Abstract
Protection against breast cancer was achieved with a DNA vaccine against murine transcription factor Fos-related antigen 1, which is overexpressed in aggressively proliferating D2F2 murine breast carcinoma. Growth of primary s.c. tumor and dissemination of pulmonary metastases was markedly suppressed by this oral DNA vaccine, carried by attenuated Salmonella typhimurium, encoding murine Fos-related antigen 1, fused with mutant polyubiquitin, and cotransformed with secretory murine IL-18. The life span of 60% of vaccinated mice was tripled in the absence of detectable tumor growth after lethal tumor cell challenge. Immunological mechanisms involved activation of T, natural killer, and dendritic cells, as indicated by up-regulation of their activation markers and costimulatory molecules. Markedly increased specific target cell lysis was mediated by both MHC class I-restricted CD8+ T cells and natural killer cells isolated from splenocytes of vaccinated mice, including a significant release of proinflammatory cytokines IFN-γ and IL-2. Importantly, fluorescence analysis of fibroblast growth factor 2 and tumor cell-induced vessel growth in Matrigel plugs demonstrated marked suppression of angiogenesis only in vaccinated animals. Taken together, this multifunctional DNA vaccine proved effective in protecting against growth and metastases of breast cancer by combining the action of immune effector cells with suppression of tumor angiogenesis.
Keywords: vaccine, tumor, metastases, antiangiogenesis
Breast cancer, one of the most common malignancies in women, is second in cancer-causing death among women between the ages of 40 and 55 years in the United States (1). This neoplasm has been studied intensively, and recently new preventive measures and therapies emerged, including immunological and genetic treatments administered as adjuvant therapy after surgery, radiation, and chemotherapy. Biotherapy produced successful results in mice with mammary carcinoma, particularly with cellular vaccines (2), DNA vaccines (3, 4), recombinant proteins (5), and adoptive immunotherapy (6).
Progression of breast cancer often is accompanied by changes in gene expression patterns, resulting in highly tumorigenic and invasive cell types (7). In this regard, the transcription factor activating protein-1 (AP-1) family defines tumor progression and regulates breast cancer cell invasion and growth and resistance to anti-estrogens (8). In addition, Fos-related antigen 1 (Fra-1), a transcription factor belonging to the AP-1 family, is overexpressed in many human and mouse epithelial carcinoma cells, including those of thyroid (9), kidney (10), esophagus (11), and breast (12). This overexpression greatly influences their morphology and motility, correlates with their transformation to a more invasive phenotype (13), and is specifically associated with highly invasive breast cancer cells (14). These findings suggest that overexpressed Fra-1 can serve as a potential target for active vaccination against breast cancer (15).
IL-18, a potent immunoregulatory cytokine, was described as an IFN-γ-inducing factor (16), which enhances cytokine production of T and/or natural killer (NK) cells and induces their proliferation and cytolytic activity (17). Tumor cells engineered to produce IL-18 are less tumorigenic (18), and systemic administration of IL-18 resulted in considerable therapeutic activity in several murine tumor models (19, 20). In addition, IL-18 enhances cellular immune mechanisms by up-regulating MHC class I antigen expression and favoring the differentiation of CD4+ helper T cells toward the T helper 1 (Th1) subtype (21). In turn, Th1 cells secrete IL-2 and IFN-γ, which facilitate the proliferation and/or activation of CD8+ cytotoxic T lymphocytes, NK cells, and macrophages, all of which can contribute to tumor regression (22). In addition, IL-18 is a novel inhibitor of angiogenesis, sufficiently potent to suppress tumor growth by directly inhibiting fibroblast growth factor 2-induced endothelial cell proliferation (23). Recently, the role of recombinant IL-18 as a biological “adjuvant” has been evaluated in murine tumor models, and its systemic administration induced significant antitumor effects in several tumor models (19, 24).
The induction of an efficacious Ag-specific immunity by DNA vaccines against self-Ag necessitates the optimization of vaccine design, including effective modalities of vaccine delivery, powerful adjuvants, and optimal antigen processing. This can be achieved by an oral vaccine delivery system using an attenuated strain of Salmonella typhimurium (dam– aroA–) (25). Thus, vaccination by gavage of these bacteria harboring plasmid DNA vaccines proved effective for DNA delivery to such secondary lymphoid tissues as Peyer's patches in the small intestine, with subsequent induction of immunity against antigens encoded by the plasmid (26). DNA immunization also can be enhanced significantly by exploiting natural pathways of antigen presentation. Thus, most peptides presented as complexes with MHC class I antigens that induce active cytotoxic T lymphocytes are derived from cytosolic proteins degraded and processed by the proteasome. Protein is targeted to this organelle by polyubiquitination, a process in which many copies of this cellular protein are covalently attached to the target protein to markedly enhance its degradation by the proteasome (27).
Here, we describe a multifunctional DNA vaccine, encoding polyubiquitinated transcription factor Fra-1 and secretory IL-18, which effectively protects against primary breast tumor growth and metastases by suppression of tumor angiogenesis and activation of T, NK, and dendritic cells.
Materials and Methods
Animals, Bacterial Strains, and Cell Lines. Female BALB/c mice, 6–8 wk of age, were purchased from The Scripps Research Institute Rodent Breeding Facility. All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The double attenuated S. typhimurium strain RE88 (aroA– dam–) was kindly provided by the Remedyne Corporation (Santa Barbara, CA). The murine D2F2 breast cancer cell line was obtained from the American Type Culture Collection and cultured in DMEM supplemented with 10% (vol/vol) FBS.
