Abstract
Human defensins form a family of small, cationic, and Cys-rich antimicrobial proteins that play important roles in innate immunity against invading microbes. They also function as effective immune modulators in adaptive immunity by selectively chemoattracting T lymphocytes and immature dendritic cells. On the basis of sequence homology and the connectivity of six conserved Cys residues, human defensins are classified into α and β families. Structures of several β-defensins have recently been characterized, confirming the disulfide connectivity conserved within the family, i.e., Cys1–Cys5, Cys2–Cys4, and Cys3–Cys6. We found that human β-defensin 3 (hBD3), a recently described member of the growing β family, did not fold preferentially into a native conformation in vitro under various oxidative conditions. Using the orthogonal protection of Cys1–Cys5 and of Cys1–Cys6, we chemically synthesized six topological analogs of hBD3 with predefined disulfide connectivities, including the (presumably) native β pairing. Unexpectedly, all differently folded hBD3 species exhibited similar antimicrobial activity against Escherichia coli, whereas a wide range of chemotactic activities was observed with these analogs for monocytes and cells transfected by the chemokine receptor CCR6. Furthermore, whereas substitution of all Cys residues by α-aminobutyric acid completely abolished the chemotactic activity of hBD3, the bactericidal activity remained unaffected in the absence of any disulfide bridge. Our findings demonstrate that disulfide bonding in hBD3, although required for binding and activation of receptors for chemotaxis, is fully dispensable for its antimicrobial function, thus shedding light on the mechanisms of action for human β-defensins and the design of novel peptide antibiotics.
Antimicrobial peptides constitute an important component of innate immunity (1). Widely distributed in plants and animals, they vary in size, structure, and amino acid composition as well as in mode of antimicrobial action (2, 3). Despite their diversity, most antimicrobial peptides are cationic, killing bacteria, fungi, and enveloped viruses presumably through disruption of their negatively charged cytoplasmic membranes. A large subgroup of homologous antimicrobial peptides is termed defensins. Among vertebrates, human defensins are the most extensively studied (4, 5).
Human defensins are cationic and Cys-rich peptides with molecular masses ranging from 3 to 5 kDa. On the basis of sequence homology and the connectivity of six conserved cysteine residues, human defensins are classified into α and β families. Although the disulfide bridges Cys1–Cys6, Cys2–Cys4, and Cys3–Cys5 are found only in α-defensins, the Cys1–Cys5, Cys2–Cys4, and Cys3–Cys6 connection is characteristic of the β-family. Despite differences in disulfide topology, the tertiary structures of human defensins from both families are quite similar as indicated by x-ray crystallography and NMR spectroscopy (6–10). Common to the overall fold is a three-stranded antiparallel β-sheet, which is constrained by three intramolecular disulfide bonds. Given the small size of defensins, disulfide bridges likely play an important role in stabilizing their tertiary structures.
Although structurally similar, α- and β-defensins are functionally distinct. Human α-defensins were first discovered as natural peptide antibiotics stored in the azurophilic granules of neutrophils and released to combat-ingested foreign microbes during phagocytosis (11–13). Other α-defensins, secreted in response to bacterial stimulation, have also been found in intestinal Paneth cells (14–16). In contrast, human β-defensins (hBDs) are found predominantly in various epithelial cells and tissues (17). hBD1, originally isolated from human blood filtrate (18), is constitutively expressed in the urogenital tract and airway, suggesting a role in protecting mucosa from microbial infection (19). hBD2, first isolated from lesions of inflamed skin, is transcriptionally up-regulated by inflammatory stimuli such as cytokines and microorganisms (20). Further, although α-defensins are antibiotic peptides effective against both Gram-positive and -negative bacteria, hBD1 and hBD2 show potent bactericidal effects primarily against Gram-negative strains (21). Notably, the antimicrobial activity of most defensins is salt-dependent, suggesting the importance of the electrostatic interactions between cationic peptides and the negatively charged phospholipid components in microbial membranes. More recently, human α- and β-defensins have been found to selectively chemoattract different subsets of T lymphocytes and immature dendritic cells, thus playing important roles as immune modulators in adaptive immunity as well (22–24). For hBDs, chemotaxis of immature dendritic cells and memory T cells results from their direct binding and activation of the chemokine receptor CCR6 whose only known chemokine ligand is MIP-3α (23).
hBD3 is a recently described member of the growing β-defensin family (21, 25–27). The amino acid sequences of the three β-defensins are as follows.
Compared with hBD1 and hBD2, hBD3 contains significantly more positively charged residues and possesses a broad spectrum of potent bactericidal activities in a salt-insensitive manner against both Gram-positive and -negative bacteria, including many drug-resistant strains (21). Despite the significant progress made in the past several years, the structure–function relationships for human defensins are largely unexplored. The sequence rules and structural determinants in human defensins that govern a great variety of biological functions and mechanisms of their action continue to remain poorly understood. Here we report that, in contrast to hBD1 and hBD2, hBD3 does not fold to a unique disulfide-linked structure in vitro under various oxidative conditions and can exist in multiple structurally different yet functionally similar forms. Further, disulfide bonds in hBD3, although required for binding and activation of the chemokine receptor CCR6, are fully dispensable for its antimicrobial activity. This observation allows the structural basis of the separate antimicrobial and chemotactic activities of β-defensins to be dissected for the first time. Our findings shed light on the mechanistic aspects of the antimicrobial action of β-defensins and may have important implications for the design of defensin-based nonchemotactic therapeutic agents to combat increasingly prevalent antibiotic resistance and emerging infectious diseases.
