Abstract
During the development of the nervous system, outgrowing axons often have to travel long distances to reach their target neurons. In this process, outgrowing neurites tipped with motile growth cones rely on guidance cues present in their local environment. These cues are detected by specific receptors expressed on growth cones and neurites and influence the trajectory of the growing fibres. Neurite growth, guidance, target innervation and synapse formation and maturation are the processes that occur predominantly but not exclusively during embryonic or early post-natal development in vertebrates. As a result, a functional neural network is established, which is usually remarkably stable. However, the stability of the neural network in higher vertebrates comes at an expensive price, i.e. the loss of any significant ability to regenerate injured or damaged neuronal connections in their central nervous system (CNS). Most importantly, neurite growth inhibitors prevent any regenerative growth of injured nerve fibres. Some of these inhibitors are associated with CNS myelin, others are found at the lesion site and in the scar tissue. Traumatic injuries in brain and spinal cord of mammals induce upregulation of embryonic inhibitory or repulsive guidance cues and their receptors on the neurites. An example for embryonic repulsive directional cues re-expressed at lesion sites in both the rat and human CNS is provided with repulsive guidance molecules, a new family of directional guidance cues.
Keywords: RGM, neogenin, regeneration, brain injury, stroke, iron metabolism
1. Discovery of repulsive guidance molecule
Much has been learnt during the past two decades about the process of guidance of neurites to their correct targets in the central nervous system (CNS). In this process, outgrowing neurites, with their motile growth cones (figure 1), rely on attractive and repulsive or inhibitory cues in their local environment, which tell them where to go. An artificial distinction is made in purely neurite growth-inhibitory molecules, predominantly active during adulthood, like the myelin-associated proteins: Nogo-A, myelin-associated glycoprotein (MAG), oligodendrocyte myelin glycoprotein (OMGp) and chondroitin sulphate proteoglycans (CSPGs), and the repulsive guidance cues acting during embryonic development. Several families of guidance molecules have been identified, with important roles during the process of wiring up the central and peripheral nervous system (PNS). The largest group of these guidance molecules is the semaphorins; this contains at least 20 different members which bind to neuropilin and plexin receptors (Tessier-Lavigne & Goodman 1996; Mueller 1999; Kruger et al. 2005). Another group of guidance molecules are the ephrins and their receptors, the Eph receptor tyrosine kinases. Chemotropic guidance cues like the netrins bind to receptors of the deleted in colorectal cancer (DCC) family and to UNC-5 receptors (UNC from uncoordinated, a phenotype observed in certain Caenorhabditis elegans mutants). Slits bind to Roundabout (Robo) receptors and the latest addition of directional guidance molecules, the RGMs (repulsive guidance molecules) have recently been shown to bind to neogenin, a receptor that binds to netrin also.
Figure 1.
A retinal growth cone growing on a laminin substratum. Axon and growth cones are stained by the F-actin marker Alexa-phalloidin. Filopodia, lamellipodia and axonal protrusions (microspikes) are clearly visible.
In this review, we will concentrate on the RGMs and will summarize numerous functions of RGMs exerted during development by extending these to the situation in adult animals and humans.
RGM, the first candidate of a topographic guidance cue, was originally described in 1990 as a glycosylphosphatidylinositol-anchored (GPI-anchor) glycoprotein with a molecular weight of 33/35 kDa having repulsive and growth cone collapse-inducing activities in the chick retinotectal system (Stahl et al. 1990). The projection of retinal ganglion cell (RGC) axons to their target organ, the optic tectum, is a suitable system to analyse axon guidance in the CNS, because retinal axons form a topographic projection in the tectum, maintaining neighbourhood relationships in the retina and the tectal target organ. In the chick embryo, nearly 2 million fibres from each eye grow towards the tectum, invade it and form synapses with their tectal target neurons. This so-called retinotectal projection is organized in the following way: retinal axons from the temporal side project to the anterior tectum, those from the nasal side project to the posterior tectum and in the dorsoventral axis, dorsal retinal axons terminate in ventral tectum and ventral retinal axons in dorsal tectum.
The question of how these topographic projections are formed remained an enigma for more than 60 years. A crucial step forward was the formulation of a testable model, the chemoaffinity hypothesis by Roger W. Sperry. Sperry's model, later modified by Gierer & Bonhoeffer, suggested that gradients of position-dependent directional cues on the surface of the optic tectum are read by ingrowing retinal axons (Sperry 1963; Gierer 1981; Bonhoeffer & Gierer 1984). However, the nature of these graded directional cues remained elusive and the development of in vitro assays, called stripe and collapse assay, proved to be suitable tools on the path to identify the graded tectal guidance cues (Walter et al. 1987a; Cox et al. 1990). In the stripe assay, membranes from anterior and posterior tectum are arranged as alternating parallel stripes. Offered as a growth substrate to outgrowing retinal axons from the temporal or the nasal retina, temporal axons prefer to grow on anterior tectal lanes, whereas the nasal axons grow equally well on both anterior and posterior stripes. The preference of temporal axons for the anterior tectal membranes, in line with their in vivo specificity, was caused by temporal-specific repellent or inhibitory cues present at higher amounts in posterior than in anterior tectal membranes (Walter et al. 1987b). The temporal-specific inhibitory activity of posterior tectal membranes was also evident in the collapse assay with posterior tectal membrane vesicles inducing collapse of temporal growth cones, but not of nasal growth cones (Cox et al. 1990). The repulsive cues could be removed by pre-treating posterior membranes with the bacterial enzyme phosphatidylinositol-specific phospholipase C (PI-PLC), proving that the temporal-specific repellent or inhibitory cues are linked to the membrane via a GPI-anchor (Walter et al. 1990). In a biochemical analysis, the first candidate was identified as a 33 kDa GPI-anchored glycoprotein, and a vesicle fraction highly enriched with this protein was very active in both stripe and collapse assays (Stahl et al. 1990). A monoclonal IgM antibody (F3D4), raised against the GPI-anchored 33 kDa protein, revealed that this protein is expressed in a gradient within the optic tectum at higher levels in posterior than in the anterior tectum and yielded first functional albeit indirect data that this protein is involved in repulsive guidance of temporal retinal axons in vitro (Muller et al. 1996). The 33 kDa protein was named RGM based on these results.
The functional activity of the 33 kDa enriched vesicle fraction, the indirect functional activity of the monoclonal antibody and the graded expression in the chick tectum stimulated the search for identifying the gene coding for RGM (Mueller 1997). After many unsuccessful biochemical endeavours, a proteomic approach was finally successful and the RGM gene was identified (Monnier et al. 2002).
2. Molecular biology of repulsive guidance molecules and neogenin
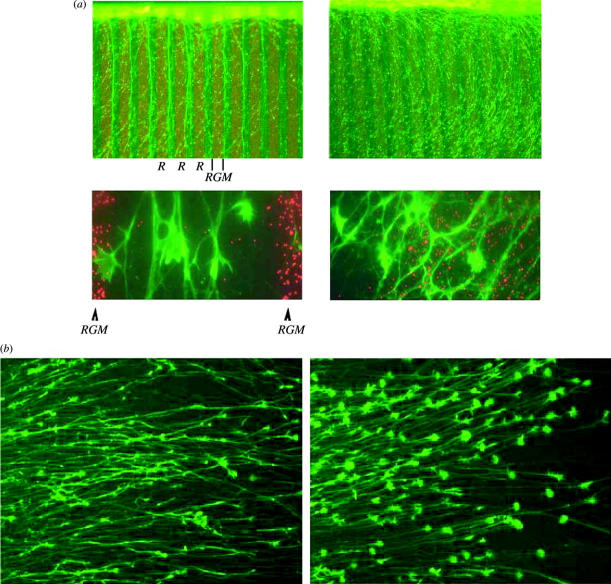
Analysis of the chick RGM gene (chRGM) revealed, despite its similarities in function and expression with the ephrins, that RGM does not share any sequence motif with the ephrins. This suggests that the function of RGM in retinotectal map formation is different from the function of the ephrins. Recombinant chRGM, having a molecular weight of 33 kDa, was active in the relevant in vitro stripe and collapse assays, in either a membrane-anchored or a soluble form (figure 2a,b). As expected, the localization of the chRGM mRNA showed the graded expression pattern in the chick tectum with higher expression levels in posterior than in anterior tectum, thereby proving that the correct gene was identified (Monnier et al. 2002).
Figure 2.
Recombinant repulsive guidance molecule (RGM) is active in both stripe and collapse assays. (a) RGM-transfected membranes (left) but not control-transfected membranes (right) are repulsive for temporal retinal axons. (b) Supernatants from RGM-transfected cells (left) but not from mock-transfected cells (right) induce collapse of temporal retinal growth cones. RGM concentration used was 10 ng ml−1.
Chick RGM is a GPI-anchored, proline- and cysteine-rich glycoprotein, consisting of 432 amino acids. Surprisingly, the reported 33 kDa of RGM turned out to be much smaller than the calculated molecular weight of the protein (49 kDa). Peptide sequencing of the N-terminus of the active chick RGM protein, however, revealed that chick RGM starts with a conserved motif (Pro-His-Leu-Arg-Thr) and the first 149 amino acids are cleaved off by an unknown protease (Monnier et al. 2002).
Orthologues of chRGM are described in vertebrates, e.g. in human (with 82% amino acid identity), mouse (82% amino acid identity), clawed frogs (Xenopus laevis with 80% amino acid identity) and zebrafish (Danio rerio with 69% amino acid identity) as well as in invertebrates, e.g. in C. elegans (33% amino acid identity). A comparison of the human, rat and chick sequences is shown in figure 3a.
Figure 3.
Amino acid sequences of repulsive guidance molecule (RGM) orthologues and homologues. (a) Amino acid sequence of human, rat and chick RGM A. (b) Amino acid sequence of human RGM A, B and C isoforms.
At least three homologues of RGM, RGM A, RGM B (DRAGON) and RGM C (hemojuvelin HJV, HFE2) are found in vertebrates (table 1). Human RGM A shares 50% amino acid identity with hRGM B and nearly 47% identity with hRGM C (figure 3b). RGM A is the most closely related RGM orthologue of chRGM (sharing 80% amino acid identity). The hRGM A gene is localized on chromosome 15q26.1 and encodes a 450 amino acid protein with a predicted molecular mass of 49 kDa. Two alternative splice forms are described, differing in a short N-terminal amino acid stretch of 16 amino acids. The human RGM B gene is localized on chromosome 5q21.1. It encodes a 478 amino acid protein with a molecular weight of 50–55 kDa and truncated versions with molecular weights of 35–40 kDa (Samad et al. 2004). The human RGM C gene is localized on chromosome 1q21.1. Several alternatively spliced variants, e.g. three different forms in human, are described and the longest mRNA isoform encodes a 426 amino acid protein of approximately 49 kDa. RGM C is detectable as a cell-associated, GPI-anchored protein and as a soluble protein in human plasma and serum (Lin et al. 2005).
