Abstract
We show that 18O evaporative enrichment of bulk leaf water in grass species can be significantly more enriched than predicted by the Craig–Gordon model, with C4 grasses considerably more enriched than C3 grasses. Our results suggest that the unanticipated 18O leaf water enrichment of grasses is attributable to the progressive evaporative enrichment along parallel veins (a function of both leaf length and interveinal distance), a pattern that does not occur in Dicotyledonous species. We propose that the differential 18O enrichment of grasses will result in distinct C18O16O biospheric signals from grassland and forest ecosystems, allowing for further partitioning of terrestrial carbon fluxes.
The evaporative isotopic enrichment of 18O in surface waters (such as lakes) was described by Craig and Gordon (1) more than three decades ago and was later applied to leaves, which also undergo an isotopic enrichment during transpiration (2–5). Application of the Craig–Gordon model to leaves from trees and herbs has been shown experimentally to be robust over a wide range of environmental conditions (4, 6–7). Capitalizing on this established relationship, Farquhar and colleagues (8) showed that the equilibration of 18O between CO2 and leaf water could be used to partition the gross fluxes of CO2 from vegetation on a global basis.
Previous studies showed that there were no differences in bulk leaf water 18O enrichment of Dicotyledonous plants possessing either the C3 or C4 photosynthetic pathways and that leaf water behaved similarly to predictions of the Craig–Gordon model (4, 6). Based on these consistent results, global modeling efforts have used the Craig–Gordon model for estimates of leaf water δ18O [δ18Owl, Standard Mean Ocean Water (SMOW) scale throughout] values that are needed to calculate the C18O16O retrodiffusing from terrestrial ecosystems to the atmosphere (8–11). However, the Craig–Gordon model of evaporative leaf-water enrichment has remained untested on grasses and other Monocotyledonous species. A fundamental difference between monocots and dicots that hinted that δ18Owl might be different is the veinal structure of leaves, with monocot leaves having parallel veins whereas dicot leaves primarily have a reticulate veinal structure. We hypothesized that grass leaves will exhibit a leaf-water 18O evaporative enrichment pattern that is not consistent with the commonly used single-water-source version of the Craig–Gordon model, because the nature of water movement along parallel veins in a grass leaf is more analogous to movement of water through a sequence of pools in series.
We investigated possible 18O differences in leaf water enrichment with a variety of C3 and C4 grasses, because grasses with different photosynthetic pathway are known to have different interveinal differences (12, 13). Our hypotheses were (i) leaf water 18O enrichment of grasses should be greater than observed in dicots and (ii) leaf water 18O enrichment of C4 grasses (narrower interveinal distances) should be greater than leaf water 18O enrichment of C3 grasses (wider interveinal distances).
Methods
Plant Material and Growth Conditions.
In our pilot experiment, we grew Lolium multiflorum (C3 grass), Muhlenbergia wrightii (C4 grass), and Helianthus annuus (C3 dicot) (n = 6 for each species) from seed under identical environmental conditions with an average midday relative humidity of 40%. In our survey experiment we used five C3 grasses and five C4 grasses (species listed in Table 1). For the survey experiment, n = 3 for each species and average midday relative humidity was 35%. The growth conditions and experimental protocol were identical for both experiments except where specified below.
Table 1.
