Abstract
Neurogenesis has recently been observed in the adult human brain, suggesting the possibility of endogenous neural repair. However, the augmentation of neurogenesis in the adult human brain in response to neuronal cell loss has not been demonstrated. This study was undertaken to investigate whether neurogenesis occurs in the subependymal layer (SEL) adjacent to the caudate nucleus in the human brain in response to neurodegeneration of the caudate nucleus in Huntington's disease (HD). Postmortem control and HD human brain tissue were examined by using the cell cycle marker proliferating cell nuclear antigen (PCNA), the neuronal marker βIII-tubulin, and the glial cell marker glial fibrillary acidic protein (GFAP). We observed a significant increase in cell proliferation in the SEL in HD compared with control brains. Within the HD group, the degree of cell proliferation increased with pathological severity and increasing CAG repeats in the HD gene. Most importantly, PCNA+ cells were shown to coexpress βIII-tubulin or GFAP, demonstrating the generation of neurons and glial cells in the SEL of the diseased human brain. Our results provide evidence of increased progenitor cell proliferation and neurogenesis in the diseased adult human brain and further indicate the regenerative potential of the human brain.
A particularly exciting development in the treatment of neurodegenerative diseases is the suggestion from both animal and human studies that transplantation of embryonic neurons or stem cells offer a potential treatment strategy for neurodegenerative disorders such as Parkinson's disease, Huntington's disease (HD), and Alzheimer's disease (1, 2). Although in recent years the transplantation of embryonic cells into the diseased human brain has emerged from the realm of the theoretical to that of the practical, it is associated with ethical, technical, and immunological problems (2). Thus, the demonstration of endogenous stem/progenitor cells in the hippocampus and the subependymal layer (SEL) of the basal ganglia in the adult mammalian brain has raised the exciting possibility that these undifferentiated cells may be able to generate neurons for cell replacement in neurodegenerative diseases such as HD. Indeed, neural stem cells in the rodent brain SEL adjacent to the caudate nucleus have recently been shown to proliferate and differentiate into neurons (3–5), suggesting they may provide a source of replacement neurons. In this regard it is especially interesting that recent studies (6) on the normal adult human brain have shown evidence of neurogenesis in the hippocampus, but no previous study has yet shown neurogenesis in the SEL of the normal or diseased human brain. Here, we have examined whether progenitor cell proliferation and neurogenesis occur in the SEL adjacent to the caudate nucleus in response to cell death in the caudate nucleus of the adult human HD brain. The results demonstrate increased progenitor cell proliferation and neurogenesis in the SEL of the HD brains indicating that the diseased human brain has the potential to repair itself.
Methods
Tissue Collection. The human brain tissue was obtained from the New Zealand Neurological Foundation Human Brain Bank at the University of Auckland. The full consent of all families was obtained at the time of autopsy and the University of Auckland Human Subjects Ethics Committee approved the study. Nine HD cases (average age = 62.8 years, range 41–74 years; average postmortem delay = 10.2 h, range 5–19 h; Fig. 1a) were examined by a neuropathologist to confirm HD pathology, to assign a pathological grade (ref. 7; Fig. 1a), and to exclude other brain pathology. No other neuropathology was found in any of the cases. Blood samples were also taken to determine the number of CAG repeats in exon 1 of the IT15 gene on chromosome 4; an expanded allele confirmed HD in all cases (Fig. 1a). Controls comprised of six neurologically normal cases (average age = 55.3 years, range 42–73 years; average postmortem delay = 14 h, range 10–18 h) that died suddenly with no known neurological disease or drug treatment and pathological examination excluded any neuropathology. The brains were removed at autopsy and the hemispheres were fixed by perfusion through the cerebral arteries, first with PBS with 1% sodium nitrite, followed by 15% formalin in 0.1 M phosphate buffer, pH 7.4. After perfusion, blocks of the caudate nucleus including the ventricular margin were dissected out and were further fixed in the same fixative for 24 h before being cryoprotected in 20% sucrose for 2–3 days, and then in 30% sucrose until equilibrated. The blocks were sectioned in the coronal plane on a freezing microtome (50-μm sections) and stored in PBS and 0.1% azide.
Fig. 1.
