Abstract
Animal interactions often involve chemical exchange but simultaneous evaluation of chemistry and behaviour has been problematical. Here we report findings from a novel method, atmospheric pressure chemical ionization-mass spectrometry (APCI-MS) coupled with manipulation of molecular-mass achieved by rearing organisms on deuterium-enhanced nutrients. This allows real-time monitoring of the occurrence and quantity of volatile chemicals released by each of two interacting individuals, in tandem with behavioural observations. We apply these methods to female–female contests in the parasitoid wasp Goniozus legneri. We show that this species emits the spiroacetal 2-methyl-1,7-dioxaspiro[5.5]undecane. Chemical release is most common in more behaviourally aggressive contests, which occur when prior resource owners successfully resist take-over by similar-sized intruder females. Volatiles released during contests are always emitted by the loser. Aggression in contests is reduced after spiroacetal release. We suggest that the spiroacetal functions as a weapon of rearguard action. We anticipate that APCI-MS, which is rapid, non-intrusive and relatively inexpensive to operate, will be widely applied in studies linking chemistry and behaviour.
Keywords: contest behaviour, chemical manipulation, real-time mass spectrometry, spiroacetal, loser emission
1. Introduction
Outcomes of direct behavioural contests for resources are commonly influenced by competitor asymmetries in intrinsic contest ability, prior ownership and resource value, as predicted by classic game-theoretic models (Maynard Smith 1982; Mesterton-Gibbons & Adams 1998; Riechert 1998). Attention has also focused on behavioural interactions between opponents within contests (Payne 1998; Riechert 1998; Briffa & Elwood 2002; Maynard Smith & Harper 2003). While it is known that interactions often involve chemicals, particularly via olfaction, their role has largely been studied in species that continuously display or deposit relatively non-volatile compounds (Gosling & Roberts 2001; Hurst et al. 2001; Nevison et al. 2003; Wyatt 2003). Studies of the exchange of more volatile chemicals have usually been constrained to trap substances onto absorbent blocks prior to analysis (e.g. by gas chromatography-mass spectrometry, GC-MS; Wyatt 2003; Gómez et al. 2005), hence missing the exact correspondence between chemical release and behavioural events. Other studies have used physiological techniques, such as electroantennography, to monitor nervous signals in sensory organs exposed to chemical stimulants (Wyatt 2003); these miss the interactive–behavioural dynamics of intact whole organisms. To date, it has thus been possible to show that chemicals are produced by one organism and detected by another but not, in general, to follow ‘a chemical conversation’ with accurate determination of the timing of, and behavioural associations with, chemical emission. There is consequently little evidence for the importance of chemicals as temporally dynamic signals, or as weapons, during animal contests (Breithaupt & Eger 2002; Monnin et al. 2002).
We provide such assessment using atmospheric pressure chemical ionization-mass spectrometry (APCI-MS; Linforth & Taylor 1997; Taylor et al. 2000; Taylor & Linforth 2003) to track olfactory events by continuously sampling the airspace around freely moving and competing individuals. We explore the chemical behaviour of the bethylid wasp Goniozus legneri Gordh (Hymenoptera: Bethylidae) a parasitoid of lepidopteran larvae that tunnel into the tissues of crops such as pistachio nuts and almonds (Steffan et al. 2001). The adult female wasp paralyses a host approximately one day before laying a clutch of eggs onto its surface. In common with other bethylids (Petersen & Hardy 1996) ‘owner’ females aggressively defend paralysed hosts against conspecific intruders, resulting in classic owner–intruder contests (figure 1). We first investigate the presence and composition of emissions by stressed G. legneri and then, using real-time analysis, the occurrence of emissions during contests between chemically manipulated, and thus distinguishable, females.
Figure 1.
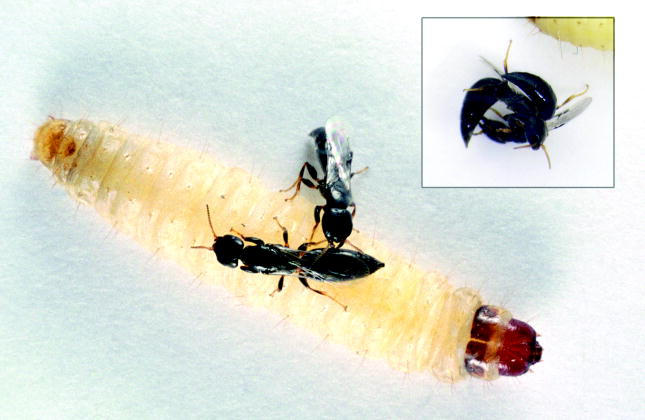
Goniozus legneri females competing for a host. One female is biting her opponent's abdomen. Such behaviour often leads to full fighting (inset; photos: Sonia Dourlot).
