Abstract
MRI studies using the manual tracing method have shown a smaller-than-normal hippocampal volume in patients with posttraumatic stress disorder (PTSD). However, these studies have yielded inconsistent results, and brain structures other than the hippocampus have not been well investigated. A recently developed, fully automated method called voxel-based morphometry enables an exploration of structural changes throughout the brain by applying statistical parametric mapping to high-resolution MRI. Here we first used this technology in patients with PTSD. Participants were 9 victims of the Tokyo subway sarin attack with PTSD and 16 matched victims of the same traumatic event without PTSD. The voxel-based morphometry showed a significant gray-matter volume reduction in the left anterior cingulate cortex (ACC) in trauma survivors with PTSD compared with those without PTSD. The severity of the disorder was negatively correlated with the gray-matter volume of the left ACC in PTSD subjects. There were no significant differences in other gray-matter regions or any of the white-matter regions between two groups. The present study demonstrates evidence for structural abnormalities of ACC in patients with PTSD. Together with previous functional neuroimaging studies showing a dysfunction of this region, the present findings provide further support for the important role of ACC, which is pivotally involved in attention, emotional regulation, and conditioned fear, in the pathology of PTSD.
Findings from neuroimaging studies of patients with posttraumatic stress disorder (PTSD) have suggested that some brain pathology plays an important role in the disorder (1, 2). Six structural MRI studies have shown that combat-related or childhood physically and/or sexually abused subjects with PTSD have a smaller-than-normal hippocampal volume (3–8). However, almost the same number of studies failed to show reduced hippocampal volume in chronically maltreated children (9–11), survivors of acute traumatic events (12), nonalcoholic combat veterans (13), and alcoholic patients (14) with PTSD. In contrast, brain structures other than the hippocampus have received less attention, although a few studies have reported whole-brain volume reduction (9), reduced total white-matter volume (7), smaller corpus callosum (9), larger superior temporal gyrus gray-matter volume (15), and attenuation of frontal lobe asymmetry (11). Therefore, it is unclear whether structural abnormality in brain structures other than the hippocampus exists in patients with PTSD.
In contrast to the emphasis on the hippocampus in previous structural MRI studies, symptom-provocation and cognitive-activation studies using functional neuroimaging have revealed greater activation of the amygdala, anterior paralimbic structures, Broca's region, and other neocortical regions and a failure of activation of anterior cingulate cortex (ACC) in response to trauma-related stimuli in individuals with PTSD (1, 2, 16). Furthermore, one study (17) showed a reduced N-acetyl-aspartate/creatine ratio in ACC in subjects with PTSD, indicating neuronal loss or dysfunction in this region. These findings suggest that PTSD may be accompanied by a hyperresponsive amygdala and an underresponsive ACC that fails to inhibit the amygdala. To our knowledge, no studies have yet examined whether ACC is structurally affected in patients with PTSD, although functional neuroimaging studies have indicated an important role of the medial prefrontal cortex including ACC in the pathophysiology of PTSD.
As stated above, there is a discrepancy between the findings from previous studies of the conventional volumetry of the structural anatomy and those that have used functional anatomy of PTSD. The former focused on the hippocampus, whereas the latter highlighted the important role of the anterior cingulate. A possible explanation for this is that the hippocampus is a clearly defined structure that lends itself to volumetric analyses. Thus, our motivation for using automated voxel-based morphometry (VBM) here is that it assesses the anatomical differences everywhere in the brain without operational bias toward those brain structures that have easily identifiable boundaries. VBM was used recently in structural MRI studies of various neuropsychiatric disorders (18). For example, VBM studies have successfully revealed regional gray-matter reductions in brain regions such as the inferior prefrontal region, insular cortex, and ACC in patients with schizophrenia compared with normal subjects; the detection of these reductions would have been difficult and/or rare by the previous region-of-interest methods (19–21). To our knowledge, however, VBM has not been used in previous studies of PTSD.
