Abstract
This paper emphasizes several characteristics of the neural control of locomotion that provide opportunities for developing strategies to maximize the recovery of postural and locomotor functions after a spinal cord injury (SCI). The major points of this paper are: (i) the circuitry that controls standing and stepping is extremely malleable and reflects a continuously varying combination of neurons that are activated when executing stereotypical movements; (ii) the connectivity between neurons is more accurately perceived as a functional rather than as an anatomical phenomenon; (iii) the functional connectivity that controls standing and stepping reflects the physiological state of a given assembly of synapses, where the probability of these synaptic events is not deterministic; (iv) rather, this probability can be modulated by other factors such as pharmacological agents, epidural stimulation and/or motor training; (v) the variability observed in the kinematics of consecutive steps reflects a fundamental feature of the neural control system and (vi) machine-learning theories elucidate the need to accommodate variability in developing strategies designed to enhance motor performance by motor training using robotic devices after an SCI.
Keywords: spinal cord injury, rehabilitation, robotics, locomotion, standing, neural control systems
1. Introduction
The title of this paper may induce a myriad of perceptions, most of which will imply physiological mechanisms related to how the adaptation of neural events within the central nervous system responds to a spinal cord injury (SCI). Clearly, after an injury of any part of the neuromuscular system, there are changes in the connectivity of those sensorimotor circuits that generate a motor task. Changes also occur during the subsequent adaptations that follow the injury. In this paper, emphasis will be on the concept of functional rather than anatomical connectivity within the spinal cord. The term functional connectivity will be used to indicate that the likelihood of a given neuron being activated is dependent on its physiological state of ‘readiness’ rather than merely on the existence of an anatomical connection. This emphasis is not to imply that changes in the actual number of synaptic connections cannot or do not occur in response to SCI. In fact, there is good evidence for the presence of such adaptations, and these changes can be associated with an improvement in motor performance capacity following an SCI (Bregman et al. 1997, 2002; Raineteau & Schwab 2001). Instead, this paper will focus on the importance of rapid, and sometimes persistent, changes in functional connectivity between a given combination of spatially and temporally linked sensory and motor circuits that are involved in the generation of posture and locomotion. A measure of functional connectivity, in the context of how we are using this term, is the probability of a specific set of neurons being activated for a given physiological state.
Many correlations have been drawn between anatomical connections and functional recovery post-SCI (Hase et al. 2002; Lee et al. 2004). However, the variability in normal stepping, even under well-controlled conditions, demonstrates the versatility and complexity in the activation of the associated spinal circuitry. We propose that as the physiological states change, the continuous adaptation in functional connectivity brings about routine variability in the activation patterns during repetitive movements, such as stepping. As a result, no two steps are generated by the same combination and sequence of neuronal activation. As the limb trajectory varies from step to step, the precise pattern of activation of the motor pools involved must also vary (figure 1). This variation is reflected in the electromyographic (EMG) signals from normal (Courtine et al. 2005a) and complete spinal animals (Lovely et al. 1990; Edgerton et al. 1992) stepping on a treadmill. Thus, even within the robust size principle of recruitment of motor neurons (Burke & Edgerton 1975; Henneman & Mendell 1981), there remains a significant level of variability in the exact combination and order of motor neurons activated within a given motor pool, and certainly across synergistic motor pools (Cope & Sokoloff 1999), to generate a specific movement.
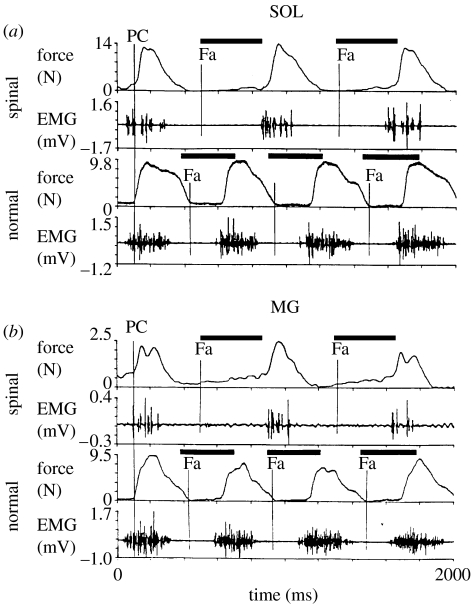
Figure 1.
Force and EMG records from the (a) soleus (sol) and (b) medial gastrocnemius (MG) muscles of a normal, intact cat and an adult spinal cat during stepping on a treadmill at 0.8 m s−1. Bold bars indicate the period of contralateral support. Compared with normal, the spinal cat exhibited a longer cycle period, a steeper decline in force beginning at mid-support, a delay in the onset of flexion at the ankle (Fa), lower peak EMG forces, and clonus in the EMG and force records of both muscles. PC, paw contact (taken from Lovely et al. 1990).
The source of this variability in stepping is undoubtedly derived from both supraspinal as well as spinal neuronal networks. It is also reasonably obvious that the variability in limb kinematics will be greater following an SCI, as recovery of stepping occurs either spontaneously or as a result of motor training. After an SCI, however, the variability in stepping is significantly reduced by motor training (de Leon et al. 1998b). We have proposed that this variability reflects the intrinsic probability of a given assembly of neurons being activated at any given time (Edgerton et al. 2001b). Thus, the underlying explanation for the presence of variability in the limb kinematics during stepping under normal conditions is that whether or not an assembly of neurons is activated is not deterministic at any given instant.
After an SCI, the probability that an appropriate combination of neurons is activated in the appropriate sequence is markedly altered. During the ‘reorganization’ of the spinal circuitry following SCI, these probabilities can become lower or higher depending, to a large degree, on the frequency with which the sensorimotor circuits experience specific patterns of activity. For example, the repetitive performance of a motor task, such as stepping, over a period of weeks increases the probability of completing a successful step (Lovely et al. 1986; Barbeau & Rossignol 1987; de Leon et al. 1998b). It appears from the results of virtually all the studies involving motor training after an SCI that the benefits of step training can be manifested as an increased probability of generating a successful step. At the systems level, a number of motor training-induced biochemical and electrophysiological changes in the spinal cord are associated with improved motor performance after an SCI (de Leon et al. 1999; Tillakaratne et al. 2000, 2002; Cote et al. 2003; Cote & Gossard 2004).
