Abstract
Adoptive transfer of antigen-specific CD25+CD4+ regulatory T cells was used to analyze the stability of their phenotype, their behavior after immunization, and their mode of suppressing cotransferred naive T cells in vivo. We found that regulatory T cells maintained their phenotype in the absence of antigen, were not anergic in vivo, and proliferated as extensively as naive CD4+ T cells after immunization without losing their suppressive function in vivo and in vitro. In vivo, the expansion of cotransferred naive T cells was suppressed relatively late in the response such that regulatory T cells expressing mostly IL-10 but not IL-2 or IFN-γ represented the dominant subset of cells. Our results reveal properties of regulatory T cells that were not predicted from in vitro studies.
Dominant mechanisms of tolerance control the autoimmune potential of self-reactive T cells in healthy individuals and animals (reviewed in refs. 1–3). Insights into the regulation of immune responses by regulatory T cells have been mostly obtained with polyclonal populations of regulatory T cells for which the role of specific antigen has been largely obscure. The impact of self-antigen on the shaping of the regulatory T cell pool came more into focus when it was observed that the coexpression of a transgenic class II MHC restricted T cell receptor (TCR) and its agonist ligand resulted in the generation of antigen-specific regulatory T cells (4). Subsequent experiments in that system confirmed the notion that thymic epithelium can have a decisive role in the formation of such cells by demonstrating that the expression of an agonist ligand on radioresistant tissue (5) and on transplanted thymic epithelium (6) was a very effective means of generating regulatory T cells, whereas the mode of generation of polyclonal regulatory T cells in normal mice still needs to be elucidated.
A subset of CD4+ T cells expressing the interleukin-2 (IL-2) receptor α-chain (CD25) recently became a major focus of interest. These CD4+CD25+ T cells were first shown by Sakaguchi and colleagues to control autoreactive T cells in vivo (7). Several characteristics of these cells have emerged from in vitro studies (reviewed in ref. 8) resulting in the notion that regulatory T cells are anergic in terms of proliferation and suppress other cells by direct cell contact, which requires neither IL-10 nor transforming growth factor-β and which results in the inability of suppressed CD4+ T cells to produce IL-2 (9–11). However, it is unclear at present how far these observations in vitro are in fact a reflection of the properties of CD4+CD25+ T cells in vivo. In some experimental systems of immune regulation by CD4+CD25+ T cells in vivo, it was found that soluble factors such as IL-4, IL-10, and transforming growth factor-β do contribute to the prevention of autoimmunity, with the role of these factors var ying between different models (12–14). Polyclonal CD25+CD4+ T cells proliferate and expand when they are transferred into rag–/– or IL-2 receptor β-deficient mice (15–17), indicating that their anergic state can be reversed under certain nonphysiological conditions. Notably, it is under exactly these lymphopenic conditions that the regulatory function of CD25+CD4+ T cells has been studied in the majority of the currently available models, at least suggesting that proliferation and suppressive function by regulatory T cells may not be mutually exclusive. A major experimental drawback of lymphopenic models of immune regulation, however, is that they provide no information on antigen-induced proliferation as observed in nonlymphopenic mice.
The present study was initiated to establish an in vivo system of antigen-specific immune regulation that is as physiological as possible to characterize the behavior of CD25+CD4+ regulatory T cells at relatively low frequency in the context of an unperturbed immune system. To this end, TCR transgenic CD25+CD4+ T cells were adoptively transferred into normal hosts either alone or in combination with naive CD4+25– T cells of identical antigen specificity. Immunization with cognate antigen was used to visualize and compare the behavior of the respective populations either alone or in combination.
Materials and Methods
Mice and Transgenic Vectors. BALB/c Thy1.2 mice were purchased from Taconic Farms. Other strains were bred in the animal facility of the Dana–Farber Cancer Institute under specific pathogen-free conditions.
Phosphoglycerate kinase-hemagglutinin (pgk-HA) mice were generated through germ-line cre-mediated recombination in mice carrying a pgk-loxβ-gallox-HA cassette vector. This vector was constructed as follows. Lox P sites were introduced 5′ and 3′ of the β-gal cDNA. The HA cDNA then was introduced downstream of the 3′ lox P site, and the construct was cloned into a eukaryotic expression vector driven by the pgk promoter. Transgenic founders (F1 FVB × 129) were backcrossed to BALB/c for at least five generations before being crossed to TS4 cre mice (18) or Whey acidic protein-cre mice (19) that had been backcrossed to BALB/c for at least four generations. Both crosses unexpectedly led to germ-line recombination of the transgene. Germ-line-recombined mice then were maintained by further backcrossing of pgk-HA × TCR-HA double-transgenic mice to BALB/c for at least four generations.
Immunization. Mice were immunized s.c. with 100 μg of peptide HA107–119 emulsified in incomplete Freund's adjuvant (IFA, Sigma–Aldrich). At the indicated time points the animals were killed, and draining (popliteal and inguinal) and distant (mesenteric) lymph nodes were harvested for analysis. The tumor cell line CT26 HA-EGFP has been described elsewhere (20).