In the current study we used VBM to explore structural brain differences in the gray matter as well as the white matter between victims of the Tokyo subway sarin attack with and without PTSD. Based on findings from previous neuroimaging studies, we hypothesized that patients with PTSD would demonstrate volume reduction in medial temporal regions (including the hippocampus) and medial prefrontal cortices (including ACC). The Tokyo subway sarin attack was caused by terrorists belonging to a cult named Aum Shinrikyo in Japan on March 20, 1995. Approximately 5,500 victims were exposed to sarin, a poisonous gas, and 12 of the victims died (22). We recruited those victims, with or without PTSD, who had never received psychiatric treatment for PTSD caused by the attack. In addition, these subjects had little history of psychotropic treatment and no history of alcohol and substance abuse that could be significant confounding factors in neuroimaging studies of PTSD.
Methods
Subjects and Clinical Evaluation. Thirty-six subjects were recruited from a group of victims of the Tokyo subway sarin attack who were treated in the emergency room for acute sarin intoxication with a follow-up visit at St. Luke's International Hospital (Tokyo). Diagnostic interviews and magnetic resonance scans were performed between 2000 and 2001, 5–6 years after the incident. The participants completed the revised impact-of-event scale (23, 24) and were interviewed by trained psychiatrists (T.S., M.S., M.T., and S.F.) using the clinician-administered PTSD scale (CAPS) structured interview [refs. 25 and 26; Japanese version translated by Asukai et al. (27)] (Table 1). All the subjects were also screened for the presence or absence of neuropsychiatric disorders by trained psychiatrists using the mini-international neuropsychiatric interview (28). These interviews were performed on the same day as magnetic resonance scanning.
Table 1. Subject characteristics and clinical measures.
|
PTSD (n = 9)
|
non-PTSD (n = 16)
|
t tests
|
|||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | df | t value | P |
| Age | 44.6 | 16 | 44.4 | 14 | 23 | -0.2 | 0.99 |
| Male/female | 5/4 | 10/6 | |||||
| Education, years | 12.8 | 2.0 | 13.8 | 2.2 | 23 | 1.09 | 0.29 |
| SES* | 2.6 | 1.0 | 2.1 | 0.8 | 23 | -1.17 | 0.25 |
| Parental SES* | 3.2 | 1.0 | 2.9 | 0.7 | 23 | -0.86 | 0.4 |
| Impact of event scale (revised) | 23 | 54 | 11.9 | 11 | 20 | -2.23 | 0.037 |
| CAPS total (lifetime) | 62.2 | 18 | 9.44 | 7.8 | 23 | -10.1 | <0.001 |
| Cocentrations of serum cholinesterase | 110 | 29 | 141 | 32 | 15 | 1.79 | 0.093 |
SES was assessed by using the Hollingshead scale. Higher scores indicate lower status
Of the 36 participants, 9 were diagnosed as having PTSD related to the attack [1 man currently had PTSD, and 8 (4 men and 4 women) had a history of PTSD). Ten filled more than one but not all the criteria of the three-symptom clusters of PTSD including re-experiencing of the event, hyperarousal, and numbing. We excluded these “partial” PTSD subjects because their inclusion would blur the difference in brain morphology between the PTSD subjects and controls. One subject with current alcohol dependence was also excluded to avoid possible confounds of alcohol/drug misuse on brain structure. The remaining 16 victims have never had PTSD (10 men and 6 women) nor any history of neuropsychiatric disorders. Thus, the final set of subjects in this study included 9 victims with PTSD and 16 victims without PTSD.
Three of the nine victims diagnosed as having PTSD had mental comorbidity: current major depression (n = 1), current (n = 1) and history of (n = 1) panic disorder with agoraphobia. The prevalence of psychiatric comorbidity did not differ significantly between groups (depression, P = 0.36; panic disorder, P = 0.12; Fisher's exact test). Although two of the nine victims diagnosed to have PTSD had received benzodiazepines for 2–4 months 2–4 years ago due to insomnia or general anxiety, the other 23 victims had never received psychiatric treatment before participating in this study. None of the 25 subjects had a history of neurological illness, serious head trauma with any known cognitive consequences or loss of consciousness for >5 min, and alcohol/substance abuse or dependence. None of the 16 victims without PTSD had a family history of axis I disorder in their first-degree relatives.
The socioeconomic status (SES) and parental SES were assessed by using the Hollingshead scale (29). Victims with and without PTSD did not differ in age, gender, duration of education, SES, and parental SES (Table 1). All subjects were right-handed based on the Edinburgh inventory (30); we determined the laterality index >0.8 as the cutoff for right-handedness. Because acute sarin exposure modulates the cholinergic pathways (31), the concentrations of serum cholinesterase were evaluated to assess the severity of the acute sarin intoxication in the emergency room, which was not significantly different between the two groups (Table 1). The ethical committee of the University of Tokyo Hospital approved of this study. After a complete explanation of the study to the subjects, written informed consent was obtained from all participants.
