Abstract
Microspheres are potential candidates for the protein drug delivery. In this work, we prepared polymer-coated starch/bovine serum albumin (BSA) microspheres using co-axial electrohydrodynamic atomization (CEHDA). First, starch solution in dimethyl sulphoxide (DMSO) was prepared and then an aqueous solution of BSA was added to it to make a starch–BSA solution. Subsequently, this solution was made to flow through the inner capillary, while the polymer, polydimethylsiloxane (PDMS), flowed through the outer capillary. On collection, filtration and subsequent drying, near-monodisperse microspheres of 5–6 μm in size were obtained. The microspheres were characterized by Fourier-transform infrared (FT-IR) spectroscopy and scanning electron microscopy. Cumulative BSA release was investigated by UV spectroscopy. BSA structure and activity was preserved in the microspheres and its release in 0.01 M phosphate buffered saline (PBS) was studied over a period of 8 days. There was an initial burst with 32 wt% of total BSA released in 2 h. Overall 75 wt% of BSA was released over a 7 day period.
Keywords: starch, co-axial electrohydrodynamic atomization, microspheres, protein release
1. Introduction
Microparticulate controlled release systems prepared from degradable polymers have been widely investigated for the delivery of drugs over the past two decades (Jeffery et al. 1993; Yan et al. 1994; Chen et al. 1997). The emphasize now is to exploit these systems for peptide (Ruiz et al. 1989) or protein (Boury et al. 1997; Crotts & Park 1997; Slager & Domb 2002) delivery. The oral and localized delivery of peptides and proteins is a major challenge. The two major barriers in oral delivery are enzymatic degradation and permeation across the gastrointestinal epithelial (Ameye et al. 2000). The delivery of protein drugs in a controlled manner can be utilized in many applications, including hormone treatment, immuno- and tumour-treatment, cardiovascular and thromobolytic treatment, and immunization (Lee 1995).
Among the different encapsulation techniques, the multiple emulsion method is the most used so far with poly(lactic-glycolic) acid (PLGA) the most studied polymer for controlled release (Ogawa et al. 1988). In this method, initially a primary water-in-oil (w/o) emulsion is prepared to permit the dispersion of the protein in the wall material containing organic phase. A second step requires the formation of a (water-in-oil)-in-water (w/o/w) emulsion in an aqueous phase containing a surface-active agent. Finally, the organic solvent is extracted, leading to the formation of solid microspheres. The exposure of a protein to various factors unfavourable for stability such as organic solvents and polymer degradation may promote deactivation during this process. For example, the microencapsulation of the enzyme, carbonic anhydrase, within PLGA microspheres causes severe non-covalent aggregation upon exposure to the water/oil interface (Sah 1999; de Weert et al. 2000; Li et al. 2000), while the encapsulated enzyme was severely hydrolysed within fast degrading PLGA due to an acidic microenvironment generated from polymer degradation (Crotts & Park 1997).
Starch is a naturally occurring biopolymer. Native starch consists of two polymers of glucose, i.e. linear amylase and branched amylopectin (Young 1984). Starch microspheres are suitable carriers for protein delivery systems due to their biocompatibility and biodegradability and they get easily metabolized and eliminated from the body. The starch matrix also helps to preserve the protein activity (Sievert & Pomeranz 1989). Modified starch has been intensively used for pharmaceutical applications (Wierik et al. 1996; Lenaerts et al. 1998; Chebli & Cartier 2000; Tuovinen et al. 2003; Pohja et al. 2004). Starch microspheres have been also frequently used as a formulation material for nasal administration (Holmberg et al. 1994; Heritage et al. 1996).
Electrohydrodynamic atomization (EHDA) is a process, which can generate near-monodisperse droplets whose size can be varied between a few to hundreds of micrometers (Ganan-Calvo et al. 1997; Jayasinghe et al. 2004). A liquid is injected through a capillary tube and a potential difference of several kilovolts is applied between the capillary and the ground electrode. This causes the liquid meniscus at the capillary exit to develop a conical shape, commonly referred to as a Taylor cone, although there are many manifestations of it. A thin jet with a high charge density emanates from the cone apex and subsequently breaks down into tiny droplets, independent of the diameter of the capillary tube (Clopeau & Prunet-Foch 1994). Its most well-known application has been in mass spectroscopy, where EHDA has been successfully exploited to produce multiply charged gas phase ions of large biomolecules in a liquid phase (Fenn et al. 1989). Co-axial EHDA (CEHDA) is a variant of conventional EHDA. In this process, two concentric capillaries are used, and two different immiscible liquids can be pushed through these two capillaries (Loscertales et al. 2002).
