Abstract
Axon growth is a highly regulated process that requires stimulating signals from extracellular factors. The extracellular signals are then transduced to regulate coordinately gene expression and local axon assembly. Growth factors, especially neurotrophins that act via receptor tyrosine kinases, have been heavily studied as extracellular factors that stimulate axon growth. Downstream of receptor tyrosine kinases, recent studies have suggested that phosphatidylinositol-3 kinase (PI3K) regulates local assembly of axonal cytoskeleton, especially microtubules, via glycogen synthase kinase 3β (GSK-3β) and multiple microtubule binding proteins. The role of extracellular signal regulated kinase (ERK) signalling in regulation of local axon assembly is less clear, but may involve the regulation of local protein translation. Gene expression during axon growth is regulated by transcription factors, among which cyclic AMP response element binding protein and nuclear factors of activated T-cells (NFATs) are known to be required for neurotrophin (NT)-induced axon extension. In addition to growth factors, extracellular matrix molecules and neuronal activity contribute importantly to control axon growth. Increasingly, evidence suggests that these influences act to enhance growth via coordinating with growth factor signalling. Finally, evidence is emerging that developmental versus regenerative axon growth may be mediated by distinct signalling pathways, both at the level of gene transcription and at the level of local axon assembly.
Keywords: axon growth, signal transduction, development, regeneration
1. Introduction
The extension of an axon is a highly coordinated process that involves gene expression in the nucleus, protein synthesis in the soma and probably along the growing axon itself, anterograde transport of raw materials, and assembly of cytoskeleton and insertion of membrane in the growing process (figure 1). During the past two decades, neuroscientists have discovered numerous extracellular cues that regulate axon extension. Major work is now underway to reveal the intracellular signalling processes that mediate responses to these cues and result in axon growth. Meanwhile, in largely parallel work in the field of cell biology, there has been an explosion of knowledge about regulation of the cytoskeleton organization during cellular morphological responses in a variety of settings. In both areas, the development of mouse genetic models is beginning to unravel the in vivo functions of the relevant extracellular and intracellular mediators.
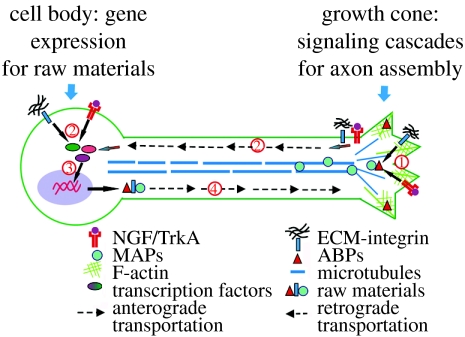
Figure 1.
A coordinated genetic programme and local signalling cascade regulate axon growth. To mediate efficient axon assembly, extracellular axon growth promoting factors, such as neurotrophins and ECMs, (1) activate signalling cascades locally at the axon that eventually converge to regulate actions of actin binding proteins (ABPs) and microtubule associated proteins (MAPs). These cytoskeletal-associated molecules then mediate axon assembly via orchestrated modulation of actin and microtubule polymerization. At the same time, (2) activated signalling mediators can be retrogradely transported from the axon to the cell body. After arriving at the soma, these signal mediators initiate (3) activation of a set of transcription factors that control axon growth. Extracellular factors may also activate transcription factors directly at the soma to induce gene expression. Finally, gene expression mediated by these transcription factors and subsequent protein translation produce the raw materials for new axons, including cytoskeletal elements. (4) These raw materials are then transported anterogradely and incorporated into the growing axon.
Despite these advances, our understanding of intracellular signalling pathways that mediate axon growth is still fragmentary. The main reason is that much of the signalling and cytoskeletal work has been done in non-neuronal cells and its relevance to neurons is unclear. Furthermore, the first generation of genetic models in many cases has been uninformative due to embryonic death of gene-targeted mice, or functional compensation mediated by molecules closely related to those that have been targeted. However, within the past few years, the development of new cell transfection technologies has dramatically impacted our ability to study intracellular signalling in neurons both in vitro and in vivo. Furthermore, perfection of methods of fusing green fluorescent protein (GFP) and its spectral variants to relevant proteins and the development of molecular biosensors have provided ways of studying signalling events specifically in the axon and at the growth cone. Finally, the generation of large numbers of mouse lines expressing the recombinase Cre in specific tissues and cell populations allows genetic mutations relevant to signal transduction pathways and cytoskeletal proteins to be restricted to neurons.
One of the major challenges in the field is to develop a framework that integrates the massively expanding knowledge of neuroscience, biochemistry and cell biology relevant to intracellular control of axon extension. We will attempt to do this in this article by reviewing recent findings at the interface of work on neuron-specific extracellular cues and receptors (neuroscience), signal transduction pathways (biochemistry) and cytoskeletal regulation (cell biology). We will focus on extension or ‘growth’ of the axon. The extensive literature on the directionality of axon growth (axon guidance) and its underlying signalling pathways has been the subject of many recent reviews (e.g. Guan & Rao 2003).
There are two occasions when axons extend over significant distances. One is during embryonic development when projection neurons in particular extend long axons to reach their appropriate synaptic partners. These axons then continue to lengthen in keeping pace with the overall developmental increase in size of the organism. The other occasion is after peripheral nerve injury when axons may extend over very long distances, in some cases re-innervating even remote target tissues. In mature mammals, neurons of the central nervous system (CNS) are of course unable to regenerate. One issue that remains unclear is whether the intracellular mechanisms that regulate developmental and peripheral nervous system (PNS) regenerative axon growth is relevant to CNS neurons and can be harnessed to promote CNS regeneration after injury. This question will be addressed in the second part of this review.
A number of models are in widespread use for studying intracellular mechanisms of axon growth. The most popular ones are embryonic PNS neurons and PC12 cells. Among all the PNS neuronal populations, sympathetic and sensory neurons are the best studied because neurotrophins, the first family of molecules identified that induce axon growth, potently promote axon growth from these neurons. During the past two decades, a large number of studies have addressed signalling mechanisms underlying axon growth stimulated by neurotrophins in these two neuronal populations. PC12 cells, a cell line of adrenal medullary origin, differentiate into neurons in response to nerve growth factor (NGF) treatment, and have been used widely to investigate NGF signalling pathways (see Vaudry et al. 2002 for a review). Of course there is uncertainty as to how faithfully PC12 cells reflect signalling events that occur in primary cultured neurons. More recently, improved cell culture methods have allowed detailed study of axonal growth of some populations of CNS neurons, particularly retinal ganglion cells (RGCs), hippocampal neurons, and cerebellar granule cells. Importantly, these CNS neuronal populations also respond to neurotrophins, so there is overlap in many of the signalling pathways to be discussed. Much less attention has been paid to signalling mechanisms mediating growth of adult axons, but the regenerative axon growth of adult dorsal root ganglion (DRG) neurons can readily be studied in vitro after a ‘pre-conditioning’ lesion (PCL). Thus, cultured PCL adult DRG neurons are increasingly used as a model system for studying regenerative axon growth.
2. Efficient axon growth requires extracellular signals
Axon extension is controlled by intracellular machinery that assembles cytoskeletal elements and membrane components into new axons. A critical question is whether this axon assembly machinery can operate autonomously in terminally differentiated neurons to mediate axon extension, or whether an intracellular signalling cascade activated by extracellular factors is necessary to power the machinery and drive axon growth. It has been a challenge to develop appropriate preparations to address this issue, especially since many extracellular factors mediating axon extension also support cell survival. Recent advances in understanding the molecular mechanisms of neuronal apoptosis have made it possible to separate cell survival from axon growth. Such studies have provided a clear conclusion. Although the basal activities of intracellular signalling molecules (e.g. kinases, second messengers, etc.) can mediate limited axon extension in the absence of extracellular factors, evidence from reduced cell culture preparations strongly suggests that extracellular axon growth promoting signals are required for long distance axon extension. Embryonic DRG neurons from mice lacking pro-apoptotic protein Bax do not die in the absence of NGF. However, they only grow rudimentary axons without NGF stimulation, less than 20% of NGF-stimulated growth over a 72 h period (Lentz et al. 1999; Marcus et al. 2002). An even more dramatic example is that RGCs over-expressing anti-apoptotic protein Bcl-2 do not extend axons at all when cultured at very low density in serum-free medium (Goldberg et al. 2002a), even though they survive very well. Together, these studies strongly suggest that extracellular signals are absolutely necessary to induce efficient axon extension from neurons. It is worth pointing out that many situations where there appears to be extensive axon growth in the absence of extracellular matrix or growth factor stimulation occur when neurons are cultured at high density or conditioned medium is added.
Neurotrophins (NTs) are the most extensively characterized group of extracellular factors that induce axon growth. NGF induces robust axon growth from sympathetic ganglion cells, and NGF and NT3 induce robust axon growth from sensory neurons by activating their corresponding receptors (TrkA, TrkC). Brain-derived neurotrophic factor (BDNF) promotes axon growth from RGCs and hippocampal neurons via its receptor TrkB. It is important to emphasize that although neurotrophins have spectacular axon growth promoting effects in vitro, their role in vivo is not fully established. Several recent studies have approached this issue. For developing sensory neurons, NGF is clearly required for axon growth and arborization in the skin. Mice with both TrkA and Bax null alleles show marked reductions in cutaneous innervation and major reductions in numbers of axons in cutaneous nerves in the absence of any significant cell death (Patel et al. 2000). Recent reexamination of NT-3 null mice showed that proximal axon growth from sympathetic neurons requires NT-3 provided by the vasculature well before axons exhibit NGF-dependent axon growth in target fields (Kuruvilla et al. 2004). Injecting highly specific anti-neurotrophin antibodies in ovo demonstrates neurotrophin dependence of axon extension in the hindlimb (Tucker et al. 2001). Together, these data confirm important roles of neurotrophins in the regulation of several stages of axon growth from developing PNS neurons in vivo. In addition, hepatocyte growth factor (HGF) signalling has been shown to regulate sensory axon growth in vivo and both the glial cell line-derived neurotrophic factor (GDNF) family and HGF have been shown to have regulatory effects on motor neuron axon growth in vivo (Ebens et al. 1996; Maina et al. 1997; Haase et al. 2002).
Of key importance is that all of these factors act via receptor tyrosine kinases (RTKs), suggesting that RTKs and their downstream signalling mediators play crucial roles in the intracellular control of axon growth. Indeed, at the molecular level, many in vitro studies have established that Raf-ERK and PI3K downstream of RTKs are key signalling mediators required for neurotrophin-mediated axon growth (see discussion below). Nevertheless, some questions remain about roles of RTK signalling in regulating axon extension. First, the initial phase of axon growth of peripheral sympathetic and sensory neurons in vivo is clearly neurotrophin independent (O'Connor & Tessier-Lavigne 1999; Kuruvilla et al. 2004). Second, whether axon extension of CNS projection neurons requires neurotrophin or tropomyosin-related kinase (Trk) signalling in vivo is unknown. Finally, in vitro the IL6 family of cytokines can promote axon growth of retinal ganglion neurons, presumably via non-RTK dependent mechanisms (e.g. Goldberg et al. 2002a). Whether axon growth in these settings shares some of the intracellular mechanisms outlined below is not yet known.