Figure 1.
Materials and Methods
Solid-Phase Peptide Synthesis (SPPS). Synthetic hBD1, hBD2, and hBD3 were prepared on solid phase by using a custom-modified chemistry tailored from the published in situ neutralization protocols for Boc SPPS (28). All peptides were purified to homogeneity by RP-HPLC and their molecular weights verified by electrospray ionization MS (ESI-MS). α-aminobutyric acid (Abu) is often considered to be isosteric to Cys (29, 30) and therefore was our preferred amino acid used to remove disulfide bridges in hBD3. When assessed by circular dichroism spectroscopy, [Abu]-hBD3, i.e., disulfide-devoid hBD3 in which Abu replaces all six Cys residues, was found largely unstructured in aqueous solution. For detailed descriptions of syntheses, purification, and characterizations, see the supporting information, which is published on the PNAS web site, www.pnas.org.
Folding and Disulfide Formation. The standard folding protocol used in our study involves a rapid 6-fold dilution of fully reduced peptides, dissolved at 1.5 mg/ml in 6 M GuHCl, into a final buffer solution containing 0.1 M NaHCO3, 1 M GuHCl, 3 mM cysteine, and 0.3 mM cystine, pH 8.1. The folding reaction typically proceeded at room temperature overnight in a sealed vial with gentle stirring. In the case of hBD3, additional folding protocols were also used, including air oxidation and DMSO oxidation. Air oxidation was carried out by dissolving samples at 0.6 mg/ml in 50 mM phosphate buffer containing 1 M GuHCl, pH 7.5, followed by gentle stirring in an open-air container. Oxidation in 20% DMSO at pH 8.1 was performed essentially as described (31).
Chemotactic Activity Assay. Mononuclear cells were isolated from human peripheral blood or bone marrow of normal donors by routine Ficoll–Paque density gradient centrifugation. The migration of monocytes and CCR6-transfected human embryonic kidney (HEK)293 cells was assessed with a 48-well microchemotaxis chamber technique as described (32). The incubation time was 1.5 h for monocytes and 5 h for transfectant HEK293 cells. The cells were suspended in, and all peptides were diluted with, culture medium RPMI 1640 containing 1% BSA. Chemotactic activity is measured as the optimal concentration of test compound at which the highest chemotactic index value is obtained.
Antimicrobial Activity Assay. Escherichia coli ATCC 25922 was grown to midlogarithmic phase in Mueller–Hinton broth II and then diluted to 1 × 106 colony-forming units per milliliter in 10 mM potassium phosphate, pH 7.4. One hundred microliters of cells was incubated in the presence of different concentrations of peptides for 2 h at 37°C. The cells were then diluted serially in the same buffer, spread on LB plates, and incubated for 18 h at 33°C, and the colonies were counted. Bactericidal activity is expressed as the ratio of colonies counted to the number of colonies on a control plate. The LD50 is the concentration of protein at which 50% of the viable cells are killed. Experimental errors are generally within one doubling dilution.
Results
Folding of hBD3 Is Ambiguous and Yields Many Species with Similar
Antimicrobial Activity. The most commonly used strategy to fold
disulfide-containing proteins involves redox-controlled disulfide shuffling
using reduced and oxidized thiol pairs in the presence of low concentrations
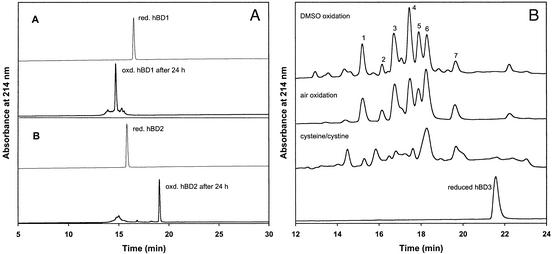
of denaturants. As shown in Fig.
1A, folding of both hBD1 and hBD2 proceeded nearly
quantitatively under these conditions. MS analysis of the predominately
populated species in both cases showed a loss of 6 mass units as compared with
corresponding reduced forms, indicative of formation of three disulfide
bridges. The native  pairing of the β topology in the folded hBD1
and hBD2 was verified by mass mapping of peptide fragments generated by
proteolytic digestion as well as Edman degradation (see supporting
information). In contrast, folding of hBD3 yielded numerous oxidized species,
the relative amounts of which depended on the conditions used
(Fig. 1B). Isolation
and ESI-MS analysis of many of these species gave rise to identical masses of
5,155.5 Da, as expected for oxidized hBD3 with three
pairing of the β topology in the folded hBD1
and hBD2 was verified by mass mapping of peptide fragments generated by
proteolytic digestion as well as Edman degradation (see supporting
information). In contrast, folding of hBD3 yielded numerous oxidized species,
the relative amounts of which depended on the conditions used
(Fig. 1B). Isolation
and ESI-MS analysis of many of these species gave rise to identical masses of
5,155.5 Da, as expected for oxidized hBD3 with three  bonds formed
(5,161.5 Da for the reduced form). This finding suggests that a mixture of
chemically identical and yet structurally distinct hBD3 molecules, each
containing three disulfides but with different
bonds formed
(5,161.5 Da for the reduced form). This finding suggests that a mixture of
chemically identical and yet structurally distinct hBD3 molecules, each
containing three disulfides but with different  connectivities, were
generated. Oxidation using 20% DMSO or in the presence of air resulted in
species better resolved by HPLC. We noted that the cysteine/cystine system led
to formation of some partially oxidized, two-disulfide-bridged hBD3 molecules,
thus yielding an HPLC elution profile different from those obtained under air
and/or DMSO oxidation
conditions.¶
connectivities, were
generated. Oxidation using 20% DMSO or in the presence of air resulted in
species better resolved by HPLC. We noted that the cysteine/cystine system led
to formation of some partially oxidized, two-disulfide-bridged hBD3 molecules,
thus yielding an HPLC elution profile different from those obtained under air
and/or DMSO oxidation
conditions.¶
Fig. 1.