MRI Acquisition. The MRI data were obtained by using a 1.5-T scanner (General Electric Signa Horizon Lx, version 8.2, GE Medical Systems, Milwaukee, WI). For volume analysis, three-dimensional Fourier-transform spoiled gradient recalled acquisition with steady state was used because it affords excellent contrast between the gray and white matter in the evaluation of brain structures. The repetition time was 35 msec, the echo time was 7 msec with one repetition, the nutation angle was 30°, the field of view was 24 cm, and the matrix was 256 × 256 (192) × 124. The voxel dimensions were 0.9375 × 0.9375 × 1.5 mm. A trained neuroradiologist (H. Yamada or O.A.) evaluated the MRI scans and found no gross abnormalities in any of the subjects.
MRI Data Analysis. Image analysis was performed by using analyze pc 3.0 (Mayo Foundation, Rochester, MN) and spm 99 software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London) running in matlab 6.1 (Mathworks, Sherborn, MA). In analyze, image data were resampled by using an algorithm to make them isotropic, with the sides measuring 0.9375 mm, and then stored. Image processing by the spm 99 software was based on the method reported by Suzuki et al. (21) and similar to those used in the studies by Ashburner and Friston (32) and Good et al. (33). Briefly, images first were spatially normalized into the standard space of Talairach and Tournoux (34). Normalized images then were segmented into the gray matter, white matter, cerebrospinal fluid, and skull/ scalp compartments by using an automated and operator-independent process. The segmentation of normalized magnetic resonance images in spm 99 uses a clustering algorithm identifying a voxel density of a particular tissue type combined with a priori knowledge of the spatial distribution of these clusters in healthy subjects (35). The segmentation step also incorporates an image-density nonuniformity correction (32) to address image-density variations caused by different positions of cranial structures within the MRI head coil. The spatially normalized segments of the gray and white matters were smoothed with a 12-mm full-width, half-maximum isotropic Gaussian kernel to accommodate individual variability in the sulcal and gyral anatomy. By smoothing the data, the partial-volume effect was used to create a spectrum of gray- or white-matter intensities. Gray- or white-matter density is equivalent to the weighted average of the gray- or white-matter voxels located in the volume defined by the smoothing kernel. Because previous studies showed a fair correlation between the regional gray- or white-matter density identified with VBM and their volumes measured by the conventional manual tracing method (18, 21, 36–38), the regional gray- or white-matter density can be considered to represent the local amount of gray or white matter.
Statistical Analysis. Statistical comparison between the two groups was performed by using an analysis of covariance model for global normalization (39), which removes global gray- or white-matter density differences for each subject and normalizes the segmented brain images to the same total amount of gray or white matter while preserving regional differences in gray- or white-matter density. Age and gender were also treated as confounding covariates. To test hypotheses with respect to regionally specific group effects, the estimates were compared by using two linear contrasts (more or less gray or white matter in patients than controls) (40). The resulting set of voxel values for each contrast constituted a statistical parametric map of the t statistic [SPM(t)]. The SPM(t) values were transformed to the normal distribution [SPM(z)] and with a threshold at P < 0.001. The significance of each region was estimated by distributional approximations from the theory of random Gaussian fields. This characterization was in terms of the probability that the peak height observed could have occurred by chance over the entire volume analyzed. Significance levels were set at corrected P < 0.05. Small-volume correction for multiple comparisons was used for regions that had been predicted in advance [hippocampus: 20 × 30 × 26-mm (8.2 resels) regions at center (±26, -24, -8) in each hemisphere, respectively, which showed volume reduction in previous volumetric studies; ACC: 28 × 45 × 45-mm (32.1 resels) regions at center (0, 18, 18) covering bilateral ACC, which showed dysfunction or neuronal loss in previous neuroimaging studies]. These criteria for the significance threshold were based on those of Maguire et al. (41) and Kubicki et al. (38). The comparisons were made between the victims with (n = 9) and without (n = 16) PTSD.
