Abstract
Semaphorins are developmental axon guidance cues that continue to be expressed during adulthood and are regulated by neural injury. During the formation of the nervous system, repulsive semaphorins guide axons to their targets by restricting and channelling their growth. They affect the growth cone cytoskeleton through interactions with receptor complexes that are linked to a complicated intracellular signal transduction network. Following injury, regenerating axons stop growing when they reach the border of the glial-fibrotic scar, in part because they encounter a potent molecular barrier that inhibits growth cone extension. A number of secreted semaphorins are expressed in the glial-fibrotic scar and at least one transmembrane semaphorin is upregulated in oligodendrocytes surrounding the lesion site. Semaphorin receptors, and many of the signal transduction components required for semaphorin signalling, are present in injured central nervous system neurons. Here, we review evidence that supports a critical role for semaphorin signalling in axon regeneration, and highlight a number of challenges that lie ahead with respect to advancing our understanding of semaphorin function in the normal and injured adult nervous system.
Keywords: axon growth inhibition, axon guidance, intracellular signalling, peripheral nervous system and central nervous system injury, neuromuscular junction, semaphorin
1. Introduction
It is now generally accepted that developing axons navigate to their targets by sensing attractive and repulsive molecules through receptors expressed on their growth cones. Binding of guidance molecules to receptors on the growth cone plasma membrane leads to the activation of intracellular signalling cascades. This results in dynamic changes in the cytoskeleton and subsequent directional axon extension and target recognition (Goodman 1996; Guan & Rao 2003; Huber et al. 2003). During the past 10–15 years, proteins instrumental in guiding developing axons by repulsive mechanisms have been proposed to also function as regulators of (structural) plasticity and inhibitors of axon regeneration. This idea is based on the initial observations that several repulsive axon guidance cues continue to be expressed in the adult nervous system and/or are induced following central nervous system (CNS) injury (De Winter et al. 2002a; Koeberle & Bahr 2004). This review will focus on our current understanding of the role of semaphorins, one of the largest families of axon guidance molecules (figure 1; Fiore & Puschel 2003), in adult nervous system regeneration. A summary of studies on the expression of semaphorins in various central and peripheral lesion paradigms (§2) will precede a detailed review of the remarkable progress that has been made with elucidating the complex intracellular signalling pathways downstream of semaphorins and a discussion of their potential as targets for encouraging axon regeneration (§3). In §4, we will discuss future directions and speculate on potential strategies for counteracting the axon growth inhibitory effects of semaphorins following neural injury.
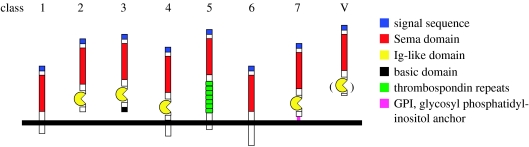
Figure 1.
Semaphorins. The semaphorin gene family has been categorized into eight classes on the basis of sequence similarity and structural features. Semaphorins exist as secreted (classes 2, 3 and V) and membrane-associated proteins (classes 1, 4, 5, 6 and 7). Classes 1 and 2 contain invertebrate semaphorins, classes 3–7 vertebrate semaphorins, and class V viral semaphorins.
Semaphorins comprise a large family of secreted and membrane-associated proteins that can signal axon repulsion and/or attraction (figure 1; Fiore & Puschel 2003). Vertebrate secreted semaphorins (Sema3s) are among the best-characterized semaphorin family members and signal through neuronal receptor complexes that contain neuropilins (NPs) and plexins as ligand binding and signal transducing subunits, respectively (Raper 2000). In contrast, several membrane-associated semaphorins bind directly to plexins and do not require NPs as co-receptors (figure 2). Cell adhesion molecules, Off-track, Met receptor, VEGF-R2 and ErbB-2 have been identified as (modulatory) components of specific semaphorin receptor complexes. In addition, semaphorin receptors unrelated to plexins and NPs have been identified, such as Tim-2 and CD72 (figure 2; Castellani & Rougon 2002; Pasterkamp & Kolodkin 2003; Kruger et al. 2005).
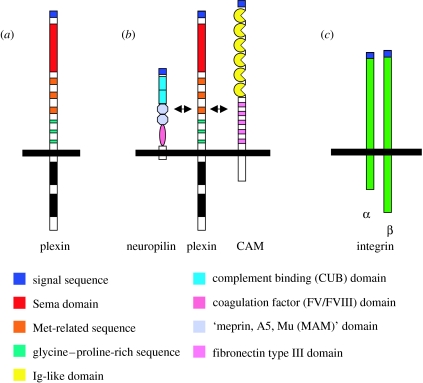
Figure 2.
Semaphorin receptors. Functional semaphorin receptors in the vertebrate nervous system: (a) semaphorins belonging to classes 4–7 and Sema3E can directly interact with plexins, (b) secreted class 3 semaphorins can signal through a neuropilin/plexinA (cell adhesion molecule (CAM)) complex and (c) Sema7A requires a β1-integrin for effects on axon outgrowth. α, alpha subunit, β, beta subunit.
Theories on semaphorin function in neural trauma have been formulated based on the cellular context in which semaphorins, their receptors and intracellular signalling molecules are (re)expressed following injury. At least four possible functions have been postulated. First, semaphorins may contribute to the inhibitory properties of oligodendrocyte-lineage cells and act in concert with the classical myelin-associated inhibitors Nogo, myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp). Multiple membrane-associated semaphorins including Sema4D, Sema5A and Sema6A are expressed by oligodendrocytes, and Sema4D is upregulated in oligodendrocytes located in the vicinity of CNS lesions (Cohen et al. 2003; Moreau-Fauvarque et al. 2003; Goldberg et al. 2004; Kerjan et al. 2005). Second, secreted class 3 semaphorins (Sema3s) may act as repulsive signals in neural scar tissue since they are expressed in meningeal fibroblasts that populate the core of the scar (Pasterkamp et al. 1998a, 1999; De Winter et al. 2002b). This idea is supported by the observation that injured CNS neurons express Sema3 receptor components and several associated cytosolic signalling molecules and may therefore be sensitive to the repulsive effects of Sema3s (Pasterkamp & Verhaagen 2001; De Winter et al. 2002a). Third, blood vessels in the scar express a semaphorin receptor, neuropilin-1, and are surrounded by Sema3A-positive meningeal fibroblasts (Pasterkamp et al. 1999). In addition, several scar-associated cells express semaphorin receptors and signalling molecules (Pasterkamp et al. 1999, 2005). Thus, secreted semaphorins could be involved in instigating or modulating the formation of the scar by influencing tissue compartmentalization or neovascularization. Fourth, in the peripheral nervous system (PNS) Sema3s are regulated by injury in lesioned motor neurons, in Schwann cells of the peripheral nerve and in specialized Schwann cells at neuromuscular junctions of fast fatigable muscle fibres (Pasterkamp et al. 1998c; Scarlato et al. 2003; Ara et al. 2004; De Winter et al. 2006). Interestingly, expression of semaphorin receptor proteins and signalling molecules is unchanged following PNS injury (Pasterkamp et al. 1998c; Gavazzi et al. 2000; R. J. Pasterkamp & J. Verhaagen 2006, unpublished observations). This suggests a role for semaphorins in motor neuron regeneration and plasticity at a subset of neuromuscular junctions. Ongoing and future studies will test the validity of the proposed involvement of semaphorins in inhibiting axon regeneration, in regulating scar formation and in modulating plasticity of the neuromuscular system.
2. Semaphorin expression in the injured peripheral nervous system and central nervous system
(a) Expression in the intact nervous system
During embryogenesis, many semaphorins are expressed in non-neuronal cells along developing nerve tracts and in subpopulations of neurons. Expression is often widespread and changes rapidly as development proceeds. In the mature nervous system, expression of semaphorins is mostly restricted to specific populations of neurons (www.gensat.org/index.html). For example, the prototype semaphorin Sema3A is expressed in cranial and spinal motor neurons, entorhinal cortex stellate cells, neurons in the subiculum and amygdala, olfactory bulb mitral cells and subsets of retinal ganglion cells (RGCs) (Giger et al. 1998; Skaliora et al. 1998; de Winter et al. 2004). Sema3A expression is not detectable in glial cells in the intact adult brain, but low levels of expression are observed in the lepto-meningeal sheet (Giger et al. 1996; Pasterkamp et al. 1998b, 1999). In addition to Sema3A, several other secreted and membrane-associated semaphorins have been reported to be expressed in the adult rodent or human brain (Eckhardt & Meyerhans 1998; Xu et al. 1998; Encinas et al. 1999; Hirsch & Bahr 1999b).
