Abstract
At present, the assessment of developing tissue-engineered constructs is almost always carried out destructively using biochemical or histological methods to determine cell number, viability and tissue growth throughout the construct. Since many of these experiments are long, taking weeks or even months to complete, simple and readily applicable non-destructive methods of monitoring changes in cell metabolism, viability and tissue deposition within the construct would be invaluable; such methods could point out adverse responses during the early stages of culture. Here, we describe the use of microdialysis for detecting local changes in cellular metabolism within a tissue-engineered construct. Three-dimensional constructs consisting of bovine articular chondrocytes entrapped in an alginate gel were cultured in a bioreactor for two weeks. Glucose and lactate were monitored by microdialysis, as the major nutrient and metabolite, respectively. Concentration gradients within the construct were evident, with the highest lactate concentrations in the construct centre. The local lactate concentration was a measure of cellular metabolic activity, decreasing as cellular activity fell and increasing as cellular activity was stimulated. Nutrient starvation and cell death in the construct centre could be readily detected in constructs deliberately cultured under adverse conditions. The results show that probe measurements can give an early warning of inappropriate local metabolic changes. Such information during the growth of tissue-engineered constructs would allow either corrective action or else an early end to an unsuccessful test.
Keywords: tissue engineering, microdialysis, articular chondrocytes, alginate
1. Introduction
Tissue engineering provides a potential method for the repair of damaged or failing tissues or organs by seeding appropriate cells into a three-dimensional scaffold and culturing the resulting construct in a bioreactor under conditions which promote cell and tissue growth. At present, this approach is still in an experimental stage for virtually all tissues and organs with vigorous investigations into the role of factors such as cell source (Barry & Murphy 2004; Oakes 2004), scaffold type and organization (Woodfield et al. 2002; Grad et al. 2003; Mao et al. 2003), growth factor addition (Blunk et al. 2002), and mechanical stress in promoting tissue growth (Mauck et al. 2003; Stegemann & Nerem 2003). Currently, the assessment of a developing construct is almost always carried out destructively using biochemical or histological methods to determine cell number, viability and tissue growth throughout the construct (Obradovic et al. 2000; Martin et al. 2004). Assessment methods using only changes in volume or shape have limited value as in many cases tissue and cell growth is uneven and occurs primarily at the construct boundary (reviewed by Martin et al. 2004). Since many of these experiments are long, taking weeks or even months to complete (Mao et al. 2003; Freyria et al. 2004; Seidel et al. 2004), simple and readily applicable non-destructive methods of monitoring changes in cell metabolism, viability and tissue deposition within the construct would be invaluable; such methods could point out adverse responses during the early stages of culture. At present, non-destructive monitoring throughout the construct can be achieved using technologies such as MRI (Potter et al. 2000; Williams et al. 2003) or optical methods (Xu et al. 2003). These techniques can provide useful information on spatial variations in tissue deposition, but cannot monitor cellular activity or any biochemical parameters. Moreover, their use is limited by cost and availability. Here, we propose an alternative method of monitoring of tissue-engineered constructs using the principle of microdialysis to determine the local concentration of metabolites and tissue components in the extracellular fluid of the construct.
Microdialysis is used to collect solutes present in the extracellular fluid via an implanted microdialysis probe with a semi-permeable membrane at its tip. The probe is perfused by a buffer (perfusate) and solutes from the environment surrounding the probe diffuse through the membrane into the perfused solution. Perfusate with the solutes (dialysate) is then collected for ex situ analysis. Microdialysis has been used in vivo to monitor the local concentrations of solutes in the extracellular fluids in a number of different tissues. The first microdialysis experiments were conducted on the brain and blood plasma (Bito et al. 1966). It has since been used to study the metabolism in numerous tissues such as brain (Jones et al. 2000), muscles (Rosendal et al. 2005), tendons (Langberg et al. 2003), subcutaneous adipose tissue (Wientjes et al. 2003), lungs (Herkner et al. 2002), kidneys (Baicu et al. 2004) and liver (Nowak et al. 2003). Microdialysis is widely used for pharmacokinetic research (Tunblad et al. 2004) and has also been used to monitor cell metabolites in cell culture medium (Wu et al. 2001).
Here, we have assessed the possibility of monitoring chemical gradients within a tissue-engineered construct to determine local changes in cell metabolism. Chondrocytes were implanted in a hydrogel and cultured in a bioreactor. Changes in metabolism in response to external signals were monitored using microdialysis probes inserted into known locations within the gel; the probes enabled us to measure the changes in local levels of glucose (the main energy source) and lactate (the major metabolic product).
2. Materials and methods
2.1 Materials
Dulbecco's modified Eagle medium (DMEM) and foetal bovine serum were obtained from Gibco, Invitrogen, Paisley, UK; sodium alginate was obtained from Fluka Chemicals (Poole, UK); ascorbic acid was obtained from Wako Pure Chemicals (Osaka, Japan); CaCl2 was obtained from BDH Laboratory Supplies (Poole, UK); all other chemicals and reagents were obtained from Sigma-Aldrich (Poole, UK). 3–O–[14C]methyl-d-glucose was purchased from Amersham Pharmacia Biotech (Pollards Wood, UK). The Viability/Cytotoxicity kit was purchased from Molecular Probes Inc, Calbiochem (Cambridge, UK). Autoclavable microdialysis probes, introducers, microdialysis tubing and adapters were supplied by Royem Scientific (Luton, UK). Peristaltic pump PVC tubing of inner diameter (i.d.) 0.25 mm was supplied by Anachem (Luton, UK). Peristaltic pump silicone tubing (i.d. 2.06 mm) was supplied by Elkay Lab Products Ltd (Basingstoke, UK); Isopore polycarbonate membrane filters with pore size 0.2 μm were obtained from Millipore (Watford, UK).
2.2 Methods
2.2.1 The bioreactor
The experimental set-up to monitor lactate concentration in the alginate gel containing bovine cartilage chondrocytes is shown in figure 1a. Both the culture medium and the microdialysis probes were supplied by an 8-channel peristaltic pump Gilson Minipuls 3, Anachem (Luton, UK) which produced a pulsed flow for the microdialysis probes at 0.6–3 μl min−1 (PVC tubing, i.d. 0.25 mm) and at 30–60 μl min−1 (silicone tubing i.d. 2.06 mm) for the bioreactor. Buffer/DMEM supplying the bioreactor was not re-circulated. The bioreactor with the cell-alginate construct and the probes were perfused continuously for two weeks. The perfusate was collected using a microdialysis fraction collector (Royem Scientific Ltd., Luton, UK). The collected dialysate samples were frozen and stored at −20 °C. At the end of the experiment, they were defrosted and lactate and glucose were determined by the corresponding methods described below.