2. Some biochemical and electrophysiological changes associated with improved motor performance in spinal animals
Prior research has identified a number of biochemical and physiological changes in the spinal cord after a complete thoracic spinal cord transection in response to training of a specific motor task. Many of these changes have been reviewed recently (Edgerton et al. 2004). Briefly, the biochemical changes generally reflect an upregulation of both the glycinergic and gamma-aminobutyric acid (GABA)ergic neurotransmitter systems within the lumbosacral spinal cord. The biochemical indicators consist of an increased number of glycinergic receptors (Edgerton et al. 2001a), an increased responsiveness to strychnine administration, an agent that blocks the glycinergic receptor (de Leon et al. 1999), an increased level of glutamic acid decarboxylase (GAD67; Tillakaratne et al. 2000) and improved locomotion when blocking GABAergic inhibition with biccuculine (Robinson & Goldberger 1986a,b). However, an important observation is that the increased level of inhibition in the spinal cord after an SCI can be countered by motor training (Edgerton et al. 2001a; Tillakaratne et al. 2002; Edgerton et al. 2004).
It appears that repetition of a specific motor task can modulate the level of persistent inhibition present in the neural networks that normally generate the motor task. These effects have been demonstrated in spinal animals that have been trained to step (de Leon et al. 1998b) or stand (de Leon et al. 1998a). However, it remains unclear as to how specific excitatory versus inhibitory neural pathways are modulated by repetitive use. Repetitive use of the extensor musculature may downregulate the GAD67 associated with extensor motor neurons, and simultaneously enhance the levels of GAD67 by increasing the inhibition of flexor motor neurons (Tillakaratne et al. 2002). A limitation of these observations is that linking the level of excitation versus inhibition of specific neural pathways to specific adaptations within the different neurotransmitter systems has not been possible to date. Furthermore, there has been relatively little identification of the receptor subtypes that may be associated with the observed level of behavioural performance. These data are further limited as it is not certain whether the observed biochemical changes are simply correlated with the changes in motor performance as opposed to there being a cause and effect relationship. Further studies are needed to address these issues.
Electrophysiological changes have also been observed in chronic spinal animals, and there is strong evidence that the efficacy of selected neuromotor pathways can be modified by repetitive training of a motor task. For example, there is an improved coordination of motor pools controlling the hindlimb musculature following step training in spinal animals, as shown by EMG bursting patterns (Lovely et al. 1990). Likewise, the step training greatly improves the transmission in polysynaptic excitatory group I load pathways (Cote et al. 2003) that convey locomotor drive to extensor motor neurons, and thus could contribute to improved recovery of weight bearing during stepping in spinal animals. The mean amplitude of excitatory post-synaptic potentials has similarly been reported to increase in response to activation of skin sensory receptors located under the paw of chronic spinal cats that have been trained to step (Cote & Gossard 2004). Recent observations also indicate that improved stepping following training in complete spinal rats correlates with the peak amplitude of the segmental excitatory post-synaptic and action potentials and level of afterhyperpolarization depth of the motor neurons recruited during locomotor activity (Petruska et al. 2004).
Based on the results of these electrophysiological studies, it appears that in chronic spinal animals, a number of spinal neural pathways can respond specifically to step training. Accordingly, it is likely that after repetitive exposure of the spinal cord to a given motor task, significant alterations may occur in the transmission of many, if not all, sensorimotor pathways caudal to the lesion. Such task-dependent functional plasticity of the spinal motor infrastructure could, in turn, contribute to the decrease in the intrinsic variability in performing a motor task (de Leon et al. 1998b), i.e. there would be an increase in the probability of activating specific motor pathways, and therefore specific functional sets of neurons, to accomplish the required task (Edgerton et al. 2001b). These results suggest an improved efficacy of the interneurons that are responsible for coordinating motor pools, e.g. Ia interneurons that provide reciprocal inhibition between antagonistic motor pools. Direct measurements of decreases followed by increases in the excitability of synapses associated with Ia interneurons in response to spinal cord transection have been observed (Valero-Cabre et al. 2004a,b). On the other hand, significant increases in the excitability of lumbar monosynaptic reflexes have been reported (Thompson et al. 1998).
Thus, it seems reasonable to hypothesize that the observed biochemical and electrophysiological adaptations of spinal animals, associated with step training, are not due to an induction of a specific set of synaptic events required for the acquisition of stepping ability. Rather, these adaptations result from the increased probability of the neural circuitries within the spinal cord of generating a successful step.
3. General control demands: hierarchically designed networks
Several observations demonstrate that supraspinal control can be, and probably often is, relatively non-specific. Supraspinal input can instruct the spinal cord to walk by providing a relatively non-specific tonic input, leaving the detailed decisions of which neuronal systems have to be activated at the spinal level. Stepping can be induced in decerebrated animals via tonic stimulation of the mesencephalic locomotor region (Shik et al. 1966). Fictive locomotion can be generated via stimulation of the dorsal roots of the spinal cord (Sjostrom & Zangger 1976). Locomotion can be induced pharmacologically in complete spinal animals (Rossignol & Barbeau 1993; de Leon et al. 1999; Antri et al. 2002; Orsal et al. 2002), and chronic complete, low thoracic spinal animals can be trained to step even without any pharmacological enhancement (Lovely et al. 1986; Barbeau & Rossignol 1987; de Leon et al. 1998b). Another illustration of the non-specific nature of the signals (in this case, from the periphery) that generate walking is the locomotor-like movements that can be elicited in humans in a recumbent position by applying non-specific tonic vibration to the relaxed leg musculature (Gurfinkel et al. 1998). These widely different manipulations represent an impressive array of different techniques that change the physiological state of the spinal cord, all having a remarkably similar effect, i.e. they induce or improve stepping ability.
The generality of the supraspinal, and even spinal, commands is also apparent from the strong interrelationships among the kinematics of multiple joints within and across limbs during locomotion in intact, as well as complete and incomplete SCI, animals (figure 2). This stereotypical output implies a close link between the neuronal systems controlling each of these joints and all the musculatures associated with their dynamics. Such a high intrinsic coupling in the generation of limb oscillation also simplifies the details required by the brain to generate a complex motor task, such as stepping at a range of speeds (Bianchi et al. 1998), grades (de la Torre & Goldsmith 1990) and directions (Courtine & Schieppati 2004). Furthermore, this stereotypical output supports the concept of automaticity in the control of locomotion (Orlovsky & Feldman 1972). Briefly, automaticity is the ability to generate a range of motor tasks, such as stepping and standing, in response to highly predictable ensembles of sensory stimuli from the periphery and motor commands from the brain. Considerable automaticity remains within the spinal cord in the absence of supraspinal input.
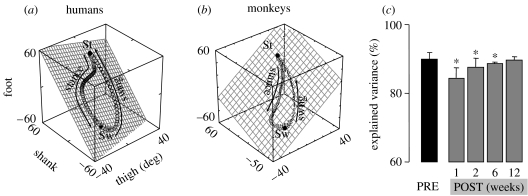
Figure 2.