2. Material and methods
(a) Real-time mass spectral analysis of volatile emissions
To monitor in real-time the volatile releases of G. legneri wasps we used an APCI-MS (Linforth & Taylor 1997; Taylor et al. 2000; Taylor & Linforth 2003). The APCI source was mounted on a Platform II mass spectrometer (Waters, Manchester, UK). The sampling system of the APCI-MS continuously draws air from the sampling point with a stream, set up at 25 ml min−1, conducted through a heated (160 °C) transfer line via a deactivated fused silica tube (1 m×0.53 mm ID). The analytes entering the source were ionized by a 4 kV positive ion corona discharge (resulting in a cascade of charge transfer), typically resulting in protonation to form MH+. The ions then entered the high vacuum region of the mass spectrometer where the ion profile is detected.
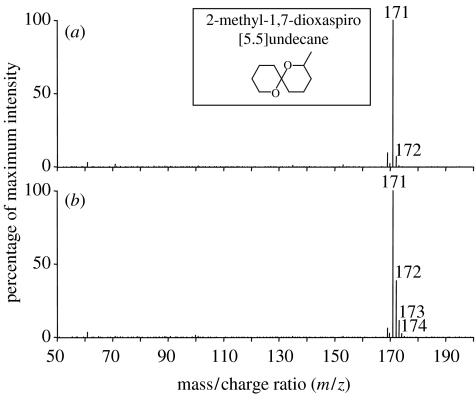
When 10 adult G. legneri were individually squashed with forceps adjacent to the sampling point of the APCI-MS, one major ion was observed in the ion profile at mass/charge ratio (m/z) 171 (figure 2a). Since APCI-MS is a soft ionization technique which typically results in the protonation of analytes (formation of MH+) with little fragmentation, the volatile compound was thought to have an original molecular weight of 170 Da. Further analysis of the gas phase above squashed wasps by gas chromatography combined with mass spectrometry showed one major peak on the chromatogram (all other peaks were less than 20% the height of this peak) matching the spectrum of 2-methyl-1,7-dioxaspiro[5.5]undecane (molecular weight 170 Da) (see below). For full-scan analysis, the mass spectrometer acquired 1 scan s−1 over mass range 25–260 Da, with the cone voltage set to 18 V. For selected-ion analysis, we monitored m/z 171, 172 and 173 with a dwell time of 0.02 s and cone voltage of 18 V. Calibration of the APCI-MS was achieved by comparing the height of the signal for tetrahydropyran (Aldrich, Gillingham, UK) with that of 2-methyl-1,7-dioxaspiro[5.5]undecane (which is commercially unavailable). Tetrahydropyran was chosen because it is structurally similar to the individual ring structures that make up the spiroacetal. In addition, it was found to give a similar signal intensity to the related bicyclic compound 1,7-dioxaspiro[5.5]undecane (figure 2a) when present at the same molarity in the gas phase.
Figure 2.
APCI-MS spectra produced from full-scan analysis of the gas phase around adult female G. legneri. (a) Undeuterated wasp (b) Wasp reared on host injected with deuterated saline showing enhanced and additional peaks at m/z 172–174, corresponding to 2-methyl-1,7-dioxaspiro[5.5]undecane molecules (see inset for structure) containing 1–3 deuterium atoms. The small peak at m/z 172 in (a) is likely due to naturally occurring C13.
(b) Chemical identification by GC-MS
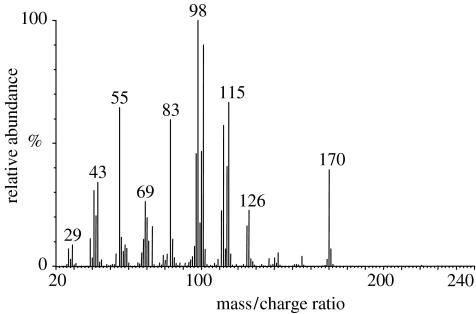
To identify the chemical released by G. legneri, 10 females were squashed and placed in a 20 ml flask sealed with a PTFE lined septum. A SPME fibre (50/30 μm, assembly Divinylbenzene/Carboxen/Polydimethylsiloxane; Supelco, Bellefonte, USA) was exposed in the flask headspace for 5 min at 22 °C. The volatile compounds on the fibre were subsequently desorbed in the injector at 240 °C for 1 min. After transfer of the volatile compounds onto the column (30 m×0.25 mm ID, BP-5, 1.0 μM film thickness; SGE, Milton Keynes, UK), the gas chromatograph (Trace GC Ultra, Thermo, Austin, USA) temperature programme started (carrier gas helium, constant flow 1.5 ml min−1). An initial temperature of 50 °C was held for 2 min and then increased at 10 °C min−1 to 230 °C. Spectra were recorded using a DSQ mass spectrometer (Thermo), scanning from m/z 20 to 250 at 2 scans s−1. The main peak observed on the GC chromatogram was at 12.22 min. The mass spectrum of this main peak (figure 3) was consistent with that of 2-methyl-1,7-dioxaspiro[5.5]undecane (Tengö et al. 1982) and the same retention time and mass spectrum were observed for an authentic standard of the compound (W. Francke 2005, personal communication).