Antibodies and Fluorescence-Activated Cell Sorter Analysis. Biotin-conjugated mAbs to CD4 (H129.19) and Thy1.1 (HIS51), phycoerythrin-conjugated mAbs to CD4 (GK1.5), CD25 (PC61), IL-2 (JES6–5H4), IL-10 (JES5-16E3), IFN-γ (XMG.1.2), tumor necrosis factor α (MP6-XT22), rat IgG1 isotype control (R3-34), and rat IgG2b isotype control (A95-1), Cy-chrome-conjugated streptavidin, mAbs to CD8 (53-6.7), and allophycocyanin-conjugated mAbs to CD4 (RM4-5), CD25 (PC61), and Thy1.2 (53-2.1) were purchased from Becton Dickinson. The mAb to the TCR-HA (6.5) was purified and conjugated with FITC in our lab. Fc-receptor-blocking mAb 2.4G2 was used as culture supernatant. Surface stainings were performed according to standard procedures at a density of 2–4 × 106 cells per 50 μl, and volumes were scaled up accordingly. Flow-cytometric analysis was performed on a FACSCalibur (Becton Dickinson) by using CELLQUEST software (Becton Dickinson).
Intracellular Cytokine Staining. Cells from the draining lymph node of immunized animals were resuspended at a density of 2 × 106 cells per ml in Iscove's modified Dulbecco's medium containing 10% FCS. Cells then were plated in 6 ml per well into six-well culture plates, 12 μl of leukocyte activation mixture with GolgiPlug (Becton Dickinson) containing phorbol 12-myristate 13-acetate, ionomycin, and brefeldin A were added, and cultures were incubated at 37°C for 6 h. Cells were harvested and incubated with 2.4G2 Fc-receptor-blocking antibody before surface staining. Cells then were fixed with Cytofix/Cytoperm buffer (Becton Dickinson) for 20 min at room temperature. Intracellular cytokine staining was performed at room temperature for 20 min in Perm/Wash buffer (Becton Dickinson). Control stainings were performed with a mixture of phycoerythrin-conjugated isotype controls.
Purification and Adoptive Transfer of Cells. Pooled cells from spleen and peripheral lymph nodes (mesenteric, axillary, brachial, popliteal, inguinal, and cervical) from pgk-HA × TCR-HA mice were subjected to erythrocyte lysis. Cells then were incubated with Fc-receptor-blocking antibody 2.4G2 and stained with anti-CD4 biotin. After incubation with streptavidin microbeads (Miltenyi Biotec, Auburn, CA), CD4+ cells were positively selected on midi-MACS columns, routinely achieving purities >95%. Cells then were stained with streptavidin-Cy-chrome, anti-CD25 phycoer ythrin, and FLUOS-labeled 6.5. CD4+CD25+6.5+ cells were sorted by using a MoFlow cell sorter (Cytomation, Fort Collins, CO). Naive TCR-HA CD4+ T cells were obtained from spleen and lymph nodes of TCR-HA rag–/– mice by magnetic enrichment for CD4 T cells.
Cells were injected into the lateral tail vein in a volume of 200 μl of PBS. Where indicated, cells were labeled with 5,6-carboxyfluorescein diacetate-succinimidyl ester (CFSE) (Molecular Probes) by incubation for 10 min at 37°C in 10 μM CFSE in PBS/0.1% BSA at a density of 1 × 107 cells per ml.
Proliferation Assays. For inhibition assays, 2 × 104 sorted or magnetically enriched CD25+ and/or CD25– CD4+6.5+ T cells were incubated with 2 × 105 irradiated (3,000 rad) BALB/c splenocytes in the presence of 5 μg/ml HA107–119 peptide in 200 μl of Iscove's modified Dulbecco's medium supplemented with 10% FCS in 96-well round-bottom plates. Where indicated, 50 units/ml recombinant IL-2 were added (Becton Dickinson). Proliferation was measured by scintillation counting after pulsing with 1 μCi per well [3H]thymidine (1 Ci = 37 GBq) for the last 16–20 h of a 90-h incubation period.
For ex vivo proliferation assays, cells from draining lymph nodes were cultured for 72–90 h in triplicates at 4 × 105 cells per well in round-bottom 96-well plates in serum-free medium (HL-1, BioWhittaker). Proliferation was measured as incorporation of [3H]thymidine, which was added for the last 18 h of culture (1 μCi per well).
Results
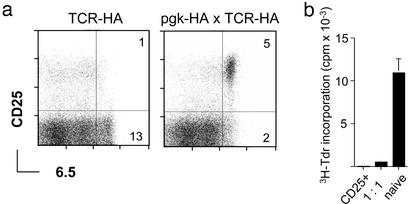
High Frequency of Antigen-Specific CD25+CD4+ Regulatory T Cells in pgk-HA × TCR-HA Mice. Mice expressing influenza-HA under the control of the ubiquitous pgk promoter, in the following referred to as pgk-HA mice, were crossed to mice expressing a transgenic TCR (TCR-HA) specific for peptide 111–119 of HA (21). Among lymph node cells of pgk-HA × TCR-HA mice, the fraction of CD4+ TCR-HA+ cells was reduced by a factor of 2 as assessed by staining with the anti-clonotypic antibody 6.5, and a distinct population of 6.5+CD25+ cells that could not be detected in TCR single transgenics was observed (Fig. 1a). Sorted 6.5+CD25+ cells from thymus (Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org) or periphery (Fig. 1b) of double-transgenic mice were anergic in vitro and suppressed the proliferation of naive 6.5+CD4+ T cells in standard coculture assays. The addition of IL-2 enabled proliferation of 6.5+CD25+ cells from both thymus and periphery (data not shown). Thymus transplantation revealed that expression of HA by radioresistant thymic epithelium was sufficient to mediate the selection of 6.5+CD25+ cells (data not shown). Thus, by these criteria, the HA-specific CD25+ cells were equivalent to polyclonal CD25+ suppressor T cells from normal mice.