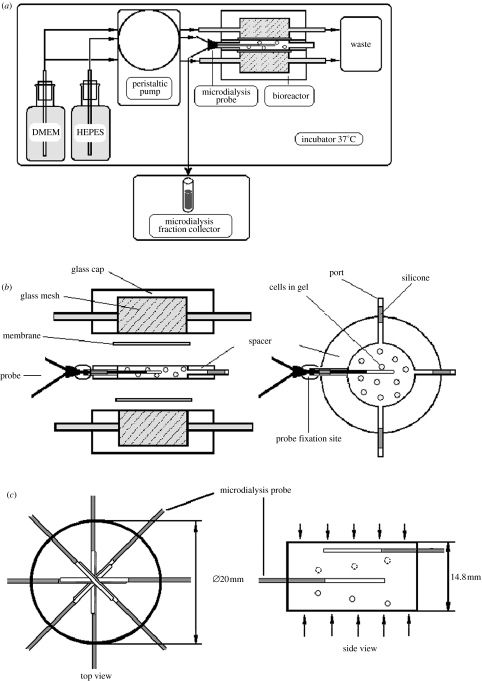
Figure 1.
(a) Schematic of the microdialysis set-up. The supply of nutrient medium to the bioreactor and of buffer to the probe was maintained using an 8-channel peristaltic pump. Dialysate was collected by a microdialysis fraction collector. Medium (waste) was collected into a 1 l container. For clarity, only one probe inserted into the spacer is shown. (b) Schematic of the bioreactor. The bioreactor consisted of two identical glass caps (od 30 mm) with the glass mash discs of i.d. 20 mm through which DMEM was passed to the cultured cells. Two Isopore polycarbonate membranes retained the gel–cell construct and separated the alginate gel from the circulating solution. Spacers (i.d. 20 mm) of different heights (4.5 and 14.8 mm), with four or eight ports for positioning microdialysis probes, controlled the construct size. (c) Schematic of probe position in the spacer. Eight probes were introduced into the gel. The centres of the probe membranes were positioned in the geometrical centre of the spacer (top view). The probes were introduced in spiral spatial order (side view). Only two probes are shown for simplicity. Ports on the spacer (circles in the schematic) were positioned in a helix along the spacer with a vertical distance of 1.64±0.17 mm between them. Arrows indicate the direction of solute diffusion into the gel.
The bioreactor itself, and reservoirs with buffer for the probe and buffer/DMEM for the bioreactor, were all kept in an incubator at 37 °C.
2.2.2 Details of the bioreactor
A glass bioreactor was developed for forming alginate gel discs and for maintaining chondrocyte cell cultures in the alginate gel (figure 1b). This particular design of bioreactor was chosen for experimental reasons to establish solute gradients and to prove the possibility of monitoring them by microdialysis. The bioreactor had both radial and horizontal symmetries, which made the multiple choice of probe positioning easier. It consisted of two identical ground glass-discs of i.d. 20 mm with a glass mesh through which DMEM was passed to the cultured cells. Two isopore polycarbonate membranes retained the gel–cell construct and separated the alginate gel from the circulating solution. Spacers (i.d. 20 mm) of different heights (4.5 mm and 14.8 mm), with four or eight ports for positioning microdialysis probes, controlled the construct size.
Before each experiment, an appropriate number of microdialysis probes (figure 1c) was introduced into the ports with the sensor of the microdialysis membrane situated along the geometrical axis (centre) of the cylindrical spacer and fixed in position using Epoxy glue and sealed using silicone glue. The spacer with fixed microdialysis probes was then assembled with other parts of the bioreactor using clamps (not shown). For experiments with chondrocyte cultures, the bioreactor was sterilized by autoclaving.
2.2.3 Preparation of chondrocyte–alginate constructs
Articular chondrocytes were isolated using collagenase digestion (Hopewell & Urban 2003) from cartilage sliced from the metacarpal–phalangeal joint of 18–24 month steers. Chondrocytes from the three joints were mixed to fill the bioreactor. The isolated cells were washed and re-suspended in DMEM supplemented with antibiotic–antimycotic solution (1% v/v). Alginate (1.2% w/v) solution was prepared in 0.9% w/v sodium chloride solution and sterilized by filtration through polyethersulphone (PES) filters pore size 0.22 μm. The cell suspension and alginate solution were mixed together to yield a final concentration 13×106–16×106 cell ml−1. The mixture was injected inside the spacer (height 14.8 mm±0.1 mm) of the assembled bioreactor using a syringe so that the cells were seeded uniformly initially. The bioreactor was then perfused with 102 mM CaCl2 for 45 min at 2 ml min−1 to cross-link the alginate (Guo et al. 1989). The CaCl2 was then replaced by DMEM supplemented with an antibiotic–antimycotic solution (1% v/v), 6% v/v foetal bovine serum and 0.5% v/v ascorbic acid. The bioreactor was perfused by DMEM solution, with supplements as required, over the course of the experiments. Two microdialysis probes were introduced into the spacer before alginate polymerization. Probe perfusion rate was 0.6–0.9 μl min−1. Dialysate was collected in a microdialysis fraction collector over 90 min periods. At the end of the experiment, the tissue-engineered construct was removed from the bioreactor, sectioned using a razor-blade, and cell viability was assessed using Viability/Cytotoxicity kit for live and dead cells.
2.2.4 Characterization of the microdialysis probes
For measurement of concentrations of low molecular weight (MW) solutes (less than 1 kDa) in alginate gels or alginate–chondrocyte constructs, autoclavable microdialysis probes of standard design (Torto et al. 1997) were used. These had a PES dialysis membrane with 15 kDa cut-off and an effective length 4 mm, outer diameter (od) 0.6 mm and a 35 mm flexible polyurethane shaft.
2.2.5 Assessment of in situ probe relative recovery and probe degradation
The dialysing properties of a microdialysis membrane are routinely expressed as its recovery for a particular solute. The recovery of the probe can be evaluated by comparing the concentration of the solute of interest in the dialysate and in the probe surroundings. The concentration of solute in the dialysate can be equal to the solute concentration in the probe surroundings, if the flow rate of perfusate equals zero. Otherwise, the concentration of the solute in the dialysate will be lower than that in the probe surroundings.
Probe recovery in our experiments was expressed as a percentage of the concentration of the solute in the probe (the dialysate) relative to that in the surrounding external solution (relative recovery). In alginate gels, it was assessed from
| (2.1) |
where Cd eq was the concentration of solute (e.g. lactate or 3-methyl-d-glucose) in the dialysate after equilibration of a gel with the solute and Cg eq was the equilibrium concentration of the solute in the gel.
In the experiments on cell–alginate gel tissue constructs, in situ probe relative recovery was assessed on the basis of the percentage of Phenol Red (PhR) in the dialysate after equilibration of the construct with DMEM containing PhR. It was important to monitor probe relative recovery during the experiment, since probe membrane could degrade due to fouling caused by protein deposition.
