Abstract
Limb-girdle muscular dystrophy types 2E and F are characterized by skeletal muscle weakness and often cardiomyopathy and are due to mutations in the genes encoding β- and δ-sarcoglycan. We previously demonstrated that loss of sarcoglycans in smooth muscle leads to constrictions of the microvasculature that contributes to the cardiac phenotype. It is unclear how vasculature abnormalities affect skeletal muscle. We injected recombinant β- or δ-sarcoglycan adenoviruses into skeletal muscles of corresponding null mice. We hypothesized that the adenoviruses would not transduce vascular smooth muscle, and we would only target skeletal muscle. Indeed, sustained expression of intact sarcoglycan–sarcospan complex was noted at the sarcolemma, neuromuscular junction, myotendinous junction, and in peripheral nerve, but not in vascular smooth muscle. Gene transfer of the corresponding deleted sarcoglycan gene preserved sarcolemmal integrity, prevented pathological dystrophy and hypertrophy, and protected against exercised-induced damage. We conclude that vascular dysfunction is not a primary cause of β- and δ-sarcoglycan-deficient muscular dystrophy. In addition, we show successful functional rescue of entire muscles after adenovirus-mediated gene delivery. Thus, virus-mediated gene transfer of sarcoglycans to skeletal muscle in combination with pharmacological prevention of cardiomyopathy constitute promising therapeutic strategies for limb-girdle muscular dystrophies.
The sarcoglycans (α, β, γ, and δ-SG) are localized at the sarcolemma of muscle fibers and form, along with sarcospan, a subcomplex within the larger dystrophin–glycoprotein complex (DGC) (1–3). The DGC is further composed of the intracellularly located protein dystrophin, which binds to the transmembrane protein β-dystroglycan, which is associated with the peripheral protein α-dystroglycan. α-Dystroglycan, in turn, binds to laminin-2, present in the skeletal muscle basement membrane (4, 5). Thus, the DGC provides a structural link between the extracellular matrix and the intracellular cytoskeleton of muscle cells. The importance of the sarcoglycans for normal skeletal muscle function is underscored by the findings that mutations in the genes for γ-, α-, β-, and δ-SG cause limb-girdle muscular dystrophy types 2C-F, respectively. Furthermore, one mutant sarcoglycan isoform causes the loss or the reduction at the muscle cell membrane of all of the other sarcoglycan proteins in the DGC (6).
In striated muscle, the sarcoglycan complex is composed of α-, β-, γ-, and δ-SG. In addition, a second sarcoglycan complex in which ε-SG replaces α-SG is also expressed in striated muscle and is predominant in smooth muscle (7–10). Loss of the sarcoglycans in vascular smooth muscle results in perturbed vascular function with the presence of vascular constrictions leading to intermittent ischemic-like events, which initiate the development of cardiomyopathy in mouse models of β- and δ-SG deficiency (11–13). Because α-SG is not expressed in smooth muscle, α-SG deficiency does not lead to cardiomyopathy. Also, the dystrophic phenotype appears less severe in α-SG-deficient mice compared to the muscular dystrophy that develops in β- and δ-SG-deficient mice. Mice lacking β- and δ-SG display large areas of necrosis as the predominant characteristic feature similar to alterations observed in tissue infarcts. In addition, arterial constrictions are detected in skeletal muscle (11, 12). Despite uniform gene disruption, the surprising heterogeneity of phenotype within muscle groups, such as these focal regions of necrosis surrounded by regions of near normalcy, suggests the possibility that vascular abnormalities are playing a primary role in these muscular dystrophies. However, the hypothesis that vascular dysfunction directly initiates muscular dystrophy has remained untested (11, 12). To address this question, we injected recombinant β- or δ-SG adenoviruses into corresponding null mice (Sgcb- or Sgcd-null, respectively). We hypothesized that the adenoviruses would not cross the perimysium, in which blood vessels run, and thus we would only target skeletal muscle. The sarcoglycans were successfully restored at the sarcolemma, neuromuscular junction (NMJ), myotendinous junction (MTJ), and peripheral nerve, but not in smooth muscle of vessels within the skeletal muscle. Despite lack of restoration in vascular smooth muscle, the dystrophic phenotype was prevented, as demonstrated by improved skeletal muscle morphology, restored sarcolemmal integrity, and reduction of muscle mass. Also, injection of δ-SG adenovirus was able to prevent the development of exercise-induced injury. We conclude that (i) disruption of the DGC in vascular smooth muscle is not a primary cause of muscular dystrophy; (ii) primary mutations in β- and δ-SG can be functionally corrected in vivo; and (iii) structures such as NMJ, MTJ, and peripheral nerve that are affected in muscular dystrophy and other neuromuscular diseases can be targets for adenoviral-mediated gene delivery.
Methods
Animals. We previously reported the generation of Sgcb-null mice, lines Q94 and Q283 (12). Here, we have also analyzed Sgcb-null mice from a third clone, Q201. Sgcd-null mice, line I74 were also described (11). All mice were on hybrid C57BL/6–129-SvJ background. Colonies were maintained in the Animal Care Unit of the University of Iowa College of Medicine, according to the animal care guidelines. The Animal Care and Use Review Committees of the University of Iowa and the Medical College of Wisconsin approved all animal procedures.
