Abstract
This study was designed to evaluate the suitability of a novel bioabsorbable material in treating bone defects. A poly(desaminotyrosyl-tyrosine-ethyl ester carbonate) (PDTE carbonate) membrane (thickness 0.2–0.3 mm) was implanted into the mandibular angle of 20 New Zealand White rabbits to cover a through-and-through defect (12×6 mm). In group 1, the defects were left unfilled but covered with membrane and in group 2 the defects were filled with bioactive glass mesh and covered with membrane, too. Controls were left uncovered and unfilled. The animals were followed for 6, 12, 24 and 52 weeks, respectively. The material was evaluated by qualitative analysis of histological reactions and newly formed bone.
We found that PDTE carbonate elicited a modest foreign body reaction in the tissues, which was uniform throughout the study. New bone formation was seen in all samples after six weeks. Group 1 had more new bone formation until 24 weeks and after this the difference settled. Based on findings of this study it was concluded that PDTE carbonate membranes have good biocompatibility and are sufficient to enhance bone growth without additional supportive matrix.
Keywords: poly(desaminotyrosyl-tyrosine-ethyl ester carbonate), bioabsorbable membrane, rabbit, mandible, reconstruction, guided bone regeneration
1. Introduction
Trauma, cancer, congenital abnormalities or infection can result in extensive tissue defects. To rehabilitate the patient, lost tissues need to be reconstructed. The problem is often primarily related to the hard tissues, because fast regenerating soft tissues often invade the originally hard tissue covered area and prevent the slow regeneration of bone tissues. Soft tissue invasion to the defect area can be prevented in several ways. The defects can be covered and stabilized with both bioabsorbable and non-absorbable membranes (Ashammakhi et al. 1995; Hurzeler et al. 1997; Baumann et al. 2002). These have shown to inhibit the soft tissue invasion to the area and this is considered the most important factor in regeneration of bone defects (Baumann et al. 2002; Pruthi et al. 2002). Today, the most frequently used material is still a non-absorbable expanded polytetrafluoroethylene, which often needs to be removed after completed healing.
The fact that non-absorbable materials often need to be removed has aroused a need for resorbable materials that do not require a second removal procedure. Hence, morbidity, risks of infections, costs and time required for treatment are reduced (Aaboe et al. 1995; St John 2003). Such bioabsorbable materials suitable for clinical use have been under extensive research since the 1970s (Kulkarni et al. 1966; Cutright et al. 1971; Cutright & Hunsuck 1972).
According to current understanding, extensive bone defects, however, require a supportive matrix for bone regeneration. The golden standard has been autologous bone transplant, which naturally provides all the necessary components for bone regeneration (Aaboe et al. 1995). Yet, the use of autologous bone transplants subjects the patient to another operation site, which also increases risk of infection, donor site morbidity, time and costs of the treatment (Howell et al. 1998; St John 2003). The aim with bioactive glass (BAG) matrices has been the mimicking of the environment created with autologous bone.
Bioabsorbable materials differ in handling properties, malleability, elasticity and absorption rates. However, according to the literature, no material has superseded the other so far (Suuronen et al. 2000). Currently, commercially available bioabsorbable polymer membranes, approved for clinical use, are polylactides (PLAs), polyglycolides (PGAs), polydioxanone and their copolymers (Ashammakhi et al. 1994; Dietz et al. 2001; Loos et al. 2002). PLAs and their copolymers are today perhaps the most commonly used and studied bioabsorbable polymers in oro-maxillofacial and orthopedic surgery (Suuronen et al. 1998; Baumann et al. 2002). By changing the composition of the polymer and the manufacturing procedure, the resorption time, handling properties and mechanical durability can be adjusted to suit the needs of the patient (Törmälä 1992; Kellomäki et al. 2003).
Tyrosine-based pseudo-peptide polymers were first introduced in 1987 by Kohn & Langer (1987). They are a relatively new category of bioabsorbable polymers; however, they have proven to be in vivo and in vitro biocompatible, biodegradable, non-toxic and non-immunogenic with good processing properties, including solubility, thermal stability and moldability (Silver et al. 1992; Hooper et al. 1998; Gupta & Lopina 2002). However, no long-term studies about in vivo degradation of the material exist. Tyrosine polycarbonates can be tailored by condensation polymerization of different esters of desaminotyrosyl-tyrosine (Ertel & Kohn 1994). A polymer carrying an ethyl ester pendent chain, poly(desaminotyrosyl-tyrosine-ethyl ester carbonate) (PDTE carbonate), has been considered the most promising derivative, stimulating cell growth more than the other hydrophobic polymers (carrying longer alkyl ester pendent chains; Ertel & Kohn 1994). PDTE carbonate has elicited bone apposition when in contact with hard tissue (Pyhältö et al. 2002).
