Abstract
Otitis media, a common and often recurrent bacterial infection of childhood, is a major reason for physician visits and the prescription of antimicrobials. Haemophilus influenzae is the cause of ≈20% of episodes of bacterial otitis media, but most strains lack the capsule, a factor known to play a critical role in the virulence of strains causing invasive H. influenzae disease. Here we show that in capsule-deficient (nontypeable) strains, sialic acid, a terminal residue of the core sugars of H. influenzae lipopolysaccharide (LPS), is a critical virulence factor in the pathogenesis of experimental otitis media in chinchillas. We used five epidemiologically distinct H. influenzae isolates, representative of the genetic diversity of strains causing otitis media, to inoculate the middle ear of chinchillas. All animals developed acute bacterial otitis media that persisted for up to 3 wk, whereas isogenic sialic acid-deficient mutants (disrupted sialyltransferase or CMP-acetylneuraminic acid synthetase genes) were profoundly attenuated. MS analysis indicated that WT bacteria used to inoculate animals lacked any sialylated LPS glycoforms. In contrast, LPS of ex vivo organisms recovered from chinchilla middle ear exudates was sialylated. We conclude that sialylated LPS glycoforms play a key role in pathogenicity of nontypeable H. influenzae and depend on scavenging the essential precursors from the host during the infection.
Keywords: ex vivo isolate, phylogeny, mass spectrometry
Carried by up to 80% of humans, Haemophilus influenzae (Hi) is a common nasopharyngeal commensal. Capsule-deficient or nontypeable (NT) Hi can cause upper and lower respiratory tract infections, the most common being episodes of otitis media (OM) in young children. On average, children experience two or more episodes of acute OM by age 2 yr (1), making OM a major cause of physician visits (24 million per year in the U.S.) and of the profligate use of antibiotics in general practice. OM causes sequelae, including impaired hearing (≈20% cases) and cognitive development (2). NTHi is also the most frequent pathogen recovered from the middle ear in children with recurrent OM (3). Although immunity against infection due to NTHi appears to develop after acute OM (4, 5), protection is strain specific, therefore permitting recurrent episodes due to distinct isolates.
We have reported that all NTHi OM isolates have the potential to incorporate sialic acid [N-acetylneuraminic acid (Neu5Ac)] into their lipopolysaccharide (LPS), and that strains expressing this sugar are more resistant to the bactericidal activity of normal human serum in vitro (6–8). Although sialylation of LPS has been implicated in bacterial virulence (9), the contribution of sialylated LPS glycoforms of Hi has not been investigated in vivo.
We have investigated the role of sialic acid as a virulence factor of NTHi in a well described chinchilla model of OM (10, 11). By comparing isogenic sialic acid-proficient and sialic acid-deficient strains, we show that sialylation of LPS is a major factor in the virulence of NTHi. In addition, we report the structural characterization of LPS glycoforms in vivo by using sensitive MS techniques and show that the heightened virulence afforded by LPS sialylation depends on the capacity of NTHi to scavenge sialic acid from the host.
Materials and Methods
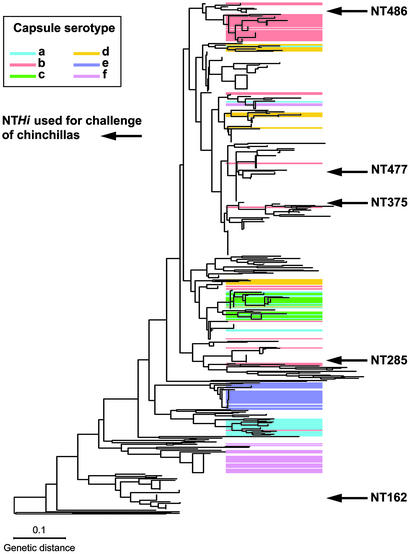
Bacterial Strains and Culture. Five genetically distinct NTHi isolates (strains 162, 285, 375, 477, and 486) were selected from a collection of 107 OM isolates of NTHi obtained by tympanocentesis (6), based on a population analysis of >500 Hi strains using ribotyping (Fig. 1). Details of the dendrogram and its validation using multilocus sequence typing have been published (12–14). Hi strains were cultured at 37°C in brain–heart infusion (BHI) broth supplemented with 10 μg/ml haemin and 2 μg/ml NAD (sBHI); BHI solidified with agar (1% wt/vol) and supplemented with 10% Levinthals reagent; or chocolate agar, supplemented with 10 μg/ml kanamycin when appropriate.
Fig. 1.
Phylogenic tree based on ribotyping of 542 Hi strains. Five isolates (162, 285, 375, 477, and 486) were selected to infect the chinchillas. Independent confirmation of this dendrogram was obtained based on capsular operon gene polymorphism analysis (15) and congruence of a ribotype-based phylogenetic tree of a subset of the type a–f isolates with results from multilocus enzyme electrophoresis analysis (16). Further validation was obtained by comparative gene sequencing of recA and 16S ribosomal RNA genes among a representative subset of 50 of the isolates and multilocus sequence typing analysis (12) of a representative set involving 51 of the NT isolates.
