Abstract
One hypothesis for the etiology of behavioral disorders is that infection by a virus induces neuronal cell dysfunctions resulting in a wide range of behavioral abnormalities. However, a direct linkage between viral infections and neurobehavioral disturbances associated with human psychiatric disorders has not been identified. Here, we show that transgenic mice expressing the phosphoprotein (P) of Borna disease virus (BDV) in glial cells develop behavioral abnormalities, such as enhanced intermale aggressiveness, hyperactivity, and spatial reference memory deficit. We demonstrate that the transgenic brains exhibit a significant reduction in brain-derived neurotrophic factor and serotonin receptor expression, as well as a marked decrease in synaptic density. These results demonstrate that glial expression of BDV P leads to behavioral and neurobiological disturbances resembling those in BDV-infected animals. Furthermore, the lack of reactive astrocytosis and neuronal degeneration in the brains indicates that P can directly induce glial cell dysfunction and also suggests that the transgenic mice may exhibit neuropathological and neurophysiological abnormalities resembling those of psychiatric patients. Our results provide a new insight to explore the relationship between viral infections and neurobehavioral disorders.
Neurobehavioral disorder is a complex disease that must arise from the actions of many genes and/or environmental factors. A number of working hypotheses propose that viral infection, as an environmental factor, contributes to the induction of neuropathological and neurophysiological disturbances, resulting in a wide range of behavioral abnormalities (1–3). Studies using animal models reveal that viruses can induce neurobehavioral disturbances predominantly through an indirect pathway that involves the release of various factors by infiltrating cells or by glial cells (3–5). This process seems to be a common pathway to neuronal injury in a wide variety of neurodegenerative disorders in which glial proliferation typically accompanies. In the case of major psychiatric disorders, however, a spectrum of neurological abnormalities associated with glial cell dysfunction is found in brains without neuronal degeneration (2, 6–8). Despite increased insight into the mechanisms of the neuropathogenesis of different viruses, viral infections or specific antigens that develop neurobehavioral abnormalities associated with psychiatric disorders have not yet been identified.
Borna disease virus (BDV) is a highly neurotropic virus that belongs to the Mononegavirales. Natural infection of BDV has now been found in a wide variety of vertebrates, suggesting that the host range of this virus probably includes all warm-blooded animals (9, 10). BDV persistently infects the CNS of many animal species and causes behavioral disturbances, such as anxiety, aggression, hyperactivity, abnormal play behavior, and cognitive deficits, reminiscent of autism, schizophrenia, and mood disorders (11–14). Thus, studies on this virus provide an important paradigm for the investigation of the mechanisms by which virus infection induces neurobehavioral disorders. Moreover, epidemiological studies have demonstrated a higher prevalence of BDV infection in psychiatric patients than in controls (15–17). These observations suggest that BDV is a human pathogen and that viral infection may play a role in the induction of certain human mental illnesses. Here we use transgenic mice expressing the phosphoprotein (P) of BDV in the CNS to understand the linkage between CNS virus infection and neurobehavioral disorders, as well as to elucidate the molecular nature of BDV neuropathogenesis. The P is abundantly expressed in infected animal brains and interferes with a multifunctional protein, HMGB1, in neuronal cells (18, 19), suggestive of a neurotropic effect of this protein in the infected CNS. We demonstrate that BDV P transgenic mice develop behavioral abnormalities resembling those in BDV-infected animals, and that the transgenic brains show severe neurobiological disturbances linked to neurobehavioral disorders.
Materials and Methods
Construction of Transgenic Mice. A BamHI–BglII fragment containing BDV P cDNA derived from MDCK/BDV cells was inserted into the BamHI linker site of a modified pGfa2Lac-1 plasmid containing a human glial fibrillary acidic protein (GFAP) promoter and the mouse protamine sequences (20). The 3.4-kb BglII fragment containing the GFAP promoter, BDV P cDNA, and the mouse protamine sequences from the construct was used for microinjection as a transgene, and ≈500 copies were microinjected into the pronuclei of fertilized C57BL/6 mouse embryos. Transgenesis was determined on tail DNA by PCR using primers specific for BDV P. Four founders were used to establish independent transgenic lines.
Behavioral Testing. For isolation-induced aggression, male resident test mice were isolated for at least 4 wk. Cages were changed once per week, but not during the week preceding the test period. Aggressive behaviors in 3- to 8-mo-old test mice were monitored during 5-min exposures to WT C57BL/6 male intruder mice that had been group-housed (three per cage). Five test sessions were conducted (one trial per day). The latency period until the first biting attack and the total number of biting attacks were recorded by videotape during each test session. For mice that failed to attack, the latency period was scored as 5 min.
A Morris-type water maze task was used to compare the cognitive abilities of transgenics and controls. The maze was a circular pool (90-cm diameter) filled with opacified water and maintained at 25°C. A round Perspex platform (12-cm diameter) was placed inside the tank at the center of the target quadrant, 1.5 cm below the water surface. Spatial training consisted of five trials (one block) per day, each trial lasting until the mouse reached the platform or 90 s, whichever came first, with 15 min between separate trials. After each trial, mice remained on the platform for 15 s. Twenty-four hours after the 20th trial, all mice were subjected to a test in which they swam for 90 s in the pool without a platform. During acquisition and trials, the movements of mice in the tank were recorded on videotape for subsequent analysis. For each trial, performance was expressed as the percentage of time spent in each of the four quadrants, and the number of crossings through the area where the platform used to be.
