Abstract
RNA polymerase II holoenzymes respond to activators and repressors that are regulated by signaling pathways. Here we present evidence for a “shortcut” mechanism in which the Snf1 protein kinase of the glucose signaling pathway directly regulates transcription by the yeast holoenzyme. In response to glucose limitation, the Snf1 kinase stimulates transcription by holoenzyme that has been artificially recruited to a reporter by a LexA fusion to a holoenzyme component. We show that Snf1 interacts physically with the Srb/mediator proteins of the holoenzyme in both two-hybrid and coimmunoprecipitation assays. We also show that a catalytically hyperactive Snf1, when bound to a promoter as a LexA fusion protein, activates transcription in a glucose-regulated manner; moreover, this activation depends on the integrity of the Srb/mediator complex. These results suggest that direct regulatory interactions between signal transduction pathways and RNA polymerase II holoenzyme provide a mechanism for transcriptional control in response to important signals.
RNA polymerase II holoenzymes figure prominently in transcriptional regulatory mechanisms in yeast and mammals. A holoenzyme purified from Saccharomyces cerevisiae contains an Srb/mediator complex associated with the carboxy-terminal repeat domain (CTD) (1, 2). The complex comprises Srb2, Srb4-Srb7, Sin4, Rox3, Rgr1, Gal11, Hrs1, and Med proteins (3), and Srb8, Srb9, and the Srb10-Srb11 CDK-cyclin may constitute a distinct subcomplex (4). Srb/mediator proteins have been implicated in the regulatory response to activators and repressors (1, 2, 4–9). In this work we have explored the possibility that the holoenzyme is also a direct target of a signal transduction pathway.
The Srb/mediator complex was genetically linked to the Snf1 protein kinase by the identification of mutations in SRB8-SRB11, SIN4, and ROX3 as suppressors of a snf1 mutation (10–12). The Snf1 protein kinase belongs to a family of conserved kinases that control gene expression and metabolism in response to stresses affecting the cellular energy supply. The mammalian homolog of Snf1, AMP-activated protein kinase, is regulated by the AMP:ATP ratio, and the yeast Snf1 kinase is activated when cells are deprived of glucose (13). The Snf1 kinase has an essential role in the transcriptional control of genes that are repressed during growth in glucose and induced in response to glucose limitation. Snf1 exerts major effects on the transcription of highly glucose-regulated genes by controlling the expression and function of activators and repressors (14). However, the shift from fermentation to respiration in response to glucose depletion also entails an at least two-fold induction of about 700 genes (15). Here we provide evidence for a direct regulatory interaction between Snf1 and the RNA polymerase II holoenzyme that could, in principle, provide a parsimonious mechanism for controlling a large array of genes.
Materials and Methods
Strains and Genetic Methods.
Wild-type S. cerevisiae strains were FY250 (MATα ura3 leu2 his3 trp1), MCY3647 (MATα ura3 leu2 his3 lys2), MCY3672 (MATα mig1Δ2∷LEU2 ura3 leu2 his3 trp1 lys2), and CTY10-5d (MATa ade2 leu2 his3 trp1 gal4 gal80 URA3∷lexAop-lacZ) (a gift of R. Sternglanz, State University of New York, Stony Brook). Isogenic mutant derivatives carry the alleles srb8Δ∷LEU2 (W. Song and M.C., unpublished work); srb9Δ = ssn2Δ2∷LEU2 (11); srb10Δ = ssn3Δ1∷HIS3 (10); srb11Δ = ssn8Δ1∷LEU2 (10); and sin4Δ∷TRP1 (16). Strains were transformed with the lexAop-lacZ reporter plasmid pSH18-18, a derivative of pLR1Δ1 (17) containing six LexA operators (a gift of S. Hanes, Wadsworth Center, Albany, NY). Synthetic complete (SC) medium lacking appropriate supplements was used to select for plasmids (18).
β-Galactosidase Assays.
β-galactosidase activity was assayed in permeabilized cells and was expressed in Miller units (18). EDTA (10 mM) was added to disperse flocculent cells in culture aliquots used for determination of cell density.
Coimmunoprecipitation Assays.
