Abstract
In the past decade there has been an explosion in our understanding, at the molecular level, of why axons in the adult, mammalian central nervous system (CNS) do not spontaneously regenerate while their younger counterparts do. Now a number of inhibitors of axonal regeneration have been described, some of the receptors they interact with to transduce the inhibitory signal are known, as are some of the steps in the signal transduction pathway that is responsible for inhibition. In addition, developmental changes in the environment and in the neurons themselves are also now better understood. This knowledge in turn reveals novel, putative sites for drug development and therapeutic intervention after injury to the brain and spinal cord. The challenge now is to determine which of these putative treatments are the most effective and if they would be better applied in combination rather than alone. In this review I will summarize what we have learnt about these molecules and how they signal. Importantly, I will also describe approches that have been shown to block inhibitors and encourage regeneration in vivo. I will also speculate on what the differences are between the neonatal and adult CNS that allow the former to regenerate and the latter not to.
Keywords: regeneration, myelin, MAG, Nogo, p75, cAMP
1. Regeneration in the adult central nervous system
Whether it is damage to the spinal cord after injury or axonal loss due to disease, the adult mammalian central nervous system (CNS), quite simply, does not spontaneously regenerate. In sharp contrast, it appears that neonatal CNS axons do indeed regenerate after being severed. This occurs for only a limited time during development, and the precise age at which this ability is lost depends on the species, which for rat, at least, is by the end of the first post-natal week (Bregman & Goldberger 1982; Kunkel-Bagden et al. 1992). What causes this loss of ability to spontaneously regenerate with development? We know from the work of Aguayo and colleagues in the early 1980s that it is not because adult CNS neurons have lost the intrinsic ability to extend axons at all, because if they are presented with a known, favourable environment, they will regenerate, albeit significantly more slowly than their younger counterparts (Richardson et al. 1980; David & Aguayo 1981; Goldberg et al. 2002). This suggests that something in the adult CNS environment, and perhaps also the response of the adult CNS to injury/disease, changes with development. That is in fact what happens—after injury the adult CNS is inhibitory for axonal regeneration. This inhibitory environment comprises the formation of a glial scar, a response to injury not observed in young animals, and the presence of inhibitory molecules present in both the scar itself and also in myelin debris. Surprisingly, the neuron's response to this environment also changes. That is to say, young neurons can quite happily extend axons through/on these inhibitors (Cai et al. 2001). In this review I will focus on these two developmental events: the change in the CNS environment and the switch in the neuron's response to that environment. I will describe what is known regarding the molecules involved, how their inhibition can be overcome to encourage regeneration and what is required to have full functional recovery after, for example, spinal cord injury.
2. The developmental change in the central nervous system environment
(a) The glial scar
The major component of the glial scar is astrocytes that have undergone reactive gliosis. In general, this occurs after injury to the brain or spinal cord when astrocytes change their morphology by enlarging and by putting out processes that interdigitate, forming a physical barrier to axonal regeneration. This change in morphology is marked by an upregulation in intermediate filaments and, indeed, increased expression of one such filament, glial fibrillary acidic protein, is used as the hallmark of a reactive astrocyte (Eng 1985). In addition, these reactive astrocytes increase expression of extracellular matrix components, most notably chondroitin sulphate proteoglycans (CSPGs), which are very inhibitory for axonal regeneration (Rudge & Silver 1990; Snow et al. 1990; McKeon et al. 1991). The formation of the glial scar is triggered by the invasion of non-CNS factors as a result of the disruption of the blood–brain barrier (BBB; Fitch et al. 1999; Preston et al. 2001). Following from this, the severity of the lesion dictates the severity of scar formation. For example, relatively minor lesions, in which the BBB is minimally disrupted, result in reactive gliosis only very close to the lesion site (Preston et al. 2001). For lesions where there is greater disruption of the BBB but in which the meninges are left intact, the reactive gliosis is much greater, with a much stronger and more widespread upregulation of inhibitory proteoglycans. In addition, a cavity now forms at the lesion centre. Finally, in those severe lesions in which the meninges are disrupted, not only is there a strong reactive gliosis and cavitation, but also fibroblasts invade the lesion core and induce the astrocytic expression of particular guidance cues that are repulsive during development, such as Slit and ephrin-B2 as well as the ephrin receptors EphB2 and EphA4 (Turnley & Bartlett 1998; Bundesen et al. 2003; Hagino et al. 2003; Goldshmit et al. 2004). The fibroblasts themselves express the repulsive guidance cue, Sema3 and axons attempting to regenerate express the Sema3 receptor, neuropilin, and are strongly repelled by the lesion core (Pasterkamp et al. 1999; De Winter et al. 2002; Jin et al. 2002). So to encourage axons to grow through the glial scar, they must not only overcome inhibitory proteoglycans, which are expressed at all lesions, but also, depending on the severity of the lesion, ephrin-B2, Slit and Sema3.
Recently, an interesting study showed considerable regeneration of corticospinal and rubrospinal axons after a hemisection lesion in the EphA4-deficient mouse (Goldshmit et al. 2004). Interestingly, there was also attenuation of reactive gliosis as marked by less hypertrophy of astrocytes and less production of CSPGs. Although EphA4 has been shown to be a receptor for ephrin B3, Eph–ephrin interactions have been shown to signal in both directions. It is conceivable then, that for inhibition of growth, EphA4 is the ligand and the signal is transduced via ephrinB3 on the axon, while on astrocytes EphA4 is the receptor and transduces the signal. The question now to be addressed is whether or not blocking Eph4A with antibodies or peptides has a similar effect on regeneration in vivo in wild-type mice as that recorded in the knockout mouse.
