Abstract
The relA gene product determines the level of (p)ppGpp, the effector nucleotides of the bacterial stringent response that are also involved in the regulation of other functions, like antibiotic production and quorum sensing. In order to explore the possible involvement of relA in the regulation of virulence of Vibrio cholerae, a relA homolog from the organism (relAVCH) was cloned and sequenced. The relAVCH gene encodes a 738-amino-acid protein having functions similar to those of other gram-negative bacteria, including Escherichia coli. A ΔrelA::kan allele was generated by replacing ∼31% of the open reading frame of wild-type relA of V. cholerae El Tor strain C6709 with a kanamycin resistance gene. The V. cholerae relA mutant strain thus generated, SHK17, failed to accumulate (p)ppGpp upon amino acid deprivation. Interestingly, compared to the wild type, C6709, the mutant strain SHK17 exhibited significantly reduced in vitro production of two principal virulence factors, cholera toxin (CT) and toxin-coregulated pilus (TCP), under virulence gene-inducing conditions. In vivo experiments carried out in rabbit ileal loop and suckling mouse models also confirmed our in vitro results. The data suggest that (p)ppGpp is essential for maximal expression of CT and TCP during in vitro growth, as well as during intestinal infection by virulent V. cholerae. Northern blot and reverse transcriptase PCR analyses indicated significant reduction in the transcript levels of both virulence factors in the relA mutant strain SHK17. Such marked alteration of virulence phenotypes in SHK17 appears most likely to be due to down regulation of transcript levels of toxR and toxT, the two most important virulence regulatory genes of V. cholerae. In SHK17, the altered expression of the two outer membrane porin proteins, OmpU and OmpT, indicated that the relA mutation most likely affects the ToxR-dependent virulence regulatory pathway, because it had been shown earlier that ToxR directly regulates their expression independently of ToxT.
Vibrio cholerae is a facultative anaerobic gram-negative bacterium and the causative agent of the severe diarrheal disease cholera. In addition to residing temporarily in the intestinal lumen of humans during the diseased state, V. cholerae has its natural niche in the aquatic environment, residing in the free-living aquatic flora found in estuarine areas and in association with crustaceans and mollusks (25). The strains of V. cholerae that cause epidemic cholera belong to serogroups O1 and O139 (3, 4, 28, 41, 50). The O1 serogroup is again divided into two biotypes, classical and El Tor (28). Strains other than O1 and O139 are known as non-O1/non-O139 vibrios.
A pathogen in its natural environment and host-associated state is subjected to a plethora of stresses, such as fluctuations in pH, salinity, osmolarity, oxygen tension, temperature, and nutritional availability. These offer selective pressure to a bacterium, eliciting various adaptive responses for its survival. The adaptive response to nutritional stress encompassing rapid and complex cellular adjustments is called the stringent response. The relA gene has been identified as the genetic determinant responsible for the stringent response to amino acid starvation in prokaryotes. The hallmark of stringent response is the RelA-catalyzed cellular increase of hyperphosphorylated guanosine nucleotides, ppGpp and pppGpp, collectively called (p)ppGpp (6), which leads to the rapid inhibition of syntheses of stable RNAs, ribosomes, and proteins, and ultimately to the arrest of cell growth (6). However, the regulation of the level of (p)ppGpp is crucial for cell viability and depends on its rates of synthesis and degradation. Degradation of (p)ppGpp is carried out in Escherichia coli and other gram-negative organisms by the product of the spoT gene, which has (p)ppGpp 3′-pyrophosphohydrolase activity (6). Furthermore, SpoT also possesses a weak (p)ppGpp synthetase activity, as revealed by the presence of a basal level of (p)ppGpp in relA null mutants that disappears in relA spoT double mutants (52).
The various cellular responses to increased (p)ppGpp concentrations have been studied in a number of microorganisms, where it was found that (p)ppGpp has a role in the synthesis and accumulation of stationary-phase sigma factor, σS (18), in antibiotic and pigment production (7, 8) and quorum sensing (49) and in developmental processes (21). Recently, it has been shown that (p)ppGpp has a role in the coordination of Legionella pneumophila virulence with entry into the stationary phase (20) and that Listeria monocytogenes relA mutants are impaired in surface-attached growth and virulence (45). The relA gene has also been cloned and characterized from a number of pathogens other than the two mentioned above, but its exact role in virulence is not known (43, 51).
At present, no information is available about the function of the relA gene in V. cholerae, and more importantly, whether it has any role in the coordination of the bacterial virulence response is not known. The virulence cascade in V. cholerae entails the activation of two transmembrane proteins, TcpP and ToxR, which act synergistically at the toxT promoter (29). ToxT then activates the expression of the two major virulence determinants of the cascade, ctxAB and tcpA (29). Due to the pioneering work done by Miller and his colleagues on ToxR (37) and because of its important role in activating the virulence genes, the regulatory cascade controlling the virulence gene expression is called the ToxR regulon. ToxR also directly regulates the expression of the outer membrane porin proteins OmpU and OmpT in a separate branch of the ToxR cascade (9). While ToxR positively regulates the expression of the OmpU protein (11), OmpT is transcriptionally repressed (31). In the present study, we report the cloning and sequencing of the relA gene of V. cholerae and the construction of a deletion-insertion relA mutant strain. Surprisingly, the relA gene mutation in V. cholerae revealed that (p)ppGpp, synthesized by RelA, plays a significant role both in vitro and in vivo in the regulation of the expression of two principal virulence factors, cholera toxin (CT) and toxin-coregulated pilus (TCP), most likely by modulating the expression of the transcriptional activator ToxR.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains, plasmids, and oligonucleotides used in this study are listed in Table 1. The C6709-R strain was a spontaneous rifampin-resistant (Rifr) mutant derived from the wild-type El Tor O1 strain C6709 (Table 1). Competition experiments showed that the C6709-R strain was as fit for growth in vitro and colonization in the suckling mouse model (see below) as the parental strain, C6709 (data not shown). Both E. coli and V. cholerae cells were routinely grown at 37°C in Luria-Bertani broth (LB) with shaking. For plate culture, LB was used with 1.5% agar. For optimal CT production by the El Tor strains of V. cholerae, AKI conditions were used (26, 27). Under AKI conditions, V. cholerae cells are grown in AKI medium without sodium bicarbonate (1.5% Bacto Peptone, 0.4% yeast extract, 0.5% NaCl) statically at 37°C for 4 h, and the culture is then shifted to overnight shaking at 37°C (26, 27). For functional assay of relA, AT medium containing the histidine biosynthesis inhibitor 3-amino-1,2,4-triazole (AT) was prepared essentially as described by Rudd et al. (42), except that it was based on M63 salt solution. For in vivo 32Pi labeling, MOPS (morpholinepropanesulfonic acid)-glucose minimal medium containing 0.4 mM phosphate, 0.4% glucose, 40 μg of each amino acid (Sigma)/ml, and 20 μg of each of the nucleosides (Sigma)/ml was used (33). Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; streptomycin, 100 μg/ml; chloramphenicol, 30 μg/ml; rifampin, 5 μg/ml; kanamycin, 50 μg/ml for E. coli and 40 μg/ml for V. cholerae. The growth kinetics of the bacterial culture was studied spectrophotometrically by measuring the optical density of the culture at 600 nm.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain or plasmid | Relevant genotype and/or phenotype | Source or reference |
|---|---|---|
| V. cholerae | ||
| 569B | Wild type (O1 classical) | 3 |
| C6709 | Wild type (O1 E1 Tor); Smr | J. J. Mekalanos |
| C6709-R | C6709 Smr Rifr | This study |
| SHK17 | C6709 ΔrelA::kan Smr Kmr | This study |
| E. coli | ||
| DH5α | F′ endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(argF-lacZYA)U169 (φ80dlacZΔM15) | Promega |
| CF1648 | Wild-type MG1655 | 52 |
| CF1652 | MG1655 relA251 Kmr | 52 |
| CF1693 | MG1655 spoT207 relA251 Kmr Cmr | 52 |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir R6K | 36 |
| Plasmids | ||
| pBluescript KS(+) | ColE1; high-copy-number cloning vector; Apr | Stratagene |
| pUC4K | Source of the kanamycin resistance gene cassette; Apr Kmr | Pharmacia |
| pGP704 | Suicide vector; pBR322 derivative with oriR6K mobRP4; Apr | 36 |
| pRELVCH | 3.21-kb PCR-amplified relA gene region of V. cholerae strain 569B in pBluescript KS(+); Apr | This study |
| pSHC4 | 1.7-kb HindIII fragment of pRELVCH cloned into the HindIII site of pBluescript KS(+); Apr | This study |
| pSH99.5 | 418-bp HindIII-PstI fragment of pRELVCH; end filled and cloned into EcoRV site of pBluescript KS(+); Apr | This study |
| pSH99.6 | 1.13-kb PstI-MluI fragment of pRELVCH; end filled and cloned into EcoRV site of pBluescript KS(+); Apr | This study |
| pSHEG17 | 1.24-kb EcoRV-HincII fragment of pRELVCH; cloned into the EcoRV site of pGP704; Apr | This study |
| pSHK17 | 660-bp NsiI fragment of the relA gene of V. cholerae present in pSHEG17 was replaced by 1.24-kb PstI fragment carrying the kanamycin resistance gene cassette of pUC4K; Apr Kmr | This study |
| Oligonucleotides | ||
| VCR-F | 5′-ACGCCACTCATAACGCGAAATT-3′ | This study |
| VCR-R | 5′-CCAAGGCTATCCCAATCAAATC-3′ | This study |
| ctxA-F | 5′-CTCAGACGGGATTTGTTAGGCACG-3′ | This study |
| ctxB-R | 5′-GGTTGCTTCTCATCATCGAACCAC-3′ | This study |
| tcpA-F | 5′-CACGATAAGAAAACCGGTCAAGAG-3′ | This study |
| tcpA-R | 5′-GAAAGGACCTTCTTTCACGTTG-3′ | This study |
| toxT-F | 5′-ACTGTCGACGCAAAGCATATTCAGAGA-3′ | This study |
| toxT-R | 5′-CGCGGATCCATACAATCGAAAATAGGA-3′ | This study |
| toxR-F | 5′-CGGGATCCATGTTCGGATTAGGACAC-3′ | This study |
| toxR-R | 5′-CGGGATCCTACTCACACACTTTGATGGC-3′ | This study |
| PMBEX1 | 5′-ACGAATTCAATGGTTGCGGTACG-3′ | This study |
Determination of intracellular (p)ppGpp concentrations.