Preparation of protein extracts and immunoprecipitation with α-HA antibody 12CA5 were as described previously (19) in buffer containing 150 mM NaCl and 0.5% Triton X-100. Proteins were separated by SDS/PAGE and were analyzed by immunoblotting with rabbit anti(α)-Snf1, monoclonal HA antibody 12CA5, or rabbit α-LexA (a gift of C. Denis, University of New Hampshire, Durham). Antibodies were detected by enhanced chemiluminescence with ECL or ECL Plus reagents (Amersham).
Preparation of Extracts for Immunoblot Analysis of LexA Fusion Proteins.
Cell pellets were mixed with sample buffer containing 4% SDS and 4% β-mercaptoethanol (50 μl of sample buffer per 1 ml of culture at OD600 of 0.5) and 0.1 g of acid-washed glass beads (0.5 mm). The samples were boiled for 3 min, were vortexed for 30 sec, were boiled and vortexed again, and were cleared by centrifugation at 12,000 × g for 1 min. Immunoblot analysis was as described above.
Results
Snf1 Kinase Increases Transcription by RNA Polymerase II Holoenzyme Bound to a Promoter.
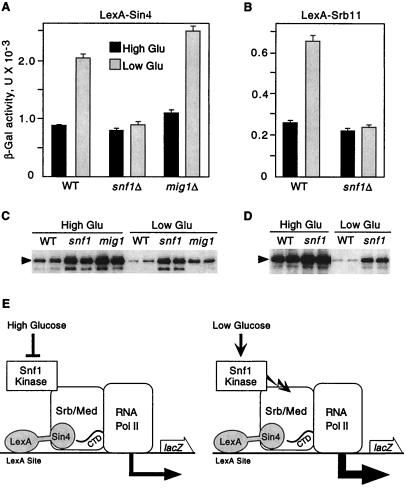
We first tested for a regulatory effect of the Snf1 kinase on transcription by the RNA polymerase II holoenzyme. Previous studies showed that LexA fusions to Srb/mediator proteins recruit the holoenzyme to a lacZ reporter with LexA binding sites and activate transcription (20). In wild-type and snf1Δ mutant cells, both LexA-Sin4 and LexA-Srb11 activated the reporter during growth in high (2%) glucose (Fig. 1 A and B). When wild-type cells were shifted to 0.05% glucose for 3 h, β-galactosidase activity increased 2.5-fold. This value represents a minimum estimate of the effect of glucose depletion because the levels of both LexA fusion proteins decreased during the shift (Fig. 1 C and D). In contrast, in the snf1Δ mutant, LexA-Sin4 and LexA-Srb11 levels remained nearly constant, but β-galactosidase activity did not increase. Control experiments with a mig1Δ mutant showed that the effect is independent of the glucose- and Snf1-regulated transcriptional repressor Mig1 (19, 21), thereby excluding any significant role for Mig1 in regulating this reporter. Thus, these findings indicate that the Snf1 kinase stimulates transcription by holoenzyme that has been recruited to a promoter (Fig. 1E).
Figure 1.
Snf1 kinase stimulates transcription by artificially recruited holoenzyme. Strains were wild-type FY250 (WT), a snf1Δ10 derivative of FY250, and MCY3672 (mig1Δ), all with the S288C genetic background. Strains were transformed with the reporter pSH18-18 and a plasmid expressing LexA-Sin4 [pSK151; derivative of pEG202 (34)] (A and C) or LexA-Srb11 [pSK34; derivative of pLexA (1–202)+PL (35)] (B and D). (A and B) Transformants were grown to mid-log phase in 2% glucose (High Glu) and were shifted to 0.05% glucose (Low Glu) for 3 h. β-galactosidase activity was assayed, and values are averages for three transformants. (C and D) Crude extracts were prepared in duplicate from the assayed transformants, and immunoblot analysis with α-LexA was carried out. Each panel shows data from a single immunoblot. (E) Fusion of an Srb/mediator component to LexA recruits the holoenzyme to the reporter and causes high-level transcription. LexA-Sin4 is depicted here. The Snf1 kinase is shown in physical contact with the Srb/mediator complex (Srb/Med) based on data shown in Fig. 2. Activation of the Snf1 kinase by the low glucose signal further increases transcription a few fold. Jagged arrow represents Snf1 kinase activity. In this figure, the holoenzyme is depicted as the target of Snf1 kinase activity, but other components of the transcriptional apparatus are also possible targets.
Snf1 Interacts with Srb10, Srb11, and Sin4 in the Two-Hybrid System.