Tyrosine-derived polycarbonates incorporate two in vivo hydrolytically labile bonds in each repeat unit, a carbonate bond that connects the monomer units and an ester bond connecting a pendent chain. Degradation rate and products of the polymer are determined by the relative hydrolysis rate of these two labile bonds. Carbonate bond is hydrolysed at a faster rate than the pendent chain ester bond (Tangpasuthadol et al. 2000a). They, however, are relatively stable and degrade only very slowly in vitro. No mass loss has been detected after 3 years in vitro degradation (Tangpasuthadol et al. 2000b). Short-term in vivo studies (570 days) have confirmed less than 5% mass loss during degradation. Calculated from the decrease in molecular weight the degradation of PDTE carbonate to 5% of its initial molecular weight would take approximately 1890 days (Hooper et al. 1998). Tyrosine-derived polycarbonates were not found to be associated in ‘acid dumping’ or the release of acidic residues found during the degradation of poly(d,l-lactic acid) (Tangpasuthadol et al. 2000b).
The degree of surface hydrophobicity is related to the length of the alkyl ester pendent chain, with the polymer carrying longer alkyl ester pendent chains being more hydrophobic (Ertel & Kohn 1994). The least hydrophobic polycarbonate (having a short ethyl ester pendent chain) has been the most suitable material. Surface hydrophobicity has proven to contribute to reduced swelling during the degradation process compared to poly(alpha-hydroxy acids) (Tangpasuthadol et al. 2000b).
Chitosan is a biodegradable, non-toxic, complex carbohydrate derivative. It has haemostatic properties which appear to function independently from the platelets and normal clotting mechanisms (Malette et al. 1983; Klokkevold et al. 1992). Chitosan is a N-deacetylated derivative of natural chitin. Chitin is a naturally abundant supporting material of shells of crustaceans and insects. The N-deacetylation is almost never complete and therefore chitin that becomes soluble in organic solvents is called chitosan. Chitosan has a close structural resemblance to cellulose. Chitosan is also a highly basic polysaccharide (Ravi Kumar 2000).
The aim of the present study was to evaluate the use of tyrosine derivative polymer membrane and BAG mesh fixed with chitosan in the treatment of artificially created defects in rabbit mandible. Bioactive mesh was studied because it was assumed to improve the healing of the defects. In this study, we evaluated the suitability of the material by means of histology and new bone formation.
2. Materials and methods
This study was approved by The Research Animal Committee of University of Helsinki and the Provincial Administrative Board, according to Finnish law.
2.1 Materials
Batches of PDTE carbonate were supplied by Integra LifeSciences Corporation (New Jersey, USA). These polycarbonates having Mw from 200 000 to 220 000 D (weight average molecular weight) were prepared according to previously published procedures (Kohn 1993; Fiordeliso et al. 1994). Materials were stored in the form of powder at −18 °C temperature prior to processing in airtight containers. Three days before processing, powder was ground in liquid nitrogen to eliminate larger particles. The homogenized powder was dried in a vacuum chamber at 53 °C for 48 h. Solid plates (85×85×3.3 mm) were compression molded at 165 °C from the raw material powder. Molded plates were biaxially oriented in one phase to a draw ratio of 2.2×2.2 at 75 °C with a plate-stretching machine Karo IV (Brueckner GmbH, Siegsdorf, Germany) resulting to thicknesses of 0.2–0.3 mm.
BAG 13-93 (Vivoxid Ltd, Turku, Finland) was spun to fibres, which were prepared to mesh fixed with 3 wt% chitosan (Chitech, Medicarb, Sweden). Samples were sized 12×6×(2–4) mm. BAG composition in weight percentages was Na2O 6%, K2O 12%, MgO 5%, CaO 20%, P2O5 4% and SiO2 53%.
Manufacturing of all the specimens was done at the Institute of Biomaterials (Tampere University of Technology, Tampere, Finland). All samples were sterilized with gamma irradiation, minimum dose 2.5 Mrad. (Willy Rütsch AG, Kernen-Rommelshausen, Germany).
2.2 Animals
Twenty adult female New Zealand White rabbits (HsdPoc strain) weighing 2500–3000 g were used as experimental animals. No preoperative fasting was required. The animals were divided in to three groups (n=6) according to the intended healing time (6, 12 and 24 weeks) and two controls for 52 weeks. National guidelines for the care of laboratory animals were followed.
