Abstract
An example of alternative male strategies is seen in diandric protogynous (female first) hermaphrodites, where individuals either mature directly as male (primary males) or first reproduce as female and then change sex to male (secondary males). In some sex-changing fishes, the testes of primary males appear anatomically similar to those of non-sex-changing species, whereas the testes of secondary males have anatomical evidence of their former ovarian function. Here, we provide evidence that in the bluehead wrasse, Thalassoma bifasciatum, these strikingly different male phenotypes arise from differences in the ontogenetic timing of environmental sex determination, timing that can be experimentally altered through changes in the social circumstances. Juveniles differentiated almost exclusively as females when reared in isolation, regardless of whether they were collected from a reef with a high proportion of primary males or from a reef with a low proportion of primary males. In contrast, one individual usually differentiated as a primary male when reared in groups of three. Our results indicate that primary males of the bluehead wrasse are an environmentally sensitive developmental strategy that has probably evolved in response to variation in the reproductive success of primary males in populations of different sizes.
Keywords: developmental plasticity, sex determination, alternative mating strategies, coral reef fish
1. Introduction
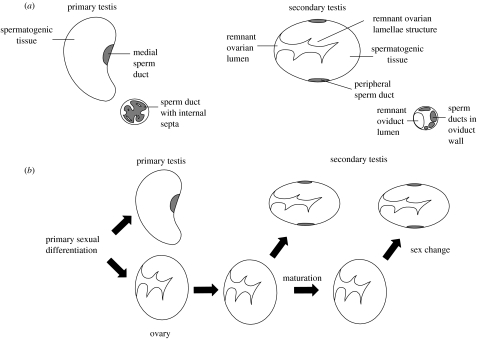
Unveiling the mechanisms controlling the expression of alternative phenotypes is an important goal of evolutionary biology (Via et al. 1995; Gross 1996; Shuster & Wade 2003; West-Eberhard 2003). Over 40 years ago, Reinboth (1962) observed that in many sex-changing fishes, there are two ways to become a male (a condition known as diandry): some individuals develop directly into males (primary males) and others reproduce first as females and then change sex to male later in their life (secondary males). In some wrasses (Labridae) and parrotfishes (Scaridae), the testes of primary males appear similar to those of non-sex-changing species, whereas the testes of secondary males retain evidence of their former ovarian function (figure 1a; Reinboth 1970; Robertson & Warner 1978; Warner & Robertson 1978). These striking anatomical differences have often been assumed to be the result of genetic sex determination (i.e. primary males are distinct from females and secondary males owing to allelic differences at a few loci of major effect; Reinboth 1970; Robertson & Choat 1974; Smith 1975; Warner & Hoffman 1980a; Charnov 1982). An alternative explanation is that individuals possess environmentally sensitive developmental reaction norms (Roff 1996; Schlichting & Pigliucci 1998; Suzuki & Nijhout 2006) that determine their capacity to become either primary males, or females that subsequently become secondary males. In this perspective, individuals take one developmental route or the other depending on the environmental cues they experience at the time of primary sexual differentiation and their inherited sensitivity to those cues.
Figure 1.
Anatomy and development of primary and secondary males in the bluehead wrasse. (a) Discriminating anatomy of the primary and secondary testes and the posterior sperm duct. Primary males have a solid testis, with sperm collected in a medial vas deferens that leads to the genital papilla through a single duct; virtually identical anatomy is seen in many non-sex-changing species. In secondary male testes, spermatogenic crypts proliferate in the ovarian lamellae that protrude into the now-unused ovarian lumen, so the ovarian structure often remains apparent. Sperm are transported through ducts in the periphery of the gonad, leading to a ring of sperm ducts embedded in the wall of the terminal oviduct. (b) Ontogeny of primary and secondary testis. Depending on the social context, individuals may become male very early in the juvenile stage. These individuals will develop a primary testis. Individuals that become male after the ovary has formed will develop a secondary testis, regardless of whether the individual embarks on the male pathway before or after maturity.