The strains were screened for patterns of (p)ppGpp accumulation essentially as described by Cashel (5), with the following changes. An overnight bacterial culture grown in MOPS-glucose minimal medium containing 2 mM phosphate and other supplements (as stated above) was washed and resuspended in low-phosphate (0.4 mM) MOPS-glucose minimal medium supplemented with nucleosides and amino acids except serine and grown to an optical density of 0.2 at 600 nm. For detection of (p)ppGpp in rich medium, the cells were resuspended in MOPS-glucose minimal medium supplemented with nucleosides and all the amino acids (33). At this stage, the cells were uniformly labeled with [32P]H3PO4 (100 μCi/ml) (BRIT, Mumbai, India), and amino acid starvation was simulated by the addition of 500 μg of dl-serine hydroxamate (SHMT)/ml as described previously (33). For extraction of (p)ppGpp from V. cholerae cells, the method described by Ojha et al. (39) was followed. Briefly, samples were withdrawn at various time intervals, centrifuged and washed with 10 mM Tris (pH 8.0) buffer, resuspended in the same buffer containing 20 μg of lysozyme (Sigma)/ml, and kept on ice for 20 min. Lysis of the cells was done with 1% sodium dodecyl sulfate (SDS), and (p)ppGpp was extracted with an equal volume of 2 M formic acid and analyzed by one-dimensional polyethyleneimine-coated thin-layer chromatography (TLC). The TLC plate (Merck) was developed with 1.5 M KH2PO4 buffer (pH 3.4), air dried, and exposed to X-ray film (Kodak) for 24 h at −70°C for autoradiography. The authenticity of ppGpp was determined by its comigration with ppGpp from the 32P-labeled formic acid extracts of various E. coli strains (Table 1) used as controls. The alkaline hydrolysis of ppGpp (39) was done to further confirm the existence of the nucleotide. For alkaline hydrolysis of ppGpp, the formic acid extract was immediately neutralized with NH4OH, followed by treatment with 0.3 M KOH and then with 0.1 M BaCl2 at 37°C for 1 h.
DNA manipulations and cloning of the relA gene of V. cholerae.
Standard molecular biological methods were followed for chromosomal and plasmid DNA preparations, electroelution of DNA fragments, restriction enzyme digestion, DNA ligations, bacterial transformation, conjugation, agarose gel electrophoresis, and Southern blotting unless stated otherwise (2). All restriction enzymes and nucleic acid-modifying enzymes were purchased from New England Biolabs and were used as suggested by the manufacturer. For random priming of DNA, [α-32P]dCTP was obtained from Amersham Life Science. Two synthetic oligonucleotides, VCR-F and VCR-R (Table 1), were designed using the whole genome sequence of V. cholerae O1 El Tor strain N16961 (available in the genome database of The Institute for Genomic Research) and were used in PCR to amplify the relA homolog of V. cholerae from the classical strain 569B. In a final volume of 20 μl of PCR, 100 ng of V. cholerae chromosomal DNA (as a template), 250 μM deoxynucleoside triphosphates, 20 pmol (each) of the primers VCR-F and VCR-R, 1.5 mM MgCl2, 1 μl of dimethylsulfoxide, and 2.5 U of Taq polymerase (Gibco-BRL) were added. PCR was done with a GeneAmp PCR system 9700 (Perkin-Elmer). A 3.21-kb amplicon was obtained using the following PCR conditions: one cycle at 94°C for 1 min, followed by 30 cycles of 10 s of denaturation at 94°C, 30 s of annealing at 55°C, and 3 min of elongation at 72°C. The amplification reaction was completed with an additional cycle of 7 min at 72°C. The PCR amplicon was electroeluted from an agarose gel, and the purified 3.21-kb DNA fragment was cloned into an EcoRV-digested, 3′ thymidine overhang-generated pBluescript KS(+) vector DNA (Table 1), essentially as described by Marchuk et al. (32a) to generate the recombinant plasmid pRELVCH (Table 1).
Nucleotide sequencing and analysis.
DNA sequencing was done by using the ABI PRISM Dye Terminator Cycle Sequencing kit (Perkin-Elmer) and the ABI PRISM automatic DNA sequencer (model 377). DNA sequence data were compiled and analyzed by using the DNASIS computer program. Amino acid sequence homology searching was done using the BLASTX program (1).
Construction of the relA deletion mutant of V. cholerae.
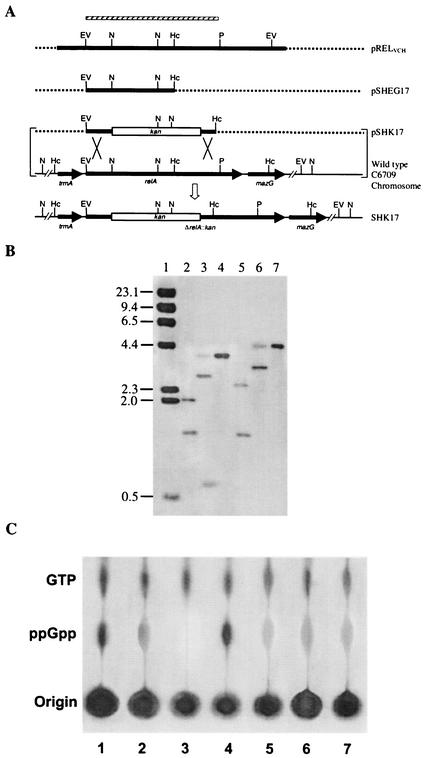
To inactivate the relA gene of V. cholerae O1 El Tor strain C6709 (Table 1), the plasmid pRELVCH (Table 1) was digested with the enzymes EcoRV and HincII, and the resulting 1.24-kb fragment, carrying the N-terminal region of the relA gene (Fig. 2), was gel purified and cloned into the EcoRV site of the suicide vector pGP704, generating the plasmid pSHEG17 (Table 1), which was propagated in E. coli strain SM10λpir (Table 1). For creating a deletion-insertion allele, pSHEG17 was digested with the enzyme NsiI to remove a 660-bp internal fragment of the relA gene of V. cholerae (Fig. 2), which was replaced with a 1.24-kb PstI-cut kanamycin resistance gene block of the vector pUC4K (Table 1). The resulting recombinant plasmid, pSHK17, was then mobilized conjugally from E. coli SM10λpir into the streptomycin-resistant wild-type V. cholerae strain C6709. Transconjugants were selected on streptomycin-kanamycin plates, where the wild-type relA gene of V. cholerae was replaced with its deletion-insertion allele ΔrelA::kan as a double-crossover event. The transconjugants were screened for pGP704 or the kanamycin cassette by colony hybridization using linearized pGP704 DNA or the 1.24-kb kanamycin gene block, respectively, as a probe. The presence of the new ΔrelA::kan allele in the V. cholerae C6709 chromosome (Table 1) was confirmed by Southern blot hybridization analysis (see Fig. 2) and also by sequencing using the primer PMBEX1 (Table 1). The V. cholerae relA mutant strain thus constructed was designated SHK17.