The CSPGs in the glial scar include brevican, phosphocan, aggrecan, neurocan and NG2, which has also been shown to be expressed by oligodendrocyte progenitor cells (Henderson et al. 1981; Dou & Levine 1994; Jones et al. 2002). Proteoglycans consist of a protein core linked to a sulphated glycosaminoglycan (GAG) chain by four sugar moieties. They differ not only in the protein core but also in length, number and pattern of sulphate of the side chains (Margolis & Margolis 1993). The GAG portion of the molecules was shown to be the inhibitory portion, because if it is removed from reactive astrocytes in culture with the enzyme chondroitinase, they were no longer inhibitory for regeneration. Importantly, application of the same enzyme in vivo after lesion resulted in considerable axonal regeneration in the spinal cord and some functional recovery (Bradbury et al. 2002). Interestingly, although the neuronal receptors for CSPGs are not yet known and little is known regarding their signalling pathway, they have been shown to activate the small GTPase, Rho, and this activation is necessary for their inhibitory effects (Borisoff et al. 2003; Monnier et al. 2003). Myelin inhibitors also signal through Rho, which points to a common target whereby inhibition by both CSPGs and myelin inhibitors can be blocked simultaneously. Another approach that has been shown to block the inhibition by both CSPGs and myelin is to elevate neuronal cAMP (Chierzi et al. 2005). Manipulations of the signalling cascades as a possible therapy are discussed in more detail below.
Unlike the mature CNS, young CNS axons will spontaneously regenerate after injury (Ferretti et al. 2003). The stage of development when the switch from being able to regenerate to not being able to do so depends on the species and on the particular neural tract, but is in all cases neonatal. For example, in the chick spinal cord, the ability to regenerate is lost between E11 and E15, whereas for the rat it is in the first post-natal week (Bregman & Goldberger 1982; Shimizu et al. 1990; Kunkel-Bagden et al. 1992; Hasan et al. 1993). Although no real scar forms after injury at the ages when spontaneous regeneration does occur, astrocytes still undergo a reaction to injury (McKeon et al. 1991). In sharp contrast to the adult, however, reactive astrocytes from these very young animals are very permissive for axonal growth. While it is not known what the precise molecules that promote growth are, it has been shown that these young reactive astrocytes do not express inhibitory CSPGs. The reason for this difference in reactive gliosis in young and old astrocytes is not known, but it has been suggested that not only is there less haemorrhage in young animals after injury but also the immune cells and the factors they secrete may be different (Ferretti et al. 2003; Silver & Miller 2004).
(b) Inhibition of regeneration by myelin
In both the CNS and peripheral nervous system (PNS), myelination is one of the last events to occur during development (Morell 1984). The growth cone of the nascent axon has either reached its target, or at least is well past the region of the nerve tract that undergoes myelination. Hence, when first growing towards their targets, young axons never encounter myelin, and, consequently, the inhibitors therein. Intact myelin is of course beneficial as it insulates the axon and allows salutatory conduction to take place. After injury or disease, when axons are severed, myelin is inevitably disrupted and rather than being tightly wrapped around the axon, myelin membrane fragments are strewn throughout the injury site. The newly formed growth cone of the transected axon now comes into contact with this myelin debris, growth stops and the axon will most likely retract (Ramon y Cajal 1928). This is the scenario in the CNS, but in the PNS the picture is quite different as the reaction to injury is very different. In the CNS, Wallerian degeneration (degeneration of the distal axon and clearing of debris, including myelin) is very slow, taking months or years, and it is questionable if it is ever complete. In addition, a glial scar forms. In contrast, Wallerian degeneration is very rapid in the PNS, and although there are inhibitors in PNS myelin, they are cleared very rapidly, along with the distal axon. No glial scar forms in the PNS; instead, Schwann cells de-differentiate and become permissive for axonal regeneration, before going on to re-myelinate the regenerated axon. Regeneration and the environment of the adult PNS is more reminiscent of axonal growth during development in either the CNS or PNS (Scherer & Salzer 2001).
(i) The inhibitors
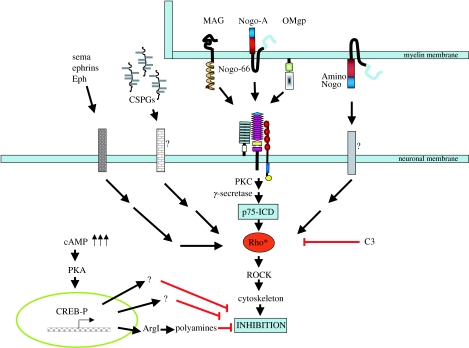
To date three myelin-associated proteins have been clearly demonstrated to be inhibitory for regeneration (figure 1). These are the three very different proteins: Nogo, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp) (Filbin 2003).
Figure 1.
Inhibitors of axonal regeneration, their receptors and how they signal. Three myelin inhibitors, MAG, Nogo-66 and OMgp, all interact with the NgR/Lingo/p75 (TROY) receptor complex. The receptors for Amino-Nogo and CSPGs are not known. All the inhibitors exert inhibition by activating Rho. Inactivation of Rho or elevation of cAMP each overcome all the inhibitors simultaneously. The cAMP effect is CREB- and transcription-dependent. ArgI is up-regulated, resulting in an increase in polyamine synthesis. Polyamines can block inhibition.
Nogo
Nogo was identified as an antigen of the IN-1 monoclonal antibody, which had been shown 10 years prior to the cloning of Nogo to encourage axonal regeneration both on myelin in culture and in the CNS in vivo (Caroni & Schwab 1988; Schnell & Schwab 1990; Bregman et al. 1995). Nogo is a member of the reticulon family of proteins, which, except for Nogo, are found only in the endoplasmic reticulum (Chen et al. 2000; GrandPre et al. 2000; Prinjha et al. 2000). Nogo exists in three isoforms, A, B and C; of these Nogo-A is enriched in oligodendrocytes, Nogo-B in neurons and Nogo-C is found largely outside the nervous system (GrandPre et al. 2000; Huber et al. 2002; Wang et al. 2002c). Nogo-A is reported to have at least two inhibitory domains, a 66-amino acid sequence, referred to as Nogo-66, which is common to all three isoforms, and a sequence found in the amino terminus which is unique to Nogo-A and is termed Amino-Nogo (Oertle et al. 2003). Although it carries no signal sequence, Nogo-A has been localized to both the inner- and the outermost wrap of the myelin sheath (GrandPre et al. 2000; Huber et al. 2002; Wang et al. 2002c). At least two topologies have been proposed for Nogo-A: one in which both Amino-Nogo and Nogo-66 are extracellular and the other in which only Nogo-66 is exposed at the surface (Oertle et al. 2003).