2.3 Surgical procedure
Preoperatively, the animals received trimethoprim–sulfadiazine (Duoprim vet, Schering-Plough, Brussels, Belgium) 0.3 mg kg−1 subcutaneously (s.c.) for infection phrophylaxis. Anaesthesia was induced with medetomidine (Domitor, Orion Pharma, Turku, Finland) 300 μg kg−1 and ketamine (Ketaminol vet, Intervet International, Boxmer, The Netherlands) 25 mg kg−1 (s.c.).
Mandible was shaved on both sides and skin was rinsed and scrubbed with chlorhexidine digluconate (Klorhexol 5 mg ml−1, Leiras, Turku, Finland). A skin incision was made along the inferior border of the rabbit's mandible. A periosteal flap was lifted and standardized bicortical through-and-through defects (12×6 mm, 72 mm2; figures 1 and 2) were created with an oscillating saw bilaterally in the mandibular angle. On the left side (group 1), the membrane (sized 20×25 mm; figure 3) was mounted and wrapped around the inferior border of the mandibular angle and fixed superior to the defect with absorbable sutures through holes drilled in the mandible. On the right side (group 2), the defect was filled with BAG mesh prior to fixation of the membrane. The periosteal flap was sutured over the implanted membrane and the incision was closed in layers with absorbable sutures (Vicryl 3-0, Ethicon, Somerville New Jersey, USA).
Figure 1.
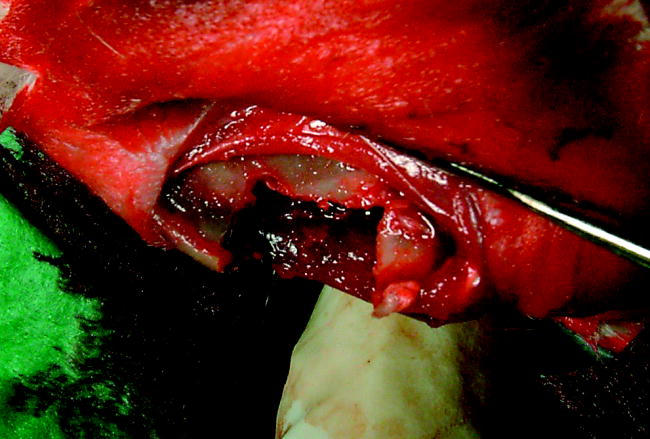
The defect before placement of the PDTE carbonate membrane.
Figure 2.
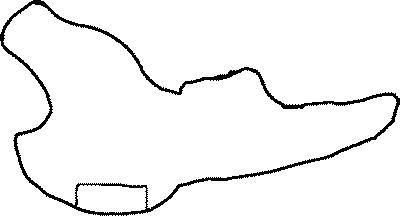
Schematic of the defect site. The box at the bottom indicating the removed piece of bone.
Figure 3.
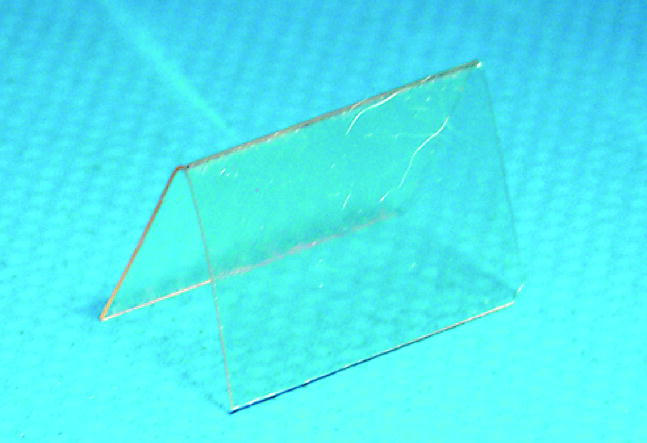
Folded PDTE carbonate membrane before implantation.
In the control animals (52 weeks), identical defects were created, but two of them were filled just with BAG and two were left uncovered and unfilled and the periosteal flap was sutured over the defect. In all defects blood clot formation was ensured. For postoperative pain control, the animals received 0.02–0.05 mg kg−1 (s.c.) buprenorphinum (Temgesic, Schering-Plough, Brussels, Belgium) immediately after the operation and every 12 h for the next 2 days. For euthanasia, pentobarbital (Mebunat, Orion Pharma, Turku, Finland) 30 mg kg−1 was used intravenously (i.v.).
