Abstract
Overproduction of IL-6, a proinflammatory cytokine, is associated with a spectrum of age-related conditions including cardiovascular disease, osteoporosis, arthritis, type 2 diabetes, certain cancers, periodontal disease, frailty, and functional decline. To describe the pattern of change in IL-6 over 6 years among older adults undergoing a chronic stressor, this longitudinal community study assessed the relationship between chronic stress and IL-6 production in 119 men and women who were caregiving for a spouse with dementia and 106 noncaregivers, with a mean age at study entry of 70.58 (SD = 8.03) for the full sample. On entry into this portion of the longitudinal study, 28 of the caregivers' spouses had already died, and an additional 50 of the 119 spouses died during the 6 years of this study. Levels of IL-6 and health behaviors associated with IL-6 were measured across 6 years. Caregivers' average rate of increase in IL-6 was about four times as large as that of noncaregivers. Moreover, the mean annual changes in IL-6 among former caregivers did not differ from that of current caregivers even several years after the death of the impaired spouse. There were no systematic group differences in chronic health problems, medications, or health-relevant behaviors that might have accounted for caregivers' steeper IL-6 slope. These data provide evidence of a key mechanism through which chronic stressors may accelerate risk of a host of age-related diseases by prematurely aging the immune response.
A growing body of evidence has implicated caregiving as a risk factor for health. Compared with noncaregivers, men and women who provide care to a spouse with a stroke or dementia report more infectious illness episodes (1), they have poorer immune responses to influenza virus and pneumococcal pneumonia vaccines (2–4), their wounds heal more slowly (5), they are at greater risk for developing mild hypertension (6, 7), and they may be at greater risk for coronary heart disease (8). Moreover, a prospective longitudinal study found that the relative risk for all-cause mortality among strained caregivers was 63% higher than noncaregiving controls (9). In this study, we provide evidence of one core pathway behind the diverse health risks associated with caregiving and other chronic stressors: overproduction of IL-6, a key proinflammatory cytokine that appears to enhance morbidity and mortality among older adults (10).
Recent medical literature has highlighted a spectrum of age-associated diseases whose onset and course may be influenced by proinflammatory cytokines, including cardiovascular disease, osteoporosis, arthritis, type 2 diabetes, certain cancers, Alzheimer's disease, periodontal disease, and frailty and functional decline. The link to cardiovascular disease, the leading cause of death, has attracted the greatest attention; the association with IL-6 is related in part to the central role that this cytokine plays in promoting the production of C-reactive protein (CRP), an important risk factor for myocardial infarction (10–12). For example, high concentrations of CRP predicted the risk of future cardiovascular disease in apparently healthy men (12).
CRP and IL-6 have other important health consequences in addition to their role in cardiovascular disease. Elevated levels of CRP and IL-6 predicted the development of type 2 diabetes in a 4-year follow-up period in healthy women after adjustments for key risk factors; among women in the highest vs. lowest quartiles, the relative risk for developing diabetes was 7.5 for IL-6 and 15.7 for CRP (13). In another study, elevated serum IL-6 levels predicted future disability in older adults, a finding that may reflect the effects of the cytokine on muscle atrophy, and/or the pathophysiologic role played by the cytokine in particular diseases (14). Proinflammatory cytokines, including IL-6, may slow muscle repair after injury and accelerate muscle wasting (15); indeed, IL-6 and CRP also play a pathogenic role in a range of diseases associated with disability among the elderly (osteoporosis, arthritis, and congestive heart failure, among others) (14).
Production of IL-6 and other proinflammatory cytokines can be directly stimulated by depression and other negative emotions and stressful experiences (16–20). Indeed, both physical and psychological stressors can provoke transient increases in proinflammatory cytokines (21, 22). Additionally, negative emotions contribute to greater risk for infection, prolonged infection, and delayed wound healing (1–5), all processes that can fuel sustained proinflammatory cytokine production. Thus, stressors can directly affect the cells of the immune system and modulate the secretion of proinflammatory cytokines. Accordingly, we argue that distress-related immune dysregulation may be one central mechanism behind a large and diverse set of health risks associated with caregiving and other chronic stressors. In this study, we tested the hypothesis that caregivers would show a steeper increase in IL-6 levels over time than noncaregiving controls. Additionally, we assessed the question of whether the cessation of caregiving would have beneficial consequences for IL-6 levels.
Materials and Methods
Participants. The 225 participants (119 caregivers and 106 controls) were part of a longitudinal project on caregiving, stress, and health in older adults (1, 2). The spousal dementia caregivers were recruited from local dementia evaluation centers in area hospitals, neurologists' referrals, Columbus Alzheimer's Disease Association support groups and monthly newsletter, and respite care programs. On entry into the study, caregivers were spending a mean ± SD of 9.72 ± 7.70 h per day in caregiving-related activities and reported they had been providing care for 4.91 ± 3.63 years.
Control participants were recruited through a variety of sources including newspaper advertisements, notices posted in senior citizen centers, and referrals from other participants; potential control participants who reported any caregiving activities were excluded. During recruitment we excluded caregivers or controls with immunologically related health problems such as cancer or recent surgeries and those taking any medications with broad immunological consequences.