Table 1.
Nomenclature, features and chromosomal location of repulsive guidance molecule (RGM) family members.
| original name | synonyms | chromosomal location (humans) |
|---|---|---|
| RGM A | RGM | chr. 15 |
| RGM B | DRAGON | chr. 5 |
| RGM C | hemojuvelin/DRAGON-like muscle (DL-M) | chr. 1 |
None of the RGMs shows significant homology to any other protein in the database. Common features of the RGMs are a N-terminal signal peptide, an RGD (Arg-Gly-Asp) site, a tri-peptide sequence which has been proposed to play a role in integrin-mediated cell adhesion, a partial von Willebrand factor type D domain (vWF-typeD; including a highly conserved proteolytic cleavage site), a hydrophobic domain of yet unknown function and the C-terminal end necessary for attaching the protein to the cell membrane via a GPI-anchor (figure 4; Monnier et al. 2002). The sequence score for the addition of the GPI-anchor differs among the RGM family members, raising the possibility that not all members are processed with the same efficiency. In agreement, RGM A and RGM C are efficiently secreted, whereas mouse RGM B was described to be more abundantly localized in the endoplasmic reticulum-/golgi-compartment of RGM B-transfected COS cells (a cell line from African green monkey kidney) (Niederkofler et al. 2004). Another group reported on cell surface localization of RGM B/DRAGON (Samad et al. 2004). Based on the data obtained with chick RGM, it was assumed that RGM A requires specific proteolytic processing for its repulsive and neurite growth-inhibiting activity. The high conservation of the amino acid sequence at the chRGM cleavage site at residue 149 (corresponds to amino acid 168 in human RGM A; FGDPHL) in all RGM members and throughout the animal kingdom first suggested that an unknown protease is responsible for this cleavage. Recent data obtained with recombinant human RGM C/hemojuvelin, however, indicate that RGM proteins also possess autocatalytic activity or carry an intrinsically unstable bond, thus regulating their activities. Zhang et al. (2005) found a conserved peptide sequence (Phe-Gly-Asp-Pro-His-Leu) including an acid-labile aspartic acid–proline bond and showed that incubation of purified RGM C/hemojuvelin at pH 5.5 increased cleavage of this bond and yielded higher amounts of the 33 kDa fragment. As mentioned earlier, this autoproteolytic/instability cleavage site is highly conserved, and could represent a mechanism whereby the activity of the RGM proteins is regulated by pH changes or autoproteolytic activity. Whether this cleavage of the RGM proteins is necessary for binding to the RGM receptor or whether it enhances binding strength remains to be shown.
Figure 4.
Schematic drawing of repulsive guidance molecule protein.
In an expression cloning strategy using a fusion protein of alkaline phosphatase–chick RGM (amino acids 28–403), neogenin was identified as a single clone out of 480 000 independent clones of a mouse adult brain library expressed in COS-7 cells (Rajagopalan et al. 2004). Originally, neogenin was isolated from embryonic chicken cerebellum as a DCC homologue (Vielmetter et al. 1994). DCC was originally characterized as a candidate tumour suppressor gene contributing to the malignant phenotype of colorectal cancer in humans (Fearon et al. 1990). Later, it was shown that DCC also participates in axonal guidance. This was already suggested through its homology with the C. elegans UNC-40 protein, the receptor of UNC-6, a ligand structurally related to laminin (Keino-Masu et al. 1996) and involved in the guidance of axons and migrating neurons (Chan et al. 1996). The human homologue of UNC-6, netrin-1, binds DCC, resulting in growth cone expansion, stimulation of neurite outgrowth and commissural axon guidance along a netrin gradient (Keino-Masu et al. 1996; Shekarabi et al. 2005). Netrin-1 also binds to neogenin, but the exact role in axon guidance for the neogenin/netrin-1 interaction has not yet been proven.
The neogenin gene (NEO1) is located on human chromosome 15q22.3 and encodes a 1461 amino acid, glycosylated protein of approximately 190 kDa. Two alternatively spliced forms of neogenin are found in chicken, both differing by a 159 bp sequence in the cytoplasmic domain, which interestingly is coding for an amino acid stretch with a high degree of histidines and prolines (Meyerhardt et al. 1997). Three additional splice variants, which differ in the extracellular domain, are described in mice (Keeling et al. 1997).
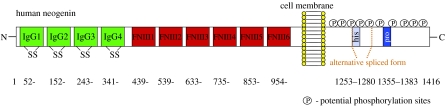
The structural conformation places neogenin and DCC into the neuronal cell adhesion molecule family (N-CAM) of the immunoglobulin (Ig) superfamily (figure 5). The extracellular domain of human neogenin displays common features of this family, containing four V-like Ig like domains and six fibronectin type-III (FN-III) like domains. Eight potential asparagine N-linked glycosylation sites are present in the extracellular domain. The cytoplasmic domain contains 338 amino acids with 14 potential phosphorylation sites.
Figure 5.
Schematic drawing of the repulsive guidance molecule receptor neogenin.
Overall, the amino acid sequence of neogenin is highly conserved in vertebrates, e.g. in mouse (91% amino acid identity) and chicken (86% amino acid identity) and in invertebrates, e.g. in C. elegans (UNC-40, 31% amino acid identity) and Drosophila melanogaster (frazzled, 31% amino acid identity; Chan et al. 1996; Kolodziej et al. 1996). Comparison of human neogenin and human DCC has revealed that both proteins have the identical domain structure and 50% amino acid identity. Therefore, it is very likely that DCC and neogenin diverged from a common ancestor. A high degree of similarity is found between the fourth FN-III and the fourth Ig domain, suggesting that the netrin-binding site on DCC and neogenin involves one of these domains. The cytoplasmatic domains of both proteins are less conserved, with only 37% identity at the amino acid level. A much higher amino acid identity (97%) is, however, observed in the P3 domain of the intracellular domains of neogenin and DCC, a region shown to be responsible for focal adhesion kinase (FAK) binding to both proteins (Ren et al. 2004). Besides this small homologous region, the cytoplasmatic domain of neogenin shows no similarity with any other protein in the database.
The binding affinity of RGM (Rajagopalan et al. 2004) to neogenin (Kd=230 pM) is much higher compared to netrin-1 (Kd=2 nM). How neogenin coordinates the binding of both ligands is currently not known. Netrin-1 has been reported to bind to the FN-III-like domain of DCC and, in a similar way, RGM has been reported to bind to the FN-III domains of neogenin (Geisbrecht et al. 2003; Rajagopalan et al. 2004). Netrin-1 and RGM share no sequence similarity and although both ligands might bind to the same neogenin region, their binding could still differ, with netrin-1 binding to another FN-III repeat than RGM. Whether RGM and netrin-1 interact is currently not known, but such receptor-dependent or -independent interact could have important synergistic or antagonistic consequences (Rajagopalan et al. 2004).
3. Signal transduction of repulsive guidance molecule proteins
Many repulsive or inhibitory axon guidance cues work by stimulating the Rho-GTPase pathway (Mueller et al. 2005). Rho-GTPases belong to the Ras superfamily of GTP-binding proteins and function like molecular switches being active in GTP-bound state and inactive in the GDP-bound state (Burridge & Wennerberg 2004). Three different types of regulatory proteins influence the ratio of GTP-Rho versus GDP-Rho, and thereby determine their cellular activity. Guanine nucleotide exchange factors (RhoGEFs) stimulate the exchange of GDP for GTP, and activate the Rho proteins. GTPase-activating proteins, RhoGAPs, enhance the intrinsic GTPase activity of Rho-GTPases and inactivate Rho proteins and the guanine nucleotide dissociation inhibitors (RhoGDIs) keep the Rho protein in the GDP bound state and sequester it in the cytosol, preventing its activation. On the basis of work done in fibroblasts, three functionally different Rho proteins have been described with RhoA being responsible for stress fibre formation and focal complex formation, Rac inducing lamellipodia and membrane ruffles and CDC42 inducing filopodia (Hall 1998). In growing neurites, these archetypical members of the Rho family exert similar functions (Mackay et al. 1995) and a role of RhoA activation in growth cone collapse was shown for many different repulsive and inhibitory proteins, like ephrin-A5, semaphorin-3A, wnts, CSPGs, Nogo-A, MAG and OMGp (Mueller 1999; Huber et al. 2003; Govek et al. 2005). RGMs induce growth cone collapse and act as repulsive guidance cues for growing neurites and similarly ephrin-A5 induces collapse of retinal growth cones by activating the RhoA–Rho kinase pathway (Wahl et al. 2000; Shamah et al. 2001). Blocking this pathway by a bacterial membrane-permeable Rho inhibitor, the C3 transferase, or by the well-known Rho kinase inhibitor, Y-27632, prevents completely the RGM-induced growth cone collapse and its repulsive guidance activity. Owing to the inhibition of both the ephrins and the RGMs in stripe assays with native tectal membranes, repulsive activity of these membranes is neutralized by the inhibitors of the RhoA–Rho kinase pathway (B. K. Mueller & S. Wahl 2000, unpublished work) and it is possible that a functional RhoA–Rho kinase pathway is crucial for proper formation of the retinotectal map. It is not known how the RhoA–Rho kinase pathway is activated by binding of RGM to neogenin. Rho activators like the RhoGEFs, ephexin and Vav2, playing an important role in ephrin-A1-induced growth cone collapse in mouse retinal axons (Cowan et al. 2005; Sahin et al. 2005) might be involved, but no data have been generated yet to suggest their involvement downstream of RGM–neogenin.
Here again, another similarity occurs between the ephrins and the RGMs in the crucial elements of the signal transduction pathway, underlying the repulsive or inhibitory function of these guidance cues.