Vector Construction and Protein Expression. Two constructs were made based on the pIRES vector (Invitrogen). The first, pUb-Fra-1, was comprised of polyubiquitinated, full-length murine Fra-1. The second, pIL-18, contained murine IL-18 with an Igκ leader sequence for secretion purposes. The empty vector with or without ubiquitin served as a control. Protein expression of Fra-1 and IL-18 was demonstrated by Western blotting with a polyclonal rabbit anti-murine Fra-1 antibody (Santa Cruz Biotechnology) and a monoclonal anti-mouse IL-18 antibody (R & D Systems). Bioactivity of murine IL-18 in cell supernatants was measured by an ELISA (R & D Systems) using the production of IFN-γ in KG-1 lymphoma cells as an indicator (28).
Transformation and Expression of S. typhimurium with DNA Vaccine Plasmids. Attenuated S. typhimurium (dam–, aroA–) was transformed with DNA vaccine plasmids by electroporation. Freshly prepared bacteria (1 × 108) at midlog growth phase were mixed with plasmid DNA (2 μg) on ice in a 0.2-cm cuvette and electroporated at 2.5 KV, 25 μF, and 200 Ω. The bacteria were transformed with the following plasmids: empty vector, pUb, pUb-Fra-1, pIL-18, or both pUb-Fra-1 and pIL-18 combined, indicated as pUb-Fra-1/pIL-18. After electroporation, resistant colonies harboring the DNA vaccine gene(s) were cultured and stored at –70°C after confirmation of their coding sequence.
Detection of Enhanced GFP (EGFP) Expression. EGFP expression by aroA– dam– S. typhimurium was used to obtain direct evidence for DNA transfer from the bacterial carrier to Peyer's patches and to establish that protein expression took place efficiently and successfully. Mice were administered 1 × 108 bacteria by gavage and killed 24 h thereafter, and biopsies were collected from the small intestine. After washing, they were checked for EGFP expression in Peyer's patches by confocal microscopy or saved for further hematoxylin/eosin staining.
Immunization and Tumor Cell Challenge. Five groups of BALB/c mice (n = 8) were immunized three times at 2-wk intervals by gavage with 100 μl of PBS containing 1 × 108 doubly mutated S. typhimurium harboring either empty vector, pUb, pUb-Fra-1, pIL-18, or pUb-Fra-1/pIL-18. One week thereafter, mice were challenged either s.c. into the right flank with 1 × 106 D2F2 breast cancer cells or i.v. with 0.5 × 106 cells to induce primary tumor or experimental pulmonary metastases, respectively. The s.c. tumors were measured for width and length, and then volume was determined according to (W2 × L)/2. Pulmonary tumor metastases were examined and scored by a visual evaluation assessing the percentage of lung surface covered by fused metastases as follows: 0 = 0%, 1 = <20%, 2 = 20–50%, 3 = >50%.
Cytotoxicity Assay. Cytotoxicity was measured and calculated by a standard 51Cr-release assay with splenocytes harvested 2 wk after challenge with 0.5 × 106 D2F2 cells, and subsequent culture for 3 d at 37°C in complete T-STIM culture medium (Becton Dickinson). Both 51Cr-labeled D2F2 and Yac-1 cells were used as targets at various effector-to-target cell ratios.
Flow Cytometry. Activation markers of T and NK cells and CD80 and CD86 costimulatory molecules were measured by two-color flow cytometric analysis with a BD Biosciences FACScan. T cell activation was determined by staining freshly isolated splenocytes from successfully vaccinated mice with anti-CD8-FITC or anti-CD3-FITC Abs in combination with phycoerythrin (PE)-conjugated anti-CD25, CD11a, CD28, or CD69 Abs (PharMingen). Activation of NK cell markers was measured with FITC-labeled anti-NK-1.1 Abs in combination with PE-conjugated anti-DX5 Abs. Costimulatory molecules on antigen-presenting cells (APCs) were detected by PE-conjugated anti-CD80 or CD86 Abs in combination with FITC-labeled CD11c Abs.
Cytokine Release Assay. Flow cytometry was used for detection of intracellular cytokines and the ELISPOT assay to measure single-cell cytokine release. Splenocytes, collected 2 wk after D2F2 tumor cell challenge, were cultured for 24 h in complete T cell medium with irradiated D2F2 cells and assayed according to instructions provided by the manufacturer (BD Bioscience). Plates were read by IMMUNOSPOT SCANALYSIS, and digitalized images were analyzed for areas in which color density exceeded background by an amount based on a comparison with experimental wells.
Evaluation of Antiangiogenic Effects. Two weeks after the last vaccination, mice were injected s.c. in the sternal region with 500 μl of growth factor-reduced Matrigel (BD Biosciences) containing 400 ng/ml murine fibroblast growth factor 2 (PeproTech, Rocky Hill, NJ) and irradiated (1,000 Gy) D2F2 tumor cells (1 × 104/ml). In all mice, except for two controls, endothelium tissue was stained 6 d later by i.v. injection into the lateral tail vein of 200 μl of fluorescent Bandeiraea simplicifolia lectin I, Isolectin B4 at 0.1 mg/ml (Vector Laboratories). Thirty minutes later, Matrigel plugs were excised and evaluated macroscopically, and then Lectin-FITC was extracted by RIPA lysis buffer (0.15 mM NaCl/0.05 mM Tris·HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) from 100-μg plugs to be quantified by fluorimetry at 490 nm. Background fluorescence found in the two noninjected control mice was subtracted in each case.