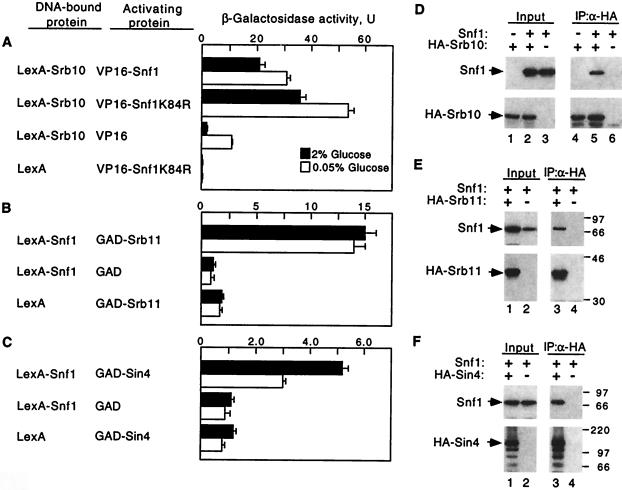
To test for physical interaction between Snf1 and Srb/mediator proteins in vivo, we first used the two-hybrid system (22). Protein fusions to the LexA DNA-binding domain (LexA87) were tested in combination with fusions to the viral VP16 or yeast Gal4 activation domain (GAD) for ability to activate a reporter with LexA binding sites (Fig. 2 A–C). LexA87-Srb10 interacted with both VP16-Snf1 and the mutant VP16-Snf1K84R, which is an inactive kinase because the invariant lysine in the ATP-binding site is replaced by arginine (23). LexA87-Snf1 interacted with GAD-Srb11 and also, weakly, with GAD-Sin4. Some or all of these interactions may be indirect and bridged by other holoenzyme components. In each case, interaction was detected both in glucose-grown cells and after a shift to low glucose. Thus, Snf1 interacts with Srb/mediator proteins in vivo, and Snf1 catalytic activity is not required.
Figure 2.
Snf1 interacts with Srb/mediator proteins. (A–C) Two-hybrid interactions were assayed in strains CTY10-5d (integrated lexAop-lacZ) (A) and FY250 carrying the lexAop-lacZ reporter pSH18-18 (B and C). Proteins were expressed from pSH2-1 (36), pACTII (37), pVP16 (38), pSK39 (10), pRJ79 (25), pRJ80 (26), pSK36 (10), pRJ56, which expresses LexA87-Snf1 from vector pSH2-1, and pIT229, which expresses GAD-Sin4 from pACTII. All LexA proteins contained the LexA87 moiety. The Srb10 and Snf1 fusion proteins are functional kinases. β-galactosidase activity was assayed in mid-log cultures grown in selective media containing 2% glucose and shifted to 0.05% glucose for 3 h. Values are averages for three to six transformants. In control experiments, VP16-Snf1 did not interact with LexA-Glc7 (26) or LexA-Ctk2. (D–F) Coimmunoprecipitation assays were carried out by using FY250 snf1Δ10 expressing Snf1 from pSK117 (19) and expressing HA-Srb10, HA-Srb11, and HA-Sin4 from pSK84, pSK86, and pWS98, respectively, which are derivatives of pWS93 (39). In D, the control strain lacking Snf1 carried the parent vector of pSK117 (lanes 1 and 4). Combinations designated as lacking HA-tagged protein instead expressed LexA87-Srb10 (10), LexA87-Srb11 (10), or LexA87-Sin4 (39). Protein extracts were prepared from cells grown in 2% glucose. Proteins (100 μg) were immunoprecipitated with α-HA, were separated by SDS/PAGE in 10% polyacrylamide, and were immunoblotted with α-Snf1. Precipitation of HA-tagged proteins was confirmed by reprobing with α-HA. The input proteins (10 μg) were similarly analyzed. The presence of the control LexA fusions in the input and their absence from the immunoprecipitates was confirmed by probing with α-LexA (not shown). Size markers are indicated in kilodaltons.
Snf1 Coimmunoprecipitates with Srb10, Srb11, and Sin4.