An important principle that governs intracellular regulation is that axon growth stimulated by extracellular factors requires both the activation of a genetic programme to provide the raw materials for axon formation (figure 1, see discussion below), and a signalling cascade acting locally in the growing axon to regulate the assembly of cytoskeletal proteins. This requirement of local signalling has been amply demonstrated by experiments using Campenot chambers, a technique that separates neuronal cell bodies from their axons in different compartments (Campenot 1982a,b). Studies of sympathetic neurons cultured using this technique have revealed that the presence of neurotrophins in the cell body compartment is not sufficient to support axon growth into the side compartment. Furthermore, axons in the side compartment cannot be maintained if NGF is removed, even if NGF is present in the central compartment. These results clearly indicate that local neurotrophin signalling is absolutely required to mediate axon growth, and that molecular events activated at the soma are not sufficient.
3. Role of PI3K pathway in regulation of local axon assembly
In the past few years, major progress has been made in understanding specific aspects of PI3K and extracellular signal regulated kinase (ERK) regulation in axon extension. Campenot chamber studies have shown that both pathways are required for neurotrophin-induced axon assembly (Atwal et al. 2000). Thus, application of pharmacological inhibitors of PI3K or ERK to the side compartment blocks axon extension into the side chamber. The PI3K pathway, in particular, is involved in several neurotrophin-induced local events that control axon morphogenesis. For instance, Gallo & Letourneau (1998) showed that PI3K activity was necessary for axon branch formation induced by local NGF application, and Ming et al. (1999) showed that NGF gradient-induced axon turning also required PI3K signalling. Both axon branching and turning involve reorganization of axonal cytoskeleton, including microtubules and actin filaments. Thus, an important question is how PI3K mediates neurotrophin signals to the axonal cytoskeleton that control axon assembly. PI3K can also regulate axon morphogenesis by controlling a number of processes that are important for axon growth. For instance, the PI3K–Akt pathway is well known to regulate local protein translation via the mTor pathway, which has recently been shown to play an important role in regulation of axon regeneration (Verma et al. 2005). Moreover, PI3K has been shown to regulate the endocytosis of the RTKs that is crucial for intracellular transduction of neurotrophin signals (Kuruvilla et al. 2000; York et al. 2000). In this review, we will mainly focus on newer studies that strongly suggest direct regulation of axonal cytoskeleton by PI3K signalling.
In both neuronal and non-neuronal cells, a number of signalling molecules, such as Akt, integrin-linked kinase (ILK), and GSK-3, act downstream of PI3K and have the potential to regulate cytoskeletal proteins (figure 2). Moreover, it is well known that PI3K regulates the small GTPases Rac and Cdc42 (Raftopoulou & Hall 2004), both of which are key regulators of cytoskeletal reorganization. Importantly, multiple recent studies have demonstrated that PI3K is activated at the leading edge of the growth cone, co-localizing with polymerizing actin filaments, suggesting that PI3K plays a key role in regulation of growth cone protrusion (Shi et al. 2003; Zhou et al. 2004). Although most of our knowledge regarding the regulation of actin dynamics by PI3K signalling comes from studies of non-neuronal cells undergoing migration, the work in non-neuronal cells may provide a clue as how PI3K regulates actin rearrangements at the growth cone. In non-neuronal cells, PI3K regulates actin filaments mainly via Rac and Cdc42 (figure 2). Rac is well known for its function in regulation of actin polymerization during lamellipodial protrusion via activation of the WAVE protein complex and subsequent activation of the Arp2/3 complex (see Smith & Li 2004 for a review). Over-expression of an active form of Rac in non-neuronal cells enhances membrane ruffling and cell motility (Nobes & Hall 1995). In contrast to Rac, Cdc42 is known to control filopodia formation via WASP family proteins and the Arp2/3 complex (see Higgs & Pollard 2001 for a review). In addition, a recent study showed that Cdc42 could also mediate filopodia formation via another effector, mammalian Diaphanous-related forming 3 (Drf3 or mDia2, Peng et al. 2003). However, unlike Rac, the role of Cdc42-mediated actin reorganization in cell migration is less clear. Indeed, most of the studies connecting Cdc42 to cell migration are related to its role in regulation of cell polarity that mainly involves membrane dynamics and microtubule rearrangement (e.g. Etienne-Manneville & Hall 2001).
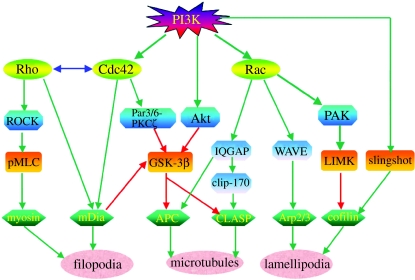
Figure 2.
Regulation of the axon cytoskeleton by PI3K signalling. Small GTPases, including Rac, Cdc42 and Rho, are major downstream mediators of PI3K signalling that regulate the cytoskeleton. Both Rac and Cdc42 are activated by neurotrophin signalling downstream of PI3K. Activated Rac can activate WAVE proteins and subsequently the Arp2/3 complex to promote actin polymerization that mediates lamellipodia formation. Rac can also activate PAK and LIM kinase (LIMK), which phosphorylates and inactivates the actin depolymerization factor cofilin. Cofilin is activated by dephosphorylation via the phosphatase Slingshot directly downstream of PI3K. Appropriate regulation of cofilin activity is important for actin polymerization. Cdc42 is mainly involved in regulation of actin filopodial formation, probably via the actin binding protein mDia. In addition to regulation of actin filaments, both Rac and Cdc42 regulate microtubule assembly and dynamics. Activated Rac at the lamellipodial leading edge can capture and stabilize dynamic microtubules via the interaction of its effector IQGAP1 with the microtubule plus end tracking proteins (+TIPs) Clip-170 and CLASP. Cdc42 may regulate microtubule dynamics via a conserved cell polarity pathway, Par3/6-aPKC, which in turn inactivates GSK-3 and promotes microtubule assembly via another +TIP, APC. There is also cross talk between Rac and Cdc42 mediated microtubule regulatory pathways. IQGAP1 can interact with APC downstream of Rac, whereas GSK-3 inactivation downstream of Cdc42 also regulates the CLASP–microtubule interaction. Another kinase, Akt, may also regulate GSK-3 activity downstream of PI3K. Although Rho is not directly regulated by PI3K, both Rac and Cdc42 can regulate Rho activity, and vise versa. Rho can regulate actin dynamics via actin-based motor protein myosin II. Activation of Rho kinase (ROCK) downstream of Rho leads to phosphorylation of myosin light chain (MLC) that is important for myosin II activation. Rho has also been shown to inactivate GSK-3 via mDia. Green arrows indicate activation, and red arrows indicate inhibition.
Although both Rac and Cdc42 are regulated by PI3K in neuronal cells (Yamaguchi et al. 2001; Aoki et al. 2005), whether the downstream pathways and molecular mechanisms that regulate neuronal actin filaments are similar to those in non-neuronal cells is unknown. A prior genetic study in Drosophila showed that Rac activity is required for axon growth (Ng et al. 2002). However, a recent genetic study in Drosophila elegantly showed that Rac-mediated actin reorganization mainly plays a negative role in axon growth via the regulation of p21-activated kinase (PAK), its downstream target LIM kinase, and the actin binding protein cofilin (Ng & Luo 2004). By using genetic rescue approaches, this study suggests that the positive role of Rac in axon growth shown previously is probably unrelated to its function in regulating actin filaments. More interestingly, the negative and positive roles of Rac in regulation of axon growth in Drosophila were shown to be mediated by different Rac guanine nucleotide exchange factors (GEFs), the upstream activators of Rac.
Unlike Rac, over-expression of active Cdc42 in neurons promotes filopodia formation and axon elongation (Brown et al. 2000). In addition, Cdc42 has recently been suggested to be involved in regulating growth cone filopodia extension (Robles et al. 2005). However, these studies do not rule out the possibility that the effect of Cdc42 on axon growth may not be directly linked to its function in regulation of actin organization. A direct pathway that links Cdc42 to growth cone actin filaments is still missing. In addition to Rac and Cdc42, PI3K has recently been shown to regulate Slingshot, a phosphatase that activates cofilin (Nishita et al. 2004). Whether this pathway also regulates axon extension remains unknown. Lastly, a very recent study demonstrates that Akt, the major mediator of PI3K signalling, regulates actin organization and cell motility via a novel protein, Girdin (Enomoto et al. 2005). In summary, the important roles of PI3K signalling in controlling actin filaments in non-neuronal cells predict similarly important regulation of lamellipodia and filopodia at the growth cone. However, many details of PI3K regulation of actin filaments in neurons that are relevant to axon extension remain to be studied.
4. GSK-3β and axonal microtubule regulation
A recent study by Zhou et al. (2004) has also enumerated a pathway that links PI3K signalling to axonal microtubule assembly. In that study, Zhou et al. (2004) showed that in an extending axon, PI3K is only activated at the distal tip and growth cone even though NGF-TrkA interaction occurs along the whole axon shaft. The spatially localized PI3K signalling is then conveyed downstream through a similarly localized inactivation of glycogen synthase kinase 3β (GSK-3β). Lastly, these two spatially coupled kinases control axon assembly by regulating a microtubule plus end binding protein, adenomatous polyposis coli (APC). Two later studies also showed that inactivation of GSK-3β downstream of PI3K is a crucial step in axon specification and growth of hippocampal neurons (Jiang et al. 2005; Yoshimura et al. 2005). It thus appears that GSK-3β is a key kinase regulating axon morphogenesis in both PNS and CNS neurons.
GSK-3β has multiple reported functions in the nervous system, ranging from neural development to neurodegeneration (see Meijer et al. 2004 for a review) consistent with its wide and high-level distribution in the brain. In resting cells, GSK-3β is usually active and suppresses cellular activity. Upon stimulation with extracellular factors, such as growth factors, it is inactivated through phosphorylation of the serine-9 residue via PI3K signalling (see Woodgett 2001 for a review). It is widely accepted that Akt is one mediator of the serine-9 phosphorylation and inactivation of GSK-3β downstream of PI3K signalling (Cross et al. 1995). Recent data have suggested that ILK and atypical protein kinase C (PKC), both of which are regulated by PI3K, may regulate GSK-3β phosphorylation as well (Delcommenne et al. 1998; Kanzaki et al. 2004). GSK-3β activity can also be blocked by Dishevelled proteins, the major components of the Wnt signalling (see Woodgett 2001 for a review). Unlike the PI3K pathway, interaction of Wnts with their receptor and the subsequent activation of Dishevelled blocks the activity of GSK-3 by recruiting frequently rearranged in advanced T-cell lymphomas (FRAT) to GSK-3. Binding of FRAT with GSK-3 blocks the interaction between GSK-3 and its substrates in the Wnt complex, such as APC and β-catenin, without affecting the kinase activity of GSK-3 (see Woodgett 2001 for a review). Wnts have also been demonstrated to have morphological effects on axons (Lucas & Salinas 1997; Krylova et al. 2002; Lyuksyutova et al. 2003).
