig. 5D). Occasional individuals with stronger phenotypes (similar to those obtained with Pnr-Gal4) are recovered (not shown), suggesting that leading-edge cells are determinant for the spreading of the imaginal tissue.
Figure 5.
dpp signaling is necessary for the spreading of imaginal discs and for filopodia formation. (A and B) The overexpression of UAS-TkvDN with Pnr-Gal4 induces a strong cleft phenotype, where heminota are kept well apart (A) but joined by naked cuticle (B). (C and D) The overexpression of UAS-TkvDN with MZ980-Gal4 produces a mild cleft phenotype (C). The intervening cuticle shows distinct polarity defects (D). (E) After the overexpression of UAS-TkvDN with Pnr-Gal4, filopodia are not present at the leading edge of imaginal tissue (arrowheads), and imaginal cells are pulled together into bunches (arrows).
In contrast to JNK activity-deficient animals, interfering with dpp signaling does not appear to affect the integrity of the larval epidermis. The lack of spreading of the imaginal sheets in the absence of Tkv activity appears to be due to extreme compacting of the actin cytoskeleton at the leading edge (Fig. 6E). No filopodia appear to be generated from the imaginal epithelium, and imaginal cells progressively are pulled together, causing bunching of the epidermis. This phenotype is reminiscent of the cellular defects of embryos mutant for tkv. In these embryos, during embryonic dorsal closure, epidermal cells appear to elongate correctly, but they become misdirected, generating a phenotype of epidermal bunching (ref. 31; E.M.-B. and A. Martínez-Arias, unpublished results). Taken together, these data indicate that dpp signaling during thorax closure is involved in the maintenance of cell polarity and the control of the actin cytoskeleton.
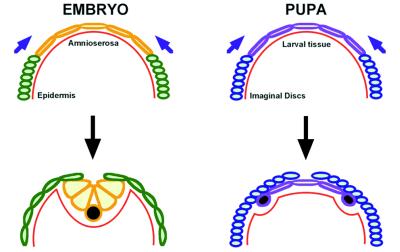
Figure 6.
Comparison of embryonic dorsal closure and imaginal thorax closure. Embryonic dorsal closure proceeds through the stretching of epidermal cells (green) and the simultaneous contraction of the apical ends of amnioserosa cells (orange). Amnioserosa and epidermis remain continuous during the whole process and keep contact with the basal lamina (red). Once dorsal closure is concluded, amnioserosa cells delaminate and undergo apoptosis (black). During imaginal thorax closure, imaginal cells (blue) detach from the basal lamina (red) and crawl over the larval cells (purple), emitting filopodia. Larval cells delaminate from the borders of the larval sheet and undergo apoptosis (black) once the imaginal cells leave them behind.
Discussion
Eversion, Spreading, and Fusion of Imaginal Tissues.
Imaginal disc epithelia have the general characteristics of other epithelia in Drosophila and in other organisms. The discs have a basal surface lined with a fibrous basal lamina and an apical surface at which cells are connected at their ends by a series of specialized junctions, including zonula adherens, gap, and septate junctions (reviewed in ref. 32).
Before eversion, the squamous cells of the peripodial epithelium are folded and adhere to the basal lamina. Just before eversion takes place, the cells detach from the basal lamina; the epithelial cells then columnarize and the accompanying contraction forces the discs to evert through the peripodial stalks (33, 34). Stalk widening and disc eversion appear to result from microfilament contraction, which leads to dramatic changes in cell shape, rather than from changes in membrane adhesiveness.
There are some differences between dorsal closure and imaginal spreading (Fig. 6). During embryonic closure, the amnioserosa and the epidermal cells keep their relative positions constant, and despite occasional delaminations, amnioserosa cells remain in place until the very end of the process. They detach from the overlying epidermis only upon closure completion, become dispersed into the body cavity, and undergo apoptosis (35). By contrast, during disc spreading, imaginal cells crawl over the larval epidermis (Fig. 2). In this process, larval cells are left below and behind and eventually delaminate from the edges.
The Role of the Cytoskeleton.