Observed and predicted values of δ18Owl for bulk leaf water
| Plant | Observed δ18Owl, ‰ | Craig–Gordon-predicted δ18Owl, ‰ |
|---|---|---|
| Agropyron desertorum (C3) | 7.7 ± 1.6 | 7.8 |
| Agrostis stolonifera (C3) | 9.2 ± 2.8 | 8.1 |
| Alopecurus pratensis (C3) | 8.3 ± 3.6 | 7.6 |
| Bromus inermis (C3) | 8.2 ± 1.7 | 7.6 |
| Lolium multiflorum (C3) | 8.0 ± 1.2 | 7.8 |
| Andropogon gerardii (C4) | 15.1 ± 3.7 | 8.0 |
| Bouteloua curtipendula (C4) | 12.7 ± 0.3 | 7.6 |
| Eragrostis curvula (C4) | 12.4 ± 4.2 | 8.0 |
| Leptochloa dubia (C4) | 14.2 ± 2.8 | 7.8 |
| Muhlenbergia wrightii (C4) | 9.0 ± 2.6 | 8.1 |
| C3 mean | 8.3 ± 0.5a | 7.8 |
| C4 mean | 12.6 ± 3.2b | 7.8 |
Each value represents a mean of three plants and the associated standard deviation. C3 and C4 means are significantly different at P < 0.01. Predicted δ18Owl values (SMOW scale) are based on a single-water-source Craig–Gordon model. Sample conditions were: δ18Oatm w vapor = −27‰; relative humidity = 35%; air temperature = 25°C; leaf temperatures varied but were not significantly different from the mean of 27°C. Sample conditions for pilot experiment (data in text): δ18Oatm w vapor = −24‰; δ18Osource = −15.6‰; relative humidity = 40%; air temperature = 35°C; leaf temperatures were C3 dicot = 34°C, C3 grass = 35°C, and C4 grass = 37°C.
Plants for all experiments were grown in the greenhouse in a manner that kept the root systems completely submerged in an isotopically constant water source, whereas the aerial portions of the plants experienced greenhouse conditions. Plants were grown from germination in 1-liter pots in a sterilized soil/perlite/vermiculite (1:1:1) mixture, and the pots were placed in 190-liter open-top plastic chambers (stock tanks; Rubbermaid, Wooster, OH). To avoid evaporative enrichment and to keep source water constant, a flow-through system was developed in which the chambers were fitted with water entry and exit ports. Tap water was gravity fed to the chambers by a system of hoses from a 658-liter buffer tank. Closed-cell foam was placed at water level to further assure that no evaporative enrichment of source water occurred. The water level within the chambers was kept constant and at a level that submerged all but the top 1 cm of soil in each pot. Source water was oxygenated by two aquarium pumps, with the air flow separated to ten diffusers distributed evenly throughout the bottom of the chambers. A nutrient solution was supplied to the plants once a week by addition to the large buffer tank. Oscillating fans were placed next to the chambers to mitigate the propagation of leaf boundary layer differences between species. Wet bulb/dry bulb thermocouples were situated at plant height to measure air temperature and relative humidity throughout the experiments. A light sensor (Li-Cor, Lincoln, NE) was placed at plant height to measure irradiance, which averaged approximately 500 μmol⋅m−2⋅s−1 in the pilot experiment and 1,000 μmol⋅m−2⋅s−1 in the survey experiment, both for a 16-h photoperiod. Thermocouples and light sensor readings were recorded at 15-min intervals with a datalogger (model 21X;Campbell Scientific, Logan, UT).
Sample Collection.
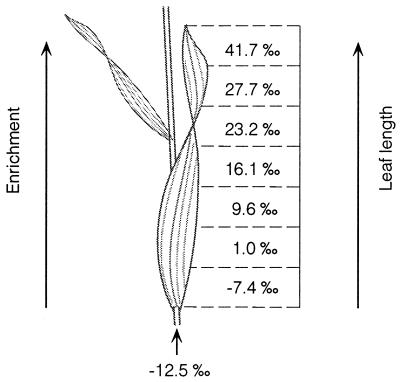
Immediately before sampling leaf material, water samples of inlet and outlet water were taken to ensure no evaporative enrichment was occurring within the chambers. During this time, water vapor in air was collected by drawing air through an ethanol dry ice trap. Leaf temperature was measured before sampling leaf material. In the pilot experiment, samples were taken for leaf water δ18O once per week for a 3-week period. In the survey experiment, leaf water was sampled at one time period. With the exception of Fig. 2, all leaf water δ18O values represent the entire leaf blade from ligule to tip.
Figure 2.