With two different ligands, RGM and netrin-1, binding to neogenin and exerting opposing repulsive or attractive axon guidance functions, questions focus on the signal transduction pathway downstream of neogenin. As mentioned earlier, neogenin and DCC bind FAK via their highly homologous intracellular p3 domain and FAK has been shown to be involved in netrin-1-stimulated outgrowth of cortical axons (Liu et al. 2004; Ren et al. 2004). Netrin-1 binding to DCC induces activation of FAK and the cytoplasmic tyrosine kinase fyn, and both are involved in netrin-1-mediated axon outgrowth and attractive turning (Liu et al. 2004). In addition, a cell-signalling complex consisting of Cdc42, Rac1, Pak1 and N-WASP is recruited to DCC by netrin-1 binding and induces Rac activation with subsequent growth cone expansion of commissural neurons (Shekarabi et al. 2005). The model proposed suggests that upon netrin-1 binding to DCC, a complex of the above proteins is build up at the scaffold protein Nck1, which is constitutively bound to DCC (Li et al. 2002). FAK, bound to DCC, recruits and stimulates the Fyn and Src tyrosine kinases and a currently unknown GEF is assumed to activate Cdc42 and Rac, thereby explaining the observed cellular phenotype of netrin-1-stimulated expansion of the growth cone structure (Shekarabi et al. 2005). It is not known whether netrin-1 binding to neogenin stimulates the same pathway. The high amino acid identity of the p3 domains suggest that this might be true, and it is therefore crucial to understand how the competition of RGM and netrin-1 for binding to neogenin results in the activation of the RhoA–Rho-kinase pathway or the Cdc42-Rac-Pak1 pathway. Potential candidates of RhoGEFs involved in the RGM–neogenin pathway are PDZ-RhoGEF (PSD-95, discs large, 20-1-Rho guanine nucleotide exchange factor) and LARG (leukaemia-associated RhoGEF). These RhoGEFs are phosphorylated by FAK (Chikumi et al. 2002) and are shown to link plexin-B activation by the growth collapse inducing Sema 4D to RhoA activation (Swiercz et al. 2002). These few findings suggest that identifying the RhoGEFs downstream of neogenin is important to understand repulsive and attractive axon guidance mechanisms, mediated by RGM and netrin-1, respectively.
4. Functional roles of repulsive guidance molecule proteins and neogenin in axon guidance
As mentioned earlier, chicken RGM was shown to be an axon guidance molecule of the retinotectal system; it is repulsive for RGC axons from the temporal half of the retina and induces collapse of their growth cones. The in vivo role in retinotectal map formation is addressed in RGM A knockout mice. Expression of RGM A in the wild-type superior colliculus, the mammalian homologous structure of the non-mammalian optic tectum, revealed that contrary to the expression gradient of chick RGM in the tectum, RGM A showed no graded expression along the anterior–posterior axis, in contrast to RGM B, which exhibited higher expression levels in posterior than in anterior mouse tectum (Niederkofler et al. 2004). Gene inactivation of RGM A does not reveal any clear mapping or pathfinding errors and it is possible that RGM B with its graded expression pattern in the mouse tectum is more important for mapping than RGM A. On the other side, ephrins and their receptors are shown to be important for retinotectal map formation (Cheng et al. 1995; Drescher et al. 1995; McLaughlin & O'Leary 2005). Ephrins are membrane-anchored guidance molecules (mouse, eight different members), which are grouped into two classes based on their mode of membrane anchorage: A-ephrins are GPI-anchored molecules whereas B-ephrins possess a transmembrane domain. Receptors of the ephrins form the largest family of receptor tyrosine kinases, the Eph receptor tyrosine kinases (mouse, 14 different members) and are grouped into two subfamilies: the Eph-A receptors, binding preferentially A-ephrins, and Eph-B receptors binding preferentially B-ephrins. Cross-binding between subfamilies has been reported, and binding redundancy with many different ephrin ligands binding to one Eph receptor and vice versa with binding of one ligand to several different Eph receptors is also a common feature of the ephrin–Eph system (McLaughlin & O'Leary 2005). Like RGM, ephrin-A2 and ephrin-A5 also show a graded expression pattern in the tectum opticum and many independent in vivo data with knockout and transgenic mice (Frisen et al. 1998; Feldheim et al. 2000, 2004; McLaughlin & O'Leary 2005) suggest that the ephrins and their receptors are important players in establishing the topographic retinotectal projection in vertebrates. The functional redundancy due to the presence of ephrins and another RGM family member, mRGM B, in the mouse tectum might explain that a retinotectal mapping phenotype was not observed in RGM A knockout mice. Additional in vivo studies are required to reveal the exact function of RGMs in topographic map formation in vertebrates since in a recent meeting presentation describing RGM B knockout mice, no aberrant phenotype in mapping along the anterior–posterior axis of the mouse superior colliculus is reported (Salie et al. 2004). With both single RGM A and B knockout mice showing no mapping phenotype along the ap-axis, the role of RGM A and B in retinotectal map formation is currently not clear and will require knockout of both genes to get a disturbed mapping phenotype. Still, however, functional similarities between A-ephrins and RGMs are obvious: both types of ligands are repulsive for temporal retinal axons, both types of ligands are graded along the anterior–posterior axis and temporal axons express higher levels of their receptors, EphA3/EphA5/EphA6 receptors and neogenin, respectively (Rajagopalan et al. 2004; McLaughlin & O'Leary 2005). In addition, ephrin-A2 and ephrin-A5 are also found on the surface of retinal axons, with nasal axons showing higher expression levels than temporal axons (Hornberger et al. 1999). Identical to ephrin-A2 and ephrin-A5, chick nasal retinal axons carry higher levels of RGM than temporal retinal axons (B. K. Mueller 2002, unpublished work). Such an axonal expression of repulsive guidance cues modifies sensitivity of the retinal axons, as shown for ephrin-A2 and ephrin-A5 in the chick retinotectal system (Hornberger et al. 1999), and this type of sensitivity tuning might also occur in repulsive axon guidance by RGM and its receptor neogenin. The retinal fibres carrying more neogenin also express more RGM at their surface. An alternative explanation for coexpression of repulsive ligand and its receptor on the same growth cone was developed recently on work done with spinal motor neurons (Marquardt et al. 2005). Similar to embryonic retinal axons, developing spinal growth cones express EphA receptors and ephrin-A ligands on the same growth cone, segregated into distinct membrane domains and inducing opposing activities like axon attraction and axon repulsion. In this way, growing motor axons can simultaneously be attracted by binding of EphA receptors to the growth cone ephrin-A and repelled by binding of ephrin-A to the growth cone EphA receptors (Marquardt et al. 2005). If we assume that attractive, neurite growth-stimulating activities are mediated by a more active Cdc42/Rac1 pathway, whereas the repulsive, inhibitory activity is mediated by a more active RhoA pathway, then the lateral compartmentalization of activators of these pathways in a single growth cone needs to be examined. In this regard, ephexin and VAV2 are very interesting candidates of RhoGEFs, because they have the potential of activating both Cdc42/Rac1 and RhoA pathways in a single growth cone (Cowan et al. 2005; Sahin et al. 2005).
It remains to be proven if such a segregation of RGM and also its neogenin receptor occurs in single growth cones and if such mechanisms are more important, then fine-tuning induced by cis interactions of receptor and ligand in the same membrane plane.
Neogenin was the only receptor candidate identified in the expression cloning approach, but other proteins interacting with RGM A and B have recently been identified.
These RGM interaction partners are members of the bone morphogenetic protein (BMP) family and both BMP2 and BMP4 have been reported to bind to RGM A or RGM B (Babitt et al. 2005; Samad et al. 2005). Both RGMs signal via BMP receptors, ALK3, ALK6 and Smad 1; no clear differences in the interaction of the RGM family members with the BMPs have been detected by the authors and both RGMs have been reported to enhance BMP signalling (Babitt et al. 2005; Samad et al. 2005). The role of BMP proteins in retinotectal map formation is currently not clear. Ventroptin is a secreted molecule, expressed in a ventral high, dorsal low, nasal high and temporal low, double-gradient along the dorsal–ventral and the nasal–temporal axes of the embryonic chick retina, and it was reported to inhibit or antagonize BMP2 and BMP4 signalling (Sakuta et al. 2001; Takahashi et al. 2003). RGM is also present in chick retina and was reported to enhance BMP2 and BMP4 signalling. At the level of the BMPs, RGM and ventroptin act antagonistically, but it is currently not known how such a molecular BMP2/4 antagonism affects intraretinal axon guidance and retinotectal map formation.
A repulsive function of RGM A was also described in another study. In the developing mouse hippocampus, RGM A has been shown to be involved in the formation of afferent connections in the dentate gyrus (Brinks et al. 2004). RGM A protein is present in the inner molecular layer of the dentate gyrus, whereas fibres from the entorhinal cortex terminate in the outer molecular layers. These fibres are repelled by RGM A in stripe assays and their growth is inhibited on RGM A expressing HEK293 cells. In the presence of function-blocking polyclonal RGM A antibody, entorhinal fibres are no longer inhibited by RGM A-HEK293 cells, suggesting that RGM A is important for termination of entorhinal fibres in the outer molecular layers of the dentate gyrus. This was confirmed in an organotypic entorhinal cortex–hippocampus co-culture system, where the function-blocking RGM A antibody resulted in massive aberrant projections of entorhinal fibres, abolishing their layer-specific termination pattern, and it suggests that RGM A exerts a repulsive guidance function in rodent cortex (Brinks et al. 2004). All these data are in agreement with the role of RGM A and neogenin in axon guidance in vertebrates. However, clear in vivo evidence for their exact role is still lacking and awaits further experiments in zebrafish, chick and mouse embryos.
5. Role of repulsive guidance molecules and neogenin in early nervous system development
The expression of RGM early in the development of zebrafish (Samad et al. 2004), chick (Matsunaga et al. 2004) and mouse (Niederkofler et al. 2004; Oldekamp et al. 2004; Samad et al. 2004; Schmidtmer & Engelkamp 2004) embryos has fostered the search for the functions of these molecules besides axon guidance. In chick embryos, chRGM as well as its receptor neogenin are expressed as early as E2.5 in the chick neural tube (Matsunaga et al. 2004). In the mouse, mRGM A and B are expressed in the developing and post-natal nervous system, but mRGM C was not detected in four out of five studies (Niederkofler et al. 2004; Oldekamp et al. 2004; Samad et al. 2004; Schmidtmer & Engelkamp 2004; but Martinez et al. 2004). Expression of mRGM A and B is first found at the tips of the neural fold at E8.5–9.5 just at the time when the neural tube will start to close (Niederkofler et al. 2004). At the same time, a weak expression of neogenin is found throughout the embryo and the expression persists in the developing structures in and outside the nervous system (Gad et al. 1997). At later developmental stages, cells in the ventricular zone are highly mRGM A-positive, whereas mRGM B is expressed more laterally in early post-mitotic neurons. Such strong and mostly non-overlapping expression of mRGM A and B was found in the embryonic brain and persisted in several brain regions after birth albeit at a reduced level (Niederkofler et al. 2004; Oldekamp et al. 2004; Samad et al. 2004; Schmidtmer & Engelkamp 2004). A first insight into the function of mRGM A and B in the developing brain became available by the study of mRGM A knockout mice: 50% of the homozygous animals show a defect in the closure of neural fold to the neural tube at the cephalic but not the spinal cord level resulting in an exencephalic phenotype with major morphological defects of dorsal brain structures. Analysis of proliferation and apoptosis in these structures did not reveal alterations in comparison to wild-type mice (Niederkofler et al. 2004).