Myelin-associated glycoprotein
Unlike Nogo, MAG was identified about 20 years before it was shown to be inhibitory for axonal growth (Quarles 1983; McKerracher et al. 1994; Mukhopadhyay et al. 1994). It is a member of the immunoglobulin (Ig)-superfamily, containing five Ig-like domains in its extracellular sequences. The two MAG isoforms, large (L) and small (S), differ only in their cytoplasmic sequences (Lai et al. 1987; Salzer et al. 1987). MAG is a sialic acid binding protein, and is a member of the Siglec (sialic acid binding lectins) family of proteins (Siglec 4; Kelm et al. 1994; Crocker et al. 1998). It binds specifically to the gangliosides GT1b and GD1a. The sialic acid binding site for MAG has been mapped to Arg118 in the first Ig-domain (Tang et al. 1997). However, it would appear that sialic acid binding alone is not sufficient to effect inhibition by MAG, because a truncated form of the protein, consisting of only MAG Ig-domains 1–3, can bind neurons in a sialic acid-dependent manner but does not inhibit neurite outgrowth. This suggests that the inhibitory domain is carried by, or at least requires the presence of, MAG Ig-domains 4 and 5 (Tang et al. 1997). In the PNS, MAG represents about 0.1% of the total myelin protein, and is found in both the inner and outer loops of the sheath, as well as in regions of uncompacted myelin at the nodes of Ranvier. In the CNS, MAG is more than 10-fold more abundant, and is found only at the inner loop and in the uncompacted loops at the nodes (Trapp 1988, 1990).
Oligodendrocyte myelin glycoprotein
Like MAG, OMgp was first described many years before it was shown to be inhibitory (Mikol & Stefansson 1988; Kottis et al. 2002; Wang et al. 2002b). It is a glycosyl phosphatidyl inositol (GPI)-linked protein and carries a conserved leucine-rich repeat (LRR) and a C-terminal LRR. It is a relatively minor component in both CNS and PNS myelin, and may indeed be expressed in more abundance by other non-myelinating cells (Huang et al. 2005). Recently, OMgp expression was described in cells that have processes that extend to the node of Ranvier. There is evidence to suggest that these OMgp-containing processes prevent aberrant sprouting from the node in the intact nervous system (Huang et al. 2005). OMgp is believed to be localized in myelin to uncompacted regions.
Development guidance cues as inhibitors of regeneration in the adult
Recently, the inhibitory guidance cue, ephrin-B3 has been shown to be expressed by adult oligodendrocytes and to associate with myelin when extracted. There is a modest improvement in neurite growth on myelin when ephrin-B3 is blocked (Benson et al. 2005). Two other repulsive guidance cues of the semaphorin family, Sema5A and Sema4D, have been shown to be expressed by mature oligodendrocytes in vivo (Moreau-Fauvarque et al. 2003; Goldberg et al. 2004). The contribution these inhibitors make to inhibition by myelin has not yet been reported. Netrin is another guidance cue that is expressed by mature oligodendrocytes (Manitt et al. 2001). Netrin is bifunctional and can function as either an attractive or a repulsive cue to axon growth (Colamarino & Tessier-Lavigne 1995). It is not known if netrin contributes to the effect of myelin on axon growth, either positively or negatively.
(ii) The receptors
The first receptor for any of the myelin-associated inhibitors to be described was for Nogo-66. It was identified as a GPI-linked protein and was termed Nogo-66 receptor (NgR). It is predicted to contain eight LRRs and a flanking LRR that is rich in cysteines (Fournier et al. 2001). The second inhibitory domain on Nogo-A, Amino-Nogo, does not interact with this receptor. Surprisingly, however, both MAG and OMgp were subsequently shown to interact with NgR to exert their inhibitory action (Domeniconi et al. 2002; Kottis et al. 2002; Liu et al. 2002; Wang et al. 2002b). This was unexpected because these three proteins bear no obvious sequence similarity or even domain similarity. However, when the crystal structure of NgR was solved it was apparent that there were a number of ‘binding platforms’ that were predicted to be able to bind a diverse group of ligands (Barton et al. 2003). There are two conflicting reports of regeneration in the NgR-deficit mouse—one report finds extensive regeneration and the other none (Kim et al. 2004; Zheng et al. 2005). It is possible that other receptors exist for Nogo-66, MAG and OMgp. Indeed, two other NgR isoforms have been described, one of which has been shown to specifically bind MAG (Barton et al. 2003; Venkatesh et al. 2005). The binding partner for Amino-Nogo has yet to be described.
Because NgR is a GPI-linked protein, it cannot transduce the inhibitory signal across the membrane; a transducing partner was needed in the receptor complex. Revelation of the transducing component in the receptor complex as the well-known neurotrophin receptor p75, was also a surprise (Wang et al. 2002a; Wong et al. 2002). However, even before NgR was identified, p75 was implicated in transducing the signal for MAG, through MAG's ability to bind specific gangliosides and so cluster and activate p75 (Yamashita et al. 2002). Although it has been shown that the ganglioside binding capability of MAG is not necessary for it to inhibit neurite outgrowth (Tang et al. 1997), it has been reported that if GT1b and GD1a are absent or blocked, MAG does not inhibit neurite outgrowth (Vinson et al. 2001; Vyas et al. 2002). This apparent discrepancy in findings may arise from differences in how the neurite outgrowth assay is conducted and how MAG behaves when expressed by live cells compared to when immobilized as a substrate. It is of note, however, that when gangliosides are clustered with antibodies, in the absence of MAG, inhibition occurs (Vinson et al. 2001; Yamashita et al. 2002). This demonstrates that gangliosides can activate the p75 pathway that signals inhibition. The question remains as to whether this ever occurs in vivo. It is, however, highly likely that gangliosides can augment the signalling of inhibitory molecules, regardless of whether or not they are necessary for the effect.