PCNA immunoreactivity is increased in the subependymal layer in HD cases. (a) Table summarizing the age, postmortem delay, pathological grade, CAG trinucleotide repeat length in the IT15 gene, and PCNA grade for each HD case is shown. For each HD case, the level of PCNA immunoreactivity in the subependymal layer was assessed by using a qualitative five-point grading scale (PCNA grade). (b) Compared with the control brain, the thickness of the subependymal layer (SEL) and the number of PCNA+ cells in the SEL are increased as the pathological grade of HD increases (grade 1 to grade 3). In each case, the boundary of the SEL with the caudate nucleus (CN) is indicated by an arrow. (EP, ependymal layer; LV, lateral ventricle). (c) Graph showing a significant correlation between pathological grade and PCNA grade in the HD cases (Spearman rank correlation, r = 0.8972, P ≤ 0.003). (d) Graph showing a significant correlation between CAG trinucleotide repeat length in the IT15 gene and PCNA grade in the HD cases (Spearman rank correlation, r = 0.8168, P ≤ 0.02). (Scale bar, 80 μm.)
Immunohistochemistry. All of the free-floating sections that were stained for proliferating cell nuclear antigen (PCNA) were first incubated in a citric acid solution, containing citric acid (Na3 salt) and Na2HPO4 (pH 4.5), overnight before undergoing a standard antigen retrieval protocol. All sections were incubated in a 50% methanol solution with 1% H2O2 to block endogenous peroxidases in the tissue. Proliferating cells were detected with a mouse anti-PCNA antibody (Santa Cruz Biotechnology; diluted 1:500). Experiments were also performed by using two other antibodies raised against PCNA (rabbit polyclonal anti-PCNA, Santa Cruz Biotechnology; diluted 1:1,000, and mouse monoclonal anti-PCNA, Chemicon; diluted 1:500). All three antibodies demonstrated identical patterns of labeling in human tissue. Neurons were identified by using a mouse anti-βIII-tubulin antibody (Sigma; diluted 1:500) and an antineuronal nuclei (NeuN) antibody (Chemicon; diluted 1:1,000). Glial cells were detected by using anti-glial fibrillary acidic protein (GFAP) (DAKO; diluted 1:5,000) and anti-vimentin (VIM) (Boehringer Mannheim, Philadelphia; diluted 1:500) antibodies. Goat anti-mouse (Sigma; diluted 1:500) and goat anti-rabbit (Sigma; diluted 1:500) secondary antibodies were applied to the sections in a species-specific manner for 12 h; the sections were then incubated for 3 h in ExtrAvidin (Sigma; diluted 1:1,000) and 3,3 diaminobenzidine chromagen was used to produce latent staining. Sections were washed in PBS and 0.2% Triton X-100 (3 × 10 min) between each blocking and antibody step. The sections were mounted onto chrome alum-coated glass slides and dehydrated in ascending grades of alcohol to xylene and were coverslipped by using Hystomount (Hughes and Hughes, Somerset, U.K.) mounting media.
For fluorescent triple labeling the sections underwent the same pretreatment as diaminobenzidine-stained sections; sections were serially incubated with either the rabbit or mouse anti-PCNA antibody (diluted 1:100) for 48 h, followed by mouse anti-βIII-tubulin (diluted 1:250), or rabbit anti-GFAP (diluted 1:500) for a further 48 h. The sections were then incubated in a pooled solution of fluorescent chromagens, anti-mouse Alexa 594 (Molecular Probes; diluted 1:200), and anti-rabbit FITC (Sigma; diluted 1:100) for 12 h. Sections were subsequently incubated in Hoechst 33258 stain (for 30 min, Sigma; concentration of 8 μg/ml) to label DNA in the cell nucleus. Sections were washed (3 × 10 min) after each blocking and antibody step. The sections were mounted and coverslipped with Citifluor (Agar Scientific, Essex, U.K.). The fluorescent-labeled sections were imaged by using a confocal laser scanning microscope (Leica TCS SP2) equipped with UV, argon, argon/krypton, and helium/neon lasers. Each fluorescent label was imaged serially to eliminate detection of bleedthrough and other artifactual fluorescence. The confocal images were captured in a Z-series with an interslice gap of 1 μm.
For terminal deoxynucleotidyltransferase-mediated dATP nick end labeling (TUNEL) staining, 16-μm cryostat cut caudate nucleus sections were mounted onto chrome alum-dipped slides before being processed by using our standard protocol as described by Butterworth et al. (8).
Bright-field images were taken with a digital camera on a light microscope (Leica, Deerfield, IL) and the images were captured in photoshop (Adobe Systems, San Jose, CA). All of the figures were compiled by using pagemaker (Adobe Systems).