In this work, we demonstrate for the first time the capabilities of the CEHDA technique for the preparation of polymer-coated microspheres containing a protein, and the subsequent controlled release of the protein. Starch/bovine serum albumin (BSA) microspheres coated with polydimethylsiloxane (PDMS) were prepared using CEHDA for controlled release of BSA. In our previous work (Pareta et al. 2005) we have shown that the EHDA does not have any significant effects on the activity of BSA, which is used as a model protein to demonstrate our method. This process has several advantages; it is a simple, single-step preparation process of near-monodisperse microspheres of a few micrometers in size, without exposure of the protein to harmful organic solvents, like dichloromethane, which causes denaturation.
2. Materials and methods
2.1 Materials
Resistant starch (donated by National Starch, UK) was used for this investigation. Resistant starch is not easily hydrolysed by enzymes in the human gastrointestinal system and passes to the large bowel (Sievert & Pomeranz 1989). BSA (lyophilized powder, Sigma-Aldrich, Poole, UK) was chosen as a model protein. One gram of starch was dissolved in 5 ml of dimethyl sulphoxide (DMSO; ≥99.0 purity, supplied by Sigma-Aldrich, Poole, UK) at 140 °C for 40 min under constant magnetic stirring in an oil bath. The starch solution was left to cool to room temperature. BSA solution was prepared separately by adding 0.1 g BSA in 5 ml of de-ionized water. One millilitre of BSA solution was added to 4 ml of starch solution at room temperature to prepare starch/BSA solution for CEHDA. PDMS (supplied by Univar Ltd., Greenwich, UK) with a molecular weight of 1000 was used to coat the microspheres.
2.2 Surface tension
Surface tensions of the starch–BSA solution and PDMS were measured using a Kruss tensiometer K9 (standard Wilhelmy's plate method). Also the interfacial surface tension between the starch–BSA solution and PDMS was measured using the Kruss tensiometer, the Wilhelmy's plate method was again used and the plate was lowered to the interface and measurements were taken according to the Kruss tensiometer instructions.
2.3 Co-axial electrohydrodynamic atomization
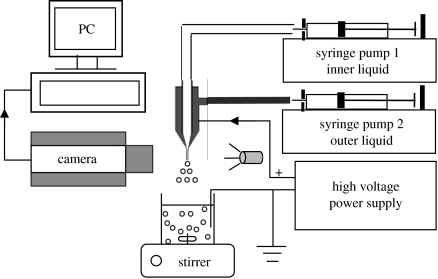
The experimental set-up used to perform CEHDA is shown in figure 1. The inner and outer liquids were uniformly and continuously pushed by an infusion pump (Harvard Apparatus PHD 4400) through a 10 ml plastic syringe to stainless steel capillaries. The outer capillary had outer and inner diameters of 1.25 and 0.85 mm, respectively. The inner capillary had outer and inner diameters of 0.81 and 0.51 mm, respectively. Silicone tubing of appropriate size was used to connect the capillaries and the syringes. A LEICA S6D colour camera was used to observe the jet modes and capture images. A high voltage d.c. power supply (Glassman Europe) was connected with the capillaries as shown in figure 1 in order that an electric field can be applied between the capillaries and the grounded collection point.
Figure 1.
Schematic of equipment set-up used for the co-axial electrohydrodynamic atomization experiments.
2.4 Preparation of polymer-coated microspheres
The starch and BSA solution was pumped in the inner capillary at the flow rate of 7.5×10−10 m3 s−1. PDMS was pumped in the outer capillary at 1×10−10 m3 s−1. A potential difference of 5.5 kV was applied between the capillaries and the grounded collection container, which contained PDMS in acetone. These flow rates and voltage were chosen as they resulted in the stable cone-jet mode, which is well known to generate a narrow droplet size-distribution. However, a range of experiments was also performed at different combinations of voltages and flow rates to demonstrate that the microsphere size-distribution can be varied by changing these process control parameters. The microspheres were collected in acetone containing a small amount of PDMS (0.5 ml PDMS in 500 ml of acetone). The acetone was vigorously stirred by using a magnetic stirrer. The distance between the tip of the capillary and the collection beaker was 15 mm. The acetone solution containing microspheres was filtered under vacuum using a Whatman filter paper with a retention size of 3 μm (Sigma Aldrich, Poole, UK). The microspheres were washed with an excess of acetone to completely remove DMSO. After filtration, the particles were dried at ambient temperature for 48 h before performing BSA release studies.