The diandric, protogynous wrasse Thalassoma bifasciatum (bluehead wrasse) has proven to be a useful model for understanding the proximate mechanisms and selective advantage of sex change (Warner 1975; Charnov 1982; Warner 1984a; Warner & Swearer 1991; Perry & Grober 2003), but even in this species it is not known whether the development of primary males is influenced by environmental conditions. It has previously been reported that the proportion of primary males in populations of the bluehead wrasse is closely correlated with population size, such that reefs with small populations have proportionally fewer primary males than those with larger populations (Warner & Hoffman 1980a; Warner 1984a). This uneven distribution of primary males appears to be adaptive because large territorial males (most of which are secondary males) monopolize breeding opportunities on reefs with small populations and, consequently, the reproductive success of small primary males is close to zero on these reefs (Warner & Hoffman 1980a,b). In contrast, territory defence becomes uneconomic in large populations (Warner & Hoffman 1980b) and small primary males have relatively high reproductive success where the population is large (Warner 1984b). Individuals rarely move between reefs after settlement; therefore, the occurrence of different proportions of primary males on the nearby reefs with different population sizes suggests that either (i) individuals destined to be primary males choose to settle on certain reefs or (ii) the development of primary males is influenced by the social environment experienced by juveniles after they were recruited to the reef. These alternatives have not previously been experimentally tested.
Bluehead wrasse spawn pelagic eggs and the larvae remain in the plankton for several weeks before they make the transition to reef-based juveniles. If the social conditions experienced on the reef influence the proportion of individuals that become primary males, we predicted that: (i) newly settled juveniles collected from reefs with very different proportions of primary males would differentiate into primary males at approximately the same frequency if exposed to identical social conditions; and (ii) newly settled juveniles collected from the same reef would differentiate into primary males with different frequencies if exposed to different social conditions. To test these predictions, we first examined the relationship between population size and the proportion of primary males on reefs at St Croix, US Virgin Islands. Having established that there was a significant relationship between population size and the proportion of primary males present on neighbouring reefs, we collected newly settled juveniles from reefs with different proportions of primary males and reared them in two social arrangements: (i) new settlers from a reef with a high proportion of primary males and those with a low proportion of primary males were reared under identical conditions, and in isolation from each other; and (ii) new settlers from the reef with a high proportion of primary males were reared either alone or in groups. Juveniles were reared until sexual differentiation was complete, and the numbers of primary males that developed in each treatment were compared to test whether primary male induction was sensitive to social conditions experienced by juveniles. If the social environment is important for primary male development in the bluehead wrasse, this would be the first demonstration that fundamental differences in testicular anatomy between alternative male phenotypes can come about through differences in the ontogenetic timing of environmental sex determination.
2. Material and methods
(a) Study species and location
This study was conducted in the Caribbean island of St Croix at the same locations where research on adult sex change in the bluehead wrasse has previously been conducted (e.g. Warner & Swearer 1991; Godwin et al. 2000; Perry & Grober 2003). This species has two different colour phases. Females and primary males exhibit a dull initial-phase coloration (figure 2a). Large territorial males, most of which are secondary males, exhibit a bright terminal-phase coloration (figure 2b). Females usually become brightly coloured when they change sex to a secondary male. Primary males can also develop terminal-phase coloration if they become large enough to defend a territory.
Figure 2.

Initial- and terminal-phase coloration of the bluehead wrasse. (a) Initial-phase colour fish can be either females or primary males. Initial-phase males either group-spawn with females or parasitize the spawning of territorial males. (b) Territorial pair-spawning males, most of which are secondary males, exhibit a bright terminal-phase coloration. (Photograph courtesy of Ken Clifton.)
(b) Population surveys
The proportions of initial-phase primary males in the populations of 22 patch reefs in Tague Bay and the contiguous reef in Jacks Bay were estimated in May 2004, immediately before the main period of settlement that occurs in June–September. Patch reefs in Tague Bay ranged in size from 20 to 4600 m2 and were separated from each other by tens to hundreds of metres (see Gladfelter & Gladfelter (1987) for a map). A sample of the adult population (20–80 fish) from each reef was collected using a lift net and sexed by standard technique (Warner & Hoffman 1980a; Warner & Swearer 1991). Initial-phase males were distinguished by the release of sperm when gentle pressure was applied to the abdomen. Individuals that did not release sperm were classified as female. This method of sexing was validated as 100% effective by macroscopic examination of the gonads of a random sample of collected fish (N=32). We assumed that all initial-phase males had a primary testis. A small number of initial-phase males may have had secondary testes (Warner & Robertson 1978); however, this number is likely to be trivial because no initial-phase males with secondary testis were detected in 138 fish (29–49 mm standard length (SL)) that were histologically examined from Tague Bay and Jacks Bay (P. Munday 2004, unpublished data), and only one male with a secondary testis was found in a sample of 26 initial-phase males (38–72 mm SL) from neighbouring Puerto Rico (Shapiro & Rasotto 1993).