Representative distribution of bulk leaf water δ18Owl values of grass leaf sections for greenhouse-grown plants. For this specific grass blade (Miscanthus sinensis), the stem source water δ18O value was −12.5‰; all other sampling parameters were the same as in Table 1. All grass species mentioned in this study were sampled in the same manner and showed similar results.
Anatomical Measurements.
Immediately before sampling for leaf water in the survey experiments, leaf blade length was measured. Using a second set of leaves, measurements of interveinal distance were made by excising the whole, fully expanded leaves of three plants per species and removing the middle, widest portion of the leaf. The middle portions were free-hand sectioned into 1-mm sections and placed in Petri dishes filled with a H2O/Clorox solution (80:20) for ≈1 h until fully cleared. Sections were mounted in glycerol, and interveinal distance was measured with a light microscope coupled with a drawing tube and bit pad (Donsanto Microplan II, Natuck, MA).
Isotope Analysis.
Leaf water was extracted by cryogenic distillation as detailed in Ehleringer and Osmond (14). For δ18O analysis of all water samples the equilibration technique of Socki et al. (15) was used with the following modifications. Water (20–500 μl) was introduced by syringe into a 6-ml headspace vial and sealed with a 20-mm Hycor Septum (Alltech, Deerfield, IL). Before injection of water, the headspace vial was evacuated and backfilled to atmospheric pressure (measured at the time of vial preparation) with air of 9:1, N2:CO2. The CO2 and water samples were allowed to equilibrate for 48–72 h within the headspace vials. A 250-μl gas-tight lockable syringe (VICI Precision Sampling, Baton Rouge, LA) was used to inject 50–150 μl of air from the headspace vial into the helium carrier stream of a Varian GC (model 3300) through a split/splitless on-column injector (approximately a 1:10 split). The sample was carried through a 50-m plot column (Porapak-Q) and on through a 1:2 fixed-post column split interface to the MS (model 252; Finnigan-MAT, San Jose, CA) in continuous flow mode. Before injection of the sample into the column, the syringe was filled and evacuated with a “presample” to purge the syringe. After purging the syringe with the presample, the syringe was filled with a sample and overpressurized to ensure that the needle dead space volume was cleansed before injection. After every 10 samples, we analyzed one each of two laboratory standards. The two laboratory water standards were analyzed in volumes from 10 to 500 μl with this modified H2O:CO2 equilibration headspace vial technique and showed no significant variation related to water volume. Precision for all isotope analysis was 0.2‰.
Results and Discussion
In applying the Craig–Gordon model to leaves, two primary assumptions are made: (i) the leaf represents a single well-mixed pool into which water enters from the petiole and from which water exits solely by evapotranspiration, and (ii) the leaf is at isotopic steady-state, where the isotopic composition of the transpiration stream is identical to the water entering the leaf through the petiole. The isotopic composition of water in the leaf is then a function of known fractionation factors that are controlled by leaf temperature, the evaporative gradient, the leaf boundary layer, and the isotopic composition of atmospheric water vapor. All previous studies using the Craig–Gordon model to predict leaf water δ18O have shown the model to work well, or in some cases the predicted δ18Owl was more enriched than what was actually observed (4–7, 16, 17). The explanation for the latter observation is that the leaf does not represent a perfectly mixed pool and that some contribution of unenriched vein water in bulk leaf water tends to decrease the observed 18O enrichment. If the primary assumptions are met and accurate measures of all parameters necessary to calculate predictions are made, the δ18O of leaf water cannot exceed predictions of the Craig–Gordon model.