2.5 Determination of droplet size distribution
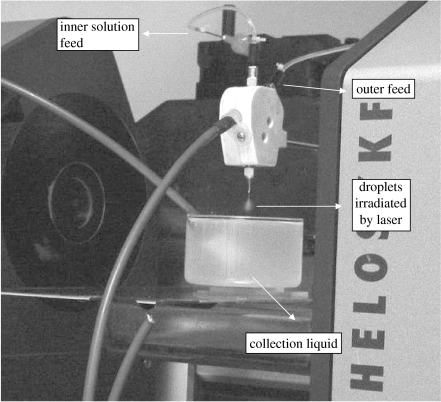
Droplets formed by CEHDA were measured using a computer controlled Sympatec Helos (helium laser optical spectrometer) Model Vario KF sizing system (Sympatec Ltd, System-Partikel-Technik, Bury, UK). The laser system was incorporated in the CEHDA set-up with the same conditions for the preparation of the microspheres (figure 2). The 15 mm diameter laser beam was approximately 10 mm below the capillary exit as in the case of the EHDA experiments. The lens used for this measurement has a droplet detection capability in the size range 0.5–175 μm. The droplet size distribution is estimated by fitting the data obtained to the Fraunhofer scattering model. The average droplet size produced by CEHDA was obtained from time-resolved data by calculating the volume-weighted average over 10 s.
Figure 2.
Droplet size distribution measurement during CEHDA.
2.6 Determination of microsphere size and morphology
The morphology and size of the encapsulated microspheres were determined using a Jeol JSM-6300 scanning electron microscope operating in the secondary electron mode with an accelerating voltage of 10 kV and working distance of 15 mm. The microspheres were dried under vacuum, mounted on aluminum stubs and sputter coated with gold for 120 s prior to microscopy.
2.7 Determination of BSA content in the microspheres
The BSA content was determined as described by Woo et al. (2001), with slight modification. Twenty milligram of microspheres were hydrolysed in a mixture of 0.9 ml of 1 M NaOH and 0.2 ml phosphate buffered saline (PBS) and centrifuged at 4000 r.p.m. for 30 min at room temperature. After hydrolysis, 0.9 ml of 1 M HCl was added to neutralize the sample solution. BSA concentration was determined by the well-known Bradford assay method, comparing with BSA standards.
2.8 In vitro BSA release from the microspheres
Five hundred milligrams of microspheres were weighed and placed in 4 ml of 0.01 M PBS adjusted to pH 7.4 in 5 ml polypropylene test tubes, which were incubated at 37 °C with occasional shaking. At designated time intervals, 0.1 ml of samples were collected and replaced by the same amount of fresh PBS. The samples were assayed using Bradford's reagent (Sigma-Aldrich, Poole, UK) and a U-2010 spectrometer operating at a wavelength of 595 nm with 2 nm slit width. This assay is based on the binding of the protein-specific dye, coomassie brilliant blue to proteins (Bradford 1976). All experiments were performed in triplicate.
2.9 FT-IR spectroscopy
Fourier-transform infrared (FT-IR) studies were performed in transmission mode on a Shimadzu FT-IR 8300 spectrometer. Eight scans were averaged at 4 cm−1 resolution in the range of 500–4000 cm−1. Small quantities of starch, BSA and microspheres were mixed with paraffin and placed between two NaCl transparent discs to obtain the spectra. The peaks for paraffin are distinguishable at 2920, 2850, 1458 and 1377 cm−1 and are not removed from the spectra. Protein amide I region [1700–1615 cm−1] of the microspheres spectra was carefully studied and compared with the BSA spectra (Lambert et al. 1998; Carrasquillo et al. 2001). Similarly, the FT-IR spectra of the microspheres was studied for PDMS and DMSO presence.
3. Results and discussion
BSA was chosen as the model protein in this investigation as it easily forms aggregates by thiol–disulphide interchange and thus is a good indicator of any change in its activity (Costantino et al. 1997). PDMS stays localized at the injected site and is not very susceptible to bacterial infection and thus forms good polymer for targeted drug delivery (Maeda et al. 2002; Kajihara et al. 2001). Also PDMS can be used in oral drug delivery as it can act as protective layer against the enzymatic degradation of microspheres in gastrointestinal epithelial. Starch does not dissolve in water and thus DMSO was utilized to dissolve starch as it is highly miscible with water, and thus an aqueous solution of BSA can be added to it to prepare the required starch–BSA solution. DMSO has been used to prepare microspheres (Park et al. 1998) and it has also been shown to stabilize partially folded conformations of proteins (Bhattachariya & Balaram 1997).