Construction of Mutant Strains Unable to Synthesize Sialylated LPS Glycoforms. The sialylation of LPS glycoforms by strain 486 depends only on the Lic3A sialyltransferase (7, 17) and therefore inactivation of lic3A by insertion of a kanamycin resistance cassette (7) completely prevented expression of sialylated glycoforms. Isogenic sialic acid-deficient mutants of NTHi strains 162, 285, 375, 477, and 486 were constructed by mutating the gene encoding CMP-Neu5Ac synthetase (siaB) as described (6). We confirmed the genotype of all mutants by using PCR and Southern hybridization (6, 7). The LPS phenotypes were confirmed as described (6).
Animal Challenge. A chinchilla model of acute OM using direct inoculation of 50–10,000 colony-forming units (cfu) of WT or the sialic acid-deficient mutant (siaB and lic3A for 486; siaB for 162, 285, 375, and 477) was used (10, 11). Strains were grown overnight in sBHI broth, with kanamycin when appropriate, at 37°C. Before inoculation, 100 μl of the culture was diluted into 10 ml of fresh sBHI, grown to midlogarithmic phase, then diluted in Gey's balanced salt solution.
Initial studies were performed by inoculating 50 cfu of WT NTHi strain 486 or its isogenic lic3A mutant in 0.1 ml of Gey's balanced salt solution (GBSS) directly into both middle ears (through the superior bullae). Middle ears of each chinchilla were examined at intervals over a 26-day period with direct and indirect otomicroscopy. The middle ears were examined via 3- to 5-mm holes created in the superior bullae through which the contents were sampled by using an angiocatheter for quantitative cultures. When middle ear fluid was present, 10 μl was collected and diluted 1:10 into GBSS and three further consecutive dilutions were prepared, 1:100, 1:103, and 1:104. One hundred microliters of each dilution were plated onto chocolate agar plates, supplemented with kanamycin where appropriate. On the basis of this dilution series, the lowest detectable number of organisms is 100 cfu/ml. Thus, for samples obtained from chinchillas where no growth was detected (zero cfu), a value of log100 = 2 was assigned. In addition, undiluted middle ear effusions or washings were plated onto chocolate agar. If middle ear fluid was absent, the middle ear was flushed with 0.5 ml of GBSS and the contents sampled as described above. Direct and indirect ear examination was continued until the middle ear was culture negative on two consecutive evaluations. The remainder of ex vivo samples was frozen at –80°C in 2% phenol for analysis of LPS by electrospray ionization (ESI)-MS or for sequence analysis of tetranucleotide repeats.
Capillary Electrophoresis (CE)–Electrospray Ionization–MS Analysis of O-Deacylated LPS Samples. Samples from the in vitro grown inocula and those obtained directly from the middle ear of chinchillas were frozen in 2% phenol, then processed in an identical manner. LPS was extracted from these samples as follows: phenol was removed by low-speed centrifugation and washing with water. The bacterial cell membrane was disrupted with proteinase K followed by successive treatments of DNase and RNase to release LPS, which was O-deacylated in situ with anhydrous hydrazine (18). Lyophylized samples were dissolved in ammonium acetate solution (1 M) and analyzed directly on a Crystal Model 310 CE instrument (ATI Unicam, Boston) coupled to an API 3000 triple quadrupole mass spectrometer (MDS/Sciex, Thornhill, ON, Canada), as described (19). Mass spectra were acquired with dwell times of 3.0 ms per step of 1 m/z unit in full-mass scan mode. Precursor ion monitoring of chinchilla middle ear effusion samples was carried out in the negative ion mode by using nitrogen as a target gas at collision energies of typically 120 eV (laboratory frame of reference), for specific detection of glycoforms that generate fragment ions at m/z 220 from pyrophosphoethanolamine (PPEtn)-containing glycoforms, m/z 290 from glycoforms containing terminal Neu5Ac, and m/z 951 from the O-deacylated lipid A component (19).
PCR and DNA Sequencing of Tetranucleotide Repeats. PCR amplification of NTHi DNA was carried out by using locus-specific primers for 1-min periods of denaturation (94°C), annealing (50°C), and polymerization (72°C) for 30 cycles. For confirmation of the presence of disrupted alleles in mutant strains, primers siaBa/SiaBb for siaB and lic3a/L3B1 for lic3A were used as described (6, 7). Before determining the number of tetranucleotide repeats, the 5′ portions of phase variable genes were amplified by using the following primers: lic2A, lic2A121F (5′-ACTGAACGTCGCAAACAT) and lic2P (5′-TTCTCAAGTTTAACTGGC); lic3A, lic3a, and lic3r (5′-CTGCACACATATAAACGC); lgtC, 6024F (5′-CAGCAAAGGCATTGACTG) and lgt2 (5′-CTGACTCACAAACGGGC). Sequencing reactions were carried out by cycle sequencing by using the same primers and ABI Big Dye Sequencing (Applied Biosystems) reagents and were analyzed on an ABI377 autosequencer.