A number of questions remain about how GSK-3 activity is regulated in PI3K and Wnt signalling pathways. First, when wild type GSK-3s were replaced by mutants that cannot be phosphorylated at their N-terminus in vivo, the resulting mutant mice developed normally with no overt phenotype in the nervous system, although a defect in insulin signalling was present (McManus et al. 2005). Second, mice that lack all three isoforms of FRAT proteins show no obvious phenotype that is related to the disruption of Wnt function (van Amerongen & Berns 2005; van Amerongen et al. 2005). Together, these results raise the possibility that alternative pathways exist that may play important roles in regulation of GSK-3 function in both PI3K and Wnt signalling. Indeed, a conserved cell polarity pathway, Cdc42-Par3/6-aPKC has been shown to regulate GSK-3 activity and polarized migration of astrocytes (Etienne-Manneville & Hall 2003). A similar pathway has also been shown to control axon-dendrite specification of hippocampal neurons in vitro (Shi et al. 2003). However, whether this polarity pathway is involved in regulation of axon extension via GSK-3 is still a mystery. Interestingly, genetic elimination of Par6 in Drosophila neurons does not show defects in axon growth and specification (Rolls & Doe 2004). This latter result suggests an evolutionarily developed role of this polarity pathway in regulation axon morphogenesis.
The ability of GSK-3β to control axon assembly largely relies on its function in regulating a number of microtubule binding proteins (MBPs). Axon assembly is achieved by microtubule polymerization (mononer addition) and stabilization (see Zhou & Cohan 2004 for a review). At the same time, a subset of microtubules at the distal axon must remain dynamic for efficient axon assembly. Interestingly, recent studies have shown that GSK-3 can regulate all three aspects of microtubule assembly via controlling different MBPs (see Zhou & Snider 2005 for a review). Collapsin response mediator protein 2 (CRMP-2), a recently identified protein that binds to tubulin dimer and promotes microtubule polymerization, has been shown to be negatively regulated by GSK-3 phosphorylation (Yoshimura et al. 2005). Similarly, the ability of two microtubule plus end binding proteins, APC and CLIP-associated proteins (CLASP) to stabilize microtubules is also abolished by GSK-3 phosphorylation (Akhmanova et al. 2001; Zhou et al. 2004). Finally, MAP-1b that functions to maintain microtubule dynamics is also regulated by GSK-3 (Trivedi et al. 2005). However, this latter function is activated by GSK-3 phosphorylation, which is the opposite to the regulation of other MBPs mentioned above. Taken together, these studies suggest that GSK-3 has a central role in the regulation of microtubule assembly during axon growth.
At present, we are still in the early stages of understanding how GSK-3 integrates signals from extracellular cues to control microtubule assembly during axon extension. For instance, inhibition of GSK-3 upon extracellular stimulation allows both CLASP and APC to bind to the microtubule plus ends and stabilize polymerized microtubules. Surprisingly, binding of APC to microtubule plus ends mediates axon growth induced by neurotrophins, whereas the CLASP–microtubule interaction is necessary for the repulsive effect of slit proteins on growing axons (Lee et al. 2004a). How this divergent effect is achieved downstream of GSK-3 signalling is unknown. Future studies that compare and contrast how APC and CLASP regulate axonal microtubule dynamics will be required to solve this intriguing question.
In addition to GSK-3, PI3K may also regulate microtubule dynamics via Rac/Ccd42 and their effector IQGAP1. In fibroblasts, activated Rac1 at the cell leading edge can capture and stabilize microtubules via the interaction between IQGAP1 and a microtubule plus end tracking protein Clip-170 (Fukata et al. 2002). However, whether a similar process occurs in nerve growth cones to regulate axonal microtubules is unknown.
5. Role of ERKs in the regulation of axon assembly
ERKs are major signalling mediators activated by neurotrophins and other extracellular factors that are important to the regulation of axon growth. Specifically, ERK phosphorylation is induced by neurotrophins, netrin (Forcet et al. 2002), semaphorin 7A (Pasterkamp et al. 2003) and cell adhesion molecules (CAM; e.g. Schmid et al. 2000) during development. Most effort in the past was focused on how signalling pathways activated by these extracellular factors regulate pERK. Surprisingly, since ERK phosphorylation is one of the most widespread readouts in all of biology, our knowledge about how ERK signalling is transduced to its downstream targets to control axon growth is still rudimentary. In addition to its well-known role in regulation of gene expression in the nucleus (see below), ERK signalling is also required for local axon assembly induced by neurotrophins (Atwal et al. 2000). Until now, however, few studies have directly linked ERK signalling to the axonal cytoskeleton. This is particularly surprising in view of the fact that microtubule associated protein 2 (MAP-2) was one of the first known substrates of ERK (Ray & Sturgill 1987).
Recent studies from Goold & Gordon-Weeks (2005) have identified MAP-1b as a potential downstream target of ERK via GSK-3 during neurotrophin-induced axon growth. Goold & Gordon-Weeks (2005) showed that phosphorylation and activation of MAP-1b by GSK-3 is positively regulated by ERK activity downstream of NGF. A similar pathway downstream of netrin appears to have been implicated in neuronal migration (Del Rio et al. 2004). Although whether this ERK–GSK-3–MAP-1b pathway is necessary for axon growth remains to be proved, this result suggests a potential link between ERK and axonal microtubule dynamics.
In addition to regulation of axonal microtubules, ERK signalling is also involved in regulating axonal actin filaments, as inhibition of ERK in growing axons with pharmacological inhibitors induces actin depolymerization and growth cone collapse (Atwal et al. 2003). There is still no clear idea of how ERK signalling regulates actin filaments in the growth cone. Recent studies have shown that ERK regulates local protein translation at the nerve growth cone in response to extracellular cues (Campbell & Holt 2003), thus suggesting a potential mechanism for ERK to control axon assembly. In support of this idea, increasing evidence shows that the synthesis of many cytoskeletal related proteins involved in axon growth and guidance is locally regulated in the axons. For instance, mRNAs of β-actin (Bassell et al. 1998), the actin binding protein cofilin (Willis et al. 2005) and GAP43 (Smith et al. 2004), which are important regulators of actin dynamics during axon assembly, are all localized in axons, especially at the growth cones. The transport of β-actin mRNA to the axon is significantly enhanced after neurotrophin stimulation (Zhang et al. 1999). However, whether translation of these proteins locally at the axons depends on ERK signalling still remains to be tested. Interestingly, local protein synthesis regulated by ERK is also required for growth cone collapse in response to the repulsive cue semaphorin 3A (Campbell & Holt 2003). Indeed, a recent study from Wu et al. (2005) showed that local synthesis of RhoA is necessary for growth cone collapse in response to semaphorin 3A. Although not tested in the latter study, a plausible idea is that ERK is involved in regulation of RhoA translation in growth cones upon semaphorin stimulation. In addition to regulating protein synthesis, ERK signalling has recently been shown to be the target of the activated proteasome, a mediator of the axon degeneration process (MacInnis & Campenot 2005) via protein degradation. Together, these studies suggest that a main function of ERK in axons is to maintain the level of proteins that are required for axons to respond to extracellular cues during axon growth and guidance via regulation of protein synthesis and degradation.
One function of this local regulation of protein supply by ERK signalling is that it allows neurons to properly adjust their responses to the changing environment and ensure efficient control of axon growth and guidance. For example, cycles of desensitization and re-sensitization occur when axons navigate long distance in a gradient of guidance cues. Evidence shows that protein synthesis and ERK signalling are required for the re-sensitization process (Ming et al. 2002). A potential explanation is that a sufficient protein supply provided by ERK regulated protein synthesis and degradation is necessary for axons to respond to the guidance cue gradient sustained over long-range navigation. The proteins regulated by ERK and local synthesis could range from raw materials, such as actin filaments, to receptors and signalling mediators of the guidance cues.
6. ECM-Integrin signalling during axon growth
Besides neurotrophic growth factors, extracellular matrix proteins (ECMs), such as laminin and fibronectin, and CAMs, such as L1 and neural cell adhesion molecule (NCAM), also regulate axon growth both in vivo and in vitro. In this review, we mainly focus on ECMs. Studies of both CNS and PNS neurons showed that ECM proteins significantly potentiate axon growth induced by growth factors (Goldberg et al. 2002a; Liu et al. 2002). However, ECMs do not induce axon elongation from purified developing RGCs in the absence of peptide neurotrophic factors (Goldberg et al. 2002a). Moreover, isolated embryonic DRG neurons only grow rudimentary axons on laminin in the absence of NGF (Lentz et al. 1999; Liu et al. 2002; Marcus et al. 2002). Thus, the major function of ECM-integrin signalling is to enhance the axon growth promoting effects of neurotrophic factors.
Exactly how ECMs promote axon growth is still not clear. It is accepted that the major effects of ECMs are mediated via different integrins, the surface receptors of ECMs, even though some ECMs also bind non-integrin receptors (Powell & Kleinman 1997). One classical function of ECMs is to provide a substrate for axon growth. Axon extension is pioneered by growth cone protrusion. Studies using snail ganglion neurons suggest that the rate of leading edge protrusion is regulated by actin polymerization at the growth cone leading edge and retrograde actin flow that moves assembled actin filaments away from the leading edge (see Zhou & Cohan 2004 for a review). As a result, decrease in retrograde actin flow will promote growth cone leading edge protrusion and axon growth. Since integrins interact with actin filaments, the binding of ECMs with integrin leads to the coupling of ECMs with actin filaments. This engagement of ECMs with actin filaments is able to serve as a clutch that slows down actin retrograde actin flow and therefore results in growth cone leading edge protrusion. More importantly, extracellular factors that promote axon growth, such as NGF, have been shown to induce the clustering of integrins at the growth cone leading edge (Grabham & Goldberg 1997). How integrins are connected to the actin filaments in the growth cone is not clear. Recent studies have shown that actin motor protein myosin X is able to bind integrins (Zhang et al. 2004). This result strongly suggests that myosin X may serve as a link between ECM-integrin and actin filaments undergoing retrograde flow. In addition to myosin X, a recent study of myosin II null neurons (Turney & Bridgman 2005) also suggests that integrin signalling may regulate axon growth via controlling myosin II-mediated contractile activity.
Besides acting at the growth cone mechanically by directly linking ECMs with cytoskeletal elements, integrins are well known to mediate signalling events inside the cells. In non-neuronal cells, integrin signalling is best known for its role in cooperating with growth factor signalling to regulate cellular functions, such as survival and proliferation (see ffrench-Constant & Colognato 2004 for a review). One way that integrin signalling cross talks with growth factor signalling is to potentiate the activation of growth factor signalling (figure 3). This function can be achieved by regulation of the growth factor receptors via either direct modification, such as phosphorylation or gene expression (see ffrench-Constant & Colognato 2004 for a review). Integrin activation can also recruit growth factor receptors into specific microdomains, such as lipid rafts, and thus affect growth factor signalling (Baron et al. 2003). The result of such cross talk is the increase in efficiency of growth factor signalling. For instance, the efficient activation of ERK upon growth factor stimulation depends on ECM–integrin interaction in some non-neuronal cells (see Howe et al. 2002 for a review).