Results
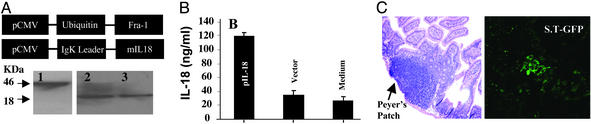
Vectors Encoding Genes for Ub-Fra-1 or IL-18 Express the Respective Bioactive Protein. We successfully constructed the eukaryotic expression vectors based on the pIRES vector backbone, namely pUb-Fra-1 and pIL-18 (Fig. 1A). Protein expression of pUb-Fra-1 and pIL-18 was demonstrated by transient transfection of each vector into COS-7 cells and by performing Western blots of the respective cell lysates (pUb-Fra-1 or pIL-18) and supernatants (pIL-18) with anti-Fra-1 and anti-IL-18 Ab, respectively. All constructs produced protein of the expected molecular mass with IL-18 being expressed in its active form at 18 kDa (Fig. 1A, lane 2) and Fra-1 as a 46-kDa protein (Fig. 1A, lane 1). Protein expression of IL-18 was also detected in the culture supernatant of transfected cells (Fig. 1A, lane 3). Importantly, the biofunctional activity of IL-18 was demonstrated by ELISA in supernatants of cells transfected with pIL-18 (Fig. 1B).
Fig. 1.
Vector construction map, protein expression, bioactivity, and targeting of expression constructs. (A) The coding sequence of full-length, murine Fra-1, fused with polyubiquitin at the N terminus, was inserted into the pIRES plasmid (pUb-Fra-1). A second plasmid, pIL-18, contained the entire coding sequence of murine IL-18 with an Igκ leader sequence. Protein expression by pUb-Fra-1 and pIL-18 was demonstrated by Western blotting. Blots are shown at either pUb-Fra-1 (lane 1) or pIL-18 (lane 2) and of culture supernatant from pIL-18-transfected COS-7 cells (lane 3). (B) Bioactivity of IL-18 (ng/ml) determined by ELISA in supernatants of KG-1 lymphoma cells that transfected with pIL-18. The error bars indicate mean standard deviation of multiple assays. (C) Expression of EGFP activity in Peyer's patches was determined in mice immunized with 108 aroA– dam– bacteria transformed with pEGFP (S.T-GFP) by gavage. Fluorescence expression of EGFP was detected by confocol microscopy (Right). Hematoxylin/eosin staining of mouse Peyer's patches is shown (Left).
S. typhimurium Transfer Expression Vectors to Mouse Peyer's Patches. DNA encoding pUb-Fra-1 and pIL-18 was successfully released from the attenuated bacteria and entered Peyer's patches in the small intestine (Fig. 1C). The DNA was subsequently transcribed by APCs, processed in the proteasome, and presented as MHC–peptide complexes to T cells. To this end, mice were administered by gavage 1 × 108 dam–, aroA– attenuated S. typhimurium. After 24 h these animals were killed and biopsies were collected from the thoroughly washed small intestine. In fact, the doubly attenuated bacteria harboring EGFP (S.T-GFP) exhibited strong EGFP fluorescence (Fig. 1C). This finding suggested not only that such bacteria can transfer the target gene to Peyer's patches, but also that plasmids encoding each individual gene can successfully express their respective proteins. Importantly, these doubly attenuated bacteria do not survive very long because neither EGFP activity nor live bacteria could be detected in immunized animals after 72 h (data not shown). However, EGFP expression was detected in adherent cells, most likely APCs, such as dendritic cells and macrophages from Peyer's patches after oral administration of S. typhimurium harboring the eukaryotic EGFP expression plasmid. Taken together, these findings suggest that both plasmid transfer to and protein expression in eukaryotic cells did take place.
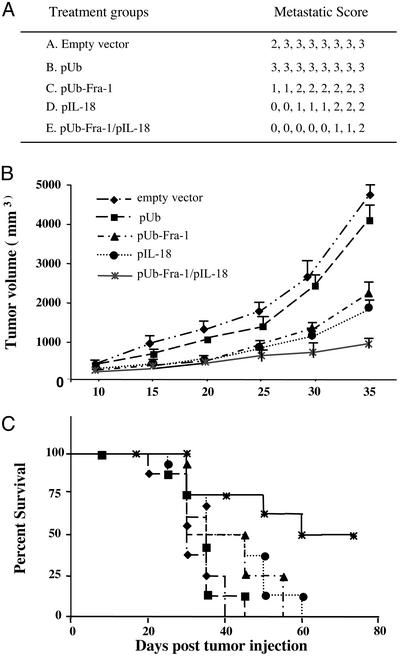
Tumor-Specific Protective Immunity Against Breast Cancer Is Induced by the DNA Vaccine. We proved our hypothesis that an orally administered DNA vaccine encoding murine Ub-Fra-1 and secretory IL-18, carried by attenuated S. typhimurium, can induce protective immunity against s.c. tumors and pulmonary metastases of D2F2 breast carcinoma. Thus, we observed marked inhibition of both s.c. tumor growth and disseminated experimental pulmonary metastases in BALB/c mice challenged 1 wk after the third vaccination with pUb-Fra-1/pIL-18 by either i.v. (Fig. 2A) or s.c. (Fig. 2B) injection of D2F2 murine breast cancer cells. In contrast, all animals vaccinated with only the empty vector (pIRES) or the vector encoding only ubiquitin (pUb), carried by attenuated bacteria, uniformly revealed rapid s.c tumor growth and extensive dissemination of pulmonary metastases. Importantly, the life span of 60% of successfully vaccinated BALB/c mice (5/8) was tripled in the absence of any detectable tumor growth up to 11 wk after tumor cell challenge (Fig. 2C).
