Abstract
The FhuA outer membrane protein of Escherichia coli actively transports ferrichrome, albomycin, and rifamycin CGP 4832, and confers sensitivity to microcin J25, colicin M, and the phages T1, T5, and φ80. Guided by the FhuA crystal structure and derived predictions on how FhuA might function, mutants were isolated in the cork domain (residues 1 to 160) and in the β-barrel domain (residues 161 to 714). Deletion of the TonB box (residues 7 to 11) completely inactivated all TonB-dependent functions of FhuA. Fixation of the cork to turn 7 of the barrel through a disulfide bridge between introduced C27 and C533 residues abolished ferrichrome transport, which was restored by reduction of the disulfide bond. Deletion of residues 24 to 31, including the switch helix (residues 24 to 29), which upon binding of ferrichrome to FhuA undergoes a large structural transition (17 Å) and exposes the N terminus of FhuA (TonB box) to the periplasm, reduced FhuA transport activity (79% of the wild-type activity) but conferred full sensitivity to colicin M and the phages. Duplication of residues 23 to 30 or deletion of residues 13 to 20 resulted in FhuA derivatives with properties similar to those of FhuA with a deletion of residues 24 to 31. However, a frameshift mutation that changed QSEA at positions 18 to 21 to KKAP abolished almost completely most of FhuA's activities. The conserved residues R93 and R133 among energy-coupled outer membrane transporters are thought to fix the cork to the β-barrel by forming salt bridges to the conserved residues E522 and E571 of the β-barrel. Proteins with the E522R and E571R mutations were inactive, but inactivity was not caused by repulsion of R93 by R522 and R571 and of R133 by R571. Point mutations in the cork at sites that move or do not move upon the binding of ferrichrome had no effect or conferred only slightly reduced activities. It is concluded that the TonB box is essential for FhuA activity. The TonB box region has to be flexible, but its distance from the cork domain can greatly vary. The removal of salt bridges between the cork and the barrel affects the structure but not the function of FhuA.
The FhuA protein of Escherichia coli actively transports ferrichrome, the antibiotics albomycin and rifamycin CGP 4832, and microcin J25 across the outer membrane. It also serves as receptor for colicin M and the phages T1, T5, φ80, and UC-1 (5). The FhuA protein consists of 22 antiparallel β-sheets that form a β-barrel into which a globular domain is inserted from the periplasmic side. The globular domain seems to close the β-barrel channel and prevent entry of even small molecules and was for this reason designated the “cork” (12) or “plug” (24). Ferrichrome, the natural substrate of FhuA, binds in a cavity located well above the outer membrane lipid bilayer. Five amino acid residues of the cork and 6 of the β-barrel are less than 4 Å away from bound ferrichrome and probably serve as ferrichrome binding sites (12). It is thought that opening of the FhuA channel requires movement of the cork, resulting in a connection between the cavity exposed to the cell surface and the region exposed to the periplasm. Although binding of ferrichrome to FhuA moves the cork about 2 Å towards ferrichrome, this does not open the channel. A much stronger structural alteration occurs in the pocket that is exposed to the periplasm. A short helix, the switch helix (residues 24 to 29) is unwound and E19 and W22 move 17 Å away from their former α-carbon position, which might facilitate the binding of FhuA to TonB (12), as has been found by chemical cross-linking of FhuA to TonB, which is enhanced in vivo upon the binding of ferrichrome (15, 27).
Energy provided by the cytoplasmic membrane in the form of the proton motive force (3) is required for all FhuA activities except infection by phage T5. The TonB-ExbB-ExbD protein complex is thought to transduce the energy from the cytoplasmic membrane to the outer membrane. An N-proximal region of FhuA, the TonB box (residues 7 to 11), interacts with a region around residue 160 of TonB, as shown by mutations in the TonB box that are suppressed by mutations in TonB (15, 31).
A similar suppression analysis has revealed the same interacting regions in the BtuB vitamin B12 transport protein and in TonB (19). Moreover, in vivo a segment of the TonB box of BtuB is chemically cross-linked via disulfide bonds with a segment around residue 160 of TonB (7). Cross-linking at several positions is increased when BtuB is loaded with vitamin B12, and the cross-linking pattern changes in mutants containing amino acid substitutions in BtuB that impair TonB-dependent BtuB activity (7). Furthermore, site-directed spin labeling and electron paramagnetic resonance spectroscopy of BtuB have suggested that the TonB box of unloaded BtuB is fixed to the β-barrel (25). Binding of vitamin B12 to BtuB converts this segment into an extended and highly dynamic structure that likely extends into the periplasm to interact physically with TonB. In vivo disulfide formation was also shown between region 160 of TonB and the TonB box of the ferric citrate outer membrane transport protein (29), which supports the interaction of the transporter TonB box with region 160 of TonB.
In the study reported here, regions for which a functional role could be predicted from the crystal structure of FhuA were mutagenized. These regions were the switch helix, the TonB box, the segment between the TonB box and the cork proper that is inserted into the β-barrel (approximately residues 19 to 160), and interacting regions of the cork and the β-barrel.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. coli strains and plasmids used are listed in Table 1. Cells were grown in TY medium (10 g of Bacto Tryptone [Difco Laboratories] liter−1, 5 g of yeast extract liter−1, 5 g of NaCl liter−1) or NB medium (8 g of nutrient broth liter−1, 5 g of NaCl liter−1, pH 7) at 37°C. To reduce the available iron of the NB medium, 2,2′-dipyridyl (0.2 mM) was added (NBD medium). Ampicillin (40 μg/ml) was added when required.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype and/or phenotype | Reference |
|---|---|---|
| Strains | ||
| HK97 | F−araD139 lacU169 rpsL150 relA1 flbV5301 deoC1 ptsF25 rbsR aroB thi fhuE::Mu53 fhuA | 21 |
| CH1857 | aroB malT tsx thi fhuACDB tonB | 4 |
| AB2847 | aroB malT tsx thi | 18 |
| MB98 | aroB malT tsx thi ΔfhuA | This study |
| Plasmids | ||
| pHK763 | pT7-6 fhuA wild type | 23 |
| pHK570 | pHSG576 fhuA wild type | 20 |
| pFhuA711 | pT7-6 fhuA (Δ7-11) | This study |
| pFhuAG16 | pT7-6 fhuA (A16G) | This study |
| pFhuAFs19 | pT7-6 fhuA (A16G QSEA18-21KKAP) | This study |
| pFhuA1320 | pT7-6 fhuA (Δ13-20 A21D W22R) | This study |
| pFhuAP15A | pT7-6 fhuA (P15A) | This study |
| pFhuAP17A | pT7-6 fhuA (P17A) | This study |
| p76SH1 | pT7-6 fhuA (W22G) | This study |
| p76SH2 | pT7-6 fhuA (A25G) | This study |
| p76SH3 | pT7-6 fhuA (A26E) | This study |
| p76SH4 | pT7-6 fhuA (T27P) | This study |
| p76SH5 | pT7-6 fhuA (A29G) | This study |
| p76SH6 | pT7-6 fhuA (Δ24-31 Q32P) | This study |
| p76SH7 | pT7-6 fhuA (d23-30) | This study |
| p76SH9 | pT7-6 fhuA (T54R) | This study |
| p76SH10 | pT7-6 fhuA (A577E) | This study |
| p76SH13 | pT7-6 fhuA (Δ24-31 Q32P V11D) | This study |
| p76SH14 | pT7-6 fhuA (Δ24-31 Q32P I9P) | This study |
| p76SH15 | pT7-6 fhuA (V11D) | This study |
| p76SH16 | pT7-6 fhuA (I9P) | This study |
| pFE6 | pT7-6 fhuA (R93L) | This study |
| pFE7 | pT7-6 fhuA (R93P ΔH89) | This study |
| pFE8 | pT7-6 fhuA (G94P) | This study |
| pFE9 | pT7-6 fhuA (R133A) | This study |
| pFE10 | pT7-6 fhuA (R133H) | This study |
| pFE11 | pT7-6 fhuA (R93L R133A) | This study |
| pFE12 | pT7-6 fhuA (G134D) | This study |
| pFE14 | pT7-6 fhuA (G146D) | This study |
| pFE15 | pT7-6 fhuA (G147D) | This study |
| pFE17 | pT7-6 fhuA (E522A) | This study |
| pFE18 | pT7-6 fhuA (E522R) | This study |
| pFE19 | pT7-6 fhuA (R93L E522R) | This study |
| pFE21 | pT7-6 fhuA (E571A) | This study |
| pFE22 | pT7-6 fhuA (E571R) | This study |
| pFE23 | pT7-6 fhuA (R93L E571R) | This study |
| pFE29 | pT7-6 fhuA (R93P) | This study |
| pFE30 | pT7-6 fhuA (R93E) | This study |
| pFE34 | pT7-6 fhuA (R93E E522R) | This study |
| pFE36 | pT7-6 fhuA (K67A E68A E522R) | This study |
| pFE37 | pT7-6 fhuA (K67A E68A E571R) | This study |
| pFE50 | pT7-6 fhuA (T27C) | This study |
| pFE53 | pT7-6 fhuA (P533C) | This study |
| pFE60 | pT7-6 fhuA (T27C P533C) | This study |
| pT7-6 | ColE1 Ampr T7 gene 10 promoter | 33 |
Recombinant DNA techniques.