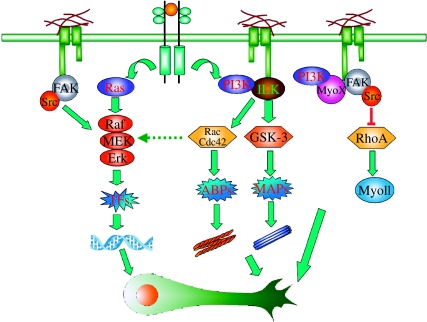
Figure 3.
ECM-integrin signalling coordinates with growth factor signalling to regulate efficient axon growth. PI3K and Raf-MEK-ERK are two major signalling pathways downstream of receptor tyrosine kinases (RTKs) activated by neurotrophic factors. Integrin signalling cascades activated upon ECM binding can interact with both of these pathways. Downstream of PI3K, integrin linked kinase (ILK) that binds to the β subunit of integrins is required for GSK-3 inactivation and the subsequent regulation of microtubule dynamics via various microtubule associated proteins (MAPs). ILK is also required for Rac/Cdc42 activation downstream of PI3K to regulate actin dynamics via actin binding proteins (ABPs). Integrin signalling may also directly regulate actin dynamics and growth cone motility by controlling two actin-based motor proteins, myosin X and myosin II. Interestingly, myosin X binds directly to the integrin β subunit and is also under the control of PI3K. Together, coordination between the integrin and PI3K pathways regulates local growth cone motility and axon assembly. ECM-integrin signalling can also regulate the Raf-MEK-ERK pathway downstream of RTK activation. Specifically, FAK and Src activation upon ECM-integrin engagement have been shown to activate the Raf-MEK-ERK pathway and the subsequent regulation of transcription factors. There is also evidence that Rac/Cdc42 is able to cross talk with the Raf-MEK-ERK pathway and regulate gene expression. In summary, integrin signalling interacts with PI3K and Raf-MEK-ERK pathways to regulate both the genetic programme and local signalling during neurotrophic factor-induced axon growth.
Whether similar coordination between integrin and growth factor signalling exists in neurons is less clear. It has been shown that co-activation of growth factor and integrin signalling is necessary for full activation of focal adhesion kinase (FAK), which in turn is required for axon growth of PC12 cells (Ivankovic-Dikic et al. 2000). However, whether the activation of ERK and PI3K, two major pathways downstream of growth factor signalling, depend on integrin activation in primary neurons is unknown. In addition to FAK, it will be interesting to see what other pathways that are crucial for growth factor-induced axon growth, require the activation of integrin signalling. For instance, inactivation of small GTPase Rho is believed to be a necessary step for neurotrophin-induced axon growth. Integrins are well known for their function to inactivate Rho via the activation of RhoGAP (Arthur & Burridge 2001). Although neurotropin receptor p75 has been shown to mediate Rho inactivation induced by neurotrophins, whether integrin signalling participates in this process is not clear.
In addition to ECMs, integrins can be trans-activated by other ligands that have integrin-binding domains. A recent study showed that semaphorin 7A, which contains a known integrin-binding motif RGD, is able to induce axon growth (Pasterkamp et al. 2003). Surprisingly, the axon promoting effect of semaphorin 7A is independent of its traditional receptor, plexinC1, as plexinC1 null neurons showed no semaphorin 7A-induced axon growth defect. In line with its ability to bind integrin with the RGD sequence, semaphorin 7A-induced axon growth depends on integrin activation. Consistent with this idea, FAK, a direct target of integrin, was activated by semaphorin 7A. Moreover, binding of semaphorin 7A with integrin also activates ERK, which is necessary for semaphorin 7A-induced axon growth. Together, these results provide an unexpected role of integrins to mediate axon growth in response to semaphorin. Although integrin is the key mediator of semaphorin 7A effect, this result does not rule out that other receptors may also be involved to coordinate with integrin signalling in promoting axon growth.
Finally, CNS inhibitory molecules chondroitin sulfate proteoglycans (CSPGs) are suggested to block axon growth by interfering with integrin signalling. Over-expression of integrins has been shown to promote axon growth over CSPGs (Condic 2001). Thus, understanding how integrin signalling contributes to enhance growth factor-induced axon growth would help us develop ways to promote axon regeneration over the inhibitory CNS substrates.
In summary, in vitro evidence suggests that the role of integrin signalling in axon growth during development is to coordinate with growth factor signalling either mechanically or via signalling cross talk. However, it should be emphasized that most studies of integrin signalling have been performed in vitro under defined conditions. Surprisingly, mice lacking β1 integrin show only mild defects in peripheral axon growth during development (Raftopoulou & Hall 2004). One potential explanation for this result is the compensatory effects of other integrin isoforms that share similar downstream signalling pathways. Future studies that eliminate multiple integrin isoforms simultaneously or common downstream signalling mediators will be needed to elucidate the functions of integrin signalling in regulating developmental axon growth in vivo.
7. Activity dependent regulation of axon growth
In addition to neurotrophic factors and ECMs discussed above, neuronal activity also plays a crucial role in regulation of axon growth in both CNS and PNS neurons. In cultured RGCs, electrically active neurons extend much longer axons than inactive cells in response to peptide axon growth factors (Goldberg et al. 2002a). Further studies of both RGCs and hippocampal neurons showed that the electrical activity enhances axon growth by upregulation of BDNF receptor TrkB at the cell membrane via the elevation of intracellular levels of cAMP (Meyer-Franke et al. 1998; Goldberg et al. 2002a). As a result, electrically stimulated neurons show enhanced responses to BDNF and extend longer axons. In addition to regulating axon growth via gene expression, electrical activity can also locally modulate competitive axon growth between adjacent neurons by interacting with neurotrophin signalling (Singh & Miller 2005). In compartmentalized cultures of sympathetic neurons, local depolarization of axons on one side significantly enhances the growth of the depolarized axons, and at the same time represses the growth of the unstimulated axons. Local depolarization activates the CAMKII-MEK-ERK pathway, which then converges with local neurotrophin signalling to enhance axon extension. At the same time, electrically activated neurons secret BDNF to repress axon growth of unstimulated neurons via p75 receptor.
Although these studies demonstrated clearly the important role of electrical activity in promoting axon growth of both CNS and PNS neurons in vitro, they did not address whether neuronal activity normally regulates axon growth in vivo. A recent genetic study in zebrafish showed that activity-based competition between neighbouring neurons also regulates axon growth of RGCs in vivo (Hua et al. 2005). When the activities of a subset of neurons were suppressed by expression of an exogenous potassium channel or a dominant-negative SNARE protein, axon growth of these neurons was significantly inhibited. Interestingly, the inhibition is relieved when the axons of the adjacent neurons are also silenced. This result is consistent with the above in vitro study that signals derived from the active axons suppress the growth of the inactive axons. Finally, loss of neuronal activity after nerve injury may also affect successful axon regeneration via reduced responsiveness to extracellular cues. Indeed, electrical stimulation can significantly accelerate axon regeneration and enhance the specificity of re-innervation of the appropriate targets by both motor and sensory axons after a peripheral nerve injury (Al-Majed et al. 2000; Brushart et al. 2005).
In summary, both in vitro and in vivo results strongly support the idea that neuronal activity can modulate local axon growth during development. Although our understanding of how electrical activity regulates axon growth is incomplete, available evidence suggests that the intracellular mechanism shares features in common with neuronal growth factor signal transduction.
8. Genetic control of axon growth
Unlike the situation with axon guidance where extracellular cues presumably act locally at the axon, long-range axon extension requires gene transcription in the nucleus. Some growth promoting signals are presented at the distal axon and must be transported back to the cell body to mediate axon growth. Others may be present all along the axon or in proximity to the cell soma. It is well accepted that activation of specific transcription factors and the subsequent gene expression downstream of neurotrophin signalling pathways is necessary for efficient and sustained axon growth. However, we know surprisingly little about which transcription factors are activated downstream of extracellular factors. It is particularly surprising in the era of expression profiling that we know even less about the gene expression patterns regulated by the transcription factors that are specific for axon growth.
Perhaps the best-characterized transcription factor regulated by neurotrophins is cyclic AMP response element binding protein (CREB). CREB and its closely related family members, ATF-1 and CREM, bind to cis-acting elements (CREs) of many growth factor-regulated genes. Upon neurotrophin stimulation, CREB is activated by phosphorylation at its Ser133, which then recruits its co-activators, such as CBP (CREB binding protein), to control transcription initiation. Phosphorylation and activation of CREB can be achieved via multiple kinases, including PKA, ribosomal S6 kinases (RSKs), Akt and CAMKs (see Lonze & Ginty 2002 for a review). In the context of neurotrophin signalling, it is believed that CREB is mainly activated by RSKs downstream of the Ras-Raf-ERK pathway. Inhibition of the ERK pathway significantly blocked NGF-induced CREB Ser133 phosphorylation in PC12 cells (Xing et al. 1998). However, it has also been shown that hippocampal neurons with a defect in PLC-γ signalling downstream of TrkB receptor had diminished CREB activation without obvious changes in ERK signalling (Minichiello et al. 2002), arguing against the role of the ERK pathway in regulation of CREB of hippocampal neurons in the neurotrophin pathway.
Despite this controversy about how CREB is regulated in the neurotrophin signalling pathway, it is clear that many neurotrophin-regulated genes have CREB binding sites in their regulatory regions (see Lonze & Ginty 2002 for a review), suggesting that CREB is an important nuclear signalling mediator of neurotrophin functions. Indeed, early in vitro studies using compartmentalized cultures demonstrated that CREB activity is necessary for neuron survival mediated by target-derived neurotrophins (Riccio et al. 1999). Expression of a dominant negative CREB mutant induced apoptosis of sympathetic neurons, whereas expression of a constitutively activated CREB mutant was sufficient to promote neuron survival after NGF withdrawal. CREB null mice are early postnatal lethal and associated with significant apoptosis in peripheral neuronal populations. In order to examine axon growth in these animals, neuronal death was prevented by crossing in a null mutation for the pro-apoptotic protein Bax, or using caspase inhibitors (Lonze et al. 2002). In this study, CREB null DRG and sympathetic ganglion neurons extended much shorter axons than those of wild type cells in the presence of NGF and caspase inhibitors, indicating CREB-mediated gene expression is necessary for neurotrophin-induced axon growth. Importantly, in vivo data suggested that CREB might play an important role in regulation of axon growth independent of neurotrophins, as an axon growth defect was observed at early developmental stages prior to the time when sensory neurons are dependent on neurotrophins for survival and growth. In summary, from this study of CREB null mice, it is clear that CREB plays a crucial role in mediating neurotrophin-induced axon growth of PNS neurons. Hopefully additional information about CREB regulation of axon growth will be forthcoming soon from mice with conditional elimination of CREB in neurons, and from identification of CREB regulated genes that are important for axon growth.