FIG. 2.
(A) Schematic diagram (not to scale) showing the strategy for the construction of a relA deletion-insertion mutant strain, SHK17, of V. cholerae O1 El Tor. Restriction maps of the relA regions of the parental and mutant strains are shown. The thick lines represent gene regions, and the dashed lines represent vector DNA. The arrows indicate the direction of transcription of a gene. The thin lines indicate intergenic regions or chromosomal DNA. The hatched bar represents the EcoRV-PstI fragment of the relAVCH gene used as a probe in Southern hybridization studies, as shown in panel B. The plasmid pSHK17, containing the kanamycin resistance gene cassette (kan) within the relAVCH gene, was introduced into strain C6709 by conjugation, and the recombinant (shown by an open arrow), in which the ΔrelA::kan allele had replaced the wild-type relA, was isolated as described in Materials and Methods. Restriction enzyme sites: EV, EcoRV; Hc, HincII; N, NsiI; P, PstI. (B) Confirmation of the relA mutation in SHK17 as a double-crossover event by Southern analysis. The EcoRV-PstI fragment (shown in panel A) of the relA gene of V. cholerae was used for hybridization studies. Chromosomal DNA was digested with different restriction enzymes. For a detailed analysis of the autoradiogram, see the text. Lanes 2 to 4, wild-type C6709 DNA digested with HincII, NsiI, and EcoRV, respectively; lanes 5 to 7, SHK17 DNA digested with HincII, NsiI, and EcoRV, respectively. In lane 1, λ DNA digested with HindIII was run as a molecular size marker, and the sizes are indicated (in kilobase pairs) on the left. (C) Failure of (p)ppGpp accumulation in the relA mutant strain SHK17 upon amino acid starvation. 32Pi-labeled cells were grown either in MOPS-glucose minimal medium with amino acid starvation induced by the addition of 500 μg of SHMT/ml (lanes 1 to 5) or in rich medium (lanes 6 and 7), formic acid extracts of the cells were prepared, and aliquots were loaded on a polyethyleneimine-coated TLC plate. The spots were developed as described in Materials and Methods. Lanes: 1 to 3, E. coli strains CF1648 (wild type), CF1652 (ΔrelA), and CF1693 (ΔrelA ΔspoT), respectively; 4 and 6, V. cholerae strain C6709 (wild type); 5 and 7, SHK17 (ΔrelA::kan).
Assay of CT by GM1-ELISA.
CT production was estimated in V. cholerae culture supernatants, sonicated cell lysates, or fluid collected from rabbit ileal loops by GM1-enzyme-linked immunosorbent assay (GM1-ELISA) as described previously (24). Dilutions of purified CT (Sigma) at known concentrations were used to estimate the amount of CT in samples. ELISA was done using a rabbit polyclonal antiserum against the purified B subunit of CT and anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Gibco-BRL). The color intensity was measured at 492 nm in an ELISA reader (Bio-Rad).
RNA preparation and Northern blot analysis.
For isolation of total cellular RNA, V. cholerae cells were grown at 37°C under AKI conditions, which are optimum for the detection of ctxAB, tcpA, toxT, and toxR transcripts of El Tor strains, as reported previously (34). Total RNA was extracted between 6 and 10 h after growth under AKI conditions (34) and purified by using guanidinium isothiocyanate as described elsewhere (2). RNA samples (∼20 μg/well) were electrophoresed in duplicate in 1% agarose-2.1 M formaldehyde-MOPS gels, and one part was stained with ethidium bromide and visualized with UV light to confirm equal loading of all samples. The other part of the gel was used for Northern blot experiments. The probes used were PCR amplified utilizing the ctxA-F and ctxB-R primers for the ctxAB genes, tcpA-F and tcpA-R primers for the tcpA gene, and toxT-F and toxT-R primers for the toxT gene (Table 1). The PCR conditions were as follows: one cycle of initial denaturation at 94°C for 5 min, followed by 30 cycles of 1 min of denaturation at 94°C, 30 s of annealing at 55°C, and 1 min of elongation/kb at 72°C. The amplification reaction was completed with an additional cycle of 7 min at 72°C. Quantification of the mRNA bands was done using Quantity 1 software (Bio-Rad).
RT-PCR assay.
Reverse transcriptase (RT) PCR experiments were carried out as follows. Equal amounts of total cellular RNA from all experimental samples were first treated with RNase-free DNase I (Life Technologies) and incubated at room temperature for 15 min to eliminate any contaminating DNA from the RNA sample. The reaction was terminated by adding 2.5 mM EDTA, and the DNase I enzyme (Life Technologies) was then heat inactivated at 65°C for 10 min. The RT reaction was carried out using the Superscript RT-PCR kit (Life Technologies), as directed by the manufacturer, in a GeneAmp PCR system (model 9700; Perkin-Elmer). Briefly, to 8 μl of the sample containing 200 ng of the DNase-treated RNA, 1 μl (10 pmol) of each of the gene-specific primers, 2× reaction mixture (a buffer containing 0.4 mM [each] deoxynucleoside triphosphate-2.4 mM MgSO4), and 1 μl of RT/PLATINUM Taq Mix were added. First-strand cDNA synthesis was carried out at 45°C for 30 min, followed by incubation at 94°C for 2 min to inactivate the RT, reactivate the Taq DNA polymerase, and denature the RNA-cDNA hybrid. PCR amplification of DNA was done for 40 cycles, each cycle consisting of denaturation at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min/kb. A final extension of 7 min was given at 72°C. Then, the cDNA samples were loaded on a 1.5% agarose gel along with the appropriate DNA size markers, and the gel was visualized under a UV transilluminator. To confirm the absence of contaminating template DNA in RNA samples, a PCR was run for each of the tested samples, using only Taq DNA polymerase (Life Technologies) and keeping the cycling parameters the same. Lack of amplification in the absence of RT confirmed that the PCR products were generated only from cDNAs. Quantification of the cDNA bands was done using Quantity 1 software.
Isolation of outer membrane proteins.
V. cholerae C6709 and its isogenic mutant SHK17 grown under AKI conditions were harvested and sonicated, and the resulting cell lysate was separated into subcellular fractions as described previously (13). The outer membrane fraction was solubilized, and outer membrane proteins, together with whole-cell lysate proteins, were analyzed by SDS-12.5% polyacrylamide gel electrophoresis (PAGE), followed by staining with Coomassie blue or by Western blot analysis. To ensure equal loading of the protein samples, the total protein concentrations in the samples were determined using a commercial protein determination kit (Bio-Rad). The sizes of proteins were estimated using commercially available (Genei, Bangalore, India, or Invitrogen) protein molecular weight standards. Contamination in the outer membrane preparation due to inner membrane was always <5% as determined by cytochrome assay, as described previously (13).
Immunological detection of OmpU, OmpT, and TcpA.