(b) Secreted semaphorins and central nervous system injury: expression in neural scar tissue
The cellular composition of the neural scar that forms as a result of penetrating brain and spinal cord injuries is complex (Silver & Miller 2004). The formation of neural scar tissue is a normal neural wound healing response that prevents further spread of damage to the uninjured parts of the nervous system. Following injury, meningeal fibroblasts and blood-borne cells invade the centre of the lesion and astrocytes around the lesion core become hypertrophic. Interactions between astrocytes and meningeal fibroblasts result in the formation of a new glia limitans and in the restoration of the blood–brain barrier (Shearer & Fawcett 2001). The fibrotic core of the scar is characterized by the presence of an extensive meshwork of extracellular matrix (ECM) consisting of collagen and chondroitin sulphate proteoglycans (CSPGs). Axons of injured CNS neurons are unable to cross the fibrotic scar and develop swollen endings (also known as dystrophic endbulbs) just proximal to or within the zone of reactive astrocytes (Tom et al. 2004). Meningeal fibroblasts invading the lesion core express the secreted semaphorins Sema3A, Sema3B, Sema3C, Sema3E and Sema3F (Pasterkamp et al. 1999; De Winter et al. 2002b). Double labelling studies reveal that the cells that express these Sema3 transcripts co-express proteins typical of fibroblasts (fibronectin and vimentin) and do not co-localize with markers of astrocytes (glial fibrillary acidic protein, GFAP), macrophages/microglia (ED1, OX42), oligodendrocytes (myelin basic protein (MBP), Gal-C) and Schwann cells (S100). In addition, Sema3-positive cells have a morphology that is congruent with previous descriptions of fibroblasts in the neural scar (Hirsch & Bahr 1999a) and form typical strands that are clearly derived from the semaphorin-positive lepto-meningeal sheet close to the primary lesion site.
Are injured adult neurons sensitive to the predicted repulsive actions of meningeal fibroblast-derived Sema3s in the lesion core? Several lines of evidence suggest that indeed injured neurons sense and respond to repulsive semaphorins. First, intact and injured adult CNS and PNS neurons express semaphorin receptors and associated intracellular signalling proteins. Second, sprouting sensory axons are responsive to Sema3-mediated axon repulsion in vitro and in vivo. Third, conditioning peripheral nerve injuries that allow dorsal root ganglia (DRG) axons to regenerate centrally do not promote regenerative axon growth through Sema3-expressing scar tissue (Wang & Strittmatter 1996; Tanelian et al. 1997; Pasterkamp et al. 1998b,c, 2000, 2001; Reza et al. 1999; Gavazzi et al. 2000; Owesson et al. 2000; De Winter et al. 2002b; Lindholm et al. 2004; Tang et al. 2004; Agudo et al. 2005). Although these results suggest that at least a subset of injured adult CNS neurons is inhibited by Sema3s, future studies employing genetically manipulated mice or other means of blocking semaphorin receptor/intracellular signalling will be needed to show unambiguously that injured neurons sense and respond to semaphorins following neural injury in vivo.
(c) Secreted semaphorins and PNS injury: control of neuromuscular plasticity?
Injured peripheral neurons are capable of regeneration and reinnervation of their distant target cells in skin and muscle. Schwann cells in the distal portion of the injured nerve support axonal regeneration by upregulating the expression of neurotrophic factors and cell adhesion molecules. This regeneration-promoting effect of Schwann cells is also clear following transplantation of peripheral nerve pieces in the injured CNS (Hirsch & Bahr 1999a). Traditionally, most studies on the mechanisms regulating peripheral nerve regeneration have focused on the expression of proteins that promote axon regeneration. However, understanding the role of neurite growth inhibitory proteins in the injured PNS may be equally important.
Adult motor and sensory neurons continue to express the receptors for secreted semaphorins, i.e. NP-1, NP-2 and several plexins (Pasterkamp et al. 1998c; Gavazzi et al. 2000; De Winter et al. 2002b; Lindholm et al. 2004). Furthermore, adult motor, but not sensory, neurons display persistent expression of Sema3A (Pasterkamp et al. 1998c). Interestingly, peripheral and central lesions of motor neuron axons affect Sema3A expression in opposite ways (Pasterkamp et al. 1998c; Lindholm et al. 2004). Peripheral nerve lesions at mid-thigh level result in a decline in Sema3A expression in motor neurons, while NP-1 continues to be expressed in motor neurons and is induced in small diameter DRG neurons (Pasterkamp et al. 1998c; Gavazzi et al. 2000). In contrast, lesions of the ventral funiculus, which lead to transection of the most proximal intraspinal segment of motor axons, result in upregulation of Sema3A and Sema3F in motor neurons. In line with observations following penetrating spinal cord and brain injuries, Sema3A is induced in the neural scar after ventral funiculus lesions (Lindholm et al. 2004). Ventral funiculus lesions result in very poor regeneration of motor axons, while peripheral lesions are followed by vigorous spontaneous regeneration. The different regulation of semaphorin expression following these two types of motor axon injuries may contribute to this differential regenerative outcome.
Sema3A expression has been studied extensively in relation to the plasticity of neuromuscular synapses. Different subsets of neuromuscular synapses differ markedly in their ability to display anatomical plasticity. Nerve terminals that innervate slow (subtype I) muscle fibres sprout vigorously following blockage of synaptic transmission. In contrast, motor nerve terminals innervating fast-fatigable (subtype IIb/x) muscle fibres fail to exhibit extra-synaptic sprouting. Interestingly, Sema3A is differentially expressed in terminal Schwann cells (TSCs) of different populations of muscle fibres: postnatal, regenerative and paralysis induced remodelling of neuromuscular connections in rat gastrocnemic muscles is accompanied by increased expression of Sema3A in TSCs of subtype IIb/x muscle fibres, but not in TSC at subtypes I and IIa muscle fibres (De Winter et al. 2006). This is the first known molecular difference between TSCs on neuromuscular junctions of different subtypes of muscle fibres. Overall, these results hint at the exciting possibility that TSCs not only support nerve terminal growth, but also restrict nerve terminal plasticity at specific neuromuscular junctions by secreting (a) chemorepulsive proteins.
(d) Are secreted semaphorins a component of the extracellular matrix?
Although numerous studies have used in situ hybridization to examine semaphorin mRNA expression in the intact and injured nervous system, the corresponding protein distribution patterns remain largely unknown. A few recent studies have begun to analyse Sema3 protein expression in the human and rat brain. In the human subiculum, Sema3A protein shows a punctate surface distribution on cell bodies and dendrites (Good et al. 2004). In the human cerebellum, Sema3A displays a punctate distribution around Purkinje cells, suggestive of secretion of Sema3A from Purkinje cell dendrites into the ECM or association of Sema3A with presynaptic terminals originating from other sources (Eastwood et al. 2003). The same antibody produces a quite different, intracellular distribution in rat Purkinje cells (Eastwood et al. 2003). In a third study, Sema3A was shown to be expressed in numerous neurons located throughout the basal ganglia and thalamus (Majed et al. 2006). This is surprising since these neurons do not express Sema3A mRNA (Giger et al. 1998). Sema3F shows a vesicular distribution in human cell lines and is associated with processes and nerve terminals in adult human brain (Hirsch & Bahr 1999b). Overall, it is difficult to draw definitive conclusions about the localization of Sema3s in nervous system tissue. One possible explanation for the inconsistent results outlined above is that the various Sema3 antibodies, in particular those directed against the highly conserved sema-domain, recognize multiple semaphorins. The available evidence should therefore be interpreted with great caution, but hints at an association of Sema3s with intracellular organelles, nerve terminals, cell surfaces and the ECM.
Support for a cell surface distribution of Sema3s also comes from bioassays on the inhibitory properties of meningeal fibroblasts. Sema3A is a major meningeal fibroblast-derived neurite growth-inhibitory factor in vitro and appears to be presented not in a soluble form but as a substrate-bound molecule associated with the cell membrane or ECM (Niclou et al. 2003). In co-cultures of astrocytes and meningeal fibroblasts, these two cell types cluster together and form more or less distinct territories as seen in the neural scar. Postnatal DRG neurons plated on these co-cultures grow quite well on the astrocytic compartment but do not cross the interface between astrocytes and meningeal fibroblasts (Shearer et al. 2003). This indicates that inhibitory factors are localized at the cell membrane or in the ECM rather than freely diffusing in the medium. Perturbation of Sema3 signalling by application of NP-2 antibodies partially overcomes the repellent effect of the meningeal boundary (Shearer et al. 2003). Taken together, this suggests that secreted Sema3s expressed by meningeal fibroblasts are presented to growth cones in a substrate-bound form.