Spreading and fusion of epidermal cells could be directed by different mechanisms. In adult vertebrates, cells at the edge of cutaneous wounds extend lamellipodia and drag themselves forward. In contrast, in vertebrate embryos, wound-edge cells remain blunt-faced and the force to draw the wound edges together seems to be provided by a purse-string-like contraction of a thick cable of actin at the leading edge (36). This mechanism also applies to some developmental processes, such as Xenopus gastrulation (37) and the late stages of C. elegans ventral enclosure (38). In Drosophila, purse-string contraction appears to be the mechanical force leading embryonic dorsal closure. Actin and non-muscle myosin accumulate at the leading edge of the epithelium and mutations in zipper (the gene coding for non-muscle myosin) (26) or hep (15) that abolish actin and non-muscle myosin accumulation yield dorsal-open phenotypes.
Our data suggest that the spreading of the imaginal epithelium is active and led by cells at the boundary, although a contribution of the rest of the cells of the epithelia may be possible. Forward locomotion of imaginal cells probably will involve contraction of intracellular actomyosin filaments. We also observe extensive, thick filopodia connecting cells to the contralateral heminota (Fig. 3). These multibranched filopodia, which protrude out of leading-edge cells, expand over the larval surface and eventually form actin bridges. Upon contact, they seem to exert a mechanical force, pulling the imaginal tissues together.
The mechanical role of thick filopodia involved in imaginal spreading is conserved in other developmental processes such as gastrulation in the sea urchin embryo and the epiboly of the C. elegans hypodermis. In sea urchin, primary and secondary mesenchyme cells extend filopodia as they move, making contacts with the ectoderm (39). During ventral enclosure in the nematode, leading cells display actin-rich filopodia and treatment with cytochalasin D immediately halts the process (38).
The Recognition of Epithelia.
One important characteristic of epithelial fusion in a developmental context is the precise recognition of the contralateral parts. During embryonic dorsal closure in Drosophila, a perfect match links the anterior and posterior compartments of each segment across the midline (40). During pupariation, the spreading of the imaginal tissues results in the alignment of notal landmarks along the anterior–posterior axis. When rare mismatches occur, anterior cells never match to posterior ones; rather, they meet anterior cells of distinct contralateral segments (41). This accurate identification suggests that different positional values must be present in different cells at the leading edge and, importantly, a mechanism should exist that allows perception of these differences at a distance.
Thorax closure starts at the anterior end of the wing disc, proceeds through the most posterior region, and, finally, fills the gap (Fig. 1). This regulated cadence also has been observed in an independent study (K. Usui, personal communication). Timing also is regulated during embryonic dorsal closure. In this process, spreading and fusion proceed from both ends of the embryo, showing segmental periodicity (42).
A mechanism that directs migration or spreading is contact guidance. Contact guidance in discs could be mediated by filopodial tracts making appropriate informative contacts at the contralateral discs. This situation would be reminiscent of sea urchin gastrulation, where thin filopodia are involved in cell–cell signaling (43). This function also has been suggested for cytonemes, actin-rich, thin filopodia present on Drosophila imaginal discs (44).
JNK and dpp Signaling Have Different Roles in Morphogenesis.
In addition to morphological similarities, genetic evidence points to a similar molecular mechanism, led by the JNK signaling cascade, directing embryonic dorsal and imaginal thorax closure.
During imaginal closure, JNK signaling affects the adhesion between imaginal and larval epidermal cells. In mutant conditions for this pathway, larval cells detach and prematurely degenerate, disrupting the continuity of the epithelium, which appears to be necessary for spreading and fusion. This defect also has been observed during embryonic dorsal closure. In embryos, after loss of both maternal and zygotic hep functions, amnioserosa cells detach from each other and from the epidermal cells and undergo premature cell death (E.M.-B. and A. Martínez-Arias, unpublished results). Thus, JNK signaling appears to affect both the cytoskeleton and cell adhesiveness.
The activity of JNK signaling is manifested by the expression of puc, which is detected in imaginal leading-edge cells and in a subset of cells of the peripodial membrane that ultimately will adopt a border position. Thus, the JNK pathway appears to determine the leading cells in the moving epithelia and to set up the borders between columnar and squamous epithelia (6). Alternatively, puc expression (and JNK signaling) could just reflect a particular physiological state of border cells.