Western blot analysis was performed with rabbit polyclonal antisera against V. cholerae OmpU and OmpT (kindly provided by K. E. Klose, University of Texas Health Science Center, San Antonio, Tex.) followed by goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma). The blots were developed with nitroblue tetrazolium and BCIP (5-bromo-4-chloro-3-indolylphosphate) substrates by standard techniques (2). For TcpA protein detection, bacterial-cell pellets obtained from 10 ml of culture grown under AKI conditions were resuspended in 100 μl of phosphate-buffered saline (10 mM sodium phosphate buffer, pH 7.0, containing 100 mM NaCl). The resuspended cells were lysed by repeated freeze-thawing, and the total protein contents were adjusted to the same value according to protein determinations. Samples (5 μl) were placed directly on polyvinylidene-difluoride membranes (Amersham-Pharmacia), and TcpA was assayed by in situ reaction with anti-TcpA monoclonal antibody (kindly provided by J. Sanchez, Universidad Autonoma del Estado de Mexico [UAEM], Cuernavaca, Morelos, Mexico), goat anti-mouse immunoglobulin G-alkaline phosphatase conjugate (Pierce), and nitroblue tetrazolium-BCIP substrates using standard techniques (2).
Motility assay.
Bacterial colonies were stabbed into LB motility plates containing 0.3% Bacto Agar (Difco) and incubated for 10 to 14 h at 37°C. The diameter of each motility zone was measured and compared with that of the parental strain present on the same plate (17).
In vivo CT production using a ligated rabbit ileal loop model.
In vivo CT production by the wild-type V. cholerae C6709 or its isogenic relA mutant SHK17 was assayed by using the ligated rabbit ileal loop model essentially as described previously (12). Fluid accumulation (FA) in the ligated rabbit ileal loop was taken as a measure of the toxinogenicity of the strain. Briefly, rabbits were starved for 48 h prior to surgery and given water ad libitum. A rabbit was anesthetized, and a laparotomy was performed. The small intestine was tied off in alternating 6- and 2-cm segments proximally to the mesoappendix. An inoculum of 1 ml of V. cholerae cells containing ∼5 × 106 CFU was introduced into each 6-cm segment, while one loop was inoculated with normal saline (0.9% NaCl) as a negative control. The intestine was returned to the peritoneal cavity, and the incision was closed. After 16 to 18 h, the animal was sacrificed and the small intestine was removed. The fluid accumulated in each loop was separately collected in sterile test tubes, measured, and expressed as milliliters per centimeter, i.e., the ratio of loop fluid volume to loop length. The intestinal fluid was centrifuged at 8,000 rpm (Eppendorf) for 10 min at 4°C to separate the bacterial cells as a pellet. The supernatant was collected, filtered through a 0.22-μm-pore-size membrane (Millipore, Bangalore, India), and assayed for CT by GM1-ELISA (24) as described above. The bacterial cell pellet was washed twice with phosphate-buffered saline and finally resuspended in the same solution, followed by sonication and centrifugation at 10,000 rpm (Eppendorf) for 20 min at 4°C to remove cell debris, and the cellular lysate was assayed for CT. Each strain was tested in at least five individual animals.
In vivo infant mouse colonization assay.
The infant mouse colonization assay was performed essentially as described elsewhere (40, 46). Briefly, mixtures of the parental strain, C6709-R (C6709 Rifr), and the mutant strain SHK17 (Kmr) were coinoculated into 3- to 5-day-old suckling mice in a peroral inoculum ratio of ∼108 mutant to 108 wild-type V. cholerae cells. After 18 to 20 h of in vivo colonization, the small intestine was isolated and homogenized, and the mutant/wild-type ratio was determined (40) by plating dilutions on Vibrio selective thiosulfate-citrate-bile salt-sucrose agar plates containing appropriate antibiotics, and the competitive index (40) was calculated. In vitro competition was carried out in 5 ml of LB inoculated with the same mixtures and grown at 37°C overnight.
Nucleotide sequence accession number.
The DNA sequence reported in this study has been deposited in GenBank under accession number AF 175295.
RESULTS
Cloning of the relA homolog of V. cholerae and its DNA sequence analyses.
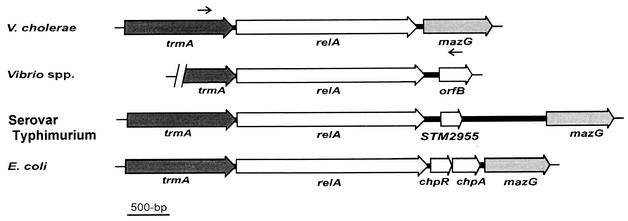
The 3.21-kb relA locus of V. cholerae (Fig. 1) was PCR amplified from the genomic DNA of the classical O1 strain 569B using the oligonucleotides VCR-F and VCR-R (Table 1) as shown in Fig. 1, and the amplified fragment was cloned as described in Materials and Methods. The recombinant plasmid thus generated was designated pRELVCH (Table 1). The entire 3.21-kb insert DNA was sequenced after several subclones were constructed (Table 1). The sequence analysis of the 3.21-kb DNA revealed only one complete open reading frame (ORF) of 2.22 kb from nucleotide positions 399 to 2615. A similarity search using the BLAST program with the nonredundant peptide sequence database revealed 100% identity at the amino acid level with the deduced RelA protein of El Tor strain N16961 (23), as well as a high degree of similarity to all known RelA homologs of other bacteria (6, 16, 38). The result indicates that the cloned fragment containing the 2.22-kb ORF is most likely a relA-like gene, and it has been designated relAVCH. However, the predicted RelA protein of V. cholerae consists of 738 amino acid residues compared to the 744 residues of Vibrio sp. strain 14 (16), Salmonella enterica serovar Typhimurium (38), and E. coli (38). Thus, the putative relA ORF of 2.22 kb should code for a protein with a molecular mass of ∼81 kDa. Analysis of the upstream and downstream nucleotide sequences of relAVCH revealed the presence of trmA- and mazG-like genes (Fig. 1), respectively, indicating that the relA locus of V. cholerae has a genetic organization similar to those of other gram-negative organisms (6, 16, 38). Complementation and functional analyses (5, 33, 42) of pRELVCH using E. coli wild-type CF1648 and its relA mutant strains CF1652 and CF1693 (Table 1) confirmed that the cloned insert in pRELVCH indeed carries a V. cholerae relA homolog whose product is responsible for the synthesis of (p)ppGpp (details of this work will be published elsewhere).
FIG. 1.
Genetic organization of the relA region of the V. cholerae chromosome and comparison with those of different gram-negative organisms. The large arrows represent ORFs. The thick and thin lines represent intergenic regions and chromosomal DNA, respectively. The small arrows indicate the positions of the two primers, VCR-F and VCR-R, used to amplify the relA gene region (3.21 kb) of V. cholerae. The trmA gene codes for an RNA methyltransferase (23); mazG codes for a protein of unknown function in V. cholerae (23) and E. coli (38), but in S. enterica serovar Typhimurium it codes for a pyrophosphatase (38); STM2955, chpA, and chpR code for a PemK-like growth inhibitor, for a suppressor of ChpA, and for a putative transcriptional regulator, respectively.
Disruption of the relA gene and its effect on (p)ppGpp accumulation.