How are Sema3s tethered to the cell membrane/ECM? It has been hypothesized that the C-terminal basic region present in Sema3s could mediate their binding to negative charges on the cell surface and ECM (Luo et al. 1993). Interestingly, GFP-tagged Sema3A associates with the cell surface of cultured neurons and is released in the medium by treatment with excess glycosaminoglycans or a glycosaminoglycan-degrading enzyme (chondroitinase ABC, chABC). This indicates that Sema3s may be connected to the ECM through binding of CSPGs (De Wit et al. 2005). To date, there is no direct evidence that proteoglycans modulate axon guidance by Sema3s, but there are some indications that heparan sulphate proteoglycans (HSPGs) can potentiate Sema3A-mediated growth cone collapse. Addition of heparin to Sema3A in a collapse assay results in enhanced growth cone collapse, while heparin alone has no effect. This suggests that association of Sema3A with heparan sulphate can potentiate Sema3A signalling (De Wit et al. 2005). Whether or not heparan sulphate can modulate Sema3s activity in vivo remains to be determined. Examples from the developing nervous system provide evidence that rather than proteoglycan expression per se, it is the presence of differentially localized chondroitin sulphate-binding molecules that confers specific inhibitory or stimulatory activity (Emerling & Lander 1996). Interestingly, an impressive study by Kantor et al. (2004) shows that HSPGs and CSPGs convert the transmembrane Sema5A in an attractive or an inhibitory axon guidance cue, respectively. Whether other classes of semaphorins are affected by proteoglycans in a similar fashion remains to be determined.
Are the above described semaphorin–proteoglycan interactions likely to occur following injury? The expression pattern of Sema3A mRNA in relation to neurite growth inhibitors, i.e. CSPGs, tenascin-C and myelin-derived inhibitors, has been studied in transection lesions of the spinal dorsal columns (Pasterkamp et al. 2001). CSPG and tenascin-C expression overlap with Sema3A-positive cells in the meninges and in the dorsolateral cap of scar tissue that is mostly comprised of meningeal fibroblasts. The area of expression of tenascin-C and CSPG extends deeper into the ventral (astrocytic) aspect of the lesion where no Sema3A positive cells were present. Conditioning lesions of the sciatic nerve enhance the growth state of ascending sensory neurites (Neumann & Woolf 1999) and promote their sprouting into areas of CSPG expression but fail to induce growth into CSPG-Sema3A positive areas of the scar (Pasterkamp et al. 2001). This observation suggests the exciting possibility that Sema3A interacts with CSPGs in the neural scar to form a highly repulsive protein complex. In light of these observations it is interesting that chABC treatment promotes regeneration of spinal axons (Bradbury et al. 2002) and reinstates synaptic plasticity in the visual cortex after the critical period (Pizzorusso et al. 2002). The release of cell surface bound Sema3A following chABC treatment in vitro (De Wit et al. 2005) may imply that this inhibitory cue is also released from the GAG chains of CSPG in the scar. The putative release of Sema3A from CSPGs in a neural scar could be a mechanism that contributes to the beneficial effect of chABC treatment on axon regeneration.
(e) Transmembrane semaphorins and central nervous system injury: oligodendrocyte-associated inhibition?
Oligodendrocytes express a number of myelin-associated inhibitory proteins, including Nogo, MAG and OMpg (Filbin 2003; McGee & Strittmatter 2003; He & Koprivica 2004; Schwab 2004). Recently, membrane-bound semaphorins have been added to the list of oligodendrocyte-associated inhibitors. Together with the classical myelin-associated inhibitors these semaphorins are thought to inhibit axonal regeneration in the injured CNS. Sema4D, a transmembrane semaphorin also known as CD100, is expressed in a subpopulation of oligodendrocytes and is upregulated transiently after spinal cord injury in oligodendrocytes in white matter areas close to the lesion site (Moreau-Fauvarque et al. 2003). Plexin-B1, a Sema4D receptor, displays very limited expression in the adult brain. Remarkably, CD72, a Sema4D receptor originally identified in the immune system, is broadly expressed in the mature CNS and could thus mediate the inhibitory effect of Sema4D on adult neurons (Moreau-Fauvarque et al. 2003). Sema5A is a member of the thrombospondin repeat-containing semaphorins (class 5) expressed in oligodendrocytes and oligodendrocyte precursor cells in the optic nerve (Goldberg et al. 2004). Sema5A induces the collapse of cultured RGC growth cones and inhibits neurite growth from RGCs. Axon outgrowth on optic nerve explants is enhanced by application of Sema5A function blocking antibodies. In contrast to the upregulation of Sema4D after a spinal cord lesion, expression of Sema5A does not change after optic nerve axotomy but its constitutive expression may be sufficient to render the optic nerve inhibitory for regenerative outgrowth. The role of Sema5A after CNS injury is particularly intriguing in view of the recent finding that Sema5A is a bi-functional molecule that is growth-inhibitory in the presence of CSPGs and growth-permissive in the presence of HSPGs (Kantor et al. 2004). Since optic nerve lesions lead to the induction of CSPG expression at the lesion site this may result in a relative increase in inhibitory Sema5A–CSPG molecular complexes at the site of injury.
Our picture of the injury-induced (changes in) expression of membrane-bound semaphorins is still far from complete. Given the large number of membrane-associated semaphorins that has been identified and studied in developmental processes, we expect that more information will become available on their expression, localization and function following neural injury in the near future.
3. Intracellular signalling components: targets for promoting central nervous system regeneration?
During the past 10–15 years, considerable progress has been made in unravelling the intracellular signal transduction pathways that function downstream of repulsive semaphorins and their receptors (for review see Castellani & Rougon 2002; Pasterkamp & Kolodkin 2003; Kruger et al. 2005). Interestingly, the sustained sensitivity of sprouting adult axons to Sema3A in vivo (Tanelian et al. 1997; Tang et al. 2004) supports the idea that these intracellular signalling pathways also operate in injured adult neurons and may represent valuable targets for blocking semaphorin repulsion following injury. Here, we review some of the recent progress in understanding repulsive semaphorin signalling and discuss the potential of individual semaphorin signalling components as targets for reducing semaphorin-mediated axon repulsion in the injured CNS (figure 3).
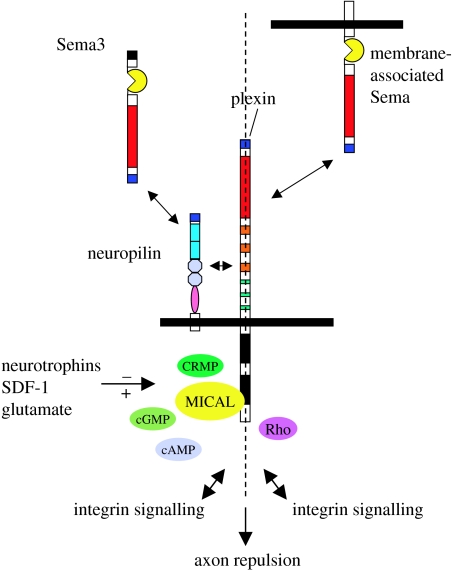
Figure 3.
Intracellular semaphorin signalling. Signalling molecules such as Rho, CRMP and MICAL may serve as targets for reducing the repulsive actions of vertebrate secreted (Sema3) and membrane-associated semaphorins in the injured CNS. See text for more detail.
(a) RhoGTPases
Binding of semaphorins to their receptors triggers a series of intracellular signalling events that ultimately affect the growth cone's actin cytoskeleton. One of the key regulators of actin dynamics is the Rho family of monomeric GTPases. Both RhoGTPases, including Rac, RhoA, RhoD and Rnd1, and the proteins that regulate their activity, such as guanine exchange factors (GEFs) and GTPase-activating proteins (GAPs), have been implicated in semaphorin signalling (Castellani & Rougon 2002; Pasterkamp & Kolodkin 2003; Kruger et al. 2005). Interestingly, semaphorin receptors themselves serve as GAPs. The Sema3A and Sema4D receptors plexinA1 and plexinB1, respectively, directly stimulate the intrinsic GTPase activity of R-Ras, a member of the Ras superfamily of small GTP binding proteins. This activity requires the interaction of plexins with another small GTP binding protein, Rnd1 (Oinuma et al. 2004; Negishi et al. 2005; Pasterkamp 2005; Toyofuku et al. 2005).
Several studies have explored Rho as a potential target for encouraging CNS regeneration. RhoGTPases and their downstream effector proteins are expressed in lesioned neurons, and blockage of Rho, by treatment with C3 transferase or Rho kinase (ROCK) inhibitors (Y27632), induces impressive anatomical and functional recovery in different models of spinal cord injury (Cai et al. 1999; Lehmann et al. 1999; Dergham et al. 2002; Fournier et al. 2003; Sivasankaran et al. 2004; Bertrand et al. 2005). Rho is thought to function downstream of several, but not all (e.g. class 3), classes of semaphorins (Castellani & Rougon 2002; Pasterkamp et al. 2003; Kruger et al. 2005). Current therapeutic strategies aimed at inactivating Rho or its downstream effectors are, therefore, likely to neutralize the repulsive effects of only a subset of repulsive semaphorins.