2.2.6 Analysis of dialysate: determination of PhR concentration
Phenol Red was added to the perfusing medium used and its concentration in the probe was monitored to determine the extent of membrane fouling. To determine the total concentration of PhR, the HPhR− form (yellow) in the dialysate was converted to PhR2− (red) by adding 2 μl of 5 M NaOH to 40–50 μl of dialysate (final pH 12.5). Absorbance of the samples was read at 540 nm on a plate reader (TECAN GENios Tecan, Reading, UK).
2.2.7 Analysis of dialysate: determination of lactate and glucose
Lactate and glucose concentrations were measured by a standard enzymatic procedure (Sigma procedures no. 735 and 510, respectively; Sigma Chemical Co (Poole, UK)). Briefly, a reagent containing lactate oxidase and colourless chromogen precursors was added to the sample in a proportion sample : reagent 1 : 10. The enzymatic reaction was based on conversion of the lactic acid by oxidase to pyruvate and hydrogen peroxide, H2O2. The H2O2 formed catalysed oxidative condensation of the chromogen precursors to produce a coloured dye. Absorbance was read at 620 nm and was proportional to the original lactic acid concentration. The method of glucose determination was based on conversion of glucose by glucose oxidase to gluconic acid and H2O2. Hydrogen peroxide was then converted by peroxidase to H2O in the presence of phenol-4-amino-phenazone. The latter accepting the electrons converted to a quinoneimine dye and its absorbance at 540 nm was proportional to the original lactic acid concentration. A sample was mixed with the reagent in proportion 1 : 10 and read after 15 min. Absorbance of each solute was calibrated against a standard curve, produced from the known concentrations of lactic acid or glucose in N-(2-hydroxyethyl)piperazine-N′-2-ethanesulphonic acid (HEPES) or blank medium with components presented in the sample. Absorbance was read using a plate reader (TECAN GENios Tecan, Reading, UK).
2.2.8 Measurement of the apparent diffusion coefficient in alginate gel
To determine the diffusion coefficients (Dapp) of lactate and 3-methyl-glucose, the bioreactor, 14.8 mm high was constructed using the 8-port spacers (figure 1c). The ports on the spacer were positioned in a helix along the spacer vertically and the ports were spaced in the vertical direction with a distance of 1.64±0.17 mm between them. Probes were introduced and alginate (without cells) was polymerized as described above. After gel polymerization, the CaCl2 solution was replaced by buffer containing 25 mM HEPES, 150 mM NaCl, 1.8 mM CaCl2, 15 mg l−1 PhR and the tracer molecules, 10 mM lactate or 0.06 μCi ml−1 3-methyl-glucose. The same buffer, but with no added tracers, was used for probe perfusion. The probe perfusion rate was 3.1 μl min−1. Dialysate was collected by a microdialysis fraction collector over 5 min time intervals. At time zero, flow on one side of the diffusion cell was stopped so that transport of tracer was from the other side only. Dialysate was collected over 27.5 h and the concentration of the solutes in it was determined by the appropriate assay.
To calculate the apparent diffusion coefficient of the solutes in a gel from measurements by microdialysis probes, eight time points from 2.4 to 5.1 h were chosen. The solute concentration at the side of the gel where flow had ceased was zero over this time. Dapp of lactate and 3-methyl-glucose were determined from the concentration-gradients measured at each time point (Winlove & Parker 1984).
2.2.9 Measurement of the partition coefficient
A 14.8 mm cell-free alginate gel was perfused to equilibrium with lactate and/or 3-methyl-glucose, i.e. until the concentration of solute in the dialysate monitored by all eight probes reached a maximum and constant value which characterizes the equilibration of all diffusion processes between the buffer supplied to the gel and the gel itself, and between the gel and the probe perfusate. After equilibration, the gel was removed from the bioreactor and dissolved as described by Bibby & Urban (2004) and the concentration of the solute in the gel was determined biochemically or by radio-isotope measurement as appropriate.
The partition coefficient (K) was calculated from
| (2.2) |
where Cg was the concentration of the solute in the gel on a wet weight basis, obtained from its concentration in the dissolved gel, and Cs was the concentration of the solute in the bioreactor feed buffer.
2.2.10 Comparison of experimental and fitted curves
Microdialysis probes were used to monitor the diffusion of lactate and 3-methyl-glucose into bioreactors using a 4 mm spacer. The probe was positioned in the centre of the spacer so that it was equidistant (2 mm) from each surface. The probe perfusion rate was 1.5 μl min−1. Dialysate was collected by a microdialysis fraction collector with 15 min time intervals. An alginate gel, formed inside the spacer, was fed from the top and bottom by a solution containing a mixture of both lactate and radioactive glucose.
The concentration of the solutes in the gel at equilibrium was related to that in solution by:
| (2.3) |
where Cs was the concentration of the solutes in the feed solution and K was defined as the partition coefficient of the corresponding solute.
The relative recovery was estimated from:
| (2.4) |
where Cd was solute concentration in the dialysate and R was defined as the relative recovery of the probe. The ratio Cg /Cg eq was plotted against ti, where ti was the time required for a solute to reach the concentration Cg in the middle of the gel.
The solution to the diffusion equation for diffusion into a plane sheet from a constant source is (Crank 1975):
| (2.5) |
where l is the distance between the symmetrical plane and the surface of the gel, C0 is the initial concentration within the region −l<x<l where probe was situated, t is the diffusion time, x is the distance from the surface of the gel and C is the concentration at the point x at time t. C1 was taken as the concentration of a solute on the surfaces of the gel (boundary condition, held constant) and D was an apparent diffusion coefficient defined experimentally as described in §2.2.8.
If C0=0, for a probe situated in the middle of the gel where x=0, with t=ti, and C=Ci=100×Cd/R at a given ti, equation (2.5) becomes
| (2.6) |
The calculated curve is then plotted as Ci/C1 versus ti.
3. Results
3.1 Measurement of solute diffusion through a cell-free alginate gel
To determine the efficiency of microdialysis probes for monitoring local metabolite concentrations in a three-dimensional-construct, diffusion through an inert alginate gel (no embedded cells) was assessed for glucose as the main cell nutrient and for lactate as the main metabolic product. One surface of the gel was perfused with a glucose/lactate solution and changes in concentration throughout the gel were determined using probes implanted at known distances from the perfused surface.