Fig. 1.
High frequency of HA-specific regulatory CD4+CD25+ T cells in pgk-HA × TCR-HA mice. (a) Expression of the transgenic TCR (mAb 6.5) versus CD25 on gated CD4 T cells from lymph nodes of TCR-HA single-transgenic versus pgk-HA × TCR-HA double-transgenic mice. Numbers in the dot plots indicate the percentage of gated cells within the respective quadrants. (b) CD25+ CD4 T cells from pgk-HA × TCR-HA mice were anergic and suppressed the proliferation of naive CD4 T cells from TCR-HA rag–/– mice in vitro. Sorted CD4+CD25+6.5+ cells from pgk-HA × TCR-HA mice and naive CD4+6.5+ T cells from TCR-HA rag–/– mice were incubated either alone or together (ratio 1:1) in the presence of BALB/c splenocytes and HA-peptide for 90 h. Proliferation was measured as incorporation of [3H]thymidine (3H-Tdr) added for the last 20 h.
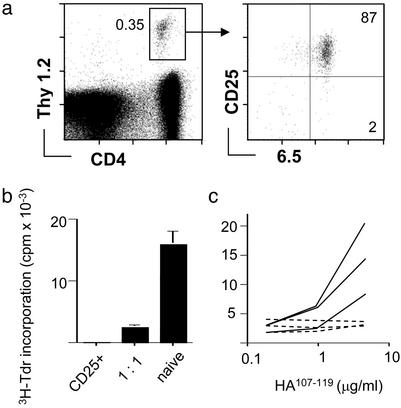
Suppression of Anti-HA Responses in Vivo After Transfer of CD25+ Regulatory T Cells. 6.5+CD25+ CD4 T cells from pgk-HA × TCR-HA Thy1.2+ mice were transferred into Thy1.1+ hosts. The frequency of donor-derived cells among host cells was similar when equal numbers of either CD25+ cells from pgk-HA × TCR-HA mice or naive 6.5+CD25– cells from TCR-HA rag–/– mice were transferred. Fourteen days after transfer, most (>85%) donor-derived cells still expressed the CD25 marker, and their number appeared stable within this time frame (data not shown). Reisolated cells were still anergic and suppressed naive 6.5+ CD4 T cells in vitro (Fig. 2 a and b), indicating that they represented a lineage rather than antigen-dependent effector cells.
Fig. 2.
Transferred CD4+CD25+6.5+ T cells retain their phenotypic and in vitro regulatory properties in the absence of antigen and suppress endogenous anti-HA-specific T cells after immunization. (a) CD4+CD25+6.5+ T cells (4 × 106) from Thy1.2+/+ pgk-HA × TCR-HA mice were transferred into BALB/c Thy1.1+/+ mice. Six days after transfer, the recipients were killed. Peripheral lymph nodes and spleen were pooled and stained for CD4, Thy1.2, CD25, and 6.5. The frequency of donor-derived cells among CD4 T cells is shown together with the sorting gate used for reisolation of cells. (Right) Purity of reisolated cells. (b) Reisolated Thy1.2+/+ cells 6 days after transfer were tested for their proliferative response after stimulation with HA-peptide and their suppressive potential when cocultured with naive cells as described for Fig. 1. (c) Recipients of 3 × 105 CD4+CD25+6.5+ T cells (dashed lines) or untreated BALB/c mice (solid lines) were immunized with HA-peptide (100 μg) in IFA. Eight days later, draining lymph node cells were harvested and stimulated in vitro with titrated amounts of HA-peptide for 90 h. Incorporation of [3H]thymidine (3H-Tdr) within the last 20 h was measured. The graph shows the data for three immunized mice of each group representative for three independent experiments.
We next asked whether transferred 6.5+CD25+ cells could inhibit the response of endogenous HA-specific T cells. Recipients of 3 × 105 6.5+CD25+ cells, corresponding to a frequency of approximately 1 in 3,000 CD4+ T cells, or noninjected control mice were immunized with peptide HA107–119 in IFA. Although draining lymph node cells from control animals proliferated vigorously when restimulated in vitro, no proliferation was detected with cells from recipients of regulatory T cells (Fig. 2c).
The colon carcinoma cell line CT26-HA, which stably expresses HA (20), was used to test whether transferred 6.5+CD25+ cells influenced the growth of an HA-expressing tumor by suppressing the antitumor response. Subcutaneous inoculation of normal BALB/c mice with CT26-HA leads to the induction of anti-HA CD4 and CD8 T cell responses (20). Transfer of 6.5+CD25+ cells allowed for accelerated growth of the tumor (Fig. 8, which is published as supporting information on the PNAS web site). Furthermore, although in tumor-bearing normal mice or mice that had received naive CD25– 6.5+ CD4 T cells a strong ex vivo proliferative response was observed, such a recall response was absent with cells from recipients of CD25+ 6.5+ T cells irrespective of whether naive cells had been cotransferred (Fig. 8). Thus, transferred CD25+ cells had potent suppressive activity in vivo.