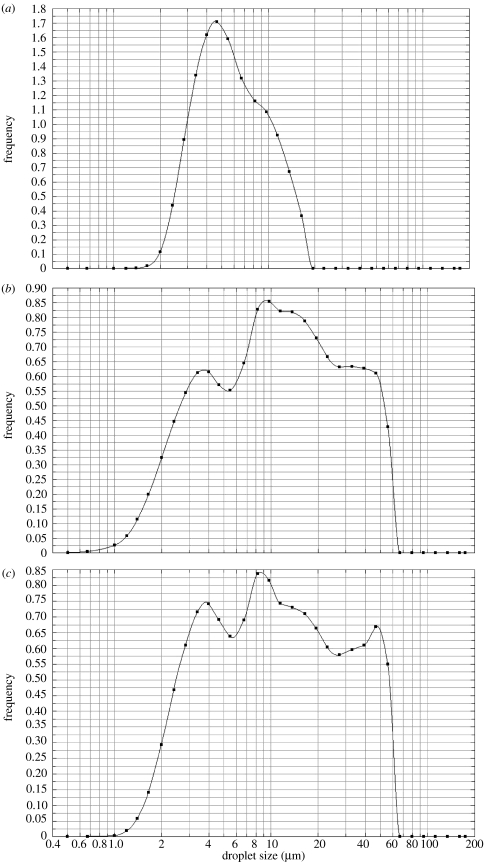
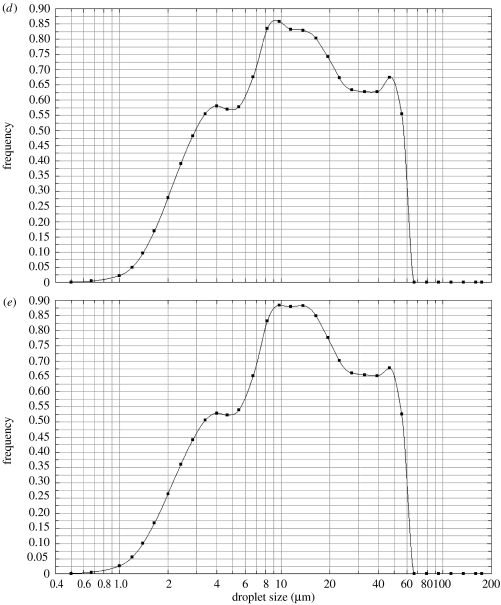
Aqueous solutions do not electrospray in stable cone-jet mode singularly and tapered capillaries have been used to assist in the generation of stable cone-jet mode in such cases (Lopez-Herrera et al. 2004). Our investigations showed that the starch–BSA solution does not generate a stable jet when subjected to EHDA using a single capillary, in contrast the co-axial method where polymer flows in the outer capillary and creates a driving interface (Lopez-Herrera et al. 2003) was successful in obtaining the stable cone-jet mode. Droplet size measurement was used as a tool to identify the process parameters at which near-monodisperse microspheres could be prepared. Parameters like the applied potential difference and flow rate have a substantial effect on the electrospraying mode and hence on droplet size distribution, which subsequently affects the microspheres. We determined the parameters, which resulted in least size distribution (figure 3a) and prepared the microspheres at these conditions. Other graphs in figure 3 show the effect of parameter variation on droplet size distribution. Figure 3b shows the effect of applied potential difference on droplet size distribution. Similarly, flow rate in the outer capillary was varied in figure 3c, inner capillary flow rate and applied potential difference were varied in figure 3d, and all three parameters were varied in figure 3e. In figure 3b–e, the EHDA mode is not stable cone-jet, secondary droplets (satellites) and the spindle mode prevails, which explains the wide size-distribution of droplets obtained in these experiments (Clopeau & Prunet-Foch 1994). The thickness of the outer layer, in our case the polymer coating, can be varied uniformly by varying the flow rate in the outer capillary, provided the EHDA mode is stable cone-jet (Lopez-Herrera et al. 2003).
Figure 3.
Droplet size distribution measured during CEHDA; (a) Qi=7.5×10−10 m3 s−1, Qo=1×10−10 m3 s−1 and V=5.5 kV, (b) Qi=7.5×10−10 m3 s−1, Qo=1×10−10 m3 s−1 and V=6.5 kV, (c) Qi=7.5×10−10 m3 s−1, Qo=0.75×10−10 m3 s−1 and V=5.5 kV, (d) Qi=9×10−10 m3 s−1, Qo=1×10−10 m3 s−1 and V=6.0 kV and (e) Qi=9×10−10 m3 s−1, Qo=1.5×10−10 m3 s−1 and V=7 kV. Q and V denote flow rate and applied potential difference, respectively. i and o indicates inner and outer, respectively.