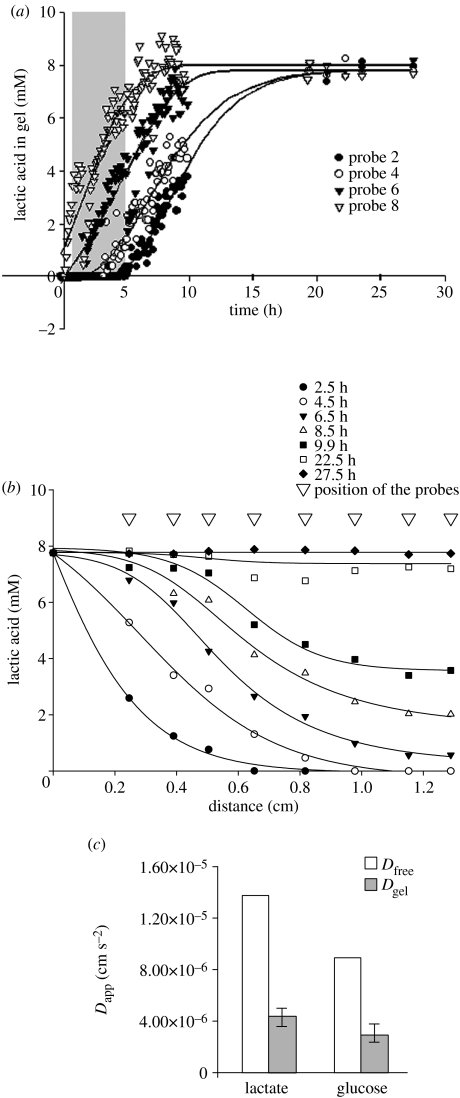
Figure 2a represents typical results from monitoring the diffusion of lactate through an alginate gel. Results are shown for a gel 14.8 mm deep formed in a bioreactor and implanted with eight microdialysis probes numbered consecutively with probe 8 closest to the perfused surface and probe 1 furthest from it (data from probes 1, 3, 5 and 7 is not shown for clarity). Lactate concentrations rose in all probes with increase in perfusion time; an increase in lactate concentration could be detected very rapidly in probe 8, but only after 5 h in probe 2 which was 11.5 mm from the perfused surface. Concentrations of all probes reached a plateau at 8.0 mg ml−1 giving a partition coefficient for lactic acid in the gel of 0.88±0.08; the relative recovery for the probes was 43.0±6%.
Figure 2.
Measurement of solute diffusion through an alginate gel. (a) Changes in lactate concentrations measured in eight probes inserted into a cell-free alginate gel after perfusion of the gel with a glucose/lactate solution. Time points ranging from 0.5 to 5 h (filled area) were used for calculation of an apparent diffusion coefficient. Points on the plateau were used to evaluate relative recovery. (b) The data were replotted to show the spatial distribution of lactate throughout a gel at different time points using information on the distance of the probes from the perfused surface. (c) The apparent diffusion coefficients (Dapp) of lactate and glucose compared with their diffusion coefficients in aqueous solution (Dfree). Values for Dfree were from Lide (1995) and recalculated using the relation D37=D25(T37/T25)(η25/η37), where T is absolute temperature and η is viscosity of water (Nicholson & Tao 1993).
In figure 2b, this data was replotted to show the spatial distribution of lactate throughout the gel at different time points in relation to the distance of the probes from the perfusate surface. In a 14.8 mm deep gel, lactate reached diffusion equilibrium across the entire gel within 27 h of the start of perfusion across the surface.
Figure 2c shows the apparent diffusion coefficients (Dapp) of lactate and glucose in comparison to their diffusion coefficients in aqueous solution (Lide 1995; Dfree). This apparent diffusion coefficient takes account of both the solute diffusivity through the gel and the diffusion resistance of the probe. Apparent diffusion coefficients were calculated from the data shown in figure 2a using eight time points ranging from 0.5 to 5 h (figure 2a, filled area). Within this period of time, diffusion could be regarded as semi-infinite and one-dimensional as the solute had not reached the far edge of the gel construct. The results show the values of Dapp were around 40% of the values of Dfree for both lactate and glucose.
3.2 Comparison of measured and predicted concentrations for diffusion into a cell-free alginate gel
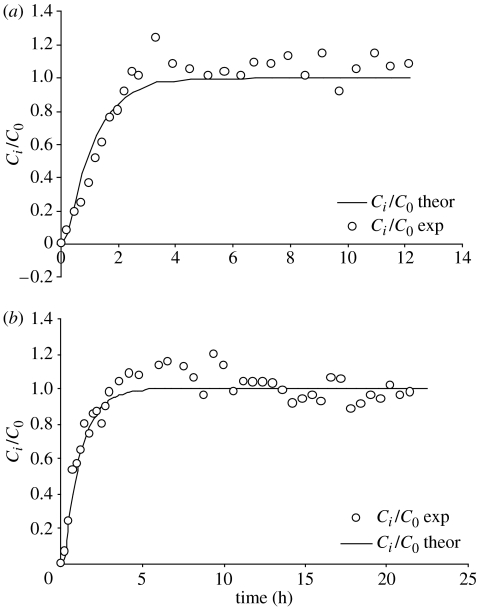
Results obtained by measuring changes in concentrations of glucose and lactate with time as these solutes diffused into a bioreactor containing a cell-free alginate gel were compared with those calculated from diffusion theory. Concentrations were measured using a microdialysis probe positioned in the centre of the gel (figure 3). Theoretical curves for diffusion of lactate (figure 3a) and glucose (figure 3b) into the centre of the bioreactor were generated using Dapp (solid lines). The experimental data were in good agreement with the predicted values.
Figure 3.
Comparison of measured and predicted concentrations for lactate and glucose diffusion into an alginate gel. The diffusion of (a) lactate and (b) glucose into an alginate gel in a bioreactor was monitored by a microdialysis probe positioned in the centre of a gel of 20 mm diameter and 4.5 mm thickness. The theoretical curves (solid lines) for diffusion of lactate and glucose in the centre of a bioreactor were generated using Dapp.
3.3 Monitoring of cell-seeded constructs
Local concentrations of lactate in alginate gels seeded with chondrocytes were monitored using embedded microdialysis probes. Perturbations in perfusate concentrations were introduced to determine if consequent changes in metabolism could be observed. In addition, probes were introduced at different depths within the gel to detect the concentration gradients of nutrients and metabolites which develop as a consequence of the balance between rate of supply and rate of cellular activity (Obradovic et al. 1999; Zhou et al. 2004).