To estimate population size, the number of fish remaining on the reef was counted before releasing the captured sample. Two divers made two separate estimates of abundance, and the mean of the four surveys was combined with the known number of fish in the sample to calculate the population size for each reef.
Since the intense recruitment season may alter population size, especially on reefs that initially had small populations, we resurveyed the population size and estimated the proportion of initial-phase males again on three of the small population reefs in September 2004.
(c) Common garden experiment
New settlers were collected from Reef 21 in Tague Bay (4.7% of initial-phase adults were primary males) and the contiguous reef in Jacks Bay (37% of initial-phase adults were primary males) between 28 May and 5 June 2004. Jacks Bay and Tague Bay are within a few kilometres of each other at the eastern end of St Croix. Fish that had settled to the reef within the previous two weeks were collected by hand net, placed in plastic bags and transferred to a flow-through seawater system. An array of 9 l plastic buckets, individually supplied with a continuous flow of fresh, unfiltered seawater, was stocked with either one or three fish each. Twenty-four single fish from Tague Bay and 27 single fish from Jacks Bay were established. Fifteen triplets were established using fish from Jacks Bay. The density of juveniles on the contiguous reef in Jacks Bay is considerably higher than those on the patch reefs in Tague Bay (0.2 versus 0.005 m−2 for the 15–20 mm total length (TL) size class; J. W. White 2003, unpublished data); therefore, the additional crowding of Jacks Bay fish in the triplets is representative of naturally greater crowding at this site. All fish were less than 20 mm TL (mean 13.4 mm TL, range 12.0–14.7, N=8 haphazardly selected fish) at the start of the experiment.
Juveniles were fed freshly hatched Artemia nauplii each morning and commercial larval fish food each afternoon. Commercial flake food was substituted for the larval diet as the juveniles grew. Buckets containing triplets received approximately three times as much food as those containing singletons. Juveniles were raised until they reached approximately 35 mm SL or until the completion of the experiment in the first week of September 2004. Whole fish were fixed in 10% seawater–formalin in preparation for histological analysis of the gonads. Fish that died during the experiment were also retained for histology. The portion of the body containing the gonads was removed from each fish, transversely sectioned at 5 μm, stained with haematoxylin and eosin and viewed using light microscopy.
In a sample of T. bifasciatum from Puerto Rico, Shapiro & Rasotto (1993) found that nearly all juveniles had fully differentiated primary testis or ovary by 27–30 mm SL. A similar pattern of gonadal ontogeny was observed in a sample of T. bifasciatum juveniles (N=35) collected from Jacks Bay in June 2004. While primary males were observed at lengths as small as 25 mm SL, the proportion of primary males in the sample increased to an asymptote at 29 mm SL. All juveniles greater than or equal to 29 mm SL exhibited either a testis or a fully differentiated ovary. Therefore, 29 mm SL was considered the minimum size for unambiguous identification of sexual identity.
Nineteen of the original 24 single fish from Tague Bay and 24 of the original 27 single fish from Jacks Bay were available for histology at the end of the experiment. Eight fish either escaped during the course of the experiment or died and were not recovered when turbid water entered the flow-through seawater system when a hurricane passed close to the island. All available fish were examined histologically, but only those that had reached a minimum of 29 mm SL were included in the analysis.
In the high-density treatment, agonistic behaviour caused mortality of subordinates in some replicates. In four of the original 15 replicates, only one fish survived and was single for at least 40 days prior to the end of the experiment. These four replicates were excluded from the analysis.
(d) Analysis
A χ2-test was used to compare the number of primary males and females that developed in the sample of new settlers collected from Reef 21 and Jacks Bay and grown in isolation from each other. It was not appropriate to use a χ2-test to compare the number of primary males among individuals reared alone versus in groups, because observations from groups were not independent. A binomial probability test was used to gauge whether significantly more primary males developed in groups compared to singletons. We used the observed frequency of females in singletons to calculate the probability of becoming a female when reared alone (pfemale=number of females/number of singletons). We then tested whether the number of triplets containing at least one primary male differed from that expected if males and females differentiated at the same rate observed in singletons (where ).