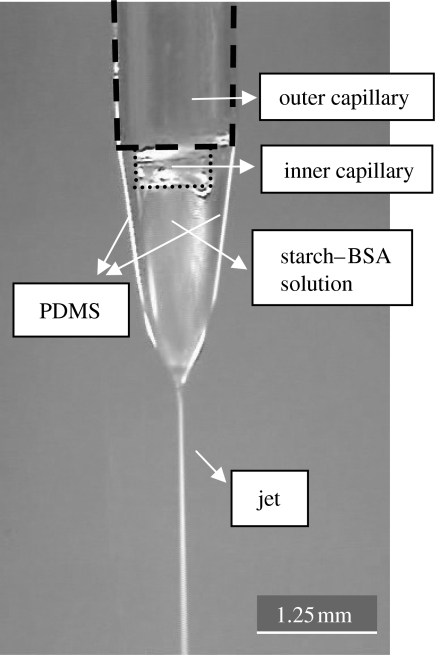
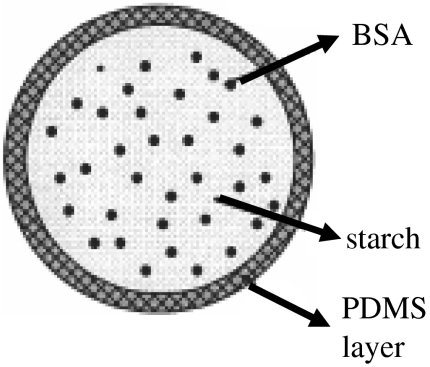
The microspheres were collected in stable cone-jet mode, as shown in figure 4. The inner capillary was kept slightly below the outer capillary as it was observed that it aids stable cone-jet mode electrospraying. The expected geometry of the microspheres is shown in figure 5, with PDMS coating the starch–BSA. The distance between the capillaries and the collecting liquid is also important, since it is necessary that the compound jet emerging from the capillaries should break into droplets before hitting the liquid surface in order to obtain uniform size microspheres, otherwise agglomerated clusters were generated. The collecting liquid was stirred to stop the microspheres from forming agglomerates. The increase in BSA concentration in the solution increases its electrical conductivity by a large extent (Pareta et al. 2005) and thus the EHDA mode changes and makes it harder to achieve cone-jet mode. Also on increasing the BSA concentration, the jet length increases and very thin starch–BSA fibres can be collected.
Figure 4.
Co-axial EHDA in cone-jet mode.
Figure 5.
Proposed geometry of microspheres.
The important question of whether the PDMS can coat and remain on the exterior of the microspheres depends on the miscibility of PDMS and the starch–BSA solution and the surface and interfacial tensions. We found that PDMS and starch–BSA solution used in this work were totally immiscible. The well-known Young–Dupré relation below helps to establish whether PDMS will form a stable coating.
| (3.1) |
where σsolution and σPDMS are the surface tensions of the starch–BSA solution and PDMS, respectively. σinterface denotes the interfacial surface tension and θ is the contact angle. For complete wetting θ=0 and therefore if σinterface<σsolution−σPDMS, PDMS forms a stable coating on the starch–BSA microspheres (Baier 1970). Table 1 shows the surface and interfacial tensions measured and from these results it is clear that the inequality explained above holds and PDMS coats the microspheres containing the protein.
Table 1.
Surface and interfacial tensions of the relevant samples.
| sample | surface tension (mN m−1) |
|---|---|
| starch–BSA solution | 36 |
| PDMS | 19 |
| starch–BSA solution–PDMS interface | 8 |
Figure 6 shows the morphology and size of the microspheres and most of the microspheres are approximately 5–6 μm in size and these results tally well with the droplet size-distribution (figure 3a). Although continuous stirring restricts agglomeration formation, it can happen while filtering and the subsequent drying of microspheres, and therefore careful handling is required. The protein encapsulation efficiency, η, was calculated as
| (3.2) |
and was found out to be 70%.
Figure 6.
Scanning electron micrographs of the PDMS-coated starch–BSA microspheres.
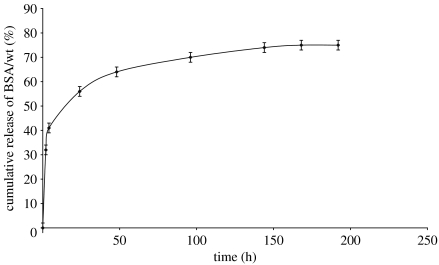
The BSA release from the microspheres was studied over 8 days and the cumulative release trend is shown in figure 7. Around 32 wt% of the BSA was released in the first 2 h. Overall 75% of the BSA was released in 7 days time period. Figure 7 shows that the BSA released plateaus at this level. The unreleased BSA is assumed to be entrapped near the centre of the microspheres and since the resistant starch does not absorb water easily to leach out BSA, this can be expected. The initial burst of BSA release probably comes from that present near the surface in the starch–BSA microspheres. If a polymer with higher molecular weight is used to coat the microspheres this initial burst can be reduced and more controlled BSA release can be obtained.
Figure 7.
BSA release from the microspheres in 0.01 M phosphate buffered saline as a function of time.