Western Blotting. Fresh frozen caudate nucleus sections from human control and HD brains, with the SEL en bloc, were cut on at 30 μm on a cryostat and the ependymal layer (EP) and SEL were selectively dissected from the caudate nucleus. The SEL samples were homogenized in homogenization buffer [150 mM sucrose/15 mM Hepes, pH 7.9/60 mM KCL/5 mM EDTA/1 mM EGTA, and one Complete minitablet protease inhibitor mixture (Roche Diagnostics) per 10 ml]. Triton X-100 was added (1% final concentration) and incubated on ice for 1 h and the samples underwent centrifugation (Heraeus Biofuge, Stratos, Germany) for 10 min at 14,000 rpm. The supernatant was retained and the protein content was quantified by using the Bio-Rad protein assay. Fifty micrograms of protein in Laemli sample loading buffer was loaded onto a 15% acrylamide gel and were separated electrophoretically. The proteins were then transferred to polyvinylidine difluoride membrane (Hybond-P, Amersham Pharmacia) and immunoblotted with mouse anti-PCNA (diluted 1:1,000). Chemiluminescence detection reagents (ECL Plus; Amersham Pharmacia) enabled visualization of peroxidase reaction products.
Results
We compared the number of proliferating progenitor cells in the SEL of control (n = 6) and HD (n = 9) human brains by using antibodies to the cell-cycle marker PCNA, which labels cells in the S phase of cell division (9, 10). Whereas we detected a small number of PCNA+ cells in the SEL of control human brains, the number of PCNA+ cells was considerably increased in HD brains (Fig. 1b). Using a 5-point qualitative PCNA grading scale (0 = small, + = few, ++ = moderate, +++ = many, and ++++ = large numbers of PCNA+ cells), the thickness of the SEL and the number of PCNA+ cells in the SEL in control and HD brains were rated by an observer blind to the case numbers. The results showed a statistically significant difference in the thickness of the SEL and in the number of PCNA+ cells in the SEL between the control and HD brains (Mann–Whitney U test, control median = 0.5; HD median = 2.0; U = 52.5; P < 0.0008; Fig. 1 a and b). Statistical analysis revealed that the grade of PCNA staining in HD cases significantly correlated with the HD neuropathological grade (ref. 7; Spearman rank correlation P < 0.003; Fig. 1c), i.e., increased numbers of PCNA+ cells correlated with increased pathological grade. Statistical analysis also demonstrated a significant correlation between the grade of PCNA staining in HD cases and the number of CAG repeats in the expanded allele of the HD IT15 gene (Spearman rank correlation P < 0.02; Fig. 1 a and d). To exclude the possibility that the increased density of progenitor cells was due to shrinkage of the caudate nucleus, we measured the ventral to dorsal length of the SEL in HD and control brains at rostral, central, and caudal levels (all brains were processed identically). Statistical analysis of the measurements demonstrated no significant reduction in SEL length in the HD brains compared with control brains (15.78 ± 3.0 mm HD; 16.63 ± 3.36 mm control). No correlation was found between the PCNA grade and the age, sex, or postmortem delay of the cases.
To examine whether there was any variation in the density of progenitor cells in the SEL at different levels of the caudate nucleus, we took sections from selected rostral, central, and caudal regions of the caudate nucleus. Comparable numbers of PCNA+ cells were detected in all three regions of the SEL overlying the caudate nucleus.
Because it has been suggested that the PCNA antibody not only detects dividing cells but also cells that are undergoing DNA repair or apoptosis (11), we performed TUNEL histochemical studies on the SEL and caudate nucleus from control and HD brains to examine the number of cells undergoing repair or apoptotic DNA fragmentation. Very few TUNEL+ cells were present in the SEL in all HD cases examined. However, within the caudate nucleus there was a large number of TUNEL+ cells, as reported by Butterworth et al. (8), but relatively few PCNA+ cells, suggesting that the PCNA antibody, in our studies, is labeling proliferating cells. The specificity of the PCNA antibody was also examined by subjecting SEL samples, extracted from control and HD brains, to Western blot analysis, and were immunoblotted with the PCNA antibody. In each case a single clear band (Fig. 2) was demonstrated at 36 kDa, which is the calculated weight of the PCNA protein. No bands were detected in Western blots when the primary antibody was omitted from the experiment. Likewise, when the primary antibody was omitted from the human section experiments, no immunostaining was revealed.
Fig. 2.
Western immunoblotting with the PCNA antibody using human control (three cases) and HD (four cases) SEL homogenates demonstrates the specificity of the PCNA antibody by a single strong band at 36 kDa.