Fig. 2.
Effect of the pUb-Fra-1/pIL-18-based DNA vaccine on primary tumor growth and metastases. Each experimental group (n = 8) of BALB/c mice was vaccinated by oral gavage as described in Materials and Methods.(A) Suppression of experimental pulmonary metastases of D2F2 breast carcinoma. Results are expressed as metastatic scores, i.e., percentage of lung surface covered by fused tumor foci. (B) Tumor growth was analyzed in mice challenged s.c. with 1 × 106 D2F2 tumor cells 1 wk after the last vaccination in each treatment or control group. (C) Survival curves represent results for eight mice in each of the treatment and control groups. Surviving mice were tumor free unless otherwise stated.
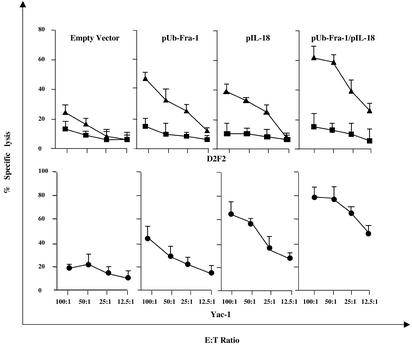
MHC Class I-Restricted Tumor-Specific Cytotoxic T Lymphocytes and NK Cells Kill D2F2 Breast Cancer Cells in Vitro. Immunization with our DNA vaccine induces tumor-specific immunity capable of killing breast cancer cells in vitro either by MHC class I Ag-restricted CD8+ T cells or NK cells. The data depicted in Fig. 3 indicate that CD8+ T cells isolated from splenocytes of mice immunized with the vaccine encoding pUb-Fra-1/pIL-18 were effective in killing D2F2 breast cancer cells in vitro at different effector-to-target cell ratios. In contrast, controls such as CD8+ T cells isolated from mice immunized with only the empty vector carried by attenuated S. typhimurium produced solely background levels of tumor cell lysis (Fig. 3). The CD8+ T cell-mediated killing of D2F2 cells was specific because syngeneic prostate cancer target cells (RM-2) lacking Fra-1 were not lysed (data not shown). Importantly, the CD8+ T cell-mediated tumor cell lysis was MHC class I Agrestricted, because addition of 10 μg/ml anti-H-2Kd/H-2Dd Ab specifically inhibited lysis of D2F2 cells (Fig. 3).
Fig. 3.
Cytotoxicity induced by CD8+ T and NK cells. Splenocytes were isolated from BALB/c mice after vaccination with experimental or control DNA vaccines 2 wk after challenge with D2F2 tumor cells and analyzed for their cytotoxic activity in a 51Cr-release assay at different effector-to-target cell ratios. (Upper) Specific lysis mediated by CD8+ T cells against D2F2 target cells (▴), which was blocked by an anti-MHC-class I Ab (H-2Kd/H-2Dd)(▪). (Lower) Lysis mediated by NK cells (•) against Yac-1 target cells. Each value shown represents the mean of eight animals.
NK cells also were effective in tumor cell killing in a standard 4-h 51Cr-release assay using NK-specific Yac-1 cells as targets for splenocytes from BALB/c mice immunized and challenged with D2F2 breast cancer cells. Lysis can also be achieved with breast cancer target cells, however, to a considerably lesser extent. Only immunization with the combined vectors, pUb-Fra-1/pIL-18, or pIL-18 alone led to significant lysis of Yac-1 target cells by NK cells, in contrast to control immunizations that were ineffective (Fig. 3).
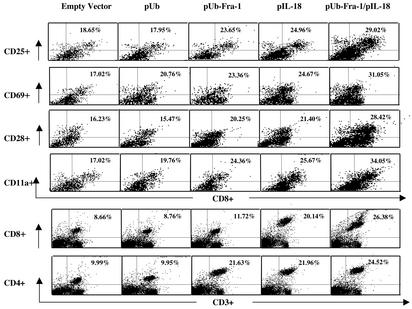
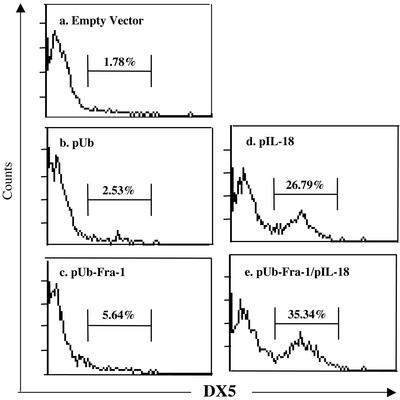
Activation of T, NK, and Dendritic Cells. Interactions between IL-18 and active T helper 1 cells and NK cells are critical for achieving both optimal Ag-specific T cell and NK cell responses. The vaccine harboring either pUb-Fra-1/pIL-18 or pIL-18 alone up-regulated the expression of T or NK cell activation markers, respectively. This was evident from an increase in expression of CD25, the high-affinity IL-2R α-chain, CD69, an early T cell activation antigen, and CD11a, which is important for the initial interaction between T cells and dendritic cells, and regular T cell markers CD4+ and CD8+ (Fig. 4). Additionally, it is known that NK cells can play a partial role in antitumor immune responses (29). For this reason, spleen cells obtained from mice successfully immunized with DNA vaccines along with the control groups were assayed with anti-DX5 antibody (Fig. 5), demonstrating that this regimen dramatically increased DX5 expression on NK cells, which is especially important for NK cytotoxity.