A neural tube defect has also been reported in zebrafish after morpholino antisense knockdown of the neogenin gene, despite the different mode of neural tube formation (Mawdsley et al. 2004). Therefore, it can be speculated that the interaction of mRGM A and neogenin is necessary for neural tube closure in mice or lumen formation of the neural tube rod in zebrafish by a mechanism obviously not related to cell proliferation and apoptosis. In mice, mRGM B cannot substitute for mRGM A at the cephalic level, but is sufficient to allow neural tube closure at lower levels of the developing neural tube. While the broad expression of neogenin throughout the developing CNS would allow to attribute the effects of mRGM A and B to this receptor, a notable exception is found in the PNS: mRGM B is heavily expressed in the developing dorsal root ganglia (Niederkofler et al. 2004; Oldekamp et al. 2004; Samad et al. 2004; Schmidtmer & Engelkamp 2004) and cranial nerve ganglia (Oldekamp et al. 2004), but neogenin is absent in the PNS with the exception of the trigeminal ganglion (Gad et al. 1997). Therefore, one has to postulate a so far unknown receptor for mRGM B beside neogenin in these structures or a homophilic self-interaction. While the former conclusion cannot be ruled out, the latter is strengthened by the studies performed by Samad and colleagues. mRGM B (DRAGON) was expressed recombinantly on HEK cells, where it enhanced adhesion of dissociated dorsal root ganglion neurons. Interestingly, mRGM A, termed mRGM in this study, had an opposite effect. Since dorsal root ganglion neurons heavily express mRGM B, a homophilic interaction was suspected and was confirmed by the authors in biochemical experiments with tagged mRGM B (Samad et al. 2004). Mice deficient in mRGM B were described in a recent poster presentation: they die three weeks post-natally, but no defects in sensory motor functions or nervous system development were disclosed (Salie et al. 2004).
6. Repulsive guidance molecule and neogenin: a ligand/dependence receptor couple
An additional insight into the function of RGM and neogenin in nervous system development was discovered by a study wherein the expression of RGM and neogenin was enhanced or suppressed in the chick neural tube (Matsunaga et al. 2004). Whereas electroporation of cRGM at E1.5 in the area of the developing dorsal metencephalon, the mesencephalon, and the caudal diencephalon did not affect cell number, neogenin overexpression reduced the cell number 24 h later at E2.5. This was accompanied by a concomitant strong increase in the number of TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling)-positive cells and of cells staining positive for activated caspase 3 indicating cell death by apoptosis. To confirm the involvement of caspases, the authors investigated the cleavage of neogenin by caspases in vitro and detected a processing by caspase 3. Mutation of the caspase cleavage site resulted in a total loss of cell death-inducing activity in vivo, confirming that cell death observed with wild-type neogenin is caspase-dependent and most probably apoptotic. In accordance, the simultaneous overexpression of the bacculovirus caspase-inhibitory protein p35 completely blocked neogenin-induced cell death. Interestingly, simultaneous coexpression of RGM completely suppressed neogenin-induced apoptosis also, and this effect could not be mimicked by the expression of netrin-1, another ligand for neogenin. On the other hand, suppression of chRGM expression by electroporation of siRNA resulted in apoptotic cell death similar to neogenin overexpression. Suppression of neogenin expression by siRNA was without effect on cell number. Similar results were also obtained in cell culture (Matsunaga et al. 2004). Given that the mechanisms leading to caspase 3 activation were unknown in this setup, these data together suggest a pro-apoptotic role for the receptor neogenin if it is not bound to its ligand chRGM. ChRGM and neogenin share this property with other ligand/receptor pairs involved in axon guidance in the nervous system, e.g. netrin-1/DCC, NGF/p75NTR. Besides these, other receptors like patched (Ptc), UNC-5 homologue, integrin receptors (αvβ3, α5β1) and the androgen receptor have been identified (Mehlen & Mazelin 2003; Matsunaga & Chedotal 2004) and termed ‘dependence receptors’ to indicate that their expression creates a state of dependence of the cells in the presence of their ligand (Bredesen et al. 2004; Mehlen & Fearon 2004). The observation that chRGM and neogenin might belong to this group would fit to their expression in developing brain and other organs, where control of differentiation and cell number is of utmost importance. An involvement of neogenin in tumorigenesis, especially the enhanced expression of neogenin in oesophageal squamous cell carcinoma specimens and the corresponding cell lines (Hu et al. 2001) and its potential contribution to breast cancer (Srinivasan et al. 2003) formation, would also be in line with the function in controlling cell number. On the other hand, considering the results in the chick neural tube, one could expect that an enhanced expression of neogenin would render the cells death-prone and would not lead to proliferation. An expression of RGM leading to survival and potentially inducing a hyper-proliferative status in these systems has yet to be demonstrated. In the knockout mice for mRGM A, which is the mRGM species with the highest homology to chick RGM, no alteration in cell number or signs of apoptosis could be found (Niederkofler et al. 2004). Further studies are warranted to confirm the data in the chick before it can be concluded that RGM and neogenin act as a ligand/dependence receptor pair in general.
A high expression of neogenin is found in many cancer tissues, including many tumour cells that have downregulated DCC (Meyerhardt et al. 1997). Thus, it was suggested that neogenin plays an integral role in the regulation of terminal differentiation programs and/or cell migration events. This implies that the biological functions of DCC and neogenin are distinct. In accordance, neogenin expression levels in areas of neuronal maturation in the brain increase in contrast to the DCC expression levels (Vielmetter et al. 1994; Barallobre et al. 2005).
7. Role of repulsive guidance molecules in adult vertebrate CNS
Injury to the human brain or spinal cord has usually devastating consequences and pharmacotherapeutic approaches, if available, are of limited benefit for the victims. At injury sites, numerous inhibitors like Nogo-A, MAG, OMGp and CSPGs are present in CNS myelin, myelin debris or in the scar tissue and prevent any constitutive regenerative neurite growth, thereby being partially responsible for the lesion-induced functional deficits. As a response to the injury, the CNS re-expresses many repulsive or neurite growth-inhibitory proteins at or adjacent to the injury site. Examples for such a re-expression of developmental inhibitory repellent molecules are semaphorins 4D, 3A and 6B, ephrin-B2 and RGMs (Miranda et al. 1999; Pasterkamp et al. 1999; De Winter et al. 2002; Moreau-Fauvarque et al. 2003; Kury et al. 2004; Schwab et al. 2005a,b). In the healthy adult human brain, immunohistochemistry with a pan-RGM antibody revealed that RGM proteins are located on perikarya of some neurons, chorioid plexus, smooth muscle of the vasculature, endothelial cells and on oligodendrocytes and white matter fibre tracts (Schwab et al. 2005a). A similar staining pattern was also observed in the rat brain, suggesting that RGM proteins could act in concert with myelin-associated inhibitors Nogo-A, MAG and OMGp to restrict or prevent plasticity of neurites in the adult mammalian CNS (Schwab et al. 2005b). This evolutionary conserved expression pattern of RGM proteins provides evidence that RGM proteins are potential inhibitors of neuroregeneration and it was therefore important to analyse their expression in the injured or stroke-damaged human brain. With this aim, sections of human brains from 21 patients with a clinical history and with neuropathologically defined focal cerebral ischaemia (FCI) and from 25 patients with traumatic brain injury (TBI) were analysed for RGM expression using a polyclonal pan-RGM antibody (Schwab et al. 2005a). In the FCI victims, whose post-infarction survival time varied from 6 h to 38 months, RGM accumulated in the infarct core, peri-infarctional areas of the penumbra and haemorrhagic areas. Lesion-associated cellular RGM staining was confined to neurons, few reactive astrocytes and invading leukocytes. In these areas, RGM-positive cells accumulated already 1 day after stroke, reached maximal numbers 1.5–2.5 days later and remained elevated for weeks or months after stroke. During the early period after the ischaemic damage (up to 2.5 days), RGM was found on neurons, leukocytes (granulocytes, monocytes, lymphocytes) and in blood vessels (endothelial and vascular smooth muscle cells). Later on (1–7 days after stroke), RGM-positive cells extravasated outside the vascular walls into the focally lesioned parenchyma (Schwab et al. 2005a). The presence of RGM on extravasating lymphocytes and in cellular components of the brain vasculature is a hint that RGM could play a role in the massive permeability changes induced by an ischaemic stroke. The molecular mechanism of how RGM is involved in these permeability changes could be a potential inactivation of the relevant vasculature-specific integrins and the RGM-induced activation of the Rho pathway, which is known to increase vessel permeability (Van Hinsbergh & Van Nieuw Amerongen 2002).
In stroke patients surviving the initial insult, a glial scar forms at the lesion and a pseudo-lamina or neo-laminar structures are formed, where RGM is no longer associated with cellular surfaces, but with extracellular, fibril-like deposits. In the newly formed glial scar and the mature scar in infarct-damaged human brain, massive accumulation of RGM proteins occurs and this was also observed in victims dying from brain injury. Their post-injury survival times varied from minutes to 5 years and the RGM localization pattern was nearly identical to the RGM-staining pattern of the FCI victims. Most notably in one case, where the TBI victim died 12 h after injury, RGM-positive cells accumulated already at the lesion site (figure 6), suggesting that the mechanisms of invasion of RGM-positive cells are extremely rapid. In the patient with 5-year survival time, extracellular, fibrillar RGM was found in the scar tissue (figure 6) and this is an indication that injury-induced accumulation of extracellular scar-associated RGM is very stable and contributes to the regeneration-inhibitory potential of this important barrier (Schwab et al. 2005a). Initially after insult or injury, RGM accumulates at damage sites and is associated with cell surfaces. Soon after, RGMs or fragments of it are found in the extracellular matrix of the lesion and it is not known how such a release from the cell surface is mediated. Several opportunities exist in how GPI-anchored RGMs or RGM fragments might be shed off from the cell surface. Autoproteolytic or instability cleavage, favoured by a pH drop, has been described for RGM and produces the more active GPI-anchored RGM, releasing 168 amino acid N-terminal fragment of human RGM A (Zhang et al. 2005). It is not known whether this N-terminal fragment has repulsive or neurite outgrowth inhibitory activity. Owing to the CNS injury and CNS ischaemia-associated tissue acidosis (Siesjoe 1992; Marmarou et al. 1993), it is assumed that the drop in pH induces cleavage of RGM. It is therefore likely that the RGM proteins accumulating at lesion sites in humans suffering from brain injury or stroke lack the amino-terminal 168 amino acids.