We next tested for coimmunoprecipitation of Snf1 with Srb/mediator proteins from cell extracts. A snf1Δ strain was cotransformed with plasmids expressing Snf1 and hemagglutinin (HA) epitope-tagged Srb10, Srb11, or Sin4. Sin4 represents a subset of proteins distinct from Srb8-Srb11, as Sin4 resides in a subcomplex of the holoenzyme comprising Rgr1, Gal11, Hrs1, and Med2 (3, 6, 24). The HA-tagged protein was immunoprecipitated, and the precipitate was subjected to immunoblot analysis with anti-Snf1. The Snf1 protein coimmunoprecipitated with HA-Srb10, HA-Srb11, and HA-Sin4 (Fig. 2 D–F). In control experiments, a LexA87 derivative was expressed instead of the HA-tagged protein, and no Snf1 was detected after precipitation with HA antibody, indicating specific dependence on the HA-tagged protein (Fig. 2 D, lane 6, and E and F, lanes 4). In addition, no signal was detected when no Snf1 was expressed (Fig. 2D, lane 4). These results, together with the two-hybrid data, provide strong evidence that Snf1 interacts with the Srb/mediator complex in vivo.
Binding of a Hyperactive Snf1 Kinase to a Promoter Stimulates Transcription in a Glucose-Regulated Manner.
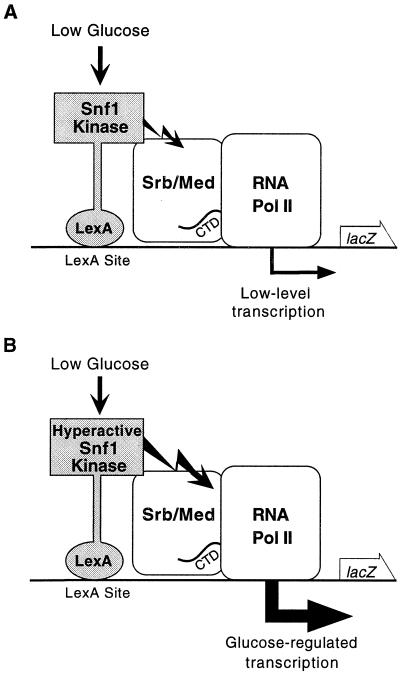
Previous studies have shown that interactions between a protein bound to a promoter and Srb/mediator components can recruit the holoenzyme to activate transcription (20). We therefore tethered LexA-Snf1 to a lexAop-lacZ reporter with the rationale that this would result in selective recruitment of Snf1-associated holoenzymes and enable us to more directly assess the regulatory input of Snf1 (Fig. 3A). Although quantitative assays did not show significant activation by LexA-Snf1 (Fig. 2 B and C), filter assays for β-galactosidase activity, which are more sensitive, revealed activation in glucose-limited cells. A faint blue color developed after lengthy incubation with X-gal, and assays of the kinase-dead LexA-Snf1K84R showed that the intensity of the color depended on Snf1 activity (data not shown), consistent with evidence that the Snf1 kinase activity stimulates transcription. We therefore tested a mutant form of Snf1 with much elevated catalytic activity, called Snf1G53R. We found that the hyperactive LexA-Snf1G53R activates strongly when cells are limited for glucose.
Figure 3.
Effects of the Snf1 kinase on transcription of a reporter by RNA polymerase II holoenzyme. (A) LexA-Snf1, when bound to LexA sites in the promoter of a lacZ reporter, causes low-level transcription, detectable only in filter lift assays (data not shown). (B) The hyperactive kinase LexA-Snf1G53R, when activated by the low glucose signal, strongly stimulates transcription, yielding glucose-regulated reporter transcription. Srb/Med, Srb/mediator complex. Jagged arrow represents Snf1 kinase activity. The holoenzyme is depicted here as the target of Snf1 kinase activity, but other components of the transcriptional apparatus are also possible targets.
The SNF1-G53R mutation was isolated as a suppressor that partially restores SUC2 expression in a mutant lacking Snf4, the stimulatory subunit for the kinase (23). The mutation alters the glycine at position 53 to arginine (subdomain I begins at residue 55), and arginine or lysine is found at this position in plant homologs of Snf1. In immune complex assays, the kinase activity of Snf1G53R is greatly elevated relative to that of wild-type Snf1 but is still stimulated by Snf4, and Snf1G53R function is glucose-regulated (23). Thus, the mutant kinase is hyperactive but otherwise similar to the wild-type Snf1.