The El Tor strain C6709 was chosen for this study because this biotype of V. cholerae is the current pandemic strain (28) and there are data available from various virulence-related studies with the strain (15, 30, 35). Database searches and alignment of the RelA protein of V. cholerae with those of other organisms (38) revealed that the catalytic domain, or the (p)ppGpp-synthesizing region of RelA, is highly conserved and putatively lies in a stretch of 200 amino acids between residues 170 and 370 near the N-terminal region (data not shown), which comprises ∼600 nucleotides. We constructed a deletion-insertion allele, ΔrelA::kan, in which a 660-bp internal region of relA overlapping the putative region coding for the catalytic domain was replaced by a kanamycin resistance gene cassette. This allele was recombined into the chromosome of the wild-type V. cholerae O1 El Tor strain C6709 as described in Materials and Methods. The V. cholerae relA mutant strain thus constructed was designated SHK17. Southern hybridization analyses of the mutant SHK17 and of the wild-type parent strain, C6709, using HincII, NsiI, and EcoRV enzymes confirmed that the relA mutant was generated by a double-crossover event. Hybridization of a HincII digest of the wild-type chromosomal DNA using the EcoRV-PstI fragment of the relAVCH gene (Fig. 2A) as a probe showed two bands of 2.05 and 1.3 kb (Fig. 2B), and the result is consistent with the V. cholerae El Tor whole-genome sequence data (23). In the case of the mutant, the 2.05-kb HincII band of the wild-type strain was replaced with a 2.63-kb hybridizing fragment (Fig. 2B). The increase in size of this HincII band in SHK17 is 0.58 kb, which is due to the insertion of the 1.24-kb kanamycin resistance gene cassette in place of the 0.66-kb internal NsiI fragment (see below) of the relAVCH gene (Fig. 2A), as described in Materials and Methods. The other HincII fragment (1.3 kb) showed no change in size in SHK17, since the two HincII sites (23) generating this fragment are present downstream of the site of allelic exchange (Fig. 2A). Similar analysis using the other restriction enzyme, NsiI, provided further evidence of the authenticity of the constructed relA mutant strain SHK17. The restriction map of the relAVCH gene indicates that there are two NsiI sites within it (Fig. 2A). As a result, when this enzyme was used for Southern analysis, three fragments of the wild-type genome with sizes of 3.8, 2.9, and 0.66 kb hybridized with the relAVCH probe (Fig. 2B). Since the ΔrelA::kan allele was created by replacing the 0.66-kb NsiI internal fragment of the relAVCH gene, no signal was obtained in this region when the NsiI-digested genome of SHK17 was probed with relAVCH (Fig. 2B). Furthermore, in SHK17, only two NsiI bands of 4.4 and 3.3 kb lit up in the autoradiograph (Fig. 2B). These bands can only arise when the ΔrelA::kan allele replaces the wild-type relAVCH. The generation of these two new NsiI bands in SHK17 could be mapped easily, since the kanamycin resistance gene cassette (1.24 kb) itself contains two NsiI sites (GenBank accession number X06404) in very close proximity (only 266 bp from each other), as shown in Fig. 2A. The calculated map distances of the NsiI sites present upstream and downstream of the relA locus of the El Tor chromosome (reference 23 and this study) and within the kanamycin resistance gene were found to be identical to the sizes of the bands determined from the autoradiogram (Fig. 2B). Hybridization analysis using EcoRV also supported the results obtained with the enzymes HincII and NsiI. The entire relA gene of El Tor is located within a 3.8-kb EcoRV fragment (Fig. 2A), as obtained from its genome sequence data (23). Thus, the EcoRV-digested genome of the wild-type El Tor strain C6709 gave a single band in the 3.8-kb region when a portion of the relA gene was used as a probe (Fig. 2B). However, in the case of the mutant strain SHK17, the band was shifted to a 4.4-kb region (Fig. 2B). Here, the increase in band size was ∼0.58 kb, which is highly consistent with the result obtained from our HincII analysis described above. Altogether, our analysis strongly suggests that the SHK17 mutant strain was generated by replacing the wild-type relAVCH by a ΔrelA::kan allele.
The mutant strain SHK17 thus developed was selected for further studies. SHK17 was found to exhibit growth sensitivity to amino acid starvation induced by the addition of SHMT (33) and AT (42) (data not shown). Moreover, following amino acid starvation, SHK17 also did not show any significant (p)ppGpp accumulation compared to its isogenic wild-type strain C6709 (Fig. 2). As controls, we used several E. coli strains (Table 1), as shown in Fig. 2. These observations are consistent with earlier reports (16, 32), where such inhibition of growth and failure of (p)ppGpp accumulation were also exhibited by the relA mutants of other organisms upon amino acid starvation. The presence of a basal level of (p)ppGpp in the relA null mutant may be due to the weak (p)ppGpp synthetase activity of the spoT gene product (52), also displayed in other gram-negative organisms (6, 16). This hypothesis was further supported when the extracts of C6709 and its isogenic relA mutant SHK17, grown in amino acid-supplemented rich medium (described in Materials and Methods), were used as controls (Fig. 2C, lanes 6 and 7). These controls showed amounts of a basal level of (p)ppGpp essentially similar to that of the mutant strain SHK17 grown under amino acid-starved conditions. Although V. cholerae possesses the spoT gene (23), whether its product has a weak (p)ppGpp synthetase activity remains to be explored.
RelA acts as a positive regulator of virulence gene expression.
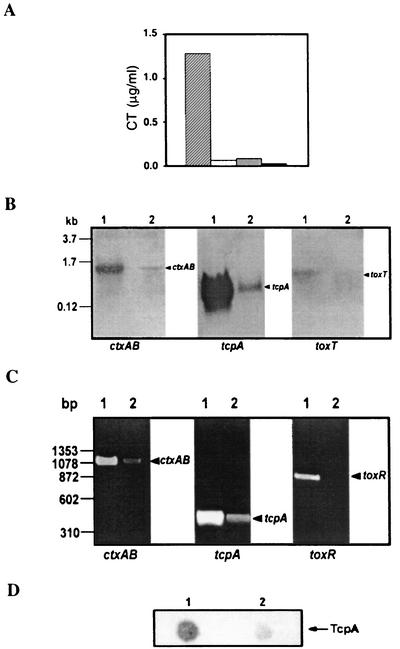
Since (p)ppGpp controls multiple cellular processes in prokaryotes, we were interested to determine whether it has any role in the regulation of expression of the major virulence factors of V. cholerae. To explore this possibility, we initially compared the levels of CT production in culture supernatants of both the wild-type El Tor C6709 strain and its relA mutant SHK17. Interestingly, the amount of CT in the culture supernatant of strain SHK17 was found to be significantly lower (∼90% reduction) than that of the wild-type strain, C6709, when both strains were grown under similar in vitro conditions favorable for optimal CT production (Fig. 3). To check whether the low level of CT in the culture supernatant of SHK17 was due to a defect in its secretion, we estimated the amount of CT in sonicated whole-cell lysates of both the parental and mutant strains. In both cases, no CT was detected (Fig. 3), indicating that the production and not the secretion of CT was actually affected in the SHK17 mutant strain. To assess whether the decrease of CT production in SHK17 was at the level of transcription, Northern blot analysis was carried out using the ctxAB genes as a probe, where total cellular RNA was prepared from cells of the wild-type and the relA mutant strain grown under AKI conditions. RNA blot analysis revealed that the production of ctxAB transcripts was severely affected in SHK17 compared to the wild-type strain, C6709 (Fig. 3). Consistent with this result, our RT-PCR experiment using RNAs from the isogenic pair C6709 and SHK17 (extracted under conditions similar to those used for the Northern blot experiment) showed that there was a significant decrease in the expression of ctxAB transcripts in the mutant strain compared to the wild type (Fig. 3). The above-mentioned evidence clearly demonstrates that RelA has a role in the regulation of production of CT in V. cholerae.
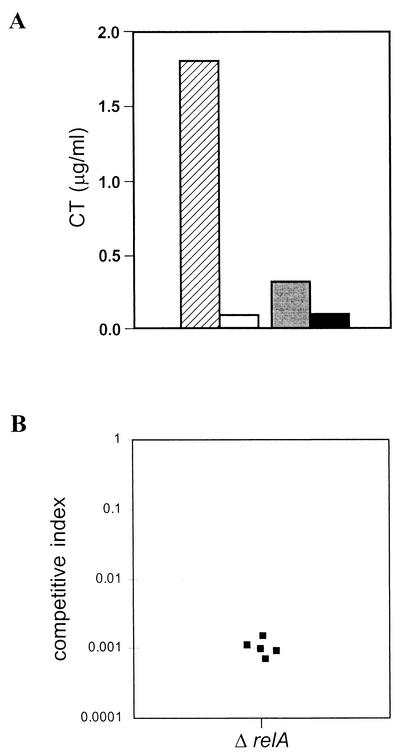
FIG. 3.