Recombinant Adenoviral Vectors. Human β- and δ-SG sequences were amplified by PCR and subcloned into the pAdCMVpA adenovirus shuttle vector. The β- and δ-SG constructs were then incorporated into an adenovirus vector through standard methods of homologous recombination with Ad5 backbone dl309 by the University of Iowa Gene Transfer Vector Core. First-generation recombinant viruses were purified as described (12, 14).
Adenoviral Vector Administration. Sgcb- and Sgcd-null pups (2- to 4-day-old) were anesthetized via hypothermia by placement on ice-cooled aluminum foil for 1–2 min. Infectious particles (1–2 × 109) of Ad5 cytomegalovirus containing human β- or δ-SG cDNA diluted in a final volume of 10 μl in 0.9% NaCl were injected percutaneously into each skeletal muscle of the corresponding null mice by using an insulin syringe. Pups were reintroduced to the mother and kept in quarantine for 5 days. All pups survived after injections.
Immunohistochemical Analysis. Stainings were performed as described (12) with Abs against β-dystroglycan (15); α-, β-, γ-, and δ-SG (8, 14, 16, 17); sarcospan (18), and neuronal nitric oxide synthase (nNOS) (19). The smooth muscle actin Ab was from Sigma. Secondary Abs were conjugated with FITC or Cy3 (Jackson ImmunoResearch) or Alexa Fluor 488 (Molecular Probes). For staining of NMJs, samples were simultaneously incubated with FITC-conjugated α-bungarotoxin (Molecular Probes). Sections were observed under an MRC-600 laser scanning confocal microscope (Bio-Rad) or a Leica DMRXA fluorescence microscope (Leica, Deerfield, IL).
Biochemical Analysis. Glycoprotein preparations were performed as described (12) and analyzed by using Abs against α-SG (αSarc/20A6); β-SG (βSarc/5B1); α-dystroglycan (IIH6) (20); α2 subunit of the dihydropyridine receptor (21); δ-SG (14) and sarcospan (18).
MRI. Before imaging, mice were anesthetized with 87.5 mg/kg ketamine and 12.5 mg/kg xylazine and surgically instrumented with vein catheters for the administration of gadolinium (Gadodiamide, Omniscan; Nycomed, Oslo). All experiments were performed at the Medical College of Wisconsin on a 3-T Bruker Medspec MR imaging system (Bruker, Billerica, MA). Mice were placed in a 10-cm three-axis local gradient coil, together with a 2 × 4-cm quadrature transmit/receive radiofrequency surface coil. After obtaining sagittal scout images, five to seven 2-mm axial slices were chosen to image the tibialis anterior. T1-weighted spin-echo images were acquired after 20 min after administration of 0.2 mmol/kg gadolinium.
Treadmill Exercise. Animals were exercised by using the Omnipacer treadmill (model LC4/M-MGA/AT, Accuscan Instruments, Columbus, OH). Eight-week-old WT, Sgcd-null, and Sgcd-null mice injected with δ-SG adenovirus were exercised at a 15° downward angle with increasing speed up to 19 m/min, allowed to rest, and then the procedure was repeated. All mice were injected with Evans blue dye (EBD) i.p. 5 h before exercise. Mice were killed 24 h after exercise, and sections of muscles were studied for EBD uptake and β-dystroglycan and α-SG expression. Quantification of EBD-positive stained areas in sections of skeletal muscle was done by using the scion image program (Scion, Frederick, MD). The percentage of positive-stained areas was calculated by dividing the area of staining by the total area of the analyzed skeletal muscle section.
Results
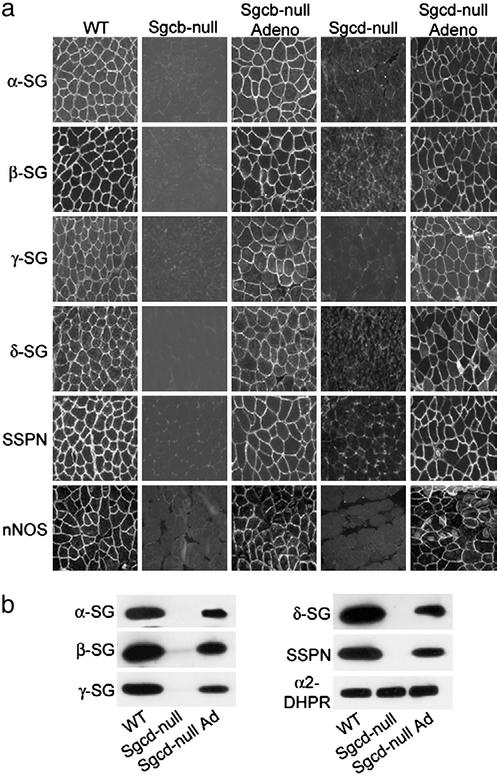
Restoration of DGC upon β- and δ-SG Gene Transfer. We injected 2- to 4-day-old Sgcb- or Sgcd-null pups directly into the tibialis anterior, quadriceps femoris, hamstring (gracilis, semitendinosus, semimembranosus, biceps femoris), and calf (gastrocnemius and soleus) muscles with adenoviruses containing the human β- or δ-SG cDNAs, respectively, under the control of the cytomegalovirus promoter. Renewed expression of the corresponding sarcoglycan in tibialis anterior, quadriceps femoris, hamstring, and calf muscles was coincident with the rescue of the entire sarcoglycan–sarcospan complex to the sarcolemma based on α-, β-, γ-, δ-SG, and sarcospan-specific immunofluorescence (Fig. 1a and data not shown). This result is in accordance with previous data from gene transfer experiments of β-SG in Sgcb-null mice (12). The DGC also directly or indirectly binds accessory proteins, including nNOS and Grb2 (22, 23). Sarcolemma localization of nNOS was severely reduced in Sgcb- and Sgcd-null muscle but maintained at the sarcolemma after gene transfer (Fig. 1a).