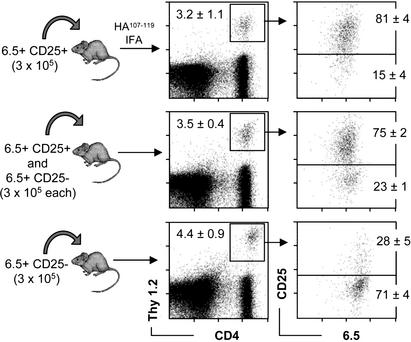
Antigen-Driven Expansion of CD25+CD4+ T Cells in Vivo. We next aimed to visualize and compare the behavior of antigen-specific regulatory and naive T cells after immunization. A control group received 3 × 105 6.5+CD25– cells only and was immunized the following day. On day 8 after immunization, ≈4% of draining lymph node CD4 T cells were donor-derived, and the majority of these cells was CD25– (Fig. 3). In nondraining lymphoid compartments, <0.05% of CD4 T cells were Thy1.2+, similar to the values observed in nonimmunized recipients (data not shown). Thus, HA-specific “naive” T cells expectedly increased ≈100-fold in the antigen-exposed lymph node. A second group of animals received 3 × 105 6.5+CD25+ cells and was immunized and analyzed as described above. Surprisingly, we found a distribution of donor-derived cells in these animals very similar to that observed in recipients of naive cells. Approximately 3% of CD4 T cells in the draining lymph nodes were donor-derived, whereas in distant lymphoid compartments, <0.05% of CD4 T cells were Thy1.2+ (data not shown). Contrary to recipients of naive cells, these cells mostly expressed the CD25 marker (Fig. 3). A third group received both 6.5+CD25+ and naive 6.5+CD25– cells. Eight days after immunization the proportion of donor-derived cells among CD4 T cells in the draining lymph node was again ≈3%, and the majority of these cells expressed high levels of CD25, as was observed in mice that had received 6.5+CD25+ cells only (Fig. 3).
Fig. 3.
Accumulation of CD4+CD25+6.5+ T cells or naive CD4+CD25–6.5+ T cells in the draining lymph nodes after immunization. (a) Thy1.1+/+ recipients of CD4+CD25+6.5+ T cells, CD4+CD25–6.5+ T cells, or both types of cells were immunized with HA-peptide as described for Fig. 2. Draining lymph node cells were harvested on day 8 after immunization and stained for CD4, Thy1.2, CD25, and 6.5. (Left) Numbers in the dot plots indicate the percentage of donor-derived cells among CD4+ T cells (mean of four animals). (Right) The dot plots show the expression of the transgenic TCR (6.5) versus CD25 on gated CD4+Thy1.2+ cells. Numbers within the dot plots indicate the percentage of gated cells in the respective quadrant (mean of four animals).
The almost identical frequencies of donor-derived cells in the draining lymph nodes of recipients of either regulatory or naive T cells implied a similar homing/expansion pattern of both cell types, a surprising finding considering the absence of an in vitro recall response in recipients of CD25+ T cells (see Fig. 2c). To confirm this apparent discrepancy, we restimulated draining lymph node cells from the three groups with HA-peptide in vitro. Cells from recipients of naive cells showed a very strong response, whereas neither recipients of 6.5+CD25+ cells alone nor recipients of mixed populations displayed any antigen-specific in vitro proliferation (data not shown).
To address whether antigen-driven expansion in vivo altered the properties of 6.5+CD25+ cells in vitro, we sorted these cells from draining lymph nodes of immunized recipients and compared them with 6.5+CD25+ cells taken directly from pgk-HA × TCR-HA mice. When stimulated in vitro, both populations were completely anergic with respect to proliferation, and provision of IL-2 restored some proliferation in both types of cultures, yet less efficiently with “expanded regulators” (data not shown). When titrated in the standard coculture assay, expanded regulators were ≈4-fold more efficient suppressors than “nonexpanded regulators” (Fig. 9, which is published as supporting information on the PNAS web site).
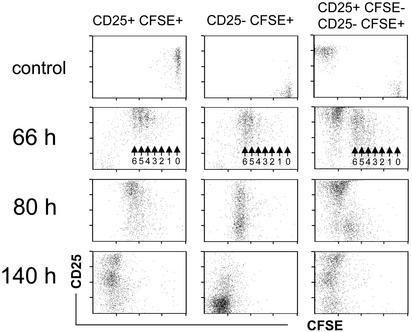
CD25+ Regulatory T Cells Do Not Affect the Initial Expansion of Naive Cells After Immunization. To address whether the accumulation of 6.5+CD25+ cells in the draining lymph nodes was a result of proliferation rather than homing, we labeled Thy1.2 6.5+CD25+ cells with CFSE before adoptive transfer. A control group received CFSE-labeled naive 6.5+CD25– cells. Animals were immunized as described before, and the phenotype of donor-derived cells in the draining lymph nodes was followed in a kinetic fashion by gating on CD4+Thy1.2+ cells. As early as 66 h after immunization, the majority of donor-derived cells in the draining lymph nodes of both groups of animals had cycled more than four times (Fig. 4). The progeny of 6.5+CD25+ cells had further up-regulated CD25, and CD25 was also expressed by the progeny of naive cells at this point in time, as expected. Eighty hours after immunization, cells in both groups of animals had undergone further divisions, the exact numbers of division no longer being discernable. The progeny of 6.5+CD25+ as well as of 6.5+CD25– cells appeared to divide in a “synchronized” wave (Fig. 4), probably indicating that the immunization protocol induced a transient window of productive antigen presentation in the draining lymph node. Donor-derived cells in the draining lymph nodes of both types of recipients had lost their CFSE-label 140 h after immunization. The progeny of 6.5+CD25+ cells contained cells that had returned to initial levels of CD25 expression, whereas others maintained elevated levels. By contrast, the progeny of naive CD25– cells had mostly lost expression of CD25. Both findings were in accord with our previous observation (compare with Fig. 3).
Fig. 4.