(A) CT production in V. cholerae C6709 and SHK17 under AKI conditions. CT was assayed by the GM1-ELISA method in the culture supernatant of C6709 (hatched bar) or SHK17 (open bar) or the sonicated cell lysate of C6709 (shaded bar) or SHK17 (solid bar). The data represent the average of three independent experiments, each done in duplicate. (B) Northern blot analysis of ctxAB, tcpA, and toxT, as indicated, in V. cholerae C6709 (lanes 1) and SHK17 (lanes 2). C6709 and SHK17 cells were grown under AKI conditions, and total cellular RNAs were prepared, electrophoresed, transferred to a nylon membrane, and hybridized with the ctxAB, tcpA, or toxT gene as shown. Molecular size markers are indicated on the left. (C) Detection of ctxAB, tcpA, and toxR transcripts of V. cholerae C6709 (lanes 1) and SHK17 (lanes 2) by RT-PCR. The strains were grown under AKI conditions, and total cellular RNAs were prepared and subjected to RT-PCR. The samples were run on a 1.5% agarose gel and visualized after being stained with ethidium bromide. DNA molecular size markers (HaeIII digests of φX174 DNA) are indicated on the left. (D) Immuno-dot-blot analysis to detect TcpA in V. cholerae C6709 (lane 1) and SHK17 (lane 2). Whole-cell lysates of the strains grown under AKI conditions were spotted on a polyvinylidene difluoride membrane and immunoblotted with anti-TcpA monoclonal antibody.
In addition to CT, TcpA is also an important virulence factor of V. cholerae, and it is well established that the expressions of both of these proteins are coregulated (29). Thus, we expected that TcpA production in SHK17 should also be affected. To test this possibility, RT-PCR was carried out, and we found that there was a significant decrease in production of tcpA transcripts in the mutant compared to the wild-type V. cholerae (Fig. 3). This was further confirmed by our Northern blot experiment using total cellular RNA of the wild-type and mutant strains with the tcpA gene as a probe. Here, also we found a notable reduction in the number of transcripts in SHK17 compared to the wild type (Fig. 3). We also performed an immunoblot experiment using cell extracts prepared from V. cholerae cultures grown under conditions similar to those mentioned above. As expected, the relA mutant SHK17 showed a severe reduction in the production of TcpA compared to its parental strain, C6709 (Fig. 3). Taken together, these data strongly suggest that like CT, expression of TcpA is also RelA dependent.
Since disruption of relA of V. cholerae led to decreased CT and TcpA production, we wanted to explore whether the expression of ToxT, the main regulator of the expression of CT and TcpA (14), is also altered in the mutant strain SHK17. To test this, we performed an RNA blot experiment to check for the expression of toxT transcripts of wild-type and relA mutant strains of V. cholerae. It was found that the toxT transcript level of SHK17 was clearly reduced compared to that of the wild type (Fig. 3). As mentioned previously, the expression of toxT is dependent on two important membrane-bound transcriptional activators, ToxR and TcpP (29). The above-mentioned results led us to question whether disruption of relA could also affect the expression of ToxR and TcpP. However, in the present study, we determined only the status of ToxR, the most important regulator of the virulence cascade (37). RT-PCR was employed again to detect any difference between the transcript levels in the wild-type and SHK17 cells. As shown in Fig. 3, the toxR transcripts of SHK17 cells were barely detectable compared to its strong and significant signal in the wild-type strain, C6709. Therefore, it appears that the reduction in production of CT and TcpA in the relA mutant SHK17 was probably due to the decreased levels of their transcriptional regulators, ToxR and ToxT.
RelA influences the expression of outer membrane porin proteins OmpU and OmpT.
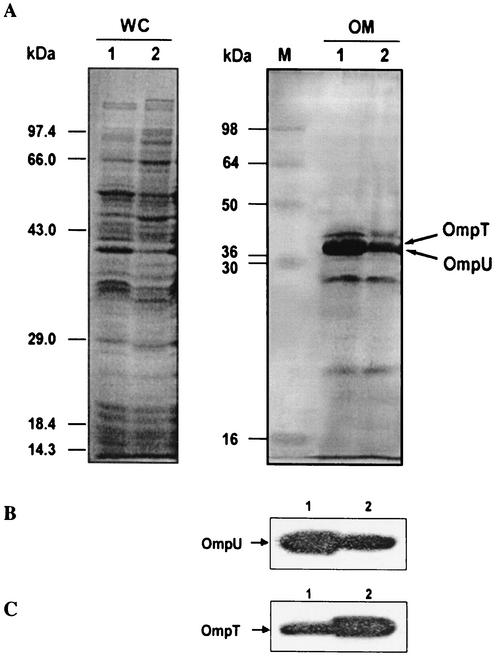
It is well established that ToxR directly regulates OmpU (38 kDa) and OmpT (40 kDa), the two porin proteins of V. cholerae, in a ToxT-independent manner (9). While OmpU is positively regulated, OmpT expression is repressed (11, 31, 36). Since toxR transcripts were found to be decreased in the relA mutant SHK17, we hypothesized that OmpU and OmpT expression should also be affected. To address this issue, whole-cell and outer membrane proteins from both the wild-type and SHK17 mutant V. cholerae, grown under AKI conditions, were separated by SDS-PAGE and analyzed. Differences between the major outer membrane protein profiles of the wild type and the relA mutant of V. cholerae were evident, as shown in Fig. 4. Furthermore, a prominent band in the 38-kDa region was found to be down regulated in the mutant strain (Fig. 4). To find out whether the 38-kDa band was that of OmpU, an immunoblot analysis was done using rabbit polyclonal antiserum against the V. cholerae OmpU protein. The anti-OmpU antiserum reacted only with the 38-kDa bands of both the wild type and SHK17, indicating that the band that was down regulated in the mutant SHK17 was indeed that of V. cholerae OmpU (Fig. 4). The expression of the OmpT protein was also similarly assessed with rabbit polyclonal antiserum against the OmpT protein of V. cholerae. As expected, the OmpT level was increased in the mutant strain SHK17 compared to its parental strain (Fig. 4). Since the expression of ToxR-regulated OmpU and OmpT proteins is also affected upon disruption of the relA gene of V. cholerae, it is probable that the virulence cascade is affected at the toxR level.
FIG. 4.
RelA affects the major outer membrane proteins in V. cholerae. (A) Whole-cell (WC) lysate or outer membrane (OM) fraction was prepared from the wild-type strain, C6709 (lanes 1), and its ΔrelA mutant, SHK17 (lanes 2). Equal amounts of protein samples of the strains were separated by SDS-PAGE (12.5% gel) and stained with Coomassie blue for visualization. M, molecular mass markers. (B and C) The OM fractions of C6709 (lanes 1) and SHK17 (lanes 2) were subjected to Western blot analysis using rabbit polyclonal antiserum against the V. cholerae protein OmpU (B) or OmpT (C).
RelA affects motility of V. cholerae.
Expression of virulence factors and motility in V. cholerae are intimately linked by an as-yet-uncharacterized mechanism (17, 22). Since the production of both CT and TcpA is drastically reduced in the relA deletion mutant in V. cholerae, it was of interest to compare the motilities of the mutant strain and the wild type. The motility of the V. cholerae relA mutant was found to be reduced by ∼80% compared to that of the wild type. Thus, it appears that the disruption of relA also impairs the motility of V. cholerae. Why relA mutation leads to the reduction of both the motility and the expression of major virulence factors when previous reports (17) showed that these two mechanisms are reciprocally regulated is unknown and will require further investigation.
Disruption of relAVCH affects in vivo CT production.
To examine the effect of V. cholerae relA mutation on the production of CT in vivo, we used a ligated rabbit ileal loop model (12). This in vivo model has an advantage, as it allows comparative studies of the parental strain and its isogenic mutant concurrently in the same animal and thus avoids variations among individual animals. About 106 CFU of V. cholerae cells were introduced into each ligated rabbit ileal loop, and the FA ratio was determined as described in Materials and Methods. While the average FA ratio of the wild-type strain, C6709, was 1.5, that of the relA mutant SHK17 was only 0.2. The data indicate that, similar to in vitro laboratory conditions, a mutation in relA of C6709 has an effect under in vivo situations on the production of the principal virulence factor, CT, since accumulation of a large quantity of fluid in the ligated loop is considered to be due mainly to the enterotoxigenic effect of the toxin (12). To further substantiate our in vivo rabbit ileal loop results, we determined the amount of CT present in the intestinal fluid generated by the wild-type strain, C6709, and its relA mutant SHK17 as described in Materials and Methods. As expected, the amount of CT estimated in intestinal fluid accumulated by SHK17 was very small compared to that of its parental strain, C6709 (Fig. 5A). To rule out the possibility that the CT is retained within the cells of the mutant SHK17, we estimated the toxin level in the sonicated cell lysate and compared it with that of the parental strain. No toxin was detected in the lysates of either C6709 or SHK17 (Fig. 5A).