After several weeks of growth in the pilot experiment, we sampled bulk leaf water (from ligule to leaf blade tip in the grasses) δ18O values and other source and atmospheric parameters necessary to evaluate the Craig–Gordon model (7) 1 day a week for 3 successive weeks. The δ18Owl value for the C4 grass (14.7 ± 3.0‰) was significantly and consistently more enriched than that of the C3 grass (7.2 ± 2.0‰; P < 0.01), which was significantly more enriched than the C3 dicot (3.9 ± 1.0‰; P < 0.01) under midday growth conditions. The observed δ18Owl values for the dicot leaves were consistent with predictions of the Craig–Gordon model (4.1‰). However, the observed δ18Owl values in both grasses were consistently more enriched than those predicted by the Craig–Gordon model: 7.2‰ versus 4.7‰ for the C3 grass, and 14.7‰ versus 6.1‰ for the C4 grass. The differences in predicted values among plants reflected measured differences in leaf temperatures.
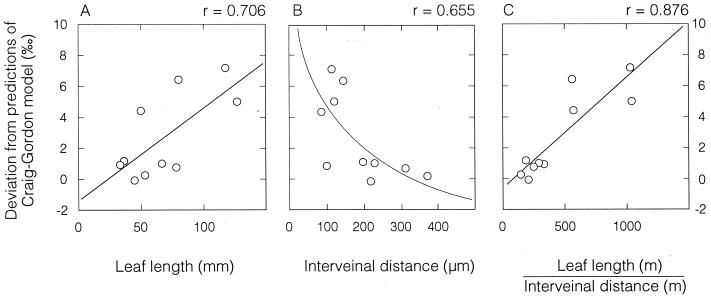
After 6 weeks of growth in the survey experiment, we sampled bulk leaf water δ18O values and all other parameters as detailed above. The patterns observed were similar to those of the pilot experiment: Whole-leaf δ18Owl values of all C4 grasses were >4‰ more enriched than all C3 grasses, and the observed whole-leaf δ18Owl of all but one grass species were more enriched than predicted Craig–Gordon values (Table 1). In analyzing the residuals (observed minus predicted values or deviations from a single-source-water version of the Craig–Gordon model), we observed a significant positive correlation with leaf length and a significant negative correlation with interveinal distance (Fig. 1 A and B; P < 0.01). When the residual was plotted versus leaf length divided by interveinal distance (Fig. 1C), there was a highly significant correlation that explained most of the deviation of the bulk grass blade δ18Owl from the Craig–Gordon model (r = 0.876; P < 0.001).
Figure 1.
The relationships between δ18Owl residuals (difference between the observed and predicted δ18Owl values) versus grass leaf morphological characteristics. (A) Residual versus leaf length (P < 0.01). (B) Residual versus interveinal distance (P < 0.01). (C) Residual versus leaf length divided by interveinal distance (P < 0.001). Each point represents a different grass species and is the mean of three plants.
To assess the pattern of 18O enrichment in grasses, whole grass blades were collected and sectioned from base to tip, and the δ18Owl value was measured in each section. A large and distinct progressive δ18Owl enrichment was observed along the length of the grass leaf (Fig. 2). The base-to-tip δ18Owl difference for this grass blade was 49‰. All grass species analyzed exhibited a similar pattern of progressive enrichment, with some base-to-tip differences exceeding 50‰. Such large and distinct 18O enrichment patterns did not occur in dicot leaves (17).
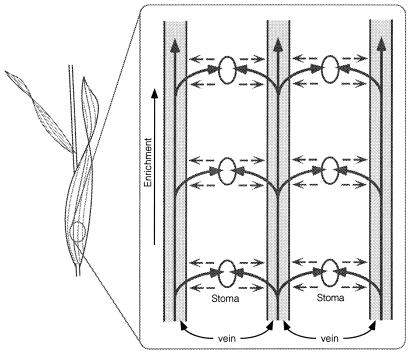
We propose a simple explanation that accounts for both the progressive enrichment and the effect of interveinal distance on grass δ18Owl (Fig. 3). As water moves out of leaf veins and toward the stomatal apertures, leaf water becomes evaporatively 18O-enriched. As a result, an 18O gradient develops between the stomatal complex (region of evaporatively enriched water) and vein (water source) as expected by the Craig–Gordon model. Despite the large advective transpirational flux toward the stoma, back diffusion of 18O-enriched leaf water toward the vein should occur [Peclet effect (18)] and any back diffusion ultimately enriches vein water. This enriched vein water travels up the leaf providing source water to the next stomatal complex, and the vein water is further enriched (explaining the observation in Fig. 2). We also expect that at any particular point along a grass blade, the extent to which vein water mixes with water from evaporative sites should be a function of the distance from the vein to the evaporative site (back diffusion path length). If interveinal distances are short, there is more mixing, and the vein water enrichment should be greater for every leaf segment. Interveinal distances are known to be significantly shorter in C4 grasses than in C3 grasses (12, 13). Consistent with this pattern, we observed that δ18Owl values were more enriched in C4 grasses.