For this study we analyzed frozen plasma samples from 6 consecutive years for all individuals in the cohort who were at least 55 years old (only seven fell below this threshold) and had provided at least two blood samples across the 6 years; 60% of the sample had eight or more blood samples, reflecting the fact that most participants had provided two samples from two different time points within each year. As expected, a number of the caregivers' spouses died during the course of the longitudinal study; by continuing to follow the caregivers, we were provided with valuable data on caregiver functioning after caregiving activities had ended (23). We also regularly added new caregivers and controls to the cohort across the longitudinal data collection. As described below, the statistical methods used allowed participants to be measured at different intervals and different numbers of times.
The 65 men and 160 women in this sample ranged in age from 55 to 89 on entry into this portion of the study (mean = 70.58, SD = 8.03). The caregiver and control cohorts did not differ in the proportion of women, χ2(1) = 1.14, P = 0.29; age, F(1,123) = 1.64, P = 0.20; ethnicity, χ2(1) = 1.36, P = 0.24; or education, χ2(4) = 5.81, P = 0.21. The mean education in both groups was partial college, and we used education as a proxy for socioeconomic status because many of our caregivers were older women who had not worked outside the home. The comparability of caregivers and controls had been closely monitored during recruitment to assure that the cohorts were indistinguishable on these key dimensions. When they entered this phase of data collection, the 225 participants included 91 spouses who were currently providing care for a spouse, 28 former caregivers whose spouse had died by this point in the longitudinal study (mean time since death = 33.71 months, SD = 19.00), and 106 controls (68 married, 17 widowed, 17 separated or divorced, 4 never married). Although the groups did differ in marital status at entry, χ2(3) = 26.35, P < 0.001, the inclusion of fewer intact marriages among the controls worked against confirmation of the experimental hypothesis: intact marriages are associated with lower rates of morbidity and mortality and better immune function (24). The Ohio State University Biomedical Research Review Committee approved the project; all participants gave written informed consent before participation.
Assessment of Stress, Depressive Symptoms, Loneliness, and Health-Related Behaviors. The 10-item Perceived Stress Scale, administered each time blood was drawn, assessed the degree to which subjects perceived their daily life during the prior week as unpredictable, uncontrollable, and overloading (25). Subjects rated each item from 0 (never) to 4 (very often).
The Beck Depression Inventory (BDI) provided information on the severity of depressive symptoms (26). The 13 items on the short BDI cover affective, cognitive, and vegetative symptoms.
Participants' self-reported loneliness was measured by using a shortened version of the New York University Loneliness Scale (27). The three-item version used in this study assessed how often participants felt lonely, how lonely they felt, and how lonely they thought they were compared with other people their own age. Scores ranged from 3 to 18, with higher scores indicating a greater sense of loneliness.
We collected health-related data to assess the possibility that relationships between caregiving and IL-6 levels might simply reflect the contribution of other variables. Plasma albumin levels and body mass data provided information on nutritional status. Health questions from the Older Adults Resources Survey (28) assessed other underlying diseases. Several studies have found excellent agreement between self-reports and hospital or physician records for specific conditions of interest to us, including myocardial infarction, stroke, and diabetes (29, 30). Assessment of health-related behaviors included recent medication use, hours of sleep in the last 3 days, and recent alcohol intake (31). Two questions assessed exercise (32).
Plasma IL-6 Levels. All blood samples were drawn between 8 and 11 a.m. to control for diurnal variation. Plasma samples frozen at -40°C from caregivers and controls were thawed. IL-6 levels were assayed by using a Quantikine High Sensitivity Immunoassay kit (R & D Systems) per kit instructions. Samples were run neat in duplicate, and all samples for an individual were run in the same assay.
Statistical Analyses. Analyses used the yearly means of the twice-yearly IL-6 blood samples for increased stability; the average correlation between pairs of blood samples within a year was r = 0.81 (range = 0.72 to 0.89, P < 0.001). IL-6 data were log-transformed (base 10) to normalize the distributions before analyses (14). All subsequent references to IL-6 refer to the log-transformed variable.
Given these repeated measures on IL-6 for each individual, the primary objective of the statistical analyses was to assess individual and group differences in the pattern of change in IL-6 and determine whether any such differences were related to other relevant variables. Multilevel models (33) were used to address these questions. In such models, the outcome variable, here IL-6, is expressed as a function of time, represented by age in this study. In our models, IL-6 was represented as a linear function of age, with each individual having a unique intercept and slope. Age was scaled so that the intercept represented the predicted level of IL-6 at age 55, and slope represented the predicted change in IL-6 per year of aging. Intercepts and slopes can be specified as either random, meaning they vary across individuals, or fixed, meaning they are the same for all individuals. Analysis using a fully random model yields estimates of the mean and variance of intercepts and slopes and their covariance. In the present context, such information was obtained simultaneously for each group: caregivers and controls. These models can be extended by introducing additional variables (e.g., health behaviors) that serve as predictors of intercepts and slopes; this approach provides a mechanism for determining whether individual or group differences in slope might be accounted for by particular variables of interest.