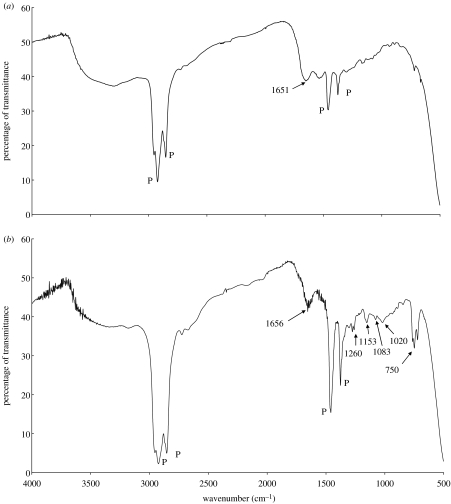
The amide I peak occurs at 1651 cm−1 for BSA, and is shifted slightly to 1656 cm−1 in the FT-IR spectra of the microspheres, as shown in figure 8a and b. This might be due to interaction between the BSA present on the surface and PDMS. The peaks at 750 and 1260 cm−1 shows Si–CH3 vibrational stretch and the peak at 1083 cm−1 shows the Si–O–Si vibrational anti-symmetric stretch, which confirms the presence of PDMS. The peak at 1020 is due to the cyclic and primary alcohol function group present in the starch chain. No peak for S=O was found which indicates the DMSO was washed away with acetone or very little was left in order to be detected. The paraffin peaks have been distinguished by marking P on the figure.
Figure 8.
FT-IR spectra of (a) BSA and (b) microspheres. P indicates paraffin peaks.
4. Conclusions
Polymer-coated starch–protein microspheres have been prepared by using a novel process, which incorporates co-axial electrohydrodynamic forming. The microspheres prepared were monodisperse and the protein, BSA, is intact and preserved during the process. About 75% of the protein was released over a period of 7 days. This single-step and relatively simple process does not expose proteins to harmful organic solvents and is a procedure, which can be exploited to prepare biodegradable microspheres for protein drug release.
Acknowledgments
R.P. wishes to acknowledge part funding from Queen Mary, University of London (QMUL). The authors wish to thank Dr Mike Watkinson (School of Biological and Chemical Sciences, QMUL) for useful discussions and Dr Hongbo Zhang (Mech. Eng. UCL) for assistance with droplet size analysis.
References
- Ameye D, Voorspoels J, Remon J.P, Demeester J, de Smedt S.C. Optimization of an in vitro procedure for the determination of the enzymatic inhibition potency of multifunctional polymers. J. Control. Release. 2000;68:413–417. doi: 10.1016/s0168-3659(00)00274-1. doi:10.1016/S0168-3659(00)00274-1 [DOI] [PubMed] [Google Scholar]
- Baier R.E. Surface properties influencing biological adhesion. In: Manly R.S, editor. Adhesion in biological systems. Academic Press; New York: 1970. pp. 15–48. [Google Scholar]
- Bhattachariya S, Balaram P. Effects of organic solvents on protein structures: observation of a structured helical core in hen egg-white lysozyme in aqueous dimethylsulfoxide. Proteins Struct. Funct. Genet. 1997;29:492–507. doi: 10.1002/(sici)1097-0134(199712)29:4<492::aid-prot9>3.0.co;2-a. doi:10.1002/(SICI)1097-0134(199712)29:4<492::AID-PROT9>3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- Boury F, Marchais H, Proust J.E, Benoit J.P. Bovine serum albumin release from poly(α-hydroxy acid) microspheres: effects of polymer molecular weight and surface properties. J. Control. Release. 1997;45:75–86. doi:10.1016/S0168-3659(96)01547-7 [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. doi:10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Carrasquillo K.G, Stanley A.M, Aponte-Carro J.C, de Jesus P, Costantino H.R, Bosques C.J, Griebenow K. Non-aqueous encapsulation of excipient-stabilized spray-freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. J. Control. Release. 2001;76:199–208. doi: 10.1016/s0168-3659(01)00430-8. doi:10.1016/S0168-3659(01)00430-8 [DOI] [PubMed] [Google Scholar]
- Chebli C, Cartier L. Effect of some physical parameters on the sustained drug-release properties of substituted amylase matrices. Int. J. Pharm. 2000;193:167–173. doi: 10.1016/s0378-5173(99)00332-4. doi:10.1016/S0378-5173(99)00332-4 [DOI] [PubMed] [Google Scholar]
- Chen L, Apte R.N, Cohen S. Characterization of PLGA microspheres for the controlled delivery of IL-1α for tumor immunotherapy. J. Control. Release. 1997;43:261–272. doi:10.1016/S0168-3659(96)01496-4 [Google Scholar]
- Clopeau M, Prunet-Foch B. Electrohydrodynamic spraying functioning modes: a critical review. J. Aerosol Sci. 1994;25:1021–1036. doi:10.1016/0021-8502(94)90199-6 [Google Scholar]