(A) Folding of hBD1 and hBD2 aided by cysteine/cystine. Fully reduced, purified peptides were analyzed by C18 RP-HPLC (thin lines) with a linear gradient of 5–65% acetonitrile over 30 min. Peptides folded after 24 h (thick lines) were analyzed under the same chromatographic conditions. (B) Folding of hBD3 under various oxidative conditions: DMSO, air, and cysteine/cystine. Analyses by C18 RP-HPLC were carried out using a linear gradient of 20–35% acetonitrile over 30 min.
Seven fractions from the DMSO oxidation reaction (Fig. 1B, numbered 1–7) with identical masses of 5,155.5 Da were collected and subjected to bactericidal assay by using E. coli ATCC 25922. Surprisingly, all of the fractions exhibited similar bactericidal activity with an LD50 value of ≈0.1 μg/ml (Table 1). The two synthetic hBD1 and hBD2 samples were also analyzed as positive controls, resulting in LD50 values of 5.0 and 0.6 μg/ml, respectively. These findings led us to postulate that the antimicrobial activity of hBD3 is independent of how disulfide bridges are paired in the molecule. To test this hypothesis, topological analogs of hBD3 with various predefined disulfide connectivities were synthesized for definitive functional as well as structural characterizations.
Table 1. Bactericidal activity of hBD-1, hBD-2, and DMSO-oxidized species against E. coli ATCC 25922.
|
DMSO-oxidized hBD3 peaks
|
||||||||
|---|---|---|---|---|---|---|---|---|
| hBD1 | hBD2 | 1 | 2 | 3 | 4 | 5,6 | 7 | |
| LD50, μg/ml | 5.0 | 0.6 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
Peaks 5 and 6, although well resolved on analytical HPLC (see Fig. 1B), were pooled as a mixture on preparative C18 column and therefore assayed as such. LD50 is defined as the peptide concentration at which 50% of the viable cells were killed under the assay conditions.
HBD3 Antimicrobial Activity Is Independent of Disulfide Connectivity. We chemically synthesized six topological analogs of hBD3 with predefined disulfide connectivities, three containing the Cys1–Cys5 pairing characteristic for the β-defensin family, and the remaining three the Cys1–Cys6 bond conserved in the α-defensin family. The synthetic strategy using reversible protection of selected Cys residues by acetamidomethyl (Acm) groups is illustrated in Fig. 2A. For hBD3 with six Cys residues, there are a total of 15 possible isoforms with distinctive disulfide pairings excluding partially oxidized variants. Protection of two of six Cys residues by Acm reduces the number of oxidation products from 15 to 3, thus significantly simplifying the separation and subsequent determination of the disulfide connectivity. As shown in Fig. 2 A and B, six desired oxidation products, a–f, each containing two disulfide bridges, were obtained from two fully reduced Cys1(Acm)/Cys5(Acm)- and Cys1(Acm)/Cys6(Acm)-hBD3 peptides. The oxidation reaction was carried out in the presence of cysteine/cystine at pH 8.1. Before carrying out the full-scale protocol, fractions a–f purified by preparative HPLC were subjected to enzymatic digestion with trypsin and chymotrypsin to determine disulfide connectivity. Although MS identification of tryptic fragments from species c and f led to unambiguous assignment of Cys1–Cys5/Cys2–Cys3/Cys4–Cys6 and Cys1–Cys6/Cys2–Cys3/Cys4–Cys5, respectively, the remaining four species (a, b, d, e) yielded a chemically identical two-disulfide-bridged tryptic fragment of 1,846.9 Da. The ambiguity was resolved by subsequent enzymatic digestion with chymotrypsin. All of the common and unique fragments generated by trypsin and chymotrypsin were identified and accounted for by analytical RP-HPLC and ESI-MS (see supporting information). Interestingly, two species (b, e) with disulfide topologies native in α- and β-defensins had the lowest folding yields.
Fig. 2.