Fig. 1.
Restoration of the sarcoglycan–sarcospan complex and nNOS at the sarcolemma upon adenovirus-mediated expression of β- and δ-SG. (a) Quadriceps muscle cryosections from WT, Sgcb-null, Sgcb-null mice injected with recombinant β-SG adenovirus, Sgcd-null, and Sgcd-null mice injected with recombinant δ-SG adenovirus were stained with Abs against α-SG, β-SG, γ-SG, δ-SG, sarcospan (SSPN), and nNOS. (b) Glycoprotein preparations from quadriceps muscle of WT, Sgcd-null, and Sgcd-null mice injected with δ-SG adenovirus were analyzed after 16 days by SDS/PAGE and immunoblotting by using Abs against α-SG, β-SG, γ-SG, δ-SG, and sarcospan (SSPN) as indicated. Abs against α2-dihydropyridine receptor (DHPR) demonstrate equal loading.
In agreement with the immunofluorescence data, Sgcd-null muscle injected with δ-SG adenovirus exhibited renewed expression of the sarcoglycan–sarcospan complex as revealed by immunoblot analysis of glycoprotein preparations of quadriceps muscle (Fig. 1b). The association between the sarcoglycan complex and α-dystroglycan was also reestablished (data not shown). Together, these results provide strong evidence that the integrity of the DGC has been restored in Sgcb- and Sgcd-null muscle expressing the corresponding β- and δ-SG adenoviruses.
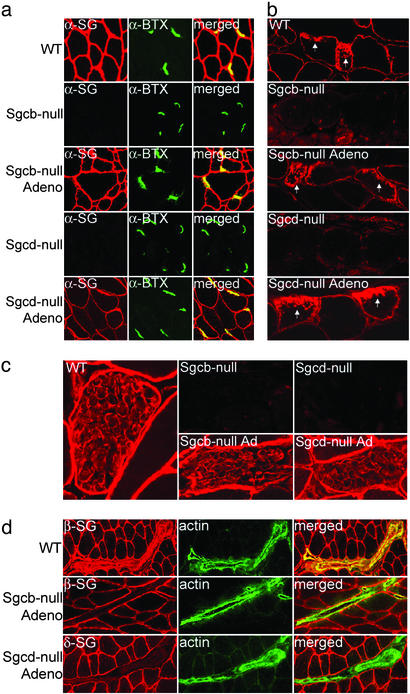
Reexpression of Sarcoglycans into Neuromuscular Junctions, Myotendinous Junctions, and Schwann Cells. We have previously shown that the sarcoglycans can be restored at the sarcolemma after adenovirus mediated α-SG gene transfer to α-SG-deficient mice (24). However, it has remained untested whether gene transfer can target specialized muscle membranes and other cell-types within skeletal muscle. For example, the sarcoglycans are normally expressed at the NMJ where they constitute a subcomplex within the utrophin–glycoprotein complex (25). Double stainings with FITC-coupled α-bungarotoxin, which binds acetylcholine receptors, revealed that the sarcoglycans were restored to the NMJs after β- or δ-SG gene transfer (Fig. 2a). Likewise, the sarcoglycans were re-established at the MTJ (Fig. 2b). A sarcoglycan complex is also present in the peripheral nerve and this complex is absent in Sgcb- and Sgcd-null mice (ref. 26; Fig. 2c). After β- or δ-SG gene transfer, the sarcoglycans were restored at the Schwann cell plasma membrane of the corresponding null mice (Fig. 2c).
Fig. 2.
Sarcoglycan expression is restored at the NMJ, MTJ, and Schwann cells, but not vasculature, on β- and δ-SG gene transfer. (a) Sections from skeletal muscle containing NMJ of WT, Sgcb-null, Sgcb-null mice injected with recombinant β-SG adenovirus, Sgcd-null, and Sgcd-null mice injected with recombinant δ-SG adenovirus were doubly stained with α-SG Ab (α-SG, red) and fluorescein α-bungarotoxin (α-BTX, green). (b) Sections from skeletal muscle of WT, Sgcb-null, Sgcb-null mice injected with recombinant β-SG adenovirus, Sgcd-null, and Sgcd-null mice injected with recombinant δ-SG adenovirus were stained with β-SG Abs. Arrows indicate MTJs. (c) Sections of skeletal muscle containing peripheral nerve of WT, Sgcb-null, Sgcb-null mice injected with recombinant β-SG adenovirus, Sgcd-null, and Sgcd-null mice injected with recombinant δ-SG adenovirus were stained with Abs against β-SG. (d) Sections from skeletal muscle containing smooth muscle of WT, Sgcb-null, and Sgcd-null mice injected with the corresponding sarcoglycan adenovirus were doubly stained with β-SG (β-SG, red) or δ-SG (δ-SG, red) and smooth muscle actin (actin, green) Abs.