Results of fitting a given model also include a deviance statistic that allows for testing differences between models when one model is a special case of another. For example, it is possible to test the difference between a model specifying that controls and caregivers have the same mean slope vs. a model, allowing those means to be different. Results of this particular test will be of primary interest in the present study. For such tests, the difference between the deviance statistics for two models provides a χ2 statistic, with degrees of freedom equal to the difference in the number of parameters estimated in the two models being compared. Multilevel analyses were conducted by using lisrel 8.53 software.
To assess the possibility that relationships between caregiver status and IL-6 might simply reflect the contribution of health problems, medications, or health habits, two different kinds of analyses were conducted. Group differences were assessed cross-sectionally each year on a series of relevant variables. In addition, a series of multilevel models investigated the possibility that group differences in rate of change in IL-6 were a function of health problems or health habits.
Results
Change in IL-6. We modeled change in IL-6 levels with a two-group linear multilevel model, with measurement occasion as the level-1 unit, participant as the level-2 unit, and age as the predictor of IL-6. Age was centered at 55 (the youngest age used in the sample) so that intercepts could be interpreted as predicted levels of IL-6 at that age. A model with both intercepts and slopes specified as random parameters demonstrated a significant linear trend over age for caregivers, mean slope (± SEM) = 0.018(0.004), P < 0.001, but not for controls, mean slope = 0.005(0.004), P = 0.157, and no significant parameter variances or covariances. Intercepts showed significant interindividual variability in the control group, variance = 0.197(0.070), P = 0.005, but not in the caregiver group, variance = 0.108(0.065), P = 0.093.
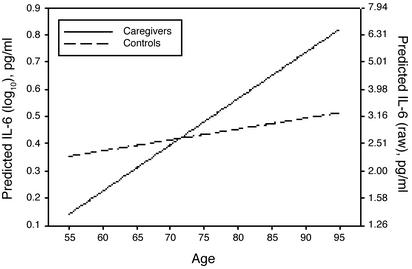
Because slopes did not vary significantly in the initial model, slope was respecified as a fixed parameter. Intercept was allowed to vary freely because its nonsignificant variance in the caregiver group was largely caused by the selection of 55 as the intercept age. The resulting model showed no significant decrement in fit as compared with the model with random slopes, χ2(4) = 2.255, not significant. The predicted rate of change in IL-6 for caregivers, slope = 0.017, was approximately four times as large as that for controls, slope = 0.004. A test of the equality of slopes between caregivers and controls compared the fit of a model with the slope parameters constrained to equality across groups vs. a model without such a constraint. Slopes were significantly different between groups, χ2(1) = 5.408, P = 0.02. The fitted functions for both caregivers and controls representing IL-6 as a linear function of age are presented in Fig. 1.
Fig. 1.
Modeled change in IL-6 in caregivers vs. noncaregivers. The slope for caregivers, mean = 0.017(0.004), P < 0.001, was significant, whereas that for controls, mean = 0.004(0.003), P = 0.212, was not. Ninety-five percent confidence intervals for slopes were {0.009, 0.025} for caregivers and {-0.002, 0.010} for controls. The two slopes were significantly different from one another, χ2(1) = 6.66, P = 0.01, and caregivers' average rate of increase was about four times greater than that of noncaregivers.
As noted earlier, 28 of the caregivers' spouses had already died at the beginning of this portion of the longitudinal study, and an additional 50 of the 119 spouses died during the 6 years of this study. Additional analyses addressed the question of whether caregivers' steeper IL-6 slopes compared with controls might be related to loss of the spouse. For example, it was possible that cessation of caregiving substantially reduced the IL-6 slope, reflecting the end of caregiving-related stressors. To investigate these possibilities, we defined and compared mutually exclusive groups of former and current caregivers. Former caregivers were defined as those participants with at least three annual valid IL-6 measurements after the loss of a spouse; current caregivers included all other caregivers. This approach produced groups of 40 former caregivers and 68 current caregivers. Importantly, for the purposes of these analyses, only measurements taken before the spouse's death were retained for current caregivers, and only measurements taken after the spouse's death were retained for former caregivers. These time periods were chosen because of our interest in assessing the longer-term sequelae of caregiving beyond what would be expected for normal bereavement-related distress (35, 36).
IL-6 data from these groups were analyzed in a two-group multilevel model with random intercepts and slopes. The mean intercept for former caregivers, 0.023(0.112), P = 0.839, and the mean intercept for current caregivers, 0.165(0.098), P = 0.091, did not differ significantly, χ2(1) = 0.912, P = 0.34. The mean slope for former caregivers, 0.021(0.009), P = 0.015, and the mean slope for current caregivers, 0.015(0.006), P = 0.014, also did not differ significantly, χ2(1) = 0.321, P = 0.571. A model with fixed slopes showed a similar pattern of effects. Thus, there were not statistically significant differences between former and current caregivers in either absolute levels or change in IL-6 over the course of this study.
Demographic Data. In accord with other researchers, we found that men had higher levels of IL-6 than women, significantly so for 3 of the 6 years of data collection. However, when controlling for age [males were significantly older than females at entry into the study, F(1,123) = 30.88, P < 0.0001], a significant difference was found for only 2 years.