2.4 Sample preparation and analysis
All samples were fixed in 70% alcohol and embedded in methylmetacrylate blocks (Schenk 1965). Five micrometre thick sections were prepared with Leica SM2500 heavy-duty sliding microtome and stained both with Masson–Goldner thrichrome and haematoxylin and eosin. All sections were analysed by a single pathologist with a light microscope to record cellular reactions, membrane placement, new bone formation and localization. Samples obtained from the animals were radiographed as described earlier by the authors (Asikainen et al. 2005). The radiographic results from weeks 12 to 24 have been described earlier (Asikainen et al. 2005) and only 52 week samples are included in this study.
3. Results
3.1 6 weeks
One animal from this group was lost due to anaesthesia complications at three weeks when radiographs of the defects were carried out. Clinically, no signs of infection were seen in any animal. After dissection a small clear fluid filled cyst was detected in the left mandible of one rabbit (membrane only). However, histological inspection revealed inflammatory cell infiltrate with lymphocytes and plasma cells in the membrane surrounding capsule in three out of five cases in the membrane only group and two out of five cases in the BAG+membrane group. No foreign body reaction with giant cells was present in any sample.
In all five cases new bone formation was detected in the membrane only group inside the membrane at the site of defect. Only in one case new bone formation was seen at the site opposite to the defect. In the membrane+BAG group new bone formation was seen in three out of five cases. In one case the inflammatory cell infiltrate with lymphocytes and plasma cells was notably greater than in others. In this case no new bone formation was detected at either site of the membrane. In one case new bone formation was seen only at the outside of the membrane.
3.2 12 weeks
Clinical inspection revealed no signs of infection in any animal. Inflammatory cell infiltrate with lymphocytes and plasma cells was present in three out of six, in membrane only, and two out of six in BAG+membrane. In one animal, at both sites, there was notably more inflammatory infiltrate with lymphocytes, plasma cells and occasional macrophages than in others. No foreign body reaction with giant cells was present in any sample.
New bone formation in the membrane only group was detected at the outside of the membrane in all six cases, at the defect site in three out of six cases (figure 4). In the membrane+BAG mesh group new bone formation was seen in three out of five cases on the outside and in one inside the membrane (one sample was lost from histological studies due the problems in polymerization of the methylmetacrylate).
Figure 4.
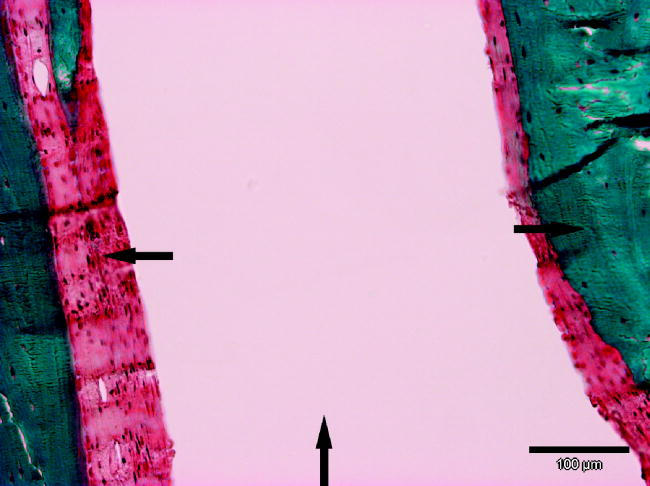
The PDTE carbonate membrane has become surrounded with bone after 12 weeks of healing. Note the absent foreign-body reaction with giant cells to the implant. In the surrounding capsule only fibroblasts are seen. The formed fibrous capsule is less than 100 μm thick where fixation has been adequate. Arrow up indicating the membrane, arrow right the bone and arrow left fibrous capsule.
3.3 24 weeks
No signs of infection were clinically evident in any animal. Inflammatory cell infiltrate with lymphocytes and plasma cells was seen in the membrane group in one of six cases and in BAG mesh+membrane group two of six cases. No foreign body reaction with giant cells was present in any sample.
New bone formation was seen in the membrane group at the inside in three out of six cases and at the outside in all but one case. In the membrane+BAG mesh group at the inside of the membrane new bone was detected in five out of six cases and at the outside in four out of six cases.
3.4 52 weeks
In the control animals identical defects were created but two of them were filled just with BAG and two were left uncovered and unfilled. No signs of infection were clinically evident in any animal. Histologically, no inflammatory infiltrate was seen. Also, no foreign body reaction with giant cells was present in any sample. No new bone formation was detected in any sample, either. Radiologically defects were not ossified and the edges of the defects were rounded (figure 5a,b). This confirms the critical size of the defect.
Figure 5.