3.3.1 Effects of glucose concentration perturbations and changes in osmolarity
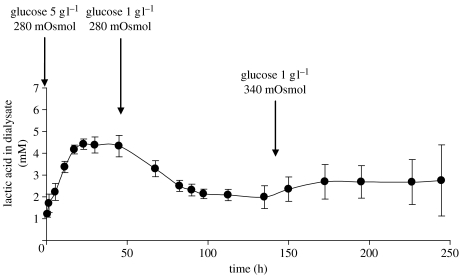
Figure 4 shows the results of a typical experiment to determine whether the effects of perturbations in glucose concentration in the feed to the bioreactor could be detected. Lactate levels were monitored in a bioreactor seeded with chondrocytes at 13 million cells g−1 gel. Results are shown for a probe positioned at a distance of 2.5 mm from the surface of a gel 5 mm thick. The level of lactate stabilized within 50 h of seeding. The glucose content of the medium supplied to the construct was then reduced from 5 to 1 g l−1. In response, lactate concentrations in the probe fell and stabilized after about 100 h at 48.0% of the initial level under 5 g l−1 glucose. Osmolarity, shown previously to stimulate lactic acid production rate (Lee et al. 2002), was then increased from 280 to 340 mOsmol by NaCl addition (Hopewell & Urban 2003); this resulted in a 23.4% increase in lactate concentration. The changes in lactate concentration evident in the dialysate could not be detected in the feed perfusate waste (not shown).
Figure 4.
The effect of glucose and osmolarity changes on cell metabolism detected by monitoring lactate concentrations in the centre of an alginate gel. The initial concentration of the cells in the gel (diameter 20 mm, thickness 14.8 mm) was 13×106 cells cm−3. Solutes diffused from both sides of the gel to the gel centre. A probe was introduced at 2.5±0.5 mm distance from the surface of the gel. The medium supplied to the bioreactor contained initially 5 g l−1 glucose and its osmolarity was 280 mOsmol. Within 2 days of the start of culturing, lactate concentrations in the dialysate stabilized. The concentration of the glucose in the medium was then changed to 1 g l−1 and lactate concentrations in the dialysate decreased. After the cell metabolism was stabilized, the osmolarity was increased to 340 mOsmol and changes in cellular metabolism were again detected: lactate concentrations in the probe increased by 23.4%. Dialysate samples were collected from the probe every 30 min during the first hour of the experiment, every 90 min from 2 to 70 h, and every 95 min thereafter. The concentrations of the samples were averaged over 5 h periods. The points on the graph represent the mean±s.d. of 3–5 sample concentrations over 7.5±2.8 h periods.
3.3.2 Effect of high cell densities and consequent cell death
Figure 5 represents typical results of monitoring the lactate and glucose levels using two probes in a bioreactor (thickness 13.5±0.1 mm) seeded evenly with a cell concentration of 15 million cells g−1 gel. These conditions were chosen to determine whether we could detect the nutrient deprivation and cell death expected in the centre of a construct of such dimensions and cell density, perfused with standard DMEM (1 g l−1 glucose; Horner & Urban 2001). One probe was thus positioned near the perfused surface; the second probe was set in the centre of the gel.
Figure 5.
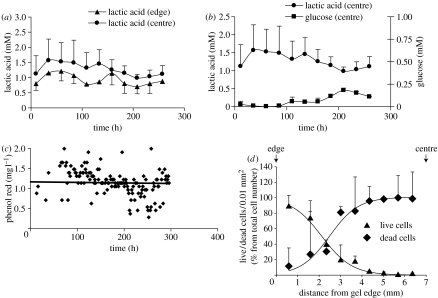
Use of probes to detect cell death in the centre of a gel. (a) Cells were introduced into a gel, diameter 20 mm, thickness 13.5±0.1 mm, at an initial concentration of 15×106 cells cm−3. Solutes diffused from both sides of the gel to the gel centre. Two probes (‘edge’ probes) were introduced symmetrically, each at 3.2±0.1 mm from the nearest surface of the gel; two more probes (‘centre’ probes) also symmetrically, at 5.8±0.1 mm from the nearest surface of the gel. The medium supplied to the bioreactor contained initially 1 g l−1 glucose, 25 mM HEPES and its osmolarity was 380 mOsmol. The concentration of HEPES in the medium was subsequently increased to 50, 75 and 100 mM in order to maintain pH 7.4. Dialysate samples were collected from the probe every 99 min. The concentrations of 15 samples were averaged over 24.75 h periods. (b) Local glucose and lactic acid concentrations were determined by the probe in the centre of the bioreactor. Lactic acid local concentration correlated negatively with the level of glucose (r=−0.74). (c) Measurement of probe fouling by monitoring the Phenol Red concentration in the dialysate. Results show that the Phenol Red concentration in both probes remained constant over the period of the experiment (250 h). (d) The cell viability profile after two weeks of culturing in the bioreactor showing that viable cells were only seen within a 5 mm zone at the edges of the gel.
The concentration of lactic acid in the centre of the bioreactor was higher than at the edges at all times (figure 5a; p<0.05), as expected in an avascular tissue or construct (Selard et al. 2003).
Glucose concentrations in the bioreactor were monitored in parallel with lactic acid concentrations (figure 5b). As a consequence of depletion of glucose by the cells, the concentration of glucose in the centre of the bioreactor was close to zero for the initial 4 days of incubation. Lactic acid concentrations in the central probe over this period were correspondingly high, and the HEPES concentration had to be increased to 100 mM to prevent acidification of the construct.
After 4 days incubation, the concentration of glucose in the central area began to rise; this rise coincided with a fall in lactic acid concentration in the same probe (r=−0.74). The concentrations in the central probe finally stabilized but at a lower lactate and higher glucose level than seen during the initial incubation period. No changes in lactate or glucose concentration could be detected in the feed perfusate waste (not shown).
3.3.3 Monitoring for fouling
In order to assess whether the fall in lactate concentration in the dialysate was due to fouling of the probe, PhR concentrations were also monitored. Phenol Red is used as a pH indicator and is of low MW (354 Da) and can readily penetrate into the probe. The concentration of PhR used as a tracer to assess probe fouling was determined for all probes used during the experiment. A typical curve of PhR concentration in the dialysate is shown in figure 5c. The concentration of PhR was similar in both probes and the decline was less than 2% per 24 h. The fall in lactate concentration in the central probe was thus not due to fouling.
3.3.4 Measurement of viable cell density
After two weeks, the construct was removed from the bioreactor and assessed for cell viability. The cell viability profile after two weeks of culturing is shown in figure 5d. Virtually no live cells were seen in the construct centre, i.e. the region where glucose concentrations had initially fallen to very low values. The viability at the edge of the construct was more than 85%.
4. Discussion
The results presented here demonstrate that microdialysis provides a non-destructive means of monitoring metabolic changes within cell-seeded three-dimensional constructs cultured in a bioreactor. Microdialysis was able to detect local changes in concentrations of metabolites within the construct (figure 2) and hence revealed differential responses to environmental perturbations (figure 4). Moreover microdialysis provided a non-destructive means of detecting changes in metabolite concentrations in the construct centre which were indicative of nutrient deprivation and cell death (figure 5).