Ethnicity was categorized as African American or non-African American, related to the small number of other minority participants. IL-6 was consistently higher for African American than for non-African American participants, significantly so for 4 of the 6 years of data collection. The means ± SD for African Americans vs. non-African Americans, respectively, were 0.46(0.555) vs. 0.380(0.370), t(151) = -0.630, P = 0.530 for year 1; 0.588(0.467) vs. 0.408(0.381), t(176) = -2.055, P = 0.04 for year 2; 0.680(0.489) vs. 0.448(0.419), t(156) = -2.36, P = 0.020 for year 3; 0.620(0.469) vs. 0.380(0.345), t(158) = -2.86, P = 0.005 for year 4; 0.588(0.414) vs. 0.447(0.416), t(156) = -1.36, P = 0.177 for year 5; and 0.700(0.441) vs. 0.438(0.372), t(124) = -2.43, P = 0.016 for year 6.
To assess longitudinal effects related to ethnicity, we specified race as a predictor of IL-6 intercept and slope. Ethnicity predicted individual differences in intercept, -0.406(0.147), P = 0.006, such that non-African Americans had a lower mean intercept (at age 55) than did African Americans. However, ethnicity did not predict slope, 0.011(0.008), P = 0.176.
Further multilevel models included gender and ethnicity when caregiver status was used to predict slope. Neither gender nor ethnicity was significantly associated with slope, whereas caregiver status remained a significant predictor when these demographic variables were included in the model.
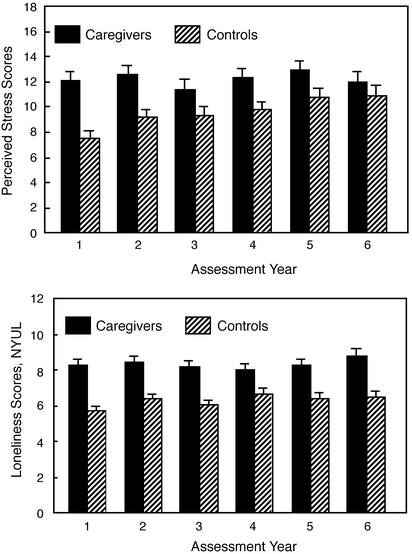
Stress, Depressive Symptoms, and Loneliness. Caregivers reported greater stress than controls across each of the 6 yearly aggregated Perceived Stress Scale assessments, with group differences that were P = 0.05 or larger for all but year 6 (Fig. 2 Upper). Further analyses comparing stress in current and former caregivers did not show significant differences in any of the 6 years, all P > 0.18.
Fig. 2.
Group differences in perceived stress scores (Upper) and loneliness [New York University Loneliness Scale (NYUL); Lower] by year. Caregivers consistently reported more stress, with group differences that were P = 0.05 or larger for all but year 6, with 1–6, respectively: t(157) = 4.70, P < 0.001; t(178) = 3.69, P < 0.001; t(165) = 1.98, P = 0.05; t(169) = 2.56, P = 0.01; t(168) = 2.01, P = 0.046; and t(143) = 0.97, P = 0.33. Similarly, caregivers consistently reported higher loneliness, with significant differences across all 6 years of the study, with 1–6 respectively: t(137) = 5.50, P < 0.001; t(167) = 4.84, P < 0.001; t(153) = 4.58, P < 0.001; t(169) = 3.02, P = 0.003; t(161) = 4.04, P < 0.001; and t(135) = 4.34, P < 0.001.
Caregivers reported significantly greater loneliness than controls in each of the 6 years of data collection (Fig. 2 Lower). Subsequent analyses comparing loneliness in current and former caregivers did not show significant differences in any of the 6 years; indeed, with the exception of year 1, t(79) = -1.52, P = 0.80, the differences between current and former caregivers were very small, t < 1, for all other years.
Further analyses addressed the question of whether the observed group difference in change in IL-6 might be reflecting group differences in severity of stress or depressive symptoms or loneliness rather than caregiver status per se. None of these variables alone significantly predicted IL-6 slope. When caregiver status and any of these variables were used to predict slope, caregiver status again predicted slope, whereas the other variables did not. Thus, the evidence suggested that the caregiver-related differences in IL-6 slope were not simply a function of differences in stress, depressive symptoms, or loneliness between caregivers and noncaregivers.
Health-Related Data. Further analyses assessed the possibility that the relationships between caregiver status and IL-6 might simply reflect the contribution of health habits associated with higher IL-6 and/or chronic health problems. Analyses yielded no significant group differences within any of the 6 years in the proportion of participants in each group who reported specific chronic health conditions that have been associated with higher IL-6, including stroke, diabetes, and cancer (14). It should be noted that base rates for each were low related to our exclusion criteria at study entry. A significantly higher proportion of caregivers (22.5%) than controls (7.7%) answered yes to the Older Adults Resources Survey question about heart trouble in year 1, χ2(1) = 6.72, P = 0.01, but the proportions were not different in any subsequent years, P > 0.34. Similarly, a significantly higher proportion of caregivers (49.5%) than controls (33.8%) answered yes to the Older Adults Resources Survey question about hypertension in year 5, χ2(1) = 4.21, P = 0.04, and a marginally higher proportion did so in year 6, χ2(1) = 3.49, P = 0.06, but not in any other years, P > 0.21.