Results
Animal Challenge Studies. We investigated the role of sialic acid as a virulence factor of NTHi in the chinchilla model of OM by comparing the virulence of WT NTHi strains to their respective isogenic sialic acid-deficient mutants.
After direct inoculation of ≈50 organisms of WT strain 486 into both ears of 13 chinchillas, all developed bilateral OM characterized by inflammatory exudate and recovery of NTHi from the middle ear. Large numbers of organisms (geometric mean of five animals 6.8 × 106 SE ± 2.4 cfu/ml) were evident by 2 days after inoculation and persisted for at least 16 days. Nasopharyngeal cultures also grew NTHi, presumably as a result of contiguous spread along the eustachian tube to the nasopharynx. In contrast, a lic3A mutant of strain 486 was essentially avirulent. In 15 of the 17 chinchillas inoculated with ≈50 cfu of the lic3A mutant of strain 486, there was complete absence of inflammation of the middle ear and negative cultures of the middle ear, and nasopharynx. NTHi was recovered transiently from the middle ear (geometric mean: 3.7 × 103 SE ± 1.9 cfu/ml) in two animals on day 5. Reinoculation of one of the isolates recovered on day 5 into two additional chinchillas did not result in middle ear infection. In additional studies in which the inoculum size of the lic3A mutant used to infect the three chinchillas was increased to 2,000 organisms, none of the chinchillas developed signs of OM or positive middle ear cultures.
To obtain confirmation of the contribution of sialylation of LPS in the pathogenesis of OM, we targeted a different gene, siaB. An inoculum of up to 10,000 organisms of the siaB mutant of strain 486 failed to produce any evidence of OM in six chinchillas compared with the acute and persistent infection once again observed in three chinchillas inoculated with ≈100 cfu of WT strain 486.
Next, we sought to investigate whether LPS sialylation was critical for virulence in other NTHi strains epidemiologically and genetically distinct from 486. These strains (162, 285, 375, and 477) have been shown to differ in the molecular details of their LPS structures, but all have the potential to make sialylated glycoforms (refs. 6 and 8; unpublished data). Although growth rates of WT and siaB mutants in vitro were equivalent, in vivo each of the siaB mutants was attenuated, two strains (162 and 285) being completely avirulent (Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). However, even in the three of nine chinchillas that showed evidence of infection with 375 siaB and 477 siaB mutant strains, OM was less severe, and there was more rapid resolution of disease.
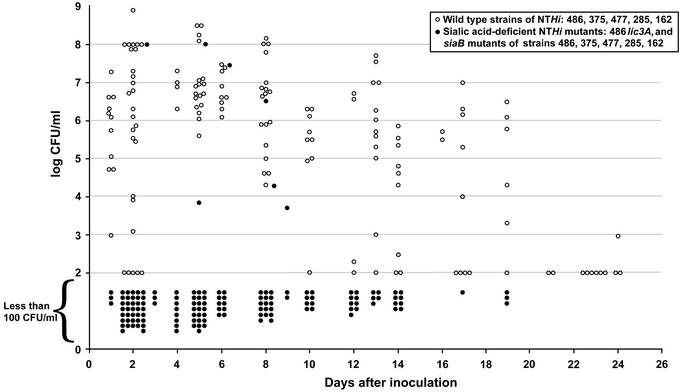
In summary, using five genetically diverse NTHi strains, mutations resulting in the inability to synthesize sialylated LPS glycoforms caused attenuation of NTHi, completely so in all but 5 of 45 animals inoculated with either lic3A or siaB mutants (Fig. 2 and Table 1). In contrast, in all 33 chinchillas inoculated with WT strains, there was purulent middle ear disease associated with high density of organisms that persisted for up to 3 wk.
Fig. 2.
Summary of viable counts (log10) of Hi, y axis, from cultures of fluid obtained over a period of 26 days (x axis) after inoculation of 50–100 Hi at day 0. Open circles represent quantitative cultures from individual samples of middle-ear fluid after inoculation with one of five representative Hi strains (see Fig. 1): 486, 375, 477, 285, or 162. Filled circles indicate the results of chinchillas inoculated with the isogenic siaB mutants. The lowest detectable number of organisms is 100 cfu/ml (logarithm 100 = 2). Thus, for all samples obtained from chinchillas where organisms were not detected (zero cfu), a value of log100 was assigned, and these data points are plotted within the area labeled “Less than 100 cfu/ml.”