Coupling in the generation of limb movements during walking in humans and monkeys. (a) When plotted in a three-dimensional space, the angular oscillation of thigh, shank and foot segment with respect to the direction of gravity, i.e. elevation angles, covaries close to a plane, both during (a) human and (b) monkey locomotion. The gait loop evolves in the counterclockwise direction. The onset of stance (St) and swing (Sw) are indicated. (c) The degree of coupling among limb movements is evaluated by applying a principal component (PC) analysis on the elevation angles of hindlimb segments (thigh, shank and foot). Mean (s.d.) values of the variance explained by the first PC during treadmill locomotion performed pre-lesion (PRE) and 1, 2, 6 and 12 weeks after a unilateral lesion to the thoracic dorsolateral column (POST) is shown for three monkeys. Asterisks indicate significant difference between pre- and post-lesion values. The high variance accounted for by the first PC reflects the high degree of coupling in the neuronal systems that generate the oscillation of the limbs during stepping both in intact and spinal cord-injured animals (adapted from Courtine et al. 2005a).
One source of such automaticity is found in the organization of the spinal circuits generating the motor patterns for walking. Stimulation of such neural circuits, often referred to as central pattern generators (CPGs), produces rhythmic alternating flexor and extensor activities in several vertebrate models, e.g. lamprey eels, neonatal rats or adult cats (Arshavsky et al. 1997; Grillner 2002; MacKay-Lyons 2002) that mimic locomotion. However, the structural organizations of these neural circuitries in mammals are not yet fully understood. Nonetheless, even within the most automated action from CPGs, automatic adjustments can be made by varying levels of hierarchical control. For example, extensor or flexor muscle activity can be altered independently during fictive locomotion without affecting the ongoing locomotor rhythm (Lafreniere-Roula & McCrea 2005). This observation suggests that the spinal-generated rhythmical input drives multiple pattern formation modules, and activities of these modules can be modified by other sources, e.g. sensory or supraspinal inputs. Such hierarchical control would introduce both stereotypical and ongoing adjustments (variability) in the motor output to adjust locomotor kinematics. This hierarchal control can range from volitional circuits to extreme automaticity, i.e. CPGs. Although there is still no direct evidence for the existence of locomotor spinal circuits that display CPG properties in humans, Dimitrijevic et al. (1998) reported that non-patterned electrical stimulation of the posterior structures of the lumbar spinal cord in subjects with complete, long-term SCI can induce rhythmic, alternating stance and swing phases of the lower limbs.
This predictability of a stereotypic stepping pattern may seem contradictory to the concept noted earlier that variability in stepping reflects an important feature of the neural control system. However, it should be recalled that spinal interneurons receive input from supraspinal, as well as from spinal, sources, and from the periphery. Nevertheless, given the apparent hierarchical organization of the neuronal systems that generate motor patterns and interpret sensory information as implied earlier, there are multiple combinations and levels of neuronal control systems that underlie the variability observed during stepping while simultaneously maintaining a very high probability of success from step to step (figure 3).
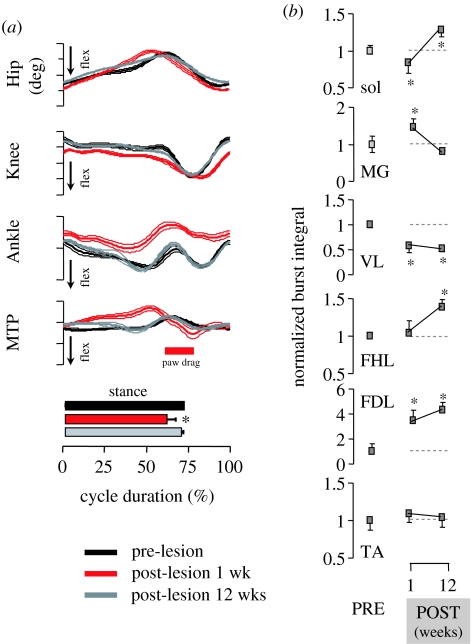
Figure 3.
(a) Mean (s.d.) waveforms of each joint angle for the hindlimb ipsilateral to the lesion side recorded during treadmill locomotion (0.45 m s−1) before (pre-lesion) and 1 and 12 weeks after (post-lesion) a unilateral interruption of the lateral CST in the thoracic spinal cord of adult Rhesus monkeys (n=2). The horizontal bars at the bottom indicate the mean (s.d.) value of the stance phase duration. (b) Mean (s.d.) values of EMG burst integrals for all recorded muscles. Sol, soleus; MG, medial gastrocnemius; VL, vastus lateralis; FHL, flexor hallucis longus; EDL, extensor digitorum longus; TA, tibialis anterior. Values are normalized to the pre-lesion baseline (dashed lines) computed as the mean value of muscle activity during pre-lesion locomotion. Asterisks indicate significant difference between pre- and post-lesion values (adapted from Courtine et al. 2005b).
It is worth noting that recovery of locomotion kinematics after an incomplete SCI in the monkey to levels observed pre-lesion (figure 3) does not imply re-establishment of pre-lesion muscle synergies, but instead reflects novel activation patterns of interneurons and motor pools that may be possible as a result of the hierarchal features noted earlier (Courtine et al. 2005b). It has also been found that new motor patterns underlie the learning of foot kinematics that are similar to those of non-disabled individuals during treadmill stepping following training in humans with an incomplete or complete SCI (Wernig et al. 1995; Grasso et al. 2004). Such findings provide evidence that successful stepping, as defined kinematically, can be achieved through activation of a variety of spinal motor neurons, and that there is no fixed locomotor circuitry for the generation of stepping in mammals, including primates.
Besides being able to accommodate the control that can be exerted by the brain on specific interneuronal assemblies or motor pools, even more direct neural connections must be present to control specific muscle units or combinations of units (figure 4). It is generally accepted, at least in primates, that there are direct projections from the corticospinal tract (CST) to spinal motor neurons (cortico-motoneuronal connections), although a small portion of the corticospinal projections actually represent a direct target to motor neurons (Lacroix et al. 2004). The cortical projection to the spinal cord can be an important source of modulation in the production of skilled locomotor movements, such as stepping over an obstacle or the precise positioning of the paw during walking in cats and primates (Lawrence & Kuypers 1968; Georgopoulos & Grillner 1989; Drew et al. 2002; Courtine et al. 2005a,b). Theoretically, corticospinal input to subsets of interneurons that control specific combinations of muscle units in specific motor pools (Fetz et al. 2000; Drew et al. 2002; Lemon et al. 2004) could allow precise voluntary activation and frequency control of groups of motor units (Kuypers 1978).