Proliferation of adoptively transferred CD4+CD25+6.5+ T cells or CD4+CD25–6.5+ T cells in the draining lymph nodes after immunization. CFSE-labeled CD4+CD25+6.5+ T cells from pgk-HA × TCR-HA mice (3 × 105) (Left) or CFSE-labeled naive CD4+CD25–6.5+ T cells from TCR-HA rag–/– mice (3 × 105) were transferred into BALB/c Thy1.1 mice. Two days later, recipients were immunized with 100 μg of HA-peptide in IFA. Controls were immunized with IFA without peptide. Mice were killed at the indicated time points after immunization, and draining lymph node cells were harvested and stained for CD4, Thy1.2, and CD25. The dot plots show the expression of CD25 versus CFSE fluorescence intensity on gated donor-derived cells (CD4+Thy1.2+). Numbered arrows within the dot plots (66 h) indicate the number of divisions of CFSE-labeled cells. Note that all dot plots (except controls, where ≈200 events are shown) show 800–1,000 CD4+Thy1.2+ events and thus do not represent the frequency of these cells among host CD4 T cells. (Right) Representative analysis of a cotransfer of 3 × 105 CFSE-labeled naive CD4+CD25–6.5+ T cells from TCR-HA rag–/– mice and 3 × 105 unlabeled CD4+CD25+6.5+ T cells from pgk-HA × TCR-HA mice into BALB/c Thy1.1 recipients.
We next performed cotransfer experiments with regulatory and CFSE-labeled naive T cells (Fig. 4 Right). No major difference in the early (66-h) pattern of division was observed as compared with transfer of naive cells alone. Later, when the CFSE label was lost, it was again obvious that the progeny of regulatory cells, as identified by the CD25+ or CD25++ phenotype, represented the predominant donor-derived cell type in the draining lymph node (Fig. 4, compare with Fig. 3).
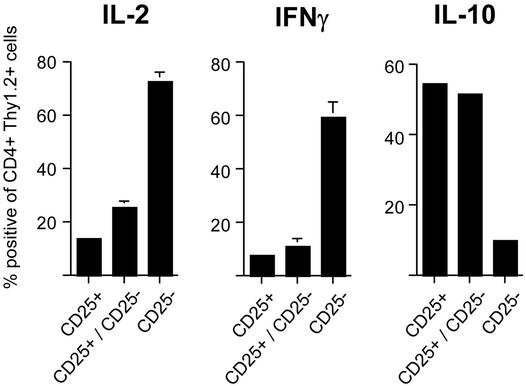
Inverse Cytokine Profile of Expanded Regulators and Activated Naive CD4 T Cells. The production of cytokines by HA-specific CD4 T cells under the different conditions was tested. Three groups of Thy1.1 animals were injected with Thy1.2+6.5+CD25+ T cells, Thy1.2+6.5+CD25– naive T cells, or both, and animals were immunized as described before. Eight days later, cytokine production by donor-derived cells was determined after brief restimulation in vitro. In recipients of 6.5+CD25– naive cells only, a large fraction (>60%) of Thy1.2+ cells produced IL-2 and IFN-γ, whereas among the progeny of 6.5+CD25+ cells (expanded regulators) only few cells produced either cytokine (Fig. 5 and Fig. 10, which is published as supporting information on the PNAS web site). By contrast, a high proportion of cells produced IL-10. Among cells derived from mice that had received equal numbers of regulatory and naive HA-specific CD4 T cells, the cytokine profile closely resembled that seen in recipients of regulatory T cells alone, again suggesting that the regulatory T cells dominated the response after the 8-day period.
Fig. 5.
Cytokine production of transferred CD4+CD25+6.5+ T cells or CD4+CD25–6.5+ T cells. (a) Transferred into Thy1.2 recipients were 3 × 105 CD4+CD25+6.5+ T cells, CD4+CD25–6.5+ T cells, or both. Mice were immunized with HA-peptide in IFA, and draining lymph node cells were harvested 8 days after immunization. Cells were restimulated in vitro with phorbol 12-myristate 13-acetate/ionomycin for6hinthe presence of brefeldin A before surface staining for CD4 and Thy1.2, fixation, and intracellular staining for the indicated cytokine. The frequencies of cytokine-positive cells among gated CD4+Thy1.2+ draining lymph node cells of the indicated groups of animals are shown (for original data see Fig. 10).