The isolation of plasmids, the use of restriction enzymes, ligation, agarose gel electrophoresis, and transformation were carried out using standard techniques (30). All genetic constructions were examined by DNA sequencing by the dideoxy chain termination method with fluorescence-labeled or unlabeled nucleotides (Auto Read sequencing kit; Pharmacia, Biotech, Freiburg, Germany) and an ALF sequencer (Pharmacia).
Site-directed mutagenesis and construction of strains.
Some of the fhuA mutants were constructed as described previously by PCR of the 2.8-kb fhuA-containing plasmid pHK763 by using mismatch primers which code for restriction sites for further subcloning (4); other fhuA mutants were constructed with a QuikChange site-directed mutagenesis kit (Stratagene, Europe; Amsterdam, The Netherlands). All of the mutated fhuA fragments were sequenced to verify the mutations. Sequences of the mutagenic oligonucleotide primers and a description of the mutagenesis procedure are available upon request.
Strain MB98 ΔfhuA was constructed using the technique described by Datsenko and Wanner (11), which resulted in a derivative of strain AB2847 in which only the signal sequence of fhuA was left on the chromosome. The fhuCDB gene cluster was transcribed under the control of the fhuA promoter.
Protein analytical methods.
E. coli HK97 transformed with one of the various plasmids was grown in NB medium at 37°C. Cells were harvested by centrifugation at an optical density at 578 nm of 0.6.
Outer membranes were prepared by lysing cells with lysozyme-EDTA, followed by solubilization of the cytoplasmic membrane with 0.2% Triton X-100 and differential centrifugation (17). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Serva blue.
Trypsin digestion was performed by incubating 10 μl of an outer membrane preparation with 1.1 μl of trypsin (0.3 mg/ml) for 30 min at 37°C. Digestion was stopped by the addition of 1.2 μl of trypsin inhibitor (1.5 mg/ml) and incubation for 5 min at room temperature. Electrophoresis sample buffer (13 μl) was added, and the samples were boiled for 5 min. The proteins were then separated by SDS-PAGE and stained with Serva blue. For preincubation with ferrichrome, 1 μl of ferrichrome (10 mM) was added to 10 μl of outer membrane preparations and the mixture was incubated for 30 min at 37°C before trypsin digestion as described above.
Phenotype assays.
All assays were performed with freshly transformed E. coli K-12 strains. The sensitivity of cells to the FhuA ligands (phages T1, T5, and φ80 and colicin M, rifamycin CGP4832, and albomycin) was tested by spotting various dilutions, as indicated in the table legends, on TY plates overlaid with 3 ml of TY soft agar containing 108 cells of the strain to be tested.
Growth promotion was tested by placing filter paper disks containing 10 μl of various concentrations of ferrichrome on NBD agar plates overlaid with 3 ml of NB soft agar containing 108 cells of the strain to be tested. The diameter and the growth density around the filter paper disk were determined after incubation overnight. The assays were performed at least in triplicate.
Transport assays.
E. coli K-12 strains HK97 aroB fhuA fhuE, CH1857 aroB fhuACDB tonB, and MB98 aroB ΔfhuA freshly transformed with the plasmids to be tested were grown overnight on TY plates. Cells were washed and suspended in transport medium (M9 salts [26], 0.4% glucose), and the cell density was adjusted to an optical density at 578 nm of 0.5. Free iron was removed by adding 25 μl of 10 mM nitrilotriacetate, pH 7.0, to a 1-ml cell suspension. After incubation for 5 min at 37°C, transport was started by adding 10 μl of 100 μM [55Fe3+]ferrichrome. Samples of 100 μl were withdrawn, and cells were harvested on cellulose nitrate filters (pore size, 0.45 μm; Sartorius AG, Göttingen, Germany) and washed twice with 5 ml of 0.1 M LiCl. The filters were dried, and the radioactivity was determined by liquid scintillation counting. In binding assays, a 150-fold surplus of nonradioactive ferrichrome was added after 17 min to show the specificity of ferrichrome binding. For reducing conditions, 10 μl of 1,4-dithio-l-threitol (DTT; 10 M) was added together with nitrilotriacetate 5 min prior to the transport assay.
Structure analysis.
Structures were analyzed with Weblab Viewer Pro 3.5 (Molecular Simulations Inc.).
RESULTS
The switch helix is not essential for FhuA activity.