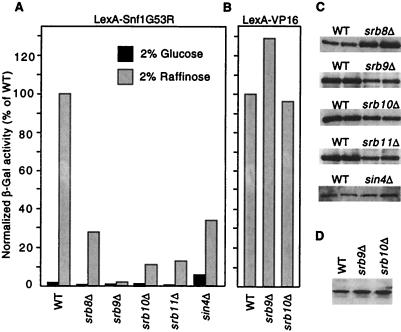
LexA-Snf1G53R strongly activated transcription of a lexAop-lacZ reporter when cells were deprived of glucose, either by a shift from 4% to 0.05% glucose (Table 1) or by growth in raffinose (Fig. 4A). Direct binding to the promoter was required because LexA-Snf1G53R did not activate transcription of the control reporter lacking LexA operators [pLR1Δ1 (ref. 17; data not shown)]. The Mig1 repressor has no significant role in regulating this activation (Table 1). Thus, this system constitutes a simple and efficient glucose-regulated promoter, in which Snf1 kinase activity triggers transcription (Fig. 3B).
Table 1.
Transcriptional activation by DNA-bound LexA-Snf1G53R
| Relevant genotype | Expressed protein | β-Galactosidase activity
|
|
|---|---|---|---|
| 4% Glu | 0.05% Glu | ||
| WT | LexA-Snf1 | <1 | <1 |
| WT | LexA-Snf1G53R | <1 | 240 |
| WT | LexA-Snf1G53R,K84R | <1 | <1 |
| snf4Δ | LexA-Snf1G53R | <1 | <1 |
| reg1Δ | LexA-Snf1G53R | 1,420 | 2,940 |
| reg1Δ | LexA | <1 | <1 |
| WT (pSH18-18) | LexA-Snf1G53R | 1 | 250 |
| mig1Δ (pSH18-18) | LexA-Snf1G53R | 2 | 440 |
Wild-type (WT) strain CTY10-5d (lexAop-lacZ) and snf4Δ3∷TRP1 or reg1Δ∷URA3 derivatives were transformed with pIT469, pRJ216, and pIT514, which express LexA fusions to Snf1, Snf1G53R, and Snf1G53R,K84R, respectively, and the vector pEG202 (34). Two strains of the S288C genetic background, wild-type FY250, and a mig1Δ mutant were transformed with both pRJ216 and the lexAop-lacZ reporter pSH18-18. Transformants were grown to mid-log phase in selective synthetic medium with 4% glucose (Glu) and were shifted to 0.05% glucose for 3 h. Values are average β-galactosidase activity for at least three transformants. Standard errors were <15%. Immunoblot analysis confirmed the stability of LexA-Snf1G53R in the snf4Δ strain.
Figure 4.
Mutations in Srb/mediator genes reduce transcriptional activation by LexA-Snf1G53R. (A) Strains were cotransformed with the reporter pSH18-18 and pIT498 or pRJ216, which express LexA-Snf1G53R from the vectors pBTM116 (22) and pEG202 (34), respectively. Isogenic pairs were strain MCY3647 (wild-type, WT) and an srb8Δ derivative carrying pRJ216; FY250 (WT) and srb9Δ, srb10Δ, and srb11Δ disruptants carrying pIT498; and FY250 and a sin4Δ disruptant carrying pRJ216. β-galactosidase activity was assayed in cells grown to mid-log phase in 2% glucose or 2% raffinose plus 0.05% glucose. Values are averages for 3–10 transformants and are expressed as percent of the activity in the isogenic wild type grown in raffinose. Standard errors for raffinose-grown cultures were <20%. Elevated β-galactosidase activity was also observed in glucose-grown sin4Δ cells expressing LexA. (B) Strains used in A were transformed with a plasmid expressing LexA-VP16 (pLexA-VP16; a gift of S. Hollenberg, Oregon Health Sciences University, Portland, OR) and pSH18-18 or the related reporter plasmid 1840, which has only one LexA binding site (identical to 1145; ref. 40). β-galactosidase activity was assayed in cells grown in 2% raffinose plus 0.05% glucose as above. Results were similar for both reporters; data shown are averages of five transformants carrying 1840. (C) Crude extracts were prepared from two of the raffinose-grown WT and mutant transformants that were assayed in A. Proteins (15 μl) were separated by SDS/PAGE in 8% polyacrylamide and were immunoblotted with α-LexA to detect LexA-Snf1G53R. (D) Levels of LexA-VP16 protein in transformants were similarly assessed by immunoblotting.