The Vascular Smooth Muscle Sarcoglycan Complex Is Not Reconstituted on Gene Transfer. The smooth muscle sarcoglycan complex (ε-, β-, γ-, and δ-SG) is normally expressed in vascular smooth muscle where it colocalizes with smooth muscle actin (Fig. 2d). However, it has remained unclear whether smooth muscle in blood vessels can be transduced by i.m. injected adenoviral vectors. On β- or δ-SG gene transfer, the sarcoglycans were only reconstituted at the sarcolemma but not in the vascular smooth muscle, as revealed by double staining with smooth muscle actin (Fig. 2d).
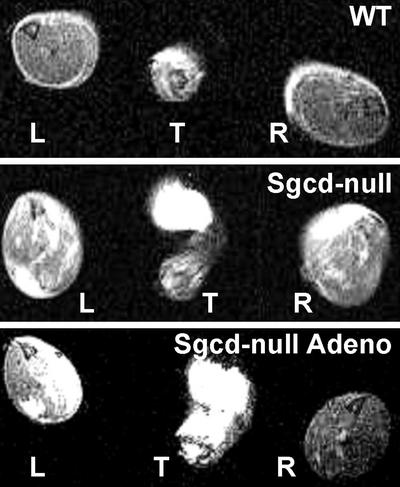
Sarcolemmal Integrity Is Restored on δ-SG Gene Transfer. After gene transfer (10 wk), sarcolemmal membrane permeability was assessed in vivo by using MRI of infused gadolinium, a contrast agent that binds reversibly to intracellular serum albumin. Absence of the sarcoglycan complex results in altered membrane permeability, which allows extracellular serum albumin and other serum proteins to cross into skeletal muscle fibers (11, 12, 17). T1-weighted spin-echo images obtained from the tibialis anterior muscles of noninjected Sgcd-null mice showed extensive areas of muscle damage as detected by uptake of the contrast agent. Interestingly, there was significantly less uptake in the injected muscle in all injected animals (Fig. 3). Together, these data demonstrate that the compromised sarcolemmal integrity in Sgcd-null mice can be reversed by restoration of the sarcoglycan complex. Similar results were previously obtained by using adenovirus-mediated α-SG gene transfer to α-SG-deficient mice (24).
Fig. 3.
Adenovirus-mediated gene transfer of δ-SG prevents uptake of gadolinium in tibialis anterior muscle. Contrast agent-enhanced MRI of WT (Top), noninjected (Middle), and right-leg-injected Sgcd-null (Bottom) mice. Cross section images through the left (L) and right (R) tibialis anterior muscles were taken 20 min after injection of gadolinium. T denotes the tail of the mouse. Note lack of uptake of contrast agent in WT muscles; dramatic uptake in Sgcd-null muscles (white areas) and major decrease in enhancement levels in right-side-injected muscles.
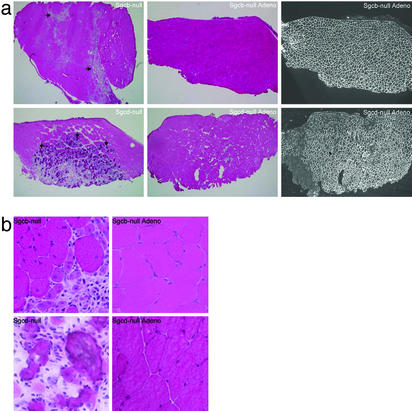
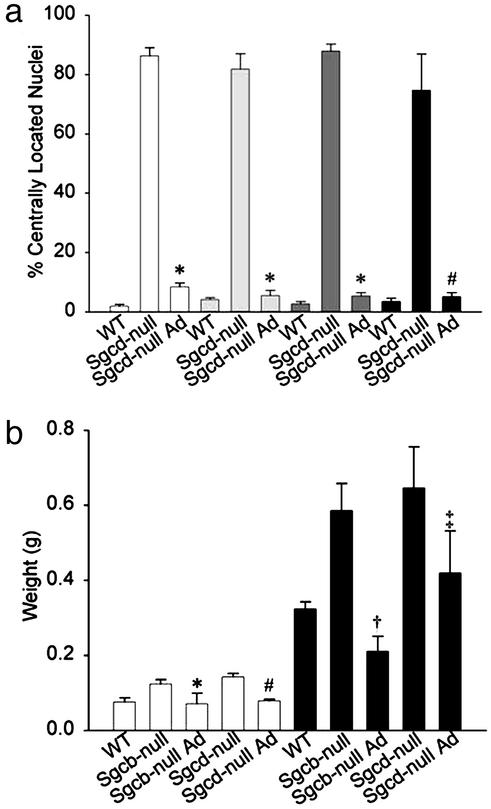
Human β- and δ-SG Gene Transfer Prevents Dystrophic Pathology of Skeletal Muscles. We next examined the morphology of injected and contralateral noninjected muscles of 35 Sgcb- or Sgcd-null mice 4–35 wk after injection. Histological features of the dystrophic muscle in Sgcb- and Sgcd-null mice include internally placed nuclei, large areas of necrosis, and dystrophic calcification (11, 12). One single injection of adenovirus into the tibialis anterior and hamstring muscles typically resulted in a 70–100% transduction of muscle fibers as revealed by immunofluorescence (Figs. 4a and 5 and data not shown). Injections into quadriceps femoris muscle resulted in transduction of vastus lateralis, but not rectus femoris (12, 24) with an efficiency of transduction of ≈40–80% (Fig. 5 and data not shown). Transduction efficiency of calf muscles was also ≈40–80% (Fig. 5 and data not shown). The dystrophic phenotype was prevented in transduced muscles, exhibited by absence of