(b) Collapsin response mediator protein
One of the first cytosolic proteins shown to link semaphorin receptor complexes to the actin cytoskeleton is collapsin response mediator protein (CRMP)-2, a member of a small family of cytosolic phosphoproteins (Goshima et al. 1995; Wang & Strittmatter 1996). A number of different in vitro approaches have firmly established the role of CRMPs in semaphorin-induced (growth cone) collapse. CRMPs can associate with tubulin heterodimers, F-actin and several cytosolic proteins, including CRMP-associated molecule (CRAM), α2-chimaerin, kinesin-1, phospholipaseD2, Numb and Fes/Fps tyrosine kinase. Sequential phosphorylation of CRMP-2 by cyclin-dependent kinase-5 (Cdk-5) and glycogen synthase kinase3β (GSK3β) is believed to be critical for Sema3A-induced growth cone collapse (Schmidt & Strittmatter in press). Furthermore, a recent study by Kaibuchi and colleagues suggests that phosphorylation of CRMP-2 by GSK3β is also critical for the regulation of neuron polarity (Yoshimura et al. 2005). The effects of CRMPs on neuron polarity and axon growth are thought to result from direct interactions between CRMPs and the microtubule cytoskeleton (Arimura et al. 2004).
CRMP expression has been observed in different populations of PNS and CNS neurons following injury (Pasterkamp et al. 1998b; De Winter et al. 2002b; Suzuki et al. 2003). In addition, adenoviral vector-mediated overexpression of CRMP-2 in lesioned hypoglossal motor neurons accelerates peripheral nerve regeneration, probably through effects on axon growth/elongation (Suzuki et al. 2003). Whether increasing CRMP levels could also help to facilitate regeneration of CNS projections is currently unknown. It should be noted, however, that increases in CRMP expression in injured CNS neurons may also render them more sensitive to repulsive semaphorins expressed by CNS scar tissue. Recent insights into how the phosphorylation state of CRMP proteins relates to their cellular function (Schmidt & Strittmatter in press) may allow for the design of CRMP molecules that stimulate axon growth but do not affect axon responsiveness to repulsive semaphorins. Neuronal application of mutated CRMP proteins that can mediate axon growth but are unable to transduce axon repulsion may help to stimulate regenerative axon growth.
In addition to intact and injured neurons, CRMPs are expressed by oligodendrocytes in CNS white matter regions. In addition to CRMPs, oligodendrocytes express a broad spectrum of semaphorins, NPs, plexins and molecule interacting with CasL (MICAL; Ricard et al. 2000, 2001; Spassky et al. 2002; Cohen et al. 2003; Moreau-Fauvarque et al. 2003; Goldberg et al. 2004). In line with these expression patterns, semaphorins belonging to different subclasses, including class 3, can induce process retraction in oligodendrocyte progenitors, and oligodendrocytes and can also orient the migration of oligodendrocyte progenitors in vitro (Ricard et al. 2000, 2001; Cohen et al. 2003; Giraudon et al. 2004; Goldberg et al. 2004). Whether CRMPs are involved in mediating semaphorin-induced effects on oligodendrocyte morphology is unknown. Also, it remains to be determined whether neural injuries affect oligodendroglial CRMP expression.
(c) Cyclic nucleotides
Cyclic nucleotides, such as 3′,5′-cyclic adenosine monophosphate (cAMP) and 3′,5′-cyclic guanosine monophosphate (cGMP), have strong regulatory effects on growth cone behaviour. Depending on the intracellular concentration of cyclic nucleotides, an axon guidance molecule can act as a repellent or attractant for the same growth cone. Elevation of cGMP in Xenopus neurons converts Sema3A-induced repulsive growth cone responses into attraction, while changes in cAMP have no effect (Song et al. 1997). However, cGMP-dependent switching of Sema3A repulsion into attraction can be blocked by cAMP antagonists (Song et al. 1998), suggesting an interplay between the different cyclic nucleotide pathways. In line with this idea, recent evidence suggests that the ratio of cAMP and cGMP may be critical for determining the polarity of turning responses (Nishiyama et al. 2003).
Two recent studies have begun to shed light on how physiological changes in cyclic nucleotide levels may affect semaphorin signalling: (i) the Drosophila A-kinase anchoring protein (AKAP) Nervy physically interacts with plexinA and couples this plexin to protein kinase A (PKA). It is thought that Nervy-tethered PKA is activated by high cAMP levels to inhibit plexin signalling by phosphorylating plexin and/or downstream signalling components (Terman & Kolodkin 2004) and (ii) the Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-mediated axon repulsion in vivo (Ayoob et al. 2004). Although it is currently unknown whether Gyc76C is truly a semaphorin receptor component, this observation may provide a functional link between local cGMP production and semaphorin-mediated axon repulsion.
Similar to RhoGTPases, cAMP has been targeted to enhance functional regeneration in the injured CNS. The initial observation that increases in cAMP may render cultured neurons less sensitive to the actions of several growth inhibitory proteins (Cai et al. 1999; Neumann et al. 2002; Qiu et al. 2002; Nikulina et al. 2004) supported the exciting possibility that functional CNS regeneration may be achieved by manipulating neuronal cAMP signalling. Indeed, several independent studies have shown that administration of cAMP analogues or rolipram following injury stimulates regeneration of axonal projections in the rat spinal cord (Neumann et al. 2002; Qiu et al. 2002; Lu et al. 2004; Nikulina et al. 2004; Pearse et al. 2004) and regeneration of the rat optic nerve (Cui et al. 2003). It should be noted, however, that conditioning nerve injuries which allow DRG axons to regenerate their centrally projecting neurites, an effect thought to be dependent on increases in endogenous cAMP, do not promote regenerative growth through Sema3-positive scar tissue (Pasterkamp et al. 2001). This is consistent with the original observation that cAMP has only a small effect on semaphorin responses in Xenopus turning assays (Song et al. 1998). More recent evidence suggests, however, that cAMP may be able to repress repulsive semaphorin responses rather then converting them into attraction. In addition, the ratio of cAMP/cGMP rather than the absolute levels of the individual cyclic nucleotides appears to be important in determining growth cone responses (Castellani & Rougon 2002; Pasterkamp & Kolodkin 2003; Bashaw 2004). Therefore, further studies are needed to better comprehend the modulation of semaphorin signalling by cyclic nucleotide pathways in vivo and to determine whether or how cyclic nucleotides can be targeted to specifically relieve repulsive semaphorin signals in the injured CNS.
(d) Molecule interacting with CasL (MICAL)
In a search for plexin-interacting proteins, MICAL (Suzuki et al. 2002) was identified (Terman et al. 2002). MICALs comprise a small family of phylogenetically conserved multidomain cytosolic proteins. Drosophila (D) MICAL binds the intracellular region of the Sema-1a receptor plexinA, and D-MICAL–plexinA interactions are required in vivo for Sema-1a-induced motor axon repulsion in the developing Drosophila neuromuscular system (Terman et al. 2002). In vertebrates, three MICAL genes have been identified (MICAL-1, -2 and -3; Suzuki et al. 2002; Terman et al. 2002; Weide et al. 2003; Fischer et al. 2005; Pasterkamp et al. 2005). Interestingly, both vertebrate and invertebrate MICALs contain a NH2-terminal flavoprotein monooxygenase domain of about 500 amino acids (Nadella et al. 2005; Siebold et al. 2005). Site-directed mutagenesis of the D-MICAL monooxygenase region attenuates repulsive Sema-1a signalling in vivo while monooxygenase inhibitors neutralize Sema3-mediated axon repulsion in vitro (Terman et al. 2002; Pasterkamp et al. 2005). These observations suggest that the MICAL monooxygenase enzyme participates in repulsive semaphorin signalling.