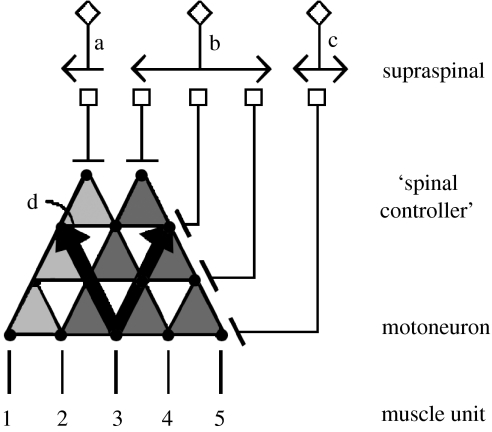
Figure 4.
Cartoon depicting several features of the sensorimotor control of movement. The cartoon illustrates the possibility of a supraspinal control centre with neurons projecting to control level neurons (‘spinal controllers’ of movements of differing complexities) that would project to a group of synergistic motor pools, muscles and muscle units. In cases illustrated by the projection of neuron a or neuron b, specific control of a small group of motor units might be unnecessary in executing a generalized motor programme to control stepping. The numbers 1–5 denote five muscle units. The dots embedded in the triangles represent individual neurons. Activation of neuron a would result in muscle units 1–4 being recruited. Neuron b would recruit muscle units 2–5, whereas neuron c would recruit only muscle unit 5. On the other hand, there can be even more selective control of motor units as illustrated with neuron c. At least for some muscle groups in some species, there may be direct supraspinal connections to some motor pools as well as the more generalized command neurons that exert more general control signals among motor pools. Two sets of divergent triangles are illustrated to point out the flexibility in modulating the set of muscles that may be recruited for a given movement. One can also view the upright triangles in the reverse direction (see arrows projecting upward, labelled as d), symbolizing a single sensory receptor projecting rostrally and diverging markedly, thus illustrating a single sensory receptor that could provide excitatory or inhibitory input to a large number of neurons within the spinal cord. This sensory information, in turn, may further diverge or even converge to specific supraspinal locations. The diverging circuits that enable different levels of control of multiple muscles also provide a means of detailed conscious control of fine movements, while also providing mechanisms for executing more general and predictable tasks, even when they are considerably complex.
4. Hierarchical command combined with ‘smart’ sensory control
The progression from a supraspinal motor command to the detailed control of hundreds of thousands of muscle fibres reflects a phenomenal rostral-to-caudal anatomical and physiological divergence. Although convergence of inputs from cortical cell populations onto motor neuron assemblies exists, there is also a remarkable divergence in how the supraspinal drive can affect various interneuronal circuits (figure 4). Similarly, projections of the signals from specific sensory receptors in the periphery to the spinal cord networks and brain are highly divergent. For example, a single muscle spindle alerts thousands of neurons within the spinal cord (and even more in the brain) that a signal related to the physical environment of the mechanoreceptor has been generated (Scott & Mendell 1976). It is inevitable that all the sensory information from the periphery project to the networks of neurons within the spinal cord. At the same time, it is apparent that the signals from multiple receptors merge and project at some level to common, as well as unique, combinations of neurons within the spinal cord. Every unique combination of neurons can, in turn, readily recognize complex and very specific sensory patterns that can trigger the appropriate motor responses for a given pattern of sensory information. In other words, a given pattern of sensory information provides very specific and recognizable information to the neurons that eventually generate the appropriate motor response to that given ensemble of sensory information. To what degree this extensive divergent and convergent information from the periphery is processed and integrated within the spinal networks prior to relaying this information to the brain is unknown, but it is readily apparent that these complex patterns of sensory input provide a continuous stream of critical information for the ongoing control of specific motor responses, such as stepping and standing.
These observations are not meant to imply that the neural circuits within the spinal cord segments have a lesser role than the brain circuits in controlling stepping. For example, the ability of the spinal circuitry to generate rhythmic motor patterns that mimic locomotion without any sensory input, i.e. fictive locomotion, is clear (Grillner 2003). This fictive central pattern generation, however, cannot make any adjustments to changes in its environment and, therefore, there are no mechanisms to change stepping frequency, modulate the appropriate level of load bearing, or adjust to any, even slight, perturbation. In fact, not only does the afferent input provide the spinal locomotor circuits with information related to unexpected events, but the ongoing sensory flux also contributes substantially to the activation of motor neurons, even under normal walking conditions (Pearson 2004). On the other hand, this central pattern generation, combined with its massive online sensory information processing capability, can effectively generate motor tasks such as stepping and standing without input from the brain (Edgerton et al. 2004).
5. Sensory modulation of motor tasks
What is the evidence that the sensory information derived from the limbs during posture and locomotion represent a critical and primary influence on motor control in complete spinal animals? Several experiments demonstrate that sensory information can define most details of all postural and locomotor movements. Administration of strychnine, which blocks glycinergic inhibition, at a dose that did not generate spontaneous rhythmic motion of the hindlimbs, facilitated consistent, full weight-bearing treadmill stepping of the hindlimbs in chronic complete spinal cats that otherwise could not step (de Leon et al. 1999). In addition, the rate of stepping was modulated to accommodate the speed of the treadmill belt. These results demonstrate that the spinal cord was not induced to generate rhythmic activity and stepping by strychnine itself, but that strychnine changed the physiological state of the spinal neural circuits so that the sensory information could be processed and transformed with sufficient accuracy to control locomotion over a range of speeds and levels of loading. Similar observations have been made after the administration of quipazine, a serotonergic agonist, to mice having a complete SCI (Fong et al. 2003, 2005). When combined, these results clearly demonstrate that the sensory input associated with standing and stepping generates successful and remarkably adaptive control of posture and locomotion in the absence of supraspinal input. Under these conditions, this adaptive control cannot be solely attributed to central pattern generation, i.e. repetitive cycles of flexion and extension. A very important additional feature of the neural circuitry that generates these patterns is its ability to interpret the sensory input in a manner that becomes meaningful to the success of the hindlimbs in responding to its environment.
Other observations support the importance of the interaction between CPG and sensory processing. Results similar to those described for strychnine earlier were observed when the dorsum of the lumbosacral spinal cord of complete mid-thoracic spinal rats (Ichiyama et al. 2005) and cats (Gerasimenko et al. 2003, 2005) was stimulated via epidural electrodes. In this case, a tonic general stimulation of modest intensity applied to the dorsum of the spinal cord did not induce any rhythmic, step-like motion. When the hindlimbs were placed on a moving treadmill belt, however, the animals stepped at a rate consistent with the speed of the treadmill belt. Previous experiments have also demonstrated that complete spinal cats receiving tonic electrical stimulation of the dorsum of the lumbar spinal cord can step backwards when their hindlimbs are placed on a treadmill belt moving forward (Gerasimenko et al. 2003). These data demonstrate that detailed complex signals that drive motor pools in a highly coordinated fashion can be derived from very general patterns of stimuli to the lumbosacral spinal cord. Furthermore, these experiments clearly indicate that the sensory information provided to the spinal cord essentially defines the type of motor task that will be performed, as well as the characteristics of the motor pattern associated with the task.