Most older adults take some medication; of greatest concern were those medications that have been associated with alterations in IL-6, particularly the statins and estrogen. Analyses showed no differences in the proportion of caregivers and controls reporting use of statins, P > 0.41, or the proportion of women on estrogen, P > 0.34. There were no significant differences between groups in the proportion reporting regular use of aspirin or other over-the-counter analgesics, P > 0.19, beta blockers, calcium channel blockers, or diuretics, all P > 0.29.
Participants were considered smokers if they reported smoking during any year they were in the study. Although caregiver status was marginally related to smoking status, χ2(1) = 3.54, P = 0.06, this relationship reflected the fact that fewer caregivers smoked than controls (3% vs. 9%, respectively). Group differences were assessed each year in body mass index (BMI), exercise, number of chronic health problems, serum albumin, and white blood cell count; there were no significant group differences for any year on any of these variables. In the case of alcohol intake, caregivers reported 2.49 ± 4.37 drinks during the week before assessment in year 4, compared with 1.31 ± 2.41 in controls, t = 2.17, P = 0.03; whereas caregivers had consistently higher values across the 6 years of data, differences in the other 5 years were not significant, P > 0.10, and mean weekly consumption in both groups was similarly low, i.e., fewer than three drinks per week at all time points for both groups.
Other researchers have shown that higher levels of IL-6 levels are associated with greater body fat and a higher BMI (34). In our data, BMI is associated with IL-6 in terms of the intercept, 0.023(0.005), P < 0.001; that is, heavier individuals do have higher levels of IL-6. However, this effect does not change when caregiver status is included as a predictor in the model; i.e., the conditional effect of BMI on intercept = 0.023(0.005), P < 0.001. Moreover, BMI is not related to the differences in slope between caregivers and controls; thus, the effect of BMI (alone) on slope = -0.000(0.001), P = 0.418, and the effect of caregiver status (with BMI) on slope = 0.014(0.005), P = 0.004, whereas the effect of BMI (with caregiver status) on slope = -0.001(0.001), P = 0.294.
Sleep disturbances are associated with alterations in IL-6 (37), and sleep differences between caregivers and controls have frequently been reported (8). Caregivers reported less sleep over the preceding 3 nights at each measurement occasion than controls, with differences in the first 2 years of this study, t(118) = -2.33, P = 0.02, and t(128) = -1.98, P = 0.05, without significant differences in the latter 4 years, P > 0.40. However, both the effect of sleep (alone) on the intercept, 0.000(0.013), P = 0.981, and the effect of sleep (with caregiver status) on the intercept, 0.001(0.013), P = 0.963, were not significant. Similarly, sleep loss (alone) did significantly affect the slope, 0.002(0.001), P = 0.196, whereas caregiver status (with sleep loss) had a significant effect on slope, 0.013(0.005), P = 0.009, and sleep loss (with caregiver status) did not have a significant effect on slope = 0.002(0.001), P = 0.153.
A further series of multilevel models investigated the possibility that the group difference in rate of change in IL-6 could be accounted for by group differences in any of the health problems or health habits described above. These analyses are pivotal, because they are much more powerful than cross-sectional analyses or strategies such as ANOVA in that they analyze six repeated measures, use a specific model for the pattern of change, and allow for individual and group differences in the parameters of the model. Only two variables were significant predictors of slope by themselves: participants who reported greater alcohol consumption had a steeper increase in IL-6 slope, 0.002(0.001), P = 0.009, and more intensive exercise was associated with slower age-related increases in the IL-6 slope, -0.002(0.001), P < 0.001. Most importantly, caregiver status was always a significant predictor of slope, even when these or other key health behaviors were included in the model. Thus, despite the fact that the number of analyses we conducted to evaluate other health-related variables substantially inflated the possibility of finding significance where null findings were desirable, we found no evidence that the differences in IL-6 patterns between caregivers and controls were simply a function of chronic health problems, medications, or health habits.
Comment
IL-6 is associated with a variety of age-related illnesses (10, 13–15, 38). Accordingly, the finding that caregivers' average rate of increase in IL-6 was about four times as large as that of control participants has notable implications for morbidity and mortality. Indeed, these data provide important evidence of a key mechanism through which chronic stressors may have potent health consequences for older adults, accelerating risk of a host of age-related diseases.
What might be the consequences of these differences over time? Epidemiological studies of individuals 65 or older have found that the highest quartile had values >3.19 pg/ml (39, 40). As one illustration of risk, participants in the upper quartile had a 2-fold greater risk of death compared with the lowest quartile (39). Applying this value to the data from our model suggests that caregivers would on average cross that line around age 75, whereas controls would cross sometime after age 90.