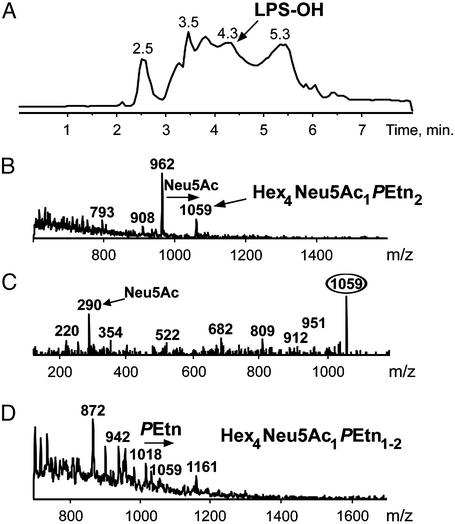
Characterization of LPS Glycoforms Expressed in Vivo. We investigated the LPS structure and patterns of sialylation during experimental infection of chinchillas by directly analyzing ex vivo organisms. Previous studies of sialic acid expression have shown qualitative and quantitative variation in the molecular details of the sialylation of LPS glycoforms (6–8), and at least three sialyltransferases have been described (7, 20, 21). Expression of sialylated LPS glycoforms would appear to depend on an exogenous source of sialic acid or its nucleotide-activated form, CMP-Neu5Ac (7, 20). We have shown that the phase-variable gene, lic3A, encodes an α2,3-sialyltransferase. This enzyme mediates addition of Neu5Ac from CMP-Neu5Ac to a lactose extension from the inner-core unit (Fig. 3), the only sialic acid-containing LPS phenotype found in NTHi strain 486 (17). We have found CE coupled to ESI-MS to be a particularly sensitive tool for profiling LPS glycoforms on O-deacylated samples (18, 19). When WT NTHi strains 486 and 375 were grown in sBHI lacking exogenous sialic acid before inoculation into chinchillas, no sialic acid could be detected in the LPS (see below). To determine whether the WT strains were expressing sialylated glycoforms in vivo during infection, we evaluated samples of exudate taken directly from the infected middle ear cavity. LPS was obtained by using a microextraction procedure (18) and O-deacylated by treatment with anhydrous hydrazine (19) rendering LPS amenable for analysis by CE-ESI-MS analysis. When WT NTHi strain 486 is grown on sBHI media without an exogenous source of sialic acid, the extracted O-deacylated LPS (LPS-OH) corresponded to the fully characterized glycoform populations containing four hexose residues (Hex4) (7, 17) (Fig. 4A). No sialylated glycoforms were detectable. A similar profile was observed for LPS-OH from the lic3A and siaB mutants, irrespective of whether they were grown in the presence or absence of sialic acid (data not shown). Sialylated LPS glycoforms were observed in exudates from the middle ears of chinchillas at 4 and 6 days after inoculation (Fig. 4). Ions caused by sialyllactose-containing Hex4 LPS species from the 2-keto-3-deoxyoctulosonic acid (KDO)-phosphate series of glycoforms were observed at m/z 764 [(M-4H)4–], 1,018 [(M-3H)3–], and 1,527 [(M-2H)2–] (Fig. 4B). These ions were not detectable in LPS from in vitro grown bacteria used to infect the animals (Fig. 4A). After 6 days, ions caused by this sialylated glycoform were only just detectable above noise (Fig. 4C). Precursor ion monitoring at m/z 220 was used to improve the limits of detection by providing a sensitive probe to select for ions from the KDO-PPEtn series of glycoforms, which produce a PPEtn fragment (m/z 220). Thus, triply charged ions corresponding to the lactose- and sialyllactose-containing Hex4 glycoforms with two phosphoethanolamine (PEtn) groups (Hex4PEtn2) could be observed 5 (Fig. 5B) and 8 days after inoculation. The presence of terminal Neu5Ac in the sialyllactose glycoform was confirmed by tandem MS where collisional activation of the ion at m/z 1,059 gave Neu5Ac as the major fragment ion (m/z 290) (Fig. 5C). Precursor ion monitoring for ions giving rise to the fragment at m/z 290 (Neu5Ac) permitted detection of the sialylated Hex4 glycoforms from both the KDO-phosphate (m/z 1,018) and KDO-PPEtn (m/z 1,059) series (Fig. 5D). Precursor ion monitoring at m/z 220 (PPEtn) was more sensitive than that at m/z 290 (Neu5Ac) for detecting LPS-OH ions in the heterogeneous mixtures obtained from chinchilla ear washings (Fig. 4, compare signal-to-noise ratio of spectra Fig. 5 B and D).
Fig. 3.