6. Implications of synaesthesia for rehabilitation
Synaesthesia is the merging of different modes of sensation received by the nervous system. Each mode of sensation, e.g. hearing, seeing or touching, is generally thought to be very closely linked with specific types of sensory receptors providing information to areas of the brain that have the capability to process sound, light or mechanical perturbation, respectively. There are many examples of how sensory modes can be merged or exchanged with respect to a predictable perception generated by a sensor. For example, Cytowic (2002) described a subject who was born blind, but later regained vision. After the vision was restored, this individual had difficulty in seeing an object without touching it with his hands. For example, when he saw a gorilla at a zoo, he could not understand its posture and movements until he had felt a statue of a gorilla. There are also impressive examples of utilizing this synaesthetic capability to rehabilitate individuals who had their vestibular system destroyed by medication. Individuals who have extreme difficulty in standing and walking as a result of a pharmacologically induced loss of vestibular function can rapidly regain excellent control by substituting the vestibular information with the output from an accelerometer placed on the head. In these cases, the electrical output from the accelerometer was passed via a wire leading to the surface of the tongue (Tyler et al. 2003). In some way, the subject's tongue was able to ‘calibrate’ the accelerometer output with visual and, presumably, head, neck, trunk and lower limb proprioceptive signals, functionally merging the information so that virtually normal posture and locomotion could be sustained. Furthermore, it is interesting that once the accelerometer device was removed, the renewed control of posture and movement was maintained for days or even weeks. Essentially, the brain of this patient was able to substitute electrical signals derived from an accelerometer and integrate this information into the circuitry that coordinates the musculature of the head, neck, trunk and lower limbs that performs postural and locomotor tasks.
With respect to the topics of this paper, the concept of synaesthesia may be important in several ways when developing strategies to recover sensorimotor function. Perhaps the most important point from these observations on synaesthesia is the degree to which the brain can reorganize its function, even in individuals without any detectable neural dysfunction. This raises the question as to what extent we can learn to substitute one sensory mode for another in facilitating recovery of function after an SCI. Following a severe, functionally incomplete SCI, for example, to what extent can the brain reorganize itself to use the small number of intact fibres to functionally project signals to the spinal cord below the lesion? In other words, can a residual source of control from the brain be modified to control a function that is different from its normal action? A second important point that can be derived from these examples of synaesthesia is that two modes of sensory information can be substituted, or at least merged, to improve sensorimotor function.
Another example of functional sensorimotor reorganization after an injury is the perception of the phantom limb, with a subject sensing the presence, and even the touch, of an arm that has been amputated (Kuiken et al. 2004). Subjects who have had an arm amputated can learn to control prosthetic devices using the EMG signals derived from intact muscles of the shoulder or from shoulder muscles that have been re-innervated with nerve branches which originally innervated muscles controlling hand and wrist movements. Interestingly, touching the skin overlying these re-innervated muscles gives the subject the sensation of touching the skin overlying the hand or wrist, i.e. the region that it normally innervates.
All these observations indicate that the potential for reorganization of sensorimotor function after an SCI has not been fully realized as a rehabilitative strategy. Combining this potential for plasticity with new technologies, such as virtual reality and smart robotic devices, seems to be a feasible and logical direction for future efforts in enhancing recovery of sensorimotor function following a wide range of neuromotor disorders. For example, robotic devices can be used to provide more precise and versatile training to SCI subjects (mentioned later).
7. An engineering perspective on the importance of variability in a control system
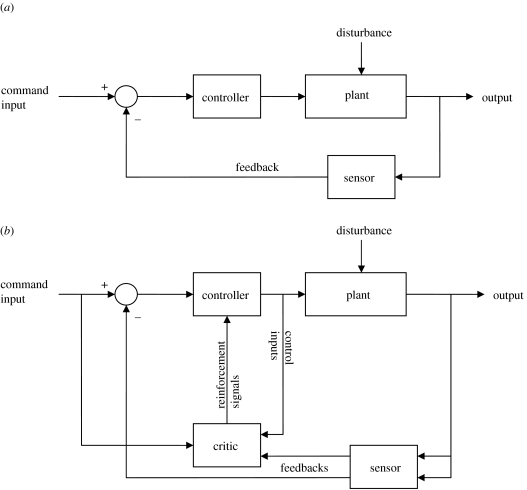
The automaticity in the control of locomotion as well as the importance of sensory feedback in neuromuscular systems (mentioned earlier) resemble that of a simple mechanical control system, where the goal is to use feedback to guide the performance of a movement such that the error between the command input and the resulting output is minimized (Zhou et al. 1996; figure 5a). Although such controllers have proven to be versatile and reliable in the engineering world, it is unlikely that biological neuromuscular systems are controlled in such a manner. Firstly, such a control system cannot adapt to changes in the environment and can become unstable quickly when there are large disturbances. Secondly, such a control system lacks the ability to learn to improve its performance from past experience. In contrast, the vertebrate spinal cord neural circuitry is capable of adjusting to disturbances (Barbeau et al. 2002) and learning from repetitive training (Edgerton et al. 1992; de Leon et al. 1998a,b), even in the absence of supraspinal control.
Figure 5.
(a) Block diagram for a simple mechanical controller. In neuromuscular systems, the controller would be the motor neurons, the plant would be the muscles and the sensor would be all the proprioceptive feedbacks to the motor neurons. The information provided by the sensor is a negative feedback (denoted by the − sign) and is used to minimize the error between the output of the plant and the command input, a positive input (denoted by the + sign), the command input. In controls, the disturbance generally refers to unmodelled dynamics of the plant. However, in neuromuscular systems, this would represent perturbations that the system might encounter. (b) Block diagram for an adaptive controller incorporating reinforcement learning. In neuromuscular systems, the controller, plant and sensor will be the same as in (a). The critic will be the input from all the interneurons, e.g. Ia, Ib, Renshaw cells, etc. affecting the efficacy and excitability of the motor neuron (the controller), which is represented by the reinforcement signals.
Another robust property of the normal neural control system of locomotion is variability. Even with this variability, the success of hundreds or even thousands of steps can be predicted in the uninjured individual under normal circumstances. Following an SCI, this variability in both the activation patterns and the resulting kinematics of the hindlimbs increases, and the probability of generating consecutive successful steps will be quite low and, in many cases, near zero. Step training reduces the variability in the kinematics of the limb motions (de Leon et al. 1998b). Presumably, increasing the occurrence of a given pattern of sensory information associated with load-bearing stepping increases the probability of pattern recognition by the neural networks that are linked to the sensory patterns. In addition, frequent occurrence increases the probability of generating a predictable kinematic pattern whenever this sensory pattern is recognized. In essence, the likelihood of a given set of neurons being activated in a given condition may change from near randomness to one that is highly predictable, reflecting properties that are typical features of learning systems.