Fig. 4.
Up-regulated expression of T cell activation molecules. Two-color flow cytometric analyses were performed with single-cell suspensions of splenocytes obtained from immunized mice. Anti-CD25, CD69, CD28, and CD11a Ab were used in PE-conjugated form in combination with FITC-conjugated anti-mouse mAb directed against CD8+ T cells. PE-labeled anti-CD8 and anti-CD4 Abs were used in combination with FITC-conjugated anti-mouse mAb CD3. Each value represents the mean for four mice. Differences between the two control groups (empty vector and Ub) were statistically significant when compared with the three treatment groups (P < 0.05).
Fig. 5.
Increased expression of NK cell marker after administration of DNA vaccine. Fluorescence-activated cell sorting analysis of splenocytes with anti-DX5 mAb revealed the activation of NK cells after DNA vaccination. The experimental setting is similar to that of Fig. 4. Percentages refer to cells gated scored positive for DX5 expression. A representative histogram plot is shown for each group with the value depicting the mean for four mice. Differences between the two control groups (a and b) and two treatment groups (d and e) were statistically significant (P < 0.05).
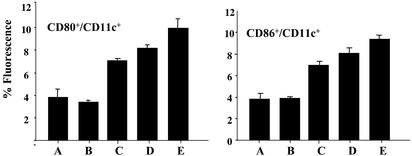
Furthermore, T cell activation is known to critically depend on up-regulated expression of costimulatory molecules CD80 and CD86 on dendritic cells to achieve optimal ligation with CD28 expressed on activated T cells. In this regard, fluorescence-activated cell sorting analyses of splenocytes obtained from syngeneic BALB/c mice, successfully immunized with the DNA vaccine, indeed demonstrated that we accomplished this particular task successfully, as the expression of CD80 and CD86 was up-regulated 2- to 3-fold over controls (Fig. 6).
Fig. 6.
Up-regulation of costimulatory molecules by DNA vaccine. In the same experiment as that depicted in Fig. 4, two-color flow cytometric analyses were performed with single-cell suspensions prepared from mouse splenocytes obtained 30 d after tumor cell challenge. Splenocytes were stained with FITC-labeled anti-CD11c Ab in combination with PE-conjugated anti-CD80 or CD86 Abs. Shown is the percent fluorescence of cell surface expressions of these two costimulatory molecules in each group (n = 4) (mean + SD). Differences between the two control groups (A and B) and the three treatment groups (C–E) were statistically significant (P < 0.05).
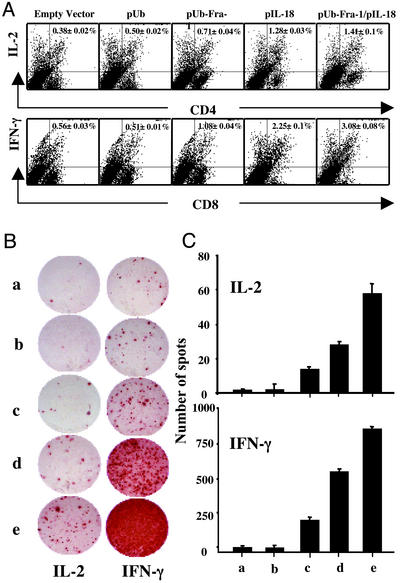
T Cell Activation Is Indicated by Increased Secretion of Proinflammatory Cytokines. The release of proinflammatory cytokines IL-2 and IFN-γ from T cells is a well-known indication of T cell activation in secondary lymphoid tissues. When we measured these two cytokines, both intracellularly with flow cytometry (Fig. 7A) and at the single-cell level by ELISPOT (Fig. 7 B and C), we found that vaccination with the pUb-Fra-1/pIL-18 plasmid and subsequent challenge with tumor cells induced a dramatic increase in IFN-γ and IL-2 release over that of splenocytes from controls.
Fig. 7.
Induction of cytokines at the intracellular and single T cell level. (A) Cytokines at the intracellular level were determined in splenocytes obtained 2 wk after tumor cell challenge and stained with FITC-anti-CD4 or CD8 Abs. Cells were fixed, permeabilized, and subsequently stained with PE-labeled anti-IFN-γ or anti-IL-2 Abs to detect the intracellular expression of these cytokines. Representative dot plots are shown for each group with the value depicting the mean for eight mice. (B) Production of IFN-γ and IL-2 was verified at the single-cell level by measuring reproduction in individual T cells by the ELISPOT assay. A representative ELISPOT assay is shown as spot formation per well induced by empty vector (a), pUb (b), pUb-Fra-1 (c), pIL-18 (d), and pUb-Fra-1/pIL-18 (e). (C) The mean spot distribution of each well in each experimental and control group is shown (n = 4, mean ± SD). Differences between the two control groups (a and b) and the three treatment groups (c–e) are statistically significant with treatment group (e) being most significant (P < 0.001).