The biological role of vertebrate MICALs is currently unknown. Expression analysis and yeast interaction experiments hint at a role downstream of Sema3s and their receptors, plexinAs (Terman et al. 2002; Pasterkamp et al. 2005). This idea gains further support from the observation that certain green tea polyphenols with the ability to block flavoprotein monooxygenases, (−)-epigallocatechin gallate (EGCG) and (−)-epicatechin (EC) (Abe et al. 2000a,b), abrogate Sema3A- and Sema3F-mediated repulsion of rat sensory axons in vitro (Terman et al. 2002; Pasterkamp et al. 2005). Vertebrate MICALs are expressed during neural development, in the adult nervous system and following injury. Contusion and hemisection injuries of the rat spinal cord induce expression of all three MICAL genes in meningeal fibroblasts that contribute to CNS scar tissue. In addition, MICAL-1 and -3 are expressed in oligodendrocytes in intact spinal cord white matter and in oligodendroglia present around the site of injury (Pasterkamp et al. 2005; figure 4). Although their role in non-neuronal cells in the intact and injured CNS requires further study, MICALs could function to transduce the effects of semaphorins on oligodendrocytes and meningeal fibroblasts. The concomitant expression of semaphorins, their receptors and associated cytosolic signalling molecules in oligodendroglia and meningeal fibroblasts suggests that semaphorins may act in an auto- and/or paracrine fashion in these cell types. Alternatively, oligodendroglia and meningeal fibroblasts could respond to semaphorins released in their environment. For example, Sema3s released from fibroblasts in the lesion core in the injured spinal cord may function to affect the migration and morphology of MICAL-positive oligodendrocytes surrounding the lesion and vice versa.
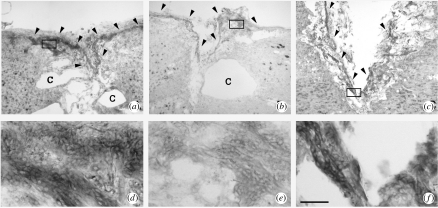
Figure 4.
MICALs are expressed in the injured rat spinal cord. Horizontal sections of a spinal cord lesion site at 56 days following overhemisection injury were subjected to in situ hybridization for MICAL-1 (a, d), MICAL-2 (b, e), or MICAL-3 (c, f). Panels d–f are higher magnifications of the boxed areas in a–c, respectively. MICALs are expressed in small, elongated meningeal cells in the lesion core and adjacent meningeal sheath (arrowheads in a–c) and in oligodendrocytes surrounding the lesion (not shown). c, cavity. Scale bar, 360 μm (a–c), 45 μm (d–f). Modified from Pasterkamp et al. (2005) with permission from Elsevier.
In line with the idea that injured adult neurons express Sema3 signalling components, expression of all three MICAL genes was found in corticospinal tract neurons following spinal hemisection and contusion injuries (Pasterkamp et al. 2005). Sustained MICAL neuronal expression following CNS injury, coupled with the potential for Sema3s to mediate glial scar inhibition of regenerative axon growth, suggest that MICALs may serve as therapeutic targets for decreasing axon responsiveness to scar-derived Sema3s following spinal cord injury. Strategies aimed at inactivating MICAL (monooxygenase enzymatic) activity may, therefore, help to paralyse repulsive semaphorin signalling. The robust effect of the monooxygenase inhibitor flavonoid EGCG on Sema3A- and Sema3F-mediated sensory neuron repulsion in vitro suggests that monooxygenase inhibitors may be used to reduce Sema3 signalling following injury (Pasterkamp et al. 2005; figure 5). However, the molecular mechanism(s) by which EGCG affects Sema3 signalling requires further research. Interestingly, EGCG also has neuroprotective and anti-inflammatory effects (Levites et al. 2001; Lee et al. 2003; Koh et al. 2004; Mandel et al. 2004). Another beneficial characteristic of EGCG in light of its potential application following neural injury is its access to the brain and other organs following peripheral administration owing to the ability of this flavonoid to efficiently cross the blood–brain barrier (Nakagawa & Miyazawa 1997; Suganuma et al. 1998). Future studies will determine whether EGCG can be used as a multiple-approach therapeutic agent for treating CNS injury.
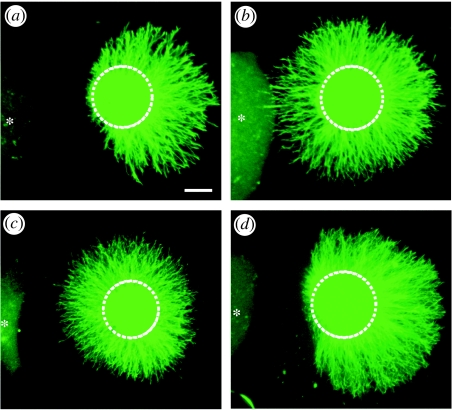
Figure 5.
Flavoprotein monooxygenase inhibitors block Sema3A-induced sensory axon repulsion. E14 rat DRG explants were co-cultured with 293 cells expressing Sema3A (asteriks) and grown for 48 h in the presence of (a) vehicle or (b–d) an inhibitor. (a) Sema3A repels rat sensory neurons. The monooxygenase inhibitors EC (b) and EGCG (c), but not the xanthine oxidase inhibitor allopurinol (d), inhibit this Sema3A-dependent axon repulsion. Modified from Terman et al. (2002) with permission from Elsevier.
(e) Intracellular modulation of semaphorin signalling
Our limited understanding of the signalling pathways downstream of repulsive semaphorins makes it difficult to predict with precision where intracellular semaphorin signalling pathways intersect with other signalling cascades. Nevertheless, several studies have begun to provide clues as to how semaphorin signalling may be modulated through interactions with other intracellular signal cascades. Intracellular signalling pathways that modulate semaphorin signalling could be excellent targets for manipulating semaphorin responsiveness following injury.
(i) Neurotrophins
Neurotrophic factors are widely used to promote CNS regeneration in vivo. It has become clear that in addition to their axon outgrowth promoting effects, neurotrophic factors may positively influence axon regeneration by changing the responsiveness of regenerating fibres to outgrowth inhibitory molecules. Neurotrophin-3 (NT-3), nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) modulate growth collapse responses to Sema3A (Tuttle & O'Leary 1998; Dontchev & Letourneau 2002). Both NGF and BDNF can protect embryonic chick DRG growth cones from Sema3A-induced collapse in a concentration-dependent manner (Dontchev & Letourneau 2002). Vice versa, Sema3F antagonizes NGF-induced TrkA signalling (Atwal et al. 2003). It is not precisely clear how and where NT and Sema3 signalling pathways intersect, but pharmacological studies suggest roles for the cyclic-nucleotide-dependent kinases PKA and PKG, phosphatidylinositol-3 kinase (PI-3), mitogen-activated protein kinase kinase (MEK) and Rho-associated kinase (Tuttle & O'Leary 1998; Ming et al. 1999; Dontchev & Letourneau 2002; Atwal et al. 2003).
(ii) Integrins
Several reports have shown that semaphorins and plexins regulate integrin function in cell adhesion, migration and axon outgrowth (Pasterkamp et al. 2003; Serini et al. 2003; Barberis et al. 2004). Recent evidence suggests that MICALs and/or R-Ras may be involved in cross-talk between these signalling cascades. MICAL was originally identified as a molecule that interacts with CasL (Suzuki et al. 2002). CasL is important for β1-integrin-induced signal transduction and cytoskeletal reorganization. This may indicate that semaphorin (axon repulsion) and integrin pathways (cell adhesion) converge on CasL. It is believed that sequestering of CasL by MICAL may locally decrease cell adhesion and thereby allow semaphorin-induced collapse. Similarly, binding of Sema3A and Sema4D to plexinA1/neuropilin-1 and plexinB1, respectively, leads to binding and inactivation of R-Ras (Oinuma et al. 2004; Toyofuku et al. 2005). Since R-Ras is a central player in integrin signalling this may mean that plexins shut down integrin signals by binding and inactivating this small GTPase.
(iii) Stromal cell-derived factor and glutamate
The chemokine stromal cell-derived factor (SDF)-1 and the neurotransmitter glutamate modulate the responsiveness of axons to Sema3s. The modulatory effects of SDF-1 are mediated through CXCR4 and a pertussis toxin-sensitive G-protein coupled signalling pathway that induces an elevation of cAMP (Chalasani et al. 2003). Similarly, the effects of glutamate are mediated by the metabotropic glutamate receptor 1 (mGluR1) and a pathway that involves the pertussis toxin-sensitive activation of PKA and inactivation of Rho (Kreibich et al. 2004).
Our understanding of how semaphorin signalling pathways are modulated by other unrelated signalling pathways and vice versa, is still rudimentary. However, further insights into this process of molecular cross-talk may provide unique opportunities for designing therapeutic strategies aimed at tackling multiple aspects of regeneration failure simultaneously (e.g. increasing both axonal growth and neuronal survival, and decreasing the inhibitory properties of the injured CNS by targeting one signalling pathway).