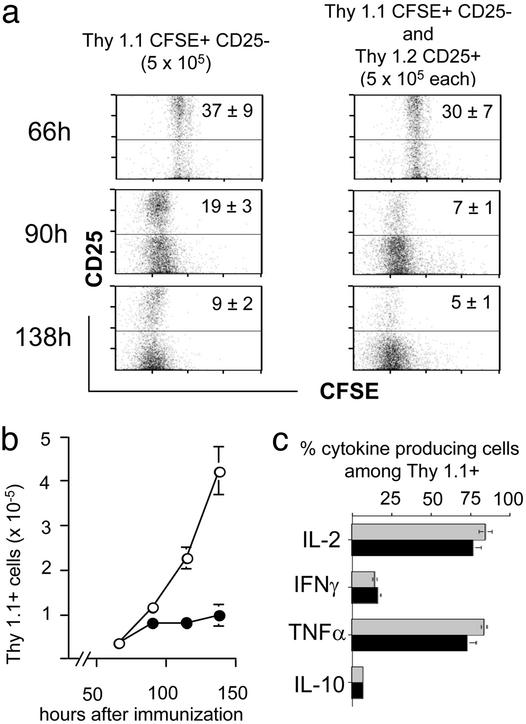
Regulatory CD25+ T Cells Suppress the Late Expansion of Naive CD4 T Cells. To visualize more conclusively the influence of regulatory T cells on naive cells at different phases, we adoptively transferred CFSE-labeled Thy1.1+6.5+CD25– T cells into Thy1.2 recipients that had either received Thy1.2+6.5+CD25+ regulatory T cells or not. Gating on Thy1.1+ CD4 T cells then was used to analyze the expansion of the naive T cells after immunization. The initial recruitment of cells into the response, their proliferation rate, and the early expansion up to 90 h after the immunization were almost identical in both groups (Fig. 6 a and b, compare with Fig. 4). At 90 h after immunization, however, a plateau in the number of Thy1.1+ cells among CD4 T cells was reached in animals that had received regulatory T cells (Fig. 6b). In contrast, in the absence of regulatory T cells the progeny of naive cells further expanded. Notably, even in the presence of regulatory T cells the majority of Thy1.1+ cells continued to cycle between 90 and 138 h (Fig. 6a), although their number did not increase any further. Also, in the presence of regulatory T cells, a small yet distinct fraction of cells appeared to fall behind the bulk of dividing cells, discernable as a “smear” into higher CFSE intensities. Interestingly, at all time points analyzed, the fraction of cells among the progeny of naive cells expressing CD25 was lower in the presence of regulatory T cells (Fig. 6a).
Fig. 6.
Expansion, CD25 expression, and cytokine production after immunization of adoptively transferred naive CD4+CD25–6.5+ T cells in the presence or absence of CD4+CD25+6.5+ regulatory T cells. CFSE-labeled naive CD4+CD25–6.5+ T cells (5 × 105) sorted from Thy1.1+ TCR-HA mice (rag+/+) were adoptively transferred into BALB/c Thy1.2 recipients (Left) or BALB/c Thy1.2 recipients that had in addition received an equal number of CD4+CD25+6.5+ T cells from Thy1.2+ pgk-HA × TCR-HA mice (Right). Mice were immunized as described before, and draining lymph node cells were harvested at the indicated time points after immunization. (a) Expression of CD25 versus CFSE fluorescence intensity on gated (CD4+Thy1.1+) progeny of naive CD4+CD25–6.5+ T cells. Numbers within the upper quadrants indicate the frequency of CD25+ cells among CD4+Thy1.1+ cells (mean of four per group). Note the “smearing” into lower-division numbers in the presence of regulatory T cells (Right). (b) Absolute number in the draining lymph nodes of the progeny of naive CD4+CD25–6.5+ T cells after immunization in the presence (filled circles) or absence (open circles) of CD4+CD25+6.5+ regulatory T cells (mean of four per group). The number at 0 h (i.e., without immunization) was <0.1 × 105. (c) Draining lymph node cells were harvested on day 8 after immunization, stimulated in vitro with phorbol 12-myristate 13-acetate/ionomycin for 6 h in the presence of brefeldin A, and stained for CD4, Thy1.1, and the respective cytokine as indicated. The frequency of cytokine-producing cells among gated Thy1.1+CD4+ T cells is shown. Gray bars, recipients of naive CD4+CD25–6.5+ T cells alone; black bars, recipients of naive CD4+CD25–6.5+ T cells plus CD4+CD25+6.5+ regulatory T cells (mean of four per group). TNFα, tumor necrosis factor α.
We determined the cytokine production 8 days after immunization by Thy1.1+ CD4 T cells from the two groups of mice, i.e., in either normally expanded or “suppressed” progeny of naive CD25– cells (Fig. 6c). The fraction of producers of IL-2, IL-10, IFN-γ, or tumor necrosis factor α was not affected significantly by suppression of their expansion through the presence of regulatory T cells, which indicated that indeed dominant expansion of regulatory T cells rather than “immune deviation” was the explanation for the observations depicted in Fig. 5.
Discussion
Most of our current understanding of the biology of suppressor T cells is derived from lymphopenic in vivo models or has been derived from the standard in vitro assay. Both types of systems have inherent limitations because of the abnormal behavior of lymphocytes in a lymphopenic environment (sometimes incorrectly referred to as homeostatic proliferation) and the questionable significance of in vitro observations in general. Our aim was to establish a model in which the behavior of antigen-specific CD4+CD25+ regulatory T cells and their influence on naive T cells after antigenic stimulation could be visualized in vivo in the context of an unperturbed immune system.
Within the time frame analyzed, the phenotype and function of 6.5+CD25+CD4+ T cells was stable after transfer into an antigen-free host. After immunization, 6.5+CD4+CD25+ T cells, despite their anergy in vitro, accumulated in the draining lymph nodes very much like naive T cells. CFSE labeling demonstrated that this accumulation was due to proliferation rather than preferential retention at the antigen-exposed site. These data are different from observations made with CD25+ and CD25– regulatory T cells from mice that express high levels of antigen on hematopoietic cells (6), where persistent exposure to antigen may render regulatory T cells incapable of in vivo proliferation.
When reisolated, expanded regulators were anergic and exhibited an enhanced suppressive capacity in vitro, reminiscent of data showing that polyclonal CD4+CD25+ T cells after IL-2-mediated expansion in vitro displayed an augmented suppressive potency (22, 23). Along the same line, it had been shown that, after transfer into IL-2 receptor β–/– mice (16) or rag–/– mice (17), polyclonal CD4+CD25+ T cells expand in vivo and retain their in vitro suppressive properties. In the latter studies, however, it was not clear whether expansion was driven by homeostatic rather than antigen-specific mechanisms.