FIG. 5.
In vivo CT production and intestinal colonization by V. cholerae C6709 and SHK17. (A) CT was measured in ileal loop fluid of C6709 (hatched bar) or SHK17 (open bar) or in sonicated cell lysate of C6709 (shaded bar) or SHK17 (solid bar). The data represent the average of five independent experiments, each done in duplicate. (B) RelA is required for intestinal colonization in V. cholerae. A suckling mouse assay was performed as described in Materials and Methods. The parental strain, C6709-R (C6709 Rifr), was coinoculated with SHK17 (ΔrelA Kmr). The competitive index is the ratio of output mutant to wild type (recovered from the small intestine) divided by the ratio of input mutant to wild type (inoculated into the mouse); thus, if a mutant strain has no colonization defect, the competitive index will be close to 1 (40). Each data point represents an individual mouse.
Disruption of relAVCH affects intestinal colonization.
To determine whether RelA has any role in intestinal colonization, we performed an in vivo competition experiment in suckling mice (40, 46). The relA mutant SHK17 (Kmr) was coinoculated with its isogenic parental strain, C6709-R (C6709 Rifr), into 3- to 5-day-old suckling mice as described in Materials and Methods. In this competition assay, SHK17 was found to be significantly attenuated in colonizing the suckling mouse small intestine relative to the wild type, as indicated by its average in vivo competition index of 0.001 (Fig. 5B). V. cholerae strains with an equivalent ability to adhere would be expected to exhibit a competition index of ∼1.0. In an in vitro competition assay, there was no marked observable difference between the mutant and parental strains (data not shown), indicating that the in vivo colonization defect of SHK17 was not caused by a general growth defect. It is well documented that TcpA is absolutely essential for successful colonization in this model (47, 48). The intestinal colonization defect of the relA mutant of V. cholerae is most likely due to reduced levels of tcpA gene expression in vivo, similar to that found under laboratory conditions in this study.
DISCUSSION
In an attempt to explore the possible involvement of the relA gene in the regulation of expression of the virulence factors in V. cholerae, we cloned and characterized the gene relAVCH from the organism. We show that the cloned gene codes for a RelA homolog by the high amino acid sequence similarity of the RelA protein with those of other organisms (38). Furthermore, a deletion-insertion mutant strain of V. cholerae, SHK17, constructed in this study also failed to accumulate (p)ppGpp upon amino acid starvation (Fig. 2). All these observations strongly suggest that relAVCH functions in a manner similar to that of the relA genes of other gram-negative organisms (6, 16, 43).
Interestingly, our findings in this study demonstrate RelA-dependent regulation of the expression of the prime virulence factors CT and TCP in the El Tor biotype of V. cholerae O1. In vitro expression of CT and TCP in El Tor vibrios has been shown to require AKI conditions (26, 27). Recently, Medrano et al. (34) provided insight into the molecular basis for control of CT and TCP production in El Tor under AKI growth conditions by analyzing the expression of the major virulence determinants toxR, toxT, ctxAB, and tcpA. In the present study, when we assayed the production of CT and TCP in the relA mutant El Tor V. cholerae strain SHK17 grown under AKI conditions, we found, to our surprise, severe defects in the expression of these factors compared to that in the parental strain, C6709 (Fig. 3). Consistent with our in vitro results, we found that the relA mutant strain SHK17 produced significantly less CT in rabbit ileal loops (Fig. 5A) and was severely attenuated in colonizing the infant mouse gut (Fig. 5B). The in vivo defect in colonization shown by SHK17 appears to be due to the low level of production of TcpA (47, 48), the principal colonization factor of V. cholerae (28, 29). Thus, our data from in vivo experiments also support a positive role for RelA-dependent regulation of the expression of the prime virulence factors CT and TCP. The current model for virulence gene regulation in V. cholerae is that of a cascade in which ToxR modulates the expression of another important regulator, ToxT, and ToxT in turn directly controls the expression of several virulence genes of V. cholerae. Thus, ToxR plays a pivotal role in the pathogenesis of V. cholerae. Lee et al. (30) recently highlighted the functional importance of ToxR in virulence gene regulation in El Tor, in which they have shown an absolute requirement of ToxR for the induction of CT during infection. In this study, we provide several pieces of indirect evidence that RelA may affect the virulence cascade at the ToxR level in El Tor; these are (i) very low levels of production of the ToxR-activated major virulence factors CT and TcpA in the relA mutant SHK17 compared to those in the parental strain; (ii) reduced production of the toxT transcripts in the relA mutant, which is directly under the control of ToxR; and (iii) a decreased level of ToxR-activated OmpU and an increased level of ToxR-repressed OmpT in SHK17. At present, it is not known whether RelA, via its effector molecule (p)ppGpp, has any direct role in the regulation of transcription of toxR. Proof of this hypothesis will necessitate construction of a relAVCH toxR double mutant, and such studies are in progress in our laboratory.
It might be argued that the effects of the disruption of relAVCH are due to polar effects on downstream genes rather than to a direct effect of relAVCH disruption. To eliminate this possibility, we analyzed the genetic organization of the downstream region of the relA locus of V. cholerae biotype El Tor (23). It was found that, although the immediate downstream gene, mazG, is transcribed in the same direction as relAVCH, the genes are not in one operon, since mazG has its own promoter (23). Furthermore, the stop codon of relA is followed by a distinct hairpin loop structure characteristic of a rho-independent transcriptional terminator. Additionally, mazG is followed by an ORF, which is transcribed in the opposite direction (23). This ORF is followed by two other ORFs, both with their own promoters and transcriptional-termination hairpin loop structures (23). Such a genetic organization eliminates the possibility of polarity, which occurs when disruption of an ORF within an operon affects downstream gene expression in addition to that of the targeted gene (44). Again, several lines of evidence suggest that stringent response, mediated by RelA, may play an important role in the virulence of many other bacterial pathogens (10, 20, 45). In this context, it is highly probable that the alterations in the phenotype of the V. cholerae relA mutant observed in this study are due to the disruption in the relAVCH gene alone.
From several recent studies, a picture is emerging that indicates that the pleiotropic role of RelA is mediated by its effector molecule, (p)ppGpp, which physically interacts with the RNA polymerase, and this may contribute significantly to our understanding of (p)ppGpp-mediated bacterial growth rate control (10). In view of this fact, it is likely that the alterations of virulence phenotype in SHK17 obtained in the present study are also mediated by the (p)ppGpp effector molecule. It is possible that (p)ppGpp interacts in a similar fashion with the RNA polymerase of V. cholerae El Tor cells grown under AKI conditions to directly modulate the transcription of toxR. An alternative explanation is that (p)ppGpp could be involved directly in the activation of the transcription of each virulence gene studied, with or without the involvement of a superimposed regulatory loop. Again, considering the fact that (p)ppGpp is involved in the regulation of many important transcriptional factors of prokaryotes, which are induced under a variety of stress conditions (10), there are several possible ways by which (p)ppGpp could exert a positive regulatory effect on the ToxR regulon in V. cholerae in an indirect manner. One such important transcription factor is the gram-negative sigma factor σS, the activity of which has been shown to be induced by (p)ppGpp in other organisms (10, 18, 49). The rpoS gene has also been implicated in the pathogenesis of V. cholerae (35), although the status of CT and TCP production has not been reported in an rpoS mutant background. Alternatively, it is possible that (p)ppGpp represses the transcription of an inhibitor of the ToxR regulon, which, due to decreased levels of (p)ppGpp in the mutant, then becomes “derepressed” and consequently inhibits the transcription of toxR.
Virulence in most bacterial species is an acquired property; the genetic determinants conferring this trait are likely to be restricted to pathogens and would not be present in closely related nonpathogenic species (19). It is now well established that this theory is also applicable to V. cholerae strains, in which the incorporation of two important pathogenicity islands, CTXφ and VPIφ, provide pathogenic potential to nonpathogenic strains (15). Thus, any horizontally acquired gene cluster carrying virulence determinants is forced to use or adapt to the existing regulatory circuits of the host cell for their expression. Thus, it is not surprising that the horizontally acquired phage-encoded CTX and TCP operons in V. cholerae have acquired their regulation, either positive or negative, through the most abundant and highly conserved regulatory genes, such as toxR, aphA, aphB, hns, and cyaA, located elsewhere on the primordial nonpathogenic V. cholerae genome. Like toxR, relA is present in both pathogenic and nonpathogenic strains of vibrios (unpublished observation). Thus, it appears that even before RelA became involved in regulating the expression of phage-encoded virulence genes via ToxR there already existed a definite “cross talk” between RelA and ToxR in the nonpathogenic strains of V. cholerae for the regulation of other physiological functions not related to virulence.