The loss of dpp signal during embryonic dorsal closure causes the pulling together of the leading edges of segments into bunches (31). This phenotype appears to be due to defects in the leading-edge cytoskeleton, resulting in a misregulated contraction. Similar defects are seen after interfering with dpp signaling during imaginal disc spreading. Bunches develop at the leading edge, resulting in the excessive contraction of the epithelium.
The role of dpp in imaginal and embryonic fusion appears to be conserved in related developmental processes. During palate fusion in vertebrates, the transforming growth factor β3, a homologue of Dpp, is expressed in the cells that will form the palate suture. Mutations in transforming growth factor β3 cause palate clefts in homozygous null mice. In these mice, in contrast to wild-type animals, filopodia-like structures are not present on the surface of the medial-edge epithelial cells (45). Thus, dpp appears to be involved in the regulation of cytoskeleton dynamics along the leading edge of epithelia, although we do not know how its activity is implemented.
Acknowledgments
We are grateful to E. Hafen, E. Wieschaus, C. Extavour, and members of the laboratory for critical reading. We also thank K. Usui and P. Simpson for communicating results before publication. E.M.-B. is a Research Scientist at the Consejo Superior de Investigaciones Científicas. J.C.P.-P. held a Residencia de Estudiantes-Ayuntamiento de Madrid Studentship. This work was supported by grants of the Dirección General de Investigación Cientifica y Técnica and Comunidad de Madrid and an institutional grant from Fundación Ramón Areces.
Abbreviations
- JNK
Jun N-terminal kinase
- GFP
green fluorescent protein
- APF
after puparium formation
- Dpp
decapentaplegic
References
- 1.Bard J. Morphogenesis: The Cellular and Molecular Processes of Developmental Anatomy. Cambridge, U.K.: Cambridge Univ. Press; 1992. [Google Scholar]
- 2.Cohen B, Simcox A, Cohen S M. Development (Cambridge, UK) 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- 3.Fristrom D, Fristrom J W. In: The Development of Drosophila melanogaster. Bate M, Martínez-Arias A, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 843–897. [Google Scholar]
- 4.Fristrom D, Fristrom J W. Dev Biol. 1975;43:1–23. doi: 10.1016/0012-1606(75)90127-x. [DOI] [PubMed] [Google Scholar]
- 5.Poodry C A, Schneiderman H A. Wilhelm Roux Arch Entwicklungsmech Org. 1971;168:1–9. doi: 10.1007/BF00582000. [DOI] [PubMed] [Google Scholar]
- 6.Agnes F, Suzanne M, Noselli S. Development (Cambridge, UK) 1999;126:5453–5462. doi: 10.1242/dev.126.23.5453. [DOI] [PubMed] [Google Scholar]
- 7.Zeitlinger J, Bohmann D. Development (Cambridge, UK) 1999;126:3947–3956. doi: 10.1242/dev.126.17.3947. [DOI] [PubMed] [Google Scholar]
- 8.Zeitlinger J, Kockel L, Peverali F A, Jackson D B, Mlodzik M, Bohmann D. EMBO J. 1997;16:7393–7401. doi: 10.1093/emboj/16.24.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riesgo-Escovar J R, Hafen E. Genes Dev. 1997;11:1717–1727. doi: 10.1101/gad.11.13.1717. [DOI] [PubMed] [Google Scholar]
- 10.Martín-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky A M, Martínez-Arias A. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou X S, Goldstein E S, Perrimon N. Genes Dev. 1997;11:1728–1737. doi: 10.1101/gad.11.13.1728. [DOI] [PubMed] [Google Scholar]
- 12.Glise B, Noselli S. Genes Dev. 1997;11:1738–1747. doi: 10.1101/gad.11.13.1738. [DOI] [PubMed] [Google Scholar]
- 13.Kockel L, Zeitlinger J, Staszewski L M, Mlodzik M, Bohmann D. Genes Dev. 1997;11:1748–1758. doi: 10.1101/gad.11.13.1748. [DOI] [PubMed] [Google Scholar]