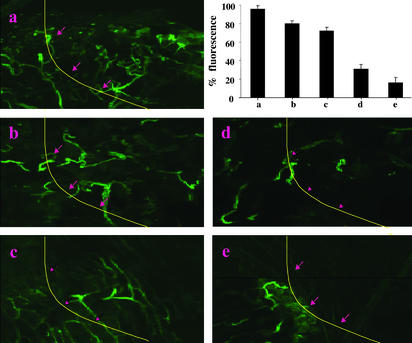
Suppression of Angiogenesis Is Induced by DNA Vaccine. We could demonstrate distinct suppression of angiogenesis induced by the pUb-Fra-1/pIL-18 DNA vaccine in a Matrigel assay. This was evident from the marked decrease in vascularization after vaccination, as evaluated by relative fluorescence after in vivo staining of endothelium with FITC-conjugated lectin. Differences were visible macroscopically, as shown in Fig. 8, depicting representative examples of Matrigel plugs removed from vaccinated mice 6 d after their injection. FITC-lectin staining clearly revealed suppression of angiogenesis, as indicated by a significantly decreased vascularization in Matrigel plugs only after vaccination with the vector encoding pUb-Fra-1/pIL-18 and to a somewhat lesser extent with pIL-18 alone, but not with vaccines encoding only pUb-Fra-1, pUb, or the empty vector control (Fig. 8).
Fig. 8.
Suppression of tumor angiogenesis. Antiangiogenesis was determined by the Matrigel assay. Quantification of vessel growth and staining of endothelium was determined by fluorimetry or confocal microscopy, respectively, using FITC-labeled Isolectin B4. The line and arrows (a–e) indicate the inside borders of the Matrigel plug. Matrigel was implanted into mice vaccinated with empty vector (a), pUb (b), pUb-Fra-1 (c), pIL-18 (d), or pUb-Fra-1/pIL-18 (e). The average fluorescence of Matrigel plugs from each group of mice is depicted by the bar graphs (n = 4; mean + SD). Comparison of control groups (a and b) with treatment groups (d and e) indicated statistical significance (P < 0.05). (Magnification: ×200.)
Discussion
Our data suggest that peripheral T cell tolerance against the Fra-1 transcription factor was broken by our DNA vaccine, fused with mutant polyubiquitin, and modified by cotransformation with a gene encoding secretory murine IL-18. This resulted in a prominent cellular immune response by CD4+ T cells, CD8+ T cells, and NK cells, tightly controlled by up-regulation of IFN-γ (29). Secretion of IFN-γ is further restricted by the availability of IFN-γ-inducing cytokines such as IL-2, IL-12, and tumor necrosis factor α, which are secreted from accessory cells after activation. IL-18 is another IFN-γ regulator (17), reported also to be a potent antiangiogenic cytokine, both in vitro and in vivo (30). Our vaccine design was successful because activation of both T and NK cells was significantly augmented. In fact, CD8+ T cell activation was indicated by critically dependent up-regulation of costimulatory molecules CD80 and CD86 on APCs, resulting in optimal ligation with CD28 on activated T cells. Indeed, our data indicate up-regulation of these costimulatory molecules on CD11c+ and MHC class II Ag-positive APCs, suggesting that their capability for presentation of tumor-specific Ag was significantly enhanced.
The marked increase in proinflammatory cytokines IFN-γ and IL-2 also demonstrated T cell activation as did the up-regulation of CD25, especially because it occurred together with increased production of IL-2 by activated T cells. Importantly, tumor angiogenesis was effectively suppressed only in mice immunized with pUb-Fra-1/pIL-18 and to a lesser extent with pIL-18 alone as indicated by suppression of vessel formation and regression of growing blood vessels.
Our success in eliciting an effective CD8+ T cell-mediated MHC class I Ag-restricted tumor protective immunity with a completely autologous oral DNA vaccine was most likely aided by our efforts to optimize antigen processing in the proteasome with polyubiquitination (31, 32). Support for this contention comes from our findings indicating that a DNA vaccine encoding murine Fra-1 lacking in ubiquitin was considerably less effective in inducing tumor protective immunity (data not shown). In contrast, the polyubiquitinated DNA vaccine was clearly capable of inducing tumor-protective immunity against a lethal challenge of D2F2 breast cancer cells.
One of the more critical aspects of DNA vaccine design is the selection of an optimally effective carrier to deliver the target gene to secondary lymphoid organs, such as Peyer's patches, in the small intestine. Live, attenuated bacterial carriers that harbor eukaryotic expression plasmids encoding Ag, combined with powerful adjuvants, are attractive vehicles for oral delivery of vaccines. Current DNA vaccine delivery vehicles include nonreplicating attenuated strains of intracellular bacteria like S. typhimurium, Listeria monocytogenes, and Mycobacterium bovis as well as Bacillus Calmette Guerin. These DNA vaccine delivery vehicles were reported to induce a broad spectrum of both mucosal and systemic immune responses. Moreover, the use of this natural route of entry could prove to be of benefit because many bacteria, like Salmonella, egress from the gut lumen via the M cells into Peyer's patches (26) and migrate eventually into lymph nodes and spleen, thus allowing natural targeting of DNA vaccines to inductive sites of the immune system.