Locomotor activity was assessed in an activity cage made of a cubicle of clear Perspex (54 × 50 cm2, 37 cm high; Ugo Basile, Comerio, Italy). The cage consisted of two blocks facing each other that contained an infrared ray array of emitters and sensors. The system could be used to automatically monitor the horizontal or vertical movements of the animals by counting the number of times each animal crossed the infrared beams. Mice were placed in the cage for 10 min (per trial), and the beam crossings were counted during that period. Statistical significance was assayed by ANOVA, followed by Fisher's post hoc test.
Immunohistochemistry. For the immunostaining of transgenic brains, deparaffinized thin sections (4 μm) were incubated with trypsin solution for 30 min at 37°C, except for sections used for GFAP staining. The sections were then treated with 0.05% HIO4 to quench endogenous peroxidase activity and were washed extensively in Tris-buffered saline. After a blocking step, the sections were incubated with rabbit anti-P polyclonal antibody (1:1,000; ref. 21), anti-GFAP antibody (Zymed), and/or antisynaptophysin monoclonal antibody (1:10, Chemicon). For detection of transgene product, primary antibodies were detected with biotinylated goat anti-rabbit IgG and avidin-biotin peroxidase complex (Vector Laboratories) by using diaminobenzidine/H2O2. For double staining using P and GFAP antibodies, FITC-conjugated donkey anti-goat and Cy3-conjugated donkey anti-rabbit IgG antibodies (1:500, Jackson ImmunoResearch) were used. For synaptophysin, specific reactions were visualized with a Fast Red Substrate System (DAKO).
For a quantitative analysis of the synaptophysin levels in the brains, the area of interest was captured by Nikon E600 microscope and charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan) under the same optical and lighting conditions. The average of optical density of positive signals was measured in six different fields of each specific region, including the cerebellum and molecular layer of the hippocampus, in at least four sections for each animal by MACSCOPE software (Mitani, Fukui, Japan). This software established two threshold densities (minimum and maximum) for the signals and extracted the pixel intensity within the thresholds to determine the optical density in selected fields. The densities beyond the thresholds were measured as backgrounds.
Semiquantitative RT-PCR Analysis of Serotonin Receptors. Semiquantitative RT-PCR for serotonin (5-HT) receptors was performed with primers 5-HT1A (5′-ACCATCTACTCCACTTTCGGCG-3′, sense; 5′-TTCACTGTCTTCCTCTCACGGG-3′, antisense); 5-HT1B (5′-AGGAGCAGGGTATTCAGTGCG-3′, sense; 5′-TGTCCAGCGTCCAGTGACCG-3′, antisense); 5-HT2C (5′-CCTACGCCGTCAAACCCTG-3′, sense; 5′-GCCTTCCCACAAAGCACCGACAG-3′, antisense). Amplification was conducted in the linear range. The amplification products were resolved on 1.5% agarose gels. The images on the agarose gels were captured electronically, and the intensity of each band was quantified by using nih image. The relative ratios of 5-HT receptors to GAPDH mRNA (primers 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′) levels were calculated and were expressed as a percentage of the mean value found in nontransgenic mice.
Results
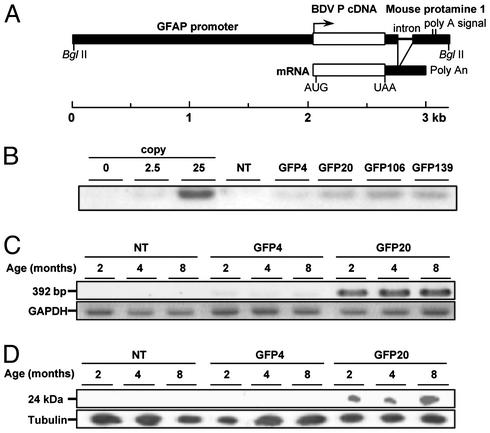
Generation of Transgenic Mice. Transgenic mice expressing BDV P were generated under the control of a human GFAP promoter (Fig. 1A). GFAP is expressed predominantly in astrocytes (22), which are one of the targets of BDV infection. Four founders were identified by PCR analysis of lysates from tail biopsies and used to establish separate transgenic lines. By DNA blot hybridization, a variable number of transgene copies were detected within these lines (Fig. 1B). We used lines GFP4 and GFP20 for further analyses, because the expression levels of P mRNA and protein in the CNS were significantly different between the two lines (Fig. 1 C and D); the expression in line GFP20 (high expressor line) was significantly higher than that in line GFP4 (low expressor line).
Fig. 1.