Can our observations in vivo be reconciled with current hypotheses on the action of regulatory T cells based on in vitro data? Generally accepted hallmarks of inhibition by CD4+CD25+ T cells in vitro are that (i) these cells are anergic, (ii) anergy and suppression can be broken by the addition of high amounts of exogenous IL-2, and (iii) inhibition is contact-dependent (reviewed in refs. 3 and 8). We consider it likely that a yet-to-be-defined milieu in vivo, only one component of which may or may not be IL-2, would allow for the antigen-driven proliferation of CD4+CD25+ T cells. It is not clear at present whether similarly high quantities of IL-2 as used in vitro are available in particular microenvironments in vivo. The cotransfer data presented here indicate a somewhat unexpected dynamics of suppression in vivo in that the regulators “outgrew” the progeny of CD25– cells, which demonstrates that proliferation and suppressive function of regulatory T cells are not mutually exclusive in vivo.
It has been suggested that immune regulation may act at least in part through competition for growth factors and space (24, 25). CD25+ regulatory T cells, which produce IL-2 only poorly or not at all, may use IL-2 and other growth factors produced by neighboring cells for their own expansion and thus deplete these factors in the local microenvironment. We found that during their expansion, 6.5+CD4+CD25+ T cells further up-regulated CD25, which should allow for a very efficient consumption of IL-2. By contrast, the presence of regulatory T cells negatively affected CD25 expression on progeny of naive T cells. This reduction in CD25 expression may indicate IL-2 “starvation,” because IL-2 regulates its high-affinity receptor via a feedback mechanism (26). We are aware that a model that is based solely on IL-2 competition would postulate a role different from the role of IL-2 in T cell apoptosis and down-modulation of T cell responses (27–29) and could not explain the function of CD25 negative regulatory T cells (6, 30, 31).
A contribution of competition for growth factors to the suppressive action of CD25+ regulatory T cells in vivo is not necessarily contradictory to in vitro findings that have been interpreted to indicate a “contact-dependent” mechanism. Thus, regulatory T cells may much more efficiently consume such factors, perhaps but not necessarily produced by the responder itself, when they are in the close vicinity of cells to be suppressed. Some quantitative considerations illustrate that the capacity of suppressor T cells to expand in vivo indeed may be an essential feature of immune regulation: Although the “standard” in vitro assay artificially generates frequencies of regulators and responders of at least 1 in 10, it seems reasonable to assume that the frequency of regulatory CD4+CD25+ T cells of a given specificity within a normal T cell repertoire is significantly lower. We consider it highly unlikely that under these circumstances a mechanism of suppression that depends on contact or close proximity can be immediately effective for instance when a self-antigen becomes exposed due to tissue damage. This scenario would explain why, under our experimental conditions (i.e., a frequency of 1 in 3000), naive cells were initially recruited into the early response irrespective of the presence of regulators of identical antigen specificity. The suppressive effect appeared to kick in once the regulatory population had reached a critical size, which may allow for a certain proximity/close contact of regulators and responders. Alternatively or in addition, “priming” of regulatory T cells may be necessary to unfold their full suppressive potential. The enhanced suppressive potency in vitro of expanded regulators argues in favor of such a scenario. On the side of suppressed cells, premature transition into activation-induced cell death may be a contributing factor, in particular in view of our observation that the number of the progeny of naive cells reached an early plateau in the presence of suppressors although the majority of cells continued to cycle. This finding would be consistent with the notion that some suppressed cells may die rather quickly. However, preliminary analyses did not reveal an increase in annexin V-positive cells among the progeny of naive T cells in the presence of regulatory T cells (data not shown).
Thus far, our data do not provide evidence for an “infectious” mechanism of immune regulation in vivo by CD25+ regulatory T cells in that they may not only limit the expansion of naive cells but also may influence the effector functions (cytokine production) of suppressed T cells beyond the phase of acute suppression, as has been described recently in vitro for human CD4+CD25+ T cells (32). Furthermore, the role of IL-10 in the model presented here needs to be addressed further. IL-10 has been shown to be dispensable for the suppressive effect of CD4+CD25+ T cells in vitro (9, 10, 23) and in specific models in vivo (33), however, certain models of “lymphopenia-driven” autoimmunity suggest an important role for this suppressive cytokine (12, 15, 34).
Taken together, the data presented here visualize a more dynamic in vivo behavior of antigen-specific CD4+CD25+ regulatory T cells than previously assumed in that these cells readily expand after antigenic stimulation. It is of interest to note that Gavin et al. (17) recently reported contradicting observations in another TCR transgenic model after immunization with antigen emulsified in complete Freund's adjuvant. It remains open in how far factors such as prior antigenic experience, antigen dose, TCR affinity, or the number of transferred cells may account for such apparent discrepancies. With respect to the mode of antigen delivery, however, we should stress that CD25+ regulatory T cells proliferated similar to naive cells irrespective of whether mice were immunized with peptide in IFA, with lipopolysaccharide-stimulated peptide-pulsed dendritic cells, or injected intravenously with peptide in PBS (our unpublished results). Based on our observations we propose that antigen-driven expansion of regulatory T cells is an essential feature of antigen-specific immune regulation because it establishes frequencies of regulatory T cells in an antigen-exposed microenvironment that may be critical for efficient suppression.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant 1RO1AI53102 (to H.v.B.), Department of Defense Breast Cancer Research Program Grant DAMD 170210361 and National Institutes of Health Grant F33 (to K.K.), and the Irvington Institute of Immunological Research (L.K.).