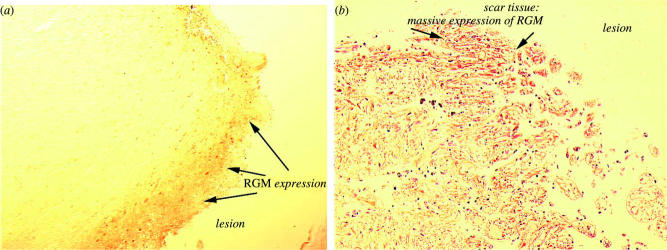
Figure 6.
Repulsive guidance molecule (RGM) proteins are localized in fresh and mature lesion sites in injured human brain. In a patient dying from traumatic brain injury, after 12 h, (a) RGM-positive cells have already accumulated at the lesion site. The lesion is located in the right frontobasal part of the brain and the patient died from severe brain oedema. In older lesions, (b) scar tissue has formed at the injury site and extracellular RGM immunoreactivity is associated with fibrillar structures. This patient died 5 years after suffering a brain injury in the left and right frontobasal part of the brain and cause of death was pulmonary embolism.
Besides the cleavage site, RGM C proteins have been reported to possess a furin cleavage site between amino acids 335 and 336 and this cleavage site could also produce a secreted form of the RGM proteins. In addition, GPI-anchor hydrolysing enzymes, the most important eukaryotic variant of the bacterial PI-PLC enzymes, the GPI-PLD enzyme, could release GPI-anchored proteins. GPI-PLC-like activities have been described in mouse brain membranes, one of these associated with myelin (Fouchier et al. 1990). The identity of the protein having such GPIase activity (releasing GPI-anchored proteins) is not known, but one potential candidate was recently identified as angiotensin-converting enzyme (ACE; Kondoh et al. 2005). In inflammatory demyelinating CNS and PNS diseases, myelin induces release of ACE from macrophages (Constantinescu et al. 2000) and the macrophages are known to accumulate at CNS injury sites (Leskovar et al. 2000). In addition, secreted splice variants of the RGM proteins might be produced, lacking the GPI-anchor. Ample opportunities exist to release RGM proteins from the cell surface and it is currently not known whether such a release of RGM proteins and fragments of it results in its increase in blood and cerebrospinal liquid. The closely related family member, RGM C/hemojuvelin, is detectable in large amounts in plasma and serum of healthy humans as two protein bands with 33 and 16 kDa (Lin et al. 2005). It is not known whether RGM A or RGM B proteins are present in blood of healthy humans, but it is assumed that their presence in human blood could be associated with pathological human CNS conditions.
Comparing cellular and extracellular RGM immunostaining patterns, two remarkable features have been noticed. First, no matter what the type of damage is, both focal ischaemic stroke and brain injury induce very similar patterns of cell surface and extracellular RGM accumulation at or around the lesion site in humans and adult rats and it remains to be shown whether similar RGM staining patterns are observed in human neurodegenerative diseases. Second, the presence of RGM proteins in the developing and mature glial scar, which prevents any regenerative invasion of sprouting neurites, is remarkable and together with RGM localization on myelinated fibre tracts and oligodendrocytes suggests that neutralizing RGM proteins in the two most important barriers (CNS myelin, glial scar) for neuroregeneration in humans is a promising strategy towards functional recovery.
The RGM receptor, neogenin, is widely expressed in the adult CNS and spinal cord and is distributed predominantly in the grey matter (Manitt et al. 2004). Netrin-1, another ligand of neogenin, is also found in the spinal cord in both grey and white matter and blocking of RGM proteins could therefore free neogenin from RGM to bind netrin-1 (Manitt et al. 2004). As the netrin-1/neogenin interaction stimulates neurite growth (Barallobre et al. 2005), such prevention of binding of RGM to neogenin is envisioned to stimulate neurite growth. Therefore, inhibition of RGM in spinal cord injury (SCI) has the advantage of not only neutralizing a potent neurite growth inhibitor, but also to allow stimulation of neurite growth via binding of netrin-1 to neogenin.
Besides binding of RGM A to neogenin and inducing neurite growth inhibition, the binding of RGM A or B to BMP2 and 4 could represent another obstacle to successful neuroregeneration and functional recovery. Usually, BMP expression is relatively low in most regions of adult CNS, but rapid increases in the expression of BMP members (BMP2, BMP6 and BMP7) have been reported in response to injury and insult (Lai et al. 1997; Martinez et al. 2001; Hall & Miller 2004; Setoguchi et al. 2004). In vitro, BMP2 and BMP4 have been described as repressors of oligodendrocyte development, because they induce a shift in lineage commitment of oligodendrocyte precursors (OPs) towards the astrocytic lineage. In addition, BMP4 prevents or blocks maturation of immature oligodendrocytes (See et al. 2004). Demyelination is commonly observed after SCI and a rapid remyelination could improve functional recovery. This is apparently prevented by upregulated BMP family members, due to their negative effects on lineage commitment and maturation of OP cells, thereby decreasing the number of oligodendrocytes available for rapid remyelination. Whether RGM proteins are involved in this process is currently not known, but the rapid upregulation of RGM proteins at the lesion or insult site and their expression by oligodendrocytes suggest that this might be the case.
It is currently not clear why RGMs and other repulsive guidance cues accumulate at lesion sites in adult mammalian CNS. Can this be regarded as an embryonic imprinting pattern in adult CNS tissues to direct and guide regenerating fibres beyond the lesion or is it a reaction to prevent excessive sprouting and aberrant wiring? Why is such a response at best only partial and why does it lack any rejuvenating response on the injured or damaged fibres? The latent inhibition of injured fibres manifested as formation of retraction bulbs, structures similar in appearance to collapsed growth cones in vitro, poses additional questions: will it be possible to reactivate the crucial and the most efficient mechanism of neurite growth and of neurite pathfinding, the elaboration of a powerful neuronal growth cone? Comparing both embryonic and adult neurons when they respond to inhibitory or repulsive guidance cues like the RGMs will help us to understand the lack of successful regeneration in humans after CNS injury.
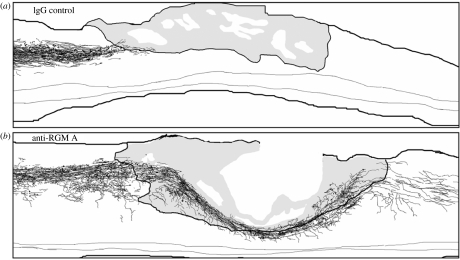
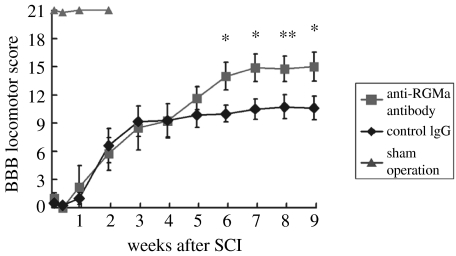
As mentioned earlier, RGM proteins are inhibitory or repulsive for growing neurites, and in the adult mammalian CNS they are found in both fresh and mature lesions, scar tissue and myelinated fibre tracts. Their neutralization by an antibody should therefore improve regenerative growth of injured fibres and functional recovery. This assumption was proven by Hata and colleagues in a recent paper (Hata et al. 2006). They raised polyclonal antibodies against an RGM A-specific peptide, which recognized the protein as a 33 kDa band in a rat CNS myelin preparation and in homogenates of the lesion tissue. In neurite growth experiments with cerebellar granule cells, the antibody blocked the inhibitory activity of RGM A resulting in increased neurite growth. When added to a CNS myelin substrate, this antibody significantly improved neurite outgrowth and after immunodepletion of RGM A from CNS myelin, the neurite growth inhibitory activity of myelin was strongly reduced. All these in vitro data suggested that RGM A is an important constituent of CNS myelin and inactivation of RGM A was expected to improve regeneration and functional recovery in a rat SCI model (Hata et al. in press). To test this hypothesis, adult rats underwent a moderate SCI (hemisection at thoracal level) and the RGM A antibodies or a control antibody were administered via pump for two weeks post-injury. Ten weeks after SCI, tracing of the corticospinal tract (CST) revealed large differences between RGM A antibody and control antibody-treated animals. In control antibody-treated animals, no regenerating CST fibres were found caudal to the lesion and only few fibres managed to extend for a short distance into the lesion or scar tissue (figure 7a). In contrast to the control antibody, the RGM A antibody induced re-growth of hundreds of fibres into the lesion and beyond the lesion and the longest fibres were found 10 mm caudal to the lesion (figure 7b), suggesting that the RGM A antibody not only neutralized myelin-associated RGM A, but also lesion- or scar tissue-associated RGM A (Hata et al. in press). The extensive re-growth or sprouting of fibres in the RGM A antibody-treated animals correlated well with their improved functional recovery. While the RGM A antibody-treated animals reached a BBB score of 15 on an average, the control antibody-treated animals reached a BBB score of only 10.9 on an average, a large and significant difference between both groups (figure 8). The improved functional recovery became evident only five weeks after injury, when many regenerating or regrowing fibres have passed the lesion site (figure 8). Taking into account that the size of the transection lesions in these rats was 3–3.5 mm in width along the rostro-caudal axis and assuming that regenerating axons grow 100 μm per day (Kerschensteiner et al. 2005), 30–35 days are necessary for the injured fibres to get beyond the lesion and synapse with spinal neurons. Interestingly, this is precisely the time frame when RGM A antibody-treated rats and control rats began to diverge in their behavioural scores, and this indicates that the RGM A antibody treatment in spinally injured rats induces functional recovery in a very different way from other neuroregenerative drugs like Nogo-A antibodies, NgR antagonists, and Rho kinase inhibitors. For all these drugs, the largest improvement in functional recovery occurred within the first two weeks of post-injury (Grandpré et al. 2002; Tanaka et al. 2004; Liebscher et al. 2005) and no further significant improvement was observed four weeks post-injury. Obviously, local sprouting in these cases is more important than long distance regeneration, which might be the primary mechanism of action for the RGM A antibody. Whatever the mechanism is, underlying functional recovery, RGM A has proven as a clear example of an embryonic directional guidance cue, reappearing in the adult CNS of humans and rats and acting as a repulsive or inhibitory cue, thereby recapitulating molecular processes of embryonic development.
Figure 7.