In this study, we concentrated on monitoring the small molecules glucose and lactic acid as the most direct indicators of chondrocyte energy metabolism. Cartilaginous cells produce ATP primarily by glycolysis and thus produce lactic acid at high rates (Otte 1991; Lee & Urban 1997). Local lactic acid concentration is thus a rapid and direct indication of the rate of cellular metabolism. By monitoring metabolism within the construct by microdialysis, we were able to show that chondrocytes reacted to a stimulatory signal, namely an increase in osmolarity (Lee et al. 2002; Hopewell & Urban 2003) by increasing metabolic activity, detectable by an elevation of lactic acid concentration (figure 4) in the construct centre. Lactic acid concentrations, however, also regulate extracellular pH (Diamant et al. 1968), and if metabolic rates are high compared to rates of lactic acid diffusion from the construct—as seen in figure 5—the centre of the construct can become acidic, with adverse effects on effects on matrix production (Gray et al. 1988; Razaq et al. 2003) and even cell viability (Bibby & Urban 2004). Figure 5a for instance shows that in circumstances where a high cell density promoted lactic acid production and a large construct size limited diffusional loss, the concentration of lactic acid in the centre of the construct rose to unacceptably high levels during culture. Conversely, the rise of glucose concentration in the centre of the construct after 4 days of incubation, coinciding with a fall in lactic acid concentration, probably represented a decrease in total metabolism in this region following cell death from nutrient deprivation.
Since glucose is the main energy source for chondrocytes, availability of glucose is a necessary condition for cell function. Local monitoring of glucose in a bioreactor can thus indicate whether the cells are supplied with their basic nutritional needs. Here, we showed that from early in the culture period in constructs loaded to a high cell density (figure 5), most of the glucose supplied was consumed by the cells at the edges of the construct and glucose was virtually undetectable at the centre of the bioreactor. After 85 h of culture, as cells in the centre began to die, thus decreasing nutritional demand there, glucose levels in the centre of the construct began to rise. Glucose levels stabilized after 200 h of culture, when demand from the remaining viable cells towards the surface of the construct balanced the amount of glucose supplied in the perfusing medium (Horner & Urban 2001). Cell death in the centre of the bioreactor was confirmed by a live/dead histological assay at the end of the culture (figure 5d). These tests showed that microdialysis can detect changes in cellular metabolism over hours or days of culture (figures 4 and 5). Monitoring metabolite concentrations in this way would, for instance, allow modification of culture conditions before the construct was irreversibly damaged.
In this study, we have described the use of microdialysis to monitor low MW solutes of metabolic importance, such as lactic acid and glucose. However, microdialysis has the potential for monitoring other soluble markers of cellular metabolism and turnover. Here, we used a probe of 15 kDa MW cut-off as we were interested in small solutes (less than 200 MW). However, we have shown that microdialysis, using a probe with a higher MW cut-off (100 kDa), can be used to monitor production of soluble proteins by cells in three-dimensional structures in vitro (Miller et al. 2004), and others have shown that a similar system can be used for measurement of soluble proteins such as collagen pro-peptides or growth factors in tissues in vivo (Langberg et al. 1999; Heinemeier et al. 2003). Thus, unlike most other methods of non-destructive monitoring such as MRI or optical techniques, microdialysis has the flexibility to monitor a wide variety of chemical species produced by the cells. The limitations arise partly from the sensitivity of the method; as concentrations within the probe are lower than in the interstitial fluid, concentrations of minor species may not be readily detectable. Microdialysis is also unsuitable for detecting rapid transients in rates of metabolism or solute concentrations (Bungay et al. 2001) because of the time interval required for solutes to diffuse through the probe membrane and flow to the point of collection. Nevertheless, microdialysis has been used successfully for measuring relatively rapid responses (minutes) in tissues (Boutelle & Fillenz 1996). Moreover, since cartilaginous cells can survive for many hours without nutrients (Bibby & Urban 2004), a relatively slow response time may be adequate for detecting and correcting adverse metabolic changes in cultures of cartilaginous cells.
Probe fouling is a recognized problem in microdialysis (Lindefors et al. 1989; Torto et al. 1997). It could occur due to blockage of the microdialysis membrane pores by protein deposition during long-term culturing. In short-term experiments (hours), it is usually possible to neglect membrane fouling. Nevertheless, for long-term culturing, when protein deposition could occur, it is necessary to distinguish a decrease of monitored molecules caused by a fall of metabolic cell activity from a decrease caused by fouling. Membrane fouling can be assessed on the basis of the change of probe recovery during the experiment. Phenol Red seems to be a good candidate for monitoring of possible changes in probe recovery, because it is routinely present in DMEM at constant concentration and is not consumed by the cells during the experiment. We could not detect any appreciable fouling during two weeks of construct monitoring. The PhR molecule (MW 354.4 Da) is bigger than glucose (MW 180.2 Da) and lactic acid (MW 90.0 Da), the substances whose concentrations we are primarily interested in. Thus, we could assume that the recoveries for glucose and lactate were constant during the experiment.
The proposed novel application of microdialysis to monitor three-dimensional tissue constructs could be beneficial for manufacturing of tissue grafts for clinical purposes. Microdialysis probes could be introduced into most tissue-engineering scaffolds as stainless steel introducers are commercially available. They can be used for monitoring tissue culture within most types of bioreactors too. Microdialysis probe insertion after gel polymerization or into the cell-seeded scaffolds could cause a local microtrauma, as occurs in tissue (Anderson et al. 1994; Krogstad et al. 1996). This could possibly have a short-term effect in affecting the measurements. Nevertheless, it was shown that probe insertion did not disturb monitored parameters or cause tissue inflammation (Lonnroth & Smith 1990; Krogstad et al. 1996; Mei et al. 1996).
The results presented here show that probe measurements can give an early warning of inappropriate local metabolic changes. Changes in local lactate and glucose concentrations, as markers of a decrease in cellular activity, were evident within 24 h. Such information during the growth of tissue-engineered constructs would allow either corrective action or else an early end to an unsuccessful test. Although we used 15 kDa MW cut-off probe in this study, microdialysis probes with membranes of pore size ranging from 15 to 3000 kDa MW cut-off are commercially available. Therefore, it is feasible to monitor high MW molecules such as products of matrix protein turnover and growth factors using this technique.
5. Conclusion
The results presented have shown how microdialysis probes can be used to monitor the spatial and temporal distribution of the local environment within three-dimensional tissue-engineered constructs. We also developed an in situ calibration technique to identify membrane fouling and probe blocking. Using a chondrocytes-seeded alginate construct as an example, we demonstrated that the spatial distribution and transient behaviour of the nutrients and metabolites within the three-dimensional tissue construct can be successfully monitored, and that fouling of the microdialysis probe is not significant.
The microdialysis system is a powerful, mobile but relatively cheap technique. Using microdialysis membrane probes with different pore size, it should be feasible to monitor a wide range of metabolites and even macromolecules produced by cells in tissue-engineered constructs. Its applications for monitoring tissue culture parameters and tissue formation in other types of engineered tissues and bioreactors should be actively explored.