4. Perspectives
Several challenges lie ahead with respect to advancing our insight into the role of repulsive semaphorins in the intact and injured adult nervous system. First, expression studies of most semaphorins have relied on in situ hybridization, while their protein localization remains largely unknown. This is the result of a lack of specific and reliable semaphorin antibodies. Determining the precise intracellular and extracellular distribution of individual secreted and membrane-associated semaphorin proteins using well-characterized antibodies will be crucial to understand their role in axon plasticity, regeneration and degeneration. Furthermore, live imaging studies employing XFP-tagged semaphorin proteins will be required to reveal their axonal and dendritic transport and secretion and deposition characteristics at extracellular sites. This is particularly important in view of the proposed interaction of these repulsive molecules with the ECM, e.g. during the inhibition of axon regeneration or while modulating synaptic plasticity. Second, the demonstration of a causal relationship between the expression of semaphorins and the failure of axon regeneration has only just begun. Several approaches can be envisioned here: genetic ablation of specific semaphorin, semaphorin receptor or intracellular signalling cue genes, antibody perturbation or pharmacological inhibitor studies. The ECM appears to be a particularly important target for reducing the inhibitory nature of the injured CNS since multiple inhibitory molecules may be affected simultaneously by interfering with the chondroitin moieties of the proteoglycans or by inhibiting the synthesis of collagen in the ECM (Bradbury et al. 2002; Klapka et al. 2005). Targeting the ECM will also help to reveal the relative contribution of the different inhibitory factors (Nogo, MAG, OMpg, CSPG, semaphorins) to the failure of CNS regeneration and should lead the way to more effective and realistic interference strategies. Third, Sema3F modulates synaptic transmission in the adult mouse hippocampus (Sahay et al. 2005). This finding illustrates the diverse functional repertoire of these guidance molecules and suggests involvement in both the formation and synaptic efficacy of neural circuits. Finally, single-nucleotide polymorphisms in Sema5A and the Sema3 receptor plexinA2 have recently been linked to Parkinson disease and schizophrenia, respectively (Maraganore et al. 2005; Mah et al. 2006). This nominates these genes as novel susceptibility factors in these diseases and warrants more work on these molecules in relation to adult brain function and neurodegeneration. As the biological functions of semaphorins continue to be dissected at multiple levels we are likely to answer fascinating questions about their involvement in neuronal plasticity, regeneration and neurological disease and come up with ways to modulate semaphorin function to treat injury or disease.
Acknowledgments
Work in the laboratory of R.J.P. is supported by the Netherlands Organization for Scientific Research, The Human Frontier Science Program Organization, The Dutch Brain Foundation, The National Alliance for Research on Schizophrenia and Depression, and the ABC Genomics Centre Utrecht. Work on semaphorins in the laboratory of J.V. is supported by the International Spinal Research Trust (London), the Dutch Brain Foundation, the Prinses Beatrix Fonds and the Netherlands Institute for Neuroscience.
Footnotes
One contribution of 13 to a Theme Issue ‘The regenerating brain’.
References
- Abe I, Kashiwagi K, Noguchi H. Antioxidative galloyl esters as enzyme inhibitors of p-hydroxybenzoate hydroxylase. FEBS Lett. 2000a;483:131–134. doi: 10.1016/s0014-5793(00)02100-1. 10.1016/S0014-5793(00)02100-1 [DOI] [PubMed] [Google Scholar]
- Abe I, Seki T, Umehara K, Miyase T, Noguchi H, Sakakibara J, Ono T. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem. Biophys. Res. Commun. 2000;268:767–771. doi: 10.1006/bbrc.2000.2217. 10.1006/bbrc.2000.2217 [DOI] [PubMed] [Google Scholar]
- Agudo M, Robinson M, Cafferty W, Bradbury E.J, Kilkenny C, Hunt S.P, McMahon S.B. Regulation of neuropilin 1 by spinal cord injury in adult rats. Mol. Cell Neurosci. 2005;28:475–484. doi: 10.1016/j.mcn.2004.10.008. 10.1016/j.mcn.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Ara J, Bannerman P, Hahn A, Ramirez S, Pleasure D. Modulation of sciatic nerve expression of class 3 semaphorins by nerve injury. Neurochem. Res. 2004;29:1153–1159. doi: 10.1023/b:nere.0000023602.72354.82. 10.1023/B:NERE.0000023602.72354.82 [DOI] [PubMed] [Google Scholar]
- Arimura N, Menager C, Fukata Y, Kaibuchi K. Role of CRMP-2 in neuronal polarity. J. Neurobiol. 2004;58:34–47. doi: 10.1002/neu.10269. 10.1002/neu.10269 [DOI] [PubMed] [Google Scholar]
- Atwal J.K, Singh K.K, Tessier-Lavigne M, Miller F.D, Kaplan D.R. Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogen-activated protein kinase kinase signaling: a mechanism for growth cone collapse. J. Neurosci. 2003;23:7602–7609. doi: 10.1523/JNEUROSCI.23-20-07602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoob J.C, Yu H.H, Terman J.R, Kolodkin A.L. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J. Neurosci. 2004;24:6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. 10.1523/JNEUROSCI.1104-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love C.A, Jones E.Y, Comoglio P.M, Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 2004;18:592–594. doi: 10.1096/fj.03-0957fje. [DOI] [PubMed] [Google Scholar]
- Bashaw G.J. Semaphorin signaling unplugged; a nervy AKAP cAMP(s) out on plexin. Neuron. 2004;42:363–366. doi: 10.1016/s0896-6273(04)00258-2. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Winton M.J, Rodriguez-Hernandez N, Campenot R.B, McKerracher L. Application of rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 2005;25:1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005. 10.1523/JNEUROSCI.3931-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E.J, Moon L.D, Popat R.J, King V.R, Bennett G.S, Patel P.N, Fawcett J.W, McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin M.T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Castellani V, Rougon G. Control of semaphorin signaling. Curr. Opin. Neurobiol. 2002;12:532–541. doi: 10.1016/s0959-4388(02)00357-4. 10.1016/S0959-4388(02)00357-4 [DOI] [PubMed] [Google Scholar]
- Chalasani S.H, Sabelko K.A, Sunshine M.J, Littman D.R, Raper J.A. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J. Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.I, Rottkamp D.M, Maric D, Barker J.L, Hudson L.D. A role for semaphorins and neuropilins in oligodendrocyte guidance. J. Neurochem. 2003;85:1262–1278. doi: 10.1046/j.1471-4159.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- Cui Q, Yip H.K, Zhao R.C, So K.F, Harvey A.R. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol. Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. 10.1016/S1044-7431(02)00037-4 [DOI] [PubMed] [Google Scholar]
- De Winter F, Holtmaat A.J, Verhaagen J. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv. Exp. Med. Biol. 2002a;515:115–139. doi: 10.1007/978-1-4615-0119-0_10. [DOI] [PubMed] [Google Scholar]
- De Winter F, Oudega M, Lankhorst A.J, Hamers F.P, Blits B, Ruitenberg M.J, Pasterkamp R.J, Gispen W.H, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 2002b;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- De Winter F, Cui Q, Symons N, Verhaagen J, Harvey A.R. Expression of class-3 semaphorins and their receptors in the neonatal and adult rat retina. Invest. Ophthalmol. Vis. Sci. 2004;45:4554–4562. doi: 10.1167/iovs.04-0173. 10.1167/iovs.04-0173 [DOI] [PubMed] [Google Scholar]
- De Winter F, Vo T, Stam F.J, Wisman L.A.B, Bar P.R, Niclou S.P, Van Muiswinkel F.L, Verhaagen J. The expression of the chemorepellent Semaphorin3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display a limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol. Cell Neurosci. 2006;32:102–117. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol. Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. 10.1016/j.mcn.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell W.D, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontchev V.D, Letourneau P.C. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J. Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood S.L, Law A.J, Everall I.P, Harrison P.J. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol. Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. 10.1038/sj.mp.4001233 [DOI] [PubMed] [Google Scholar]
- Eckhardt F, Meyerhans A. Cloning and expression pattern of a murine semaphorin homologous to H-sema IV. Neuroreport. 1998;9:3975–3979. doi: 10.1097/00001756-199812010-00038. [DOI] [PubMed] [Google Scholar]
- Emerling D.E, Lander A.D. Inhibitors and promoters of thalamic neuron adhesion and outgrowth in embryonic neocortex: functional association with chondroitin sulfate. Neuron. 1996;17:1089–1100. doi: 10.1016/s0896-6273(00)80242-1. 10.1016/S0896-6273(00)80242-1 [DOI] [PubMed] [Google Scholar]