Construction of BDV P transgenic mice. (A) GFAP promoter-driven transgene encoding BDV P (see Materials and Methods). (B) Southern blot analysis of the tail DNA samples from P transgenic mice. DNA samples (10 μg) were subjected to DNA blotting with a digoxigenin-labeled BDV P probe. As controls, plasmid-derived P cDNA corresponding to 0, 2.5, or 25 copies per diploid was also used. NT, nontransgenic. (C) Detection of P mRNA in the brains by RT-PCR. Total RNAs were isolated from the brains of 2-, 4-, and 8-mo-old mice. As a control for RNA input, the levels of GAPDH were assayed (see Materials and Methods). (D) Immunoblot analysis of the brains of transgenic mice. The lysates (10 μg of protein per lane) from the brains of transgenic mice (2, 4, and 8 mo old) were detected by P polyclonal antibody. As a control for protein input, the level of expression of tubulin was measured.
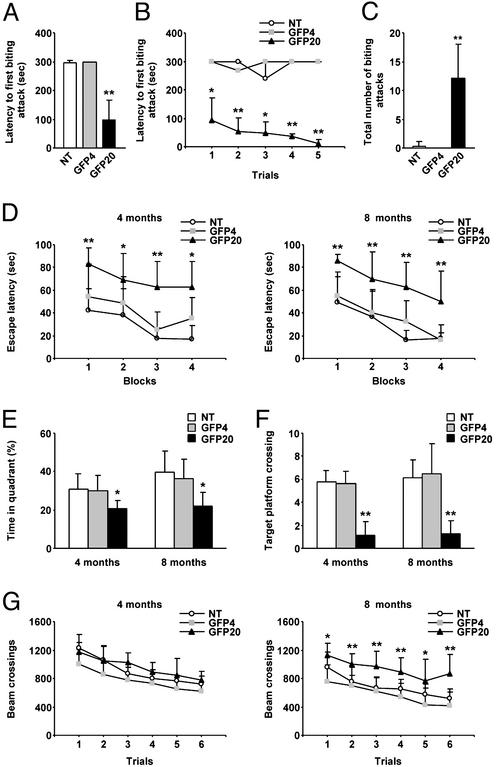
Behavioral Analysis of Transgenic Mice. The transgenic mice developed normally and were apparently normal as adults. To characterize the effects of P expression in the CNS, we assessed the behavioral alterations in P transgenic mice in terms of aggressive behavior, cognitive ability, and locomotor activity. To investigate aggressive behavior in these mice, we measured the offensive intermale fighting among the mice by using a resident–intruder paradigm. Four-month-old GFP20 resident males attacked an intruder more rapidly than did the GFP4 and the nontransgenic residents [n = 6, 99.5 ± 66.0 s (GFP20) compared with 297.2 ± 6.94 s (nontransgenic), P < 0.001; Fig. 2A]. Two-way ANOVA revealed that the biting attack latencies of GFP20 mice were consistently short during the trial blocks (F2,30 = 183.7, P < 0.001), whereas no significant effect of trial block on the latencies was found in the testing (F4,30 = 0.975, P = 0.435) (Fig. 2B). The number of biting attacks was also significantly higher in line GFP20 [n = 6, 12.16 ± 5.84 times (GFP20) compared with 0.33 ± 0.81 times (nontransgenic), P < 0.001; Fig. 2C]. The low expressor GFP4 and control mice showed very similar and fairly constant biting attack performance over the course of testing. Older GFP20 mice (8–12 mo old) and another high expressor line, GFP139, also displayed aggressive behavior similar to that of the 4-mo-old GFP20 toward intruders. Interestingly, although it was not quantified, the high expressor lines showed a lack of coordination with frenzied activity; they always assumed a biting position toward human hands when the researchers attempted to pick them up by the tail, whereas the low expressor and control mice never showed such aggressive behavior.
Fig. 2.
Behavioral alterations in P transgenic mice. (A) Offensive intermale aggression of P transgenic mice. The latency to the first biting attack was measured in 4-mo-old transgenic and nontransgenic (NT) mice. The results of the first trial are shown. ANOVA revealed a significant difference among the genotypes. (B) The latency to the first biting attack during five trial blocks. (C) Number of biting attacks in 4-mo-old transgenic and control mice. (D) The latency to escape to the hidden platform in the water maze was abnormally increased in GFP20 mice. (E) The time spent in the target quadrant during a probe test. All data are mean values and ±SEM of 4- and 8-mo-old transgenic and NT mice. ANOVA revealed a significant difference among the genotypes. (F) The number of crossings through the platform area during a 90-s probe test. ANOVA revealed a significant difference among the genotypes. (G) Locomotor activities of 4- and 8-mo-old transgenic and NT mice. The number of times the mice crossed the infrared beams was counted during a trial for horizontal activity. Values are expressed as mean ± SEM (*, P < 0.01; **, P < 0.001).