necrotic areas and dystrophic calcifications (Fig. 4). In addition, significantly fewer central nuclei were noted in transduced muscles (Figs. 4b and 6a). For several muscles of Sgcb- and Sgcd-null mice (≈30-wk-old), compared to WT mice, the masses were 30–95% greater (Fig. 6b and data not shown), most likely due to pathological hypertrophy. Because high transduction efficacy (70–100%) was exhibited in tibialis anterior and hamstring muscles, we analyzed the mass of these muscles after gene transfer. Indeed, the mass of transduced Sgcb- and Sgcd-null tibialis anterior and hamstring muscles was significantly less than the mass of uninjected Sgcb- and Sgcd-null muscles (Fig. 6b). Importantly, muscle fibers remained transduced for at least 35 wk, and the morphology of transduced muscles was significantly preserved (data not shown).
Fig. 4.
Adenovirus-mediated expression of β- and δ-SG protects the tibialis anterior muscle from dystrophic damage. (a Left) Severe dystrophic changes including central nucleation, necrosis (horizontal arrows), and dystrophic calcification (vertical arrows) were detected by hematoxylin and eosin staining of cryosections of noninjected tibialis anterior muscles of Sgcb-null and Sgcd-null mice. (Center) Contralateral tibialis anterior muscles 1 mo after i.m. injections of β- or δ-SG adenoviruses. Note that transduced fibers were protected from dystrophic damage. (Right) Serial sections of injected muscles stained with a polyclonal Ab against α-SG. (b) Higher magnification of muscles in a.
Fig. 5.
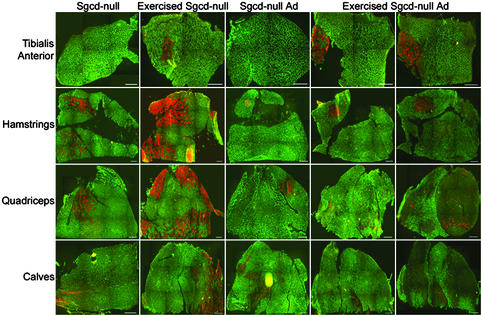
Injection of δ-SG adenovirus protects against exercised-induced injury. Composite cross-sectional images of tibialis anterior, hamstring, quadriceps, and calf muscles from Sgcd-null mice, exercised Sgcd-null mice, Sgcd-null mice injected with δ-SG adenovirus (Sgcd-null Ad), and exercised Sgcd-null mice injected with δ-SG adenovirus. Staining in green denotes β-dystroglycan (first four columns) or α-SG (last column), and staining in red denotes EBD uptake. (Bars = 220 μm.)
Fig. 6.
Adenovirus-mediated expression of δ- and β-SG protects myofibers
from central nucleation and increased muscle mass. (a) Percentage of
transduced fibers with centrally located nuclei 2 mo after adenovirus
injections into Sgcd-null tibialis anterior (□), hamstring
 , quadriceps
, quadriceps
 , and calf (▪)
muscles. *, Difference from Sgcd-null, P ≈ 0; #, difference
from Sgcd-null, P < 0.0015. (b) Tibialis anterior (□)
and hamstring (▪) muscle masses of 30-wk-old mice. *, Difference
from Sgcb-null, P < 0.026; #, difference from Sgcd-null,
P < 0.0002; †, difference from Sgcb-null and WT, P
< 0.001 and P < 0.008, respectively; ‡, difference from
Sgcd-null, P < 0.027. Statistical significance was examined by
using Student's t test.
, and calf (▪)
muscles. *, Difference from Sgcd-null, P ≈ 0; #, difference
from Sgcd-null, P < 0.0015. (b) Tibialis anterior (□)
and hamstring (▪) muscle masses of 30-wk-old mice. *, Difference
from Sgcb-null, P < 0.026; #, difference from Sgcd-null,
P < 0.0002; †, difference from Sgcb-null and WT, P
< 0.001 and P < 0.008, respectively; ‡, difference from
Sgcd-null, P < 0.027. Statistical significance was examined by
using Student's t test.