Another family of transcription factors known to have critical roles in neurotrophin-induced axon growth are the nuclear factor of activated T-cells (NFATc). NFATc proteins are transcription factors that are activated by dephosphorylation of their amino-termini via increases in intracellular calcium and the subsequent activation of phosphatase calcineurin (see Graef et al. 2001 for a review). Dephosphorylation of NFATc proteins exposes their nuclear localization sequence and thus promotes their translocation into the nucleus. The role of NFATc proteins in regulation of axon growth was an unexpected finding by examining the mice lacking 2 or 3 NFATc family members (Graef et al. 2003). In the triple NFATc mutant mice, both peripheral and central projections of sensory neurons show severe defects at E10.5. These defects were mimicked by in utero application of calcineurin inhibitors, indicating the effects of NFATc mutant on axon growth are downstream of calcineurin signalling. More importantly, in vitro studies of mutant trigeminal ganglia indicated that NFATc proteins are only necessary for neurotrophic factor and netrin-induced axon growth. Axon growth induced by culturing neurons in matrigel was independent of NFATc function, suggesting that different axon growth promoting factors use distinct transcription factors to mediate axon growth. In contrast, CREB also mediates axon growth that does not require known neurotrophins (Lonze et al. 2002). In addition, unlike CREB, which is clearly involved in regulating cell survival, NFATc proteins may not be required for the survival of sensory neurons. Thus, compared with CREB, NFATc proteins may be more specific in the regulation of neurotrophin-induced axon growth.
Similar to CREB, NFATc proteins require additional nuclear factors (NFATn) to form a functional transcription complex that is also regulated by other signalling mediators, such as PKC and ERK (see Hogan et al. 2003 for review). Thus, NFATc mediated transcription requires coordinated activation of calcium/calcineurin signalling and PKC/ERK pathways downstream of extracellular factors. A potential mediator of neurotrophins that may control NFATc-mediated transcription is PLC-γ, which activates both PKC and calcium/calcineurin signalling (see Hogan et al. 2003 for review). Indeed, inhibition of PLC-γ or depletion of calcium from intracellular store blocks BDNF-induced NFATc transcriptional activities (Groth & Mermelstein 2003). However, whether PLC-γ function is necessary for neurotrophin-induced axon growth is still controversial. Expression of mutant a TrkA that is defective in activating PLC-γ in Xenopus spinal cord neurons interferes with NGF-mediated attractive growth cone turning (Ming et al. 1999). In contrast, mutant TrkB that cannot activate PLC-γ did not seem to affect BDNF-induced axon growth of mouse sympathetic neurons (Atwal et al. 2000). The exact reason for this apparent discrepancy is not clear. One potential explanation is that PLC-γ may play a distinct role in regulation of axon guidance versus axon extension. In axon guidance, growth cones need to decipher a subtle gradient of the guidance cues, which may specifically require PLC-γ function. In contrast, the major role of the growth cone during axon extension is to provide traction force and regulate microtubule assembly. Of note is that the above studies mainly relied on mutating the binding site of PLC-γ on Trk receptors to block PLC-γ activity. Future studies using RNAi techniques may give us a more definite answer of the function of PLC-γ in regulation of axon growth.
In addition to regulating neurotrophin-induced axon growth, both CREB and NFAT also regulate other neurotrophin-mediated cellular functions, such as neuron survival and synapse remodelling, which presumably involve different sets of expressed genes from those associated axon growth. How this specificity is achieved during different developmental stages remains a mystery. Since both CREB and NFAT require other nuclear partners (co-activators or other transcription factors) to regulate transcription, one potential mechanism is that they mediate distinct patterns of gene expression by binding to different partners. For instance, during T-cell activation, NFAT can partner with c-Jun and induce a specific pattern of gene expression that characterizes active T-cells. Without c-Jun, NFAT alone then activates a different set of genes, leading to a distinct functional outcome (see Beachy et al. 2004 for a review). Another important question remaining to be answered is whether CREB- and NFAT-mediated gene expression coordinate with each other to regulate axon growth. It is also possible that CREB and NFAT regulate different molecular events that coordinate to regulate axon growth. Indeed, it has been shown recently that during presynaptic differentiation, CREB and NFAT respectively regulate synaptic vesicle accumulation and axon terminal remodelling, both of which are important events for synaptogenesis during development (Yoshida & Mishina 2005).
It is worth emphasizing that both CREB and NFAT are negatively regulated by GSK-3. Phosphorylation of CREB by GSK-3 inhibits its DNA binding ability (Grimes & Jope 2001), while phosphorylation of NFAT by GSK-3 promotes its translocation out of the nucleus, and thus inhibits its function (Beals et al. 1997). Therefore, inhibition of GSK-3 activity by neurotrophins not only promotes local axonal microtubule assembly, but also enhances nuclear translocation of transcription factors. As a result, GSK-3 inhibition may serve to integrate the genetic process at the cell bodies with the local assembly of cytoskeleton at the axons.
Finally, a recent very important study has revealed an unexpected transcriptional regulation of axon growth by cell cycle machinery. Konishi et al. (2004) has shown that anaphase promoting complex plays an important role in transcriptional regulation of axon growth. This complex is a multisubunit ubiquitin ligase that functions in mitotic cells to promote cell cycle exit via degradation of cyclins and other regulators of cell cycle progression (see Murray 2004 for a recent review). The activation of the anaphase-promoting complex requires an activator protein, Cdh1. Interestingly, both Cdh1 and anaphase promoting complex proteins are highly expressed in neurons, which are post-mitotic cells (Gieffers et al. 1999). Surprisingly, when Cdh1 was knocked down using siRNA in mouse cerebellar granule neurons, axon growth of these neurons was significantly enhanced (Konishi et al. 2004), indicating a novel function of this ubiquitin ligase complex in neurons. Since the majority of the Cdh1–anaphase-promoting complex resides in the nucleus, it must regulate axon growth via a transcription-dependent mechanism. It will be interesting in the future to identify the substrates of this ubiquitin ligase in neurons that regulate gene expression associated with axon growth. This result also raises the possibility that cell cycle progression in mitotic cells and axon growth of neurons may share similar or overlapping gene expression patterns.
9. Axon regeneration
Another situation where extensive axon elongation occurs is during peripheral regenerative axon growth induced by injury. There are important similarities and contrasts to the axon extension that occurs during the development of peripheral innervation. Similar to the situation in early development, regenerative growth requires advance of the growth cone. However, in contrast to the situation in development, the growth cone may need to traverse inhospitable terrain. Furthermore, the distances that axons must travel are a major difference between regenerative axon growth and developmental axon growth. During development, axons may reach targets, such as the neuromuscular junction (NMJ), by mid-embryonic stages. The majority of the axon growth may later be achieved via mechanical stretch (Pfister et al. 2004) as growth of the body occurs, a process independent of growth cone motility. In contrast, during regeneration, the growth cone has to traverse the entire distance and must be regulated by growth mechanisms that do not involve stretch. Even in commonly used paradigms in rodents, the distances traversed may be several centimetres.
The assembly of cytoskeletal elements is a very conserved process regulated by a common set of cytoskeletal regulating molecules across different cell types and species. Thus, in our view, it is very likely that similar pathways regulate axonal cytoskeleton assembly during both development and regeneration. However, the terrain that axons traverse during regeneration is drastically different from that during development. Therefore, it can be expected that the regenerating neurons respond to a different set of extracellular factors that induce axon assembly during regeneration compared with during development. The outcome of such changes may mean a switch of signalling pathways that act upstream of axonal cytoskeletal assembly during regeneration compared with during development.
Since neurotrophins are the major growth factors that regulate developmental axon growth, an important question is whether neurotrophins and their downstream signalling mediators are also necessary for regenerative axon growth in adult animals. Because the same peripheral adult neurons have been studied in the context of neurotrophin signalling during development, it should be straightforward to compare the neurotrophin response mechanisms with injury-induced mechanisms. Unfortunately, the original round of neurotrophin and Trk knockout mice do not survive into adulthood; therefore roles of neurotrophins and their receptors in peripheral axon regeneration cannot be tested in these animals. Several neurotrophic factors, such as NGF and GDNF, are strikingly upregulated at the distal peripheral nerve after injury, suggesting a potential role for local growth factor signalling in axon regeneration (see Fu & Gordon 1997 for a review). However, the literature on neurotrophins and regeneration is highly contradictory, with many studies reporting improved regeneration and some studies reporting inhibited regeneration. Interestingly, one micro-array study suggested that GDNF suppressed growth associated genes (Linnarsson et al. 2001). In one particularly clear-cut result, sensory axons regenerated normally in vivo when the endogenous NGF signalling was neutralized with a specific antibody (Diamond et al. 1987). Few studies have yet been performed on the role of neurotrophin signalling mediators in regeneration. One recent study strongly suggested that ERK phosphorylation is required for retrograde transport of regeneration signalling at least for a subpopulation of DRG neurons (Perlson et al. 2005). It is possible that the ERK phosphorylation shown in that study is downstream of signals other than neurotrophins. Fortunately, mice with floxed allele of ERKs have recently been reported (Hatano et al. 2003), which should allow the role of ERK signalling in axon regeneration to be determined definitively.
The abundance of laminin isoforms in the peripheral nerve pathways (Lentz et al. 1997) suggests that integrin signalling may play a key role in peripheral nerve regeneration. Indeed, there is functional evidence for the importance of integrin signalling since loss of integrin subtype α7 impedes the facial nerve regeneration to some degree after a lesion (Werner et al. 2000). As noted above, mice lacking β1 integrin do not seem to have defects in peripheral axon growth during development (Raftopoulou & Hall 2004). One possible interpretation of these data is that integrin signalling may play a more important role in regulating axon regeneration in adult animals than in axon growth during development.
Until now, few studies have compared the signalling pathways associated with regenerative axon growth with those associated with developmental axon growth. By comparing peripheral axotomy-induced regenerative axon growth from adult DRG neurons with NGF-induced axon growth from embryonic DRG neurons, we have found that integrin-induced axon assembly from regenerating adult neurons probably does not require the activities of PI3K and ERKs, both of which are key signalling mediators of axon growth from developing neurons (Liu & Snider 2001; Zhou et al. 2006). Obvious next questions are how axon growth of adult PNS regenerating neurons is regulated, and what are the genetic mechanisms underlying such regenerative axon extension.