Several lines of evidence confirm that transcriptional activation by LexA-Snf1G53R depends on the catalytic activity (Table 1). Activation was inhibited by glucose and depended on the Snf4 stimulatory subunit. Activation also increased dramatically and was no longer inhibited by glucose in mutant cells lacking Reg1, which negatively regulates Snf1 by targeting the catalytic subunit of type 1 protein phosphatase to the kinase (25–27). Finally, we generated a kinase-defective, double mutant protein with an alteration in the ATP-binding site, LexA-Snf1G53R,K84R, which did not provide Snf1 function in complementation tests or in immune complex kinase assays (data not shown). LexA-Snf1G53R,K84R did not activate transcription of the reporter (Table 1). Together, these results show that catalytic activity is responsible for high-level transcriptional activation by DNA-bound LexA-Snf1G53R.
Mutations in Srb/Mediator Genes Affect Activation by LexA-Snf1G53R.
If the physical interaction between Snf1 and Srb/mediator proteins is relevant to transcriptional activation by LexA-Snf1G53R, then disruption of the structural integrity of the Srb/mediator complex should have an effect on this activation. We therefore compared wild-type and isogenic srb8Δ-srb11Δ and sin4Δ mutant strains. Activation was reduced 3- to 50-fold in the mutants relative to the wild type (Fig. 4A), without corresponding changes in LexA-Snf1G53R protein levels (Fig. 4C). In contrast, LexA-VP16 function was not affected by srb9Δ or srb10Δ (Fig. 4 B and D), indicating that these mutations do not affect transcription indiscriminately. It was still conceivable that activation by LexA-VP16 does not depend on Srb/mediator function because VP16 is a strong activator; however, the even stronger activator LexA-GAD displayed a five-fold dependence on Srb10 (data not shown). Thus, we consider the dependence of LexA-Snf1G53R on Srb/mediator proteins to be significant.
Discussion
Previous studies have implicated the Srb/mediator complex of RNA polymerase II holoenzyme in responses to transcriptional activators and repressors. Here we present evidence that the holoenzyme is also a target of a signal transduction pathway, namely the Snf1 protein kinase pathway, which has a central role in transcriptional regulation in response to glucose depletion. We show here that Snf1 kinase activity can stimulate transcription by the holoenzyme in a glucose-regulated manner and that Snf1 interacts physically with the Srb/mediator complex of the holoenzyme. These findings appear to define a novel regulatory interaction between the Snf1 kinase and the RNA polymerase II holoenzyme.
First, we show that, in response to glucose starvation, the Snf1 kinase stimulates transcription by RNA polymerase II holoenzyme that has been artificially recruited to a promoter. Second, we show that Snf1 interacts with Srb/mediator proteins in the two-hybrid system and coimmunoprecipitates with these proteins from cell extracts. Although the interactions with the specific Srb/mediator proteins tested may be indirect, these findings constitute genetic and biochemical evidence that Snf1 interacts with the Srb/mediator complex in vivo. These contacts do not require Snf1 catalytic activity and occur in glucose-grown cells. Third, we show that the catalytically hyperactive kinase Snf1G53R, when bound to a promoter as a LexA fusion protein, activates transcription in a glucose-regulated manner, and genetic analysis confirmed that the catalytic activity of Snf1G53R is responsible. Finally, activation by LexA-Snf1G53R is reduced in mutants lacking Srb8-Srb11 or Sin4, thereby supporting the idea that activation results from interaction with the holoenzyme. Individual Srb/mediator proteins may affect the ability of DNA-bound Snf1G53R to recruit the holoenzyme or may be required for subsequent stimulatory effects on transcription.
The findings presented here suggest that Snf1 interacts with the RNA polymerase II holoenzyme in glucose-grown cells. In response to glucose deprivation, the Snf1 kinase activity stimulates transcription, presumably by phosphorylation of some component of the transcription machinery. The holoenzyme is a likely candidate, based on its physical interaction with Snf1, and it will be interesting to identify the phosphorylated protein(s).
The interaction between Snf1 and the RNA polymerase II holoenzyme provides a novel mechanism for modulating the transcriptional response to glucose depletion. We propose that this mechanism acts in concert with other Snf1-dependent mechanisms involving transcription repressors and activators but provides a “shortcut” between the kinase and the holoenzyme (Fig. 5). It seems likely that Snf1 affects holoenzyme function only at a specific subset of promoters. Different holoenzyme forms have been purified, including forms that are distinct from the Srb/mediator-containing holoenzyme (28), and it is possible that these forms exhibit promoter specificity and that only certain holoenzymes are susceptible to regulation by Snf1.