δ-SG Gene Transfer Protects Against Exercise-Induced Injury. To demonstrate functional benefit conferred by the virus-mediated expression of sarcoglycans, we exercised 8-wk-old WT, Sgcd- and Sgcd-null mice injected with δ-SG adenovirus in hind limb muscles. Skeletal muscles, in particular hamstring, quadriceps, and calf muscles, of Sgcd-null mice were susceptible to exercise-induced sarcolemmal injury as evidenced by increased uptake of EBD (Fig. 5) compared to exercised WT animals (data not shown) and nonexercised Sgcd-null muscle (Fig. 5). Quantitative image analysis indicated EBD uptake in hamstring muscles increased from 3.7 to 28.6% upon exercise whereas in quadriceps and calf muscles, EBD uptake increased from 1.8 to 18% and from 2.0 to 4.1%, respectively. Tibialis anterior muscle of Sgcd-null mice was less susceptible to exercise-induced injury (EBD uptake increased from 0.2% to 1.8%). Interestingly, δ-SG adenoviral injections were able to dramatically reduce exercise-induced injury (Fig. 5). The EBD uptake in exercised tibialis anterior, hamstring, quadriceps, and calf muscles injected with δ-SG adenovirus was 2.4%, 1.9%, 1.2%, and 0.1%, respectively.
Discussion
Muscular dystrophy represents a group of skeletal muscle diseases that are characterized by progressive muscle weakness and wasting. However, not only the muscle fiber per se is affected in muscular dystrophy but also other cell types (smooth muscle) and specialized muscle membranes (NMJ and MTJ) within skeletal muscle are involved. For example, loss of the sarcoglycans in vascular smooth muscle of Sgcb- or Sgcd-null mice is hypothesized to be necessary for the development of cardiomyopathy characterized by vascular constrictions and increased markers of ischemic damage. Also, vascular constrictions are present in skeletal muscle of these mice, but it is not clear whether they also contribute to the muscular dystrophy phenotype (11–13). To address this question, we injected recombinant β- or δ-SG adenoviruses into corresponding null mice. We assumed that the inability of adenoviral particles to cross the perimysium (24), in which many arteries run, would prevent transduction of vascular smooth muscle. One single injection of β- or δ-SG adenovirus into skeletal muscle of neonatal pups deficient in β- or δ-SG, respectively, resulted in a complete restoration of the skeletal muscle DGC at the sarcolemma and prevented the dystrophic pathology. The vascular smooth muscle DGC, on the other hand, was not restored. Because we were able to prevent muscular dystrophy, we conclude that vascular perturbations do not significantly contribute to the skeletal muscle pathology. If vascular dysfunction does not play a major role in the pathogenesis of muscular dystrophy of Sgcb- or Sgcd-null mice, why do mice deficient in α-SG (with an intact vascular smooth muscle complex) present a less severe muscle phenotype compared to mice deficient in β-or δ-SG, which have a disrupted smooth muscle sarcoglycan complex? One plausible explanation is the difference in skeletal muscle expression of ε-SG between the mutant animals. Mice lacking α-SG still express the ε-SG complex in skeletal muscle, whereas the skeletal muscle ε-SG complex is grossly perturbed in Sgcb- or Sgcd-null mice (7, 12, 17 and unpublished data). Thus, the ε-SG complex may partially compensate for the loss of the predominant α-SG-containing complex.
We herein report successfully reconstituted sarcoglycan complexes at the NMJ, MTJ, and peripheral nerves upon gene transfer. The transduction of peripheral nerve is surprising considering the fact that both blood vessels and nerves are located in the perimysium. Nevertheless, it is still possible that the virus can cross the perimysium and failure of transduction of vascular smooth muscle could be due to other factors. An alternative explanation is that the virus cannot cross the perimysium and infects the peripheral nerve by other means, possibly by infection of Schwann cell precursors or retrograde transport from the NMJ. Genes encoding for components of the NMJ are expressed in the subsynaptic nuclei, specialized myonuclei that lie beneath the NMJ (27). It was unclear whether the virus and promoter would target these nuclei. However, the successful restoration of the sarcoglycans at the NMJ suggests successful adenoviral targeting to these specialized myonuclei.
Although overt structural defects of the NMJs, MTJs, and peripheral nerves have not yet been reported in sarcoglycan-deficient animals, absence of other DGC components affects these structures (11, 12, 17, 26). Loss of dystroglycan in chimeric mice, for example, results in aberrant synapses in addition to muscular dystrophy (28). Likewise, α-dystrobrevin is important for the maturation and maintenance of the neuromuscular synapse and loss of α-dystrobrevin also results in muscular dystrophy (29, 30). Absence of integrin α7 leads to muscular dystrophy and pathological changes of the myotendinous junctions (31). Finally, lack of laminin α2 results in developmental dysmyelination of peripheral nerve in addition to severe muscular dystrophy (32–35). Thus, our data on successful adenoviral-mediated gene delivery to NMJ, MTJ, and peripheral nerve can be adapted to study these DGC components or even disparate proteins involved in disorders affecting these membrane specializations and differential cell types.