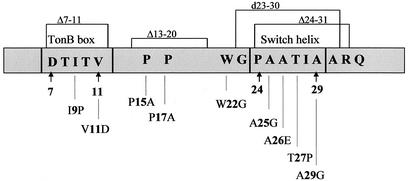
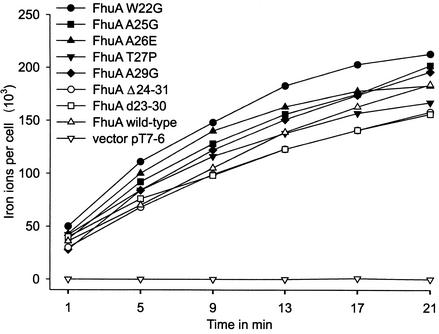
Upon binding of ferrichrome, the switch helix (residues 24 to 29) is unwound and residues 19 and 20 are translated 17 Å from their former position (12). It is thought that this large movement facilitates the interaction of FhuA with TonB because the TonB box (residues 7 to 11 in FhuA), which is not seen in the crystal structure, might be more exposed to the periplasm. This prediction was examined by introducing point mutations into the switch helix as listed in Fig. 1. In addition, residues 24 to 31 were deleted and residues 23 to 30 were duplicated (designated d23-30). The activities of the resulting mutant FhuA proteins were tested by measuring the transport rate of [55Fe3+]ferrichrome by E. coli HK97 fhuA transformed with the mutated fhuA genes cloned on the medium-copy-number vector pT7-6. Compared to wild-type FhuA encoded on the same vector as the mutant FhuA proteins, none of the mutants showed a strong reduction in the ferrichrome transport rate (Fig. 2; Table 2). Deletion or duplication of the switch helix reduced the ferrichrome transport rate to only 79 or 72% of the wild-type rate, respectively; this reduced level was similar to the level of the T27P (threonine 27 replaced by proline) mutant (77%), which was created to alter the conformation of the switch helix.
FIG. 1.
Diagram of mutations introduced into the N-terminal region of FhuA. The numbers indicate the positions of the amino acid residues in mature FhuA. Arrows indicate the start and stop sites of the TonB box (amino acids 7 to 11) and of the switch helix (amino acids 24 to 29).
FIG. 2.
Time-dependent transport of [55Fe3+]ferrichrome (1 μM) into E. coli strain HK97 fhuA fhuE aroB expressing the plasmid-encoded FhuA proteins as indicated in the figure.
TABLE 2.
Phenotypic characterization of N-proximal FhuA mutants
| Phenotypea | Fc binding (%)b | Fc transport (%)c | Sensitivity tod:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Albomycin | Microcin J25 | Colicin M | T1 | φ80 | T5 | |||
| Wild type | 100 | 100 | 5 (6, 7) | 3 (4) | 3 (4) | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| W22G | 107 | 100 | 5 (6, 7) | 3 (4) | 3 (4) | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| A25G | 83 | 92 | 5 (6, 7) | 3 (4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| A26E | 90 | 90 | 5 (6, 7) | 3 (4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| T27P | 103 | 77 | 5 (6) | 3 (4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| A29G | 42 | 96 | 5 (6) | 3 (4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| Δ24-31 | 82 | 79 | 4 (5, 6) | 2 (3, 4) | 3 (4) | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| d23-30 | 132 | 72 | 4 (5, 6) | 2 (3, 4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| A577E | 45 | 68 | 4 (5, 6) | 2 (3, 4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| T54R | 106 | 81 | 2 (3-5) | 2 (3, 4) | 3 (4) | 5 (6) | 4 (5) | 3 (4, 5) |
| A16G | 31 | 90 | 5 (6, 7) | 2 | 3 | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| A16G QSEA18-21KKAP | 0 | 1 | — | — | 1 | — | 1 (2, 3) | 2 (3) |
| Δ7-11 | 22 | 0 | — | — | — | — | — | 3 (4, 5) |
| Δ13-20 | NDe | 57 | 4 (5, 6) | 3 (4) | 3 (4) | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| P15A | ND | 100 | 4 (5, 6) | 3 (4) | 3 (4) | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| P17A | ND | 100 | 4 (5, 6) | 3 (4) | 3 (4) | 5 (6, 7) | 4 (5) | 3 (4, 5) |
| T27C | ND | 100 | 5 (6, 7) | 2 (3, 4) | 3 (4) | ND | 4 (5) | 3 (4, 5) |
| P533C | ND | 90 | 5 (6, 7) | 2 (3, 4) | 3 (4) | ND | 4 (5) | 3 (4, 5) |
| T27C P533C | ND | 0 | — | — | 3 | ND | 3 (4, 5) | 3 (4, 5) |
| I9P | 133 | 18 | — | — | 1 (2) | 4 (5, 6) | 3 (4) | 3 (4, 5) |
| I9P Δ24-31 | 97 | 0 | — | — | — | — | — | 3 (4, 5) |
| V11D | 144 | 0 | — | — | — | — | — | 3 (4, 5) |
| V11D Δ24-31 | 103 | 0 | — | — | — | — | — | 3 (4, 5) |
Wild-type and mutant fhuA alleles were expressed from pT7-6 in HK97 fhuA.
Percentages of the wild-type level of ferrichrome (Fc) binding to CH1857 fhuACDB tonB transformed with the indicated plasmids.
The transport rates (numbers of iron ions per cell per minute) were calculated in the nearly linear region between minutes 5 and 13 and compared to the ferrichrome transport rate of wild-type FhuA (100%). Fc, ferrichrome.
Sensitivities to the ligands were tested by using E. coli HK97 fhuA freshly transformed with the plasmids listed in Table 1 expressing the indicated FhuA derivatives. Sensitivities were tested by spotting 4 μl of 10-fold (colicin M and phages T1, T5, and φ80) or 3-fold (microcin J25 and albomycin) dilutions onto TY agar plates overlaid with TY top agar containing the strain to be tested. The results are given as the last of a dilution series that resulted in a clear zone of growth inhibition; e.g., 4 means that the stock solution diluted fourfold was still active. Numbers in parentheses indicate a turbid zone of growth inhibition. Tabulated values of the sensitivity tests are from a single experiment, but little variation was seen in repeated trials. —, no response.
ND, not determined.
E. coli HK97 contains an inactive FhuA protein with several point mutations and a single amino acid deletion in the β-barrel. Recently, we found that this protein can restore the FhuA activity of the FhuA derivative with residues 5 to 160 deleted (FhuAΔ5-160) by providing the missing cork. However, we found no evidence for complementation of a complete FhuA protein with mutations in the cork domain by a wild-type cork (M. Braun, F. Endriß, H. Killmann, and V. Braun, submitted for publication). To make sure that HK97 FhuA did not complement the small cork deletion derivatives, ferrichrome transport was determined in transformants of an E. coli MB98 strain that lacks the fhuA gene and transcribes the fhuCDB gene cluster under the control of the fhuA promoter. The transformants that synthesized FhuAΔ24-31, FhuAΔ13-20 (A21D W22R), FhuAd23-30, FhuA(G134D), and FhuA(G147D) displayed transport rates similar to those obtained with HK97 (data not shown), which excludes complementation of the mutant corks by the HK97 wild-type cork as the cause of the mutants' activities.
The binding of [55Fe3+]ferrichrome to cells synthesizing the mutant proteins was examined with E. coli CH1857 fhuCDB tonB, which does not transport ferrichrome across the outer membrane and the cytoplasmic membrane. All the FhuA mutants expressed by strain CH1857 showed a level of binding of ferrichrome either similar to or lower than that of wild-type FhuA. FhuA(A29G) displayed a rather low level of binding of ferrichrome, which, however, did not reduce the ferrichrome transport rate. An exception was FhuAd23-30, which displayed enhanced binding (Table 2). SDS-PAGE did not reveal an increased amount of FhuAd23-30, which might have explained the stronger binding (data not shown). Binding as opposed to transport was verified by the constant level of radioactivity associated with the cells during the entire incubation period and by the chase of radiolabeled ferrichrome with a surplus of unlabeled ferrichrome.