After nerve injury, it is generally accepted that the hostile environment provided by the adult CNS is the main barrier that prevents adult CNS neurons from re-growing. However, embryonic CNS neurons are able to extend long axons in the adult CNS environment. Moreover, several recent reports have demonstrated that isolated adult PNS neurons can grow long axons in the adult spinal cord white matter (Davies et al. 1997, 1999), suggesting that the adult CNS environment is still permissive for axon regeneration. Furthermore, genetic studies targeting major molecules that contribute to the inhibitory nature of the CNS environment have produced controversial, but in general disappointing results. Axons still regenerate poorly in the spinal cords of mice that lack all three isoforms of Nogo (Zheng et al. 2003) or the Nogo receptor (NgR) that mediates effects of all three CNS myelin-based inhibitors, including Nogos, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp) (Zheng et al. 2005). A potential explanation for these surprising results is that the intrinsic capacity of the neurons to grow axons is a prerequisite for successful regeneration. In other words, the embryonic CNS or isolated adult PNS neurons possess high intrinsic capacity for growth that allows them to extend long axons, while adult CNS neurons have significantly reduced intrinsic capacity for axon, growth. Thus, isolated adult PNS neurons can overcome some of the growth inhibition by the adult CNS environment, whereas adult CNS neurons cannot grow axons, even in the absence of major inhibitory molecules. This idea is consistent with recent findings that attenuating the inhibitory effect of spinal cord myelin by inactivating PKC or blocking inhibitory effects of the glial scar by inactivating CSPGs allow limited regeneration of the central axons from DRG neurons in the dorsal column, but not corticospinal axons in the same location (Bradbury et al. 2002; Sivasankaran et al. 2004). The importance of intrinsic axon growth capacity was best illustrated in axon regeneration of RGCs over myelin-based inhibitors. Expression of dominant negative NgR in naive RGCs has no beneficial effects on axon regeneration. However, when RGCs are primed with macrophage-derived factors to activate their intrinsic growth programme, dominant negative NgR expression greatly enhances axon regeneration over myelin-based inhibitors (Fischer et al. 2004). One molecule that has been implicated in regulation of the intrinsic capacity of axon regeneration is anti-apoptotic protein Bcl-2. Over-expression of Bcl-2 in mature RGCs significantly enhances retinal axon regeneration independently of its anti-apoptotic role (Chen et al. 1997). Further study showed that Bcl-2 decreases calcium uptake and storage at the endoplasmic reticulum (ER), and thereby leads to a larger intracellular calcium response induced by axotomy (Jiao et al. 2005). The subsequent activation of ERK and CREB may underlie the ability of Bcl-2 to promote axon regeneration.
How is the intrinsic capacity of neurons to grow axons regulated during development? By comparing axon growth of embryonic RGCs with that of postnatal cells, elegant studies from Goldberg et al. (2002b) revealed that after neurons mature, their intrinsic growth capacity is irreversibly reduced. Embryonic RGCs always grow longer axons than postnatal cells when highly purified cells are cultured in the same extracellular environment. These investigators also showed that the loss of the intrinsic ability of RGCs is not due to an internal clock within the cells, as isolated embryonic cells did not lose their growth ability after a longer period of culturing. One potential explanation for these results is that a target-derived STOP signal may mediate the loss of intrinsic growth capacity after neurons mature and form synapses with their targets. Consistent with this idea, RGCs start to lose their intrinsic growth ability at around P0 to P1, the time when they start to form synapses with amacrine cells in the retina. Indeed, co-culturing of embryonic RGCs with the amacrine cells led to decreased axon growth.
Similar target-derived STOP signals are also thought to exist in the PNS to regulate axon growth. Obviously, one effect of peripheral axotomy that induces axon growth is to separate neurons from their peripheral targets. Importantly, blocking microtubule-based retrograde transport in peripheral nerves alone without axotomy is sufficient to mimic the peripheral axotomy-induced conditioning lesion effect and led to enhanced axon growth (Smith & Skene 1997). In contrast to RGCs that show irreversibly reduced growth capacity after contacting their targets, adult DRG neurons are able to regain their intrinsic ability to grow when targets are removed. Understanding how target-derived signals differently affect the intrinsic axon growth capacity of CNS and PNS neurons is potentially a key to promote axon regeneration after CNS injury. One powerful approach will be the genetic profiling studies that compare the gene expression patterns of RGCs or adult DRG neurons with or without their corresponding target-derived signals.
How does peripheral nerve injury allow PNS neurons to regain their intrinsic capacity for axon growth? It is accepted that axotomy provokes a series of complex changes in the axotomized neuron leading to the regeneration of the axon. One important peripheral axotomy-induced change may be the upregulation of the second messenger cAMP. Two recent studies showed that adult DRG neurons primed by peripheral axotomy were able to overcome the inhibitory effects of myelin-based inhibitors, such as MAG and Nogo (Neumann et al. 2002; Qiu et al. 2002). Importantly, these studies provide convincing evidence that axotomy-triggered cAMP elevation changes the intrinsic properties of adult DRG neurons, leading to the altered response of adult neurons to inhibitory molecules.
Another important molecular event triggered by peripheral nerve injury is the rapid and sustained upregulation and activation of inducible transcription factors, which in turn change the intrinsic properties of regenerating neurons. Previous expression-profiling studies have identified a large number of genes that show upregulated expression after peripheral axotomy (Costigan et al. 2002; Tanabe et al. 2003), including genes encoding several transcription factors. Among transcription factors, c-Jun, a component of the AP-1 transcription factor complex, is the best-studied in association with axon regeneration. c-Jun is upregulated in PNS neurons but not CNS neurons after axotomy (Broude et al. 1997).
The importance of c-Jun in axon regeneration has recently been demonstrated in conditional knockouts generated by crossing c-junf/f with nestin-Cre. In conditionally c-Jun null mice, axon regeneration of facial nerve was significantly delayed (Raivich et al. 2004). In contrast, lack of c-Jun in neurons had little effect on axon growth during development, suggesting that c-Jun specifically regulates regenerative axon growth (Raivich et al. 2004). Interestingly, expression of integrins, which are known to be important in axon regeneration, was significantly decreased in the c-Jun null mice. In addition to c-Jun, a related transcription factor ATF3, is also upregulated in response to axotomy with a similar expression pattern to that of c-Jun (Tsujino et al. 2000). Dimerization of c-Jun and ATF3 can recognize the cAMP response element (CRE) binding motif (see Herdegen & Leah 1998 for a review), which may leads to the regulation of different sets of genes. Nuclear translocation of c-Jun and subsequent gene transcription requires the kinase activity of the Jun N-terminal kinases (JNKs). JNKs are also activated by axotomy concurrent with c-Jun upregulation (Kenney & Kocsis 1998). A recent study went a step further and showed that retrograde transport of JNK signalling component after nerve injury contributes to the activation of c-Jun at the nucleus (Lindwall & Kanje 2005). Interestingly, in addition to regulating c-Jun, JNK proteins can directly regulate axonal microtubules by phosphorylating MAPs, including MAP-2 and MAP-1b (Chang et al. 2003). JNK1 null mice show disrupted axon tracts of CNS neurons, such as the anterior commissure axons and spinal cord axons (Chang et al. 2003). Careful analysis of the mutant mice revealed that the observed axon abnormalities are due to a progressive degeneration of the nerve fibres in mature animals rather than defects in developmental axon growth. Consistent with the role of JNK1 in regulating microtubules, degenerated axons in JNK1 null mice are associated with loss of microtubule integrity.
Stat3, involved in cytokine signalling, is another well-studied transcription factor that is associated with the peripheral axotomy-induced axon growth (Lee et al. 2004b). Similar to c-Jun, activated Stat3 (tyrosine phosphorylated) is specifically increased in the adult DRG neurons after peripheral injury, but not after dorsal column lesion in the spinal cord (Qiu et al. 2005). Further study showed that preventing Stat3 activation via inhibiting Jak2, an upstream kinase that activates Stat3 in vivo, significantly blocked peripheral injury-induced axon growth (Qiu et al. 2005). This result indicates that Stat3 activation is necessary for increased growth ability of DRG neurons after a conditioning injury.
As discussed above, CREB and NFAT are two transcription factors involved in regulation of neurotrophin-induced axon growth during development. Whether they play any role during axon regeneration is less clear. A recent study by Gao et al. (2004) showed that CREB-mediated gene expression underlies cAMP-induced axon growth over myelin-based inhibitors after peripheral axotomy. NFAT is activated via calcineurin upon calcium increase. Since axotomy triggers calcium influx (Ziv & Spira 1993), it is possible that NFAT may play an important role in regulation of axon regeneration.
Finally, Wnt proteins were recently shown to induce axon growth from embryonic DRG neurons via the canonical Wnt pathway (Lu et al. 2004). This result suggests that TCF-4, the downstream transcription factor of canonical Wnt pathway, may be able to mediate axon growth during regeneration. Indeed, increasing evidence now links Wnt signalling to tissue regeneration in non-nervous systems, such as liver and skin (see Beachy et al. 2004 for a review). It is worth noting that all the transcription factors that we have discussed can interact with each other in the nucleus and thus integrate different signalling pathways that regulate axon regeneration. The outcome of such integration is the induction of specific patterns of gene expression.
What genes regulated by specific transcription factors are activated during axon regeneration? Does the same group of genes that regulate developmental axon growth also control regenerative axon growth of adult neurons? The answers to these questions are largely missing. Although gene profiling studies have identified many genes that are upregulated after peripheral nerve injury, such as galanin, GAP43, SPIRRA, FN14, etc. (Bonilla et al. 2002; Tanabe et al. 2003 and references therein), few of them have yet been shown to have significant function in the regulation of axon regeneration. However, the lack of developmental defects in c-Jun null mice suggests that c-Jun might mediate gene expression after nerve injury that is specific for regenerative axon growth. Indeed, some upregulated genes identified via gene profiling studies are only expressed during axon regeneration (Bonilla et al. 2002). Together, these data suggest that there might be a specific set of genes that are expressed after peripheral nerve injury to promote axon regeneration.
10. Summary and future directions
In summary, most of our knowledge about intracellular signalling that regulates axon growth derives from studies of neurotrophin-induced axon growth from PNS and CNS neurons during development and axotomy-induced axon growth of PNS neurons in maturity. Importantly, similar signalling events also regulate axon growth induced by other extracellular factors, such as netrin and the recently identified semaphorin 7A. However, it is important to acknowledge the limitations of existing paradigms. The recent work with anaphase-promoting complex and cell cycle regulation suggests that there exist critical intracellular signalling pathways regulating axon extension that have not yet been discovered.
Based on current knowledge, we can draw some conclusions. We know that axon growth is not a default property of differentiated neurons but requires specific extracellular stimulation. In addition, any extracellular factor that induces axon growth has to coordinately regulate a genetic programme at the cell body and a local signalling cascade at the axon. Moreover, the inability of adult CNS neurons to regenerate is due to a decrease in their intrinsic growth capacity after cells mature that is not reversed after axotomy, whereas adult PNS neurons are able to regain their intrinsic growth ability after nerve injury. Adult neurons that are able to regenerate probably use distinct signalling mechanisms to control axon growth compared with developing neurons. Finally, for both CNS and PNS neurons, ECM-integrin signalling is critical for efficient axon growth induced by neurotrophic growth factors.
Although much progress has been made on how axon growth is regulated by extracellular factors and the associated intracellular signalling pathways, there are still many unanswered important questions. For instance, at the genetic level, how are signals from extracellular factors conveyed to specific transcription factors that regulate axon growth? What are the genes activated by the transcription factors that specifically regulate axon growth? What are the transcription factors and genes that specifically regulate axon regeneration versus developmental axon growth? Locally at axons, how are signals from extracellular factors transduced downstream to regulate axonal cytoskeleton? More importantly, how is the genetic programme at the cell body coordinated with local signalling at the axon to regulate axon growth? Lastly, how do signals from ECM contribute to the genetic programme and local signalling to regulate axon growth? The answers to these questions will be critical to a fuller understanding of the intracellular mechanisms underlying axon growth, and for future development of ways to enhance adult CNS axon growth after injury.