The anti-repulsive guidance molecule (anti-RGM) A antibody, but not the IgG control antibody, promotes the regeneration of corticospinal tract axons after spinal cord injury. Camera lucida drawings of biotin-dextran-amine labelled corticospinal axons of a (a) control IgG-treated rat and an (b) anti-RGM A antibody-treated rat in consecutive parasagittal sections. Grey-coloured tissue corresponds to scar tissue at or adjacent to the transection injury lesion. Reproduced from the Journal of Cell Biology 2006; 173, 47–58 by copyright permission of the Rockefeller University Press.
Figure 8.
The anti-repulsive guidance molecule (RGM) A antibody promotes functional recovery after spinal cord injury. The BBB score, a neurological rating score for evaluation of hind limb function, was determined at the indicated time points after dorsal hemisection injuries. RGM A antibody-treated animals (n=9) reached a final average BBB score of 15, control antibody-treated rats reached a score of 10.9 (n=11) and the sham-operated controls (n=5) reached a score like normal healthy rats of 21. The BBB scoring curves of control- and anti-RGM A-treated animals started to diverge five weeks post-injury, with the difference increasing further at later time points. Antibody treatment was done within the first two weeks post-injury (total dose: 80 μg per rat), starting immediately after injury. Reproduced from the Journal of Cell Biology 2006; 173, 47–58 by copyright permission of the Rockefeller University Press.
8. Role of repulsive guidance molecules outside the central nervous system
Beside a strong albeit complementary expression in many regions of the developing mouse brain, mRGM A and B mRNA have been detected outside the nervous system during development. At E10.5 expression of RGM A mRNA was found associated with somitic structures (Schmidtmer & Engelkamp 2004). At E14.5, mRGM B is expressed in nasal epithelium, the digestive tract and cartilage, whereas mRGM A is expressed in limb primordia, the cochlear epithelium and both isoforms are found in the developing lung (Oldekamp et al. 2004) in the embryonic gut and enteric ganglia cells of foetal and adult gut (Metzger et al. 2005). RGM B is expressed throughout the reproductive tract and is found in testis, epididymis, ovary, uterus and pituitary (Xia et al. 2005). RGM A and RGM B are also expressed in a larger variety of organs, like heart, liver and kidney, of the adult rat (Babitt et al. 2005). mRGM C mRNA was found in differentiating muscle cells as early as E9.5 (Schmidtmer & Engelkamp 2004; Niederkofler et al. 2004) and is expressed in striated muscles and cardiac muscle in juvenile (P7) mice (Oldekamp et al. 2004) as well as in foetal and adult liver (Papanikolaou et al. 2004). Interestingly, the RGM receptor neogenin shows a widespread expression outside the CNS during embryonic development (Keeling et al. 1997). An overlap in expression with members of the RGM family during at least some stages of development is found in somites and developing skeletal muscles (between E10.5 and 14.5), in the lung as well as in cartilage and connective tissue surrounding the nasal epithelium (Gad et al. 1997). Neogenin is regarded as a candidate for the regulation of myogenesis together with its alternative ligands, members of the netrin family (Kang et al. 2004). The partial overlap of the expression of different members of the mRGM family on one site with the expression of neogenin and netrins on the other site might indicate a crosstalk between members of the different ligand families of neogenin in development outside the CNS. This scenario gains likelihood given that processes necessary for neurite outgrowth and axon guidance, e.g. re-shaping of the actin cytoskeleton, are also involved in cell differentiation and migration.
The hemojuvelin/RGM C gene on human chromosome 1q21 was recently linked to juvenile haemochromatosis (JH), an early-onset autosomal recessive disease characterized by iron overload resulting in cardiomyopathy, diabetes and hypogonadism (Papanikolaou et al. 2004). More than 15 different mutations have been described in exons 3 and 4 of hemojuvelin/RGM C in JH patients (Lanzara et al. 2004; Lee et al. 2004; Papanikolaou et al. 2004). Clinically and biochemically, the hemojuvelin/RGM C JH was very similar to JH caused by homozygous disruption of the HAMP gene (Roetto et al. 2003). The HAMP gene codes for hepcidin, a key iron-regulatory peptide, 25 amino acids in length regulating iron absorption in the small intestine, iron release from macrophages and iron transport across the placenta (Ganz 2003). Severe iron overload was observed in hepcidin knockout mice, mimicking the JH phenotype of humans (Nicolas et al. 2001). Iron overload also occurred in hemojuvelin/RGM C knockout mice particularly in the liver and heart, mimicking the human situation although cardiomyopathy, diabetes and hypogonadism were absent (Huang et al. 2005; Niederkofler et al. 2005). The clinical similarities of the hepcidin and the hemojuvelin/RGM C JH, the fact that both the loss of function HAMP and hemojuvelin knockout mice suffer severe iron overload, are clear hints that both gene products act in the same iron-regulatory pathway. Mutations in hRGM C or knockout of mRGM C drastically reduces the expression of peptide hormone, hepcidin, by a mechanism currently under investigation (Papanikolaou et al. 2004; Huang et al. 2005; Niederkofler et al. 2005). Hepcidin controls the extracellular iron concentration by regulating the iron flow into the plasma through the transmembrane protein, ferroportin (Roetto et al. 2003; Nemeth et al. 2004), such that reduction of hepcidin levels results in increased iron levels in the plasma followed by iron overload in several organs. Two recent studies gave first hints on the possible regulation of hepcidin by hemojuvelin/RGM C. In human hepatocytes, soluble hemojuvelin/RGM C, at concentrations found in human sera, suppressed hepcidin mRNA expression in a dose-dependent way, whereas cellular hemojuvelin/RGM C positively regulated hepcidin expression (Lin et al. 2005). Such a competition of soluble hemojuvelin/RGM C and membrane-attached hemojuvelin/RGM C in hepcidin regulation is unique, was not observed before in the RGM family and might also be of relevance in human brain injury and stroke, where soluble- and membrane-attached RGMs are known to exist at the same time and location.
In the second study with hemojuvelin/RGM C-transfected HEK293 cells, the RGM receptor, neogenin, was implicated as a receptor-regulating hepcidin (Zhang et al. 2005). Binding of hemojuvelin/RGM C to neogenin in HEK293 cells resulted in increased intracellular iron and ferritin levels and the most common JH point mutation in hemojuvelin/RGM C, a G320 V substitution, was apparently no longer able to interact with neogenin as shown by coimmunoprecipitation of HEK293 cells transfected with the G320V mutant with an RGM C antibody (Zhang et al. 2005).
These interesting results require a more detailed analysis of the effects of different hemojuvelin/RGM C mutants on their interaction with neogenin.
The studies mentioned earlier add the regulation of iron metabolism to the spectrum of functions exerted by the members of the RGM family. This finding might even have more widespread impact, since alterations in the level or the redox status of iron are involved in the pathophysiology of several neurodegenerative diseases, like Parkinson's disease and Alzheimer's disease (Ke & Ming Qian 2003; Doraiswamy & Finefrock 2004; Thomas & Jankovic 2004). While several investigators could not detect the RGM C mRNA in the brain by in situ hybridization and northern blot (Niederkofler et al. 2004; Oldekamp et al. 2004; Samad et al. 2004; Schmidtmer & Engelkamp 2004), a recent study (Martinez et al. 2004) reported its expression using freshly prepared mouse brain RNA in RT-PCR and mouse brain protein in western blot. Interestingly, commercially available mouse and human brain cDNA failed to show the expression of RGM C. Unless the expression of RGM C in human brain can be shown unequivocally, a potential link to brain iron metabolism and neurodegenerative diseases remains highly speculative.
9. Concluding remarks
Since the discovery of the RGM protein family, numerous functions have been ascribed to this family of proteins. Their injury- or damage-related accumulation in the human brain, their localization to two different important barriers of nerve regeneration, the CNS myelin sheets and the lesion- or damage-associated scar tissue suggested that blocking their inhibitory activity by selective antagonists will turn out to be a fruitful approach to stimulate regeneration of nerve fibres and enhance functional recovery. In an animal model of SCI, this has recently been achieved by a function-blocking RGM A-specific polyclonal antibody. RGM A can be regarded as an embryonic directional guidance cue, re-expressed at lesion sites in both humans and rats, inhibiting re-growth of injured nerve fibres and subsequently functional recovery. Besides acting as a repulsive guidance cue for growing axons during the development of embryonic CNS, the function of RGM A in the adult CNS is more inhibitory than being repulsive. Neutralization of RGM A inhibition has therefore resulted in astonishing functional recovery and is a promising new therapeutic strategy for the treatment of SCI.
Acknowledgments
The authors thank Hans Schoemaker and Gerhard Gross for their comments, their excellent feedback and insightful discussions. B.K.M. also thanks his former collaborators, Lutz Deitinghoff, Jan Schwab and Friedrich Bonhoeffer.
Footnotes
One contribution of 13 to a Theme Issue ‘The regenerating brain’.