In addition, growth tests were performed with a dilution series of ferrichrome supplied on paper disks placed on nutrient broth agar plates supplemented with 0.2 mM dipyridyl to limit the available iron (NBD plates). The growth zones of E. coli HK97 transformed with the mutated fhuA genes were identical to the growth zones of the wild-type fhuA transformant (data not shown). The sensitivity of the FhuA mutant cells to albomycin and microcin J25 was the same as the sensitivity of wild-type FhuA cells, except with the mutants expressing FhuAΔ24-31 and FhuAd23-30, which were threefold less sensitive. The sensitivities of the FhuA mutant cells to the phages T1, φ80, and T5 were indistinguishable from those of wild-type FhuA cells (Table 2). Together with the results of the transport assays, the data indicate that the FhuA segment of the switch helix does not play an essential role in FhuA activity.
The FhuA crystal structure indicates that the switch helix is fixed through select hydrophobic residues in a hydrophobic pocket formed by periplasmic turns 8 and 9 and β-strand A of the cork domain (12, 24). Alanine 577 of turn 8 is oriented such that it interacts with P24 of the switch helix (distance, 3.5 Å), and T54 of cork β-strand A interacts with A26 (distance, 3.8 Å). To abolish a hydrophobic interaction, A577 was replaced by glutamate. In accordance with the sequence of the E. coli K-12 genome (2), residues 577 and 578 read AA and not RP, as was determined previously (10). The ferrichrome transport rate and the sensitivities to albomycin and microcin J25 of mutant FhuA(A577E) were reduced, but the sensitivity to colicin M and to the phages were at the wild-type FhuA level (Table 2). In addition, FhuA(A577E) showed 45% of the wild-type ferrichrome binding level (Table 2). Fixation of the switch helix by hydrophobic interaction does not seem to be crucial for FhuA functioning. In addition, interaction within the cork could be changed without the loss of FhuA activity since FhuA(T54R) showed 81% of the wild-type ferrichrome transport rate and unaltered ferrichrome binding and phage and colicin sensitivities (Table 2).
Previously, we showed that the mutation V11D in the TonB box of FhuA renders FhuA inactive in all TonB-dependent FhuA activities but retains TonB-independent sensitivity to phage T5 (31). In the light of the unexpected results obtained with the switch helix mutants, we reexamined these findings and fully verified them. FhuA(V11D) was inactive in all TonB-dependent functions and conferred TonB-independent infection by phage T5 as wild-type FhuA did (Table 2). There is evidence that the TonB box sequences assume a certain conformation which is important for interaction with TonB and that mutations affect the conformation rather than pairwise interaction with TonB residues (8). Therefore, we tested whether deletion of the switch helix in FhuA(V11D) altered the conformation of the mutated TonB box such that its function was partially restored, with the result that FhuA(V11D Δ24-31) remained inactive. Another TonB box mutant, FhuA(I9P), displayed a residual ferrichrome transport activity. Deletion of the switch helix in mutant FhuA(I9P) completely inactivated ferrichrome transport but left residual sensitivity to colicin M and the phages T1 and φ80 and full sensitivity to phage T5 (Table 2). Apparently, the switch helix deletion further changed the conformation of FhuA(I9P) such that its residual activity was abolished. FhuA(V11D) and FhuA(I9P) bound larger amounts of [55Fe3+]ferrichrome than wild-type FhuA. The amounts of the TonB box mutant proteins were similar to the amounts of wild-type FhuA. The switch-helix-deletion derivatives of FhuA(V11D) and FhuA(I9P) bound [55Fe3+]ferrichrome at the same level as wild-type FhuA (Table 2).
For studying the properties of a mutant that lacks the entire TonB box, FhuA(Δ7-11) was constructed. This mutant protein, which was produced in wild-type amounts, was completely impaired in all TonB-dependent activities but remained T5 sensitive (Table 2). To examine whether inactivation is confined to the TonB box, we constructed FhuAΔ13-20, which contains two additional mutations, A21D and W22R. Despite these strong alterations, the mutant still transported ferrichrome with 57% of the rate of wild-type FhuA and was fully sensitive to colicin M and the phages. Additional mutations were created with the aim of altering the local conformation caused by the FhuA(P17A) and FhuA(P15A) proline replacements. The mutants exhibited wild-type properties (Table 2). In contrast, another mutant in this region, with a seemingly small alteration, FhuA(A16G), showed strongly reduced ferrichrome binding but otherwise displayed wild-type activities (Table 2). Only the sensitivity to microcin J25, to which FhuA proteins with structural changes have the most sensitive reactions, was somewhat reduced.
PCR mutagenesis unintentionally resulted in the frameshift mutation QSEA→KKAP at positions 18 to 21, and the resulting protein did not bind and transport ferrichrome (Table 2). Reduced sensitivity to phage T5, residual sensitivity to phage φ80, and weak sensitivity to colicin M indicated that the protein was in the outer membrane, but SDS-PAGE revealed smaller amounts than those produced by wild-type FhuA (data not shown). The mutated fragment seems to assume an altered conformation that interferes with correct folding. This interference in turn results in a lower level of incorporation into the outer membrane and a reduced activity.
Fixation of the switch helix to turn 7.
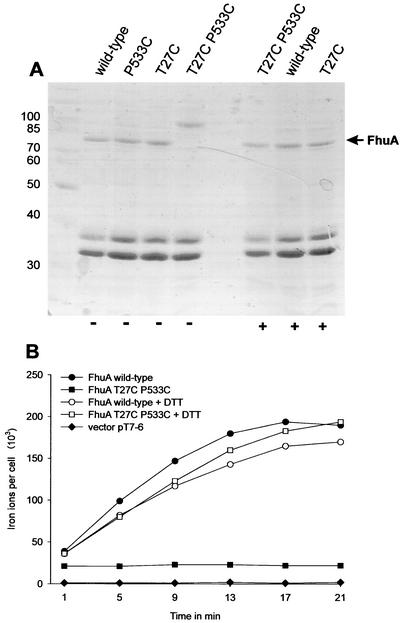
To prevent unwinding of the switch helix, followed by the movement to the opposite side of the barrel wall, cysteine residues were introduced into the switch helix (T27C) and turn 7 (P533C). Both amino acid side chains are sufficiently close to form a disulfide bridge. To examine whether disulfide bonds were formed between these cysteines, the outer membrane fraction was isolated, and the proteins were dissolved in sample buffer with and without β-mercaptoethanol and then separated by SDS-PAGE. The FhuA mutant exhibited a lower electrophoretic mobility in the nonreduced sample than in the reduced sample (Fig. 3A), which indicated the presence of a disulfide bond in the FhuA mutant. Although disulfide cross-linked proteins usually move faster than the un-cross-linked forms, the size of the cross-linked FhuA of approximately 90 kDa and the lack of cross-linked products with the C27 and C533 single mutants excludes the possibility of the presence of cross-linked FhuA dimers. No transport of ferrichrome was observed in this mutant unless the disulfide bond was reduced by DTT prior to the transport assay (Fig. 3B). The disulfide derivative of FhuA conferred no sensitivity to albomycin and microcin J25, whereas sensitivities to phage T5 and colicin M were the same as those of wild-type FhuA and sensitivity to phage φ80 was reduced 10-fold (Table 2).
FIG. 3.