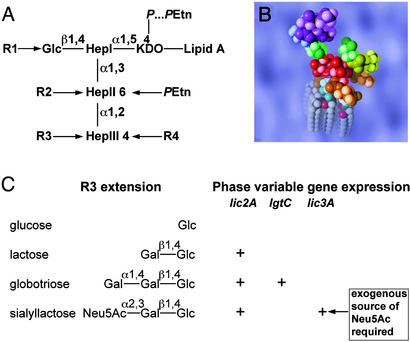
Hi LPS comprises a heterogeneous mixture of glycoforms consisting of an oligosaccharide moiety attached to a membrane anchoring lipid A component. (A) Structural model of the conserved inner core region of the oligosaccharide portion of the molecule. (B) Space-filling molecular model of the minimum energy conformer of the sialyllactose containing LPS glycoform of NTHi strain 375 calculated by a Monte Carlo method (22). The conserved l-glycero-d-manno-heptopyranosyl trisaccharide (HepI–HepIII), depicted in red, is linked to the lipid A portion of the molecule (turquoise and gray) via a phosphorylated KDO residue (brown). The triheptosyl inner-core unit is substituted by a β-d-glucopyranose residue (Glc; green) at the O-4 position of HepI and by a phosphoethanolamine residue (PEtn; brown) at the O-6 position of HepII. In NTHi 375, 285, and 162 LPS, the Glc residue is substituted at O-6 by a phosphocholine residue (R1 = PCho; yellow). In NTHi strain 486 (R1 = H), HepII is substituted at the O-3 position by an α-d-glucopyranose which, in turn, is substituted by PCho at the O-6 position (R2 = PCho-6Glc). NTHi strain 375 can also carry a PEtn group on HepIII (R4 = H or PEtn). As shown in C, the NTHi strains used in this study can exhibit oligosaccharide chain extension from HepIII (R3) through sequential addition of sugar units (6, 7). LPS glycoforms containing β-d-glucopyranose, lactose, globotriose, and sialyllactose oligosaccharide chains have been identified (6, 7, 17, 23). The relative proportions of these glycoforms in the LPS from a particular strain depend on the expression of phase variable genes lic2A, lgtC, and lic3A (7, 24).
Fig. 4.
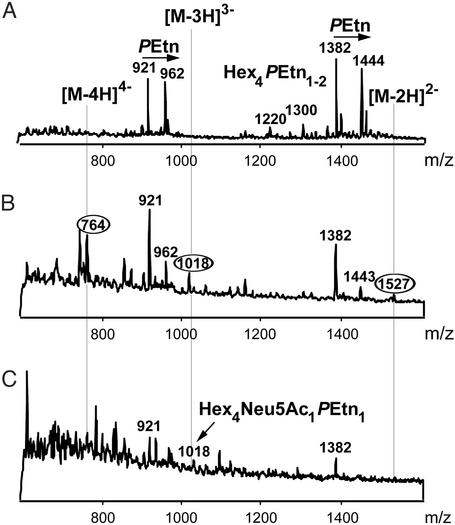
Negative ion CE-ESI-MS shows major glycoforms of LPS-OH extracted from NTHi strain 486. (A) Extracted mass spectrum for LPS-OH extracted from NTHi bacteria used to inoculate chinchillas. The mass spectrum is dominated by molecular peaks corresponding to doubly [(M-2H)2–] and triply [(M-3H)3–] charged ions. Triply charged ions at m/z 921 and 962 represent the lactose containing glycoform populations comprising four hexoses (Fig. 3A: R1 = H; R2 = phosphocholine residue-6Glc; R3 = lactose). The glycoform responsible for the ion at m/z 921 has a phosphate group at O-4 of the KDO residue, whereas that at m/z 962 carries a PPEtn group (Fig. 3A). Related doubly charged ions at m/z 1,382 and 1,444 are also observed. (B and C) Extracted mass spectra obtained from the CE-ESI-MS of exudate from the ear of a chinchilla 4 and 6 days, respectively, after inoculation with 50 WT organisms of the NTHi strain 486 described in A. In addition to ions corresponding to the Hex4 glycoforms observed in A, sialyllactose containing LPS species from the KDO-phosphate series of glycoforms are observed at m/z 764 [(M-4H)4–], 1,018 [(M-3H)3–], and 1,527 [(M-2H)2–] in B. After 6 days, ions due to the sialylated glycoforms (e.g., m/z 1,018) are only just detectable above background (C).
Fig. 5.
Negative ion CE-ESI-MS with precursor ion monitoring of O-deacylated LPS extracted from NTHi strain 486. (A) Total ion electropherogram (m/z 600–1,600) obtained from the negative ion CE-ESI-MS of exudate from the ear of a chinchilla 5 days after inoculation with 50 WT organisms of the NTHi strain 486 described in Fig. 4A. (B) Precursor ion monitoring at m/z 220 of extracted LPS-OH spectrum shown in A. Triply charged ions corresponding to the lactose and sialyllactose containing Hex4PEtn2 glycoforms are observed at m/z 962 and 1,059. (C) Collisional activation (tandem MS) of ions at m/z 1,059. Major fragment ions at m/z 290 (Neu5Ac) are observed, confirming the presence of terminal sialic acid groups. (D) Precursor ion monitoring at m/z 290 (Neu5Ac) of extracted LPS-OH spectrum shown in A. Sialylated Hex4 glycoforms from both the KDO-P (m/z 1,018) and KDO-PPEtn (m/z 1,059) series are detected.