- Costantino H.R, Shieh L, Klibanov A.M, Langer R. Heterogeneity of serum albumin samples with respect to solid-state aggregation via thiol–disulfide interchange—Implications for sustained release from polymers. J. Control. Release. 1997;44:255–261. doi:10.1016/S0168-3659(96)01528-3 [Google Scholar]
- Crotts G, Park T.G. Stability and release of bovine serum albumin encapsulated within poly(D,L-lactide-co-glycolide) microparticles. J. Control. Release. 1997;44:123–134. doi:10.1016/S0168-3659(96)01511-8 [Google Scholar]
- van de Weert M, Hoechstetter J, Hennink W.E, Crommelin D.J.A. The effect of a water/organic solvent interface on the structural stability of lysozyme. J. Control. Release. 2000;68:351–359. doi: 10.1016/s0168-3659(00)00277-7. doi:10.1016/S0168-3659(00)00277-7 [DOI] [PubMed] [Google Scholar]
- Fenn J.B, Mann M, Meng C.K, Wong S.F. Electrospray ionization for mass spectroscopy of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Ganan-Calvo A.M, Davila J, Barrero A. Current and droplet size in the electrospraying of liquids. Scaling laws. J. Aerosol Sci. 1997;28:249–275. doi:10.1016/S0021-8502(96)00433-8 [Google Scholar]
- Heritage P.L, Loomes L.M, Jianxiong J, Brook M.A, Underdown B.J, Macdermott M.R. Novel polymer-grafted starch microparticles for mucosal delivery of vaccines. Immunology. 1996;88:162–168. doi: 10.1046/j.1365-2567.1996.d01-639.x. doi:10.1046/j.1365-2567.1996.d01-639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K, Bjork E, Edman P. Influence of degradable starch microspheres on the human nasal mucosa. Rhinology. 1994;32:74–77. [PubMed] [Google Scholar]
- Jayasinghe S.N, Edirisinghe M.J, Wang D.Z. Controlled deposition of nano-particle clusters by electrohydrodynamic atomization. Nanotechnology. 2004;15:1519–1523. doi:10.1088/0957-4484/15/11/025 [Google Scholar]
- Jeffery H, Davis S.S, O'Hagan D.T. The preparation and characterization of poly(lactide-co-glycolide) microparticles 2. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm. Res. 1993;10:362–368. doi: 10.1023/a:1018980020506. doi:10.1023/A:1018980020506 [DOI] [PubMed] [Google Scholar]
- Kajihara M, Sigie T, Hojo T, Maeda H, Sano A, Fujioka K, Sugawara S, Urabe Y. Development of a new drug delivery system for protein drugs using silicone (II) J. Control. Release. 2001;73:279–291. doi: 10.1016/s0168-3659(01)00302-9. doi:10.1016/S0168-3659(01)00302-9 [DOI] [PubMed] [Google Scholar]
- Lambert J.B, Shurvell H.F, Lightner D.A, Cooks R.G, editors. Organic structural spectrospcopy. Prentice Hall; Upper Saddle River, NJ: 1998. pp. 193–196. [Google Scholar]
- Lee J.J. Biopharmaceutical properties and pharmacokinetics of peptide and protein drugs. In: Taylor M.D, Amidon G.L, editors. Peptide-based drug design. Controlling transport and metabolism. ACS professional reference book. ACS; Washington, DC: 1995. pp. 69–100. [Google Scholar]
- Lenaerts V, Moussa I, Dumoulin Y, Mebsout F, Chouinard F, Szabo P, Mateescu M.A, Cartier L, Marchessault R. Cross-linked high amylose starch for controlled release of drugs: recent advances. J. Control. Release. 1998;53:225–234. doi: 10.1016/s0168-3659(97)00256-3. doi:10.1016/S0168-3659(97)00256-3 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Yan R, Jia W, Yuan M, Deng X, Huang Z. Influence of process parameters on the protein stability encapsulated in poly-DL-lactide-poly(ethylene glycol) microspheres. J. Control. Release. 2000;68:41–52. doi: 10.1016/s0168-3659(00)00235-2. doi:10.1016/S0168-3659(00)00235-2 [DOI] [PubMed] [Google Scholar]
- Lopez-Herrera J.M, Barrero A, Lopez A, Loscertales I.G, Marquez M. Co-axial jets generated from electrified Taylor cones. Scaling laws. J. Aerosol Sci. 2003;34:535–552. doi:10.1016/S0021-8502(03)00021-1 [Google Scholar]
- Lopez-Herrera J.M, Barrero A, Boucard A, Loscertales I.G, Marquez M. An experimental study of the electrospraying of water in air at atmospheric pressure. J. Am. Soc. Mass Spectrom. 2004;15:253–259. doi: 10.1016/j.jasms.2003.10.018. doi:10.1016/j.jasms.2003.10.018 [DOI] [PubMed] [Google Scholar]