Although p75 was clearly a member of this receptor complex and could indeed transduce the signal, it is not expressed by all types of neurons; yet neurons that express no p75 are still inhibited by myelin. It was subsequently found that another member of the tumour necrosis factor receptor family, of which p75 is a member, was able to substitute for p75 in the receptor complex and transduce the inhibitory signal; this protein was a known protein termed TROY or TAJ (Park et al. 2005; Shao et al. 2005). Finally, a third component of the complex was identified as a protein called LINGO, which is believed to be involved in bringing ligand-bound NgR and p75 or TROY together (Mi et al. 2004).
The receptors for the axonal guidance molecules that are believed to contribute to inhibition of axonal growth in the adult CNS are the same as those that inhibit growth during development.
(iii) Signalling by myelin-associated inhibitors
The final changes that occur in response to a signal, regardless of whether that signal triggers axonal growth or inhibition of growth, are changes in the arrangement of the cytoskeleton. The ultimate goal is to characterize all the signal transduction steps arising from receptor–ligand interaction that culminate in a direct effect on the cytoskeleton. It therefore seems reasonable to suggest that eventually all inhibitors of regeneration must at some point in the signalling pathway converge, such that they exert the same changes on the dynamics of the cytoskeleton. One such convergence point is probably the activation of the small GTPase, Rho, as all the inhibitors described above, regardless of whether they are myelin-associated or guidance cues, and regardless of what receptor complex they initially activate, have been reported to activate Rho (Liu & Strittmatter 2001; Schmucker & Zipursky 2001; Niederost et al. 2002; Yamashita et al. 2002; Borisoff et al. 2003; Fournier et al. 2003; Monnier et al. 2003). Activated Rho changes the dynamics of the actin cytoskeleton by activating the Rho kinase, ROCK (Hall 1998). What have yet to be completely characterized are the steps before and after Rho/ROCK activation. Of the myelin-associated inhibitors, other than its ability to activate Rho, little else is known regarding the signalling initiated by Amino-Nogo. Considerably, more is known regarding signalling through the NgR receptor complex by the other myelin-associated inhibitors.
Recently, it has been shown that upon MAG binding to the NgR complex, p75 undergoes regulated intramembrane proteolysis (RIP; Domeniconi et al. 2005). It is believed that RIP begins when MAG binds by, first, the extracellular domain of p75 being released by cleavage close to the membrane by an α-secretase. This is necessary for the second step to occur, which is a protein kinase C (PKC)-dependent, γ-secretase cleavage within the membrane, RIP. This second cleavage in turn releases the C-terminus of p75 into the cytoplasm, which is necessary both to activate Rho and to effect inhibition of neurite outgrowth. It is not known if this p75 fragment then enters the nucleus to exert its effect, as do the cytoplasmic fragments of other proteins that have undergone RIP, such as Notch (De Strooper et al. 1999). Alternatively, the fragment could have a direct effect in the cytoplasm by altering the cytoskeleton. If any one of the players in the RIP of p75—α-secretase, PKC or γ-secretase—is blocked, inhibition by myelin is blocked. In addition, it is of note that if the cytoplasmic fragment of p75 is expressed in neurons, even in the absence of myelin-associated inhibitors, Rho is activated and neurite outgrowth is inhibited (Yamashita & Tohyama 2003; Domeniconi et al. 2005). Interestingly, it has been proposed that p75 in the receptor complex is associated with Rho bound to its inhibitor Rho-GDI. Upon interacting with myelin inhibitors, p75 is proposed to activate Rho by displacing Rho-GDI (Yamashita & Tohyama 2003). What is unclear, however, is whether p75 cleavage directs this proposed displacement.
More recently, a novel trans-activation of the epidermal growth factor receptor (EGFR) and its downstream effector, Erk, by myelin-associated inhibitors has been reported (Koprivica et al. 2005). If activation of the EGFR pathway is blocked, the myelin-associated inhibitors have no effect, but activation of the pathway by its own ligand, EGF, does not induce inhibition of neurite outgrowth. This demonstrates that although activation of the EGFR by myelin inhibitors is necessary to bring about inhibition of neurite growth, it is not sufficient; the implication is that in addition to the EGFR pathway, activation of the NgR receptor complex must activate a separate pathway, which is also required to effect inhibition. It is of note that CSPGs also trans-activate the EGFR pathway, which is also necessary for them to bring about inhibition (Koprivica et al. 2005).
(iv) Overcoming myelin inhibitors to promote regeneration in vivo
It may appear futile to try and encourage regeneration by blocking only the myelin-associated inhibitors without also blocking those in the glial scar. However, one study, a number of years ago, suggested that immediately after the injury the major impediments to regeneration are myelin-associated inhibitors (Huang et al. 1999). In that study, mice were immunized with myelin proteins prior to carrying out a spinal cord lesion. In the myelin-immunized mice, many axons regenerated and some appeared to be ‘pinched’, suggesting that the scar had indeed formed after the axons had re-grown. Therefore, there may be a window of opportunity, immediately after injury, when blocking myelin inhibitors may be sufficient to promote axon regeneration.
In general, there are two ways in which to overcome inhibitors in the CNS and encourage regeneration: either block the inhibitors or their receptors with antibodies or peptides, or change the neuron from within such that it no longer interprets the signal as inhibitory. Antibodies or peptides corresponding to Nogo and to the NgR have received the most attention in recent years and varying degrees of success have been reported (Bregman et al. 1995; Brosamle et al. 2000; Fournier et al. 2002; GrandPre et al. 2002; Li & Strittmatter 2003). However, it is difficult to reconcile these different studies, as success has been reported by one group with antibodies specifically to Amino-Nogo, while others have blocked only Nogo-66 or the NgR receptor. Hence, it is difficult to interpret the results when one study would argue that blocking only Amino-Nogo is sufficient while the other indicates that Nogo-66–NgR is more important. Conflicts are also reflected in the results from mice that are deficient in various combinations of Nogo isoforms or in NgR, as findings range from no improvement in regeneration, to limited regeneration, to considerable regeneration with functional recovery (Kim et al. 2003, 2004; Simonen et al. 2003; Zheng et al. 2003; Willis et al. 2005). These issues remain to be resolved. It is noteworthy that, as described above, substantial regeneration was reported in the Eph4A-deficient mouse, which complicates the situation even further (Goldshmit et al. 2004).