Figure 3.
GC-MS spectrum of the main peak observed in the analysis of G. legneri headspace. The spectrum is consistent with that of 2-methyl-1,7-dioxaspiro[5.5]undecane.
(c) Chemical marking of emissions
Goniozus legneri were reared on 30–40 mg larvae of the factitious host Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) at 27±2 °C, 70% R.H. and a 9L : 15D photoperiod. Host larvae were placed individually in glass vials with an adult female wasp. After paralysis by the wasp, host larvae were removed from the tubes and injected, using a fine hypodermic needle inserted through the dorsal integument just posterior to the head, with 5 μl of phosphate buffered saline (PBS) (solution 10×DNase RNase and protease free) in either 90% water (H2O), as a control, or 90% deuterated oxide, ‘heavy water’ (D2O). Host larvae were returned to the vial and subsequently parasitized by the wasp. The resultant offspring matured around 14 days later and the females, either ‘deuterated’ (i.e. developed on hosts injected with deuterated solution) or ‘undeuterated’ (i.e. developed on hosts injected with the control solution), were used in contest experiments. In the spectra of chemicals emitted by ‘deuterated’ females, the relative proportion of the m/z 172 ion was 40–50% of the height of m/z 171 (figure 2b); in contrast to the 8–12% observed from ‘undeuterated’ (control) females (figure 2a). Manipulation of the spiroacetal thus enables clear distinction between emissions of individual females during contests.
(d) Contest experiments: simultaneous behavioural and chemical study
We used 4–5 day old females, marked with a dot of acrylic paint (either red or yellow) on the dorsal surface of the thorax and weighed to an accuracy of 10−2 mg. ‘Owner’ females were each provided with a host larva for 24 h, which they had paralysed but not laid eggs on, whereas ‘intruder’ females had had, as adults, no previous contact with a host. Pairs of non-sibling females were selected in a way to always oppose an owner and an intruder, a red and a yellow marked female and a deuterated and an undeuterated female. Between 9 and 15 replicates were obtained for each of the possible combinations, giving a total of 51 contest replicates.
Contests were staged, following established methods (Petersen & Hardy 1996) that neither force behavioural interactions nor prevent loser retreat, in a plastic contest block made of three chambers connected by a slot and covered with clear Plexiglas (figure 4). The temperature inside the contest block was maintained at 28 °C. Owners and their hosts were placed into the central chamber and an intruder into a peripheral and initially separated chamber. After 30 min, barriers filling the slot were withdrawn sufficiently to inter-connect the three chambers. Wasp behaviour was recorded from above using a digital video camera for 30 min from when the intruder entered the central chamber. Behavioural interactions (principally chases, bites, attempted attacks with stinger, attacks and fights) and possession of the paralysed host were noted. Possession was defined (following Petersen & Hardy 1996) as being in close association with a host, remaining on or next to it, and inspecting it regularly).
Figure 4.
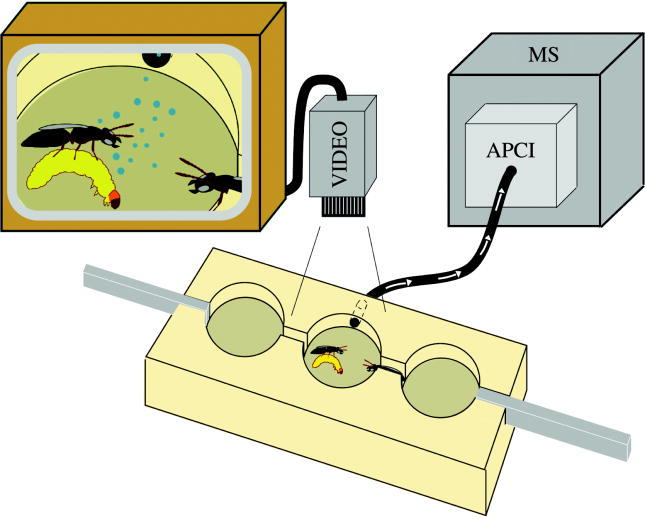
Schematic diagram of the experimental set-up showing the contest block connected to the APCI-MS. The APCI-MS continually draws air (represented by the arrows) from the central chamber and evaluates gas phase composition. A video camera above the central chamber records behavioural interactions between the competing females. A close-up of female–female interaction is shown on the screen; the bubbles represent chemical release.