Figure 3.
Schematic of progressive 18O enrichment of leaf water as expected in grass leaves. As source water (solid arrowed lines) exits a vein and moves toward a stoma, it becomes evaporatively enriched in 18O. This enrichment process creates a concentration gradient of enriched water at the stoma relative to water in the vein, which results in a back diffusion of enriched water (dashed arrowed lines). The mixing of the isotopically distinct water pools results in a slight enrichment of vein water, which then influences 18O enrichment of leaf water in the next leaf segment, where the process occurs again further enriching vein water.
The patterns of leaf water enrichment we describe here require successively enriched pools in contrast to the Craig– Gordon model, which considers only one pool. Our hypothesis for grass blade enrichment is analogous to the string-of-lakes model developed by Gat and Bowser (19):
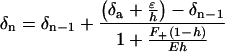 |
1 |
where δa and δn refer to the δ18O of atmospheric water vapor and water entering an evaporating surface (i.e., stoma), respectively. F+ represents the flux into a segment, and E represents the evaporative flux out of a segment. Humidity is represented by h, and ɛ = ɛeq + (1 − h)ɛk, where ɛeq and ɛk represent the equilibrium and kinetic fractionation factors.
If one assumes that E is the same for all leaf segments, then the mean δ18O value of water from all segments has the same value as predicted by the single-water-source Craig–Gordon model. Under this assumption, we do predict progressive 18O enrichment along the leaf for successive leaf segments, but the predicted δ18O values of each segment do not match observed values (Table 2). Actual E along a grass blade is known to vary (20). When we fitted E values of leaf segments to match the δ18Owl data, we obtained close agreement (Table 2). There was not a fitted value of E that accurately predicted δ18O in the terminal segment. We believe this observation is because the water volume in a leaf decreases as the leaf tapers toward the tip, a phenomenon not incorporated into the model. Note that by varying E between segments, the predicted whole-leaf water δ18Owl value is considerably more enriched than that predicted by the single-water-source Craig–Gordon model, without violating steady-state assumptions. As it stands, the Gat–Bowser model assumes complete mixing within each segment (e.g., interveinal distance of 0), but if we relax this assumption in accordance with known C3/C4 anatomical differences, species-specific differences can be incorporated into the model.
Table 2.
Observed and predicted leaf water δ18O values of leaf segments
| Leaf segment | Observed δ18O, ‰ | Craig–Gordon model-predicted δ18O, ‰ | Gat–Bowser model
|
|||
|---|---|---|---|---|---|---|
| Nonvariable E
|
Fitted E
|
|||||
| E, mmol⋅m−2⋅s−1 | Predicted δ18O, ‰ | E, mmol⋅m−2⋅s−1 | Predicted δ18O, ‰ | |||
| Base 1 | −7.4 | 11.0 | 7.14 | −7.5 | 7.40 | −7.4 |
| 2 | 1.0 | 11.0 | 7.14 | −2.4 | 12.25 | 1.0 |
| 3 | 9.6 | 11.0 | 7.14 | 3.2 | 10.60 | 9.6 |
| 4 | 16.1 | 11.0 | 7.14 | 9.4 | 6.05 | 16.1 |
| 5 | 23.2 | 11.0 | 7.14 | 16.4 | 5.70 | 23.2 |
| 6 | 27.7 | 11.0 | 7.14 | 24.6 | 2.40 | 27.7 |
| Tip 7 | 41.7 | 11.0 | 7.14 | 35.3 | 5.60 | 37.3 |
| Mean | 16.0 | 11.0 | 7.14 | 11.3 | 7.14 | 15.4 |
δ18Osource was −12.5‰, and all other environmental parameters were as mentioned in Table 1. In the Gat–Bowser model, the mean value of E for the entire leaf remains constant. However, the proportion of E within a segment was either held constant (nonvariable E) or was varied (fitted E). The calculated variations in fitted E across the length of the leaf are less than those of sugarcane (20).