The mean rate of increase in IL-6 among former caregivers did not differ from that of current caregivers even several years after the death of the impaired spouse. The absence of any notable improvement after cessation of caregiving may be related to both biological and psychological mechanisms. Stress and depression can permanently alter the responsiveness of the immune system (41–44). For example, prior stressor exposure appears to prime proinflammatory cytokine responses, such that subsequent exposure to a bacterial endotoxin, lipopolysaccharide, resulted in a larger or more rapid induction of proinflammatory cytokines in stressed rats compared with nonstressed controls (41).
The evidence that prior stress produces exaggerated proinflammatory cytokine responses to infection (41) is important in light of data discussed earlier: caregivers report more infectious illness episodes, and they have poorer immune responses to influenza virus and pneumococcal pneumonia vaccines than noncaregivers (1–4). Thus, stressed caregivers are likely to be at greater risk for infection, and their inflammatory responses to these challenges could be exaggerated and prolonged through this mechanism as well.
Although there are biological mechanisms that may lead to persistent IL-6 changes even after the cessation of caregiving, it is also important to highlight psychological processes that may also have physical repercussions. In other longitudinal data collected from our participants before and after the death of the impaired spouse, we examined changes in the same individuals over the period they were caregiving and then after the death of their spouse; we found that former caregivers' scores on measures of depression and loneliness did not “rebound” to levels comparable to noncaregivers and, in fact, remained similar to those of current caregivers up to 3 years after caregiving had ceased (23). These findings stand in contrast to evidence from longitudinal studies of spousal bereavement in the general (noncaregiver) population that have shown that depression returns to baseline within 1–2 years postloss (35, 36); thus, the changes in caregivers are not simply an artifact of bereavement. Other longitudinal studies have found that caregivers show some improvement in psychological functioning after bereavement, albeit without returning to normative levels (7, 45, 46). Indeed, the loss of social supports and consequent increased loneliness are well documented correlates of dementia caregiving (47); after providing care for 3–10 years, many former caregivers may not emerge from the experience with the same social support system that they had before the spouse's dementia. Social isolation has clear ties to morbidity and mortality (24), and thus may serve as an additional conduit for perpetuation of caregiving-related stresses.
In one large population-based study of the elderly drawn from a random, stratified sample, caregivers reported less stress during caregiving on average than we and others have found (48). Although there are key differences between the two cohorts in the way caregivers were defined as such, it is noteworthy that all-cause mortality among their randomly selected caregivers who described themselves as strained was 63% greater than that of noncaregiving controls (9). Our IL-6 findings provide one viable mechanism that could explain caregivers' substantial differences in mortality across a range of illnesses.
More broadly, our data have implications well beyond caregiving; they suggest that if other chronic stressors similarly provoke persistent distress in older adults, then they may also accelerate age-related increases in IL-6. Indeed, we found preliminary evidence of ethnicity differences, with African Americans having higher IL-6 than non-African Americans. Such changes may be one factor in the well documented racial and ethnic disparities in health (49) and deserve further investigation.
Other researchers have demonstrated that higher plasma IL-6 levels are associated with adverse health habits: values are higher in smokers than nonsmokers, in individuals who report less physical activity, in those whose sleep is impaired, and in those with a higher BMI, all behaviors that are adversely affected by stress (14, 34, 37, 50–52). Although the linkage between caregiving and IL-6 was still significant in our data after accounting for these health behaviors, chronic stressors are also likely to affect IL-6 and thus health through these pathways as well.
IL-6 is associated with a spectrum of age-associated diseases whose onset and course may be influenced by proinflammatory cytokines. The finding that caregivers' average rate of increase in IL-6 was about four times as large as that of noncaregivers suggests that a chronic stressor is capable of substantially augmenting normal age-related increases, effectively prematurely aging the immune response. These data provide important evidence of a key mechanism through which chronic stressors may have potent health consequences for older adults, accelerating risk of a host of age-related diseases.
Acknowledgments
This research was supported in part by National Institutes of Health Grants R37 MH42096 and PO1 AG11585, National Institutes of Health General Clinical Research Center Grant MO1-RR-0034, and Comprehensive Cancer Center Core Grant CA16058.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CRP, C-reactive protein; BMI, body mass index.