Spiroacetal releases were continuously monitored by the APCI-MS in selected ion mode, connected to the contest arena as shown in figure 4. The intake of the sampling line was inserted into a recess in the contest block, drawing air out via a 3.5 mm diameter hole in the chamber wall. The exact starting time of experiments was recorded on both the videotape and the ion trace to reveal correspondence between behavioural events and chemical release (figure 5a). Temporal resolution is dependent on the sampling flow of the APCI-MS (25 ml min−1) and the volume of the arena chamber (4.7 cm3): the system was calibrated by injecting 300 μl gas phase samples containing 1 mg m−3 1,7-dioxaspiro[5.5]undecane into the arena through a small hole in a Plexiglas cover. The mean delay to detection by the MS ranged from 1 to 2.1 s, depending on whether the injection was delivered close to the APCI-MS intake or at the far side of the arena (n=3 for each delivery point). During experiments, the start of signal detection was used to indicate chemical release and was matched to behavioural events, allowing for the small temporal offset. Calibration further showed that the time for the APCI-MS to reach maximum signal intensity was 2.2–3.6 s, while the peak width at half-peak height was from 4.6 to 9.6 s. This short time is consistent with a close correlation between volatile concentration in the arena and signal intensity. Within the operating range of the APCI-MS, the height of the signal is proportional to analyte concentration (Taylor et al. 2000) and this was used to determine the absolute intensity of the signal.
Figure 5.
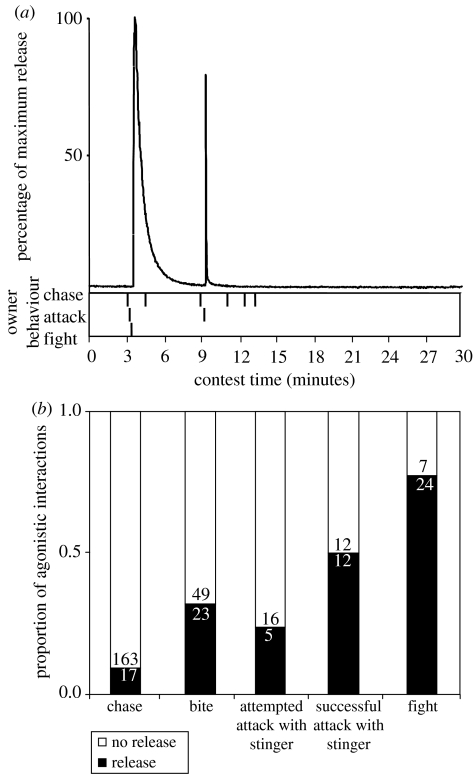
Associations between chemical release and behaviour. (a) Typical ion trace during a contest. The two peaks show that the intruder released twice in response to owner aggression (deuterium marking allowed identification of which contestant released). Some chases were not accompanied by release. The intruder did not initiate aggressive behaviour in this case (see video of the electronic supplementary material). (b) Frequencies of agonistic interactions, in order of apparently increasing escalation, with and without associated spiroacetal release. Agonistic interactions and releases are summed across intruders and owners and across 51 replicates.
After the contest, each female was individually harassed using a paintbrush to check whether they were able to release volatile chemicals: all (n=102) released the spiroacetal and the spectra of deuterated and undeuterated females were clearly distinguishable.
(e) Statistical analysis
Generalized linear modelling (in GenStat v. 7.2.0.208) was used to obtain parsimonious statistical descriptions via stepwise backward analyses. Logistic analyses were used for (proportional) contest data (Crawley 1993; Petersen & Hardy 1996; Hardy & Field 1998); these both evaluate the significance of relationships and estimate the (continuously varying) probabilities of given outcomes occurring (figure 6).
Figure 6.
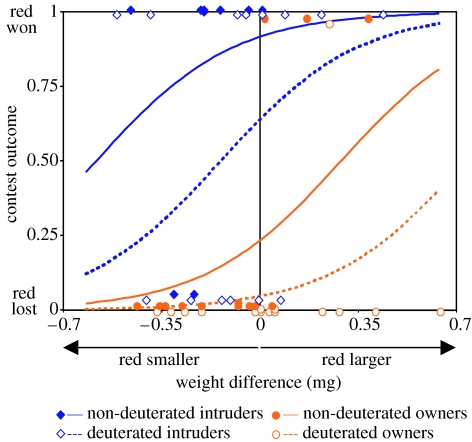
Determinants of contest outcome. The probability of the red-marked female winning, shown by the regression lines, was significantly enhanced by (i) being larger relative to the yellow-marked female (G1=6.13, P=0.013), (ii) being an intruder (G1=21.22, P<0.001) and (iii) being undeuterated (G1=5.13, P=0.024), with ownership accounting for more of the deviance (31%) than either weight (9%) or deuteration (7.6%). There were no significant interactions between these main effects. Data points are vertically displaced from their binary positions to show numbers of observations.