The 18O differences in leaf water should affect C18O16O values of gross photosynthetic CO2 fluxes, because carbonic anhydrase assures rapid equilibration of 18O in leaf water and CO2 (8). Hence, 18O leaf water differences should be reflected in the δ18O values of retrodiffused CO2 leaving the leaf and from the ecosystem. Farquhar et al. (8) showed that most of the latitudinal variation in global C18O16O is a direct result of leaf water enrichment in terrestrial vegetation and implied that C18O16O values could be used to further distinguish terrestrial from oceanic CO2 fluxes. Our observations suggest that C3-dominated grasslands and C3 forests should be different in δ18Owl. Consequently, the discrimination value of C18O16O fluxing from these ecosystems should differ according to the theory developed by Farquhar and colleagues (8). Discrimination against C18O16O in C4 plants is distinguishable from C3 plants based on the inherent differences of [CO2] in the air spaces of the leaf (8). However, based on the correlations shown here between δ18Owl and leaf anatomy, the C18O16O discrimination values of major grasslands, such as the short-grass and tall-grass C4-dominated prairies, may be distinct because of the large differences in leaf length and interveinal distance of the dominant species in these grasslands. Furthermore, the vast difference in C18O16O discrimination between C3 and C4 grasses, along with δ13C information from CO2, may allow for separation of seasonal activity shifts in C3/C4 dominance within these grasslands.
Grass-dominated ecosystems comprise nearly one-fourth of total global vegetated land area (21) and are among the most productive ecosystems. Yet there can be more than a 4-fold difference in primary productivity on a year-to-year basis (22). Given that the 13CO2 discrimination by C4 grasslands is nearly indistinguishable from oceanic discrimination (23), partitioning of the global carbon flux into grassland versus oceanic carbon sinks is difficult. The higher δ18Owl and C18O16O discrimination values predicted for grassland ecosystems might be an approach for distinguishing grassland from oceanic productivity in the global 13C18O16O flask observations.
Acknowledgments
We thank J. Berry, G. D. Farquhar, J. Gillon, H. Griffiths, C. E. Martin, C. Still, and D. Yakir for helpful comments on early drafts of this manuscript. We thank J. Roden for enlightening discussions during the early stages of this work. J. Sperry was of great assistance with the anatomical measurements. C. Cook, M. Lott, and Big Dog provided help for isotope analysis. This research was funded by the National Institute for Global Environmental Change through the U.S. Department of Energy (Cooperative Agreement No. DE-FC03–90ER61010). B.R.H was funded additionally by a National Aeronautics and Space Administration Earth System Science Fellowship.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Craig H, Gordon L I. In: Stable Isotopes in Oceanographic Studies and Paleotemperatures. Tongiorigi E, editor. Pisa, Italy: Consiglio Nazionale Delle Ricerche Laboratorio di Geologia Nucleare; 1965. pp. 9–130. [Google Scholar]