(A) Strategy for preparation of six topological analogs of hBD3. Fully reduced hBD3 peptides with Cys1/Cys5 or Cys1/Cys6 selectively protected by Acm were subjected to oxidative folding aided by cysteine/cystine, resulting in six intermediate products (a–f), each containing two disulfide bridges. After determination of the disulfide connectivity in each species, deprotection and spontaneous oxidation of Cys1/Cys5 or Cys1/Cys6 were achieved by treatment with I2 at pH 4. (B) Formation of two disulfide bridges in Cys1/Cys5- or Cys1/Cys6-protected hBD3. C18 RP-HPLC analyses were performed using a linear gradient of 5–65% acetonitrile over 30 min. All six resultant oxidation products of identical masses, a–f, were pooled for identification of the disulfide connectivity. The minor peak between species a and b was a partially oxidized product with only one disulfide bridge formed as indicated by ESI-MS. (C) Analysis of the six purified topological analogs of hBD3 with defined disulfide connectivities on C18 RP-HPLC running a linear gradient of 5–65% acetonitrile over 30 min. Note that the order of elution changes upon formation of the third disulfide bridge.
Deprotection and spontaneous oxidation of Cys1/Cys5 or Cys1/Cys6 in species a–f whose disulfide topology had been previously determined were achieved through treatment with I2 at acidic pH according to the published protocols (33). Final products were verified by ESI-MS, yielding an observed mass value of 5,155.5 Da, in agreement with the expected value of 5,155.2 Da, calculated on the basis of average isotope compositions of fully oxidized hBD3. To demonstrate chromatographic purity, samples of the six topological analogs of hBD3 were analyzed on RP-HPLC (Fig. 2C).
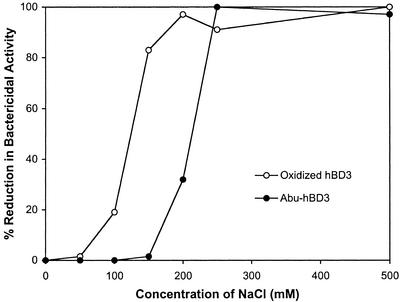
The same antimicrobial assay described earlier was performed for the six topological analogs of hBD3 along with [Abu]-hBD3 by using E. coli ATCC 25922. The data are tabulated in Table 2. The LD50 values for the six disulfide-linked analogs range from 0.02 to 0.08 μg/ml, confirming that different connectivities of three disulfide bridges in hBD3 had little effect on its antimicrobial activity. Notably, removal of three disulfide bridges in hBD3 resulted in an LD50 value of 0.04 μg/ml, demonstrating unequivocally that disulfide bonds are not required for the antimicrobial activity of hBD3. We also compared salt dependence of the antimicrobial activity of [Abu]-hBD3 and a disulfide-bridged hBD3 molecule. The linear form of hBD3 appeared more salt-resistant than the disulfide-linked molecule (Fig. 3).
Table 2. Bactericidal activity of [Abu]-hBD3 and topological analogs of hBD3 against E. coli ATCC 25922.
|
Six topological analogs of hBD3
|
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | [Abu]-hBD3 | |
| LD50, μg/ml | 0.08 | 0.06 | 0.03 | 0.08 | 0.05 | 0.02 | 0.04 |
Fig. 3.
Salt dependence of antimicrobial activity of linear and disulfide bridged hBD3 against E. coli. Oxidized hBD3 and [Abu]-hBD3 were assayed against E. coli ATCC 25922 at a fixed concentration of 20 μg/ml, as described in Materials and Methods, except that NaCl at concentrations noted in the figure were included in the assay buffer (10 mM potassium phosphate, pH 7.4).
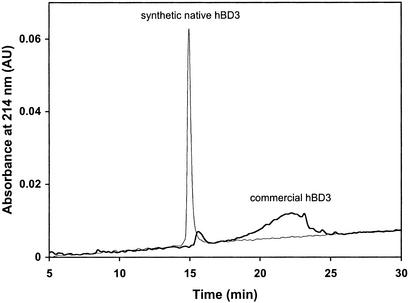
Recently, the solution structure of a commercially available recombinant hBD3 has been reported, showing a similar fold to hBD1 and hBD2 (10). We noted that the disulfide topology of hBD3 used in the NMR study was not chemically determined, nor were the disulfide restraints used in the structure calculation independently observed from β-proton nuclear Overhauser effects. To determine whether the recombinant hBD3 adopts the native disulfide pairing, we obtained a sample from the same commercial source (PeproTech, Rocky Hill, NJ) and analyzed it along with our highly pure synthetic hBD3 with the defined β topology on C18 RP-HPLC (Fig. 4). Although our material eluted as a sharp and symmetric peak, the recombinant preparation showed vast heterogeneity, with no detectable component overlapping with our synthetic species. The apparent discrepancy calls into question the structural identity of the recombinant hBD3 and underscores the difficulty with which the polypeptide folds into the native conformation in vitro.
Fig. 4.
Comparative analysis of synthetic hBD3 with the β topology and commercial hBD3 (1 μg each) on a Vydac C18 RP column (4.6 × 150 mm). The chromatographic data were collected using a linear gradient of 20–35% acetonitrile over 30 min.