The cognitive abilities of P transgenic mice were examined by using a Morris-type water maze test (Fig. 2D). Although no significant differences in escape latency were found between GFP4 and control mice at both 4 and 8 mo of age, the high expressor line GFP20 revealed a significant delay in escape latency. Two-way ANOVA revealed significant effects of the genotype and the trial block on the escape latency (n = 10, genotype F2,84 = 29.639, P < 0.001, trials F3,84 = 8.565, P < 0.001 at 4 mo; genotype F2,92 = 45.017, P < 0.001, trials F3,92 = 17.273, P < 0.001 at 8 mo), whereas the effects of genotype × trial interaction did not (F6,84 = 0.484, P = 0.818 at 4 mo; F6,92 = 0.352, P = 0.906 at 8 mo). These results indicated that, although GFP20 mice showed a significant delay in escape latency over the trials, the transgenic mice mastered the task at learning rates similar to those of GFP4 and control mice. After the last training day, all mice were given a probe trial. The test revealed a significant reduction in spatial retention in GFP20 mice, whereas GFP4 and control mice showed a clear preference for the target quadrant (Fig. 2E). Line GFP20 spent 22.02 ± 6.92% of the time in the target quadrant compared with 39.68 ± 11.08% for the controls (P = 0.0055 for 4-mo-old mice, P < 0.001 for 8-mo-old mice). GFP20 mice also showed a significant reduction in the number of crossings through the area where the platform used to be [1.25 ± 1.16 times (GFP20) compared with 6.1 ± 1.59 times (nontransgenic), P < 0.001; Fig. 2F]. These data revealed that spatial reference memory in the GFP20 mice was significantly impaired.
The locomotor abilities of the mice were analyzed by using an activity cage. Two-way ANOVA showed significant effects of the genotype (n = 8, F2,138 = 45.365, P < 0.001) and trial (n = 8, F5,138 = 11.368, P < 0.001) in the horizontal activity of 8-mo-old GFP20 mice (Fig. 2G). GFP4 and control mice showed very similar activity over the course of testing. Furthermore, the maximum swimming speed of the transgenic mice was not significantly different from that of control mice at both 4 and 8 mo of age (data not shown). These observations suggested that the GFP20 mice developed hyperactivity and that the cognitive deficits in this line were not due to physical disabilities.
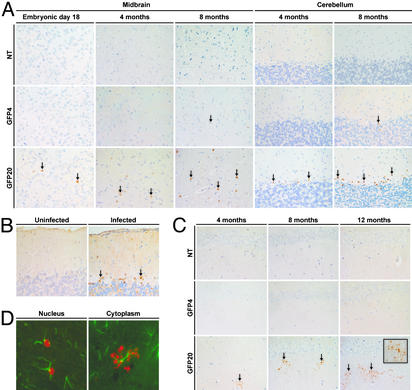
Transgene Expression in the Brains. To understand the behavioral alterations in the high expressor mice, we investigated the neuropathology of the transgenic mice. The GFP20 mice clearly produced the transgene product in the brain as early as embryonic day 18 (Fig. 3A). In association with development, the number of immunoreactive cells increased, and the positive cells were found to be widely distributed in the brains of GFP20 mice. In 3- to 4-mo-old GFP20 mice, the transgene product was readily detected in Bergmann glial cells of the cerebellum (Fig. 3A). A similar staining pattern of P was frequently found in rat brains infected with BDV (Fig. 3B). On the other hand, weak P-positive signals appeared in the low expressor line, GFP4, only at 8 mo of age. The expression of P in the GFP4 brains was fairly stable at low levels during the observation period. Of note, the immunohistological study revealed alteration in the staining pattern of P in GFP20 brains. At 4 mo of age, P began to be observed in the neuropil of the hippocampus as small dots with a punctate staining pattern (Fig. 3C). The level of neuropil staining obviously increased with age in the GFP20 mice (Fig. 3C). Immunostaining with P and GFAP antibodies revealed that the P was found in the nucleus of GFAP-positive cells during the early adulthood of GFP20 mice, whereas cytoplasmic staining of the protein was identified in mice >4 mo old (Fig. 3D). The punctate staining was associated with the cell processes of astrocytes, suggesting a possibility for the deposition and accumulation of P in the cytoplasm of astrocytes in the hippocampal neuropil in the aged GFP20 mice.
Fig. 3.
Expression of BDV P in transgenic brains. (A) Brain sections were immunostained with an antibody to BDV P. The positive signals are indicated by arrows. (B) The P-positive signals in Bergmann glial cells of the cerebellum of persistently BDV-infected rats (8 wk after neonatal infection, arrows). (C) The P signals were detected in the hippocampal neuropil of the high expressor brains after 4 mo of age (arrows). The stained areas increased with the age of the mice. NT, nontransgenic. (D) Double staining of GFP20 brain sections with P (red) and GFAP (green) antibodies revealed that P was present not only in the nucleus (Left) but also in the cytoplasm (Right: hippocampal neuropil region of 6-mo-old mice) of GFAP-positive cells.
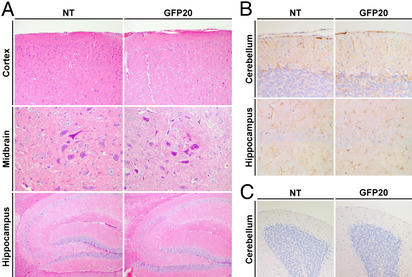
On the other hand, in the transgenic brains, neither reactive astrocytosis nor loss of large neurons such as the pyramidal neurons and Purkinje cells was observed by the hematoxylin and eosin and GFAP stainings, even in the aged high expressor lines (Fig. 4 A and B). Furthermore, the numbers of terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-positive cells were not significantly different between GFP20 and nontransgenic mice in any regions of the brains from 8-mo-old animals (Fig. 4C), indicating that neurodegenerative reactions do not occur in the transgenic mice. Importantly, although the transgenic mice developed behavioral abnormalities, GFP20 mice did not show degeneration of the hippocampus dentate gyrus neurons usually found in the brains of BDV-infected rats (Fig. 4A).