3. Results and discussion
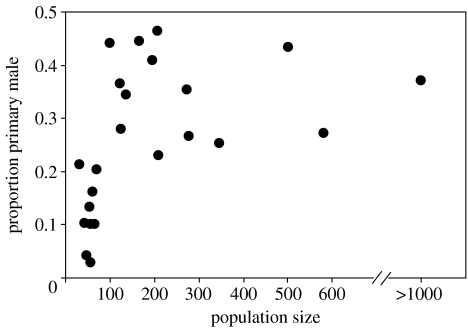
The relationship between population size and the proportion of primary males fell into two distinct categories (figure 3). Reefs with small populations (less than 100) had few primary males (3–22%), whereas reefs with larger populations (greater than 100) had many primary males (23–46%; t-test: p<0.001). Reefs with small populations never had large proportions of primary males and reefs with large populations never had very low proportions of primary males. In keeping with this pattern, the proportion of primary males increased on two small population reefs, where intense recruitment generated a large increase in population size, but not on another reef where population size remained close to 100 (table 1).
Figure 3.
Proportion of initial-phase primary males in relation to total population size on 22 patch reefs in Tague Bay and the contiguous reef in Jacks Bay (Jacks Bay is far right data point).
Table 1.
Population size and proportion of initial-phase (IP) primary males on three reefs surveyed in May and September 2004.
| May 2004 | September 2004 | |||
|---|---|---|---|---|
| reef | population size | proportion of IP primary males (%) | population size | proportion of IP primary males (%) |
| 12 | 55 | 14 | 105 | 13 |
| 21 | 48 | 4.7 | 117 | 29 |
| 9B | 44 | 12 | 137 | 58 |
Juvenile bluehead wrasse reared in isolation differentiated almost exclusively as females (table 2) and there was no difference in the proportion of primary males that developed in the juveniles collected from the two reefs (χ2-test: p=0.96), despite the different proportions of adult primary males observed on those reefs at the time of settlement. Approximately 6% of the individuals from each reef differentiated as primary males, which is close to the minimum proportion of primary males seen in natural populations (figure 3). Importantly, only one of the 17 individuals (5.9%) from Jacks Bay differentiated as a primary male, even though primary males made up 39% of the natural adult population at this location. Since the rearing environment was the same for all individuals, this result clearly suggests that primary male induction is influenced by environmental conditions.
Table 2.
Proportion of initial-phase primary males on focal reefs and in new settlers collected from those reefs and reared under identical conditions until sexual differentiation occurred.
| natural population | experimental fishes | ||||
|---|---|---|---|---|---|
| location | population size | proportion of primary males (%) | female | male | proportion of primary males (%) |
| Reef 21 | 48 | 4.7 | 15 | 1 | 6.3 |
| Jacks Bay | >1000 | 37 | 16 | 1 | 5.9 |
One individual differentiated as a full primary male in 45% (5 out of 11) of the high-density replicates. In all but one instance, it was the largest individual in the group that became male. This significant increase in primary male induction (binomial probability p=0.02) indicates that a higher-density social situation triggers more individuals to differentiate into primary males.
In one of the 11 high-density replicates, one fish first differentiated as a female before developing into a male, marked by the presence of remnant ovarian structures in the developing testis. This shows that individuals that first differentiate as females (i.e. develop an ovary) retain the capacity to become male before maturation, even though this trait was not observed in the natural population at St Croix (N=138 initial-phase fish examined histologically).
All individuals differentiated as females in the remaining five high-density replicates. The presence of some high-density replicates where primary males did not develop indicates that individuals vary in their sensitivity to density as a cue for primary male development. This is consistent with primary males being an expression of a quantitative threshold trait (Roff 1996), where genetic variation underlying the trait makes some individuals more likely to become primary males than others when exposed to the same environmental cues.