To investigate the fate of PCNA+ cells in the SEL of the diseased adult human brain, we applied single- and double-immunohistochemical labeling techniques by using antibodies against βIII-tubulin, which labels neurons early in their development (12, 13), and NeuN, a mature neuronal marker. For the detection of glial cells we used antibodies to GFAP and VIM. Comparison of the distribution of PCNA+, βIII-tubulin+, NeuN+, GFAP+, and VIM+ cells in the SEL of serial sections from the HD brains showed an even distribution of PCNA+ cells across the SEL (Fig. 3a), whereas βIII-tubulin+ and NeuN+ cells were located mainly in the region of the SEL adjacent to the caudate nucleus (Fig. 3b). GFAP+ and VIM+ cells were scattered across the SEL and caudate nucleus, but a slightly more intense GFAP+ band was detected in the region adjacent to the EP (Fig. 3c).
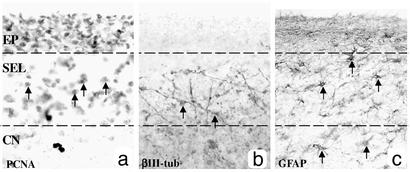
Fig. 3.
PCNA+, βIII-tubulin+, and GFAP+ cells are located in different regions within the SEL as demonstrated with bright-field immunohistochemical serial sections through the EP, SEL, and CN of a grade 2 HD brain. (a) PCNA+ cells are evenly distributed within the SEL (arrows demonstrate examples of PCNA+ cell bodies), and the EP is also densely stained. (b) βIII-tubulin+ fibers and cell bodies are present in the lower part of the SEL (arrows indicate cell bodies with immunoreactive fibers). (c) GFAP+ cells are distributed homogenously throughout the SEL and in the CN (arrows indicate examples of GFAP cell bodies).
Using fluorescence confocal microscopy, we detected colocalization of the PCNA+ cells with the early neuronal marker βIII-tubulin in the SEL of HD cases (Fig. 4 a–e). We used a Hoechst nuclear DNA stain to confirm that PCNA+ staining was located in the nuclear region of the βIII-tubulin+ cell. The demonstration that βIII-tubulin stained processes colocalized with PCNA+ cell nuclei in the HD SEL (Fig. 4 c–e) indicates that these PCNA+ cells exhibit a neuronal phenotype. The PCNA+/βIII-tubulin+ cells were located mainly in the deeper regions of the SEL adjacent to the caudate nucleus (Figs. 3b and 4a) and comprised in the order of 5% of the PCNA+ cells in the SEL.
Fig. 4.

Newly generated cells in the SEL of the HD brain exhibit either a neuronal or glial phenotype. (a) Confocal microscopy demonstrates that the SEL of the HD brain contains newly generated cells that coexpress PCNA and βIII-tubulin and stain with a Hoechst stain. The new neurons are located in the lower part of the SEL. (Scale bar, 6 μm.) (b–d) Higher magnification of the neuron, which is outlined with a box in a. (Scale bar, 3 μm.) (b) Hoechst (blue) stains the nucleus of cells in the SEL. (c) PCNA (red) labels the nucleus of a new cell and has a granular appearance (19). (d) βIII-tubulin (green) labels the cytoplasm of neurons early in their development. (e) The merged image demonstrates coexpression of PCNA and βIII-tubulin in the same cell that displays a Hoechst+ stain, indicating that neurogenesis occurs in the SEL of the HD brain. (f) Confocal microscopy demonstrates that the SEL of the HD brain also contains newly generated cells that coexpress PCNA and GFAP and stain with a Hoechst stain. These new glial cells are located in the upper part of the SEL in the HD brain. (Scale bar, 18 μm.) (g–i) Higher magnification of the glial cell, which is outlined with a box in f. (Scale bar, 9 μm.) (g) Hoechst (blue) stains the nucleus of cells in the SEL. (h) PCNA (red) labels the nucleus of the cell with punctate staining. (i) The astrocytic marker GFAP (green) labels the cytoplasm of the cell. (j) The merged image demonstrates coexpression of PCNA and GFAP in the same cell that displays a Hoechst stain, indicating the occurrence of gliogenesis. Images a–e and f–j were each obtained by using different excitation wavelengths, and the signal was detected at different emission wavelengths.