Abbreviations: TCR, T cell receptor; pgk, phosphoglycerate kinase; HA, hemagglutinin; IFA, incomplete Freund's adjuvant; CFSE, 5,6-carboxyfluorescein diacetate-succinimidyl ester.
References
- 1.Modigliani, Y., Bandeira, A. & Coutinho, A. (1996) Immunol. Rev. 149, 155–120. [DOI] [PubMed] [Google Scholar]
- 2.Le Douarin, N., Corbel, C., Bandeira, A., Thomas-Vaslin, V., Modigliani, Y., Coutinho, A. & Salaun, J. (1996) Immunol. Rev. 149, 35–53. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi, S., Sakaguchi, N., Shimizu, J., Yamazaki, S., Sakihama, T., Itoh, M., Kuniyasu, Y., Nomura, T., Toda, M. & Takahashi, T. (2001) Immunol. Rev. 182, 18–32. [DOI] [PubMed] [Google Scholar]
- 4.Jordan, M. S., Riley, M. P., von Boehmer, H. & Caton, A. J. (2000) Eur. J. Immunol. 30, 136–144. [DOI] [PubMed] [Google Scholar]
- 5.Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2, 301–306. [DOI] [PubMed] [Google Scholar]
- 6.Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. (2002) Nat. Immunol. 3, 756–763. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151–1164. [PubMed] [Google Scholar]
- 8.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389–400. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10, 1969–1980. [DOI] [PubMed] [Google Scholar]
- 10.Thornton, A. M. & Shevach, E. M. (1998) J. Exp. Med. 188, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccirillo, C. A., Letterio, J. J., Thornton, A. M., McHugh, R. S., Mamura, M., Mizuhara, H. & Shevach, E. M. (2002) J. Exp. Med. 196, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 190, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon, B. & Mason, D. (1999) J. Exp. Med. 189, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suri-Payer, E. & Cantor, H. (2001) J. Autoimmun. 16, 115–123. [DOI] [PubMed] [Google Scholar]
- 15.Annacker, O., Pimenta-Araujo, R., Burlen-Defranoux, O., Barbosa, T. C., Cumano, A. & Bandeira, A. (2001) J. Immunol. 166, 3008–3018. [DOI] [PubMed] [Google Scholar]
- 16.Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. (2002) Immunity 17, 167–178. [DOI] [PubMed] [Google Scholar]
- 17.Gavin, M. A., Clarke, S. R., Negrou, E., Gallegos, A. & Rudensky, A. (2002) Nat. Immunol. 3, 33–41. [DOI] [PubMed] [Google Scholar]
- 18.Saam, J. R. & Gordon, J. I. (1999) J. Biol. Chem. 274, 38071–38082. [DOI] [PubMed] [Google Scholar]
- 19.Wagner, K. U., Wall, R. J., St-Onge, L., Gruss, P., Wynshaw-Boris, A., Garrett, L., Li, M., Furth, P. A. & Hennighausen, L. (1997) Nucleic Acids Res. 25, 4323–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein, L., Trautman, L., Psarras, S., Schnell, S., Siermann, A., Liblau, R., von Boehmer, H. & Khazaie, K. (2003) Eur. J. Immunol. 33, 806–814. [DOI] [PubMed] [Google Scholar]
- 21.Kirberg, J., Baron, A., Jakob, S., Rolink, A., Karjalainen, K. & von Boehmer, H. (1994) J. Exp. Med. 180, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton, A. M. & Shevach, E. M. (2000) J. Immunol. 164, 183–190. [DOI] [PubMed] [Google Scholar]
- 23.Levings, M. K., Sangregorio, R. & Roncarolo, M. G. (2001) J. Exp. Med. 193, 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunther, J., Haas, W. & von Boehmer, H. (1982) Eur. J. Immunol. 12, 247–249. [DOI] [PubMed] [Google Scholar]
- 25.Barthlott, T., Kassiotis, G. & Stockinger, B. (2003) J. Exp. Med. 197, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, K. A. & Cantrell, D. A. (1985) Proc. Natl. Acad. Sci. USA 82, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenardo, M. J. (1991) Nature 353, 858–861. [DOI] [PubMed] [Google Scholar]
- 28.Kneitz, B., Herrmann, T., Yonehara, S. & Schimpl, A. (1995) Eur. J. Immunol. 25, 2572–2577. [DOI] [PubMed] [Google Scholar]
- 29.Li, X. C., Demirci, G., Ferrari-Lacraz, S., Groves, C., Coyle, A., Malek, T. R. & Strom, T. B. (2001) Nat. Med. 7, 114–118. [DOI] [PubMed] [Google Scholar]
- 30.Olivares-Villagomez, D., Wensky, A. K., Wang, Y. & Lafaille, J. J. (2000) J. Immunol. 164, 5499–5507. [DOI] [PubMed] [Google Scholar]
- 31.Stephens, L. A. & Mason, D. (2000) J. Immunol. 165, 3105–3110. [DOI] [PubMed] [Google Scholar]
- 32.Dieckmann, D., Bruett, C. H., Ploettner, H., Lutz, M. B. & Schuler, G. (2002) J. Exp. Med. 196, 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jooss, K., Gjata, B., Danos, O., von Boehmer, H. & Sarukhan, A. (2001) Proc. Natl. Acad. Sci. USA 98, 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontoux, C., Banz, A. & Papiernik, M. (2002) Int. Immunol. 14, 233–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.