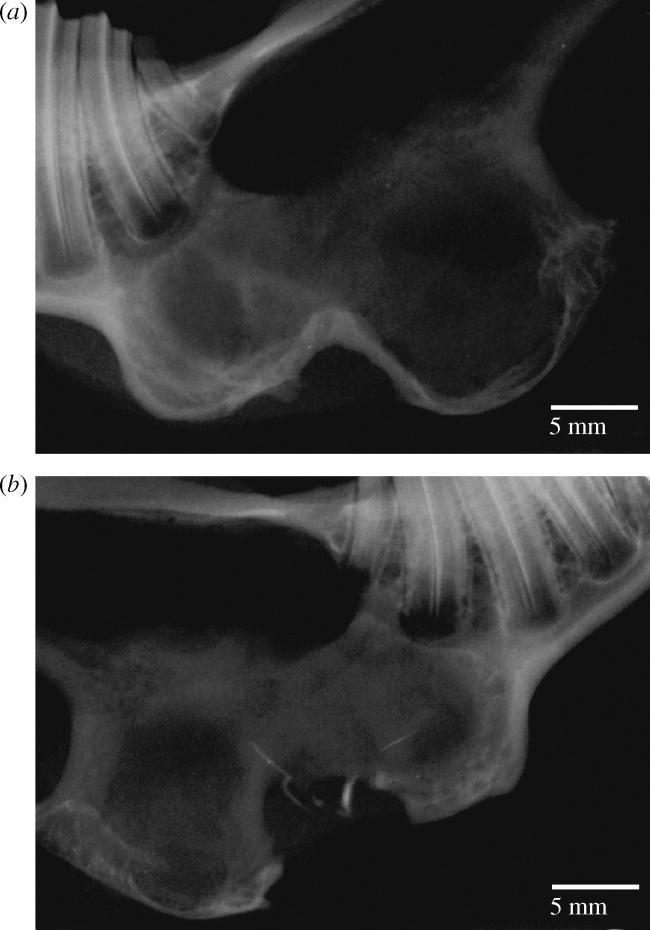
Control radiographs (a) without membrane treatment and (b) with BAG treatment only after 52 weeks. At both radiographs edges of the defects are rounded and original bony contour is not achieved.
4. Discussion
Clinically, all animals went through an uneventful postoperative healing process except one, which due to wound dehiscence, needed two additional sutures in the mandible on the first postoperative day. All animals started eating normally within 24 h after the operation. This suggests that no excessive functional damage was caused by the operation.
A common problem with any guided bone regeneration material is the handling and the correct placement of the membrane. The membrane used in this study showed excellent bending and handling properties at the operation. Edges of the membrane could be rounded with scissors and a needle, used to introduce the fixating suture, perforated the membrane easily. Blood clot formation and correct positioning of the membrane could be ensured because the membrane was transparent.
The membranes were fixated with Vicryl 3-0 sutures, because the rabbit mandible is too thin for commonly used tack or pin fixation. This, however, was found to be inadequate, since at the end of follow-up time in only five out of 18 cases the membrane was situated where it was originally placed. The fixation was considered good in all cases intraoperatively. Possible reasons for the loosening of the fixation are the holes through which the sutures were drawn. The mechanical stress between bone and membrane may have caused shearing of the sutures and lead to loosening of the fixation. In the cases where the fixation was adequate, a notably thinner fibrous capsule surrounded the membrane (figure 6). This is supporting the theory that micromovement of any implanted devices increases the granulation tissue around the device (Pyhältö et al. 2002). In some of the cases where fixation was not stable enough, M. pterygoideus medialis was seen between the membrane and mandible.
Figure 6.
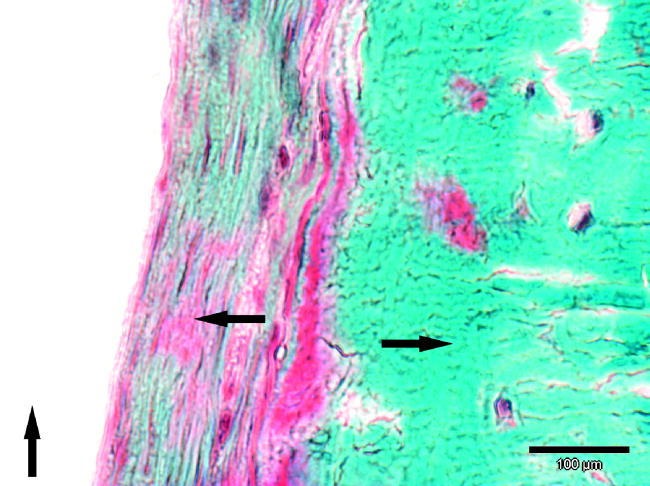
At this 24-week sample, the formed fibrous capsule between surrounding the PDTE membrane is over 100 μm thick possibly due the inadequate fixation of the membrane. Also no foreign body reaction with giant cells is seen in this sample. Arrow up indicating the membrane, arrow right the bone and arrow left fibrous capsule.