Most machine learning theories of learning systems are developed based on ‘supervised learning’, a process of learning from examples provided by a knowledgeable instructor, where the input and output examples are provided as a classified pair (Anderson et al. 1983). Although this type of learning is significant in knowledge acquisition, this alone is not adequate to explain locomotor learning. In a neuromuscular system, most of the learning processes are unsupervised and involve skill refinement. Although the idea has been around for decades, a relatively new set of learning theories called ‘reinforcement learning’ has been developed in recent years to address this problem.
In contrast to supervised learning, reinforcement learning emphasizes learning feedback that evaluates performance without providing a standard of correctness in the form of behavioural targets, i.e. reinforcement learning gives an index of how well the system performed relative to its previous trials without giving any indication of the correct response (Barto 1994). Therefore, to maximize the reward, the reinforcement-learning paradigm requires the system to actively try alternatives, evaluate the results, and then use a selection mechanism to guide the behaviour towards the best alternative. The fundamental process is analogous to ‘trial and error’. However, the search is not random or undirected. Instead, the system takes into account results acquired from previous trials to decide how and where the next increment in stepping will be taken, choosing a path that will give the highest probability for future success. In this modern concept of reinforcement learning, randomness is often utilized to create behavioural varieties, which are call explorations. The consequential actions, however, are strongly guided by the evaluation of earlier experiences and often the system will prefer an option that has produced favourable results in the past; such a move is call exploitation. As a result, reinforcement-learning algorithms are selection processes, but there must be variability in the action-generation process so that the consequences of alternative actions can be compared to select the best alternative.
By incorporating reinforcement learning into a control system, such a system is able to use feedback to evaluate the performance and improve subsequent movement by changing the controller itself (figure 5b). This is analogous to sensory inputs changing the efficacy of spinal circuitries that control locomotion as a result of training. In most artificial reinforcement-learning systems, the critic's output at any time is a number that scores the controller's behaviour; the higher the number, the better the behaviour. If the behaviour being scored is immediately preceding a subsequent unit of behaviour produced by the controller based on the critic's score, there must be enough variability in the controller's behaviour so that the critic can evaluate many alternatives for this process to work. A learning mechanism must then adjust the controller's behaviour so that it tends towards behaviours that are favoured by the critics (Sutton & Barto 1998). Applying these learning theories to the biological system, it is apparent that it is necessary to have a system where there is variability for unsupervised learning to occur.
8. Merging of sensory and robotic control
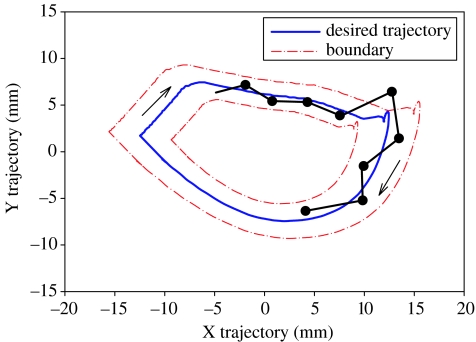
As de Leon et al. have demonstrated, robotic devices can be used as effective training tools for SCI subjects (de Leon et al. 2002a,b) and commercially available robotic orthotics, such as the Lokomat, are already available to facilitate the rehabilitative training of SCI and stroke patients, and have shown promising results (Hesse et al. 2003). One strategy for robotically training an SCI subject is to generate a fixed pattern that mimics the kinematics and trajectory of a normal step (centre blue bold line in figure 6). This idealized step could be derived by generating a mean kinematic and mechanical training trajectory based on a number of steps in an intact, normal animal (fixed trajectory training). In other words, every step would be mechanically identical. In such a situation, the spinal circuitry of a complete spinal subject would essentially have no control of the step trajectory imposed by the robotic arms. This type of control for training subjects with a complete SCI, theoretically, may be counterproductive. There may be several fundamental reasons for this to be counterproductive. Firstly, a complete robotic control does not accommodate the variability intrinsic to the neural circuits that normally generate stepping (mentioned earlier). Consequently, at any given phase of the step cycle, it is unlikely that the motor pattern, formed on the basis of the intrinsic motor pattern combined with the sensory patterns associated with each phase of the step cycle, would exactly match the idealized trajectory defined by the robot arm control. Secondly, fixed trajectory training seems to eventually cause an extensive level of habituation to the sensory information generated, to the point that there is little or no response to the invariant sensory input imposed by the robotic training device and, therefore, little or no motor output is generated, i.e. a state of ‘learned helplessness’. This would result in a less robust, rather than an enhanced, motor response (Wirz et al. 2005). Thirdly, fixed trajectory training does not allow the damaged spinal circuitry to learn to regain autonomous control, i.e. formulate high probability, temporally important sensory motor synaptic events that generate stepping.
Figure 6.
Schematic of a semi-active fixed-trajectory paradigm for step training, where the desired limb trajectory (blue) is bounded by both the inner and the outer boundaries (red). The actual trajectory (black) that the neural circuits might induce is allowed to vary within the boundary. However, once the trajectory falls outside the boundary, the robot will actively bring it back within the boundaries. The black line with periodic dots illustrates the potential positions that the intrinsic neural control might choose to generate for any given bin time. The probability that the neural control would move the limb to the exact position defined by the blue line, representing a fixed trajectory, is highly unlikely. As a result, theoretically, the neural control system is continuously disrupted by the fixed trajectory paradigm. This fixed trajectory, therefore, does not allow the neural control circuitry to respond to any of its intrinsic activation patterns, but rather forces the intrinsic circuitry to continuously respond to external perturbations. This strategy for control would seem to unnecessarily disrupt the spinal circuitry and in the process minimize or even preclude the intrinsic circuitry from interpreting relevant proprioceptive information required to generate a solution (i.e. make choices) and, thus, presumably prevent the circuitry from meaningful learning phenomena.