Fig. 4.
Neuroanatomical analysis of transgenic brains. (A and B) Brain sections from 8-mo-old nontransgenic (NT) and GFP20 mice were stained with hematoxylin and eosin (A) and GFAP antibody (B). The cerebral cortex, midbrain, cerebellum, and hippocampus regions are shown. (C) Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining was performed with brain sections from 8-mo-old NT and GFP20 mice. The cerebral cortex regions are shown.
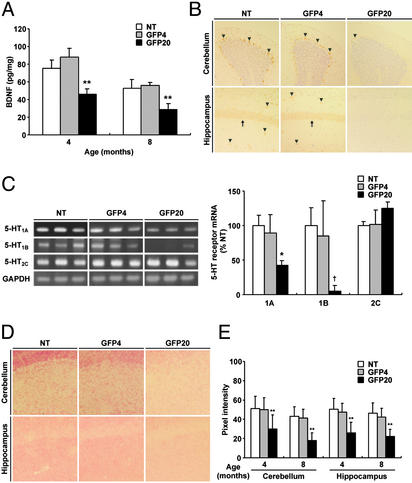
Neurobiological Abnormalities in Transgenic Brain. Previous studies have demonstrated that behavioral abnormalities could be reflected by alterations in the expression of neurotrophic factors such as brain-derived neurotrophic factor (BDNF; refs. 23–26). Therefore, we next investigated the expression of the BDNF in the transgenic mouse brains. ELISA revealed a significant reduction in the BDNF level in the hippocampus of GFP20 mice [4 mo: 46.2 ± 5.68 pg/mg (GFP20) compared with 75.6 ± 9.11 pg/mg (nontransgenic), n = 4, P < 0.001; 8 mo: 28.9 ± 6.59 pg/mg (GFP20) compared with 52.6 ± 9.79 pg/mg (nontransgenic), n = 4, P < 0.001], whereas GFP4 showed a level related to that of control mice (Fig. 5A). In situ hybridization also showed a decreased expression of BDNF mRNA in the cerebellum and hippocampus (Fig. 5B). BDNF expression in the GFP20 brains showed no recovery to the levels observed in the controls during an observation period of 14 mo.
Fig. 5.
Neurobiological abnormalities in the brains of high expressor line. (A) The ELISA system revealed a significant decrease in the BDNF levels in the hippocampus of GFP20 mice. (B) The brains of the GFP20 mice showed a reduced level of expression of BDNF mRNA (arrowheads) compared with the low expressor line, GFP4, and nontransgenic (NT) mice. Arrows indicate BDNF-positive neurons in the cornu ammonis layer (CA1). The brain sections were prepared from 8-mo-old mice. In situ hybridization was performed as described (21). (C) Expression of 5-HT receptors in transgenic and control brains. The results from three different mice in each group are shown. The intensities of amplification products were quantified. (D) Detection of synaptic formation by synaptophysin staining. The hippocampus and cerebellum regions of 8-mo-old mouse brains are shown. (E) Quantitative analysis of the synaptophysin levels in transgenic brain. Statistical significance was assayed by ANOVA, followed by Fisher's post hoc test (*, P < 0.01; **, P < 0.001; †, P = 0.02).
BDNF moderates the 5-HT system, as well as the synapse formation, at multiple levels in the CNS (23, 27, 28). Thus, we examined the expression of 5-HT receptors and synaptic density in the transgenic brains. To determine the expression of 5-HT receptor mRNA, we used semiquantitative RT-PCR analysis. We focused on the mRNAs of three 5-HT receptors (5-HT1A, 5-HT1B, and 5-HT2C receptors) that were suggested to play prominent roles in the regulation of several behaviors, including aggression (29–32). Repeated tests revealed significant decreases in the mRNA levels of 5-HT1A and 5-HT1B receptors, but not of 5-HT2C receptor, in the hippocampus of 8-mo-old GFP20 brains (n = 4, GFP20 vs. nontransgenic; 5-HT1A receptor, P = 0.007; 5-HT1B receptor, P = 0.02; 5-HT2C receptor, P = 0.116) (Fig. 5C).
To determine whether the expression of P in the transgenic brains affects synaptic formation, sections from the 4- and 8-mo-old mice were immunolabeled with synaptophysin antibody. The analysis of the optical density of positive signals clearly showed that the density of the synapses was significantly decreased in the GFP20 brains, in comparison with GFP4 and nontransgenic brains at the same age (n = 4, P < 0.001; Fig. 5 D and E). These observations indicated that transgenic expression of P in glial cells resulted in severe neurobiological abnormalities, including synaptic damage, that have also been reported in the brains of BDV-infected animals.