Our results indicate that primary males in the bluehead wrasse are the result of developmental plasticity. All juveniles first develop a rudimentary female gonad (Shapiro & Rasotto 1993) that can differentiate into either primary testis or ovary. The proportion of individuals that develop primary testes in a local population appears to depend on local environmental conditions and individual genetic sensitivity to those conditions. Individuals that differentiate as primary males apparently remain male throughout their life, whereas individuals that differentiate as females can change sex to male at some later stage (Warner & Swearer 1991). Consequently, the anatomical differences between primary and secondary males are simply the result of differences in the ontogenetic timing of environmental sex determination—individuals that become male at the time of primary sexual differentiation develop a testis that is similar to that of non-sex-changing fishes, with no evidence of prior female function, whereas the gonads of individuals that become male later in life retain evidence of their former ovarian state (figure 1b).
Developmental plasticity has probably evolved in T. bifasciatum in response to differences in the reproductive success of primary males in populations of different sizes (Warner & Hoffman 1980b; Warner 1984b), combined with the presence of post-settlement cues that indicate whether the primary male phenotype is likely to be successful or not. The local population size that a juvenile will experience upon settling from the plankton is highly unpredictable, but the number or density of individuals encountered after settlement is likely to provide a reliable indicator of the profitability of becoming a primary male. Exactly these sorts of conditions are predicted to favour the evolution of developmental plasticity (Shuster & Wade 2003; West-Eberhard 2003).
There is increasing evidence that social conditions can influence sexual differentiation at a range of stages in the ontogeny of sex-changing fishes (Munday et al. 2006). For example, Liu & Sadovy (2004a) showed that social factors influence the development of males both before and after maturation in the rockcod, Cephalopholis boenak. Comparing primary male development in the rockcod with that observed here for the bluehead wrasse provides an important insight into the complex patterns of sexual development in hermaphroditic fishes. In C. boenak, primary male testis develops from a bisexual gonad with an ovarian lumen (Liu & Sadovy 2004b). Consequently, the testicular structure of primary males is similar to that of secondary males. In contrast, differentiation of primary male testis in the bluehead wrasse occurs before there are any distinctive ovarian features in the gonad (Shapiro & Rasotto 1993). This results in a testicular anatomy of primary males that is strikingly different from that of secondary males. The unifying point in these two examples is that primary male development is triggered by social conditions in both species. This means that fundamental differences in the anatomy of primary and secondary males in some species, but not others, are not necessarily indicative of different mechanisms controlling sexual differentiation. Instead, it seems that the same mechanism (environmental sex determination) can generate a range of different anatomical arrangements depending on the structure of the juvenile gonad and the precise timing that the male pathway is taken.
An important difference between the results of Liu & Sadovy (2004a) and those observed here is that isolated juveniles always differentiated as male in C. boenak and nearly always as female in T. bifasciatum. The reason for this difference might lie in the ecology of the two species. The bluehead wrasse has two different mating tactics (Warner & Robertson 1978), whereas the rockcod appears to have just one (Liu & Sadovy 2004b). In the bluehead wrasse, large terminal-phase males (mostly secondary males) are territorial and they pair-spawn with visiting females. The smaller initial-phase primary males are non-territorial group-spawners. Territorial males prevent primary males from breeding on small reefs (Warner & Hoffman 1980a,b); therefore, females have higher reproductive success than small primary males in small populations. Limited interactions with other individuals, as experienced by singletons in our experiment, would probably provide juveniles with a reliable cue that the population is small and that they would benefit from maturing as a female rather than as a primary male.
In contrast to the bluehead wrasse, the rockcod forms small harem-like groups, where a male pair spawns with one or two smaller females (Liu & Sadovy 2004a), and there is no indication that small males exhibit alternative mating strategies, such as group spawning (Liu & Sadovy 2004b). In the absence of alternative mating tactics, the reproductive success of small males is limited by the presence of dominant males. Small males might gain some reproductive success from occasional sneak spawning with harem females, but the big reproductive pay-off would come from gaining the status of harem master. Primary males in this species may be positioning themselves to take over a group of females on the loss of a dominant male. The absence of a larger male, as experienced by singletons in Liu & Sadovy's (2004a) experiments, might trigger juveniles to mature as males, because it indicates that there is a reasonable opportunity for high reproductive success as a male.