The discrete binary response (1=won, 0=lost) was defined by red female success (Petersen & Hardy 1996) after checking that outcomes were not influenced by mark colour (red wasps won 19/51 contests; binomial test, P=0.092). Analysis of count data (number of aggressive interactions) employed log-linear models with the dispersion parameter estimated empirically to take overdispersion into account, and significance assessed using F-ratio tests (Crawley 1993). Non-parametric analyses were used when error variances did not conform to parametric assumptions.
3. Results
(a) Chemical emissions
The mass spectrum of the volatile emitted by G. legneri (figure 3) matched that of 2-methyl-1,7-dioxaspiro[5.5]undecane (molecular weight 170 Da). The volatile was not detected in the atmosphere of the living environment of the wasps but could be released from both males and females when they were harassed with the hairs of a fine paintbrush. This showed that the release of the chemical was a discrete event, under the active control of the wasps. Independent crushing of heads, thoraxes and abdomens of 10 freshly dissected females under the APCI-MS sampling point showed that the spiroacetal was released from the head, but not from the thorax or abdomen.
(b) Contest outcomes
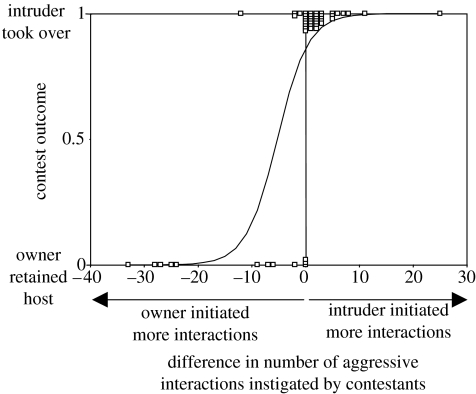
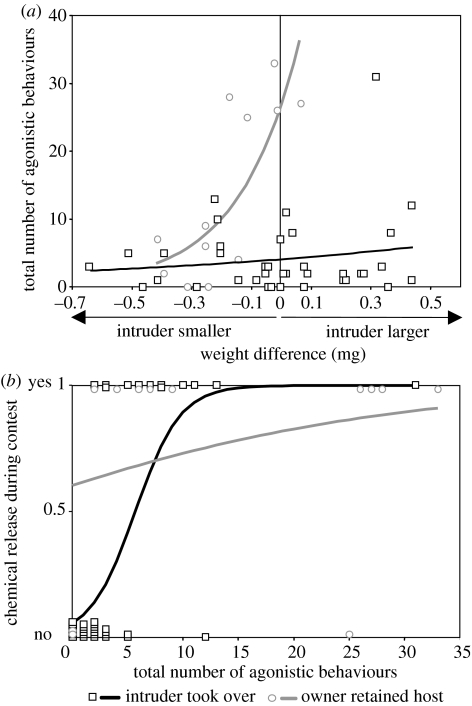
Contest outcomes were influenced by competitor asymmetries in weight, prior ownership and deuterium treatment (figure 6). Larger body weight was advantageous while prior ownership of the host reduced female contest success (figure 6). Deuterated females were disadvantaged compared to similar sized undeuterated females but the effect of deuterium was smaller than that of ownership. Contests were generally won by the female that instigated the most agonistic interactions (G1=26.28, P<0.001, figure 7). When intruders took over the host, there was no relationship between intruder-owner weight asymmetry and the number of agonistic interactions but when prior owners successfully defended their hosts, aggressive behaviour was more common when contestants were of similar weight (figure 8a).
Figure 7.
Contest outcome in relation to the difference in number of aggressive interactions (chases, bites, attacks with stinger, attempted attacks and fights) instigated by competing females (number instigated by intruder minus number instigated by owner). The fitted curve shows the probability of intruder take-over as estimated by logistic regression. Data points are vertically displaced from their binary positions to show numbers of observations.
Figure 8.
Contest outcome, agonistic behaviour and chemical release. (a) Number of agonistic behaviours during each contest in relation to owner–intruder weight difference and winner identity. Level of aggression was not influenced by size asymmetry when intruders took possession of the host (log-linear analysis, F1,37=1.29, P=0.26) but was strongly related to weight asymmetry when owners retained the host (F1,10=11.44, P=0.007). (b) Probability of spiroacetal release in relation to frequency of agonistic behaviour and winner identity. Release was more likely when agonistic interactions were more common (logistic analysis, G1=17.23, P<0.001). Chemical release was further influenced by the winner's identity (G1=7.76, P=0.005) and its interaction with the number of agonistic interactions (G1=7.79, P=0.005): release was generally common when prior owners retained their hosts and more strongly influenced by the number of agonistic interactions when intruders took over. Data points are vertically displaced from their binary positions to show numbers of observations.