Correlational analysis with the PTSD severity was performed in PTSD subjects only because of the low, restricted range of PTSD symptom severity in the non-PTSD subjects. Global gray (or white) matter, age, and gender were treated as confounding covariates, and the total score of CAPS was treated as the covariates of interest. To test hypotheses about regional specific covariate effects, the estimates were compared by using two linear contrasts (positive or negative correlation) (40). Significance levels were also set at corrected P < 0.05. Small-volume correction was also applied by using the maxima obtained by the group analysis as the center of a small volume. Furthermore, to rule out potential confounding factors that may affect VBM findings, correlational analysis was also performed with the clinical measures (age at onset, height, body weight, SES, parental SES, duration of illness, scores of the revised impact-of-event scale, and concentration of serum cholinesterase) in victims with and without PTSD separately. These clinical measures were treated as the covariates of interest, and global gray (or white) matter, age, and gender were treated as confounding covariates. Statistical significance was defined at the same threshold as in the correlational analysis with PTSD severity.
Results
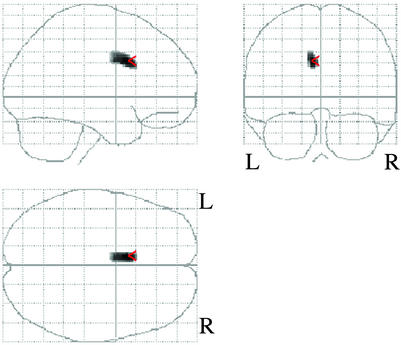
Comparison of Regional Gray or White Matter Between Victims With and Without PTSD. The area with less gray-matter density in victims with PTSD compared with those without PTSD existed within the left ACC {peak coordinate [x, y, z (mm)] = (-8, 12, 32), k = 113, T score = 5.67} (Figs. 1 and 2). The intensities in the other gray-matter regions and any of the white-matter region did not show any significant differences between the two groups. These results indicate a significant left ACC gray-matter volume reduction after controlling for age and gender effects in victims with PTSD compared with those without PTSD. We note that it was unlikely that the volume reduction of ACC in patients with PTSD is solely the consequence of the neurotoxic effect of sarin on the brain structure, because there was no significant difference in serum cholinesterase concentration between the two groups (a trend-level significance even indicated that non-PTSD subjects may have been exposed to sarin itself slightly more) (Table 1).
Fig. 1.
Results of SPM analysis of MRI data. Statistical parametric map in three orthogonal projections shows voxels where less regional gray-matter densities emerged in the victims with PTSD compared with those without PTSD.
Fig. 2.
Regional differences between the victims with and without PTSD. Areas with significantly reduced gray-matter densities in the victims with PTSD compared with those without PTSD were rendered onto orthogonal slices of the normal template magnetic resonance images.
Correlates of Gray or White Matter with Clinical Variables in Patients with PTSD. Among the patients with PTSD, there was a significant negative correlation between the total score of CAPS and the gray-matter density in the left ACC gray matter {peak coordinate [x, y, z (mm)] = (-8, 12, 28), Z score = 4.36} (Fig. 3). This result indicates that the reduced ACC gray-matter volume showed a significant inverse relationship with PTSD severity after controlling for the age and gender effects. In the other regions, gray- or white-matter density was not significantly correlated with the total score of CAPS. In victims with and without PTSD, there were no significant positive or negative correlations between regional gray- or white-matter density and age at onset, SES, parental SES, scores of the revised impact-of-event scale, and concentration of serum cholinesterase.
Fig. 3.
Left ACC volume correlations with PTSD symptoms measured by total score of CAPS.
Discussion
Morphological analysis with VBM revealed a significant regional volume reduction in the left ACC gray matter in trauma survivors with PTSD compared with those without PTSD. This reduced ACC gray-matter volume was significantly related to the severity of the disorder in PTSD subjects. The coexistence of these theoretically independent effects, i.e., the group difference in densities of ACC and the association between densities and behavioral scores within the patient group, provides compelling evidence for the importance of ACC in the pathophysiology of PTSD. In addition, we found no significant structural differences in the other regions including the medial temporal region. This study provides evidence of a volume reduction of ACC in patients with PTSD. The findings of the present study are consistent with several neuroimaging studies that have suggested dysfunction or neuronal loss in ACC in patients with PTSD (16, 17, 42–46). The results of the present and previous studies support a neuroanatomic model of PTSD that posits a failure of ACC to inhibit a hyperresponsive amygdala (16, 43, 47, 48). Furthermore, the results of this study are in line with those from published neuropsychological studies (49, 50) that showed that hippocampal functions were not impaired in PTSD after recent traumatic events, whereas performances on frontal lobe tasks such as attention and set-shifting were impaired.