- 2.Dongmann G, Nurnberg H W, Forstel H, Wagener K. Radiat Environ Biophys. 1974;11:41–52. doi: 10.1007/BF01323099. [DOI] [PubMed] [Google Scholar]
- 3.Farris F, Strain B R. Radiat Environ Biophys. 1978;15:167–202. doi: 10.1007/BF01323264. [DOI] [PubMed] [Google Scholar]
- 4.Flanagan L B, Comstock J P, Ehleringer J R. Plant Physiol. 1991;96:588–596. doi: 10.1104/pp.96.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yakir D, DeNiro M J, Gat J R. Plant Cell Environ. 1990;13:49–56. [Google Scholar]
- 6.Flanagan L B, Bain J F, Ehleringer J R. Oecologia. 1991;88:394–400. doi: 10.1007/BF00317584. [DOI] [PubMed] [Google Scholar]
- 7.Roden J S, Ehleringer J R. Plant Physiol. 1999;120:1165–1173. doi: 10.1104/pp.120.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farquhar G D, Lloyd J, Taylor J A, Flanagan L B, Syversten J P, Hubick K T, Wong S C, Ehleringer J R. Nature (London) 1993;363:439–443. [Google Scholar]
- 9.Ciais P, Tans P P, Denning A S, Francey R J, Trolier M, Meijer H A J, White J W C, Berry J A, Randall D A, Collatz G J, et al. J Geophys Res. 1997;102:5873–5883. [Google Scholar]
- 10.Ciais P, Meijer H A J. In: Stable Isotopes: Integration of Biological, Ecological, and Geochemical Processes. Griffiths H, editor. Oxford: BIOS Scientific Publishers; 1998. pp. 409–431. [Google Scholar]
- 11.Flanagan L B, Phillips S L, Ehleringer J R, Lloyd J, Farquhar G D. Aust J Plant Physiol. 1994;21:221–234. [Google Scholar]
- 12.Kawamitsu Y, Hakoyama S, Agata W, Takeda T. Plant Cell Physiol. 1985;26:589–593. [Google Scholar]
- 13.Dengler N G, Dengler R E, Donnely P M, Hattersley P W. Ann Bot (London) 1994;73:241–255. [Google Scholar]
- 14.Ehleringer J R, Osmond C B. In: Plant Physiological Ecology Field Methods and Instrumentation. Pearcy R W, Ehleringer J R, Mooney H A, Rundel P W, editors. London: Chapman & Hall; 1989. pp. 281–300. [Google Scholar]
- 15.Socki R A, Karlsson H R, Gibson E K. Anal Chem. 1992;64:829–831. doi: 10.1021/ac00031a026. [DOI] [PubMed] [Google Scholar]
- 16.Yakir D, Berry J A, Giles L, Osmond C B. Plant Cell Environ. 1994;17:73–80. [Google Scholar]
- 17.Wang X-F, Yakir D. Plant Cell Environ. 1995;18:1377–1385. [Google Scholar]
- 18.Farquhar G D, Lloyd J. In: Stable Isotopes and Plant Carbon/Water Relations. Ehleringer J R, Hall A E, Farquhar G D, editors. San Diego: Academic; 1993. pp. 47–70. [Google Scholar]
- 19.Gat J R, Bowser C. In: Stable Isotope Geochemistry: A Tribute to Samuel Epstein. Taylor H P J, O'Neil J R, Kaplan I R, editors. Special Publication No. 3. St. Louis: Geochemical Society; 1991. pp. 159–168. [Google Scholar]
- 20.Meinzer F C, Saliendra N Z. Aust J Plant Physiol. 1997;24:769–775. [Google Scholar]
- 21.Sims P L. In: North American Terrestrial Vegetation. Barbour M G, Billings W D, editors. Cambridge, U.K.: Cambirdge Univ. Press; 1988. pp. 265–286. [Google Scholar]
- 22.Knapp A K, Briggs J M, Blair J M, Turner C L. In: Grassland Dynamics: Long-Term Ecological Research in Tallgrass Prairie. Knapp A K, Briggs J M, Hartnett D C, Collins S L, editors. Vol. 1. New York: Oxford Univ. Press; 1998. pp. 193–221. [Google Scholar]
- 23.Ciais P, Tans P P, Trolier M, White J W C, Francey R J. Science. 1995;269:1098–1102. doi: 10.1126/science.269.5227.1098. [DOI] [PubMed] [Google Scholar]