Acknowledgments
This work was funded by the UK Biotechnology and Biological Science Research Council, Engineering and Biological Science Committee (grant reference E15902).
References
- Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J. Invest. Dermatol. 1994;102:807–811. doi: 10.1111/1523-1747.ep12378630. doi:10.1111/1523-1747.ep12378630 [DOI] [PubMed] [Google Scholar]
- Baicu S.C, Simmons P.M, Campbell L.H, Taylor M.J, Brockbank K.G. Interstitial fluid analysis for assessment of organ function. Clin. Transplant. 2004;18(Suppl. 12):16–21. doi: 10.1111/j.1399-0012.2004.00212. doi:10.1111/j.1399-0012.2004.00212 [DOI] [PubMed] [Google Scholar]
- Barry F.P, Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. doi:10.1016/j.biocel.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Bibby S.R, Urban J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. doi:10.1007/s00586-003-0616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito L, Davson H, Levin E, Murray M, Snider N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J. Neurochem. 1966;13:1057–1067. doi: 10.1111/j.1471-4159.1966.tb04265.x. [DOI] [PubMed] [Google Scholar]
- Blunk T, Sieminski A.L, Gooch K.J, Courter D.L, Hollander A.P, Nahir A.M, Langer R, Vunjak-Novakovic G, Freed L.E. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8:73–84. doi: 10.1089/107632702753503072. doi:10.1089/107632702753503072 [DOI] [PubMed] [Google Scholar]
- Boutelle M.G, Fillenz M. Clinical microdialysis: the role of on-line measurement and quantitative microdialysis. Acta Neurochir. Suppl. 1996;67:13–20. doi: 10.1007/978-3-7091-6894-3_3. [DOI] [PubMed] [Google Scholar]
- Bungay P.M, Dedrick R.L, Fox E, Balis F.M. Probe calibration in transient microdialysis in vivo. Pharm. Res. 2001;18:361–366. doi: 10.1023/a:1011015316327. doi:10.1023/A:1011015316327 [DOI] [PubMed] [Google Scholar]
- Crank J. The mathematics of diffusion. Clarendon Press; Oxford: 1975. pp. 43–61. [Google Scholar]
- Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24:1195–1196. doi: 10.1007/BF02146615. doi:10.1007/BF02146615 [DOI] [PubMed] [Google Scholar]
- Freyria A.M, Cortial D, Ronziere M.C, Guerret S, Herbage D. Influence of medium composition, static and stirred conditions on the proliferation of and matrix protein expression of bovine articular chondrocytes cultured in a 3-D collagen scaffold. Biomaterials. 2004;25:687–697. doi: 10.1016/s0142-9612(03)00568-4. doi:10.1016/S0142-9612(03)00568-4 [DOI] [PubMed] [Google Scholar]
- Grad S, Kupcsik L, Gorna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163–5171. doi: 10.1016/s0142-9612(03)00462-9. doi:10.1016/S0142-9612(03)00462-9 [DOI] [PubMed] [Google Scholar]
- Gray M.L, Pizzanelli A.M, Grodzinsky A.J, Lee R.C. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J. Orthop. Res. 1988;6:777–792. doi: 10.1002/jor.1100060602. doi:10.1002/jor.1100060602 [DOI] [PubMed] [Google Scholar]
- Guo J.F, Jourdian G.W, MacCallum D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect. Tissue Res. 1989;19:277–297. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Olesen J.L, Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J. Appl. Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- Herkner H, Muller M.R, Kreischitz N, Mayer B.X, Frossard M, Joukhadar C, Klein N, Lackner E, Muller M. Closed-chest microdialysis to measure antibiotic penetration into human lung tissue. Am. J. Resp. Crit. Care Med. 2002;165:273–276. doi: 10.1164/ajrccm.165.2.2106082. [DOI] [PubMed] [Google Scholar]
- Hopewell B, Urban J.P. Adaptation of articular chondrocytes to changes in osmolality. Biorheology. 2003;40:73–77. [PubMed] [Google Scholar]
- Horner H.A, Urban J.P. Volvo award winner in basic science studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. doi:10.1097/00007632-200112010-00006 [DOI] [PubMed] [Google Scholar]
- Jones D.A, Ros J, Landolt H, Fillenz M, Boutelle M.G. Dynamic changes in glucose and lactate in the cortex of the freely moving rat monitored using microdialysis. J. Neurochem. 2000;75:1703–1708. doi: 10.1046/j.1471-4159.2000.0751703.x. doi:10.1046/j.1471-4159.2000.0751703.x [DOI] [PubMed] [Google Scholar]
- Krogstad A.L, Jansson P.A, Gisslen P, Lonnroth P. Microdialysis methodology for the measurement of dermal interstitial fluid in humans. Br. J. Dermatol. 1996;6:1005–1012. doi:10.1046/j.1365-2133.1996.d01-893.x [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen L.J, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J. Physiol. 1999;521(Pt 1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. doi:10.1111/j.1469-7793.1999.00299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Boushel R, Skovgaard D, Risum N, Kjaer M. Cyclo-oxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J. Physiol. 2003;551:683–689. doi: 10.1113/jphysiol.2003.046094. doi:10.1113/jphysiol.2003.046094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.B, Urban J.P. Evidence for a negative Pasteur effect in articular cartilage. Biochem. J. 1997;321(Pt 1):95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.B, Wilkins R.J, Razaq S, Urban J.P. The effect of mechanical stress on cartilage energy metabolism. Biorheology. 2002;39:133–143. [PubMed] [Google Scholar]
- Lide D.R. CRC handbook of chemistry and physics. CRC press; London: 1995. pp. 6–257. [Google Scholar]
- Lindefors N, Amberg G, Ungerstedt U. Intracerebral microdialysis: I. Experimental studies of diffusion kinetics. J. Pharmacol. Meth. 1989;22:141–156. doi: 10.1016/0160-5402(89)90011-9. doi:10.1016/0160-5402(89)90011-9 [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Smith U. Microdialysis—a novel technique for clinical investigations. J. Intern. Med. 1990;227:295–300. doi: 10.1111/j.1365-2796.1990.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Mao J, Zhao L, De Yao K, Shang Q, Yang G, Cao Y. Study of novel chitosan–gelatin artificial skin in vitro. J. Biomed. Mater. Res. A. 2003;64:301–308. doi: 10.1002/jbm.a.10223. doi:10.1002/jbm.a.10223 [DOI] [PubMed] [Google Scholar]
- Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. doi:10.1016/j.tibtech.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Mauck R.L, Nicoll S.B, Seyhan S.L, Ateshian G.A, Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. doi:10.1089/107632703768247304 [DOI] [PubMed] [Google Scholar]
- Mei D.A, Gross G.J, Nithipatikom K. Simultaneous determination of adenosine, inosine, hypoxanthine, xanthine, and uric acid in microdialysis samples using microbore column high-performance liquid chromatography with a diode array detector. Anal. Biochem. 1996;238:34–39. doi: 10.1006/abio.1996.0246. doi:10.1006/abio.1996.0246 [DOI] [PubMed] [Google Scholar]
- Miller S, Chinkes D, MacLean D.A, Gore D, Wolfe R.R. In vivo muscle amino acid transport involves two distinct processes. Am. J. Physiol. Endocrinol. Metab. 2004;287:E136–E141. doi: 10.1152/ajpendo.00092.2004. doi:10.1152/ajpendo.00092.2004 [DOI] [PubMed] [Google Scholar]
- Nicholson C, Tao L. Hindered diffusion of high molecular weight compounds in brain extracellular microenvironment measured with integrative optical imaging. Biophys. J. 1993;65:2277–2290. doi: 10.1016/S0006-3495(93)81324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Ungerstedt J, Wernerson A, Ungerstedt U, Ericzon B.G. Hepatic cell membrane damage during cold preservation sensitizes liver grafts to rewarming injury. J. Hepatobiliary Pancreat. Surg. 2003;10:200–205. doi: 10.1007/s00534-002-0760-4. doi:10.1007/s00534-002-0760-4 [DOI] [PubMed] [Google Scholar]
- Oakes B.W. Orthopaedic tissue engineering: from laboratory to the clinic. Med. J. Australia. 2004;180:S35–S38. doi: 10.5694/j.1326-5377.2004.tb05912.x. [DOI] [PubMed] [Google Scholar]
- Obradovic B, Carrier R.L, Vunjak-Novakovic G, Freed L.E. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol. Bioeng. 1999;63:197–205. doi: 10.1002/(sici)1097-0290(19990420)63:2<197::aid-bit8>3.0.co;2-2. doi:10.1002/(SICI)1097-0290(19990420)63:2<197::AID-BIT8>3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- Obradovic B, Carrier R.L, Vunjak-Novakovic G, Freed L.E. Glycosaminoglycan deposition in engineered cartilage: Experiments and mathematical model. AICHE J. 2000;46:1860–1871. doi:10.1002/aic.690460914 [Google Scholar]
- Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z. Rheumatol. 1991;50:304–312. [PubMed] [Google Scholar]
- Potter K, Butler J.J, Horton W.E, Spencer R.G. Response of engineered cartilage tissue to biochemical agents as studied by proton magnetic resonance microscopy. Arthritis Rheum. 2000;43:1580–1590. doi: 10.1002/1529-0131(200007)43:7<1580::AID-ANR23>3.0.CO;2-G. doi:10.1002/1529-0131(200007)43:7<1580::AID-ANR23>3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- Razaq S, Wilkins R.J, Urban J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003;12:341–349. doi: 10.1007/s00586-003-0582-3. doi:10.1007/s00586-003-0582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J. Appl. Physiol. 2005;98:477–481. doi: 10.1152/japplphysiol.00130.2004. doi:10.1152/japplphysiol.00130.2004 [DOI] [PubMed] [Google Scholar]
- Seidel J.O, Pei M, Gray M.L, Langer R, Freed L.E, Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445–458. [PubMed] [Google Scholar]
- Selard E, Shirazi-Adl A, Urban J.P. Finite element study of nutrient diffusion in the human intervertebral disc. Spine. 2003;28:1945–1953. doi: 10.1097/01.BRS.0000087210.93541.23. doi:10.1097/01.BRS.0000087210.93541.23 [DOI] [PubMed] [Google Scholar]
- Stegemann J.P, Nerem R.M. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann. Biomed. Eng. 2003;31:391–402. doi: 10.1114/1.1558031. doi:10.1114/1.1558031 [DOI] [PubMed] [Google Scholar]
- Torto N, Laurell T, Gorton L, Varga G.M. A study of a polysulfone membrane for use in an in-situ tunable microdialysis probe during monitoring of starch enzymatic hydrolysates. J. Membr. Sci. 1997;130:239–248. doi:10.1016/S0376-7388(97)00030-6 [Google Scholar]
- Tunblad K, Hammarlund-Udenaes M, Jonsson E.N. An integrated model for the analysis of pharmacokinetic data from microdialysis experiments. Pharm. Res. 2004;21:1698–1707. doi: 10.1023/b:pham.0000041468.00587.c6. doi:10.1023/B:PHAM.0000041468.00587.c6 [DOI] [PubMed] [Google Scholar]
- Wientjes K.J, Grob U, Hattemer A, Hoogenberg K, Jungheim K, Kapitza C, Schoonen A.J. Effects of microdialysis catheter insertion into the subcutaneous adipose tissue assessed by the SCGM1 system. Diabetes Technol. Ther. 2003;5:615–620. doi: 10.1089/152091503322250631. doi:10.1089/152091503322250631 [DOI] [PubMed] [Google Scholar]
- Williams A, Oppenheimer R.A, Gray M.L, Burstein D. Differential recovery of glycosaminoglycan after IL-1-induced degradation of bovine articular cartilage depends on degree of degradation. Arthritis Res. Ther. 2003;5:R97–R105. doi: 10.1186/ar615. doi:10.1186/ar615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winlove C.P, Parker K.H. Diffusion of macromolecules in hyaluronate gels. I. Development of methods and preliminary results. Biorheology. 1984;21:347–362. doi: 10.3233/bir-1984-21305. [DOI] [PubMed] [Google Scholar]
- Woodfield T.B, Bezemer J.M, Pieper J.S, van Blitterswijk C.A, Riesle J. Scaffolds for tissue engineering of cartilage. Crit. Rev. Eukar. Gene Expr. 2002;12:209–236. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.40. doi:10.1615/CritRevEukaryotGeneExpr.v12.i3.40 [DOI] [PubMed] [Google Scholar]
- Wu Y.S, Tsai T.H, Wu T.F, Cheng F.C. Determination of pyruvate and lactate in primary liver cell culture medium during hypoxia by on-line microdialysis-liquid chromatography. J. Chromatogr. A. 2001;913:341–347. doi: 10.1016/s0021-9673(00)01265-6. doi:10.1016/S0021-9673(00)01265-6 [DOI] [PubMed] [Google Scholar]
- Xu X, Wang R.K, El Haj A. Investigation of changes in optical attenuation of bone and neuronal cells in organ culture or three-dimensional constructs in vitro with optical coherence tomography: relevance to cytochrome oxidase monitoring. Eur. Biophys. J. 2003;32:355–362. doi: 10.1007/s00249-003-0285-z. doi:10.1007/s00249-003-0285-z [DOI] [PubMed] [Google Scholar]
- Zhou S, Cui Z, Urban J.P. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–3924. doi: 10.1002/art.20675. doi:10.1002/art.20675 [DOI] [PubMed] [Google Scholar]