The observation that environmental sex determination can generate different patterns of gonadal anatomy in primary males has important consequences for the way we classify early developing males in labroid fishes (wrasses and parrotfishes). Males that develop before maturation have historically been divided into two groups: (i) those in which the testes show no evidence of having passed through an earlier female stage (anatomical primary males), and (ii) those in which the testes show evidence of having passed through an ovarian stage before differentiation (prematurational sex-change males). The assumption underlying this dichotomy is that there are two coexisting sexual types (gonochoristic males and hermaphroditic females) in species with anatomical primary males (e.g. the scarid genus Scarus; Robertson & Warner 1978), whereas there is only one sexual type (hermaphroditic females) in species with prematurational sex-change males (e.g. the scarid genus Sparisoma; Robertson & Warner 1978). Our results suggest that there is no fundamental difference between anatomical primary males and prematurational sex changers. The differences in testicular structure are simply related to the timing of sexual differentiation. Some species might be genetically programmed to take the primary male pathway earlier than others. This implies that from a functional perspective there is no need to distinguish between these two anatomically different types of primary males. The important issue from an evolutionary perspective is whether primary males exhibit alternative mating tactics or not. In labroid fishes, all initial-phase primary males exhibit a mating tactic different from that of territorial males. It is the success of this strategy in all but the smallest populations that maintains it in populations of these species (Warner 1984b).
The mechanism responsible for the morphological and behavioural differences between primary and secondary males of many sex-changing fishes has remained a mystery for over 40 years. We show that in the bluehead wrasse, these differences are the result of environmentally induced differences in the timing of male development. In most vertebrates, a genetically controlled switch activated during a critical window of early development directs the differentiation of testis or ovary from an otherwise indistinguishable embryo (Mittwoch 1996). A temperature-sensitive period during early life has a similar effect in other species (Crews 2003; Godwin et al. 2003). It now seems that sensitivity to social conditions experienced during early life performs the same function in an hermaphroditic species, producing either primary testes or ovaries at the time of sexual differentiation—the difference is that in protogynous hermaphrodites, the male switch can be activated again later in life to produce secondary males from individuals that first differentiated as female.
Acknowledgments
We thank K. Munday, J. Barr, S. Brander and E. Marsh for their assistance in the field. S. Shuster, P. Buston and R. Reinboth provided very helpful comments on earlier versions of the manuscript. This research was conducted with approval from the Department of Planning and Natural Resources, St Croix and was supported by the Australian Research Council, Fulbright Commission, and the Partnership for Interdisciplinary Study of Coastal Oceans (funded by the Packard and Moore Foundations).
References
- Charnov E.L. Princeton University Press; New Jersey, NJ: 1982. The theory of sex allocation. [Google Scholar]
- Crews D. Sex determination: where environment and genetics meet. Evol. Develop. 2003;5:50–55. doi: 10.1046/j.1525-142x.2003.03008.x. doi:10.1046/j.1525-142X.2003.03008.x [DOI] [PubMed] [Google Scholar]
- Gladfelter W.B, Gladfelter E.H. Fish community structure as a function of habitat structure on West Indian patch reefs. Rev. Biol. Trop. 1987;26:65–84. [Google Scholar]
- Godwin J, Sawby R, Warner R.R, Crews D, Grober M.S. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav. Evol. 2000;55:77–84. doi: 10.1159/000006643. doi:10.1159/000006643 [DOI] [PubMed] [Google Scholar]
- Godwin J, Luckenbach J.A, Borski R.J. Ecology meets endocrinology: environmental sex determination in fishes. Evol. Develop. 2003;5:40–49. doi: 10.1046/j.1525-142x.2003.03007.x. doi:10.1046/j.1525-142X.2003.03007.x [DOI] [PubMed] [Google Scholar]