The doubly mutated strain of S. typhimurium (dam–, aroA–) was used as a delivery vehicle for our DNA vaccine because it was shown to be highly attenuated and useful as a live vaccine in a murine model of infection (33). Additionally, this mutant does not cause a transient state of nonspecific immune suppression and thus is useful for delivering heterologous antigens to immune inductive sites (34). Although dam– mutants were found to be unable to cause disease in mice, transient bacteria remained after several weeks in terminal organs (35). Thus, to completely abolish the systemic presence of the bacteria, a second mutation (aroA–) was introduced that inhibits the synthesis of aromatic amino acids and causes the bacteria to die after just a few passages.
We not only demonstrated that the Fra-1 antigen targets appropriate pathways of MHC class I Ag processing and presentation, but also that an adequate cytokine milieu is generated that effectively promotes Ag-specific responses. The most prominent advantage of this vaccine carrier vehicle is its ability to directly target DNA vaccines intralymphatically to Peyer's patches, which harbor immature dendritic cells, B cells, T cells, and macrophages, i.e., most of the important effector cells necessary for an immune response induced by a DNA vaccine. In fact, Maloy et al. (36) clearly demonstrated that intralymphatic immunization enhances DNA vaccination, increasing immunogenicity by 100- to 1,000-fold while inducing strong and biologically relevant CD8+ cytotoxic T lymphocyte responses.
Taken together, our studies demonstrate that the transcription factor Fra-1 is a suitable target for induction of a T cell-mediated specific immune response against D2F2 breast cancer cells, and that the design of a DNA vaccine, especially polyubiquitination of the encoded protein immunogen and utilization of an attenuated bacterial carrier, lead to effective Ag processing and presentation, which result in effective tumor-protective immunity. The coexpression of secretory IL-18 by our vaccine acts as a powerful and natural adjuvant for further activation of both CD8+ and CD4+ T cells and NK cells, leading to the production of IFN-γ and IL-2 and importantly, to the suppression of effective angiogenesis in the tumor vasculature. It is anticipated that this multifunctional DNA vaccine may aid in the rational design of such vaccines for the immunotherapy of human breast cancer.
Acknowledgments
We thank D. Markowitz and C. Dolman for advice and technical assistance and Collette Beaton and Kathy Cairns for preparation of this manuscript. This study was supported by Department of Defense Grant DAMD17-02-1-0562 (to R.X.), Tobacco-Related Disease Research Program Grant 9RT-0017 (to R.A.R.), and E. Merck, Darmstadt-Lexigen Research Center (Billerica, MA) Grant SFP1330 (to R.A.R.). This is The Scripps Research Institute's manuscript no. 15763-IMM.
Abbreviations: Fra-1, Fos-related antigen 1; NK, natural killer; EGFP, enhanced GFP; PE, phycoerythrin; APC, antigen-presenting cell.
References
- 1.Ries, L., Eisner, M. P., Kosary, C. L., Hankey, B. F., Miller, B. A., Clegg, L. & Edwards, B. K. (2002) Seer Cancer Statistics Review 1995–2000 (National Cancer Institute, Bethesda).
- 2.Cefai, D., Morrison, B. W., Sckell, A., Favre, L., Balli, M., Leunig, M. & Gimmi, C. D. (1999) Int. J. Cancer 83, 393–400. [DOI] [PubMed] [Google Scholar]
- 3.Reilly, R. T., Gottlieb, M. B., Ercolini, A. M., Machiels, J. P., Kane, C. E., Okoye, F. I., Muller, W. J., Dixon, K. H. & Jaffee, E. M. (2000) Cancer Res. 60, 3569–3576. [PubMed] [Google Scholar]
- 4.Niethammer, A. G., Xiang, R., Becker, J. C., Wodrich, H., Pertl, U., Karsten, G., Eliceiri, B. P. & Reisfeld, R. A. (2002) Nat. Med. 8, 1369–1375. [DOI] [PubMed] [Google Scholar]
- 5.Esserman, L. J., Lopez, T., Montes, R., Bald, L. N., Fendly, B. M. & Campbell, M. J. (1999) Cancer Immunol. Immunother. 47, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granziero, L., Krajewski, S., Farness, P., Yuan, L., Courtney, M. K., Jackson, M. R., Peterson, P. A. & Vitiello, A. (1999) Eur. J. Immunol. 29, 1127–1138. [DOI] [PubMed] [Google Scholar]
- 7.Fish, E. M. & Molitoris, B. A. (1994) N. Engl. J. Med. 330, 1580–1588. [DOI] [PubMed] [Google Scholar]
- 8.Liu, Y., Ludes-Meyers, J., Zhang, Y., Munoz-Medellin, D., Kim, H. T., Lu, C., Ge, G., Schiff, R., Hilsenbeck, S. G., Osborne, C. K., et al. (2002) Oncogene 21, 7680–7689. [DOI] [PubMed] [Google Scholar]