A similar pattern of results was obtained on LPS-OH samples from chinchillas inoculated with NTHi strain 375. In the absence of an exogenous source of sialic acid, NTHi strain 375 elaborates predominantly Hex4 LPS glycoforms (Fig. 3) (6). When strain 375 was grown in the presence of Neu5Ac, the ESI-MS also showed ions from a sialyllactose-containing Hex3 glycoform (data not shown). Extracted spectra from precursor ion monitoring at m/z 220 (PPEtn) of LPS-OH from chinchillas infected with 50 cfu of NTHi 375 after 5 and 8 days revealed lactose- and sialyllactose-containing Hex3 glycoforms as well as asialyl Hex2 glycoforms with two or three PEtn groups (data not shown). Our previous structural studies suggest that the sialyllactose-containing Hex3 glycoform has the structure shown in Fig. 3B.
Analysis of the Potential Role of Phase Variation in Synthesis of Sialylated Glycoforms. Given that lic3A is a phase variable gene, a possible genetic explanation for the altered sialylation phenotype of the WT NTHi organisms in vivo is that there was strong selection for in-frame lic3A variants, the consequence of slip-page-like events involving the multiple, tandem repeats of 5′-CAAT during the infection (7). We investigated this possibility by comparing the number of tetranucleotide repeats of lic3A of in vitro grown WT NTHi, the organisms used to infect the chinchillas, and of organisms subsequently obtained from the middle-ear exudate of seven chinchillas. On sequencing the 5′ portion of lic3A containing the tetranucleotide repeats, we found that the NTHi organisms (strain 486) derived either from the inoculum or the infected exudates each had the same number of tetranucleotide repeats of 5′-CAAT. This number of repeats is permissive for correct translation of lic3A. A further possibility was that the gene lic2A (24) responsible for adding the galactose to which the sialic acid is attached is also phase variable, and its expression could have altered during the course of the experiment. Again DNA sequencing showed that the number of repeats was permissive for expression and was unchanged between the inoculum and the in vivo organisms. Thus, the sialylated phenotype of in vivo organisms apparently did not occur as a result of the selection of in-frame lic3A phase variants or any change in the lic2A gene.
Discussion
The major conclusion of this investigation is that sialylation of LPS is a critical factor in the virulence of NTHi. Using several different strains of NTHi, we constructed mutants deficient in their ability to make sialylated LPS glycoforms and showed, compared with their WT parent strains, that these mutants were profoundly attenuated in a chinchilla model of OM. Sensitive structural analytical techniques were used to analyze the LPS of the inoculated and infecting organisms during experimental infection of the middle ear of chinchillas. The results provide compelling evidence that sialylation of LPS depends on the ability of Hi to scavenge essential precursors from the host.
Although it has been known for several years that Hi LPS can be sialylated (26), the biological role of this structural feature has not been investigated in vivo. Previous in vitro studies had shown that NTHi lacking sialic acid in their LPS were more susceptible to the bactericidal activity of normal human serum (6), and we hypothesized that lack of sialylation might enhance clearance by host defenses and reduce virulence in vivo. We constructed a mutation in the sialyltransferase (Lic3A) of NTHi strain 486, because our previous studies had shown that this enzyme was essential for LPS sialylation in this strain (17). Inactivation of lic3A in strain 486 resulted in its profound attenuation when compared with the isogenic WT parent strain. It was then important to determine whether the apparently critical virulence role of LPS sialylation in strain 486 might be a characteristic of other genetically distinct NTHi strains. All isolates of NTHi studied to date incorporate Neu5Ac as a common constituent of LPS (8), but it is also known that the patterns of sialylation of LPS in Hi are complex (6, 7, 20), and at least three distinct sialyltransferases have been identified (7, 20, 21). On the basis of the complete genome sequence of Hi strain Rd, the intrinsic pathway for synthesis of sialic acid is missing several key enzymes, a finding consistent with the observation that an exogenous source of Neu5Ac is required (7, 25). However, all NTHi isolates examined to date have the siaB gene (6), and its inactivation provides a convenient strategy to construct sialic acid-deficient LPS in any NTHi strain. Thus, in addition to strain 486, we investigated the contribution of sialylation in four additional genetically diverse strains of NTHi (Fig. 1) (14), which elaborate different sialylated glycoforms. The attenuation of virulence observed in all five siaB mutant strains provides compelling evidence of the importance of sialylation irrespective of the different molecular environments in which it is located.
The mechanism by which sialylation of LPS contributes to virulence is clearly a key to understanding the pathogenesis of OM caused by NTHi and may be relevant to other diseases caused by both encapsulated and nontypeable strains. Since its detection in Escherichia coli K1, sialic acid has been implicated as a virulence factor in several bacterial, fungal, and protozoal species. As a general mechanism, sialic acid functions as an antirecognition molecule, allowing the sialylated microbe to masquerade as “self” while eluding host immune mechanisms that would otherwise rapidly clear an unsialylated strain (27). In addition to molecular mimicry, sialic acid down-regulates the insertion of the membrane attack complex of complement, thereby inhibiting bacteriolysis (28, 29). However, the relevance of these mechanisms to the virulence of NTHi in the chinchilla model must be speculative, given that chinchilla sera do not support in vitro bactericidal activity in the absence of an exogenous source of complement. Sialylation also modulates microbial interactions with host cells, for example by inhibiting phagocytosis or by sterically impeding the interaction of cell surface molecules such as adhesins and invasins (9, 30–34).