- Loscertales I.G, Barrero A, Guerrero I, Cortijo R, Marquez M, Ganan-Calvo A.M. Micro/nano encapsulation via electrified co-axial liquid jets. Science. 2002;295:1695–1698. doi: 10.1126/science.1067595. doi:10.1126/science.1067595 [DOI] [PubMed] [Google Scholar]
- Maeda M, Moriuchi S, Sano A, Yoshimine T. New drug delivery system for water-soluble drugs using silicone and its usefulness for local treatment: application of GCV-silicone to GCV/HSV-TK gene therapy for brain tumor. J. Control. Release. 2002;84:15–25. doi: 10.1016/s0168-3659(02)00236-5. doi:10.1016/S0168-3659(02)00236-5 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Yamamoto M, Okada T, Yashiki T, Shimamoto T. A new technique to efficiently entrap leuprolide acetate into microcapsules of polylactic or copoly(lactic/glycolic) acid. Chem. Pharm. Bull. 1988;36:1095–1103. doi: 10.1248/cpb.36.1095. [DOI] [PubMed] [Google Scholar]
- Pareta R, Brindley A, Jayasinghe S.N, Edirisinghe M.J. Electrohydrodynamic atomization of protein (bovine serum albumin) J. Mater. Sci. Mater. Med. 2005;16:919–925. doi: 10.1007/s10856-005-4426-z. doi:10.1007/s10856-005-4426-z [DOI] [PubMed] [Google Scholar]
- Park T.G, Lee H.Y, Nam Y.S. A new preparation method for protein loaded poly(D,L-lactic-co-glycolic acid) microspheres and protein release mechanism study. J. Control. Release. 1998;55:181–191. doi: 10.1016/s0168-3659(98)00050-9. doi:10.1016/S0168-3659(98)00050-9 [DOI] [PubMed] [Google Scholar]
- Pohja S, Suihko E, Vidgren M, Paronen P, Ketolainen J. Starch acetate as a tablet matrix for sustained drug release. J. Control. Release. 2004;94:293–302. doi: 10.1016/j.jconrel.2003.09.017. doi:10.1016/j.jconrel.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Ruiz J.M, Tissier B, Benoit J.P. Microencapsulation of peptide: a study of the phase separation of poly(D,L-lactic acid-co-glycolic acid) copolymers 50/50 by silicone oil. Int. J. Pharm. 1989;49:69–77. doi:10.1016/0378-5173(89)90154-3 [Google Scholar]
- Sah H. Protein behaviour at the water/methylene chloride interface. J. Pharm. Sci. 1999;88:1320–1325. doi: 10.1021/js9900654. doi:10.1021/js9900654 [DOI] [PubMed] [Google Scholar]
- Sievert D, Pomeranz Y. Enzyme-resistant starch. I. Characterization and evaluation by enzymic, thermoanalytical, and microscopic methods. Cereal Chem. 1989;66:342–347. [Google Scholar]
- Slager J, Domb A.J. Stereocomplexes based on poly(lactic acid) and insulin: formulation and release studies. Biomaterials. 2002;23:4389–4396. doi: 10.1016/s0142-9612(02)00179-5. doi:10.1016/S0142-9612(02)00179-5 [DOI] [PubMed] [Google Scholar]
- Tuovinen L, Peltonen S, Jarvinen K. Drug release from starch-acetate films. J. Control. Release. 2003;91:345–354. doi: 10.1016/s0168-3659(03)00259-1. doi:10.1016/S0168-3659(03)00259-1 [DOI] [PubMed] [Google Scholar]
- Wierik G.H.P.T, Bergsma J, Arends-Scholte A.W, Boersma T, Eissens A.C, Lerk C.F. A new generation starch products as excipient in pharmaceutical tablets: I. Preparation and binding properties of high surface area potato starch products. Int. J. Pharm. 1996;134:27–36. doi:10.1016/0378-5173(95)04383-7 [Google Scholar]
- Woo B.H, Jiang G, Jo Y.W, Deluca P.P. Preparation and characterization of a composite PLGA and poly(Acryloyl hydroxyethyl starch) microsphere system for protein delivery. Pharm. Res. 2001;18:1600–1606. doi: 10.1023/a:1013090700443. doi:10.1023/A:1013090700443 [DOI] [PubMed] [Google Scholar]
- Yan C, Resau L.H, Hewhtson J, Vest M, Rill W.L, Kende M. Characterization and morphological analysis of protein-loaded poly(lactide-co-glycolide) microparticles prepared by water-in-oil-in-water emulsion technique. J. Control. Release. 1994;32:231–241. doi:10.1016/0168-3659(94)90233-X [Google Scholar]
- Young A.H. Fractionation of starch. In: Whistler R.L, BeMiller J.N, Paschall R.F, editors. Starch: chemistry and technology. 2nd edn. Academic Press; London: 1984. pp. 249–283. [Google Scholar]