References
- 1.Kiecolt-Glaser, J. K., Dura, J. R., Speicher, C. E., Trask, O. J. & Glaser, R. (1991) Psychosom. Med. 53, 345-362. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser, J. K., Glaser, R., Gravenstein, S., Malarkey, W. B. & Sheridan, J. (1996) Proc. Natl. Acad. Sci. USA 93, 3043-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser, R., Sheridan, J. F., Malarkey, W. B., MacCallum, R. C. & Kiecolt-Glaser, J. K. (2000) Psychosom. Med. 62, 804-807. [DOI] [PubMed] [Google Scholar]
- 4.Vedhara, K., Cox, N. K. M., Wilcock, G. K., Perks, P., Hunt, M., Anderson, S., Lightman, S. L. & Shanks, N. M. (1999) Lancet 353, 627-631. [DOI] [PubMed] [Google Scholar]
- 5.Kiecolt-Glaser, J. K., Marucha, P. T., Malarkey, W. B., Mercado, A. M. & Glaser, R. (1995) Lancet 346, 1194-1196. [DOI] [PubMed] [Google Scholar]
- 6.Shaw, W. S., Patterson, T. L., Ziegler, M. G., Dimsdale, J. E., Semple, S. J. & Grant, I. (1999) J. Psychosom. Res. 46, 215-227. [DOI] [PubMed] [Google Scholar]
- 7.Grant, I., Adler, K. A., Patterson, T. L., Dimsdale, J. E., Ziegler, M. G. & Irwin, M. R. (2002) Psychosom. Med. 64, 477-486. [DOI] [PubMed] [Google Scholar]
- 8.Vitaliano, P. P., Scanlan, J. M., Zhang, J., Savage, M. V., Hirsch, I. B. & Siegler, I. C. (2002) Psychosom. Med. 64, 418-435. [DOI] [PubMed] [Google Scholar]
- 9.Schulz, R. & Beach, S. R. (1999) J. Am. Med. Assoc. 282, 2215-2219. [DOI] [PubMed] [Google Scholar]
- 10.Papanicolaou, D. A., Wilder, R. L., Manolagas, S. C. & Chrousos, G. P. (1998) Ann. Intern. Med. 128, 127-137. [DOI] [PubMed] [Google Scholar]
- 11.Kiechl, S., Egger, G., Mayr, M., Wiedermann, C. J., Bonora, E., Oberhollenzer, F., Muggeo, M., Xu, Q., Wick, G., Poewe, W. & Willeit, J. (2001) Circulation 103, 1064-1070. [DOI] [PubMed] [Google Scholar]
- 12.Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. (1997) N. Engl. J. Med. 336, 973-979. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan, A., Manson, J., Rifai, N., Buring, J. & Ridker, P. (2001) J. Am. Med. Assoc. 286, 327-334. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci, L., Harris, T., Guralnik, J., Tracy, R., Corti, M., Cohen, H., Penninx, B., Pahor, M., Wallace, R. & Havlik, R. (1999) J. Am. Geriatr. Soc. 47, 639-646. [DOI] [PubMed] [Google Scholar]
- 15.Cannon, J. (1995) J. Gerontol. A Biol. Sci. Med. Sci. 50, 120-123. [DOI] [PubMed] [Google Scholar]
- 16.Dentino, A. N., Pieper, C. F., Rao, K. M. K., Currie, M. S., Harris, T., Blazer, D. G. & Cohen, H. J. (1999) J. Am. Geriatr. Soc. 47, 6-11. [DOI] [PubMed] [Google Scholar]
- 17.Maes, M., Bosmans, E., De Jongh, R., Kenis, G., Vandoolaeghe, E. & Neels, H. (1997) Cytokine 9, 853-858. [DOI] [PubMed] [Google Scholar]
- 18.Lutgendorf, S. K., Garand, L., Buckwalter, K. C., Reimer, T. T., Hong, S. & Lubaroff, D. M. (1999) J. Gerontol. A Biol. Sci. Med. Sci. 54, M434-M439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maes, M., Song, C., Lin, A., De Jongh, R., Van Gastel, A., Kenis, G., Bosmans, E., De Meester, I., Benoy, I., Neels, H., et al. (1998) Cytokine 10, 313-318. [DOI] [PubMed] [Google Scholar]
- 20.Maes, M., Lin, A., Delmeire, L., Van Gastel, A., Kenis, G., De Jongh, R. & Bosmans, E. (1999) Biol. Psychiatry 45, 833-839. [DOI] [PubMed] [Google Scholar]
- 21.DeRijk, R., Michelson, D., Karp, B., Petrides, J., Galliven, E., Deuster, P., Paciotti, G., Gold, P. W. & Sternberg, E. M. (1997) J. Clin. Endocrinol. Metab. 82, 2182-2192. [DOI] [PubMed] [Google Scholar]
- 22.Zhou, D., Kusnecov, A. W., Shurin, M. R., DePaoli, M. & Rabin, B. S. (1993) Endocrinology 133, 2523-2530. [DOI] [PubMed] [Google Scholar]
- 23.Robinson-Whelen, S., Tada, Y., MacCallum, R. C., McGuire, L. & Kiecolt-Glaser, J. K. (2001) J. Abnormal Psychol. 110, 573-584. [DOI] [PubMed] [Google Scholar]
- 24.House, J. S., Landis, K. R. & Umberson, D. (1988) Science 241, 540-545. [DOI] [PubMed] [Google Scholar]
- 25.Cohen, S. & Williamson, G. M. (1988) in The Social Psychology of Health, eds. Spacapan, S. & Oskamp, S. (Sage, Beverly Hills, CA), pp. 31-67.
- 26.Beck, A. T., Steer, R. A. & Garbin, M. G. (1988) Clin. Psychol. Rev. 8, 77-100. [Google Scholar]
- 27.Rubenstein, C. M. & Shaver, P. (1982) in Loneliness: A Sourcebook of Current Theory, Research, and Therapy, eds. Peplau, L. A. & Perlman, D. (Wiley, New York), pp. 206-223.