Two strategies can also be taken to change the neuron from within: interfere with the cascade that signals inhibition or activate a parallel pathway that will overcome the inhibition signalling pathway. Both approaches have been taken with some success. As mentioned above, all signals from all the inhibitors described to date converge on Rho, an observation supported by the fact that there is considerable regeneration and functional recovery in animals treated with the Rho inhibitor C3 (Lehmann et al. 1999; Dergham et al. 2002; Winton et al. 2002). In addition, others have reported that a PKC inhibitor (Sivasankaran et al. 2004) or an inhibitor of Erk activation promotes regeneration of lesioned optic nerve (Koprivica et al. 2005). It is difficult to compare these strategies and determine which is more potent, as the nerve tracts lesioned are often different as are, in most cases, the types of lesion.
A number of groups have shown that activation of the cAMP signalling pathway in neurons not only blocks inhibition of neurite outgrowth by myelin-associated inhibitors, but also changes growth repulsion to attraction for some guidance cues, as well as CSPGs (Cai et al. 1999; Song et al. 1998; Chierzi et al. 2005). To activate this pathway, cAMP has been elevated in cultured neurons in a variety of ways: with membrane permeable, non-hydrolysable cAMP analogues; by inhibiting phosphodiesterases, the enzymes that degrade cAMP; and by prior exposure of neurons to neurotrophins, such nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) (Cai et al. 1999; Lu et al. 2004; Nikulina et al. 2004; Pearse et al. 2004). It is of note that if the neurons are exposed to the neurotrophin at the same time as the myelin inhibitor, the neurotrophin has no effect and inhibition occurs. It has been established that neurotrophins elevate cAMP by an Erk-dependent inhibition of phosphodiesterase, and signalling by myelin-associated inhibitors, by a mechanism not yet fully understood, blocks this event (Cai et al. 1999; Gao et al. 2003). However, if cAMP is elevated with neurotrophins and the cAMP pathway triggered before exposure to the inhibitors, the inhibitors have no effect. Regardless of how cAMP is elevated, transcription is triggered by activation of the transcription factor cAMP response element binding protein (CREB) and the ability of cAMP to overcome inhibition by myelin is both CREB- and transcription-dependent (Gao et al. 2004). At least one gene is known to be upregulated by cAMP that plays a role in this phenomenon (Cai et al. 2002). It is the enzyme arginase I (ArgI), which is key in the synthesis of polyamines, and either overexpression of ArgI in neurons or exogenous polyamines are able to allow growth in the presence of myelin-associated inhibitors in culture. The mechanism whereby polyamines overcome inhibition of regeneration remains to be determined. However, what is of particular importance is that cAMP overcomes inhibition through the initiation of a genetic programme, by affecting transcription in the nucleus. This presents the attractive possibility of treating neuronal cell bodies, which can be more accessible to manipulation, rather than the lesion site per se to promote axonal regeneration in vivo. Indeed, this has been done successfully with injection of dibutyryl cAMP directly into the dorsal root ganglion cell body, which is sufficient to promote regeneration of spinal dorsal column axons lesioned at the T7/T8 level (Neumann et al. 2002; Qiu et al. 2002).
3. The neuronal response to the environment
As described previously, during development, myelin is not an impediment to axonal growth because it is not present. Myelination is one of the last events to occur in development. In rodents, it begins in the ventral roots and the ventral and lateral tracts of the spinal cord at about post-natal day 2. It then proceeds in a rostrocaudal direction with the first myelin appearing in the brain at post-natal day 10 (Morell 1984; Lazzarini 2003). Consistent with the lack of myelin inhibitors, coupled with the fact that no glial scar forms after injury, spontaneous regeneration occurs in neonatal animals after spinal cord injury. Indeed, it has been shown for some regions of the nervous system that the loss in ability to spontaneously regenerate coincides with the onset of myelination (Ferretti et al. 2003). However, surprisingly, even though there are no inhibitors around, if young neurons are removed and grown on myelin, they are not inhibited (Cai et al. 2001). Depending on the age and the type of neuron, with development comes a switch in response to myelin and they become strongly inhibited from extending processes. It is not known if there is a physiological reason why young neurons are not inhibited by myelin, despite the fact that they are unlikely to encounter it. Perhaps it is as a safeguard in case growth of some axons is delayed in reaching their targets and so they would then come in contact with myelin. Regardless of whether or not there is a physiological reason for young neurons being able to grow on myelin, it is important to know mechanistically how they can grow in the presence of these inhibitors. Two possibilities are that they do not express the receptors for myelin inhibitors or that they are intrinsically different from older neurons and respond to the inhibitors in a positive manner. The latter seems to be the most likely explanation. We have shown that if young neurons that grow very well on myelin and on individual myelin inhibitors such as MAG are treated with a protein kinase A inhibitor, neurite outgrowth is now inhibited (Cai et al. 2001). Similarly, the ability of neonatal spinal axons to spontaneously regenerate is blocked by a PKA inhibitor administered in vivo. Consistent with the suggestion that cAMP/PKA signalling plays some role in the developmental switch to myelin inhibitors, endogenous cAMP levels are high in young neurons and the levels fall quite sharply with age at a time when the switch to inhibition occurs. The indication that it is the intrinsic state of the neuron that changes raises two questions: as with artificially elevating cAMP in older neurons described above, what are the downstream effectors of the cAMP/PKA pathway that allow these young neurons to grow in an inhibitory environment and spontaneously regenerate and what triggers the decrease in cAMP levels with development? For the downstream effectors, ArgI levels are also higher in young neurons than in older neurons, which is consistent with what is upregulated in older neurons when cAMP is elevated, suggesting that the mechanisms are the same. As to the trigger for the decrease in cAMP, it is tempting to speculate that myelination per se is the trigger, given that it has been shown that interaction of myelin with the neuron activates a pertussis toxin-sensitive Gi/Go protein, which could bring about a drop in cAMP (Cai et al. 1999). However, there are many events that occur during development that could affect the neuronal cAMP levels, such as a decline in neurotrophins and their receptors. The trigger(s) remains to be described.