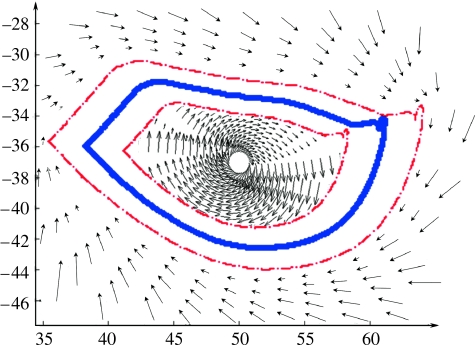
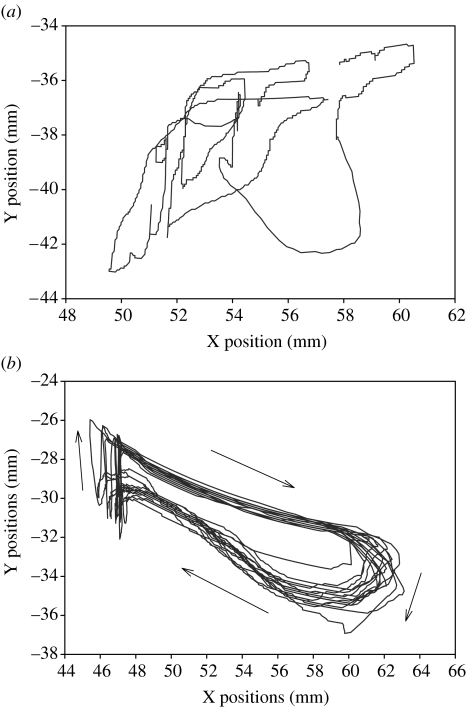
We have found a more effective method using robotics to enhance recovery of locomotor control largely by mimicking some of the basic principles used by physical therapists, such as ‘assist as needed’ training strategies. Training with robotic control algorithms that provide an assist as needed approach resulted in a greater improvement in stepping than training with a fixed pattern. We refer to this principle of robotic control as ‘soft control’, which is graphically illustrated in figure 7. Conceptually, the robotic arms generate a force field that pushes the leg towards the target trajectory when the deviation is greater than a predetermined amount. The magnitude of the force field is proportional to the distance away from the desired trajectory that the leg has moved. In essence, the robot performs similarly to a therapist providing manual assistance to a patient stepping on the treadmill with a weight-supporting device. With the ‘soft control’ approach, the greater the deviation from the desired trajectory, the more the assistance needed to correct the trajectory. When adult, complete spinal mice trained with this soft control paradigm were compared with spinal mice trained with a fixed trajectory training paradigm, the soft control trained mice stepped significantly better (figure 8). The fact that the soft control paradigm was more effective than the fixed control paradigm is consistent with the many demonstrations that variability is a critical component that characterizes all neurally controlled movements.
Figure 7.
Soft robotic control schematics on how the semi-active control paradigm for step training is implemented. A moving window (red) bounds the desired trajectory (blue) of the mouse limb during stepping. Within the window, the robotic arm allows the mouse to vary its movement. However, when the neural control desired trajectory falls outside the window, the robot will experience a convergent velocity field that actively returns the mouse's limbs back within the window. This type of soft control is thought to approximate the ‘assist as needed’ approach used by experienced therapists (modified from Cai et al. 2005).
Figure 8.
(a) Trajectory plot of the ankle of an untrained adult transected mouse without any drug administration attempting to step on a moving treadmill for 10 s at a rate of 3 cm s−1. (b) Trajectory plot of the ankle of an adult transected mouse successfully stepping on a moving treadmill for 10 s at a rate of 3 cm s−1 after four weeks of step training with quipazine (0.5 mg kg−1) daily for 10 min d−1, 5 days per week. Arrows in (b) are showing the direction of ankle movements. Note the more consistent trajectories in the trained versus untrained mouse. The untrained mouse often failed to execute any successful plantar placing steps as shown in (b).
9. Conclusion
In this paper, we have emphasized the high degree of plasticity of the functional connectivity within the spinal sensorimotor infrastructure in response to an injury and/or step training. We have pointed out that the neural processes involved in the generation of standing and stepping are extremely flexible functionally, and are unlikely to be due to a hardwired, fixed neuronal architecture. Instead, there are many possible pathways and combinations of circuits that can generate movement. This view implies that locomotor-related neural circuits are better defined as the probability of a given assembly of synapses to be activated appropriately to produce a successful step rather than simply by the presence of anatomical connectivity. Such functional flexibility in the activation of the sensorimotor circuits for stepping, in turn, would be responsible for the variability inherent to gait patterns. This variability, however, reflects a fundamental feature of the neural control system that should be recognized and accommodated in developing strategies designed to enhance motor performance by motor training using robotic devices after an SCI.
Acknowledgments
This work was supported, in part, by NIH grant NS16333.
Footnotes
One contribution of 13 to a Theme Issue ‘The regenerating brain’.
References
- Anderson J.R, Michalski R.S, Carbonell J.G, Mitchell T.M. M. Kaufmann; Los Altos, CA: 1983. Machine learning: an artificial intelligence approach. [Google Scholar]
- Antri M, Orsal D, Barthe J.Y. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. 10.1046/j.1460-9568.2002.02088.x [DOI] [PubMed] [Google Scholar]
- Arshavsky Y.I, Deliagina T.G, Orlovsky G.N. Pattern generation. Curr. Opin. Neurobiol. 1997;7:781–789. doi: 10.1016/s0959-4388(97)80136-5. 10.1016/S0959-4388(97)80136-5 [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. 10.1016/0006-8993(87)91442-9 [DOI] [PubMed] [Google Scholar]
- Barbeau H, Fung J, Leroux A, Ladouceur M. A review of the adaptability and recovery of locomotion after spinal cord injury. Prog. Brain Res. 2002;137:9–25. doi: 10.1016/s0079-6123(02)37004-3. [DOI] [PubMed] [Google Scholar]
- Barto A.G. Reinforcement learning control. Curr. Opin. Neurobiol. 1994;4:888–893. doi: 10.1016/0959-4388(94)90138-4. 10.1016/0959-4388(94)90138-4 [DOI] [PubMed] [Google Scholar]
- Bianchi L, Angelini D, Orani G.P, Lacquaniti F. Kinematic coordination in human gait: relation to mechanical energy cost. J. Neurophysiol. 1998;79:2155–2170. doi: 10.1152/jn.1998.79.4.2155. [DOI] [PubMed] [Google Scholar]
- Bregman B.S, Diener P.S, McAtee M, Dai H.N, James C. Intervention strategies to enhance anatomical plasticity and recovery of function after spinal cord injury. Adv. Neurol. 1997;72:257–275. [PubMed] [Google Scholar]
- Bregman B.S, Coumans J.V, Dai H.N, Kuhn P.L, Lynskey J, McAtee M, Sandhu F. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog. Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- Burke R.E, Edgerton V.R. Motor unit properties and selective involvement in movement. Exerc. Sport Sci. Rev. 1975;3:31–81. [PubMed] [Google Scholar]
- Cai L.L, Fong A.J, Otoshi C.K, Liang Y.Q, Cham J.G, Zhong H, Roy R.R, Edgerton V.R, Burdick J.W. Effects of consistency vs. variability in robotically controlled training of stepping in adult spinal mice. Proc. Int. Conference Rehab. Robotics. 2005;9:575–579. [Google Scholar]