The findings of the present study are interesting in light of the fact that ACC may perform a compensatory role in the regulation of emotion (51) and play an important role in attention (52). It has been proposed that an impairment of emotional modulation and gating function may underlie the neurobiology of PTSD (53). Furthermore, studies in rats have shown that lesions in ACC facilitated fear responses during the acquisition and extinction phases of a fear-conditioning task (54). Theoretically, a dysfunction in ACC could provide a failure to regulate fear responses to traumatic events in patients with PTSD (2). In addition, Pujol et al. (55) showed a relationship between anatomical variability of ACC and harm avoidance of temperament and character inventory, a temperamental disposition to fear, and anticipatory worry in healthy subjects. Moreover, Richman and Frueh (56) reported that combat veterans with PTSD were found to be high on harm avoidance and that high scores for harm avoidance were predictive of increased PTSD symptom severity. Therefore, it may be reasonable to expect that a preexisting smaller ACC gray-matter volume in patients with PTSD may indicate an individual's higher vulnerability to developing PTSD after exposure to a traumatic event. However, the design of this study cannot address the etiology of the volume reduction directly, i.e., whether it is a vulnerability marker or a consequence of the chronic effect of stress.
In the present study there were no morphological differences in the hippocampus between subjects with PTSD and those without PTSD. We propose two possible explanations for the discrepancy between our results and those of previous studies showing hippocampal volume reduction. First, the previous studies showing hippocampal volume reduction examined combat veterans or survivors of sexual and/or physical abuse with PTSD (3–8), whereas studies of PTSD subjects with acute, short-term traumatization (including the present study) reported negative findings (12). Therefore, a smaller hippocampus may be seen only in patients with highly chronic PTSD. Second, the presence or absence of substance/alcohol misuse and depression in PTSD subjects may also contribute to the inconsistency. Most PTSD subjects examined in previous studies showing hippocampal volume reduction included subjects with current alcohol dependence and/or depression (3–8). Both depression (57) and alcoholism (14) are possibly associated with a smaller hippocampal volume. Schuff et al. (13) showed that there were no differences in hippocampal volumes between PTSD patients without a recent history of alcohol abuse and control subjects. Agartz et al. (14) also reported that the hippocampal volumes in alcoholic women who had PTSD did not differ from those of the alcoholic women who did not have PTSD. In the present study, the subjects included only one PTSD patient with depression and no alcohol or substance abusers.
Here we address the methodological considerations of our study. First, the findings from this VBM study need validation through manual tracing studies of the hippocampus and ACC with the same subjects, although the validity of VBM has been confirmed in several previous studies in comparison with the conventional region-of-interest measurements (18, 21, 36, 37). It has been suggested that VBM may not detect very small and localized gray-matter volume reductions, because false-positive or false-negative VBM findings may arise from the changes in the shape or displacement of structures in the course of spatial normalization (18). Therefore, we are not able to totally rule out the possibility that some subtle differences in particular brain regions including the hippocampus remained below the sensitivity of VBM. Second, because our sample size for patients with PTSD was small, we cannot exclude the possibility that regions other than the left ACC gray matter, which were below the statistically significant level in the present examination, may show significant differences between two groups if the sample size were to be improved.
In conclusion, the present study using VBM revealed a smaller left ACC gray-matter volume in trauma survivors with PTSD than those without PTSD. Furthermore, this gray-matter volume reduction in the left ACC was significantly associated with the severity of symptoms in the patients with PTSD. These findings provide further evidence that the ACC, which is involved in attention, emotional regulation, and conditioned fear, plays an important role in the pathology of PTSD.
Acknowledgments
We gratefully thank Dr. Shin-ichi Ishimatsu (Emergency Unit, St. Luke's International Hospital) for helping us to enroll the subjects, and Drs. Yasuhiro Kawasaki, Hiroshi Matsuda, and Takashi Ohnishi for technical advice. This study was performed through Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology from the Japanese Government.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PTSD, posttraumatic stress disorder; ACC, anterior cingulate cortex; VBM, voxel-based morphometry; CAPS, clinician-administered PTSD scale; SES, socioeconomic status; SPM, statistical parametric mapping.