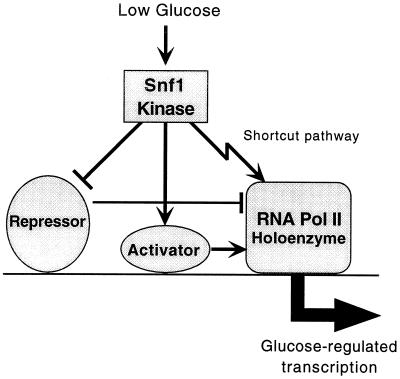
Figure 5.
Model for transcriptional regulation by the Snf1 kinase. When activated in response to glucose starvation, the Snf1 kinase positively regulates transcription by at least three mechanisms. Previous studies have shown that Snf1 both up-regulates transcription activators and inhibits transcription repressors. Evidence presented here indicates that Snf1 also stimulates the function of the RNA polymerase II holoenzyme, by phosphorylating the holoenzyme or possibly another component(s) of the transcription apparatus. At a given promoter, some or all of these mechanisms may be operative.
This shortcut pathway could contribute to regulation in vivo in several ways. It may serve to initiate a more immediate response at highly glucose-regulated promoters or simply to provide yet further amplification of the response. Such a direct regulatory interaction could also modulate transcription on a genomic scale and may have a role in the modest induction of many genes during the nutrient deprivation in the diauxic shift (15). Consistent with this possibility, genome-wide expression analysis has implicated holoenzyme components in such coordinated regulatory responses; Srb5 affects expression of pheromone response genes and Srb10 affects genes that are expressed during the diauxic shift and the dimorphic shift (29). These Srb proteins may be targets of specific signal transduction pathways.
It will be interesting to determine whether the Srb10 kinase is one of the targets of Snf1. Our data (this study; S.K., unpublished results) suggest that Snf1 does not stimulate transcription by the holoenzyme primarily by antagonism of the repressor function of Srb10 (30). However, evidence that the levels of Srb10 and Srb11 are regulated by nutrients (29, 31) suggests that the composition, as well as the function, of holoenzymes is regulated. Snf1 may have a role in this process as Snf1 activity affected LexA-Srb11 and LexA-Sin4 levels.
Mammalian RNA polymerase II holoenzymes contain homologs of Srb/mediator proteins that could potentially interact with signaling pathways. A protein that is homologous to the Ring-3 kinase, which responds to mitogenic signals (32), has been identified in a murine mediator (33) and may have a regulatory function. Direct interactions between signal transduction pathways and the holoenzyme may provide a mechanism for effecting immediate or wide-ranging transcriptional responses to important signals in eukaryotes.
Acknowledgments
We thank Valmik Vyas for helpful discussions and assistance. We thank R. Jiang for plasmids and M. Gottesman and A. Mitchell for comments on the manuscript. This work was supported by National Institutes of Health Grant GM47259 to M.C.