- Encinas J.A, Kikuchi K, Chedotal A, de Castro F, Goodman C.S, Kimura T. Cloning, expression, and genetic mapping of Sema W, a member of the semaphorin family. Proc. Natl Acad. Sci. USA. 1999;96:2491–2496. doi: 10.1073/pnas.96.5.2491. 10.1073/pnas.96.5.2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fiore R, Puschel A.W. The function of semaphorins during nervous system development. Front. Biosci. 2003;8:S484–S499. doi: 10.2741/1080. [DOI] [PubMed] [Google Scholar]
- Fischer J, Weide T, Barnekow A. The MICAL proteins and rab1: a possible link to the cytoskeleton? Biochem. Biophys. Res. Commun. 2005;328:415–423. doi: 10.1016/j.bbrc.2004.12.182. 10.1016/j.bbrc.2004.12.182 [DOI] [PubMed] [Google Scholar]
- Fournier A.E, Takizawa B.T, Strittmatter S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Stonehouse J, Sandvig A, Reza J.N, Appiah-Kubi L.S, Keynes R, Cohen J. Peripheral, but not central, axotomy induces neuropilin-1 mRNA expression in adult large diameter primary sensory neurons. J. Comp. Neurol. 2000;423:492–499. 10.1002/1096-9861(20000731)423:3%3C492::AID-CNE11%3E3.0.CO;2-L [PubMed] [Google Scholar]
- Giger R.J, Wolfer D.P, De Wit G.M.J, Verhaagen J. Anatomy of rat semaphorin III/collapsin-1 mRNA expression and relationship to developing nerve tracts during neuroembryogenesis. J. Comp. Neurol. 1996;375:378–392. doi: 10.1002/(SICI)1096-9861(19961118)375:3<378::AID-CNE3>3.0.CO;2-#. 10.1002/(SICI)1096-9861(19961118)375:3%3C378::AID-CNE3%3E3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Giger R.J, Pasterkamp R.J, Heijnen S, Holtmaat A.J, Verhaagen J. Anatomical distribution of the chemorepellent semaphorin III/collapsin-1 in the adult rat and human brain: predominant expression in structures of the olfactory–hippocampal pathway and the motor system. J. Neurosci. Res. 1998;52:27–42. doi: 10.1002/(SICI)1097-4547(19980401)52:1<27::AID-JNR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Giraudon P, et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J. Immunol. 2004;172:1246–1255. doi: 10.4049/jimmunol.172.2.1246. [DOI] [PubMed] [Google Scholar]
- Goldberg J.L, Vargas M.E, Wang J.T, Mandemakers W, Oster S.F, Sretavan D.W, Barres B.A. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J. Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. 10.1523/JNEUROSCI.4390-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P.F, Alapat D, Hsu A, Chu C, Perl D, Wen X, Burstein D.E, Kohtz D.S. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer's disease. J. Neurochem. 2004;91:716–736. doi: 10.1111/j.1471-4159.2004.02766.x. [DOI] [PubMed] [Google Scholar]
- Goodman C.S. Mechanisms and molecules that control growth cone guidance. Annu. Rev. Neurosci. 1996;19:341–347. doi: 10.1146/annurev.ne.19.030196.002013. 10.1146/annurev.ne.19.030196.002013 [DOI] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, Strittmatter S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. 10.1038/376509a0 [DOI] [PubMed] [Google Scholar]
- Guan K.L, Rao Y. Signalling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 2004;27:341–368. doi: 10.1146/annurev.neuro.27.070203.144340. 10.1146/annurev.neuro.27.070203.144340 [DOI] [PubMed] [Google Scholar]
- Hirsch S, Bahr M. Growth promoting and inhibitory effects of glial cells in the mammalian nervous system. Adv. Exp. Med. Biol. 1999a;468:199–205. doi: 10.1007/978-1-4615-4685-6_16. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Bahr M. Immunocytochemical characterization of reactive optic nerve astrocytes and meningeal cells. Glia. 1999b;26:36–46. doi: 10.1002/(sici)1098-1136(199903)26:1<36::aid-glia4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Huber A.B, Kolodkin A.L, Ginty D.D, Cloutier J.F. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. 10.1146/annurev.neuro.26.010302.081139 [DOI] [PubMed] [Google Scholar]
- Kantor D.B, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. 10.1016/j.neuron.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell K.J, Chedotal A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat. Neurosci. 2005;8:1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers F.P, Muller D, Zuschratter W, Muller H.W. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 2005;22:3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. 10.1111/j.1460-9568.2005.04495.x [DOI] [PubMed] [Google Scholar]
- Koeberle P.D, Bahr M. Growth and guidance cues for regenerating axons: where have they gone? J. Neurobiol. 2004;59:162–180. doi: 10.1002/neu.10345. 10.1002/neu.10345 [DOI] [PubMed] [Google Scholar]
- Koh S.H, et al. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology. 2004;202:213–225. doi: 10.1016/j.tox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Kreibich T.A, Chalasani S.H, Raper J.A. The neurotransmitter glutamate reduces axonal responsiveness to multiple repellents through the activation of metabotropic glutamate receptor 1. J. Neurosci. 2004;24:7085–7095. doi: 10.1523/JNEUROSCI.0349-04.2004. 10.1523/JNEUROSCI.0349-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R.P, Aurandt J, Guan K.L. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. 10.1038/nrm1740 [DOI] [PubMed] [Google Scholar]
- Lee S.Y, Kim C.Y, Lee J.J, Jung J.G, Lee S.R. Effects of delayed administration of (−)-epigallocatechin gallate, a green tea polyphenol on the changes in polyamine levels and neuronal damage after transient forebrain ischemia in gerbils. Brain Res. Bull. 2003;61:399–406. doi: 10.1016/s0361-9230(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levites Y, Weinreb O, Maor G, Youdim M.B, Mandel S. Green tea polyphenol (−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001;78:1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. 10.1046/j.1471-4159.2001.00490.x [DOI] [PubMed] [Google Scholar]
- Lindholm T, Skold M.K, Suneson A, Carlstedt T, Cullheim S, Risling M. Semaphorin and neuropilin expression in motoneurons after intraspinal motoneuron axotomy. Neuroreport. 2004;15:649–654. doi: 10.1097/00001756-200403220-00015. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones L.L, Filbin M.T, Tuszynski M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. 10.1523/JNEUROSCI.1492-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper J.A. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. 10.1016/0092-8674(93)80064-L [DOI] [PubMed] [Google Scholar]
- Mah S, et al. Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia. Mol. Psychiatry. 2006;11:471–478. doi: 10.1038/sj.mp.4001785. [DOI] [PubMed] [Google Scholar]
- Majed H.H, Chandran S, Niclou S, Nicholas R.S, Wilkins A, Wing M.G, Rhodes K.E, Spillantini M.G, Compston A. A novel role for Sema3A in neuroprotection from injury mediated by activated microglia. J. Neurosci. 2006;26:1730–1708. doi: 10.1523/JNEUROSCI.0702-05.2006. 10.1523/JNEUROSCI.0702-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Weinreb O, Amit T, Youdim M.B. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J. Neurochem. 2004;88:1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- Maraganore D.M, et al. High-resolution whole-genome association study of Parkinson disease. Am. J. Human Genet. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee A.W, Strittmatter S.M. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. 10.1016/S0166-2236(03)00062-6 [DOI] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. 10.1016/S0896-6273(00)80760-6 [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadella M, Bianchet M.A, Gabelli S.B, Barrila J, Amzel L.M. Structure and activity of the axon guidance protein MICAL. Proc. Natl Acad. Sci. USA. 2005;102:16 830–16 835. doi: 10.1073/pnas.0504838102. 10.1073/pnas.0504838102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Miyazawa T. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in the rat. J. Nutr. Sci. Vitaminol. (Tokyo) 1997;43:679–684. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- Negishi M, Oinuma I, Katoh H. R-ras as a key player for signaling pathway of plexins. Mol. Neurobiol. 2005;32:217–222. doi: 10.1385/MN:32:3:217. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf C.J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. 10.1016/S0896-6273(00)80755-2 [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. 10.1016/S0896-6273(02)00702-X [DOI] [PubMed] [Google Scholar]