(c) Relationships between chemical release and behaviour
The spiroacetal was released during 21% (40/189) of aggressive encounters. While release was associated with all classes of agonistic behaviour, its probability increased with the aggressiveness of the interaction (figure 5b): release was seldom associated with chasing (repeated measures ANOVA: F1,50=22.09, P<0.0001) or biting (F1,50=4.40, P=0.04), and the numbers of attempted and successful attacks with and without release were similar (F1,50=2.92, P=0.09 and F1,50=0.00, P=1.00, respectively). In contrast, release usually co-occurred with fights (F1,50=9.66, P=0.003), in which females violently grapple for 0.40–8.68 s until one breaks away and retreats (figure 1, video of the electronic supplementary material). Overall, the probability of release was higher when agonistic behavioural interactions were more common and further depended on whether the prior owner or the intruder eventually won the contest (figure 8b).
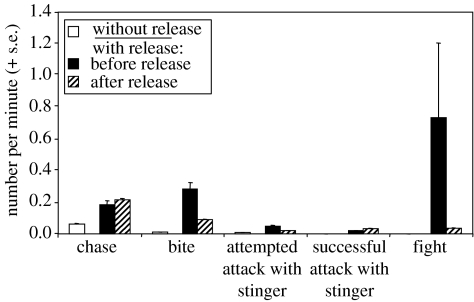
Without exception, the spiroacetal was released by females when losing an individual agonistic encounter, and in 93.6% (44/47) of instances of release the female that emitted was also the ultimate loser of the contest overall. In two contests both females emitted spiroacetal, with the last to emit being the ultimate loser. Within individual contest replicates, the occurrence of biting and fighting were reduced following spiroacetal release (Wilcoxon signed-ranks test: T=42, n=19, P=0.03 and T=11, n=14, P=0.007, respectively) whereas occurrences remained similar for other agnostic behaviours (Chase, T=104, n=21, P=0.70; Attempted attack, T=12.5, n=8, P=0.46; Attack, T=30.5, n=11, P=0.83; figure 9).
Figure 9.
Effect of spiroacetal release on subsequent behaviour. Rates of agonistic behavioural events are shown during replicates with and without chemical release. Successful attacks and fights were never observed in replicates without release. For replicates with release, rates are shown both before and after the first release.
(d) Timing and intensity of chemical release
Chemical releases during contests typically had short half-peak widths (mean±s.d., 40±25 s) and short times to reach peak intensity (12±7 s). Given the sampling flow rate and chamber volume (see §2), clearance of spiroacetal was relatively rapid, supporting the assertion that emissions were single, short-duration events. The gas phase concentration of the spiroacetal released during contests (range 0.2–18.2 mg m−3) was generally lower than during experimental stressing with a paintbrush (mean±s.e.m., 78.8±13.3 mg m−3) or squashing (mean±s.e.m., 98.3±31.2 mg m−3): such comparisons are, however, likely to be compromised because females emitting during contests were usually retreating from the arena and thus from the vicinity of the APCI-MS intake.
4. Discussion
Contests occur commonly in numerous and diverse animal taxa (Maynard Smith 1982; Mesterton-Gibbons & Adams 1998; Riechert 1998; Briffa & Elwood 2002; Maynard Smith & Harper 2003; Kokko et al. 2006). The advantage associated with large body size in G. legneri accords with observations on a congener, G. nephantidis (Petersen & Hardy 1996), and the animal contest literature in general (Riechert 1998; Kokko et al. 2006). The lower success of prior owners is an unusual observation but is corroborated by independently gathered data on this species (T. Hull 2005, personal communication) and such apparently paradoxical outcomes can be predicted by game theory under restrictive conditions (Mesterton-Gibbons & Adams 1998; Field & Hardy 2000; Kokko et al. 2006). We are currently investigating the possibility that this result may be accounted for by asymmetries in the value that owners and intruders place on winning the resource (e.g. Humphries et al. in press).
Our experiments further reveal that G. legneri produce and emit a spiroacetal, 2-methyl-1,7-dioxaspiro[5.5]undecane, a compound that it is reported in mandibular or cephalic secretions of several bee species and in the abdominal glands of the beetle Agapanthia villosoviridescens (Tengö et al. 1982; Francke & Kitching 2001). In insects, spiroacetals appear to function variously as sex, aggregation or repellent ‘spacer’ pheromones and as components of defensive secretions (Francke & Kitching 2001). In G. legneri, spiroacetal release is clearly associated with agonistic intra-specific interactions, particularly fully escalated fighting. In addition, release by artificially stressed males (which are not known to fight with conspecifics) and by females suggests that release may also deter predators or allospecific competitors. Spiroacetal release during female–female contests could reduce subsequent aggression by functioning as a signal (Maynard Smith & Harper 2003) of submission. The resolution of contests without emission, however, indicates that any signalling function is not always necessary. More likely is that the spiroacetal functions as a weapon. Several minutes of exposure to a high concentration (ca 240 mg m−3) of 2-methyl-1,7-dioxaspiro[5.5]undecane is fatal to some insects (Dettner et al. 1992). The concentrations detected during our experiments using continually flushed chambers were approximately one-tenth of those evaluated by survival-time experiments (Dettner et al. 1992). Nevertheless, concentrations may be much higher when emission occurs within small cavities and tunnels excavated by the host. We thus suggest that the spiroacetal functions as a weapon used by losers during tactical withdrawals from behaviourally intense agonistic interactions. We also suggest that this spiroacetal release has the effect of temporarily and partially incapacitating the winner that remains within the confines of the host's tunnel.