References
- 1.Pitman, R. K., Shin, L. M. & Rauch, S. L. (2001) J. Clin. Psychiatry 62, 47-54. [PubMed] [Google Scholar]
- 2.Villarreal, G. & King, C. Y. (2001) Semin. Clin. Neuropsychiatry 6, 131-145. [DOI] [PubMed] [Google Scholar]
- 3.Bremner, J. D., Randall, P., Scott, T. M., Bronen, R. A., Seibyl, J. P., Southwick, S. M., Delaney, R. C., McCarthy, G., Charney, D. S. & Innis, R. B. (1995) Am. J. Psychiatry 152, 973-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner, J. D., Randall, P., Vermetten, E., Staib, L., Bronen, R. A., Mazure, C., Capelli, S., McCarthy, G., Innis, R. B. & Charney, D. S. (1997) Biol. Psychiatry 41, 23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurvits, T. V., Shenton, M. E., Hokama, H., Ohta, H., Lasko, N. B., Gilbertson, M. W., Orr, S. P., Kikinis, R., Jolesz, F. A., McCarley, R. W., et al. (1996) Biol. Psychiatry 11, 1091-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein, M. B., Koverola, C., Hanna, C., Torchia, M. G. & McClarty, B. (1997) Psychol. Med. 27, 951-959. [DOI] [PubMed] [Google Scholar]
- 7.Villarreal, G., Hamilton, D. A., Petropoulos, H., Driscoll, I., Rowland, L. M., Griego, J. A., Kodituwakku, P. W., Hart, B. L., Escalona, R. & Brooks, W. M. (2002) Biol. Psychiatry 52, 119-125. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson, M. W., Shenton, M. E., Ciszewski, A., Kasai, K., Lasko, M. B., Orr, S. P. & Pitman, R. K. (2002) Nat. Neurosci. 5, 1242-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bellis, M. D., Keshavan, M. S., Clark, D. B., Casey, B. J., Giedd, J. N., Boring, A. M., Frustaci, K. & Ryan, N. D. (1999) Biol. Psychiatry 45, 1271-1284. [DOI] [PubMed] [Google Scholar]
- 10.De Bellis, M. D., Hall, J., Boring, A. M., Frustaci, K. & Moritz, G. (2001) Biol. Psychiatry 50, 305-309. [DOI] [PubMed] [Google Scholar]
- 11.Carrion, V. G., Weems, C. F., Eliez, S., Patwardhan, A., Brown, W., Ray, R. D. & Reiss, A. L. (2001) Biol. Psychiatry 50, 943-951. [DOI] [PubMed] [Google Scholar]
- 12.Bonne, O., Brandes, D., Gilboa, A., Gomori, J. M., Shenton, M. E., Pitman, R. K. & Shalev, A. Y. (2001) Am. J. Psychiatry 158, 1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuff, N., Neylan, T. C., Lenoci, M. A., Du, A. T., Weiss, D. S., Marmar, C. R. & Weiner, M. W. (2001) Biol. Psychiatry 50, 952-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agartz, I., Momenan, R., Rawlings, R. R., Kerich, M. J. & Hommer, D. W. (1999) Arch. Gen. Psychiatry 56, 356-363. [DOI] [PubMed] [Google Scholar]
- 15.De Bellis, M. D., Keshavan, M. S., Frustaci, K., Shifflett, H., Iyengar, S., Beers, S. R. & Hall, J. (2002) Biol. Psychiatry 51, 544-552. [DOI] [PubMed] [Google Scholar]