4. Conclusions
Is the best approach to achieving successful regeneration in the adult CNS to try and recapitulate development? The answer is not clear. Obviously, it would be a tremendous advance if axons could be encouraged to grow as fast as their young counterparts and also not to be blocked from growing by all the inhibitors described previously. Perhaps inactivation of Rho and elevating cAMP or some of its downstream effectors are steps in the right direction. The timing of treatment then becomes crucial, in that the axons need to re-grow before the glial scar matures and physically locks them in. To overcome this restriction on timing of treatment, perhaps methods could be devised to try and prevent the scar from forming, such as manipulation of the immune response. However, this in itself may present its own set of problems, in that it appears that the main function of the scar is not to stop axons regenerating but to limit damage to healthy tissue by locking in the site of injury and the immune cell invasion (Bush et al. 1999; Faulkner et al. 2004). An attractive alternative would be to induce in old animals the reactive gliosis that occurs in young astrocytes, a daunting task given the complexity of the reaction. To date the most successful strategy to encourage axons to regenerate, which in some cases achieves functional recovery and attenuation of the glial scar, has been to use a combination of treatments: transplant cells that are permissive for growth at the lesion site, as well as elevate cAMP and neurotrophins (Lu et al. 2004; Nikulina et al. 2004; Pearse et al. 2004). To this combination, inactivation of Rho, inactivation of EGFR and, importantly, digestion of CSPGs with chondroitinase should be added and maybe then many more axons can be encouraged to grow long distances. If this is achieved, the next hurdles will be giving the axon direction such that it reaches its correct target, ensuring it makes a functional synapse and finally that it is remyelinated. This then would be a true recapitulation of development.
Footnotes
One contribution of 13 to a Theme Issue ‘The regenerating brain’.
References
- Barton W.A, Liu B.P, Tzvetkova D, Jeffrey P.D, Fournier A.E, Sah D, Cate R, Strittmatter S.M, Nikolov D.B. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. 10.1093/emboj/cdg325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M.D, Romero M.I, Lush M.E, Lu Q.R, Henkemeyer M, Parada L.F. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl Acad. Sci. USA. 2005;102:10 694–10 699. doi: 10.1073/pnas.0504021102. 10.1073/pnas.0504021102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisoff J.F, Chan C.C, Hiebert G.W, Oschipok L, Robertson G.S, Zamboni R, Steeves J.D, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol. Cell. Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. 10.1016/S1044-7431(02)00032-5 [DOI] [PubMed] [Google Scholar]
- Bradbury E.J, Moon L.D, Popat R.J, King V.R, Bennett G.S, Patel P.N, Fawcett J.W, McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- Bregman B.S, Goldberger M.E. Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science. 1982;217:553–555. doi: 10.1126/science.7089581. [DOI] [PubMed] [Google Scholar]
- Bregman B.S, Kunkel-Bagden E, Schnell L, Dai H.N, Gao D, Schwab M.E. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. 10.1038/378498a0 [DOI] [PubMed] [Google Scholar]
- Brosamle C, Huber A.B, Fiedler M, Skerra A, Schwab M.E. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J. Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesen L.Q, Scheel T.A, Bregman B.S, Kromer L.F. Ephrin-B2 and EphB2 regulation of astrocyte–meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush T.G, Puvanachandra N, Horner C.H, Polito A, Ostenfeld T, Svendsen C.N, Mucke L, Johnson M.H, Sofroniew M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. 10.1016/S0896-6273(00)80781-3 [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin M.T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. 10.1016/S0896-6273(00)80681-9 [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman B.S, Filbin M.T. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan R.R, Filbin M.T. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. 10.1016/S0896-6273(02)00826-7 [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab M.E. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. 10.1016/0896-6273(88)90212-7 [DOI] [PubMed] [Google Scholar]
- Chen M.S, Huber A.B, van der Haar M.E, Frank M, Schnell L, Spillmann A.A, Christ F, Schwab M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. 10.1038/35000219 [DOI] [PubMed] [Google Scholar]
- Chierzi S, Ratto G.M, Verma P, Fawcett J.W. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur. J. Neurosci. 2005;21:2051–2062. doi: 10.1111/j.1460-9568.2005.04066.x. 10.1111/j.1460-9568.2005.04066.x [DOI] [PubMed] [Google Scholar]
- Colamarino S.A, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. 10.1016/0092-8674(95)90083-7 [DOI] [PubMed] [Google Scholar]
- Crocker P.R, et al. Siglecs: a family of sialic-acid binding lectins [letter] Glycobiology. 1998;8:v. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell W.D, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. 10.1038/19083 [DOI] [PubMed] [Google Scholar]
- De Winter F, Oudega M, Lankhorst A.J, Hamers F.P, Blits B, Ruitenberg M.J, Pasterkamp R.J, Gispen W.H, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. 10.1006/exnr.2002.7884 [DOI] [PubMed] [Google Scholar]
- Domeniconi M, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. 10.1016/S0896-6273(02)00770-5 [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao M.V, Filbin M.T. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. 10.1016/j.neuron.2005.05.029 [DOI] [PubMed] [Google Scholar]
- Dou C.L, Levine J.M. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L.F. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. 10.1016/S0165-5728(85)80063-1 [DOI] [PubMed] [Google Scholar]
- Faulkner J.R, Herrmann J.E, Woo M.J, Tansey K.E, Doan N.B, Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti P, Zhang F, O'Neill P. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev. Dyn. 2003;226:245–256. doi: 10.1002/dvdy.10226. 10.1002/dvdy.10226 [DOI] [PubMed] [Google Scholar]
- Filbin M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. 10.1038/nrn1195 [DOI] [PubMed] [Google Scholar]
- Fitch M.T, Doller C, Combs C.K, Landreth G.E, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A.E, GrandPre T, Strittmatter S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. 10.1038/35053072 [DOI] [PubMed] [Google Scholar]