To date, there have been several reports on successful adenovirus-mediated gene transfer resulting in morphologically rescued muscles in various animal models of muscular dystrophy (14, 17, 24, 36). However, few reports have demonstrated any functional benefits of muscle gene transfer. A major functional defect in dystrophic muscle of mdx mice is muscle damage and sarcolemma disruptions caused by eccentric exercise (37). Also, treadmill exercise initiates the development of cardiac muscle necrosis in young Sgcd-null mice (11). Yet, the effects of exercise on β- or δ-SG-deficient skeletal muscle had been unclear. In Sgcd-null mice, our downhill running protocol produced a large increase in percentage of dye permeable muscle fibers, compared to nonexercised Sgcd-null mice and exercised normal mice. Thus, muscle fibers of Sgcd-null mice are more vulnerable to exercise induced sarcolemma damage than those of normal mice. Importantly, we report effective prevention of exercised induced injury and sarcolemma damage by adenovirus-mediated gene transfer. The muscle masses of 30-wk-old Sgcb-null and Sgcd-null mice were significantly greater than muscle masses of WT mice. Hypertrophy constitutes an adaptation to the continuous damage to muscle fibers. Lynch et al. (38) demonstrated that muscle hypertrophy in mdx mice is highly effective in the maintenance of absolute force, although the specific force was lower than that of the control mice since muscle masses of mdx mice are greater. The decreased muscle masses of injected Sgcb-null and Sgcd-null mice thus suggest that injected mice produce normal force levels. Together, we provide the first evidence for successful functional rescue of entire muscles after adenoviral-mediated gene-delivery.
In conclusion, we have established that vascular perturbation in skeletal muscle is not a primary cause of β- and δ-SG-deficient muscular dystrophy. In cardiac muscle, however, the presence of the vascular constrictions and ischemic-like injury appears to initiate the development of cardiomyopathy. We have demonstrated that cardiomyopathy in β- and δ-SG-deficient mice can be prevented by using verapamil, a calcium channel blocker (13). Thus, early virus-mediated gene transfer of sarcoglycans to skeletal muscle in combination with pharmacological intervention constitute promising therapeutic strategies for limb-girdle muscular dystrophies associated with cardiomyopathy.
Acknowledgments
We gratefully acknowledge Beverly L. Davidson and Richard D. Anderson and the Gene Therapy Center Vector Core Facility, supported by National Institutes of Health Grant P30DK54759, for their assistance. This work was supported in part by the Muscular Dystrophy Association. K.P.C. is an Investigator of the Howard Hughes Medical Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SG, sarcoglycan; DGC, dystrophin–glycoprotein complex; NMJ, neuromuscular junction; MTJ, myotendinous junction; EBD, Evans blue dye; nNOS, neuronal nitric oxide synthase.
References
- 1.Campbell, K. P. & Kahl, S. D. (1989) Nature 338, 259–263. [DOI] [PubMed] [Google Scholar]
- 2.Ervasti, J. M., Ohlendieck, K., Kahl, S., Gaver, M. G. & Campbell, K. P. (1990) Nature 345, 315–319. [DOI] [PubMed] [Google Scholar]
- 3.Crosbie, R. H., Lebakken, C. S., Holt, K. H., Venzke, D. P., Straub, V., Lee, J. C., Grady, R. M., Chamberlain, J. S., Sanes, J. R. & Campbell, K. P. (1999) J. Cell Biol. 145, 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ervasti, J. M. & Campbell, K. P. (1993) J. Cell Biol. 122, 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung, D., Yang, B., Meyer, H. J., Chamberlain, J. S. & Campbell, K. P. (1995) J. Biol. Chem. 270, 273005–27310. [DOI] [PubMed] [Google Scholar]
- 6.Cohn, R. D. & Campbell, K. P. (2000) Muscle Nerve 23, 1456–1471. [DOI] [PubMed] [Google Scholar]
- 7.Liu, L. A. & Engvall, E. (1999) J. Biol. Chem. 274, 38171–38176. [DOI] [PubMed] [Google Scholar]
- 8.Durbeej, M. & Campbell, K. P. (1999) J. Biol. Chem. 274, 26609–26616. [DOI] [PubMed] [Google Scholar]
- 9.Straub, V., Ettinger, A. J., Durbeej, M., Venzke, D. P., Cutshall, S., Sanes, J. R. & Campbell, K. P. (1999) J. Biol. Chem. 274, 27989–27996. [DOI] [PubMed] [Google Scholar]
- 10.Barresi, R., Moore, S. A., Stolle, C. A., Mendell, J. & Campbell, K. P. (2000) J. Biol. Chem. 275, 38554–38560. [DOI] [PubMed] [Google Scholar]