- 16.Shin, L. M., Whalen, P. J., Pitman, R. K., Bush, G., Macklin, M. L., Lasko, N. B., Orr, S. P., McInerney, S. C. & Rauch, S. L. (2001) Biol. Psychiatry 50, 932-942. [DOI] [PubMed] [Google Scholar]
- 17.De Bellis, M. D., Keshavan, M. S., Spencer, S. & Hall, J. (2000) Am. J. Psychiatry 157, 1175-1177. [DOI] [PubMed] [Google Scholar]
- 18.Wright, I. C., Ellison, Z. R., Sharma, T., Friston, K. J., Murray, R. M. & McGuire, P. K. (1999) Schizophr. Res. 35, 1-14. [DOI] [PubMed] [Google Scholar]
- 19.Wright, I. C., McGuire, P. K., Poline, J. B., Travere, J. M., Murray, R. M., Frith, C. D., Frackowiak, R. S. & Friston, K. J. (1995) Neuroimage 2, 244-252. [DOI] [PubMed] [Google Scholar]
- 20.Sigmundsson, T., Suckling, J., Maier, M., Williams, S. C. R., Bullmore, E. T., Greenwood, K. E., Fukuda, R., Ron, M. & Toone, B. K. (2001) Am. J. Psychiatry 158, 234-243. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki, M., Nohara, S., Hagino, H., Kurokawa, K., Yotsutsuji, T., Kawasaki, Y., Takahashi, T., Matsui, M., Watanabe, N., Seto, H., et al. (2002) Schizophr. Res. 55, 41-54. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki, T., Morita, H., Ono, K., Maekawa, K., Nagai, R. & Yazaki, Y. (1995) Lancet 345, 980 (lett.). [PubMed] [Google Scholar]
- 23.Weiss, D. S. & Marmar, C. R. (1997) in Assessing Psychological Trauma and PTSD, eds. Wilson, J. P. & Keane, T. M. (Guilford, New York), pp. 399-411.
- 24.Asukai, N., Kato, H., Kawamura, N., Kim, Y., Yamamoto, K., Kishimoto, J., Miyake, Y. & Nishizono-Maher, A. (2002) J. Nerv. Ment. Dis. 190, 175-182. [DOI] [PubMed] [Google Scholar]
- 25.Blake, D. D., Weathers, F. W., Nagy, L. M. M., Kaloupek, D. G., Klauminzev, G. & Keane, T. M. (1990) Behav. Therapist 13, 187-188. [Google Scholar]
- 26.Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S. & Keane, T. M. (1995) J. Trauma Stress 8, 75-90. [DOI] [PubMed] [Google Scholar]
- 27.Asukai, N. & Nishizono-Maher, A. (1998) The Japanese Version of Clinician-Administered PTSD Scale (Tokyo Institute of Psychiatry, Tokyo).
- 28.Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R. & Dunbar, G. C. (1998) J. Clin. Psychiatry 59, 22-33. [PubMed] [Google Scholar]
- 29.Hollingshead, A. B. (1965) Two-Factor Index of Social Position (Yale Univ. Press, New Haven, CT).
- 30.Oldfield, R. C. (1971) Neuropsychologia 9, 97-113. [DOI] [PubMed] [Google Scholar]
- 31.Khan, W. A., Dechkovskaia, A. M., Herrick, E. A., Jones, K. H. & Abou-Donia, M. B. (2000) Toxicol. Sci. 57, 112-120. [DOI] [PubMed] [Google Scholar]
- 32.Ashburner, J. & Friston, K. J. (2000) Neuroimage 11, 805-821. [DOI] [PubMed] [Google Scholar]
- 33.Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J. & Frackowiak, R. S. (2001) Neuroimage 14, 21-36. [DOI] [PubMed] [Google Scholar]
- 34.Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain (Thieme, Stuttgart).