- Fournier A.E, Gould G.C, Liu B.P, Strittmatter S.M. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J. Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A.E, Takizawa B.T, Strittmatter S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Nikulina E, Mellado W, Filbin M.T. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J. Neurosci. 2003;23:11 770–11 777. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Deng K, Nikulina E, Mellado W, Spencer T, Barco A, Kandel E, Filbin M.T. Activation of CREB is necessary and sufficient to overcome mylein inhibitors and encourage spinal axon regeneration in vivo. Neuron. 2004;44:609–619. doi: 10.1016/j.neuron.2004.10.030. 10.1016/j.neuron.2004.10.030 [DOI] [PubMed] [Google Scholar]
- Goldberg J.L, Klassen M.P, Hua Y, Barres B.A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. 10.1126/science.1068428 [DOI] [PubMed] [Google Scholar]
- Goldberg J.L, Vargas M.E, Wang J.T, Mandemakers W, Oster S.F, Sretavan D.W, Barres B.A. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J. Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. 10.1523/JNEUROSCI.4390-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Galea M.P, Wise G, Bartlett P.F, Turnley A.M. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 2004;24:10 064–10 073. doi: 10.1523/JNEUROSCI.2981-04.2004. 10.1523/JNEUROSCI.2981-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter S.M. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. 10.1038/35000226 [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Hagino S, et al. Slit and glypican-1 mRNAs are coexpressed in the reactive astrocytes of the injured adult brain. Glia. 2003;42:130–138. doi: 10.1002/glia.10207. 10.1002/glia.10207 [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- Hasan S.J, Keirstead H.S, Muir G.D, Steeves J.D. Axonal regeneration contributes to repair of injured brainstem–spinal neurons in embryonic chick. J. Neurosci. 1993;13:492–507. doi: 10.1523/JNEUROSCI.13-02-00492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C.E, Huchet M, Changeux J.P. Neurite outgrowth from embryonic chicken spinal neurons is promoted by media conditioned by muscle cells. Proc. Natl Acad. Sci. USA. 1981;78:2625–2629. doi: 10.1073/pnas.78.4.2625. 10.1073/pnas.78.4.2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.W, McKerracher L, Braun P.E, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. 10.1016/S0896-6273(00)81118-6 [DOI] [PubMed] [Google Scholar]
- Huang J.K, et al. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. 10.1126/science.1118313 [DOI] [PubMed] [Google Scholar]
- Huber A.B, Weinmann O, Brosamle C, Oertle T, Schwab M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G.B, Inoue S, Urano T, Cho S, Ouchi Y, Cyong J.C. Induction of anti-metallothionein antibody and mercury treatment decreases bone mineral density in mice. Toxicol. Appl. Pharmacol. 2002;185:98–110. doi: 10.1006/taap.2002.9531. 10.1006/taap.2002.9531 [DOI] [PubMed] [Google Scholar]
- Jones L.L, Yamaguchi Y, Stallcup W.B, Tuszynski M.H. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm S, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. 10.1016/S0960-9822(00)00220-7 [DOI] [PubMed] [Google Scholar]
- Kim J.E, Li S, GrandPre T, Qiu D, Strittmatter S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. 10.1016/S0896-6273(03)00147-8 [DOI] [PubMed] [Google Scholar]
- Kim J.E, Liu B.P, Park J.H, Strittmatter S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. 10.1016/j.neuron.2004.10.015 [DOI] [PubMed] [Google Scholar]
- Koprivica V, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. 10.1126/science.1115462 [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao Z.C, Zhang R, Dergham P, Braun P.E. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J. Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. 10.1046/j.1471-4159.2002.01146.x [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Dai H.N, Bregman B.S. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp. Neurol. 1992;116:40–51. doi: 10.1016/0014-4886(92)90174-o. 10.1016/0014-4886(92)90174-O [DOI] [PubMed] [Google Scholar]
- Lai C, Brow M.A, Nave K.A, Noronha A.B, Quarles R.H, Bloom F.E, Milner R.J, Sutcliffe J.G. Two forms of 1B236/myelin-associated glycoprotein, a cell adhesion molecule for postnatal neural development, are produced by alternative splicing. Proc. Natl Acad. Sci. USA. 1987;84:4337–4341. doi: 10.1073/pnas.84.12.4337. 10.1073/pnas.84.12.4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R.A. Academic Press; San Diego, CA: 2003. Myelin: biology and disorders. [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter S.M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.P, Strittmatter S.M. Semaphorin-mediated axonal guidance via Rho-related G proteins. Curr. Opin. Cell Biol. 2001;13:619–626. doi: 10.1016/s0955-0674(00)00260-x. 10.1016/S0955-0674(00)00260-X [DOI] [PubMed] [Google Scholar]
- Liu B.P, Fournier A, GrandPre T, Strittmatter S.M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. 10.1126/science.1073031 [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones L.L, Filbin M.T, Tuszynski M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. 10.1523/JNEUROSCI.1492-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Colicos M.A, Thompson K.M, Rousselle E, Peterson A.C, Kennedy T.E. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J. Neurosci. 2001;21:3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R.K, Margolis R.U. Nervous tissue proteoglycans. Experientia. 1993;49:429–446. doi: 10.1007/BF01923587. 10.1007/BF01923587 [DOI] [PubMed] [Google Scholar]
- McKeon R.J, Schreiber R.C, Rudge J.S, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson D.L, Kottis V, Dunn R.J, Braun P.E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. 10.1016/0896-6273(94)90247-X [DOI] [PubMed] [Google Scholar]
- Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. 10.1038/nn1188 [DOI] [PubMed] [Google Scholar]
- Mikol D.D, Stefansson K. A phosphatidylinositol-linked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes. J. Cell Biol. 1988;106:1273–1279. doi: 10.1083/jcb.106.4.1273. 10.1083/jcb.106.4.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier P.P, Sierra A, Schwab J.M, Henke-Fahle S, Mueller B.K. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. 10.1016/S1044-7431(02)00035-0 [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P. 2nd edn. Plenum Press; New York, NY: 1984. Myelin. [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh F.S, Crocker P.R, Filbin M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. 10.1016/0896-6273(94)90042-6 [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. 10.1016/S0896-6273(02)00702-X [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney R.A, Bandtlow C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10 368–10 376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell L, Dai H, Bregman B.S, Filbin M.T. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes regeneration and functional recovery. Proc. Natl Acad. Sci. USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. 10.1073/pnas.0402595101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle T, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.B, Yiu G, Kaneko S, Wang J, Chang J, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. 10.1016/j.neuron.2004.12.040 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Giger R.J, Ruitenberg M.J, Holtmaat A.J, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell. Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. 10.1006/mcne.1999.0738 [DOI] [PubMed] [Google Scholar]