- Gross M.R. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- Liu M, Sadovy Y. The influence of social factors on adult sex change and juvenile sexual differentiation in a diandric, protogynous epinepheline, Cephalopholis boenak (Pisces, Serranidae) J. Zool. Lond. 2004a;264:239–248. [Google Scholar]
- Liu M, Sadovy Y. Early gonadal development and primary males in the protogynous epinepheline, Cephalopholis boenak. J. Fish. Biol. 2004b;65:987–1002. doi:10.1111/j.0022-1112.2004.00503.x [Google Scholar]
- Mittwoch U. Sex-determining mechanisms in animals. Trends Ecol. Evol. 1996;11:63–67. doi: 10.1016/0169-5347(96)81044-5. doi:10.1016/0169-5347(96)81044-5 [DOI] [PubMed] [Google Scholar]
- Munday P.L, Buston P.M, Warner R.R. Diversity and flexibility of sex-change strategies in animals. Trends Ecol. Evol. 2006;21:89–95. doi: 10.1016/j.tree.2005.10.020. doi:10.1016/j.tree.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Perry A.N, Grober M.S. A model for social control of sex change: interactions of behavior, neuropeptides, glucocorticoids, and sex steroids. Horm. Behav. 2003;43:31–38. doi: 10.1016/s0018-506x(02)00036-3. doi:10.1016/S0018-506X(02)00036-3 [DOI] [PubMed] [Google Scholar]
- Reinboth R. Morphologische und funktionelle zweigeschlechtlichkeit bei marinen teleostiern (Serranidae, Sparidae, Centracanthidae, Labridae) Zool. Jb. Physiol. 1962;69:405–480. [Google Scholar]
- Reinboth R. Intersexuality in fishes. Mem. Soc. Endocrinol. 1970;18:515–543. [Google Scholar]
- Robertson D.R, Choat J.H. Protogynous hermaphroditism and social systems in Labrid fish. Proc. Second Int. Coral Reef Symp. 1974;1:217–224. [Google Scholar]
- Robertson D.R, Warner R.R. Sexual patterns in the labroid fishes of the western Caribbean, II: the parrotfishes (Scaridae) Smithsonian Cont. Zool. 1978;255:1–26. [Google Scholar]
- Roff D.A. The evolution of threshold traits in animals. Q. Rev. Biol. 1996;71:3–35. doi:10.1086/419266 [Google Scholar]
- Schlichting C.D, Pigliucci M. Sinauer; Sunderland, MA: 1998. Phenotypic evolution: a reaction norm perspective. [Google Scholar]
- Shapiro D.Y, Rasotto M.B. Sex differentiation and gonadal development in the diandric protogynous wrasse, Thalassoma bifasciatum (Pisces, Labridae) J. Zool. Lond. 1993;230:231–245. [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; New Jersey, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Smith C.L. The evolution of hermaphroditism in fishes. In: Reinboth R, editor. Intersexuality in the animal kingdom. Springer; Berlin, Germany: 1975. [Google Scholar]
- Suzuki Y, Nijhout F. Evolution of a polyphenism by genetic accommodation. Science. 2006;311:650–652. doi: 10.1126/science.1118888. doi:10.1126/science.1118888 [DOI] [PubMed] [Google Scholar]
- Via S, Gomulkiewicz R, de Jong G, Scheiner S.M, Van Tienderen P.H. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 1995;10:212–217. doi: 10.1016/s0169-5347(00)89061-8. doi:10.1016/S0169-5347(00)89061-8 [DOI] [PubMed] [Google Scholar]
- Warner R.R. The adaptive significance of sequential hermaphroditism in animals. Am. Nat. 1975;109:61–82. doi:10.1086/282974 [Google Scholar]
- Warner R.R. Mating behaviour and hermaphroditism in coral reef fishes. Am. Sci. 1984a;72:128–136. [Google Scholar]
- Warner R.R. Deferred reproduction as a response to sexual selection in a coral reef fish: a test of the life historical consequences. Evolution. 1984b;38:148–162. doi: 10.1111/j.1558-5646.1984.tb00268.x. doi:10.2307/2408554 [DOI] [PubMed] [Google Scholar]
- Warner R.R, Hoffman S.G. Local population size as a determinant of mating system and sexual composition in two tropical marine fishes (Thalassoma spp.) Evolution. 1980;34:508–518. doi: 10.1111/j.1558-5646.1980.tb04840.x. doi:10.2307/2408220 [DOI] [PubMed] [Google Scholar]
- Warner R.R, Hoffman S.G. Population density and the economics of territorial defense in a coral reef fish. Ecology. 1980b;61:772–780. doi:10.2307/1936747 [Google Scholar]
- Warner R.R, Robertson D.R. Sexual patterns in the labroid fishes of the western Caribbean, I: the Wrasses (Labridae) Smithsonian Cont. Zool. 1978;254:1–27. [Google Scholar]
- Warner R.R, Swearer S.E. Social control of sex change in the bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae) Biol. Bull. 1991;181:199–204. doi: 10.2307/1542090. [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]