Although numerous studies have focused on animal contests and communication (Maynard Smith 1982; Riechert 1998; Maynard Smith & Harper 2003), technical constraints have meant that very few prior studies have been able to evaluate the temporal dynamics of chemical release associated with contests (Breithaupt & Eger 2002; Monnin et al. 2002). Without reliable correspondence between observable behaviour and chemical exudation, such as exists when some ants compete (Monnin et al. 2002), it may be possible to use a manipulative technique, such as injecting fluorescein dye into crayfish Astacus leptodactylus to make visible subsequent emissions of urinary signals during aquatic contests (Breithaupt & Eger 2002), or the chemical marking technique we develop here. Although, in G. legneri, deuteration is disadvantageous to contestant females, the effect of deuterium was smaller than that of ownership, indicating that experimental manipulation of wasp chemical composition need not obscure relationships of behavioural interest.
For small, terrestrial species, the APCI-MS technique can continuously sample the air around intact and freely moving animals and has great potential to be widely applicable. In addition to the timing of release, this technique can detect the chemical composition of emissions, quantifying simultaneously a number of different compounds, whether released individually or together. APCI-MS is much less behaviourally intrusive and less technically demanding than electroantennography (Wyatt 2003) yet analytical results are immediately available, unlike GC-MS (Wyatt 2003; Gómez et al. 2005). We envision that APCI-MS will be employed to facilitate the study of many other types of chemically related behaviour, such as mating interactions (Wyatt 2003), insect attraction to induced plant volatiles (Turlings et al. 2004) and, when coupled with chemical manipulation, mark–recapture studies (Steffan et al. 2001) in which standard techniques are invasive, time consuming, technically challenging or more expensive (Steffan et al. 2001; Turlings et al. 2004). Using APCI-MS, the current study has discovered a hitherto unknown component of parasitoid contest interactions: a volatile chemical that is emitted upon losing.
Acknowledgments
We thank G. Channell, D. Clarke, A. Damon, S. Dourlot, L. Evans, W. Francke, R. Howard, T. Hull, L. Jublot, J. Marquez and R. Romani for help. This research was funded by a Biotechnology and Biological Sciences Research Council (UK) grant (BB/C504778/1) to I.C.W.H., A.J.T. and R.S.T.L.
Supplementary Material
A 17 second clip of typical contest behaviour (1.18MB, Windows Media Audio/Video file). The yellow-marked owner attacks and repels a red-marked intruder and the time of chemical release is indicated. The associated ion trace is shown in Fig. 5A.
References
- Breithaupt T, Eger P. Urine makes the difference: chemical communication in fighting crayfish made visible. J. Exp. Biol. 2002;205:1221–1231. doi: 10.1242/jeb.205.9.1221. [DOI] [PubMed] [Google Scholar]
- Briffa M, Elwood R.W. Power of shell rapping signals influences physiological cost and subsequent decisions during hermit crab fights. Proc. R. Soc. B. 2002;269:2332–2336. doi: 10.1098/rspb.2002.2158. doi:10.1098/rspb.2002.2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley M.J. Blackwell Scientific Publications; Oxford, UK: 1993. GLIM for ecologists. [Google Scholar]
- Dettner K, Fettköther R, Ansteeg O, Deml R, Liepert C, Petersen B, Haslinger E, Francke W. Insecticidal fumigants from defensive glands of insects—a fumigant test with adults of Drosophila melanogaster. J. Appl. Entomol. 1992;113:128–137. [Google Scholar]
- Field S.A, Hardy I.C.W. Butterfly contests: contradictory but not paradoxical. Anim. Behav. 2000;59:F1–F3. doi: 10.1006/anbe.1999.1305. doi:10.1006/anbe.1999.1305 [DOI] [PubMed] [Google Scholar]
- Francke W, Kitching W. Spiroacetals in insects. Curr. Org. Chem. 2001;5:233–251. doi:10.2174/1385272013375652 [Google Scholar]
- Gómez J, Barrera J.F, Rojas J.C, Macias-Samano J, Liedo J.P, Cruz-Lopez L, Badii M.H. Volatile compounds released by disturbed females of Cephalonomia stephanoderis (Hymenoptera: Bethylidae): a parasitoid of the coffee berry borer Hypothenemus hampei (Coleoptera: Scolytidae) Fla. Entomol. 2005;88:180–187. [Google Scholar]
- Gosling L.M, Roberts S.C. Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Stud. Behav. 2001;30:169–217. doi:10.1016/S0065-3454(01)80007-3 [Google Scholar]
- Hardy I.C.W, Field S.A. Logistic analysis of animal contests. Anim. Behav. 1998;56:787–792. doi: 10.1006/anbe.1998.0833. doi:10.1006/anbe.1998.0833 [DOI] [PubMed] [Google Scholar]
- Humphries, E. L., Hebblethwaite, A. J., Batchelor, T. P. & Hardy, I. C. W. In press. The importance of valuing resources: host weight and contender age as determinants of parasitoid wasp contest outcomes. Anim. Behav.