Disulfide Bonding in hBD3 Is Important for Its Chemotactic Function. hBD1 and hBD2 chemoattract immature dendritic cells, memory T cells and CCR6-transfected HEK293 cells in a dose-dependent manner with an optimal concentration of 1,000 ng/ml (23). This value is an order of magnitude higher than that of the chemokine MIP-3α (23), a known natural ligand of CCR6. Similar assays were carried out for analogs of hBD3 with monocytes and CCR6(+) HEK293 cells. The results are shown in Table 3. The six disulfide-stabilized hBD3 analogs, although all active, demonstrated a wide range of chemotactic activities with both monocytes and HEK293. The optimal concentrations of these analogs ranged from 10 to 1,000 ng/ml for HEK293 and spanned 4 orders of magnitude for monocytes, i.e., 1–10,000 ng/ml. Significantly, absence of disulfide bridges in [Abu]-hBD3 rendered the peptide chemotactically inactive. These results strongly suggest that a disulfide-stabilized structure of hBD3 is a prerequisite for binding and activation of receptors for chemotaxis.
Table 3. Optimal peptide concentrations (ng/ml) for chemotaxis by topological analogs of hBD3 of monocytes and CCR6/HEK293 cells.
| 1 | 2 | 3 | 4 | 5 | 6 | [Abu]-hBD3 | |
|---|---|---|---|---|---|---|---|
| Monocytes | 100 | 10 | 1,000 | ≥10,000 | 1,000 | 1 | inactive at > 10,000 |
| CCR6/HEK293 | 10 | 100 | 1,000 | 1,000 | 100 | 100 | inactive at > 10,000 |
Discussion
Human defensins are endogenous antimicrobial proteins with potent and broad killing activity against various microorganisms and therefore are attractive candidates for the development of novel peptide antibiotics. Because these antimicrobial peptides are generally cationic and target the negatively charged cytoplasmic membranes of invading microbes, acquired resistance rarely occurs, presumably due to the high evolutionary costs for microbes to substantially re-engineer the charge on their membrane structures (2, 34). Despite this advantage, concerns exist that defensin-based antibiotics may exhibit potential side effects associated with their proinflammatory chemotactic properties. We have demonstrated in this report that separation of the bactericidal and chemotactic activities of hBD3 can be achieved through disulfide engineering, thus paving the way for further development of novel classes of defensin-based nonchemotactic antimicrobial therapeutics, not susceptible to existing resistance mechanisms, for the treatment of infectious diseases. Our findings also shed light on the mechanistic aspects of how these antimicrobial and chemotactic proteins work at the molecular level.
It has long been known that the folded structures of proteins and their unique biological functions are determined by their amino acid sequences (35). hBD1 and hBD2 fold to form native disulfide-linked structures in vitro, whereas hBD3 yields a complex mixture of products under similar conditions. It is plausible that an excessive number (one-third of the peptide) of positively charged residues in hBD3 may have contributed to prevent population of native-like compact structures in the folding process due to the prevention of sufficient hydrophobic packing by electrostatic repulsion. Despite the fact that synthetic hBD3 does not fold preferentially into a native conformation in vitro under various oxidative conditions examined, it is reasonable to assume that the naturally occurring hBD3, whose structure has not been experimentally determined (21), is conformationally homogeneous with a defined disulfide connectivity of, most likely, the β topology. This is understandable because protein folding in vivo is aided by various accessory molecules (36), which catalyze disulfide and peptide bond isomerization reactions, prevent aggregation of incorrectly folded or denatured species, or target them to the proteolytic degradation pathway. A notable topological analog of hBD3 is Cys1–Cys6, Cys2–Cys5, and Cys3–Cys4, which showed strong antimicrobial activity against E. coli with a LD50 value of 0.02 μg/ml, as well as potent chemotactic activity for monocytes with an optimal concentration at 1 ng/ml. This construct has been crystallized and diffracted to high resolution, and its structural determination is under way.
Functional Implications. Despite the complex mixtures obtained on oxidative folding, all oxidized species of hBD3 examined, as well as its linear analog [Abu]-hBD3, displayed similar antimicrobial activities. Why does this natural antibiotic peptide carry six Cys residues when functionally there seems no need for disulfide bonds? An answer may lie in the chemotactic properties of this polypeptide, which exhibited widely varied chemotatic activities among the disulfide isoforms. Chemotaxis of cells is a receptor-mediated biological event. Human β-defensins are known chemoattractants for memory T cells and immature dendritic cells through binding and activation of CCR6. As is the case with all chemokines (37), a defined 3D structure stabilized by disulfide bonding is required for productive binding to and activation of receptors. Therefore it is not surprising that structurally destabilized [Abu]-hBD3 does not chemoattract monocytes and CCR6(+) HEK293 cells. Interestingly, chemotaxis of monocytes and CCR6-transfected cells strongly depend on the topology of disulfide connectivities in hBD3. In some cases, markedly enhanced chemoctactic activities were observed with both monocytes and HEK293 cells as compared with MIP-3α, demonstrating that receptor binding and activation can be modulated by varying the disulfide topology of the hBD3 ligand.