- 14.Ip Y T, Davis R J. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 15.Glise B, Bourbon H, Noselli S. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 16.Riesgo-Escovar J R, Jenni M, Fritz A, Hafen E. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- 17.Sluss H K, Han Z, Barrett T, Davis R J, Ip Y T. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 18.Affolter M, Nellen D, Nussbaumer U, Basler K. Development (Cambridge, UK) 1994;120:3105–3117. doi: 10.1242/dev.120.11.3105. [DOI] [PubMed] [Google Scholar]
- 19.Calleja M, Moreno E, Pelaz S, Morata G. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- 20.Sanson B, White P, Vincent J P. Nature (London) 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- 21.Haerry T E, Khalsa O, O'Connor M B, Wharton K A. Development (Cambridge, UK) 1998;125:3977–3987. doi: 10.1242/dev.125.20.3977. [DOI] [PubMed] [Google Scholar]
- 22.Davis I, Girdham C H, O'Farrell P H. Dev Biol. 1995;170:726–729. doi: 10.1006/dbio.1995.1251. [DOI] [PubMed] [Google Scholar]
- 23.Martín-Blanco E. Curr Opin Genet Dev. 1997;7:666–671. doi: 10.1016/s0959-437x(97)80015-9. [DOI] [PubMed] [Google Scholar]
- 24.Noselli S. Trends Genet. 1998;14:33–38. doi: 10.1016/S0168-9525(97)01320-6. [DOI] [PubMed] [Google Scholar]
- 25.Martín-Blanco E. Int J Dev Biol. 1998;42:363–368. [PubMed] [Google Scholar]
- 26.Young P E, Richman A M, Ketchum A S, Kiehart D P. Genes Dev. 1993;7:29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Spencer F A, Hoffmann F M, Gelbart W M. Cell. 1982;28:451–461. doi: 10.1016/0092-8674(82)90199-4. [DOI] [PubMed] [Google Scholar]
- 28.Morimura S, Maves L, Chen Y, Hoffmann F M. Dev Biol. 1996;177:136–151. doi: 10.1006/dbio.1996.0151. [DOI] [PubMed] [Google Scholar]
- 29.Simin K, Bates E A, Horner M A, Letsou A. Genetics. 1998;148:801–813. doi: 10.1093/genetics/148.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson J B, Podos S D, Keith K, Simpson S L, Ferguson E L. Development (Cambridge, UK) 1998;125:1407–1420. doi: 10.1242/dev.125.8.1407. [DOI] [PubMed] [Google Scholar]
- 31.Ricos M G, Harden N, Sem K P, Lim L, Chia W. J Cell Sci. 1999;112:1225–1235. doi: 10.1242/jcs.112.8.1225. [DOI] [PubMed] [Google Scholar]
- 32.Poodry C A. In: The Genetics and Biology of Drosophila. Ashburner M, Wright T R F, editors. London: Academic; 1980. pp. 407–441. [Google Scholar]
- 33.Milner M J, Bleasby A J, Pyott A. Roux's Arch Dev Biol. 1983;192:164–170. doi: 10.1007/BF00848686. [DOI] [PubMed] [Google Scholar]
- 34.Milner M J, Bleasby A J, Kelly S L. Roux's Arch Dev Biol. 1984;193:180–186. doi: 10.1007/BF00848893. [DOI] [PubMed] [Google Scholar]
- 35.Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Roux's Arch Dev Biol. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- 36.Martin P, Lewis J. Nature (London) 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 37.Keller R E. J Embryol Exp Morphol. 1980;60:201–234. [PubMed] [Google Scholar]
- 38.Williams-Masson E M, Malik A N, Hardin J. Development (Cambridge, UK) 1997;124:2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 39.Gustafson T. Exp Cell Res. 1963;32:570–589. doi: 10.1016/0014-4827(63)90195-2. [DOI] [PubMed] [Google Scholar]
- 40.Martínez-Arias A. In: The Development of Drosophila melanogaster. Bate M, Martínez-Arias A, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 517–608. [Google Scholar]
- 41.García-Bellido A, Santamaría P. Genetics. 1972;72:87–101. doi: 10.1093/genetics/72.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harden N, Ricos M, Ong Y M, Chia W, Lim L. J Cell Sci. 1999;112:273–284. doi: 10.1242/jcs.112.3.273. [DOI] [PubMed] [Google Scholar]
- 43.Miller J, Fraser S E, McClay D. Development (Cambridge, UK) 1995;121:2501–2511. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- 44.Ramírez-Weber F A, Kornberg T B. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 45.Taya Y, O'Kane S, Ferguson M W J. Development (Cambridge, UK) 1999;126:3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]