New woven bone formation was seen already in the six-week group. A clear and surprising difference between the groups could be seen: in the ‘membrane only’ group all five samples showed new bone formation at the defect site and in the ‘membrane with BAG mesh’ group three out of five showed new bone formation at the defect. The difference was unexpected because BAG mesh was studied due to its previously reported osteoconductivity (Brink et al. 1997) and it was assumed to improve the healing of the defects. However, this might be explained by elevated levels of tissue inhibitory proteinases during the initial phase of degradation of the BAG used (A. J. Asikainen et al. unpublished data).
In the 12-week group, the difference in bone formation was even more clearly visible: in the ‘membrane only’ group all six samples showed new bone formation and in the ‘membrane with BAG mesh’ group new bone was seen in three samples. In cases where radiological ossification of the defect was complete, new bone formation was mainly seen at the outside of the membrane. The site treated with combination of membrane and BAG mesh seems to have healed more slowly, which is consistent with the radiological findings published earlier (Asikainen et al. 2005).
In the 24-week group, new bone formation was approximately at the same level on both sides. Compared to the radiological findings, the histological ossification of the defects proceeded as predicted from the radiographs. The membrane and BAG mesh group was healing initially slower, but at 24 weeks the difference in the ossification between the sites seems to have diminished.
No noticeable swelling was evident with tyrosine-derived polycarbonates; they do not take up more than 5% of water during any stage of degradation. The lack of water uptake means that tyrosine-derived implants maintain their profile for longer periods than derivatives of lactide or glycolide, and, hence, can be used in applications where swelling could result in failure of implantation (Hooper et al. 1998; James et al. 1999).
PDTE carbonate has not proven to cause accumulation of acidic degradation products into the implant surrounding tissues. The acid groups formed during the degradation of tyrosine-derived polycarbonates are polymer-bound and not able to diffuse into the surrounding media (Tangpasuthadol et al. 2000b) to cause pH decrease and possible osteolytic reactions around implants. This, however, is not the case with PLA and PGA.
To the best of our knowledge no previous studies concerning bioabsorbable membranes with similar experimental setup exists. The good healing and ossification obtained by using PDTE carbonate membranes are encouraging. This material, however, should be compared with more traditional membranes of PLA, PGA or PLGA to ensure the efficacy of the new membrane.
5. Conclusions
Based on this study it can be concluded that PDTE carbonate is effective in treating experimental bone defects without BAG as supportive matrix. However, care must be taken in the design of the fixation in future experiments. Properties of PDTE carbonate and results of this study suggest testing of the material in experimental applications, where the use of PLA and PGA has resulted in compromised results.
Acknowledgments
This study was supported by the State Subsidiary grant (TYH1331 and TYH4307), Finnish Dental Society Apollonia and National Technology Agency of Finland. Authors wish to thank Histola Ltd, Tampere, Finland for preparing the samples.