Footnotes
One contribution of 13 to a Theme Issue ‘The regenerating brain’.
References
- Akhmanova A, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. doi: 10.1016/s0092-8674(01)00288-4. 10.1016/S0092-8674(01)00288-4 [DOI] [PubMed] [Google Scholar]
- Al-Majed A.A, Neumann C.M, Brushart T.M, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Nakamura T, Fujikawa K, Matsuda M. Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells. Mol. Biol. Cell. 2005;16:2207–2217. doi: 10.1091/mbc.E04-10-0904. 10.1091/mbc.E04-10-0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W.T, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal J.K, Massie B, Miller F.D, Kaplan D.R. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. 10.1016/S0896-6273(00)00035-0 [DOI] [PubMed] [Google Scholar]
- Atwal J.K, Singh K.K, Tessier-Lavigne M, Miller F.D, Kaplan D.R. Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase signaling: a mechanism for growth cone collapse. J. Neurosci. 2003;23:7602–7609. doi: 10.1523/JNEUROSCI.23-20-07602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. 10.1016/S0960-9822(02)01437-9 [DOI] [PubMed] [Google Scholar]
- Bassell G.J, Zhang H, Byrd A.L, Femino A.M, Singer R.H, Taneja K.L, Lifshitz L.M, Herman I.M, Kosik K.S. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy P.A, Karhadkar S.S, Berman D.M. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. 10.1038/nature03100 [DOI] [PubMed] [Google Scholar]
- Beals C.R, Sheridan C.M, Turck C.W, Gardner P, Crabtree G.R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. 10.1126/science.275.5308.1930 [DOI] [PubMed] [Google Scholar]
- Bonilla I.E, Tanabe K, Strittmatter S.M. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J. Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E.J, Moon L.D, Popat R.J, King V.R, Bennett G.S, Patel P.N, Fawcett J.W, McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- Broude E, McAtee M, Kelley M.S, Bregman B.S. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp. Neurol. 1997;148:367–377. doi: 10.1006/exnr.1997.6665. 10.1006/exnr.1997.6665 [DOI] [PubMed] [Google Scholar]
- Brown M.D, Cornejo B.J, Kuhn T.B, Bamburg J.R. Cdc42 stimulates neurite outgrowth and formation of growth cone filopodia and lamellipodia. J. Neurobiol. 2000;43:352–364. doi: 10.1002/1097-4695(20000615)43:4<352::aid-neu4>3.0.co;2-t. 10.1002/1097-4695(20000615)43:4%3C352::AID-NEU4%3E3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- Brushart T.M, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. 10.1016/j.expneurol.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Campbell D.S, Holt C.E. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. 10.1016/S0896-6273(03)00158-2 [DOI] [PubMed] [Google Scholar]
- Campenot R.B. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Dev. Biol. 1982a;93:13–21. doi: 10.1016/0012-1606(82)90233-0. 10.1016/0012-1606(82)90233-0 [DOI] [PubMed] [Google Scholar]
- Campenot R.B. Development of sympathetic neurons in compartmentalized cultures. I. Local control of neurite growth by nerve growth factor. Dev. Biol. 1982b;93:1–12. doi: 10.1016/0012-1606(82)90232-9. 10.1016/0012-1606(82)90232-9 [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman M.H, Goldstein L.S, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. 10.1016/S1534-5807(03)00094-7 [DOI] [PubMed] [Google Scholar]
- Chen D.F, Schneider G.E, Martinou J.C, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–439. doi: 10.1038/385434a0. 10.1038/385434a0 [DOI] [PubMed] [Google Scholar]
- Condic M.L. Adult neuronal regeneration induced by transgenic integrin expression. J. Neurosci. 2001;21:4782–4788. doi: 10.1523/JNEUROSCI.21-13-04782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. 10.1186/1471-2202-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D.A, Alessi D.R, Cohen P, Andjelkovich M, Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- Davies S.J, Fitch M.T, Memberg S.P, Hall A.K, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Davies S.J, Goucher D.R, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl Acad. Sci. USA. 1998;95:11 211–11 216. doi: 10.1073/pnas.95.19.11211. 10.1073/pnas.95.19.11211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio J.A, et al. MAP1B is required for Netrin 1 signaling in neuronal migration and axonal guidance. Curr. Biol. 2004;14:840–850. doi: 10.1016/j.cub.2004.04.046. 10.1016/j.cub.2004.04.046 [DOI] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc. Natl Acad. Sci. USA. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. 10.1073/pnas.84.18.6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebens A, Brose K, Leonardo E.D, Hanson M.G, Jr, Bladt F, Birchmeier C, Barres B.A, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. 10.1016/S0896-6273(00)80247-0 [DOI] [PubMed] [Google Scholar]
- Enomoto A, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. 10.1016/j.devcel.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. 10.1016/S0092-8674(01)00471-8 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. 10.1038/nature01423 [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. 10.1016/j.tcb.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Fischer D, He Z, Benowitz L.I. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J. Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. 10.1523/JNEUROSCI.5119-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcet C, Stein E, Pays L, Corset V, Llambi F, Tessier-Lavigne M, Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447. doi: 10.1038/nature748. 10.1038/nature748 [DOI] [PubMed] [Google Scholar]
- Fu S.Y, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Fukata M, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. 10.1016/S0092-8674(02)00800-0 [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau P.C. Localized sources of neurotrophins initiate axon collateral sprouting. J. Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. 10.1016/j.neuron.2004.10.030 [DOI] [PubMed] [Google Scholar]
- Gieffers C, Peters B.H, Kramer E.R, Dotti C.G, Peters J.M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl Acad. Sci. USA. 1999;96:11 317–11 322. doi: 10.1073/pnas.96.20.11317. 10.1073/pnas.96.20.11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J.L, Espinosa J.S, Xu Y, Davidson N, Kovacs G.T, Barres B.A. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002a;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. 10.1016/S0896-6273(02)00602-5 [DOI] [PubMed] [Google Scholar]
- Goldberg J.L, Klassen M.P, Hua Y, Barres B.A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002b;296:1860–1864. doi: 10.1126/science.1068428. 10.1126/science.1068428 [DOI] [PubMed] [Google Scholar]
- Goold R.G, Gordon-Weeks P.R. The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol. Cell Neurosci. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. 10.1016/j.mcn.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Grabham P.W, Goldberg D.J. Nerve growth factor stimulates the accumulation of beta1 integrin at the tips of filopodia in the growth cones of sympathetic neurons. J. Neurosci. 1997;17:5455–5465. doi: 10.1523/JNEUROSCI.17-14-05455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef I.A, Chen F, Crabtree G.R. NFAT signaling in vertebrate development. Curr. Opin. Genet. Dev. 2001;11:505–512. doi: 10.1016/s0959-437x(00)00225-2. 10.1016/S0959-437X(00)00225-2 [DOI] [PubMed] [Google Scholar]
- Graef I.A, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree G.R. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. 10.1016/S0092-8674(03)00390-8 [DOI] [PubMed] [Google Scholar]
- Grimes C.A, Jope R.S. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J. Neurochem. 2001;78:1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. 10.1046/j.1471-4159.2001.00495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth R.D, Mermelstein P.G. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J. Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K.L, Rao Y. Signalling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- Haase G, Dessaud E, Garces A, de Bovis B, Birling M, Filippi P, Schmalbruch H, Arber S, deLapeyriere O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. 10.1016/S0896-6273(02)00864-4 [DOI] [PubMed] [Google Scholar]
- Hatano N, et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–856. doi: 10.1046/j.1365-2443.2003.00680.x. 10.1046/j.1365-2443.2003.00680.x [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah J.D. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, CREB/ATF proteins. Brain Res. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. 10.1016/S0165-0173(98)00018-6 [DOI] [PubMed] [Google Scholar]
- Higgs H.N, Pollard T.D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. 10.1146/annurev.biochem.70.1.649 [DOI] [PubMed] [Google Scholar]
- Hogan P.G, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. 10.1101/gad.1102703 [DOI] [PubMed] [Google Scholar]
- Howe A.K, Aplin A.E, Juliano R.L. Anchorage-dependent ERK signaling—mechanisms and consequences. Curr. Opin. Genet. Dev. 2002;12:30–35. doi: 10.1016/s0959-437x(01)00260-x. 10.1016/S0959-437X(01)00260-X [DOI] [PubMed] [Google Scholar]
- Hua J.Y, Smear M.C, Baier H, Smith S.J. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. 10.1038/nature03409 [DOI] [PubMed] [Google Scholar]
- Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth B.U, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat. Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. 10.1038/35023515 [DOI] [PubMed] [Google Scholar]
- Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms; critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jiao J, Huang X, Feit-Leithman R.A, Neve R.L, Snider W, Dartt D.A, Chen D.F. Bcl-2 enhances Ca2+ signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005;24:1068–1078. doi: 10.1038/sj.emboj.7600589. 10.1038/sj.emboj.7600589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Mora S, Hwang J.B, Saltiel A.R, Pessin J.E. Atypical protein kinase C (PKC{zeta}/{lambda}) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J. Cell Biol. 2004;164:279–290. doi: 10.1083/jcb.200306152. 10.1083/jcb.200306152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney A.M, Kocsis J.D. Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. J. Neurosci. 1998;18:1318–1328. doi: 10.1523/JNEUROSCI.18-04-01318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. 10.1126/science.1093712 [DOI] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley K.E, Ehler E, Henriquez J.P, Hughes S.M, Salinas P.C. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Ye H, Ginty D.D. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. 10.1016/S0896-6273(00)00061-1 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel L.S, Glebova N.O, Lonze B.E, Valdez G, Ye H, Ginty D.D. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. 10.1016/j.cell.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Lee H, Engel U, Rusch J, Scherrer S, Sheard K, Van Vactor D. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 2004a;42:913–926. doi: 10.1016/j.neuron.2004.05.020. 10.1016/j.neuron.2004.05.020 [DOI] [PubMed] [Google Scholar]
- Lee N, Neitzel K.L, Devlin B.K, MacLennan A.J. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J. Comp. Neurol. 2004b;474:535–545. doi: 10.1002/cne.20140. 10.1002/cne.20140 [DOI] [PubMed] [Google Scholar]
- Lentz S.I, Miner J.H, Sanes J.R, Snider W.D. Distribution of the ten known laminin chains in the pathways and targets of developing sensory axons. J. Comp. Neurol. 1997;378:547–561. doi: 10.1002/(sici)1096-9861(19970224)378:4<547::aid-cne9>3.0.co;2-2. 10.1002/(SICI)1096-9861(19970224)378:4%3C547::AID-CNE9%3E3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- Lentz S.I, Knudson C.M, Korsmeyer S.J, Snider W.D. Neurotrophins support the development of diverse sensory axon morphologies. J. Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol. Cell Neurosci. 2005;29:269–282. doi: 10.1016/j.mcn.2005.03.002. 10.1016/j.mcn.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Mikaels A, Baudet C, Ernfors P. Activation by GDNF of a transcriptional program repressing neurite growth in dorsal root ganglia. Proc. Natl Acad. Sci. USA. 2001;98:14 681–14 686. doi: 10.1073/pnas.251548898. 10.1073/pnas.251548898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.Y, Snider W.D. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J. Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.Y, Schmid R.S, Snider W.D, Maness P.F. NGF enhances sensory axon growth induced by laminin but not by the L1 cell adhesion molecule. Mol. Cell Neurosci. 2002;20:2–12. doi: 10.1006/mcne.2002.1107. 10.1006/mcne.2002.1107 [DOI] [PubMed] [Google Scholar]