Fluorescent confocal microscopy using antibodies against PCNA and GFAP combined with a Hoechst stain demonstrated large numbers of cells in the SEL that had intense GFAP cytoplasmic staining (Fig. 4i), with the nuclear region clearly delineated by PCNA and Hoechst staining (Fig. 4 g–i). The demonstration that the GFAP-stained cytoplasm colocalized with PCNA+ nuclei in the HD SEL (Fig. 4j) indicates that these PCNA+ cells exhibit a glial phenotype. The PCNA+/GFAP+ cells were identified mainly in the more superficial region of the SEL adjacent to the EP (Fig. 3c and 4f) and comprised ≈50% of the PCNA+ cells in the SEL.
To ensure that the fluorescence we were detecting was not simply accounted for by autofluorescence or artifact, we also imaged sections from each control and HD brain, omitting the βIII-tubulin or GFAP primary antibodies. We did not detect any βIII-tubulin or GFAP-like immunofluorescence in these tissues, but the PCNA immunofluorescence was the same as that seen in the sections that had PCNA and either βIII-tubulin or GFAP antibody treatment. We performed the same test omitting the PCNA primary antibody and were unable to detect PCNA-like immunofluorescence, but detected normal βIII-tubulin and GFAP signal, indicating that there was no crossreactivity between the primary antibodies and no bleedthrough between wavelength channels. To test for autofluorescence, we imaged sections that were not exposed to antibody treatment, with and without Hoechst staining, and also sections where the primary antibodies were omitted from the incubation. The autofluorescence that was detected from these sections was present in all wavelength channels, was homogenously spread across the section, and was not cell specific.
Discussion
Neurogenesis is a well established phenomenon in the adult brain of subhuman primates and other mammals. In addition, neurogenesis has been recently demonstrated in the normal adult human brain (6). In these studies, Eriksson et al. (6) used postmortem brain tissue from cancer patients who, as a part of their oncological treatment, had been administered the thymidine analog BrdUrd, which labels dividing cells. BrdUrd was detected immunohistochemically in the progeny of the dividing cells and subsequent analysis of this cell population in the dentate gyrus of the hippocampus revealed that they also stained positively for neuronal or glial markers.
Various models of brain injury have demonstrated that neuronal death can trigger increased neurogenesis in the adult rodent brain (4, 14, 15). However, the production of increased numbers of progenitor cells in the SEL of the adult human brain in response to neurodegenerative diseases, such as HD, has never been previously demonstrated. Here we have not only demonstrated increased numbers of progenitor cells in the SEL of the HD human brain but also that these progenitor cells take on either a neuronal or glial phenotype, as evidenced by βIII-tubulin+ or GFAP+ staining, respectively, in the SEL. Supporting these observations, the level of PCNA mRNA has been reported to increase ≈2-fold in the striatum of 12-week-old R6/2 transgenic HD mice compared with wild-type littermate controls (16).
Interestingly, whereas Eriksson et al. (6) detected BrdUrd+ cells in the normal adult human SEL, these cells did not coexpress the cell-specific markers for GFAP and NeuN. They suggested that these cells are required to migrate from the SEL before they differentiate. Our results support this hypothesis by the observation that most of the PCNA+/βIII-tubulin+ cells were located in the SEL farthermost from the ependymal layer adjacent to the caudate nucleus. In contrast PCNA+//GFAP+-labeled cells were found in the most superficial regions close to the ventricular edge.
Our findings indicate a high level of neural plasticity in the adult human brain in response to the degenerating environment that is present in HD. We also note the close relationship that the SEL has with the adjacent caudate nucleus, the part of the brain that is most severely and preferentially affected in HD, and the location of the PCNA+/βIII-tubulin+ cells very close to the caudate nucleus. Since, in early neural development, cells migrate from the SEL to form the caudate nucleus, it would not be unreasonable to suggest that these immature neurons are migrating toward the caudate nucleus in response to the neuronal cell loss occurring in the HD brain. Furthermore, the observation of increased numbers of progenitor cells in the SEL with advancing neuropathological grades of HD would indicate that the proliferation is a response to greater numbers of degenerating neurons in the caudate nucleus. It is not clear what factors signal the observed cellular proliferation and migration, but it is clear that the increase demonstrated in this study is insufficient to compensate for the progressive cell loss observed in the HD brain. A variety of growth factors (e.g., FGF-2; ref. 17) and pharmaceutical agents (e.g., lithium; ref. 18) have been shown to induce cell proliferation and neurogenesis. If the potential for endogenous neural replacement could be augmented pharmacologically with the use of exogenous growth factors or pharmaceuticals that increased the rate of neural progenitor formation, neural migration, and neural maturation, then the rate of cell loss may be slowed, and clinical improvements may be observed. We believe the discovery that the diseased adult human brain is capable of neuronal regeneration in response to neuronal loss will be of major relevance for the development of therapeutic approaches in the treatment of neurodegenerative diseases.