(A) Stained proteins after SDS-PAGE of outer membrane fractions of E. coli HK97 fhuA transformed with FhuA derivatives as indicated with (+) and without (−) β-mercaptoethanol in the loading buffer. The molecular masses of standard proteins in kilodaltons are indicated. (B) Time-dependent transport of [55Fe3+]ferrichrome (1 μM) into E. coli strain HK97 fhuA fhuE aroB expressing the plasmid-encoded FhuA proteins as indicated in the figure. To reduce the disulfide bond between C27 and C533, 100 mM DTT was added 5 min prior to the transport assay.
Sensitivity to colicin M and the TonB-dependent phages may result from a small fraction of un-cross-linked FhuA, or the cross-linked FhuA may still function as a receptor. To examine whether FhuA(T27C P533C) still interacts with TonB, pFE60 was transformed in E. coli MB98 that synthesized wild-type FhuA from the low-copy-number vector pHK570. The disulfide derivative completely inhibited ferrichrome transport of wild-type FhuA, suggesting that overexpressed FhuA(T27C P533C) bound to TonB and made it unavailable for binding to wild-type FhuA.
Interaction of the cork domain with the β-barrel domain.
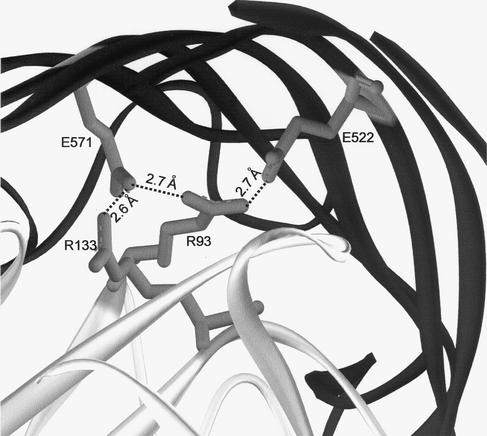
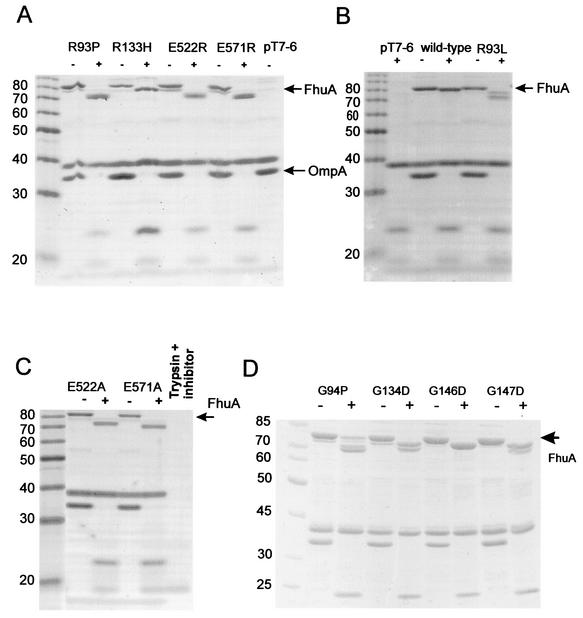
Residues R93 and R133 of the cork are close enough to residues E522 and E571 of the β-barrel to form salt bridges (Fig. 4). These residues are conserved in 26 energy-coupled outer membrane proteins, and they display the same stereochemistry in the four determined crystal structures (9, 12, 14, 24). To examine the functional relevance of the predicted salt bridges for fixing the cork to the β-barrel, R93P and R93P Δ89H mutant FhuAs, the latter constructed unintentionally by a false primer, and R93L, R93E, R133A, R133H, E522A, E522R, E571A, and E571R mutant FhuAs were constructed. The substitutions with prolines should alter the local conformation of the polypeptide chain, the nonpolar residues prevent the formation of the salt bridges, and the substitutions with the charged residues lead to the repulsion of the positively charged side chains. A conformational change in the FhuA mutant proteins was examined by incubation of the outer membrane fraction with trypsin. The amounts of the mutant FhuA proteins were similar to the amount of wild-type FhuA (Fig. 5; the R93E and R133A mutant FhuAs are not shown). Unlike with wild-type FhuA, of which trypsin cleaved only a small fragment (Fig. 5), larger fragments were released from the mutant proteins. Under the conditions used, the C-terminal end of OmpA located in the periplasm (32) was completely removed by trypsin.
FIG. 4.
Interacting sites between the cork and the barrel domain studied in this paper. The FhuA crystal structure is shown in ribbon presentation with the side chains of residues R93, R133, E522, and E571. The distances between the nitrogen atoms of R93 and R133 and the oxygen atoms of E522 and E571 are indicated.
FIG. 5.
Stained proteins after SDS-PAGE of outer membrane fractions of E. coli HK97 fhuA transformed with the FhuA mutants indicated, with (+) and without (−) trypsin digestion. The molecular masses of standard proteins in kilodaltons are indicated. (C) In the last lane, trypsin and trypsin inhibitor were applied.
It has been reported that wild-type FhuA is cleaved by trypsin at position 67 and that cleavage is prevented by ferrichrome (28). To determine the cleavage site of the mutant FhuA proteins, the FhuA(K67A, E522R) and FhuA(K67A, E571R) double mutant proteins were constructed, cleaved by trypsin, and separated by SDS-PAGE; the resulting bands were at the same electrophoretic position as those of FhuA(E522R) and FhuA(E571R), showing that K67 is not the cleavage site of the mutant proteins integrated in the outer membrane (data not shown). Among the mutant proteins tested, the addition of ferrichrome prevented the trypsin cleavage of FhuA(E522A) and FhuA(E571A) but not of FhuA(E522R) and FhuA(E571R) (data not shown).
The mutant FhuAs expressed in E. coli HK97 fhuA showed FhuA activities similar to that of wild-type FhuA, except for FhuA(R93P), FhuA(R93P ΔH89), FhuA(R133H), FhuA(E522R), and FhuA(E571R) (Table 3). Ferrichrome transport by the R93P mutant was reduced to 42%, which rendered cells nearly albomycin and microcin J25 resistant. E. coli HK97 FhuA(R93P ΔH89) was virtually insensitive to albomycin and microcin J25 and transported [55Fe3+]ferrichrome at 17% of the wild-type FhuA rate. The R93P and ΔH89 mutations probably changed the conformation of the cork locally, which affected transport but not sensitivity to the phages and colicin M. In contrast to cells expressing R133A, cells expressing R133H showed reduced transport and reduced sensitivity to phages φ80 and T5 and to colicin M.
TABLE 3.