- Lonze B.E, Ginty D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. 10.1016/S0896-6273(02)00828-0 [DOI] [PubMed] [Google Scholar]
- Lonze B.E, Riccio A, Cohen S, Ginty D.D. Apoptosis, axonal growth defects, degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. 10.1016/S0896-6273(02)00686-4 [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. 10.1016/j.cell.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Lucas F.R, Salinas P.C. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. 10.1006/dbio.1997.8734 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova A.I, Lu C.C, Milanesio N, King L.A, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. 10.1126/science.1089610 [DOI] [PubMed] [Google Scholar]
- MacInnis B.L, Campenot R.B. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol. Cell Neurosci. 2005;28:430–439. doi: 10.1016/j.mcn.2004.10.003. 10.1016/j.mcn.2004.10.003 [DOI] [PubMed] [Google Scholar]
- McManus E.J, Sakamoto K, Armit L.J, Ronaldson L, Shpiro N, Marquez R, Alessi D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. 10.1038/sj.emboj.7600633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina F, Hilton M.C, Ponzetto C, Davies A.M, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A, Zhong J, Snider W.D. Raf and Akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. 10.1016/j.tips.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson G.A, Kruttgen A, Hu M, Munro E, Hanson M.G, Jr, Reichardt L.F, Barres B.A. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. 10.1016/S0896-6273(00)80586-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. 10.1016/S0896-6273(00)80760-6 [DOI] [PubMed] [Google Scholar]
- Ming G.L, Wong S.T, Henley J, Yuan X.B, Song H.J, Spitzer N.C, Poo M.M. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. 10.1038/nature745 [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella A.M, Medina D.L, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. 10.1016/S0896-6273(02)00942-X [DOI] [PubMed] [Google Scholar]
- Murray A.W. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. 10.1016/S0092-8674(03)01080-8 [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. 10.1016/j.neuron.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson B.J, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. 10.1038/416442a [DOI] [PubMed] [Google Scholar]
- Nishita M, Wang Y, Tomizawa C, Suzuki A, Niwa R, Uemura T, Mizuno K. Phosphoinositide 3-kinase-mediated activation of cofilin phosphatase Slingshot and its role for insulin-induced membrane protrusion. J. Biol. Chem. 2004;279:7193–7198. doi: 10.1074/jbc.M312591200. 10.1074/jbc.M312591200 [DOI] [PubMed] [Google Scholar]
- Nobes C.D, Hall A. Rho, rac, cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. 10.1016/0092-8674(95)90370-4 [DOI] [PubMed] [Google Scholar]
- O'Connor R, Tessier-Lavigne M. Identification of maxillary factor, a maxillary process-derived chemoattractant for developing trigeminal sensory axons. Neuron. 1999;24:165–178. doi: 10.1016/s0896-6273(00)80830-2. 10.1016/S0896-6273(00)80830-2 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Peschon J.J, Spriggs M.K, Kolodkin A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. 10.1038/nature01790 [DOI] [PubMed] [Google Scholar]
- Patel T.D, Jackman A, Rice F.L, Kucera J, Snider W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. 10.1016/S0896-6273(00)80899-5 [DOI] [PubMed] [Google Scholar]
- Peng J, Wallar B.J, Flanders A, Swiatek P.J, Alberts A.S. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr. Biol. 2003;13:534–545. doi: 10.1016/s0960-9822(03)00170-2. 10.1016/S0960-9822(03)00170-2 [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. 10.1016/j.neuron.2005.01.023 [DOI] [PubMed] [Google Scholar]
- Pfister B.J, Iwata A, Meaney D.F, Smith D.H. Extreme stretch growth of integrated axons. J. Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. 10.1523/JNEUROSCI.1974-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S.K, Kleinman H.K. Neuronal laminins and their cellular receptors. Int. J. Biochem. Cell Biol. 1997;29:401–414. doi: 10.1016/s1357-2725(96)00110-0. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman P.N, Bregman B.S, Filbin M.T. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. 10.1016/S0896-6273(02)00730-4 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cafferty W.B, McMahon S.B, Thompson S.W. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J. Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. 10.1523/JNEUROSCI.3269-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. 10.1016/j.ydbio.2003.06.003 [DOI] [PubMed] [Google Scholar]
- Raivich G, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. 10.1016/j.neuron.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Ray L.B, Sturgill T.W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc. Natl Acad. Sci. USA. 1987;84:1502–1506. doi: 10.1073/pnas.84.6.1502. 10.1073/pnas.84.6.1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport C.M, Blendy J.A, Ginty D.D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. 10.1126/science.286.5448.2358 [DOI] [PubMed] [Google Scholar]
- Robles E, Woo S, Gomez T.M. Src-dependent tyrosine phosphorylation at the tips of growth cone filopodia promotes extension. J. Neurosci. 2005;25:7669–7681. doi: 10.1523/JNEUROSCI.2680-05.2005. 10.1523/JNEUROSCI.2680-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M.M, Doe C.Q. Baz, Par-6 and aPKC are not required for axon or dendrite specification in Drosophila. Nat. Neurosci. 2004;7:1293–1295. doi: 10.1038/nn1346. 10.1038/nn1346 [DOI] [PubMed] [Google Scholar]
- Schmid R.S, Pruitt W.M, Maness P.F. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J. Neurosci. 2000;20:4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.H, Jan L.Y, Jan Y.N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. 10.1016/S0092-8674(02)01249-7 [DOI] [PubMed] [Google Scholar]
- Singh K.K, Miller F.D. Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron. 2005;45:837–845. doi: 10.1016/j.neuron.2005.01.049. 10.1016/j.neuron.2005.01.049 [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang K.C, Zhang Y.P, Shields C.B, Xu X.M, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. 10.1038/nn1193 [DOI] [PubMed] [Google Scholar]
- Smith L.G, Li R. Actin polymerization: riding the wave. Curr. Biol. 2004;14:R109–R111. 10.1016/S0960-9822(04)00031-4 [PubMed] [Google Scholar]
- Smith D.S, Skene J.H. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.L, Afroz R, Bassell G.J, Furneaux H.M, Perrone-Bizzozero N.I, Burry R.W. GAP-43 mRNA in growth cones is associated with HuD and ribosomes. J. Neurobiol. 2004;61:222–235. doi: 10.1002/neu.20038. 10.1002/neu.20038 [DOI] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles J.A, Strittmatter S.M. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J. Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi N, Marsh P, Goold R.G, Wood-Kaczmar A, Gordon-Weeks P.R. Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J. Cell Sci. 2005;118:993–1005. doi: 10.1242/jcs.01697. 10.1242/jcs.01697 [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol. Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Tucker K.L, Meyer M, Barde Y.A. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 2001;4:29–37. doi: 10.1038/82868. 10.1038/82868 [DOI] [PubMed] [Google Scholar]
- Turney S.G, Bridgman P.C. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat. Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. 10.1038/nn1466 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Berns A. Re-evaluating the role of frat in wnt-signal transduction. Cell Cycle. 2005;4:1065–1072. [PubMed] [Google Scholar]
- van Amerongen R, Nawijn M, Franca-Koh J, Zevenhoven J, van der Gulden H, Jonkers J, Berns A. Frat is dispensable for canonical Wnt signaling in mammals. Genes Dev. 2005;19:425–430. doi: 10.1101/gad.326705. 10.1101/gad.326705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Stork P.J, Lazarovici P, Eiden L.E. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. 10.1126/science.1071552 [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd A.M, Campbell D.S, Meyer R.L, Holt C.E, Fawcett J.W. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J. Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. 10.1523/JNEUROSCI.3073-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A, Willem M, Jones L.L, Kreutzberg G.W, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J. Neurosci. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, et al. Differential transport and local translation of cytoskeletal, injury-response, neurodegeneration protein mRNAs in axons. J. Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. 10.1523/JNEUROSCI.4235-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J.R. Judging a protein by more than its name: GSK-3. Sci. STKE. 2001;100:RE12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- Wu K.Y, Hengst U, Cox L.J, Macosko E.Z, Jeromin A, Urquhart E.R, Jaffrey S.R. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. 10.1038/nature03885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Kornhauser J.M, Xia Z, Thiele E.A, Greenberg M.E. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M. RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J. Biol. Chem. 2001;276:18 977–18 983. doi: 10.1074/jbc.M100254200. 10.1074/jbc.M100254200 [DOI] [PubMed] [Google Scholar]
- York R.D, Molliver D.C, Grewal S.S, Stenberg P.E, McCleskey E.W, Stork P.J. Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol. Cell Biol. 2000;20:8069–8083. doi: 10.1128/mcb.20.21.8069-8083.2000. 10.1128/MCB.20.21.8069-8083.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Mishina M. Distinct roles of calcineurin-nuclear factor of activated T-cells and protein kinase A-cAMP response element-binding protein signaling in presynaptic differentiation. J. Neurosci. 2005;25:3067–3079. doi: 10.1523/JNEUROSCI.3738-04.2005. 10.1523/JNEUROSCI.3738-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. 10.1016/j.cell.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Zhang H.L, Singer R.H, Bassell G.J. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J. Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. 10.1083/jcb.147.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Berg J.S, Li Z, Wang Y, Lang P, Sousa A.D, Bhaskar A, Cheney R.E, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 2004;6:523–531. doi: 10.1038/ncb1136. 10.1038/ncb1136 [DOI] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. 10.1016/S0896-6273(03)00225-3 [DOI] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He X.L, Garcia K.C, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. 10.1073/pnas.0409026102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.Q, Cohan C.S. How actin filaments and microtubules steer growth cones to their targets. J. Neurobiol. 2004;58:84–91. doi: 10.1002/neu.10278. 10.1002/neu.10278 [DOI] [PubMed] [Google Scholar]
- Zhou F.Q, Snider W.D. Cell biology: GSK-3beta and microtubule assembly in axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. 10.1126/science.1110301 [DOI] [PubMed] [Google Scholar]
- Zhou F.Q, Zhou J, Dedhar S, Wu Y.H, Snider W.D. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. 10.1016/j.neuron.2004.05.011 [DOI] [PubMed] [Google Scholar]
- Zhou F.Q, Walzer M.A, Wu Y.H, Zhou J, Dedhar S, Snider W.D. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J. Cell Sci. 2006;119:2787–2796. doi: 10.1242/jcs.03016. 10.1242/jcs.03016 [DOI] [PubMed] [Google Scholar]
- Ziv N.E, Spira M.E. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. Eur. J. Neurosci. 1993;5:657–668. doi: 10.1111/j.1460-9568.1993.tb00531.x. 10.1111/j.1460-9568.1993.tb00531.x [DOI] [PubMed] [Google Scholar]