- Hurst J.L, Payne C.E, Nevison C.M, Marie A.D, Humphries R.E, Robertson D.H.L, Cavaggioni A, Beynon R.J. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. doi:10.1038/414631a [DOI] [PubMed] [Google Scholar]
- Kokko H, López-Sepulcre A, Morrell L.J. From hawks and doves to self-consistent games of territorial behaviour. Am. Nat. 2006;167:901–912. doi: 10.1086/504604. doi:10.1086/504604 [DOI] [PubMed] [Google Scholar]
- Linforth, R. S. T. & Taylor, A. J. 1997 Apparatus and methods for the analysis of trace constituents of gases. EU Patent 97305409.1.
- Maynard Smith J. Cambridge University Press; Cambridge, MA: 1982. Evolution and the theory of games. [Google Scholar]
- Maynard Smith J, Harper D. Oxford University Press; Oxford, UK: 2003. Animal signals. [Google Scholar]
- Mesterton-Gibbons M, Adams E.S. Animal contests as evolutionary games. Am. Sci. 1998;86:334–341. doi:10.1511/1998.4.334 [Google Scholar]
- Monnin T, Ratnieks F.L.W, Jones G.R, Beard R. Pretender punishment induced by chemical signalling in a queenless ant. Nature. 2002;417:61–65. doi: 10.1038/nature00932. doi:10.1038/nature00932 [DOI] [PubMed] [Google Scholar]
- Nevison C.M, Armstrong S, Beynon R.J, Humphries R.E, Hurst J.L. The ownership signature in mouse scent marks is involatile. Proc. R. Soc. B. 2003;270:1957–1963. doi: 10.1098/rspb.2003.2452. doi:10.1098/rspb.2003.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R.J.H. Gradually escalating fights and displays: the cumulative assessment model. Anim. Behav. 1998;56:651–662. doi: 10.1006/anbe.1998.0835. doi:10.1006/anbe.1998.0835 [DOI] [PubMed] [Google Scholar]
- Petersen G, Hardy I.C.W. The Importance of being larger: parasitoid intruder-owner contests and their implications for clutch size. Anim. Behav. 1996;51:1363–1373. doi:10.1006/anbe.1996.0139 [Google Scholar]
- Riechert S.E. Game theory and animal contests. In: Dugatkin L.A, Reeve H.K, editors. Game theory and animal behavior. Oxford University Press; Oxford, UK: 1998. pp. 64–93. [Google Scholar]
- Steffan S.A, Daane K.M, Mahr D.L. 15N-enrichment of plant tissue to mark phytophagous insects, associated parasitoids, and flower-visiting entomophaga. Entomol. Exp. Appl. 2001;98:173–180. doi:10.1023/A:1018718800713 [Google Scholar]
- Taylor A.J, Linforth R.S.T. Direct mass spectrometry of complex volatile and non-volatile flavour mixtures. Int. J. Mass. Spectrom. 2003;223–224:179–191. [Google Scholar]
- Taylor A.J, Linforth R.S.T, Harvey B.A, Blake B. Atmospheric pressure chemical ionisation mass spectrometry for in vivo analysis of volatile flavour release. Food Chem. 2000;71:327–338. doi:10.1016/S0308-8146(00)00182-5 [Google Scholar]
- Tengö J, Bergström G, Borgkarlson A.K, Groth I, Francke W. Volatile compounds from cephalic secretions of females in two cleptoparasite bee genera, Epeolus (Hym., Anthophoridae) and Coelioxys (Hym., Megachilidae) Z. Naturforsch. 1982;37:376–380. [Google Scholar]
- Turlings T.C.J, Davison A.C, Tamò C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2004;29:45–55. doi:10.1111/j.1365-3032.2004.0362.x [Google Scholar]
- Wyatt T.D. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour: communication by smell and taste. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A 17 second clip of typical contest behaviour (1.18MB, Windows Media Audio/Video file). The yellow-marked owner attacks and repels a red-marked intruder and the time of chemical release is indicated. The associated ion trace is shown in Fig. 5A.