Acknowledgments
We are indebted to our Director, S. Bhattacharya, for his constant support and encouragement during this work. We are grateful to U. Dasgupta for critically reading the manuscript. We thank M. Cashel, National Institutes of Health, Bethesda, Md., for the generous gift of E. coli strains CF1648, CF1652, and CF1693; John J. Mekalanos, Harvard Medical School, Boston, Mass., for kindly providing the E. coli SM10λpir and V. cholerae C6709 strains and the plasmid DNA pGP704; J. Sanchez, UAEM, for the generous gift of anti-TcpA monoclonal antibody; and K. Klose, University of Texas Health Science Center, for anti-OmpU and anti-OmpT polyclonal antisera. We thank I. Guhathakurta for excellent technical support in performing animal experiments.
This work was supported by research grant BT/PRO412/Med/09/97 from the Department of Biotechnology, Government of India. S.H. and S.N. are grateful to the Council of Scientific and Industrial Research for a research fellowship.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 3.Bhadra, R. K., S. Roychoudhury, R. K. Banerjee, S. Kar, R. Majumdar, S. Sengupta, S. Chatterjee, G. Khetawat, and J. Das. 1995. Cholera toxin (CTX) genetic element in Vibrio cholerae O139. Microbiology 141:1977-1983. [DOI] [PubMed] [Google Scholar]
- 4.Bik, E. M., A. E. Bunschoten, R. J. Willems, A. C. Chang, and F. R. Mooi. 1996. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol. Microbiol. 20:799-811. [DOI] [PubMed] [Google Scholar]
- 5.Cashel, M. 1969. The control of ribonucleic acid synthesis in Escherichia coli. J. Biol. Chem. 244:3133-3141. [PubMed] [Google Scholar]
- 6.Cashel, M., D. R. Gentry, V. J. Hernandes, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 7.Chakraburtty, R., and M. Bibb. 1997. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J. Bacteriol. 179:5854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraburtty, R., J. White, E. Takano, and M. Bibb. 1996. Cloning, characterization and disruption of a (p)ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2). Mol. Microbiol. 19:357-368. [DOI] [PubMed] [Google Scholar]
- 9.Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 10.Chatterji, D., and A. K. Ojha. 2001. Revisiting the stringent response, ppGpp and starvation signaling. Curr. Opin. Microbiol. 4:160-165. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, J. A., J. B. Kaper, and V. J. DiRita. 1998. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol. Microbiol. 29:235-246. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta, U., R. K. Bhadra, D. K. Panda, A. Deb, and J. Das. 1994. Recombinant derivative of a naturally occurring non-toxinogenic Vibrio cholerae O1 expressing the B subunit of cholera toxin: a potential oral vaccine strain. Vaccine 12:359-364. [DOI] [PubMed] [Google Scholar]
- 13.Deb, A., D. Bhattacharyya, and J. Das. 1995. A 25-kDa β-lactam-induced outer membrane protein of Vibrio cholerae. J. Biol. Chem. 270:1-7. [DOI] [PubMed] [Google Scholar]
- 14.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulation cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flardh, K., T. Axberg, N. H. Albertson, and S. Kjelleberg. 1994. Stringent control during carbon starvation of marine Vibrio sp. strain S14: molecular cloning, nucleotide sequence and deletion of the relA gene. J. Bacteriol. 176:5949-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentry, D. R., V. J. Hernandez, L. H. Nguyen, D. B. Jensen, and M. Cashel. 1993. Synthesis of the stationary-phase sigma factor sigma s is positively regulated by ppGpp. J. Bacteriol. 175:7982-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groisman, E. A., and H. Ochman. 1994. How to become a pathogen. Trends Microbiol. 2:289-294. [DOI] [PubMed] [Google Scholar]
- 20.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 21.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hase, C. C. 2001. Analysis of the role of flagellar activity in virulence gene expression in Vibrio cholerae. Microbiology 147:831-837. [DOI] [PubMed] [Google Scholar]
- 23.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmgren, J. 1973. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect. Immun. 8:851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam, M. S., B. S. Drasar, and R. B. Sack. 1994. The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review. J. Diarrhoeal Dis. Res. 12:87-96. [PubMed] [Google Scholar]
- 26.Iwanaga, M., and T. Kuyyakanond. 1987. Large production of cholera toxin by Vibrio cholerae O1 in yeast extract peptone water. J. Clin. Microbiol. 25:2314-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 28.Kaper, J. B., J. G. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klose, K. E. 2001. Regulation of virulence in Vibrio cholerae. Int. J. Med. Microbiol. 291:81-88. [DOI] [PubMed] [Google Scholar]
- 30.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 31.Li, C. C., J. A. Crawford, V. J. DiRita, and J. B. Kaper. 2000. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol. Microbiol. 35:189-203. [DOI] [PubMed] [Google Scholar]
- 32.Mach, H., M. Hecker, I. Hill, A. Schroeter, and F. Mach. 1989. Stringent control and starvation survival in Escherichia coli. Z. Naturforsch. Teil C 44:838-844. [PubMed] [Google Scholar]
- 32a.Marchuk, D., M. Drumm, A Saulino, and F. S. Collins. 1991. Construction of T-vectors, a rapid and egneral system for direct cloning of unmodified PCR products Nucleic Acids Res. 19:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Costa, O. H., M. A. Fernandez-Moreno, and F. Malpartida. 1998. The relA/spoT-homologous gene in Streptomyces coelicolor encodes both ribosome-dependent (p)ppGpp-synthesizing and -degrading activities. J. Bacteriol. 180:4123-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medrano, A. I., V. J. DiRita, G. Castillo, and J. Sanchez. 1999. Transient transcriptional activation of the Vibrio cholerae El Tor virulence regulator toxT in response to culture conditions. Infect. Immun. 67:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrell, D. S., A. D. Tischler, S. H. Lee, and A. Camilli. 2000. Vibrio cholerae requires rpoS for efficient intestinal colonization. Infect. Immun. 68:6691-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 38.Mittenhuber, G. 2001. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol. 3:585-600. [PubMed] [Google Scholar]
- 39.Ojha, A. K., T. K. Mukherjee, and D. Chatterji. 2000. High intracellular level of guanosine tetraphosphate in Mycobacterium smegmatis changes the morphology of the bacterium. Infect. Immun. 68:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provenzano, D., C. M. Lauriano, and K. E. Klose. 2001. Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence. J. Bacteriol. 183:3652-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karaswa, H. Kurazono, A. Pal, and Y. Takeda. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 42.Rudd, K. E., B. R. Bochner, M. Cashel, and J. R. Roth. 1985. Mutations in the spoT gene of Salmonella typhimurium: effects on his operon expression. J. Bacteriol. 163:534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen, K., J. Hayashi, and H. K. Kuramitsu. 2000. Characterization of the relA gene of Porphyromonas gingivalis. J. Bacteriol. 182:3302-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder, L., and W. Champness (ed.). 1997. Molecular genetics of bacteria. ASM Press, Washington, D.C.
- 45.Taylor, C. M., M. Beresford, H. A. Epton, D. C. Sigee, G. Shama, P. W. Andrew, and I. S. Roberts. 2002. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol. 184:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64:2853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tischler, A. D., S. H. Lee, and A. Camilli. 2002. The Vibrio cholerae vieSAB locus encodes a pathway contributing to cholera toxin production. J. Bacteriol. 184:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Delden, C., R. Comte, and A. M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldor, M. K., and J. J. Mekalanos. 1994. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect. Immun. 62:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wendrich, T. M., and M. A. Marahiel. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26:65-79. [DOI] [PubMed] [Google Scholar]
- 52.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980-5990. [PubMed] [Google Scholar]