- Niclou S.P, Franssen E.H, Ehlert E.M, Taniguchi M, Verhaagen J. Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth. Mol. Cell Neurosci. 2003;24:902–912. doi: 10.1016/s1044-7431(03)00243-4. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell J.L, Dai H.N, Bregman B.S, Filbin M.T. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc. Natl Acad. Sci. USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. 10.1073/pnas.0402595101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley J.R, Goshima Y, Tessier-Lavigne M, Poo M.M, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. 10.1038/nature01751 [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Owesson C, Pizzey J, Tonge D. Sensitivity of NGF-responsive dorsal root ganglion neurons to semaphorin D is maintained in both neonatal and adult mice. Exp. Neurol. 2000;165:394–398. doi: 10.1006/exnr.2000.7477. 10.1006/exnr.2000.7477 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J. R-Ras fills another GAP in semaphorin signalling. Trends Cell Biol. 2005;15:61–64. doi: 10.1016/j.tcb.2004.12.005. 10.1016/j.tcb.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Kolodkin A.L. Semaphorin junction: making tracks toward neural connectivity. Curr. Opin. Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Verhaagen J. Emerging roles for semaphorin in neural regeneration. Brain Res. Rev. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. 10.1016/S0165-0173(00)00050-3 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, De Winter F, Giger R.J, Verhaagen J. Role for semaphorin III and its receptor neuropilin-1 in neuronal regeneration and scar formation? Prog. Brain Res. 1998a;117:151–170. doi: 10.1016/s0079-6123(08)64014-5. [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, De Winter F, Holtmaat A.J, Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J. Neurosci. 1998b;18:9962–9976. doi: 10.1523/JNEUROSCI.18-23-09962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp R.J, Giger R.J, Verhaagen J. Regulation of semaphorin III/collapsin-1 gene expression during peripheral nerve regeneration. Exp. Neurol. 1998c;153:313–327. doi: 10.1006/exnr.1998.6886. 10.1006/exnr.1998.6886 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Giger R.J, Ruitenberger M.J, Holtmaat A.J, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. 10.1006/mcne.1999.0738 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Giger R.J, Baker R.E, Hermens W.T, Verhaagen J. Ectopic adenoviral vector-directed expression of Sema3A in organotypic spinal cord explants inhibits growth of primary sensory afferents. Dev. Biol. 2000;220:129–141. doi: 10.1006/dbio.2000.9627. [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Anderson P.N, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 2001;13:457–471. doi: 10.1046/j.0953-816x.2000.01398.x. 10.1046/j.0953-816X.2000.01398.x [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Peschon J.J, Spriggs M.K, Kolodkin A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. 10.1038/nature01790 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R.J, Dai H.N, Terman J.R, Wahlin K.J, Kim B, Bregman B.S, Popovich P.G, Kolodkin A.L. MICAL flavoprotein monooxygenases: expression during neural development and following spinal cord injuries in the rat. Mol. Cell Neurosci. 2005;31:52–69. doi: 10.1016/j.mcn.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pearse D.D, Pereira F.C, Marcillo A.E, Bates M.L, Berrocal Y.A, Filbin M.T, Bunge M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. 10.1038/nm1056 [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett J.W, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. 10.1126/science.1072699 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman P.N, Bregman B.S, Filbin M.T. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raper J.A. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. 10.1016/S0959-4388(99)00057-4 [DOI] [PubMed] [Google Scholar]
- Reza J.N, Gavazzi I, Cohen J. Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol. Cell Neurosci. 1999;14:317–326. doi: 10.1006/mcne.1999.0786. 10.1006/mcne.1999.0786 [DOI] [PubMed] [Google Scholar]
- Ricard D, et al. Differential expression of collapsin response mediator proteins (CRMP/ULIP) in subsets of oligodendrocytes in the postnatal rodent brain. Mol. Cell Neurosci. 2000;16:324–337. doi: 10.1006/mcne.2000.0888. [DOI] [PubMed] [Google Scholar]
- Ricard D, Rogemond V, Charrier E, Aguera M, Bagnard D, Belin M.F, Thomasset N, Honnorat J. Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsin response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/CRMP2 in Sema3a-sensitive oligodendrocytes. J. Neurosci. 2001;21:7203–7214. doi: 10.1523/JNEUROSCI.21-18-07203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Kim C.H, Sepkuty J.P, Cho E, Huganir R.L, Ginty D.D, Kolodkin A.L. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J. Neurosci. 2005;25:3613–3620. doi: 10.1523/JNEUROSCI.5255-04.2005. 10.1523/JNEUROSCI.5255-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato M, Ara J, Bannerman P, Scherer S, Pleasure D. Induction of neuropilins-1 and -2 and their ligands, Sema3A, Sema3F, and VEGF, during Wallerian degeneration in the peripheral nervous system. Exp. Neurol. 2003;183:489–498. doi: 10.1016/s0014-4886(03)00046-3. [DOI] [PubMed] [Google Scholar]
- Schmidt, E. F. & Strittmatter, S. M. In press. The CRMP family of proteins and their role in Sema3A signaling. Adv. Exp. Med. Biol. [DOI] [PMC free article] [PubMed]
- Schwab M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. 10.1016/j.conb.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Serini G, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. 10.1038/nature01784 [DOI] [PubMed] [Google Scholar]
- Shearer M.C, Fawcett J.W. The astrocyte/meningeal cell interface—a barrier to successful nerve regeneration? Cell Tissue Res. 2001;305:267–273. doi: 10.1007/s004410100384. [DOI] [PubMed] [Google Scholar]
- Shearer M.C, Niclou S.P, Brown D, Asher R.A, Holtmaat A.J, Levine J.M, Verhaagen J, Fawcett J.W. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol. Cell Neurosci. 2003;24:913–925. doi: 10.1016/j.mcn.2003.09.004. 10.1016/j.mcn.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Siebold C, et al. High-resolution structure of the catalytic region of MICAL (molecule interacting with CasL), a multidomain flavoenzyme-signaling molecule. Proc. Natl Acad. Sci. USA. 2005;102:16 836–16 841. doi: 10.1073/pnas.0504997102. 10.1073/pnas.0504997102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang K.C, Zhang Y.P, Shields C.B, Xu X.M, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. 10.1038/nn1193 [DOI] [PubMed] [Google Scholar]
- Skaliora I, Singer W, Betz H, Puschel A.W. Differential patterns of semaphorin expression in the developing rat brain. Eur. J. Neurosci. 1998;10:1215–1229. doi: 10.1046/j.1460-9568.1998.00128.x. 10.1046/j.1460-9568.1998.00128.x [DOI] [PubMed] [Google Scholar]
- Song H.-J, Ming G.-L, Poo M.-M. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. 10.1126/science.281.5382.1515 [DOI] [PubMed] [Google Scholar]
- Spassky N, de Castro F, Le Bras B, Heydon K, Queraud-LeSaux F, Bloch-Gallego E, Chedotal A, Zalc B, Thomas J.L. Directional guidance of oligodendroglial migration by class 3 semaphorins & netrin-1. J. Neurosci. 2002;22:5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nakamoto T, Ogawa S, Seo S, Matsumura T, Tachibana K, Morimoto C, Hirai H. MICAL, a novel CasL interacting molecule, associates with vimentin. J. Biol. Chem. 2002;277:14 933–14 941. doi: 10.1074/jbc.M111842200. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nakagomi S, Namikawa K, Kiryu-Seo S, Inagaki N, Kaibuchi K, Aizawa H, Kikuchi K, Kiyama H. Collapsin response mediator protein-2 accelerates axon regeneration of nerve-injured motor neurons of rat. J. Neurochem. 2003;86:1042–1050. doi: 10.1046/j.1471-4159.2003.01920.x. 10.1046/j.1471-4159.2003.01920.x [DOI] [PubMed] [Google Scholar]
- Tanelian D.L, Barry M.A, Johnston S.A, Le T, Smith G.M. Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat. Med. 1997;3:1398–1401. doi: 10.1038/nm1297-1398. [DOI] [PubMed] [Google Scholar]
- Tang X.Q, Tanelian D.L, Smith G.M. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. 10.1523/JNEUROSCI.1263-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman J.R, Kolodkin A.L. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. 10.1126/science.1092121 [DOI] [PubMed] [Google Scholar]
- Terman J.R, Mao T, Pasterkamp R.J, Yu H.H, Kolodkin A.L. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. 10.1016/S0092-8674(02)00794-8 [DOI] [PubMed] [Google Scholar]
- Tom V.J, Steinmetz M.P, Miller J.H, Doller C.M, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004;24:6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. 10.1523/JNEUROSCI.0994-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat. Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. 10.1038/nn1596 [DOI] [PubMed] [Google Scholar]
- Tuttle R, O'Leary D.D.M. Neurotrophins rapidly modulate growth cone response to the axon guidance molecule, collapsin-1. Mol. Cell Neurosci. 1998;11:1–8. doi: 10.1006/mcne.1998.0671. 10.1006/mcne.1998.0671 [DOI] [PubMed] [Google Scholar]
- Wang L.-H, Strittmatter S.M. A family of rat CRMP genes is differentially expressed in the nervous system. J. Neurosci. 1996;16:6197–6207. doi: 10.1523/JNEUROSCI.16-19-06197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide T, Teuber J, Bayer M, Barnekow A. MICAL-1 isoforms, novel rab1 interacting proteins. Biochem. Biophys. Res. Commun. 2003;306:79–86. doi: 10.1016/s0006-291x(03)00918-5. 10.1016/S0006-291X(03)00918-5 [DOI] [PubMed] [Google Scholar]
- Xu X, Ng S, Wu Z.L, Nguyen D, Homburger S, Seidel-Dugan C, Ebens A, Luo Y. Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J. Biol. Chem. 1998;273:22 428–22 434. doi: 10.1074/jbc.273.35.22428. 10.1074/jbc.273.35.22428 [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. 10.1016/j.cell.2004.11.012 [DOI] [PubMed] [Google Scholar]