Is it possible that disulfide bridges are required for hBD3 so that the antimicrobial protein can function at high salt concentrations? High salt concentrations (ionic strength) tend to decrease antimicrobial activity as a result of weakened electrostatic interactions between cationic peptides and negatively charged membranes. In fact, this has been shown to be physiologically relevant in cystic fibrosis (CF), where abnormally high NaCl in the lung inactivates hBD1, resulting in the CF-associated defect in bacterial killing (38, 39). There appears to be a correlation between the number of positive charges and the ability to retain antimicrobial activity of defensins at higher concentrations of salts (40, 41). It is plausible that a greater number of charged residues are less sufficiently screened by salts to diminish the binding to the negatively charged lipid components in membranes. We have noted in a salt-dependence assay that an increase in NaCl concentration from 50 to 150 mM caused loss by 90% of the bactericidal activity of a disulfide bridged construct of hBD3 at 20 μg/ml. However, under the same assay conditions, the activity of [Abu]-hBD3 was unaffected. This finding indicates that the linear form of hBD3 is more salt-resistant than the folded versions, suggesting that disulfide bonds in hBD3 play little role, if any, in enhancing salt resistance of the antimicrobial protein. Our result is an exception to reported findings that structural rigidity of antimicrobial peptides correlates to their salt-dependent activity, particularly at high salt concentrations (42–45).
Mechanistic Implications. Antimicrobial peptides kill microorganisms by permeabilizing and lysing the cell membranes (2). They preferentially target prokaryotic cells because bacterial membranes contain large amounts of negatively charged phospholipids, whereas the surface of mammalian cell membranes is composed of neutral zwitterionic phospholipids with abundant embedded cholesterol molecules. Although there is little disagreement over the importance of electrostatic interactions in initiating membrane association of cationic peptides as well as their selective toxicity toward microorganisms, opinions differ with regard to the subsequent events that eventually lead to the lysis of microbes (46).
The central dispute that remains to be settled is whether peptides disintegrate the membranes by forming multimeric solvent-permeable pore structures in the membranes, therefore causing leakage of intracellular components (pore-forming model), or simply by densely covering the outer leaflets of the membranes like detergents (carpet model) without forming discrete membrane channels. The pore-forming theory postulates that structural amphiphilicity, i.e., spatial segregation of clusters of cationic residues from patches of hydrophobic groups, is the most important prerequisite for stable pore formation in the membrane (2, 47, 48). Many defensins indeed form amphiphilic dimers and sometimes higher-order amphiphilic oligomers in solution. However, lack of oligomerization and amphiphilicity has also been demonstrated for some other defensin molecules (7, 49, 50), thus creating an uncertainty about the stringency of requirements of amphiphilicity for antimicrobial activity (51). In contrast, the carpet model suggests that no specific structure is needed on membrane association, because membrane disruption is not thought to involve peptide insertion into the hydrophobic core of lipid bilayers (52). Growing evidence appears to support the existence of multiple mechanisms of action for different peptides, and for a given peptide, the mechanism may also vary for different bacterial species it targets (51, 53–55). Further, the correlation between membrane permeability and antimicrobial activity does not always exist for many β-sheet antibiotic peptides (51, 52, 55, 56). In fact, it has been suggested that linear analogs and their disulfide bonded parent molecules can follow different mechanisms of action in bacterial killing (47).
We have demonstrated that hBD3 has antimicrobial activity irrespective of whether and how three disulfide bridges are paired in the molecule. Given an unusually large number of positively charged residues distributed across the sequence of hBD3, it would be difficult to envision a 3D structure of amphiphilic nature. Furthermore, hBD3 molecules constrained by disulfide bonding of different topologies as well as disulfide-devoid [Abu]-hBD3 likely differ in amphiphilicity. Therefore, our findings with hBD3 appear consistent with the carpet model for the mode of action where an overwhelming number of positive charges are sufficient to induce membrane destruction (52).
Conclusion
We have demonstrated that the antimicrobial and chemotactic activities of hBD3 can be cleanly decoupled through removal of three disulfide bonds. This finding may have important implications in designing hBD3-based novel therapeutic agents to treat infectious diseases and to combat antibiotic resistance. Comprehensive structure–activity relationship studies are needed to further elucidate the molecular basis of how these antimicrobial peptides work in innate and adaptive immunity.
Supplementary Material
Acknowledgments
We thank Drs. Rob Powell and Bryan Ericksen for helpful comments on the manuscript. This research was supported by the Institute of Human Virology, a center of the University of Maryland Biotechnology Institute (W.L.), and by the Intramural AIDS Targeted Antiviral Program of the Office of the Director, National Institute of Health (J.L.).
Abbreviations: hBD, human β-defensin; Abu, α-aminobutyric acid; Acm, acetamidomethyl; CCR6, CC chemokine receptor 6; ESI-MS, electrospray ionization MS.
Footnotes
It is worth pointing out that folded hBD1 and hBD2 showed different chromatographic behavior. Usually, folding of a globular protein containing disulfide bridges is accompanied by a shift to a shorter retention time on RP-HPLC, as a result of burial of hydrophobic residues. Although this was indeed the case for hBD1, the hBD2 molecule became more strongly retained on the C18 column after oxidative folding. Both hBD1 and hBD2 are amphiphilic in their monomeric forms, with hydrophobic patches and charged clusters situated on the opposite site of the molecule. However, comparative structural analysis indicates that the hydrophobic patch on hBD2 is significantly larger than that on hBD1. Therefore, it is plausible that the increased retention of folded hBD2 on C18 RP-HPLC is due to stronger interactions between the column media and the folded molecule.