Discussion
We have shown that glial expression of BDV P leads to behavioral abnormalities, as well as neurobiological disturbances, in transgenic mice. We have also demonstrated that neither glial cell proliferation nor neurodegenerative reaction occurs in the transgenic brains, indicating that P can directly induce neuronal cell dysfunctions leading to behavioral alterations. The presence of a similar behavioral abnormality in a separate high expressor line indicates that they are not caused by an insertional mutation with neurodevelopmental consequences. Numerous studies using the GFAP promoter have confirmed that the effects of P expression do not result nonspecifically from astrocyte expression of any foreign protein (22, 33). A specific relationship between BDV P expression and induction of neurobiological disturbances is further supported by the correlation between the intensity of abnormality and the expression level of P in different transgenic lines. As far as we tested, the low/late expressor GFP4 showed almost the same performance on behavioral tests as control mice and no neurobiological disturbances, suggesting that not only the level but also the developmental expression of P in the brains may be critical to induce behavioral abnormalities in mice.
Previous studies demonstrated that BDV persistent infection develops neurobehavioral diseases in rodent models. BDV infection in a mouse strain that lacks functional CD8+ T cells leads to persistent infection and causes learning deficits without overt neuronal loss (14). Furthermore, it is known that some strains of mice exhibit locomotor hyperactivity after BDV infection (11). In addition, neonatally BDV-infected rats show learning and memory deficits, as well as hyperactivity, in the absence of an inflammatory response (34, 35). In neonatally infected rats, an abnormal level in BDNF and 5-HT receptors was also demonstrated (36, 37). These similarities between BDV-infected animals and P transgenic mice may indicate that P is a main contributor to BDV-induced neuronal abnormalities. However, it is also true that there are significant differences between persistently BDV-infected rodents and P transgenic mice in the behavioral and neurochemical alterations. In neonatally infected rats, BDV causes significant neuroanatomical disturbances characterized by degeneration of the dentate gyrus, cortical shrinkage, and cerebellar hypoplasia (13). It has been also demonstrated that neonatally infected rats show abnormal social play behavior, but not aggressive behavior, by the intruder–resident paradigm (12). A chronic astrocytosis and microgliosis were also demonstrated in neonatally infected rat brains (38). Moreover, BDV-infected rodent brains exhibit severe abnormalities in the levels of cytokines, chemokines, neurotrophic factors, and neurotransmitters and their receptors. These discrepancies give rise to a possibility that behavioral deficits in the transgenic mice may be qualitatively different from those seen in the natural course of BDV persistent infection. It is possible that the phenotype in P transgenic mice is mainly related to effects due to glial expression of P in early life. Nevertheless, the findings in the transgenic mice could provide a good basis for the pathological role of P in BDV infection, because an abundant expression of P is always found in persistently infected brains.
Although the mechanism by which glial expression of P induces neurobiological disturbances remains to be elucidated, the lack of reactive astrocytosis and neuronal cell death in the transgenic brains suggests that P can directly affect astrocyte function. Recent evidence has shown that astrocytes are conceivably required for synapse formation, maintenance, and efficacy, as well as for the maintenance of brain homeostasis (39–41). It has also been demonstrated that astrocytes increase structurally mature, functional synapses in the CNS (42). We showed a loss of ≈50% of synaptic density in the brains of the high expressor mice at 8 mo of age, suggesting that impairment of astrocyte function in the transgenic brains could be critical enough to cause deleterious effects in neuronal functions involved in synaptic formation. The lasting dysfunctions of astrocytes and synapses must link to a variety of neurobiological deficits (40, 41). We found a significant reduction of BDNF and an unbalanced expression of 5-HT receptors in the transgenic brains. Although the direct linkage between astrocyte dysfunction and BDNF expression is obscure, synaptic alterations, in turn, could influence BDNF releases from CNS cells. The reduction of BDNF could successively modulate further disabilities of synapses, as well as 5-HT system, in the brains (23, 27, 28).
In this study, we found severe behavioral abnormalities in high expressor GFP20 mice. Interestingly, the disruption of BDNF and 5-HT receptors has been found to directly induce behavioral disorders in experimental animals (23, 24, 26, 29, 31, 43–45). The reduction of these molecules in the brains can impair long-term potentiation in the hippocampal neurons and induce behavioral deficits without degeneration of hippocampal and cerebellar development (26, 43, 46). Therefore, it is conceivable that the behavioral impairments in the P transgenic mice could be due to an abnormal level of BDNF and 5-HT receptors in the brain. On the other hand, we showed locomotor hyperactivity only in the 8-mo-old GPP20 mice. The reason the 4-mo-old mice did not show abnormal activity is unclear, but the expression level of P and/or long-term alterations in synapse and neurobiochemical environments may be involved in the impairment of neuronal functions regarding locomotor activity in mice. We demonstrated a gradual deposition of P in the transgenic brains. In this regard, it is of interest to identify the association between expression level of P in the brains and behavioral impairments in the transgenic mice.
It is well known that glial cells play important roles in raising the basic scaffolding of the brain during development (41). Given that the embryonic expression of P directly causes astrocyte dysfunctions, it is likely that the transgenic brains show a wide range of disturbances on neuronal development. The GFP4 mice, in which P expression was found only at 8 mo of age, showed almost the same phenotype as control mice. This finding may support a deleterious effect of P in the developing brains. Intriguingly, we have recently found that BDV P directly interacts with a neurite outgrowth factor, HMGB1, and inhibits its function in cultured neuronal cells (18). Considering the roles of HMGB1 in neurite outgrowth, cell migration, and cellular survival in developing brains (19), it is also possible that P can affect neuronal development by inhibiting HMGB1 function in glial cells. In this study, however, we could not detect any neuroanatomical abnormalities in the transgenic brains. A more detailed investigation regarding neuroanatomical and neurodevelopmental abnormalities should be necessary to understand the effects of BDV P during CNS development.