- 28.Fillenbaum, G. G. & Smyer, M. A. (1981) J. Gerontol. 36, 428-434. [DOI] [PubMed] [Google Scholar]
- 29.Bush, T. L., Miller, S. R., Golden, A. L. & Halle, W. E. (1989) Am. J. Public Health 79, 1554-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehoe, R., Wu, S. Y., Leske, M. C. & Chylack, L. T. (1994) Am. J. Epidemiol. 139, 813-818. [DOI] [PubMed] [Google Scholar]
- 31.Kiecolt-Glaser, J. K. & Glaser, R. (1988) Brain Behav. Immun. 2, 67-78. [DOI] [PubMed] [Google Scholar]
- 32.Washburn, R. A., Adams, L. L. & Hale, G. T. (1987) Prev. Med. 16, 626-646. [DOI] [PubMed] [Google Scholar]
- 33.Raudenbush, S. & Bryk, A. (2002) Hierarchical Linear Models: Applications and Data Analysis Methods (Sage, Thousand Oaks, CA), 2nd Ed.
- 34.Vgontzas, A. N., Papanicolaou, D. A., Bixler, E. O., Kales, A., Tyson, K. & Chrousos, G. P. (1997) J. Clin. Endocrinol. Metab. 82, 1313-1316. [DOI] [PubMed] [Google Scholar]
- 35.Harlow, S. D., Goldberg, E. L. & Comstock, G. W. (1991) Am. J. Epidemiol. 134, 526-538. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, L. W., Gallagher-Thompson, D., Futterman, A., Gilewski, M. J. & Peterson, J. (1991) Psychol. Aging 6, 434-442. [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas, A. N., Papanicolaou, D. A., Bixler, E. O., Lotsikas, A., Zachman, K., Kales, A., Prolo, P., Wong, M. L., Licinio, J., Gold, P. W., et al. (1999) J. Clin. Endocrinol. Metab. 84, 2603-2607. [DOI] [PubMed] [Google Scholar]
- 38.Ridker, P., Rifai, N., Stampfer, M. & Hennekens, C. (2000) Circulation 101, 1767-1772. [DOI] [PubMed] [Google Scholar]
- 39.Harris, T., Ferrucci, L., Tracy, R., Corti, M., Wacholder, S., Ettinger, W. J., Heimovitz, H., Cohen, H. & Wallace, R. (1999) Am. J. Med. 106, 506-512. [DOI] [PubMed] [Google Scholar]
- 40.Reuben, D. B., Ferrucci, L., Wallace, R., Tracy, R. P., Corti, M. C., Heimovitz, H. & Harris, T. B. (2000) J. Am. Geriatr. Soc. 48, 1404-1407. [DOI] [PubMed] [Google Scholar]
- 41.Johnson, J. D., O'Connor, K. A., Deak, T., Stark, M., Watkins, L. R. & Maier, S. F. (2002) Brain Behav. Immun. 16, 461-476. [DOI] [PubMed] [Google Scholar]
- 42.Johnson, J. D., O'Connor, K. A., Deak, T., Spencer, R. L., Watkins, L. R. & Maier, S. F. (2002) Psychoneuroendocrinology 27, 353-365. [DOI] [PubMed] [Google Scholar]
- 43.Dantzer, R., Wollman, E., Vitkovic, L. & Yirmiya, R. (1999) Mol. Psychiatry 4, 328-332. [DOI] [PubMed] [Google Scholar]
- 44.Maes, M., Ombelet, W., De Jongh, R., Kenis, G. & Bosmans, E. (2001) J. Affect. Disorders 63, 85-92. [DOI] [PubMed] [Google Scholar]
- 45.Schulz, R., Beach, S. R., Lind, B., Martire, L. M., Zdanuik, B., Hirsch, C., Jackson, S. & Burton, L. (2001) J. Am. Med. Assoc. 285, 3123-3129. [DOI] [PubMed] [Google Scholar]
- 46.Mullan, J. T. (1992) Gerontologist 32, 673-683. [DOI] [PubMed] [Google Scholar]
- 47.Light, E. & Lebowitz, B. D. (1989) Alzheimer's Disease Treatment and Family Stress: Directions for Research (National Institute of Mental Health, Rockville, MD).
- 48.Schulz, R., Visitainer, P. & Williamson, G. M. (1990) J. Gerontol. 45, 181-191. [DOI] [PubMed] [Google Scholar]
- 49.Williams, D. R. & Collins, C. (1995) Annu. Rev. Sociol. 21, 349-386. [Google Scholar]
- 50.Taaffe, D. R., Harris, T. B., Ferrucci, L., Rowe, J. & Seeman, T. E. (2000) J. Gerontol. A Biol. Sci. Med. Sci. 55, M709-M715. [DOI] [PubMed] [Google Scholar]
- 51.Redwine, L., Hauger, R. L., Gillin, J. C. & Irwin, M. (2000) J. Clin. Endocrinol. Metab. 85, 3597-3603. [DOI] [PubMed] [Google Scholar]
- 52.Irwin, M. (2002) Brain Behav. Immun. 16, 1-16. [DOI] [PubMed] [Google Scholar]