- 35.Ashburner, J. & Friston, K. (1997) Neuroimage 6, 209-217. [DOI] [PubMed] [Google Scholar]
- 36.Richardson, M. P., Friston, K. J., Sisodiya, S. M., Koepp, M. J., Ashburner, J., Free, S. L., Brooks, D. J. & Duncan, J. S. (1997) Brain 120, 1961-1973. [DOI] [PubMed] [Google Scholar]
- 37.Vargha-Khadem Watkins, K. E., Price, C. J., Ashburner, J., Alcock, K. J., Connelly, A., Frackowiak, R. S., Friston, K. J., Pembrey, M. E., Mishkin, M., Gadian, et al. (1998) Proc. Natl. Acad. Sci. USA 95, 12695-12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubicki, M., Shenton, M. E., Salisbury, D. F., Hirayasu, Y., Kasai, K., Kikinis, R., Jolesz, F. A. & McCarley, R. W. (2002) Neuroimage 17, 1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friston, K. J., Frith, C. D., Liddle, P. F., Dolan, R. J., Lammertsma, A. A. & Frackowiak, R. S. (1990) J. Cereb. Blood Flow Metab. 10, 458-466. [DOI] [PubMed] [Google Scholar]
- 40.Friston, K. J., Holms, A. P., Worsley, K. J., Poline, J. B., Frith, C. D. & Frackowiak, R. S. (1995) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 41.Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S. & Frith, C. D. (2000) Proc. Natl. Acad. Sci. USA 97, 4398-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bremner, J. D., Narayan, M., Staib, L. H., Southwick, S. M., McGlashan, T. & Charney, D. S. (1999a) Am. J. Psychiatry 156, 1787-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bremner, J. D., Staib, L. H., Kaloupek, D., Southwick, S. M., Soufer, R. & Charney, D. S. (1999b) Biol. Psychiatry 45, 806-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin, L. M., McNally, R. J., Kosslyn, S. M., Thompson, W. L., Rauch, S. L., Alpert, N. M., Metzger, L. J., Lasko, N. B., Orr, S. P. & Pitman, R. K. (1999) Am. J. Psychiatry 156, 575-584. [DOI] [PubMed] [Google Scholar]
- 45.Semple, W. E., Goyer, P. F., McCormick, R., Donovan, B., Muzic, R. F., Jr., Rugle, L., McCutcheon, K., Lewis, C., Liebling, D., Kowaliw, S., et al. (2000) Psychiatry 63, 65-74. [DOI] [PubMed] [Google Scholar]
- 46.Lanius, R. A., Williamson, P. C., Densmore, M., Boksman, K., Gupta, M. A., Neufeld, R. W., Gati, J. S. & Menon, R. S. (2001) Am. J. Psychiatry 158, 1920-1922. [DOI] [PubMed] [Google Scholar]
- 47.Rauch, S. L., van der Kolk, B. A., Fisler, R. E., Alpert, N. M., Orr, S. P., Savage, C. R., Fischman, A. J., Jenike, M. A. & Pitman, R. K. (1996) Arch. Gen. Psychiatry 53, 380-387. [DOI] [PubMed] [Google Scholar]
- 48.Rauch, S. L., Whalen, P. J., Shin, L. M., McInerney, S. C., Macklin, M. L., Lasko, N. B., Orr, S. P. & Pitman, R. K. (2000) Biol. Psychiatry 47, 769-776. [DOI] [PubMed] [Google Scholar]
- 49.Uddo, M., Vasterling, J. J., Brailey, K. & Sutker, P. B. (1993) J. Psychopathol. Behav. Assess. 15, 43-52. [Google Scholar]
- 50.Vasterling, J. J., Brailey, K., Constans, J. I. & Sutker, P. B. (1998) Neuropsychology 12, 125-133. [DOI] [PubMed] [Google Scholar]
- 51.Devinsky, O., Morrell, M. J. & Vogt, B. A. (1995) Brain 118, 279-306. [DOI] [PubMed] [Google Scholar]
- 52.Cohen, J. D., Botvinick, M. & Carter, C. S. (2000) Nat. Neurosci. 3, 421-423. [DOI] [PubMed] [Google Scholar]
- 53.Hamner, M. B., Lorberbaum, J. P. & George, M. S. (1999) Depress. Anxiety 9, 1-14. [PubMed] [Google Scholar]
- 54.Morgan, M. A. & LeDoux, J. E. (1995) Behav. Neurosci. 109, 681-688. [DOI] [PubMed] [Google Scholar]
- 55.Pujol, J., Lopez, A., Deus, J., Cardoner, N., Vallejo, J., Capdevila, A. & Paus, T. (2002) Neuroimage 15, 847-855. [DOI] [PubMed] [Google Scholar]
- 56.Richman, H. & Frueh, B. C. (1997) Depress. Anxiety 6, 70-77. [DOI] [PubMed] [Google Scholar]
- 57.Nurnberger, J. I., Jr., Blehar, M. C., Kaufmann, C. A., York-Cooler, C., Simpson, S. G., Harkavy-Friedman, J., Severe, J. B., Malaspina, D. & Reich, T. (1994) Arch. Gen. Psychiatry 51, 849-859. [DOI] [PubMed] [Google Scholar]