Acknowledgments
This work was supported by grants from the Health Research Council of New Zealand and the Neurological Foundation of New Zealand. M.A.C. was funded by the Neurological Foundation of New Zealand Miller Scholarship. Imaging was carried out in the Biomedical Imaging Research Unit located at the Faculty of Medical and Health Sciences.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SEL, subependymal layer; EP, ependymal layer; HD, Huntington's disease; GFAP, glial fibrillary acidic protein; PCNA, proliferating cell nuclear antigen; NeuN, neuronal nuclei.
References
- 1.Svendsen, C. N., Caldwell, M. A., Shen, J., ter Borg, M. G., Rosser, A. E., Tyers, P., Karmiol, S. & Dunnett, S. B. (1997) Exp. Neurol. 148, 135-146. [DOI] [PubMed] [Google Scholar]
- 2.Freed, C. R., Greene, P. E., Breeze, R. E., Tsai, W. Y., DuMouchel, W., Kao, R., Dillon, S., Winfield, H., Culver, S., Trojanowski, J. Q., et al. (2001) N. Engl. J. Med. 344, 710-719. [DOI] [PubMed] [Google Scholar]
- 3.Morshead, C. M., Reynolds, B. A., Craig, C. G., McBurney, M. W., Staines, W. A., Morassutti, D., Weiss, S. & van der Kooy, D. (1994) Neuron 13, 1071-1082. [DOI] [PubMed] [Google Scholar]
- 4.Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. (2002) Nat. Med. 8, 963-970. [DOI] [PubMed] [Google Scholar]
- 5.Parent, J. M., Valentin, V. V. & Lowenstein, D. H. (2002) J. Neurosci. 22, 3174-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A., Nordborg, C., Peterson, D. A. & Gage, F. H. (1998) Nat. Med. 4, 1313-1317. [DOI] [PubMed] [Google Scholar]
- 7.Vonsattel, J. G. & DiFiglia, M. (1993) J. Neuropathol. Exp. Neurol. 57, 369-384. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth, N. J., Williams, L., Bullock, J. Y., Love, D. R., Faull, R. L. M. & Dragunow, M. (1998) Neuroscience 87, 49-53. [DOI] [PubMed] [Google Scholar]
- 9.Kurki, P., Vanderlaan, M., Dolbeare, F., Gray, J. & Tan, E. M. (1986) Exp. Cell Res. 166, 209-219. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi, T. & Caviness, V. S., Jr. (1993) J. Neurocytol. 22, 1096-1102. [DOI] [PubMed] [Google Scholar]
- 11.Tomasevic, G., Kamme, F. & Wieloch, T. (1998) Mol. Brain Res. 60, 168-176. [DOI] [PubMed] [Google Scholar]
- 12.Lee, M. K., Tuttle, J. B., Rebhun, L. L., Cleveland, D. W. & Frankfurter, A. (1990) Cell Motil. Cytoskeleton 17, 118-132. [DOI] [PubMed] [Google Scholar]
- 13.Menezes, J. R. L. & Luskin, M. B. (1994) J. Neurosci. 14, 5399-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magavi, S. S., Leavitt, B. R. & Macklis, J. D. (2000) Nature 405, 951-955. [DOI] [PubMed] [Google Scholar]
- 15.Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S. & Lowenstein, D. H. (1997) J. Neurosci. 17, 3727-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luthi-Carter, R., Strand, A., Peters, N. L., Solano, S. M., Hollingsworth, Z. R., Menon, A. S., Frey, A. S., Spektor, B. S., Penney, E. B., Schilling, G., et al. (2000) Hum. Mol. Genet. 9, 1259-1271. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura, S., Takagi, Y., Harada, J., Teramoto, T., Thomas, S. S., Waeber, C., Bakowska, J. C., Breakefield, X. O. & Moskowitz, M. A. (2001) Proc. Natl. Acad. Sci. USA 98, 5874-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, G., Rajkowska, G., Du, F., Seraji-Bozorgzad, N. & Manji, H. K. (2000) J. Neurochem. 75, 1729-1734. [DOI] [PubMed] [Google Scholar]
- 19.Chan, P., Frakes, R., Tan, E. M., Brattain, M. G., Smetana, K. & Busch, H. (1983) Cancer Res. 43, 3770-3777. [PubMed] [Google Scholar]