References
- Babitt J.L, Zhang Y, Samad T.A, Xia Y, Tang J, Campagna J.A, Schneyer A.L, Woolff C.J, Lin H.Y. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J. Biol. Chem. 2005;280:29 820–29 827. doi: 10.1074/jbc.M503511200. 10.1074/jbc.M503511200 [DOI] [PubMed] [Google Scholar]
- Barallobre M.J, Pascual M, Del Rio J.A, Soriano E. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res. Brain Res. Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. 10.1016/j.brainresrev.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Bonhoeffer F, Gierer A. How do retinal axons find their targets on the tectum? Trends Neurosci. 1984;7:378–381. 10.1016/S0166-2236(84)80060-0 [Google Scholar]
- Bredesen D.E, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol. Rev. 2004;84:411–430. doi: 10.1152/physrev.00027.2003. 10.1152/physrev.00027.2003 [DOI] [PubMed] [Google Scholar]
- Brinks H, et al. The repulsive guidance molecule RGMa is involved in the formation of afferent connections in the dentate gyrus. J. Neurosci. 2004;24:3862–3869. doi: 10.1523/JNEUROSCI.5296-03.2004. 10.1523/JNEUROSCI.5296-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. 10.1016/S0092-8674(04)00003-0 [DOI] [PubMed] [Google Scholar]
- Chan S.S, Zheng H, Su M.W, Wilk R, Killeen M.T, Hedgecock E.M, Culotti J.G. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. 10.1016/S0092-8674(00)81337-9 [DOI] [PubMed] [Google Scholar]
- Cheng H.J, Nakamoto M, Bergemann A.D, Flanagan J.G. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. 10.1016/0092-8674(95)90426-3 [DOI] [PubMed] [Google Scholar]
- Chikumi H, Fukuhara S, Gutkind J.S. Regulation of G protein-linked guanine nucleotide exchange factors for Rho, PDZ-RhoGEF, and LARG by tyrosine phosphorylation: evidence of a role for focal adhesion kinase. J. Biol. Chem. 2002;277:12 463–12 473. doi: 10.1074/jbc.M108504200. 10.1074/jbc.M108504200 [DOI] [PubMed] [Google Scholar]
- Constantinescu C.S, Goodman D.B.P, Hilliard B, Wysocka M, Cohen J.A. Murine macrophages stimulated with central and peripheral nervous system myelin or purified myelin proteins release inflammatory products. Neurosci. Lett. 2000;287:171–174. doi: 10.1016/s0304-3940(00)01184-8. 10.1016/S0304-3940(00)01184-8 [DOI] [PubMed] [Google Scholar]
- Cowan C.W, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. 10.1016/j.neuron.2005.03.019 [DOI] [PubMed] [Google Scholar]
- Cox E.C, Muller B, Bonhoeffer F. Axonal guidance in the chick visual system: posterior tectal membranes induce collapse of growth cones from the temporal retina. Neuron. 1990;4:31–37. doi: 10.1016/0896-6273(90)90441-h. 10.1016/0896-6273(90)90441-H [DOI] [PubMed] [Google Scholar]
- De Winter F, Holtmaat A.J, Verhaagen J. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv. Exp. Med. Biol. 2002;515:115–139. doi: 10.1007/978-1-4615-0119-0_10. [DOI] [PubMed] [Google Scholar]
- Doraiswamy P.M, Finefrock A.E. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 2004;3:431–434. doi: 10.1016/S1474-4422(04)00809-9. 10.1016/S1474-4422(04)00809-9 [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. 10.1016/0092-8674(95)90425-5 [DOI] [PubMed] [Google Scholar]
- Fearon E.R, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Feldheim D.A, Kim Y.I, Bergemann A.D, Frisen J, Barbacid M, Flanagan J.G. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/s0896-6273(00)81060-0. 10.1016/S0896-6273(00)81060-0 [DOI] [PubMed] [Google Scholar]
- Feldheim D.A, Nakamoto M, Osterfield M, Gale N.W, DeChiara T.M, Rohatgi R, Yancopoulos G.D, Flanagan J.G. Loss-of-function analysis of EphA receptors in retinotectal mapping. J. Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. 10.1523/JNEUROSCI.0239-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier F, Baltz T, Rougon G. Identification of glycosylphosphatidylinositol-specific phospholipases C in mouse brain membranes. Biochem. J. 1990;269:321–327. doi: 10.1042/bj2690321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Yates P.A, McLaughlin T, Friedman G.C, O'Leary D.D, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. 10.1016/S0896-6273(00)80452-3 [DOI] [PubMed] [Google Scholar]
- Gad J.M, Keeling S.L, Wilks A.F, Tan A.F, Cooper H.M. The expression patterns of guidance receptors, DCC and neogenin, are spatially and temporally distinct throughout mouse embryogenesis. Dev. Biol. 1997;192:258–273. doi: 10.1006/dbio.1997.8756. 10.1006/dbio.1997.8756 [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. 10.1182/blood-2003-03-0672 [DOI] [PubMed] [Google Scholar]
- Geisbrecht B.V, Dowd K.A, Barfield R.W, Longo P.A, Leahy D.J. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J. Biol. Chem. 2003;278:32 561–32 568. doi: 10.1074/jbc.M302943200. 10.1074/jbc.M302943200 [DOI] [PubMed] [Google Scholar]
- Gierer A. Development of projections between areas of the nervous system. Biol. Cybern. 1981;42:69–78. doi: 10.1007/BF00335161. 10.1007/BF00335161 [DOI] [PubMed] [Google Scholar]
- Govek E.E, Newey S.E, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. 10.1101/gad.1256405 [DOI] [PubMed] [Google Scholar]
- Grandpré T, Li S, Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- Hall A.K, Miller R.H. Emerging roles for bone morphogenetic proteins in central nervous system glial biology. J. Neurosci. Res. 2004;76:1–8. doi: 10.1002/jnr.20019. 10.1002/jnr.20019 [DOI] [PubMed] [Google Scholar]
- Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller B.K, Yamashita T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J. Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M.R, et al. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 1999;22:731–742. doi: 10.1016/s0896-6273(00)80732-1. 10.1016/S0896-6273(00)80732-1 [DOI] [PubMed] [Google Scholar]
- Hu Y.C, Lam K.Y, Law S, Wong J, Srivastave G. Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: overexpression of ra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin. Cancer Res. 2001;7:2213–2221. [PubMed] [Google Scholar]
- Huang F.W, Pinkus J.L, Pinkus G.S, Fleming M.D, Andrews N.C. A mouse model of juvenile hemochromatosis. J. Clin. Invest. 2005;115:2187–2191. doi: 10.1172/JCI25049. 10.1172/JCI25049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A.B, Kolodkin A.L, Ginty D.D, Cloutier J.F. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. 10.1146/annurev.neuro.26.010302.081139 [DOI] [PubMed] [Google Scholar]
- Kang J.-S, Yi M.-J, Zhang W, Feinleib J.L, Cole F, Krauss R.S. Netrins and neogenin promote myotube formation. J. Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. 10.1083/jcb.200405039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–253. doi: 10.1016/s1474-4422(03)00353-3. 10.1016/S1474-4422(03)00353-3 [DOI] [PubMed] [Google Scholar]
- Keeling S.L, Gad J.M, Cooper H.M. Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene. 1997;15:691–700. doi: 10.1038/sj.onc.1201225. 10.1038/sj.onc.1201225 [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo E.D, Chan S.S, Culotti J.G, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. 10.1016/S0092-8674(00)81336-7 [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab M.E, Lichtman J.W. Misgeld T in vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005;11:572–577. doi: 10.1038/nm1229. 10.1038/nm1229 [DOI] [PubMed] [Google Scholar]
- Kolodziej P.A, Timpe L.C, Mitchell K.J, Fried S.R, Goodman C.S, Jan L.Y, Jan Y.N. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. 10.1016/S0092-8674(00)81338-0 [DOI] [PubMed] [Google Scholar]
- Kondoh G, et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat. Med. 2005;11:160–166. doi: 10.1038/nm1179. 10.1038/nm1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R.P, Aurandt J, Guan K.-L. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. 10.1038/nrm1740 [DOI] [PubMed] [Google Scholar]
- Kury P, Abankwa D, Kruse F, Greiner-Petter R, Muller H.W. Gene expression profiling reveals multiple novel intrinsic and extrinsic factors associated with axonal regeneration failure. Eur. J. Neurosci. 2004;19:32–42. doi: 10.1111/j.1460-9568.2004.03112.x. 10.1111/j.1460-9568.2004.03112.x [DOI] [PubMed] [Google Scholar]
- Lai M, Gluckman P.D, Dragunow M, Hughes P. Focal brain injury increases activin beta mRNA expression in hippocampal neurons. Neuroreport. 1997;8:2691–2694. doi: 10.1097/00001756-199708180-00011. 10.1097/00001756-199708180-00011 [DOI] [PubMed] [Google Scholar]
- Lanzara C, et al. Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood. 2004;103:4317–4321. doi: 10.1182/blood-2004-01-0192. 10.1182/blood-2004-01-0192 [DOI] [PubMed] [Google Scholar]
- Lee P.L, Beutler E, Rao S.V, Barton J.C. Genetic abnormalities and juvenile hemochromatosis: mutations of the HJV gene encoding hemojuvelin. Blood. 2004;103:4669–4671. doi: 10.1182/blood-2004-01-0072. 10.1182/blood-2004-01-0072 [DOI] [PubMed] [Google Scholar]
- Leskovar A, Moriarty L.J, Turek J.J, Schoenlein I.A, Borgens R.B. The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. J. Exp. Biol. 2000;203:1783–1795. doi: 10.1242/jeb.203.12.1783. [DOI] [PubMed] [Google Scholar]
- Li X, Meriane M, Triki I, Shekarabi M, Kennedy T.E, Larose L, Lamarche-Vane N. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J. Biol. Chem. 2002;277:37 788–37 797. doi: 10.1074/jbc.M205428200. 10.1074/jbc.M205428200 [DOI] [PubMed] [Google Scholar]
- Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. 10.1002/ana.20627 [DOI] [PubMed] [Google Scholar]
- Lin L, Goldberg Y.P, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106:2884–2889. doi: 10.1182/blood-2005-05-1845. 10.1182/blood-2005-05-1845 [DOI] [PubMed] [Google Scholar]
- Liu G, Beggs, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. 10.1038/nn1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D.J, Nobes C.D, Hall A. The Rho's progress: a potential role during neuritogenesis for the Rho family of GTPases. Trends Neurosci. 1995;18:496–501. doi: 10.1016/0166-2236(95)92773-j. 10.1016/0166-2236(95)92773-J [DOI] [PubMed] [Google Scholar]
- Manitt C, Thompson K.M, Kennedy T.E. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J. Neurosci. Res. 2004;77:690–700. doi: 10.1002/jnr.20199. 10.1002/jnr.20199 [DOI] [PubMed] [Google Scholar]
- Marmarou A, Holdaway R, Ward J.D, Yoshida K, Choi S.C, Muizelaar J.P, Young H.F. Traumatic brain tissue acidosis: experimental and clinical studies. Acta Neurochir. 1993;57(Suppl. Wien):160–164. doi: 10.1007/978-3-7091-9266-5_23. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews S.E, Carter N, Hunter T, Pfaff S.L. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. 10.1016/j.cell.2005.01.020 [DOI] [PubMed] [Google Scholar]
- Martinez G, Carnazza M.L, Di Giacomo C, Sorrenti V, Vanella A. Expression of bone morphogenetic protein-6 and transforming growth factor-beta1 in the rat brain after a mild and reversible ischemic damage. Brain Res. 2001;894:1–11. doi: 10.1016/s0006-8993(00)03140-1. 10.1016/S0006-8993(00)03140-1 [DOI] [PubMed] [Google Scholar]
- Martinez A.R, Niemelä O, Parkkila S. Hepatic and extrahepatic expression of the new iron regulatory protein hemojuvelin. Haematologica. 2004;89:1441–1445. [PubMed] [Google Scholar]