The present studies go beyond the previous demonstration of the need for an exogenous source of sialic acid by identifying the requirement for sialylation of LPS by NTHi in the pathogenesis of experimental OM. Sialylated glycoforms were identified by direct analysis using CE-ESI-MS of ex vivo organisms from middle ear washings obtained during the course of experimental middle ear infection, although sialylated glycoforms were undetectable in the inoculum. We confirmed that middle ear infection did not result from selection of phase variants in the lic3A and lic2A genes.
Sialylated LPS glycoforms were observed in samples of exudates from the middle ears of chinchillas at two time points after infection for WT strains 486 and 375. It should be emphasized that, to date, all previously published structural analyses on bacterial glycolipids have been done on material extracted from in vitro grown organisms irrespective of the bacterial species. Direct analysis of LPS structure using ex vivo organisms has not been previously described. We estimate that LPS from ≈107 bacteria is detectable by our microextraction CE-ESI-MS analytical procedure (data not shown). It is likely that LPS glycoforms were not detected by CE-ESI-MS in samples of exudate before 4 days or after 8 days because the numbers of bacteria present in those specimens were too few to allow detection of the glycoforms. In summary, we have obtained clear evidence of sialylated glycoforms from organisms isolated from chinchilla middle ears during the acute phase of infection, although sialylated glycoforms were consistently absent in the inoculum of Hi organisms used to infect the chinchillas, thus demonstrating that modification of the LPS phenotype had occurred during the course of the infection.
Supplementary Material
Acknowledgments
We express appreciation to Juhani Eskola and the Otitis Media Study Group (National Public Health Institute, Finland) for the H. influenzae OM isolates and to Edward Feil and Brian Spratt for MLST-based validation of dendrogram in Fig. 1. E.R.M., D.W.H., and G.A.R. were supported by the U.K. Medical Research Council (Program Grant). R.G. and V.B. were supported in part by National Institutes of Health National Institute on Deafness and Other Communication Disorders (NIDCD) Awards DC04583, DC05564, and DC005855 and acknowledge Dr. Thomas M. Johnson, NIDCD Program Administrator, for encouragement, enthusiasm, and support. We gratefully acknowledge financial assistance from Aventis Pasteur. Chinchilla studies were funded in part by a research grant to S.I.P. from the Shereta R. Seelig Charitable Foundation Trust.
Abbreviations: BHI, brain–heart infusion; CE, capillary electrophoresis; cfu, colony-forming unit; ESI-MS, electrospray ionization MS; Hex, hexose; Hi, Haemophilus influenzae; KDO, 2-keto-3-deoxyoctulosonic acid; LPS, lipopolysaccharide; LPS-OH, O-deacylated LPS; Neu5Ac, N-acetylneuraminic acid; NT, nontypeable; OM, otitis media; PEtn, phosphoethanolamine; PPEtn, pyrophosphoethanolamine; sBHI, supplemented BHI; Hex4, four hexose residues; siaB, CMP-Neu5Ac synthetase.
References
- 1.Teele, D. W., Klein, J. O., Rosner, B., Bratton, L., Fisch, G. R., Mathieu, O. R., Porter, P. J., Starobin, S. G., Tarlin, L. D. & Younes, R. P. (1983) J. Am. Med. Assoc. 249, 1026–1029. [PubMed] [Google Scholar]
- 2.Klein, J. O. (2000) Vaccine 19, Suppl. 1, S2–S8. [DOI] [PubMed] [Google Scholar]
- 3.Kilpi, T., Herva, E., Kaijalainen, T., Syrjanen, R. & Takala, A. K. (2001) Pediatr. Infect. Dis. J. 20, 654–662. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, J. M., Faden, H. S., Loos, B. G., Murphy, T. F. & Ogra, P. L. (1992) Int. J. Pediatr. Otorhinolaryngol. 23, 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Shurin, P. A., Pelton, S. I., Tager, I. B. & Kasper, D. L. (1980) J. Pediatr. 97, 364–369. [DOI] [PubMed] [Google Scholar]
- 6.Hood, D. W., Makepeace, K., Deadman, M. E., Rest, R. F., Thibault, P., Martin, A., Richards, J. C. & Moxon, E. R. (1999) Mol. Microbiol. 33, 679–692. [DOI] [PubMed] [Google Scholar]