References
- 1.Nizet, V., Ohtake, T., Lauth, X., Trowbridge, J., Rudisill, J., Dorschner, R. A., Pestonjamasp, V., Piraino, J., Huttner, K. & Gallo, R. L. (2001) Nature 414, 454–457. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff, M. (2002) Nature 415, 389–395. [DOI] [PubMed] [Google Scholar]
- 3.Hancock, R. E. W. & Lehrer, R. (1998) Trends Biotechnol. 16, 82–88. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer, R. I. & Ganz, T. (2002) Curr. Opin. Immunol. 14, 96–102. [DOI] [PubMed] [Google Scholar]
- 5.Ganz, T. & Lehrer, R. I. (1998) Curr. Opin. Immunol. 10, 41–44. [DOI] [PubMed] [Google Scholar]
- 6.Hill, C. P., Yee, J., Selsted, M. E. & Eisenberg, D. (1991) Science 251, 1481–1485. [DOI] [PubMed] [Google Scholar]
- 7.Hoover, D. M., Rajashankar, K. R., Blumenthal, R., Puri, A., Oppenheim, J. J., Chertov, O. & Lubkowski, J. (2000) J. Biol. Chem. 275, 32911–32918. [DOI] [PubMed] [Google Scholar]
- 8.Hoover, D. M., Chertov, O. & Lubkowski, J. (2001) J. Biol. Chem. 276, 39021–39026. [DOI] [PubMed] [Google Scholar]
- 9.Bauer, F., Schweimer, K., Klüver, E., Conejo-Garcia, J. R., Forssmann, W. G., Rösch, P., Adermann, K. & Sticht, H. (2001) Protein Sci. 10, 2470–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schibli, D. J., Hunter, H. N., Aseyev, V., Starner, T. D., Wiencek, J. M., McCray, P. B., Tack, B. F. & Vogel, H. J. (2002) J. Biol. Chem. 277, 8279–8289. [DOI] [PubMed] [Google Scholar]
- 11.Ganz, T., Selsted, M. E., Szklarek, D., Harwig, S. S., Daher, K., Bainton, D. F. & Lehrer, R. I. (1985) J. Clin. Invest. 76, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selsted, M. E., Harwig, S. S., Ganz, T., Schilling, J. W. & Lehrer, R. I. (1985) J. Clin. Invest. 76, 1436–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilde, C. G., Griffith, J. E., Marra, M. N., Snable, J. L. & Scott, R. W. (1989) J. Biol. Chem. 264, 11200–11203. [PubMed] [Google Scholar]
- 14.Jones, D. E. & Bevins, C. L. (1992) J. Biol. Chem. 267, 23216–23225. [PubMed] [Google Scholar]
- 15.Jones, D. E. & Bevins, C. L. (1993) FEBS Lett. 315, 187–192. [DOI] [PubMed] [Google Scholar]
- 16.Ayabe, T., Satchell, D. P., Wilson, C. L., Parks, W. C., Selsted, M. E. & Ouellette, A. J. (2000) Nat. Immunol. 1, 99–100. [DOI] [PubMed] [Google Scholar]
- 17.Schutte, B. C. & McCray, P. B., Jr. (2002) Annu. Rev. Physiol. 64, 709–748. [DOI] [PubMed] [Google Scholar]
- 18.Bensch, K. W., Raida, M., Mägert, H. J., Schulz-Knappe, P. & Forssmann, W. G. (1995) FEBS Lett. 368, 331–335. [DOI] [PubMed] [Google Scholar]
- 19.Zhao, C., Wang, I. & Lehrer, R. I. (1996) FEBS Lett. 396, 319–322. [DOI] [PubMed] [Google Scholar]
- 20.Harder, J., Bartels, J., Christophers, E. & Schröder, J. M. (1997) Nature 387, 861. [DOI] [PubMed] [Google Scholar]
- 21.Harder, J., Bartels, J., Christophers, E. & Schröder, J. M. (2001) J. Biol. Chem. 276, 5707–5713. [DOI] [PubMed] [Google Scholar]
- 22.Chertov, O., Michiel, D. F., Xu, L., Wang, J. M., Tani, K., Murphy, W. J., Longo, D. L., Taub, D. D. & Oppenheim, J. J. (1996) J. Biol. Chem. 271, 2935–2940. [DOI] [PubMed] [Google Scholar]
- 23.Yang, D., Chertov, O., Bykovskaia, S. N., Chen, Q., Buffo, M. J., Shogan, J., Anderson, M., Schröder, J. M., Wang, J. M., Howard, O. M. & Oppenheim, J. J. (1999) Science 286, 525–528. [DOI] [PubMed] [Google Scholar]
- 24.Yang, D., Chen, Q., Chertov, O. & Oppenheim, J. J. (2000) J. Leukocyte Biol. 68, 9–14. [PubMed] [Google Scholar]
- 25.Schutte, B. C., Mitros, J. P., Bartlett, J. A., Walters, J. D., Jia, H. P., Welsh, M. J., Casavant, T. L. & McCray, P. B. (2002) Proc. Natl. Acad. Sci. USA 99, 2129–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Jia, H., Schutte, B. C., Schudy, A., Linzmeier, R., Guthmiller, J. M., Johnson, G. K., Tack, B. F., Mitros, J. P., Rosenthal, A., Ganz, T., et al. (2001) Gene 263, 211–218. [DOI] [PubMed] [Google Scholar]