Importantly, many studies indicate that human psychiatric disorders show neurological disturbances that must arise from glial cell dysfunction and predominantly occur without neuronal degeneration (6–8). Furthermore, an abnormal level of BDNF and 5-HT receptors, as well as a reduced number of synaptic formations, has also been demonstrated in the brains of certain human mental disorders (8, 47, 48). These observations indicate that the P transgenic mice developed neuropathological and neurophysiological abnormalities in common with these human disorders, and impairment of glial cell function may exist as a common cause for the induction of neurobehavioral disorders. Our results suggest that viral infection may play a role in the development of neurobehavioral dysfunctions. Although the role of BDV infection in the induction of psychiatric disorders remains controversial, this work should promote further investigation regarding this question.
Acknowledgments
We thank Dr. Michael Brenner for providing pGfa2Lac plasmid. This article was supported in part by Special Coordination Funds for Science and Technology and by grants-in-aid, both from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Japan Society for the Promotion of Science.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BDV, Borna disease virus; P, phosphoprotein; GFAP, glial fibrillary acidic protein; BDNF, brain-derived neurotrophic factor; 5-HT, serotonin.
References
- 1.Pearce, B. D. (2001) Mol. Psychiatry 6, 634-646. [DOI] [PubMed] [Google Scholar]
- 2.Sawa, A. & Snyder, S. H. (2002) Science 296, 692-695. [DOI] [PubMed] [Google Scholar]
- 3.Weed, M. R. & Gold, L. H. (2001) Adv. Virus Res. 56, 583-626. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed, A. H., Norrby, E. & Kristensson, K. (1993) Rev. Neurosci. 4, 267-286. [DOI] [PubMed] [Google Scholar]
- 5.Kaul, M., Garden, G. A. & Lipton, S. A. (2001) Nature 410, 988-994. [DOI] [PubMed] [Google Scholar]
- 6.Arnold, S. E. (2001) Harvard Rev. Psychiatry 9, 69-76. [DOI] [PubMed] [Google Scholar]
- 7.Damadzic, R., Bigelow, L. B., Krimer, L. S., Goldenson, D. A., Saunders, R. C., Kleinman, J. E. & Herman, M. M. (2001) Brain Res. Bull. 55, 611-618. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, D. R., Pariante, C. M. & Everall, I. P. (2001) Brain Res. Bull. 55, 585-595. [DOI] [PubMed] [Google Scholar]
- 9.Rott, R. & Becht, H. (1995) Curr. Top. Microbiol. Immunol. 190, 17-30. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig, H. & Bode, L. (2000) Rev. Sci. Tech. 19, 259-288. [DOI] [PubMed] [Google Scholar]
- 11.Rubin, S. A., Waltrip, R. W., II, Bautista, J. R. & Carbone, K. M. (1993) J. Virol. 67, 548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pletnikov, M. V., Rubin, S. A., Vasudevan, K., Moran, T. H. & Carbone, K. M. (1999) Behav. Brain Res. 100, 43-50. [DOI] [PubMed] [Google Scholar]
- 13.Hornig, M., Solbrig, M., Horscroft, N., Weissenbock, H. & Lipkin, W. I. (2001) Curr. Top. Microbiol. Immunol. 253, 157-177. [DOI] [PubMed] [Google Scholar]
- 14.Sauder, C., Wolfer, D. P., Lipp, H., Staeheli, P. & Hausmann, J. (2001) Behav. Brain Res. 120, 189-201. [DOI] [PubMed] [Google Scholar]
- 15.Lipkin, W. I., Schneemann, A. & Solbrig, M. V. (1995) Trends Microbiol. 3, 64-69. [DOI] [PubMed] [Google Scholar]
- 16.Carbone, K. M. (2001) Clin. Microbiol. Rev. 14, 513-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikuta, K., Ibrahim, M. S., Kobayashi, T. & Tomonaga, K. (2002) Front. Biosci. 7, D470-D495. [DOI] [PubMed] [Google Scholar]
- 18.Kamitani, W., Shoya, Y., Kobayashi, T., Watanabe, M., Lee, B. J., Zhang, G., Tomonaga, K. & Ikuta, K. (2001) J. Virol. 75, 8742-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller, S., Scaffidi, P., Degryse, B., Bonaldi, T., Ronfani, L., Agresti, A., Beltrame, M. & Bianchi, M. E. (2001) EMBO J. 20, 4337-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner, M., Kisseberth, W. C., Su, Y., Besnard, F. & Messing, A. (1994) J. Neurosci. 14, 1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe, M., Lee, B. J., Kamitani, W., Kobayashi, T., Taniyama, H., Tomonaga, K. & Ikuta, K. (2001) Virology 282, 65-76. [DOI] [PubMed] [Google Scholar]