- 11.Coral-Vazquez, R., Cohn, R. D., Moore, S. A., Hill, J. A., Weiss, R. M., Davisson, R. L., Straub, V., Barresi, R., Bansal, D., Hrstka, R. F., et al. (1999) Cell 98, 465–474. [DOI] [PubMed] [Google Scholar]
- 12.Durbeej, M., Cohn, R. D., Hrstka, R. F., Moore, S. A., Allamand, V., Davidson, B. L., Williamson, R. A. & Campbell, K. P. (2000) Mol. Cell 5, 141–151. [DOI] [PubMed] [Google Scholar]
- 13.Cohn, R. D., Durbeej, M., Moore, S. A., Coral-Vazquez, R., Prouty, S. & Campbell, K. P. (2001) J. Clin. Invest. 107, R1–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt, K. H., Lim, L. E., Straub, V., Venzke, D. P., Duclos, F., Anderson, R. D., Davidson, B. A. & Campbell, K. P. (1998) Mol. Cell 1, 841–848. [DOI] [PubMed] [Google Scholar]
- 15.Williamson, R. A., Henry, M. D., Daniels, K. J., Hrstka, R. F., Lee, J. C., Sunada, Y., Ibraghimov-Beskrovnaya, O. & Campbell, K. P. (1997) Hum. Mol. Genet. 6, 831–841. [DOI] [PubMed] [Google Scholar]
- 16.Roberds, S. L., Anderson, R. D., Ibraghimov-Beskrovnaya, O. & Campbell, K. P. (1993) J. Biol. Chem. 268, 23739–23742. [PubMed] [Google Scholar]
- 17.Duclos, F., Straub, V., Moore, S. A., Venzke, D. P., Hrstka, R. F., Crosbie, R. H., Durbeej, M., Lebakken, C. S., Ettinger, A. J., van der Meulen, J., et al. (1998) J. Cell Biol. 142, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebakken, C. S., Venzke, D. P., Hrstka, R. F., Consolino, C. M., Faulkner, J. A., Williamson, R. A. & Campbell, K. P. (2000) Mol. Cell. Biol. 20, 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crosbie, R. H., Straub, V., Yun, H.-Y., Lee, J. C., Rafael, J. A., Chamberlain, J. S., Dawson, V. L., Dawson, T. M. & Campbell, K. P. (1998) Hum. Mol. Genet. 7, 823–829. [DOI] [PubMed] [Google Scholar]
- 20.Ervasti, J. M. & Campbell, K. P. (1991) Cell 66, 1121–1131. [DOI] [PubMed] [Google Scholar]
- 21.Ohlendieck, K. & Campbell, K. P. (1991) J. Cell Biol. 115, 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, B., Jung, D., Motto, D., Meyer, J., Koretzky, G. & Campbell, K. P. (1995) J. Biol. Chem. 270, 11711–11714. [DOI] [PubMed] [Google Scholar]
- 23.Brenman, J. E., Chao, D. S., Xia, H., Aldape, K. & Bredt, D. S. (1995) Cell 82, 743–752. [DOI] [PubMed] [Google Scholar]
- 24.Allamand, V., Donahue, K. M., Straub, V., Davisson, R. L. & Campbell, K. P. (2000) Gene Ther. 7, 1385–1391. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura, K., Ervasti, J. M., Ohlendieck, K., Kahl, S. D. & Campbell, K. P. (1992) Nature 360, 588–591. [DOI] [PubMed] [Google Scholar]
- 26.Imamura, M., Araishi, K., Noguchi, S. & Ozawa, E. (2000) Hum. Mol. Genet. 9, 3091–3100. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer, L., de Kerchove d'Exaerde, A. & Changeux, J. P. (2001) Neuron 31, 15–22. [DOI] [PubMed] [Google Scholar]
- 28.Côté, P. D., Moukhles, H., Lindenbaum, M. & Carbonetto, S. (1999) Nat. Genet. 23, 338–342. [DOI] [PubMed] [Google Scholar]
- 29.Grady, R. M., Grange, R. W., Lau, K. S., Maimone, M. M., Nichol, M. C., Stull, J. T. & Sanes, J. R. (1999) Nat. Cell Biol. 4, 215–220. [DOI] [PubMed] [Google Scholar]
- 30.Grady, R. M., Zhou, H., Cunningham, J. M., Henry, M. D., Campbell, K. P. & Sanes, J. R. (2000) Neuron 25, 279–293. [DOI] [PubMed] [Google Scholar]
- 31.Mayer, U., Saher, G., Fässler, R., Bornemann, A., Echtermeyer, F., von der Mark, H., Miosge, N., Poschl, E. & von der Mark, K. (1997) Nat. Genet. 17, 318–323. [DOI] [PubMed] [Google Scholar]
- 32.Bradley, W. G. & Jenkinson, M. (1973) J. Neurol. Sci. 18, 227–247. [DOI] [PubMed] [Google Scholar]
- 33.Madrid, R. E., Jaros, E., Cullen, M. J. & Bradley, W. G. (1975) Nature 257, 319–321. [DOI] [PubMed] [Google Scholar]
- 34.Xu, H., Christmas, P., Wu, X. R., Wewer, U. M. & Engvall, E. (1994) Nat. Genet. 8, 297–302. [DOI] [PubMed] [Google Scholar]
- 35.Sunada, Y., Bernier, S. M., Utani, A., Yamada, Y. & Campbell, K. P. (1995) Hum. Mol. Genet. 4, 1055–1061. [DOI] [PubMed] [Google Scholar]
- 36.Wakefield, P. M., Tinsley, J. M., Wood, M. J., Gilbert, R., Karpati, G. & Davies, K. E. (2000) Gene Ther. 7, 201–204. [DOI] [PubMed] [Google Scholar]
- 37.Brussee, V., Tardif, F. & Tremblay, J. P. (1997) Neuromuscul. Disord. 7, 487–492. [DOI] [PubMed] [Google Scholar]
- 38.Lynch, G. S., Hinkle, R. T., Chamberlain, J. S., Brooks, S. V. & Faulkner, J. A. (2001) J. Physiol. (London) 535, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]