- Matsunaga E, Chedotal A. Repulsive guidance molecule/neogenin: a novel ligand-receptor system playing multiple roles in neural development. Dev. Growth Differ. 2004;46:481–486. doi: 10.1111/j.1440-169x.2004.00768.x. 10.1111/j.1440-169x.2004.00768.x [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Tauszig-Delamasure S, Monnier P.P, Mueller B.K, Strittmatter S.M, Mehlen P, Chédotal A. RGM and its receptor neogenin regulate neuronal survival. Nat. Cell Biol. 2004;6:749–755. doi: 10.1038/ncb1157. 10.1038/ncb1157 [DOI] [PubMed] [Google Scholar]
- Mawdsley D.J, Cooper H.M, Hogan B.M, Cody S.H, Lieschke G.J, Heath J.K. The Netrin receptor Neogenin is required for neural tube formation and somitogenesis in zebrafish. Dev. Biol. 2004;269:302–315. doi: 10.1016/j.ydbio.2004.02.001. 10.1016/j.ydbio.2004.02.001 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary D.D. Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. 10.1146/annurev.neuro.28.061604.135714 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mazelin L. The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol. Cell. 2003;95:425–436. doi: 10.1016/s0248-4900(03)00072-8. 10.1016/S0248-4900(03)00072-8 [DOI] [PubMed] [Google Scholar]
- Mehlen P, Fearon E.R. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J. Clin. Oncol. 2004;22:3420–3428. doi: 10.1200/JCO.2004.02.019. 10.1200/JCO.2004.02.019 [DOI] [PubMed] [Google Scholar]
- Metzger M, Conrad S, Alvarez-Bolado G, Skutella T, Just L. Gene expression of the repulsive guidance molecules during development of the mouse intestine. Dev. Dynam. 2005;234:169–175. doi: 10.1002/dvdy.20506. 10.1002/dvdy.20506 [DOI] [PubMed] [Google Scholar]
- Meyerhardt J.A, Look A.T, Bigner S.H, Fearon E.R. Identification and characterization of neogenin, a DCC-related gene. Oncogene. 1997;14:1129–1136. doi: 10.1038/sj.onc.1200935. 10.1038/sj.onc.1200935 [DOI] [PubMed] [Google Scholar]
- Miranda J.D, White L.A, Marcillo A.E, Willson C.A, Jagid J, Whittemore S.R. Induction of Eph B3 after spinal cord injury. Exp. Neurol. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. 10.1006/exnr.1998.7012 [DOI] [PubMed] [Google Scholar]
- Monnier P.P, et al. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–395. doi: 10.1038/nature01041. 10.1038/nature01041 [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B.K. RGM, a repulsive guidance molecule, is involved in retinal axon guidance in vitro. In: Fujisawa H, editor. Molecular basis of axon growth and nerve pattern formation. Japan Scientific Societies Press; Tokyo, Japan: 1997. pp. 215–229. [Google Scholar]
- Mueller B.K. Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. 10.1146/annurev.neuro.22.1.351 [DOI] [PubMed] [Google Scholar]
- Mueller B.K, Mack H, Teusch N. Rho Kinase, a promising drug target for neurological disorders. Nat. Rev. Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. 10.1038/nrd1719 [DOI] [PubMed] [Google Scholar]
- Muller B.K, Jay D.G, Bonhoeffer F. Chromophore-assisted laser inactivation of a repulsive guidance molecule. Curr. Biol. 1996;6:1497–1502. doi: 10.1016/s0960-9822(96)00754-3. 10.1016/S0960-9822(96)00754-3 [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle M.S, Powelson J, Vaughn M.B, Donovan A, Ward D.M, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc. Natl Acad. Sci. USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. 10.1073/pnas.151179498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J. Neurosci. 2004;24:808–818. doi: 10.1523/JNEUROSCI.4610-03.2004. 10.1523/JNEUROSCI.4610-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J. Clin. Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. 10.1172/JCI25683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldekamp J, Krämer N, Alvarez-Bolado G, Skutella T. Expression pattern of the repulsive guidance molecules RGM A, B, and C during mouse development. Gene Expr. Patterns. 2004;4:283–288. doi: 10.1016/j.modgep.2003.11.008. 10.1016/j.modgep.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Papanikolaou G, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 2004;36:77–82. doi: 10.1038/ng1274. 10.1038/ng1274 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Giger R.J, Ruitenberg M.J, Holtmaat A.J, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. 10.1006/mcne.1999.0738 [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller B.K, Strittmatter S.M. Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. 10.1038/ncb1156 [DOI] [PubMed] [Google Scholar]
- Ren X.R, et al. Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. 10.1038/nn1330 [DOI] [PubMed] [Google Scholar]
- Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat. Genet. 2003;33:21–22. doi: 10.1038/ng1053. 10.1038/ng1053 [DOI] [PubMed] [Google Scholar]
- Sahin M, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. 10.1016/j.neuron.2005.01.030 [DOI] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura Si, Yamamoto T.S, Ueno N, Noda M. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science. 2001;293:111–115. doi: 10.1126/science.1058379. 10.1126/science.1058379 [DOI] [PubMed] [Google Scholar]
- Salie, R., Niederkofler, V. & Arber, S. 2004 In vivo functions of repulsive guidance molecule (RGM) family members in murine nervous system development. Society for Neuroscience Abstracts, Abstract 9422.
- Samad T.A, et al. Member of the repulsive guidance molecule-related family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive properties. J. Neurosci. 2004;24:2027–2036. doi: 10.1523/JNEUROSCI.4115-03.2004. 10.1523/JNEUROSCI.4115-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad T.A, et al. DRAGON, a bone morphogenetic protein co-receptor. J. Biol. Chem. 2005;280:14 122–14 129. doi: 10.1074/jbc.M410034200. 10.1074/jbc.M410034200 [DOI] [PubMed] [Google Scholar]
- Schmidtmer J, Engelkamp D. Isolation and expression pattern of three mouse homologues of chick Rgm. Gene Expr. Patterns. 2004;4:105–110. doi: 10.1016/s1567-133x(03)00144-3. 10.1016/S1567-133X(03)00144-3 [DOI] [PubMed] [Google Scholar]
- Schwab J.M, Monnier P.P, Schluesener H.J, Conrad S, Beschorner R, Chen L, Meyermann R, Mueller B.K. CNS injury-induced RGM (repulsive guidance molecule) expression in the adult human brain. Arch. Neurol. 2005a;62:1561–1568. doi: 10.1001/archneur.62.10.1561. 10.1001/archneur.62.10.1561 [DOI] [PubMed] [Google Scholar]
- Schwab J.M, Conrad S, Monnier P.P, Julien S, Mueller B.K, Schluesener H.J. Spinal cord injury induces lesional expression pattern of the repulsive guidance molecule (RGM) Eur. J. Neurosci. 2005b;21:1569–1576. doi: 10.1111/j.1460-9568.2005.03962.x. [DOI] [PubMed] [Google Scholar]
- See J, Zhang X, Eraydin N, Mun S.B, Mamontov P, Golden J.A, Grinspan J.B. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol. Cell Neurosci. 2004;26:481–492. doi: 10.1016/j.mcn.2004.04.004. 10.1016/j.mcn.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp. Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. 10.1016/j.expneurol.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Shamah S.M, Lin M.Z, Goldberg J.L, Estrach S, Sahin M, Hu L, Bazalakova M, Neve R.L, Corfas G, Debant A, Greenberg M.E. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. 10.1016/S0092-8674(01)00314-2 [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moore S.W, Tritsch N.X, Morris S.J, Bouchard J.F, Kennedy T.E. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. 10.1523/JNEUROSCI.1920-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjoe B.K. Pathophysiology and treatment of focal cerebral ischemia. Part I: pathophysiology. J. Neurosurg. 1992;77:169–184. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Sperry R.W. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl Acad. Sci. USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. 10.1073/pnas.50.4.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin G.C. Hinck L Netrin-1/Neogenin interaction stabilizes multipotential progenitor cap cells during mammary gland morphogenesis. Dev. Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. 10.1016/S1534-5807(03)00054-6 [DOI] [PubMed] [Google Scholar]
- Stahl B, Müller B, von Boxberg Y, Cox E.C, Bonhoeffer F. Biochemical characterization of a putative axonal guidance molecule of the chick visual system. Neuron. 1990;5:735–743. doi: 10.1016/0896-6273(90)90227-7. 10.1016/0896-6273(90)90227-7 [DOI] [PubMed] [Google Scholar]
- Swiercz J.M, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. 10.1016/S0896-6273(02)00750-X [DOI] [PubMed] [Google Scholar]
- Takahashi H, Shintani T, Sakuta H. Noda M CBF1 controls the retinotectal topographical map along the anteroposterior axis through multiple mechanisms. Development. 2003;130:5203–5215. doi: 10.1242/dev.00724. 10.1242/dev.00724 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yamashita T, Yachi K, Fujiwara T, Yoshikawa H, Tohyama M. Cytoplasmic p21(Cip1/WAF1) enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience. 2004;127:155–164. doi: 10.1016/j.neuroscience.2004.05.010. 10.1016/j.neuroscience.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman C.S. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. 10.1126/science.274.5290.1123 [DOI] [PubMed] [Google Scholar]
- Thomas M, Jankovic J. Neurodegenerative disease and iron storage in the brain. Curr. Opin. Neurol. 2004;17:437–442. doi: 10.1097/01.wco.0000137534.61244.d1. 10.1097/01.wco.0000137534.61244.d1 [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V.W, van Nieuw Amerongen G.P. Intracellular signalling involved in modulating human endothelial barrier function. J. Anat. 2002;200:549–560. doi: 10.1046/j.1469-7580.2002.00060.x. 10.1046/j.1469-7580.2002.00060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielmetter J, Kayyem J.F, Roman J.M, Dreyer W.J. Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation, is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J. Cell Biol. 1994;127:2009–2020. doi: 10.1083/jcb.127.6.2009. 10.1083/jcb.127.6.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller B.K. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. 10.1083/jcb.149.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membranes by temporal retinal axons. Development. 1987b;101:909–913. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- Walter J, Muller B, Bonhoeffer F. Axonal guidance by an avoidance mechanism. J. Physiol. (Paris) 1990;84:104–110. [PubMed] [Google Scholar]
- Xia Y, Sidis Y, Mukherjee A, Samad T.A, Brenner G, Woolf C.J, Lin H.Y, Schneyer A. Localization and action of Dragon (repulsive guidance molecule b), a novel bone morphogenetic protein coreceptor, throughout the reproductive axis. Endocrinology. 2005;146:3614–3621. doi: 10.1210/en.2004-1676. 10.1210/en.2004-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.S, West A.P, Jr, Wyman A.E, Bjorkman P.J, Enns C.A. Interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. J. Biol. Chem. 2005;280:33 885–33 894. doi: 10.1074/jbc.M506207200. 10.1074/jbc.M506207200 [DOI] [PubMed] [Google Scholar]