- 7.Hood, D. W., Cox, A. D., Gilbert, M., Makepeace, K., Walsh, S., Deadman, M. E., Cody, A., Martin, A., Månsson, M., Schweda, E. K., et al. (2001) Mol. Microbiol. 39, 341–350. [DOI] [PubMed] [Google Scholar]
- 8.Bauer, S. H., Månsson, M., Hood, D. W., Richards, J. C., Moxon, E. R. & Schweda, E. K. (2001) Carbohydr. Res. 335, 251–260. [DOI] [PubMed] [Google Scholar]
- 9.Vogel, U. & Frosch, M. (1999) Mol. Microbiol. 32, 1133–1139. [DOI] [PubMed] [Google Scholar]
- 10.Giebink, G. S. (1981) Rev. Infect. Dis. 3, 342–353. [DOI] [PubMed] [Google Scholar]
- 11.Karasic, R. B., Trumpp, C. E., Gnehm, H. E., Rice, P. A. & Pelton, S. I. (1985) J. Infect. Dis. 151, 273–279. [DOI] [PubMed] [Google Scholar]
- 12.Feil, E. J., Holmes, E. C., Bessen, D. E., Chan, M. S., Day, N. P., Enright, M. C., Goldstein, R., Hood, D. W., Kalia, A., Moore, C. E., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolduc, G. R., Bouchet, V., Jiang, R. Z., Geisselsoder, J., Truong-Bolduc, Q. C., Rice, P. A., Pelton, S. I. & Goldstein, R. (2000) Infect. Immun. 68, 4505–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cody, A. J., Field, D., Feil, E. J., Stringer, S., Deadman, M. E., Tsolaki, A. G., Gratz, B., Bouchet, V., Goldstein, R., Hood, D. W., et al. (2003) Infect. Genet. Evol. 3, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroll, J. S., Ely, S. & Moxon, E. R. (1991) Mol. Cell Probes 5, 375–379. [DOI] [PubMed] [Google Scholar]
- 16.Musser, J. M., Kroll, J. S., Moxon, E. R. & Selander, R. K. (1988) Infect. Immun. 56, 1837–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Månsson, M., Bauer, S. H., Hood, D. W., Richards, J. C., Moxon, E. R. & Schweda, E. K. (2001) Eur. J. Biochem. 268, 2148–2159. [DOI] [PubMed] [Google Scholar]
- 18.Li, J., Thibault, P., Martin, A., Richards, J. C., Wakarchuk, W. W. & van der Wilp, W. (1998) J. Chromatogr. A 817, 325–336. [DOI] [PubMed] [Google Scholar]
- 19.Thibault, P. & Richards, J. C. (2000) in Bacterial Toxins: Methods and Protocols, ed. Holst, O. (Humana, Totowa, NJ), pp. 327–344.
- 20.Jones, P. A., Samuels, N. M., Phillips, N. J., Munson, R. S., Jr., Bozue, J. A., Arseneau, J. A., Nichols, W. A., Zaleski, A., Gibson, B. W., et al. (2002) J. Biol. Chem. 277, 14598–14611. [DOI] [PubMed] [Google Scholar]
- 21.Cox, A. D., Hood, D. W., Martin, A., Makepeace, K. M., Deadman, M. E., Li, J., Brisson, J. R., Moxon, E. R. & Richards, J. C. (2002) Eur. J. Biochem. 269, 4009–4019. [DOI] [PubMed] [Google Scholar]
- 22.Peters, T., Meyer, B., Stuike-Prill, R., Somorjai, R. & Brisson, J. R. (1993) Carbohydr. Res. 238, 49–73. [DOI] [PubMed] [Google Scholar]
- 23.Risberg, A., Masoud, H., Martin, A., Richards, J. C., Moxon, E. R. & Schweda, E. K. (1999) Eur. J. Biochem. 261, 171–180. [DOI] [PubMed] [Google Scholar]
- 24.High, N. J., Deadman, M. E. & Moxon, E. R. (1993) Mol. Microbiol. 9, 1275–1282. [DOI] [PubMed] [Google Scholar]
- 25.Vimr, E., Lichtensteiger, C. & Steenbergen, S. (2000) Mol. Microbiol. 36, 1113–1123. [DOI] [PubMed] [Google Scholar]
- 26.Mandrell, R. E., McLaughlin, R., Aba Kwaik, Y., Lesse, A., Yamasaki, R., Gibson, B., Spinola, S. M. & Apicella, M. A. (1992) Infect. Immun. 60, 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vimr, E. & Lichtensteiger, C. (2002) Trends Microbiol. 10, 254–257. [DOI] [PubMed] [Google Scholar]
- 28.Fearon, D. T. (1978) Proc. Natl. Acad. Sci. USA 75, 1971–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ram, S., Sharma, A. K., Simpson, S. D., Gulati, S., McQuillen, D. P., Pangburn, M. K. & Rice, P. A. (1998) J. Exp. Med. 187, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil, G., Virji, M. & Moxon, E. R. (1994) Microb. Pathog. 16, 153–163. [DOI] [PubMed] [Google Scholar]
- 31.Schauer, R. (2000) Glycoconj. J. 17, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varki, A. (1993) Glycobiology 3, 97–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutishauser, U. (1998) J. Cell. Biochem. 70, 304–312. [DOI] [PubMed] [Google Scholar]
- 34.Telang, S., Vimr, E., Mahoney, J. R., Law, I., Lundqvist-Gustafsson, H., Qian, M. & Eaton, J. W. (2001) J. Infect. Dis. 184, 159–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.