- 27.Garcia, J. R., Jaumann, F., Schulz, S., Krause, A., Rodriguez-Jimenez, J., Forssmann, U., Adermann, K., Klüver, E., Vogelmeier, C., Becker, D., et al. (2001) Cell Tissue Res. 306, 257–264. [DOI] [PubMed] [Google Scholar]
- 28.Schnölzer, M., Alewood, P., Jones, A., Alewood, D. & Kent, S. B. (1992) Int. J. Pept. Protein Res. 40, 180–193. [DOI] [PubMed] [Google Scholar]
- 29.Lu, W., Apostol, I., Qasim, M. A., Warne, N., Wynn, R., Zhang, W. L., Anderson, S., Chiang, Y. W., Ogin, E., Rothberg, I., et al. (1997) J. Mol. Biol. 266, 441–461. [DOI] [PubMed] [Google Scholar]
- 30.Bigler, T. L., Lu, W., Park, S. J., Tashiro, M., Wieczorek, M., Wynn, R. & Laskowski, M., Jr. (1993) Protein Sci. 2, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tam, J. P., Wu, C. R., Liu, W. & Zhang, J. W. (1991) J. Am. Chem. Soc. 113, 6657–6662. [Google Scholar]
- 32.Falk, W., Goodwin, R. H. & Leonard, E. J. (1980) J. Immunol. Methods 33, 239–247. [DOI] [PubMed] [Google Scholar]
- 33.Tam, J. P., Lu, Y. A. & Yu, Q. T. (1999) J. Am. Chem. Soc. 121, 4316–4324. [Google Scholar]
- 34.Hancock, R. E. (1997) Lancet 349, 418–422. [DOI] [PubMed] [Google Scholar]
- 35.Anfinsen, C. B. (1973) Science 181, 223–230. [DOI] [PubMed] [Google Scholar]
- 36.Fersht, A. (1999) Structure and Mechanism in Protein Science (Freeman, New York).
- 37.Fernandez, E. J. & Lolis, E. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 469–499. [DOI] [PubMed] [Google Scholar]
- 38.Smith, J. J., Travis, S. M., Greenberg, E. P. & Welsh, M. J. (1996) Cell 85, 229–236. [DOI] [PubMed] [Google Scholar]
- 39.Goldman, M. J., Anderson, G. M., Stolzenberg, E. D., Kari, U. P., Zasloff, M. & Wilson, J. M. (1997) Cell 88, 553–560. [DOI] [PubMed] [Google Scholar]
- 40.Fujii, G., Selsted, M. E. & Eisenberg, D. (1993) Protein Sci. 2, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De-Samblanx, G. W., Goderis, I. J., Thevissen, K., Raemaekers, R., Fant, F., Borremans, F., Acland, D. P., Osborn, R. W., Patel, S. & Broekaert, W. F. (1997) J. Biol. Chem. 272, 1171–1179. [DOI] [PubMed] [Google Scholar]
- 42.Tam, J. P., Lu, Y. A. & Yang, J. L. (2000) Biochem. Biophys. Res. Commun. 267, 783–790. [DOI] [PubMed] [Google Scholar]
- 43.Tam, J. P., Lu, Y. A. & Yang, J. L. (2000) Biochemistry 39, 7159–7169. [DOI] [PubMed] [Google Scholar]
- 44.Tam, J. P., Wu, C. & Yang, J. L. (2000) Eur. J. Biochem. 267, 3289–3300. [DOI] [PubMed] [Google Scholar]
- 45.Yu, Q., Lehrer, R. I. & Tam, J. P. (2000) J. Biol. Chem. 275, 3943–3949. [DOI] [PubMed] [Google Scholar]
- 46.Blondelle, S. E., Lohner, K. & Aguilar, M. (1999) Biochim. Biophys. Acta 1462, 89–108. [DOI] [PubMed] [Google Scholar]
- 47.Matsuzaki, K. (1999) Biochim. Biophys. Acta 1462, 1–10. [DOI] [PubMed] [Google Scholar]
- 48.White, S. H., Wimley, W. C. & Selsted, M. E. (1995) Curr. Opin. Struct. Biol. 5, 521–527. [DOI] [PubMed] [Google Scholar]
- 49.Hristova, K., Selsted, M. E. & White, S. H. (1996) Biochemistry 35, 11888–11894. [DOI] [PubMed] [Google Scholar]
- 50.Trabi, M., Schirra, H. J. & Craik, D. J. (2001) Biochemistry 40, 4211–4221. [DOI] [PubMed] [Google Scholar]
- 51.Sitaram, N. & Nagaraj, R. (1999) Biochim. Biophys. Acta 1462, 29–54. [DOI] [PubMed] [Google Scholar]
- 52.Shai, Y. (1999) Biochim. Biophys. Acta 1462, 55–70. [DOI] [PubMed] [Google Scholar]
- 53.Epand, R. M. & Vogel, H. J. (1999) Biochim. Biophys. Acta 1462, 11–28. [DOI] [PubMed] [Google Scholar]
- 54.Friedrich, C. L., Moyles, D., Beveridge, T. J. & Hancock, R. E. (2000) Antimicrob. Agents Chemother. 44, 2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, L., Rozek, A. & Hancock, R. E. (2001) J. Biol. Chem. 276, 35714–35722. [DOI] [PubMed] [Google Scholar]
- 56.Wu, M., Maier, E., Benz, R. & Hancock, R. E. (1999) Biochemistry 38, 7235–7242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.