- 9.Chiappetta, G., Tallini, G., De Biasio, M. C., Pentimalli, F., de Nigris, F., Losito, S., Fedele, M., Battista, S., Verde, P., Santoro, M., et al. (2000) Clin. Cancer Res. 6, 4300–4306. [PubMed] [Google Scholar]
- 10.Urakami, S., Tsuchiya, H., Orimoto, K., Kobayashi, T., Igawa, M. & Hino, O. (1997) Biochem. Biophys. Res. Commun. 241, 24–30. [DOI] [PubMed] [Google Scholar]
- 11.Hu, Y. C., Lam, K. Y., Law, S., Wong, J. & Srivastava, G. (2001) Clin. Cancer Res. 7, 2213–2221. [PubMed] [Google Scholar]
- 12.Roy, D., Calaf, G. & Hei, T. K. (2001) Mol. Carcinog. 31, 192–203. [DOI] [PubMed] [Google Scholar]
- 13.Kustikova, O., Kramerov, D., Grigorian, M., Berezin, V., Bock, E., Lukanidin, E. & Tulchinsky, E. (1998) Mol. Cell. Biol. 18, 7095–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zajchowski, D. A., Bartholdi, M. F., Gong, Y., Webster, L., Liu, H. L., Munishkin, A., Beauheim, C., Harvey, S., Ethier, S. P. & Johnson, P. H. (2001) Cancer Res. 61, 5168–5178. [PubMed] [Google Scholar]
- 15.Darnell, J. E., Jr. (2002) Nat. Rev. Cancer 2, 740–749. [DOI] [PubMed] [Google Scholar]
- 16.Okamura, H., Tsutsi, H., Komatsu, T., Yutsudo, M., Hakura, A., Tanimoto, T., Torigoe, K., Okura, T., Nukada, Y., Hattori, K., et al. (1995) Nature 378, 88–91. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui, H., Nakanishi, K., Matsui, K., Higashino, K., Okamura, H., Miyazawa, Y. & Kaneda, K. (1996) J. Immunol. 157, 3967–3973. [PubMed] [Google Scholar]
- 18.Osaki, T., Hashimoto, W., Gambotto, A., Okamura, H., Robbins, P. D., Kurimoto, M., Lotze, M. T. & Tahara, H. (1999) Gene Ther. 6, 808–815. [DOI] [PubMed] [Google Scholar]
- 19.Micallef, M. J., Tanimoto, T., Kohno, K., Ikeda, M. & Kurimoto, M. (1997) Cancer Res. 57, 4557–4563. [PubMed] [Google Scholar]
- 20.Hashimoto, W., Osaki, T., Okamura, H., Robbins, P. D., Kurimoto, M., Nagata, S., Lotze, M. T. & Tahara, H. (1999) J. Immunol. 163, 583–589. [PubMed] [Google Scholar]
- 21.Nakanishi, K., Yoshimoto, T., Tsutsui, H. & Okamura, H. (2001) Annu. Rev. Immunol. 19, 423–474. [DOI] [PubMed] [Google Scholar]
- 22.Wigginton, J. M., Lee, J. K., Wiltrout, T. A., Alvord, W. G., Hixon, J. A., Subleski, J., Back, T. C. & Wiltrout, R. H. (2002) J. Immunol. 169, 4467–4474. [DOI] [PubMed] [Google Scholar]
- 23.Okamura, H., Tsutsui, H., Kashiwamura, S., Yoshimoto, T. & Nakanishi, K. (1998) Adv. Immunol. 70, 281–312. [DOI] [PubMed] [Google Scholar]
- 24.Osaki, T., Peron, J. M., Cai, Q., Okamura, H., Robbins, P. D., Kurimoto, M., Lotze, M. T. & Tahara, H. (1998) J. Immunol. 160, 1742–1749. [PubMed] [Google Scholar]
- 25.Stocker, B. A. (2000) J. Biotechnol. 83, 45–50. [DOI] [PubMed] [Google Scholar]
- 26.Darji, A., Guzman, C. A., Gerstel, B., Wachholz, P., Timmis, K. N., Wehland, J., Chakraborty, T. & Weiss, S. (1997) Cell 91, 765–775. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez, F., Zhang, J. & Whitton, J. L. (1997) J. Virol. 71, 8497–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawashima, M., Yamamura, M., Taniai, M., Yamauchi, H., Tanimoto, T., Kurimoto, M., Miyawaki, S., Amano, T., Takeuchi, T. & Makino, H. (2001) Arthritis Rheum. 44, 550–560. [DOI] [PubMed] [Google Scholar]
- 29.Lode, H. N., Xiang, R., Dreier, T., Varki, N. M., Gillies, S. D. & Reisfeld, R. A. (1998) Blood 91, 1706–1715. [PubMed] [Google Scholar]
- 30.Cao, R., Farnebo, J., Kurimoto, M. & Cao, Y. (1999) FASEB J. 13, 2195–2202. [DOI] [PubMed] [Google Scholar]
- 31.Xiang, R., Lode, H. N., Chao, T. H., Ruehlmann, J. M., Dolman, C. S., Rodriguez, F., Whitton, J. L., Overwijk, W. W., Restifo, N. P. & Reisfeld, R. A. (2000) Proc. Natl. Acad. Sci. USA 97, 5492–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finley, D. (2002) Nat. Cell Biol. 4, E121–E123. [DOI] [PubMed] [Google Scholar]
- 33.Heithoff, D. M., Conner, C. P., Hentschel, U., Govantes, F., Hanna, P. C. & Mahan, M. J. (1999) J. Bacteriol. 181, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heithoff, D. M., Enioutina, E. Y., Daynes, R. A., Sinsheimer, R. L., Low, D. A. & Mahan, M. J. (2001) Infect. Immun. 69, 6725–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heithoff, D. M., Sinsheimer, R. L., Low, D. A. & Mahan, M. J. (1999) Science 284, 967–970. [DOI] [PubMed] [Google Scholar]
- 36.Maloy, K. J., Erdmann, I., Basch, V., Sierro, S., Kramps, T. A., Zinkernagel, R. M., Oehen, S. & Kundig, T. M. (2001) Proc. Natl. Acad. Sci. USA 98, 3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]