Phenotypic characterization of the FhuA mutants located at the cork-β-barrel interface
| Phenotypea | Growth on ferrichromeb | Fc transport rate (%)c | Sensitivity tod:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Albomycin | Microcin J25 | Colicin M | T1 | φ80 | T5 | |||
| Wild type | 27 | 100 | 3 (4-6) | 3 (4) | 4 | 6 (7) | 5 (6, 7) | 5 (6) |
| R93L | 27 | 110 | 3 (4, 5) | 4 | 4 | 7 | 5 (6, 7) | 5 (6) |
| R93P | 27 | 42 | ((0-3)) | ((0-2)) | 3 (4) | 6 (7) | 4 (5, 6) | 5 (6) |
| R93P ΔH89 | (27) | 17 | ((0-3)) | ((0-2)) | 3 (4) | 6 (7) | 4 (5, 6) | 5 (6) |
| R93E | 27 | 93 | 3 (4, 5) | 4 (5) | 4 | 7 | 5 (6, 7) | 5 (6) |
| R133A | 27 | 92 | 4 (5, 6) | 3 (4) | 3 (4) | 6 (7) | 5 (6, 7) | 5 (6) |
| R133H | 27 | 75 | ((0, 1)) | ((0, 1)) | 2 (3) | 6 (7) | 4 | 4 (5) |
| R93L R133A | 27 | 88 | 3 (4, 5) | 2 (3) | 4 | 6 | 5 (6) | 5 (6) |
| E522A | 27 | 105 | 3 (4-6) | 3 (4) | 4 | 6 (7) | 5 (6, 7) | 5 (6) |
| E522R | — | 0 | — | — | 1 | (0-5) | ((0)) | ((0-2)) |
| R93L E522R | — | 1 | — | — | 1 (2) | (0-6) | ((0-3)) | ((0-5)) |
| E571A | 27 | 113 | 4 (5) | 3 (4, 5) | 4 | 6 (7) | 5 (6) | 5 (6) |
| E571R | — | 1 | — | — | 1 | 5 (6) | ((0)) | (0-4) |
| R93L E571R | (20) | 5 | — | — | 2 | 5 (6) | (0-2) | 3 (4, 5) |
| R93E E522R | (27) | 7 | ((0-2)) | ((0-2)) | 2 (3) | 5 (6) | ((0-2)) | 3 (4, 5) |
| G94P | 27 | 107 | 3 (4-6) | 3 (4, 5) | 4 | 6 (7) | 4 (5, 6) | 4 (5, 6) |
| G134D | 27 | 67 | ((0-2)) | ((0, 1)) | 3 (4) | 6 (7) | 5 (6) | 5 (6) |
| G146D | 27 | 91 | 1 (2-4) | 0 (1, 2) | 3 (4) | 6 (7) | 5 (6) | 5 (6) |
| G147D | 27 | 32 | ((0-2)) | ((0, 1)) | 3 | 6 (7) | (0-5) | 5 (6) |
Wild-type and mutant fhuA alleles were expressed from pT7-6 in HK97 fhuA.
Growth promotion results are expressed as the diameter of the growth zone (in millimeters) around the paper disk (diameter of the disk is not subtracted). Ten microliters of a 1 mM ferrichrome solution was placed on the paper disk and placed onto NBD plates overlaid with NB top agar containing 108 cells of the strains to be tested. Parentheses indicate a low cell density in the growth zone. —, no growth.
The ferrichrome (Fc) transport rates (numbers of iron ions per cell per minute) were calculated in the nearly linear region between minutes 5 and 13 and compared to the ferrichrome transport rate of wild-type FhuA (100%).
Sensitivities to the ligands were tested by using E. coli HK97 fhuA freshly transformed with the plasmids listed in Table 1 expressing the indicated FhuA derivatives. Sensitivities were tested by spotting 4 μl of 10-fold (colicin M and phages T1, T5, and φ80) or 3-fold (microcin J25 and albomycin) dilutions onto TY agar plates overlaid with TY top agar containing the strain to be tested. The results are given as the last of a dilution series that resulted in a clear zone of growth inhibition; e.g., 4 means that the phage stock suspension could be diluted 104-fold and the albomycin solution could be diluted 34-fold to yield a clear zone of cell lysis. Numbers in parentheses indicate turbid zones of growth inhibition; double parentheses indicate very turbid zones. Tabulated values of the sensitivity tests derived from a single experiment, but little variation was seen in repeated trials.—, no response.
The predicted repulsion between E522R and R93 and between E571R and R133 and R93 seemed to abolish the transport of ferrichrome, albomycin, and microcin J25 completely. However, the double mutant proteins FhuA(E522R R93L) and FhuA(E571R R93L), in which no repulsion between these residues could occur, were also transport deficient. Moreover, FhuA(R93E), which should be repulsed by E522 and E571, showed 93% of the FhuA wild-type ferrichrome transport activity. These data demonstrate that repulsion does not play a role in the inactivation of the FhuA mutant transport functions. The glutamate residues are not essential since their replacement by alanine resulted in fully active FhuA mutant proteins.
A remarkable property of FhuA(E522R) was that it mediated virtually no phage sensitivity (Table 3). FhuA(E571R) displayed a somewhat different phenotype in that cells were resistant to phage T5 and phage φ80 and almost fully sensitive to phage T1. In both strains, sensitivity to colicin M was 1,000-fold reduced. In contrast, FhuA(E522A) and FhuA(E571A) conferred full phage and colicin sensitivity. The FhuA(R93L E522R) double mutant was as insensitive as FhuA(E522R) to the phages and colicin M, showing that repulsion of R522 by R93 did not cause the inactivity of FhuA(E522R). The function of FhuA(E571R) for infection by phage T5 and sensitivity to colicin M was partially restored when R93 was replaced by leucine. Here it seems that repulsion of R571 by R93 reduced FhuA activity. These data support the conclusion drawn from the transport data that arginine residues at sites 522 and 571 severely reduce the β-barrel activity.
Roles of the highly conserved glycine residues of the cork domain.
Upon the binding of ferrichrome, the cork moves on one side relative to the β-barrel up to 2 Å towards ferrichrome, whereas it stays fixed on the opposite side (12). The fixed side contains amino acids that are highly conserved in TonB-dependent transport proteins, e.g., the G94, G134, G146, and G147, R93, and R133 mutant proteins analyzed above. The glycine residues that confer conformational flexibility were replaced by amino acids with more rigidly fixed side chains, and the activities of the resulting mutant FhuA proteins were examined. FhuA(G94P) displayed rather high activities (Table 3), whereas FhuA(G134D) and FhuA(G147D) exhibited reduced activities. Noteworthy are the turbid φ80 plaques formed on cells synthesizing FhuA(G147D). Trypsin cleaved the glycine substitution mutants to smaller fragments than wild-type FhuA (Fig. 5D). These results suggest that the mutations affected the FhuA structure and that, for this reason, the FhuA transport activity was reduced. However, except for that of G147D, the structural alterations were not so profound that they reduced sensitivity to colicin M and the phages.
DISCUSSION
Interaction of TonB with the TonB box of FhuA is assumed to cause long-range structural transitions throughout the entire FhuA molecule, which require highly specific interactions. For example, TonB-dependent conformational changes must occur in surface loop 4 for infection by phages T1 and φ80 (22) since the phages bind only reversibly and do not infect energy-depleted wild-type cells or tonB mutant cells (16). Likewise, there must be specific stereochemical requirements for the TonB-mediated transfer of energy to FhuA and the concerted conformational changes in the cork and the β-barrel to release ferrichrome from its binding sites and to open the channel for ferrichrome diffusion into the periplasm. In the substrate-unloaded form of the FhuA and FecA crystals, the switch helix is fixed to the hydrophobic pocket (12, 14). It is assumed that the strong translation of residues of the resolved switch helix, in particular, residues E19 and W22 by 17 Å, seen in the crystal structure upon the binding of ferrichrome (12, 13), and a similar translation observed in FecA upon the binding of (Fe3+-citrate)2 (14) facilitate or even permit functionally relevant interactions of FhuA and FecA with TonB. It is not important whether residues 24 to 29 form a helix, and in fact no helix is observed in the crystal structure of BtuB (9), but one may expect that the structural response of FhuA upon interaction with TonB of an energized cell requires a highly specific structure between the TonB box and the cork. Therefore, the most unexpected result was that deletion or duplication of the switch helix in FhuA had no strong effect on ferrichrome and albomycin transport and did not reduce the sensitivity of cells to colicin M and the phages T1 and φ80, which require the interaction of FhuA with TonB. Since FhuA remained active after deletion or duplication of the switch helix, the TonB box remained exposed to interact functionally with TonB and could confer structural changes on the cork and the β-barrel. The other deletion derivative studied, FhuA(Δ13-20) exhibited 57% of the wild-type FhuA transport rate, was threefold-less sensitive to albomycin and microcin J25, and conferred full sensitivity to colicin M and the phages T1, φ80, and T5. Even in this truncated derivative in which the TonB box was linked to residue 23, interaction of the TonB box and TonB somewhat functioned.