References
- Aaboe M, Pinholt E.M, Hjorting-Hansen E. Healing of experimentally created defects: a review. Br. J. Oral Maxillofac. Surg. 1995;33:312–318. doi: 10.1016/0266-4356(95)90045-4. doi:10.1016/0266-4356(95)90045-4 [DOI] [PubMed] [Google Scholar]
- Ashammakhi N, Mäkelä A, Vihtonen K, Rokkanen P, Törmälä P. Absorbable membranes for bone repair: an experimental study on rabbits. Clin. Mater. 1994;17:113–118. doi: 10.1016/0267-6605(94)90133-3. doi:10.1016/0267-6605(94)90133-3 [DOI] [PubMed] [Google Scholar]
- Ashammakhi N, Mäkela E.A, Vihtonen K, Rokkanen P, Törmälä P. Repair of bone defects with absorbable membranes. A study on rabbits. Ann. Chir. Gynaecol. 1995;84:309–315. [PubMed] [Google Scholar]
- Asikainen A.J, et al. Tyrosine derived polycarbonate membrane is useful for guided bone regeneration in rabbit mandibular defects. J. Mater. Sci. Mater. Med. 2005;16:753–758. doi: 10.1007/s10856-005-2613-6. doi:10.1007/s10856-005-2613-6 [DOI] [PubMed] [Google Scholar]
- Baumann A, Burggasser G, Gauss N, Ewers R. Orbital floor reconstruction with an alloplastic resorbable polydioxanone sheet. Int. J. Oral Maxillofac. Surg. 2002;31:367–373. doi: 10.1054/ijom.2001.0219. doi:10.1054/ijom.2001.0219 [DOI] [PubMed] [Google Scholar]
- Brink M, Turunen T, Happonen R.P, Yli-Urpo A. Compositional dependence of bioactivity of glasses in the system Na2O–K2O–MgO–CaO–B2O3–P2O5–SiO2. J. Biomed. Mater. Res. 1997;37:114–121. doi: 10.1002/(sici)1097-4636(199710)37:1<114::aid-jbm14>3.0.co;2-g. doi:10.1002/(SICI)1097-4636(199710)37:1<114::AID-JBM14>3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- Cutright D.E, Hunsuck E.E. The repair of fractures of the orbital floor using biodegradable polylactic acid. Oral. Surg. Oral Med. Oral Pathol. 1972;33:28–34. doi: 10.1016/0030-4220(72)90204-6. doi:10.1016/0030-4220(72)90204-6 [DOI] [PubMed] [Google Scholar]
- Cutright D.E, Hunsuck E.E, Beasley J.D. Fracture reduction using a biodegradable material, polylactic acid. J. Oral Surg. 1971;29:393–397. [PubMed] [Google Scholar]
- Dietz A, Ziegler C.M, Dacho A, Althof F, Conradt C, Kolling G, von Boehmer H, Steffen H. Effectiveness of a new perforated 0.15 mm poly-p-dioxanon-foil versus titanium-dynamic mesh in reconstruction of the orbital floor. J. Craniomaxillofac. Surg. 2001;29:82–88. doi: 10.1054/jcms.2000.0188. [DOI] [PubMed] [Google Scholar]
- Ertel S.I, Kohn J. Evaluation of a series of tyrosine-derived polycarbonates as degradable biomaterials. J. Biomed. Mater. Res. 1994;28:919–930. doi: 10.1002/jbm.820280811. doi:10.1002/jbm.820280811 [DOI] [PubMed] [Google Scholar]
- Fiordeliso J, Bron S, Kohn J. Design, synthesis, and preliminary characterization of tyrosine-containing polyarylates: new biomaterials for medical applications. J. Biomater. Sci. Polym. Ed. 1994;5:497–510. [PubMed] [Google Scholar]
- Gupta A.S, Lopina S.T. l-Tyrosine-based backbone-modified poly(amino acids) J. Biomater. Sci. Polym. Ed. 2002;13:1093–1104. doi: 10.1163/156856202320813819. doi:10.1163/156856202320813819 [DOI] [PubMed] [Google Scholar]
- Hooper K.A, Macon N.D, Kohn J. Comparative histological evaluation of new tyrosine-derived polycarbonates and poly(l-lactic acid) as a function of polymer degradation. J. Biomed. Mater. Res. 1998;41:443–454. doi: 10.1002/(sici)1097-4636(19980905)41:3<443::aid-jbm14>3.0.co;2-j. doi:10.1002/(SICI)1097-4636(19980905)41:3<443::AID-JBM14>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- Howell S.J, Sear Y.M, Yeates D, Goldacre M, Sear J.W, Foex P. Risk factors for cardiovascular death after elective surgery under general anaesthesia. Br. J. Anaesth. 1998;80:14–19. doi: 10.1093/bja/80.1.14. [DOI] [PubMed] [Google Scholar]
- Hurzeler M.B, Quinones C.R, Schupbach P. Guided bone regeneration around dental implants in the atrophic alveolar ridge using a bioresorbable barrier. An experimental study in the monkey. Clin. Oral Implants Res. 1997;8:323–331. doi: 10.1034/j.1600-0501.1997.080411.x. doi:10.1034/j.1600-0501.1997.080411.x [DOI] [PubMed] [Google Scholar]