- Pearse D.D, Pereira F.C, Marcillo A.E, Bates M.L, Berrocal Y.A, Filbin M.T, Bunge M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. 10.1038/nm1056 [DOI] [PubMed] [Google Scholar]
- Preston E, Webster J, Small D. Characteristics of sustained blood–brain barrier opening and tissue injury in a model for focal trauma in the rat. J. Neurotrauma. 2001;18:83–92. doi: 10.1089/089771501750055794. 10.1089/089771501750055794 [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore S.E, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons D.L, Walsh F.S. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. 10.1038/35000287 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman P.N, Bregman B.S, Filbin M.T. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. 10.1016/S0896-6273(02)00730-4 [DOI] [PubMed] [Google Scholar]
- Quarles R.H. Myelin-associated glycoprotein in development and disease. Dev. Neurosci. 1983;6:285–303. doi: 10.1159/000112356. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and regeneration of the nervous system. vol. 5. Oxford University Press; Oxford, UK: 1928. [Google Scholar]
- Richardson P.M, McGuinness U.M, Aguayo A.J. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. 10.1038/284264a0 [DOI] [PubMed] [Google Scholar]
- Rudge J.S, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J.L, Holmes W.P, Colman D.R. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J. Cell Biol. 1987;104:957–965. doi: 10.1083/jcb.104.4.957. 10.1083/jcb.104.4.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S.S, Salzer J.L. Axon–Schwann cell interactions during peripheral nerve degeneration and regeneration. In: Jensen K.R, Richardson W.D, editors. Glia cell development. Oxford University Press; Oxford, UK: 2001. p. 299. [Google Scholar]
- Schmucker D, Zipursky S.L. Signaling downstream of Eph receptors and ephrin ligands. Cell. 2001;105:701–704. doi: 10.1016/s0092-8674(01)00391-9. 10.1016/S0092-8674(01)00391-9 [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab M.E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. 10.1038/343269a0 [DOI] [PubMed] [Google Scholar]
- Shao Z, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. 10.1016/j.neuron.2004.12.050 [DOI] [PubMed] [Google Scholar]
- Shimizu I, Oppenheim R.W, O'Brien M, Shneiderman A. Anatomical and functional recovery following spinal cord transection in the chick embryo. J. Neurobiol. 1990;21:918–937. doi: 10.1002/neu.480210609. 10.1002/neu.480210609 [DOI] [PubMed] [Google Scholar]
- Silver J, Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Simonen M, et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. 10.1016/S0896-6273(03)00226-5 [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang K.C, Zhang Y.P, Shields C.B, Xu X.M, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. 10.1038/nn1193 [DOI] [PubMed] [Google Scholar]
- Snow D.M, Steindler D.A, Silver J. Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev. Biol. 1990;138:359–376. doi: 10.1016/0012-1606(90)90203-u. 10.1016/0012-1606(90)90203-U [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. 10.1126/science.281.5382.1515 [DOI] [PubMed] [Google Scholar]
- Tang S, Shen Y.J, DeBellard M.E, Mukhopadhyay G, Salzer J.L, Crocker P.R, Filbin M.T. Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J. Cell Biol. 1997;138:1355–1366. doi: 10.1083/jcb.138.6.1355. 10.1083/jcb.138.6.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D. Distribution of the myelin-associated glycoprotein and P0 protein during myelin compaction in quaking mouse peripheral nerve. J. Cell Biol. 1988;107:675–685. doi: 10.1083/jcb.107.2.675. 10.1083/jcb.107.2.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D. Myelin-associated glycoprotein. Location and potential functions. Ann. N. Y. Acad. Sci. 1990;605:29–43. doi: 10.1111/j.1749-6632.1990.tb42378.x. [DOI] [PubMed] [Google Scholar]
- Turnley A.M, Bartlett P.F. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport. 1998;9:1987–1990. doi: 10.1097/00001756-199806220-00013. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi P.S, Kantor D.B, Newman B.A, Mage R, Rader C, Giger R.J. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. 10.1523/JNEUROSCI.4464-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson M, Strijbos P.J, Rowles A, Facci L, Moore S.E, Simmons D.L, Walsh F.S. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J. Biol. Chem. 2001;276:20 280–20 285. doi: 10.1074/jbc.M100345200. 10.1074/jbc.M100345200 [DOI] [PubMed] [Google Scholar]
- Vyas A.A, Patel H.V, Fromholt S.E, Heffer-Lauc M, Vyas K.A, Dang J, Schachner M, Schnaar R.L. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc. Natl Acad. Sci. USA. 2002;99:8412–8417. doi: 10.1073/pnas.072211699. 10.1073/pnas.072211699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.C, Kim J.A, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. 10.1038/nature01176 [DOI] [PubMed] [Google Scholar]
- Wang K.C, Koprivica V, Kim J.A, Sivasankaran R, Guo Y, Neve R.L, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. 10.1038/nature00867 [DOI] [PubMed] [Google Scholar]
- Wang X, Chun S.J, Treloar H, Vartanian T, Greer C.A, Strittmatter S.M. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon–myelin and synaptic contact. J. Neurosci. 2002c;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, et al. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. 10.1523/JNEUROSCI.4235-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton M.J, Dubreuil C.I, Lasko D, Leclerc N, McKerracher L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 2002;277:32 820–32 829. doi: 10.1074/jbc.M201195200. 10.1074/jbc.M201195200 [DOI] [PubMed] [Google Scholar]
- Wong S.T, Henley J.R, Kanning K.C, Huang K.H, Bothwell M, Poo M.M. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. 10.1038/nn975 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. 10.1083/jcb.200202010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. 10.1016/S0896-6273(03)00225-3 [DOI] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He X.L, Garcia K.C, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. 10.1073/pnas.0409026102 [DOI] [PMC free article] [PubMed] [Google Scholar]