The point mutation A577E in the hydrophobic pocket was constructed under the assumption that the interaction with P24 would be disrupted and FhuA transport activity strongly reduced. However, compared to that of the wild type, the ferrichrome transport rate was reduced to 68%, ferrichrome binding was reduced to 45%, and sensitivity to albomycin and microcin J25 was reduced accordingly. Disruption of the predicted interaction between another interacting pair in this region, T54 and A26, by the mutation T54R retained ferrichrome transport at 81% of the wild-type level. As in the deletion derivatives the point mutations did not abolish the structural transitions which are assumed to occur in FhuA in response to an interaction with TonB. FhuA tolerates to a remarkable extent strong structural changes in the region that links the TonB box to the cork.
Additional mutations between the TonB box and the switch helix unpredictably influenced FhuA activity. The mutations P15A and P17A should exert a stronger effect on the conformation than A16G, but the FhuA activities of all three mutant proteins were only slightly reduced and by similar degrees. In contrast, the frameshift mutation QSEA→KKAP at positions 18 to 21 with an additional A16G mutation nearly abolished all FhuA activities. Apparently, the segment with the frameshift can no longer bind to TonB and/or transduce the assumed structural change to the cork and the β-barrel that is elicited by interaction with TonB.
Covalent fixation of the flexible cork segment to the β-barrel through disulfide bonds between C27 and C533 inactivated the transport activity for ferrichrome, albomycin, and microcin J25. Activity was restored when the disulfide bonds were cleaved by reduction. Lack of transport suggests that permanent fixation of the cork to the β-barrel prevents movement of the cork to open the channel of the β-barrel. The TonB box of FhuA must be flexible for TonB-coupled FhuA activity. In BtuB, flexibility has been demonstrated by electroparamagnetic resonance spectroscopy of spin-labeled cysteine residues, which revealed strong structural transitions of the TonB box from a structure fixed to the β-barrel to an extended dynamic structure upon substrate binding (25). However, in the cross-linked cysteine double mutant protein, sensitivity to colicin M was unaffected and sensitivity to φ80 was reduced only 10-fold, which indicated that TonB-dependent structural transitions required for phage infection and colicin toxicity still occurred. It is possible that the response to phage φ80 and colicin M results from a small fraction of un-cross-linked FhuA molecules which may be sufficient for phage infection and colicin M toxicity but insufficient for transport. It is also possible that TonB-dependent structural changes required for the transport of small molecules differ from those required for an interaction with large biomolecules. Since the FhuA disulfide derivative inhibited wild-type FhuA activity, it is likely that the TonB box of the disulfide derivative binds to but does not react to energized TonB.
Since changes in the structure of the FhuA segment that is exposed to the periplasm did not strongly reduce FhuA activity, we reinvestigated the previously characterized TonB box mutants FhuA(V11D) and FhuA(I9P). The inactivity of FhuA(V11D) for all TonB-dependent FhuA functions and the partial activity of FhuA(I9P) was reproduced. Since TonB box mutations might not define residues that directly interact with residues in the 160 region of TonB but change the conformation such that interaction is impaired (8), it was possible that deletion of the switch helix might partially restore the proper conformation of the TonB box mutants. However, FhuA(V11D Δ24-31) remained inactive and FhuA(I9P Δ24-31) became completely inactive for all TonB-dependent activities. The importance of the TonB box was also shown by its deletion FhuA(Δ7-11), which inactivated all TonB-dependent functions but retained fully the TonB-independent sensitivity to phage T5.
The crystal structure of FhuA reveals that residues R93 and R133 of the cork are close enough and oriented such that they can form salt bridges to residues E522 and E571 of the β-barrel. These residues, which contribute to the fixation of the cork in the β-barrel and possibly the cross talk between the two domains, are strictly conserved in TonB-dependent outer membrane transport proteins (34). Replacement of the arginine residues reduced FhuA activity most (42% of the wild-type activity) when they were replaced by proline, which changed the conformation of the polypeptide. Replacement by leucine retained full FhuA transport activity, and replacement by glutamate nearly completely retained its activity (93%). The latter result is particularly remarkable since E93 should repulse E522. In the other repulsion constructs, FhuA(R93 E522R) and FhuA(R93 R133 E571R), no transport activity remained (0 and 1%, respectively), but this was also the case with FhuA(R93L E522R) (1%) and FhuA(R93L E571R) (5%), where no repulsion occurred. Repulsion of two residues may not be sufficient to destabilize FhuA since approximately 60 hydrogen bonds and nine salt bridges are predicted to be involved in the interaction between the cork and the β-barrel (24). However, the strict conservation of these residues suggests a function in the communication between the cork and the β-barrel beyond the mere structural role. The arginine residues at positions 522 and 571 may have disrupted this communication. The glutamate residues were not essential since their replacement by alanine resulted in highly transport-active FhuA derivatives. FhuA(E522R) and FhuA(E571R) also failed to confer sensitivity to the phages, and sensitivity to colicin M was reduced 1,000-fold. Since infection by phage T5 was also impaired, the arginine residues did not, or did not only, disrupt the functional response to TonB.
Upon the binding of ferrichrome, the cork moves along one side of the β-barrel and remains fixed along the opposing side (12). Most of the cork residues in these two areas are conserved among outer membrane transporters. Replacement of the conserved glycine residues (G94, G134, G146, and G147) of the stationary side by either proline or aspartate resulted in FhuA derivatives exhibiting between 32 and 100% of wild-type FhuA activity. The flexibility which glycine residues confer to polypeptides seems to be important for some of the studied residues.
All of the examined FhuA mutant proteins, with the possible exception of FhuA(R133H) and FhuA(G146D), showed an altered electrophoretic mobility and/or an altered trypsin cleavage compared to wild-type FhuA. These properties indicate an altered conformation which, however, was not as severe as to disrupt all FhuA activities. In the cases of FhuA(R133H) and FhuA(G146D), trypsin may not uncover the structural changes.
A few mutants, FhuA(I9P) in the TonB box and FhuA(d23-30) with the duplicated switch helix, showed an enhanced binding of ferrichrome. The mutations are far from the ferrichrome binding sites (R81, G99, Q100, Y116, Y244, W246, Y313, Y315, F391, and F693) and support the concept of long-range structural transitions caused by the mutations, binding of ferrichrome, and interaction with TonB.