Abbreviations
- HA
hemagglutinin
- GAD
Gal4 activation domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140109897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140109897
References
- 1.Koleske A J, Young R A. Nature (London) 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y-J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 3.Myers L C, Gustafsson C M, Bushnell D A, Lui M, Erdjument-Bromage H, Tempst P, Kornberg R D. Genes Dev. 1998;12:45–54. doi: 10.1101/gad.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hengartner C J, Thompson C M, Zhang J, Chao D M, Liao S-M, Koleske A J, Okamura S, Young R A. Genes Dev. 1995;9:897–910. doi: 10.1101/gad.9.8.897. [DOI] [PubMed] [Google Scholar]
- 5.Koh S S, Ansari A Z, Ptashne M, Young R A. Mol Cell. 1998;1:895–904. doi: 10.1016/s1097-2765(00)80088-x. [DOI] [PubMed] [Google Scholar]
- 6.Myers L, Gustafsson C, Hayashibara K, Brown P, Kornberg R. Proc Natl Acad Sci USA. 1999;96:67–72. doi: 10.1073/pnas.96.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenblatt J. Curr Opin Cell Biol. 1997;9:310–319. doi: 10.1016/s0955-0674(97)80002-6. [DOI] [PubMed] [Google Scholar]
- 8.Carlson M. Annu Rev Cell Dev Biol. 1997;13:1–23. doi: 10.1146/annurev.cellbio.13.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Parvin J D, Young R A. Curr Opin Genet Dev. 1998;8:565–570. doi: 10.1016/s0959-437x(98)80012-9. [DOI] [PubMed] [Google Scholar]
- 10.Kuchin S, Yeghiayan P, Carlson M. Proc Natl Acad Sci USA. 1995;92:4006–4010. doi: 10.1073/pnas.92.9.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Treich I, Qian N, Kuchin S, Carlson M. Mol Cell Biol. 1996;16:115–120. doi: 10.1128/mcb.16.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balciunas D, Ronne H. Nucleic Acids Res. 1995;23:4421–4425. doi: 10.1093/nar/23.21.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardie D G, Carling D, Carlson M. Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 14.Carlson M. Curr Opin Microbiol. 1999;2:202–207. doi: 10.1016/S1369-5274(99)80035-6. [DOI] [PubMed] [Google Scholar]
- 15.DeRisi J L, Iyer V R, Brown P O. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y W, Stillman D J. Mol Cell Biol. 1992;12:4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West R W, Jr, Yocum R R, Ptashne M. Mol Cell Biol. 1984;4:2467–2478. doi: 10.1128/mcb.4.11.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose M D, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 19.Treitel M A, Kuchin S, Carlson M. Mol Cell Biol. 1998;18:6273–6280. doi: 10.1128/mcb.18.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ptashne M, Gann A. Nature (London) 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 21.Ostling J, Ronne H. Eur J Biochem. 1998;252:162–168. doi: 10.1046/j.1432-1327.1998.2520162.x. [DOI] [PubMed] [Google Scholar]
- 22.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 23.Estruch F, Treitel M A, Yang X, Carlson M. Genetics. 1992;132:639–650. doi: 10.1093/genetics/132.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Bjorklund S, Jiang Y W, Kim Y-J, Lane W S, Stillman D J, Kornberg R D. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang R, Carlson M. Genes Dev. 1996;10:3105–3115. doi: 10.1101/gad.10.24.3105. [DOI] [PubMed] [Google Scholar]
- 26.Ludin K, Jiang R, Carlson M. Proc Natl Acad Sci USA. 1998;95:6245–6250. doi: 10.1073/pnas.95.11.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz P, Alms G R, Haystead T A J, Carlson M. Mol Cell Biol. 2000;20:1321–1328. doi: 10.1128/mcb.20.4.1321-1328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang M, Jaehning J A. Nucleic Acids Res. 1997;25:4861–4865. doi: 10.1093/nar/25.24.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holstege F C P, Jennings E G, Wyrick J J, Lee T I, Hengartner C J, Green M R, Golub T R, Lander E S, Young R A. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 30.Hengartner C J, Myer V E, Liao S-M, Wilson C J, Koh S S, Young R A. Mol Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- 31.Cooper K F, Mallory M J, Strich R. Mol Cell Biol. 1999;19:3338–3348. doi: 10.1128/mcb.19.5.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denis G V, Green M R. Genes Dev. 1996;10:261–271. doi: 10.1101/gad.10.3.261. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y W, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway J W, Conaway R C, Kornberg R D. Proc Natl Acad Sci USA. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golemis E A, Serbriiskii I, Gyuris J, Brent R. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Vol. 3. New York: Wiley; 1997. p. 20.1. [Google Scholar]
- 35.Ruden D M, Ma J, Li Y, Wood K, Ptashne M. Nature (London) 1991;350:250–252. doi: 10.1038/350250a0. [DOI] [PubMed] [Google Scholar]
- 36.Hanes S D, Brent R. Cell. 1989;57:1275–1283. doi: 10.1016/0092-8674(89)90063-9. [DOI] [PubMed] [Google Scholar]
- 37.Legrain P, Dokhelar M-C, Transy C. Nucleic Acids Res. 1994;22:3241–3242. doi: 10.1093/nar/22.15.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vojtek A B, Hollenberg S M, Cooper J A. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 39.Song W, Carlson M. EMBO J. 1998;17:5757–5765. doi: 10.1093/emboj/17.19.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brent R, Ptashne M. Cell. 1985;43:729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]