- 22.Toggas, S. M., Masliah, E., Rockenstein, E. M., Rall, G. F., Abraham, C. R. & Mucke, L. (1994) Nature 367, 188-193. [DOI] [PubMed] [Google Scholar]
- 23.Lyons, W. E., Mamounas, L. A., Ricaurte, G. A., Coppola, V., Reid, S. W., Bora, S. H., Wihler, C., Koliatsos, V. E. & Tessarollo, L. (1999) Proc. Natl. Acad. Sci. USA 96, 15239-15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kernie, S. G., Liebl, D. J. & Parada, L. F. (2000) EMBO J. 19, 1290-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duman, R. S., Malberg, J., Nakagawa, S. & D'Sa, C. (2000) Biol. Psychiatry 48, 732-739. [DOI] [PubMed] [Google Scholar]
- 26.Nestler, E. J., Barrot, M., DiLeone, R. J., Eisch, A. J., Gold, S. J. & Monteggia, L. M. (2002) Neuron 34, 13-25. [DOI] [PubMed] [Google Scholar]
- 27.Eaton, M. J., Staley, J. K., Globus, M. Y. & Whittemore, S. R. (1995) Dev. Biol. 170, 169-182. [DOI] [PubMed] [Google Scholar]
- 28.Alsina, B., Vu, T. & Cohen-Cory, S. (2001) Nat. Neurosci. 4, 1093-1101. [DOI] [PubMed] [Google Scholar]
- 29.Saudou, F., Amara, D. A., Dierich, A., LeMeur, M., Ramboz, S., Segu, L., Buhot, M. C. & Hen, R. (1994) Science 265, 1875-1878. [DOI] [PubMed] [Google Scholar]
- 30.Ramboz, S., Oosting, R., Amara, D. A., Kung, H. F., Blier, P., Mendelsohn, M., Mann, J. J., Brunner, D. & Hen, R. (1998) Proc. Natl. Acad. Sci. USA 95, 14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson, R. J. & Chiavegatto, S. (2001) Trends Neurosci. 24, 713-719. [DOI] [PubMed] [Google Scholar]
- 32.Gross, C., Zhuang, X., Stark, K., Ramboz, S., Oosting, R., Kirby, L., Santarelli, L., Beck, S. & Hen, R. (2002) Nature 416, 396-400. [DOI] [PubMed] [Google Scholar]
- 33.Campbell, I. L. (1998) Brain Res. Rev. 26, 327-336. [DOI] [PubMed] [Google Scholar]
- 34.Pletnikov, M. V., Rubin, S. A., Schwartz, G. J., Moran, T. H., Sobotka, T. J. & Carbone, K. M. (1999) Physiol. Behav. 66, 823-831. [DOI] [PubMed] [Google Scholar]
- 35.Rubin, S. A., Sylves, P., Vogel, M., Pletnikov, M., Moran, T. H., Schwartz, G. J. & Carbone, K. M. (1999) Brain Res. Bull. 48, 23-30. [DOI] [PubMed] [Google Scholar]
- 36.Pletnikov, M. V., Rubin, S. A., Vogel, M. W., Moran, T. H. & Carbone, K. M. (2002) Brain Res. 944, 108-123. [DOI] [PubMed] [Google Scholar]
- 37.Zocher, M., Czub, S., Schulte-Monting, J., de la Torre, J. C. & Sauder, C. (2000) J. Neurovirol. 6, 462-477. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Dunia, D., Watanabe, M., Syan, S., Mallory, M., Masliah, E. & de la Torre, J. C. (2000) J. Virol. 74, 3441-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfrieger, F. W. & Barres, B. A. (1997) Science 277, 1684-1687. [DOI] [PubMed] [Google Scholar]
- 40.Gallo, V. & Chittajallu, R. (2001) Science 292, 872-873. [DOI] [PubMed] [Google Scholar]
- 41.Fields, R. D. & Stevens-Graham, B. (2002) Science 298, 556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ullian, E. M., Sapperstein, S. K., Christopherson, K. S. & Barres, B. A. (2001) Science 291, 657-661. [DOI] [PubMed] [Google Scholar]
- 43.Lu, B. & Figurov, A. (1997) Rev. Neurosci. 8, 1-12. [DOI] [PubMed] [Google Scholar]
- 44.Poo, M. M. (2001) Nat. Rev. Neurosci. 2, 24-32. [DOI] [PubMed] [Google Scholar]
- 45.Guillin, O., Diaz, J., Carroll, P., Griffon, N., Schwartz, J. C. & Sokoloff, P. (2001) Nature 411, 86-89. [DOI] [PubMed] [Google Scholar]
- 46.Sarnyai, Z., Sibille, E. L., Pavlides, C., Fenster, R. J., McEwen, B. S. & Toth, M. (2000) Proc. Natl. Acad. Sci. USA 97, 14731-14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eastwood, S. L. & Harrison, P. J. (2000) Mol. Psychiatry 5, 425-432. [DOI] [PubMed] [Google Scholar]
- 48.Manji, H. K., Drevets, W. C. & Charney, D. S. (2001) Nat. Med. 7, 541-547. [DOI] [PubMed] [Google Scholar]