Random PCR mutagenesis of a DNA fragment encoding the 203 N-terminal amino acids of FepA and selection with diluted colicin B, which preferentially selects for inactive FepA derivatives containing point mutations, resulted in 11 mutants with three mutations in the TonB box (residues 14 to 16), six mutations in the cork near the barrel wall, and two mutations in the extracellular loops of the cork. Selection with colicin B for spontaneous mutants yielded three of the PCR mutants, additional cork mutants, and two extracellular-loop barrel mutants (1). In some mutants, the degree of resistance was correlated with the degree of colicin binding; in other mutants, resistance could not be explained by impaired binding but must have been caused by a defect in a later step of colicin uptake. All of the colicin B-resistant mutants also showed reduced ferric enterobactin transport, except FepA(G549R), which showed a wild-type level of transport activity. These mutants and two mutants generated by site-directed mutagenesis, one in the TonB box and one in loop 7 of the barrel, support the essential function of the TonB box for FepA function and show that not only surface-exposed loops but also cork regions buried in the barrel affect binding. Cork mutations were clustered near the wall, and the randomly generated R75L, R75P, and R126H mutations are in sites that correspond to R93 and R133 of FhuA. R75 of FepA forms salt bridges to E511 and E567, just as R93 of FhuA forms salt bridges to E522 and E571; R126 of FepA forms a salt bridge to E567, as R133 of FhuA forms a salt bridge to E571. In fact, the geometry of these residues and that of eight additional neighbor residues is strictly conserved in the crystal structures of FhuA (12, 24), FepA (6), FecA (14), and BtuB (9). However, our data indicate that the salt bridges are not essential for the structure and function of FhuA, and this conclusion might also apply to FepA, FecA, and BtuB.
Mutagenesis analysis of FhuA revealed a robust molecule that tolerated many mutations without losing much of its activity. The cork mutations affected the transport of ferrichrome and sensitivity to albomycin and microcin J25 more strongly than sensitivity to colicin M and the phages. Whereas transport rates were reduced, sensitivities to colicin M and the phages frequently remained at the wild-type level. Although this finding might be influenced by the transport assay more easily revealing small differences than the colicin and phage assays, the lower transport rates were strictly accompanied by strongly reduced sensitivities to albomycin and microcin J25, which were determined in drop tests on nutrient agar plates, as were the colicin and phage assays. Therefore, we conclude that most mutations in the cork reduced sensitivity to colicin M and the phages less than they reduced transport activity.
Acknowledgments
We thank Karen A. Brune for critically reading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (grant BR 330/20-1 and funds from the Forschergruppe Bakterielle Zellhülle: Synthese Funktion und Wirkort) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Barnard, T. J., M. E. Watson, Jr., and M. A. McIntosh. 2001. Mutations in the Escherichia coli receptor FepA reveal residues involved in ligand binding and transport. Mol. Microbiol. 41:527-536. [DOI] [PubMed] [Google Scholar]
- 2.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Bradbeer, C. 1993. The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J. Bacteriol. 175:3146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, M., H. Killmann, and V. Braun. 1999. The beta-barrel domain of FhuAΔ5-160 is sufficient for TonB-dependent FhuA activities of Escherichia coli. Mol. Microbiol. 33:1037-1049. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V. 1995. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 16:295-307. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. Van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 7.Cadieux, N., and R. J. Kadner. 1999. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc. Natl. Acad. Sci. USA 96:10673-10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadieux, N., C. Bradbeer, and T. J. Kadner. 2000. Sequence changes in the Ton box region of BtuB affect its transport activities and interaction with TonB protein. J. Bacteriol. 182:5954-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimento, D. P., A. K. Mohanty, T. J. Kadner, and C. Wiener. 2003. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 10:394-401. [DOI] [PubMed] [Google Scholar]
- 10.Coulton, J. W., P. Mason, D. R. Cameron, G. Carmel, R. Jean, and H. N. Rode. 1986. Protein fusions of β-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J. Bacteriol. 165:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, A. D., J. W. Coulton, K. Diederichs, and W. Welte. 2001. The ferric hydroxamate uptake receptor FhuA and related TonB-dependent transporters in the outer membrane of gram-negative bacteria, p. 834-849. In A. Messerschmidt, R. Huber, T. Poulos, and K. Wieghardt (ed.), Handbook of metalloproteins. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 14.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 15.Günter, K., and V. Braun. 1990. In vivo evidence for FhuA outer membrane interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 274:85-88. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, R. E. W., and V. Braun. 1976. Nature of energy requirements of the irreversible adsorption of bacteriophages T1 and φ80 to Escherichia coli. J. Bacteriol. 125:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hantke, K. 1981. Regulation of ferric iron transport in Escherichia coli K-12: isolation of a constitutive mutant. Mol. Gen. Genet. 191:288-292. [DOI] [PubMed] [Google Scholar]
- 18.Hantke, K., and V. Braun. 1978. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J. Bacteriol. 135:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller, K. J., R. J. Kadner, and K. Günther. 1988. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene 64:147-153. [DOI] [PubMed] [Google Scholar]
- 20.Killmann, H., C. Herrmann, A. Torun, G. Jung, and V. Braun. 2002. TonB of Escherichia coli activates FhuA through interaction with the β-barrel. Microbiology 148:3497-3509. [DOI] [PubMed] [Google Scholar]
- 21.Killmann, H., and V. Braun. 1992. An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J. Bacteriol. 174:3479-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killmann, H., G. Videnov, G. Jung, H. Schwarz, and V. Braun. 1995. Identification of receptor binding sites by competitive peptide mapping: phages T1, T5, and φ80 and colicin M bind to the gating loop of FhuA. J. Bacteriol. 177:694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killmann, H., R. Benz, and V. Braun. 1993. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 12:3007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 25.Merianos, H. J., N. Cadieux, C. H. Lin, R. J. Kadner, and D. S. Cafiso. 2000. Substrate-induced exposure of an energy-coupling motif of a membrane transporter. Nat. Struct. Biol. 7:205-209. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Moeck, G. S., J. W. Coulton, and K. Postle. 1997. Cell envelope signaling in Escherichia coli. Ligand binding to the ferrichome-iron receptor FhuA promotes interaction with the energy-transducing protein TonB. J. Biol. Chem. 272:28391-28397. [DOI] [PubMed] [Google Scholar]
- 28.Moeck, G. S., P. Tawa, H. Xiang, A. A. Ismail, J. L. Turnbull, and J. W. Coulton. 1996. Ligand-induced conformational change in the ferrichrome-iron receptor of Escherichia coli K-12. Mol. Microbiol. 22:459-471. [DOI] [PubMed] [Google Scholar]
- 29.Ogierman, M., and V. Braun. 2003. Interactions between the outer membrane ferric citrate transporter FecA and TonB: studies of the FecA TonB box. J. Bacteriol. 185:1870-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Schöffler, H., and V. Braun. 1989. Transport across the outer membrane of Escherichia coli K-12 via FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol. Gen. Genet. 217:378-383. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer, M., I. Hindennach, W. Garten, and U. Henning. 1978. Major proteins of the E. coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur. J. Biochem. 82:211-217. [DOI] [PubMed] [Google Scholar]
- 33.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Helm, D., and R. Chakraborty. 2001. Structures of siderophore receptors, p. 261-287. In G. Winkelmann (ed.), Microbial transport systems. Wiley-VCH Verlag GmbH, Weinheim, Germany.