- James K, Levene H, Parsons J.R, Kohn J. Small changes in polymer chemistry have a large effect on the bone-implant interface: evaluation of a series of degradable tyrosine-derived polycarbonates in bone defects. Biomaterials. 1999;20:2203–2212. doi: 10.1016/s0142-9612(99)00151-9. doi:10.1016/S0142-9612(99)00151-9 [DOI] [PubMed] [Google Scholar]
- Kellomäki M, Pohjonen T, Törmälä P, editors. Self reinforced polylactides optimization of degradation and mechanical properties. Citus Books; London: 2003. [Google Scholar]
- Klokkevold P.R, Subar P, Fukayama H, Bertolami C.N. Effect of chitosan on lingual hemostasis in rabbits with platelet dysfunction induced by epoprostenol. J. Oral Maxillofac. Surg. 1992;50:41–45. doi: 10.1016/0278-2391(92)90194-5. [DOI] [PubMed] [Google Scholar]
- Kohn J. Design, synthesis, and possible applications of pseudo-poly(amino acids) Trends Polym. Sci. 1993;1:206–212. [Google Scholar]
- Kohn J, Langer R. Polymerization reactions involving the side chains of alpha-l-amino acids. J. Am. Chem. Soc. 1987;109:817–820. doi:10.1021/ja00237a030 [Google Scholar]
- Kulkarni R.K, Pani K.C, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch. Surg. 1966;93:839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- Loos B.G, Louwerse P.H, Van Winkelhoff A.J, Burger W, Gilijamse M, Hart A.A, van der Velden U. Use of barrier membranes and systemic antibiotics in the treatment of intraosseous defects. J. Clin. Periodontol. 2002;29:910–921. doi: 10.1034/j.1600-051x.2002.291006.x. doi:10.1034/j.1600-051X.2002.291006.x [DOI] [PubMed] [Google Scholar]
- Malette W.G, Quigley H.J, Gaines R.D, Johnson N.D, Rainer W.G. Chitosan: a new hemostatic. Ann. Thorac. Surg. 1983;36:55–58. doi: 10.1016/s0003-4975(10)60649-2. [DOI] [PubMed] [Google Scholar]
- Pruthi V.K, Gelskey S.C, Mirbod S.M. Furcation therapy with bioabsorbable collagen membrane: a clinical trial. J. Can. Dent. Assoc. 2002;68:610–615. [PubMed] [Google Scholar]
- Pyhältö T, Lapinsuo M, Patiala H, Pelto M, Törmälä P, Rokkanen P. Fixation of distal femoral osteotomies with self-reinforced poly(desamino tyrosyl-tyrosine ethyl ester carbonate) rods: an experimental study on rats. J. Orthop. Sci. 2002;7:549–556. doi: 10.1007/s007760200098. doi:10.1007/s007760200098 [DOI] [PubMed] [Google Scholar]
- Ravi Kumar M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. doi:10.1016/S1381-5148(00)00038-9 [Google Scholar]
- Schenk R. Zur histologischen Verarbeitung von unentkalkten Knochen. Acta Anat. 1965;60:3–19. [PubMed] [Google Scholar]
- Silver F.H, Marks M, Kato Y.P, Li C, Pulapura S, Kohn J. Tissue compatibility of tyrosine-derived polycarbonates and polyiminocarbonates: an initial evaluation. J. Long Term Eff. Med. Implants. 1992;1:329–346. [PubMed] [Google Scholar]
- St John T.A, Vaccaro A.R, Sah A.P, Schaefer M, Berta S.C, Albert T, Hilibrand A. Physical and monetary costs associated with autogenous bone graft harvesting. Am. J. Orthop. 2003;32:18–23. [PubMed] [Google Scholar]
- Suuronen R, Pohjonen T, Hietanen J, Lindqvist C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J. Oral Maxillofac. Surg. 1998;56:604–614. doi: 10.1016/s0278-2391(98)90461-x. doi:10.1016/S0278-2391(98)90461-X discussion 614–615. [DOI] [PubMed] [Google Scholar]
- Suuronen R, Kallela I, Lindqvist C. Bioabsorbable plates and screws: current state of the art in facial fracture repair. J. Craniomaxillofac. Trauma. 2000;6:19–27. discussion 28–30. [PubMed] [Google Scholar]
- Tangpasuthadol V, Pendharkar S.M, Kohn J. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part I: study of model compounds. Biomaterials. 2000a;21:2371–2378. doi: 10.1016/s0142-9612(00)00104-6. doi:10.1016/S0142-9612(00)00104-6 [DOI] [PubMed] [Google Scholar]
- Tangpasuthadol V, Pendharkar S.M, Peterson R.C, Kohn J. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part II: 3-yr study of polymeric devices. Biomaterials. 2000b;21:2379–2387. doi: 10.1016/s0142-9612(00)00105-8. doi:10.1016/S0142-9612(00)00105-8 [DOI] [PubMed] [Google Scholar]
- Törmälä P. Biodegradable self-reinforced composite materials; manufacturing structure and mechanical properties. Clin. Mater. 1992;10:29–34. doi: 10.1016/0267-6605(92)90081-4. doi:10.1016/0267-6605(92)90081-4 [DOI] [PubMed] [Google Scholar]