- Cope T.C, Sokoloff A.J. Orderly recruitment tested across muscle boundaries. Prog. Brain Res. 1999;123:177–190. doi: 10.1016/s0079-6123(08)62855-1. [DOI] [PubMed] [Google Scholar]
- Cote M.P, Gossard J.P. Step training-dependent plasticity in spinal cutaneous pathways. J. Neurosci. 2004;24:11 317–11 327. doi: 10.1523/JNEUROSCI.1486-04.2004. 10.1523/JNEUROSCI.1486-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote M.P, Menard A, Gossard J.P. Spinal cats on the treadmill: changes in load pathways. J. Neurosci. 2003;23:2789–2796. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Schieppati M. Tuning of a basic coordination pattern constructs straight-ahead and curved walking in humans. J. Neurophysiol. 2004;91:1524–1535. doi: 10.1152/jn.00817.2003. 10.1152/jn.00817.2003 [DOI] [PubMed] [Google Scholar]
- Courtine G, Roy R.R, Hodgson J, McKay H, Raven J, Zhong H, Yang H, Tuszynski M.H, Edgerton V.R. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus) J. Neurophysiol. 2005a;93:3127–3145. doi: 10.1152/jn.01073.2004. 10.1152/jn.01073.2004 [DOI] [PubMed] [Google Scholar]
- Courtine G, Roy R.R, Raven J, Hodgson J, McKay H, Yang H, Zhong H, Tuszynski M.H, Edgerton V.R. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta) Brain. 2005b;128:2338–2358. doi: 10.1093/brain/awh604. 10.1093/brain/awh604 [DOI] [PubMed] [Google Scholar]
- Cytowic R.E. MIT Press; Cambridge, MA: 2002. Synesthesia: a union of the senses. [Google Scholar]
- de la Torre J.C, Goldsmith H.S. Collagen-omental graft in experimental spinal cord transection. Acta Neurochir (Wien) 1990;102:152–163. doi: 10.1007/BF01405432. 10.1007/BF01405432 [DOI] [PubMed] [Google Scholar]
- de Leon R.D, Hodgson J.A, Roy R.R, Edgerton V.R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998a;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon R.D, Hodgson J.A, Roy R.R, Edgerton V.R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 1998b;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- de Leon R.D, Tamaki H, Hodgson J.A, Roy R.R, Edgerton V.R. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- de Leon R.D, Kubasak M.D, Phelps P.E, Timoszyk W.K, Reinkensmeyer D.J, Roy R.R, Edgerton V.R. Using robotics to teach the spinal cord to walk. Brain Res. Brain Res. Rev. 2002a;40:267–273. doi: 10.1016/s0165-0173(02)00209-6. 10.1016/S0165-0173(02)00209-6 [DOI] [PubMed] [Google Scholar]
- de Leon R.D, Reinkensmeyer D.J, Timoszyk W.K, London N.J, Roy R.R, Edgerton V.R. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog. Brain Res. 2002b;137:141–149. doi: 10.1016/s0079-6123(02)37013-4. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M.R, Gerasimenko Y, Pinter M.M. Evidence for a spinal central pattern generator in humans. Ann. NY Acad. Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. 10.1111/j.1749-6632.1998.tb09062.x [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res. Brain Res. Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. 10.1016/S0165-0173(02)00200-X [DOI] [PubMed] [Google Scholar]
- Edgerton V.R, Roy R.R, Hodgson J.A, Prober R.J, de Guzman C.P, de Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J. Neurotrauma. 1992;9(Suppl. 1):S119–S128. [PubMed] [Google Scholar]
- Edgerton V.R, et al. Retraining the injured spinal cord. J. Physiol. 2001a;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. 10.1111/j.1469-7793.2001.0015b.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton V.R, Roy R.R, De Leon R. Kluwer Academic Publishers; Boston, UK: 2001b. Neural Darwinism in the mammalian spinal cord. Spinal cord plasticity: alterations in reflex function. [Google Scholar]
- Edgerton V.R, Tillakaratne N.J, Bigbee A.J, de Leon R.D, Roy R.R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. 10.1146/annurev.neuro.27.070203.144308 [DOI] [PubMed] [Google Scholar]
- Fetz E.E, Perlmutter S.I, Prut Y. Functions of mammalian spinal interneurons during movement. Curr. Opin. Neurobiol. 2000;10:699–707. doi: 10.1016/s0959-4388(00)00160-4. 10.1016/S0959-4388(00)00160-4 [DOI] [PubMed] [Google Scholar]
- Fong, A. J. et al 2003 Effects of quipazine and robotic training on spinal mice Washington, DC: Society of Neuroscience Abstract Program No. 498.20.
- Fong A.J, Cai L.L, Otoshi C.K, Reinkensmeyer D.J, Burdick J.W, Roy R.R, Edgerton V.R. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 2005;25:11 738–11 747. doi: 10.1523/JNEUROSCI.1523-05.2005. 10.1523/JNEUROSCI.1523-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A.P, Grillner S. Visuomotor coordination in reaching and locomotion. Science. 1989;245:1209–1210. doi: 10.1126/science.2675307. [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y.P, Avelev V.D, Nikitin O.A, Lavrov I.A. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2003;33:247–254. doi: 10.1023/a:1022199214515. 10.1023/A:1022199214515 [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y.P, Lavrov I.A, Bogacheva I.N, Shcherbakova N.A, Kucher V.I, Musienko P.E. Formation of locomotor patterns in decerebrate cats in conditions of epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2005;35:291–298. [PubMed] [Google Scholar]
- Grasso R, Ivanenko Y.P, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, Lacquaniti F. Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain. 2004;127:1019–1034. doi: 10.1093/brain/awh115. 10.1093/brain/awh115 [DOI] [PubMed] [Google Scholar]
- Grillner S. The spinal locomotor CPG: a target after spinal cord injury. Prog. Brain Res. 2002;137:97–108. doi: 10.1016/s0079-6123(02)37010-9. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. 10.1038/nrn1137 [DOI] [PubMed] [Google Scholar]
- Gurfinkel V.S, Levik Y.S, Kazennikov O.V, Selionov V.A. Locomotor-like movements evoked by leg muscle vibration in humans. Eur. J. Neurosci. 1998;10:1608–1612. doi: 10.1046/j.1460-9568.1998.00179.x. 10.1046/j.1460-9568.1998.00179.x [DOI] [PubMed] [Google Scholar]
- Hase T, Kawaguchi S, Hayashi H, Nishio T, Mizoguchi A, Nakamura T. Spinal cord repair in neonatal rats: a correlation between axonal regeneration and functional recovery. Eur. J. Neurosci. 2002;15:969–974. doi: 10.1046/j.1460-9568.2002.01932.x. 10.1046/j.1460-9568.2002.01932.x [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell L.M. Handbook of physiology. The Nervous System. Motor Control, section 1. vol. II, pt. 1, ch. 11. American Physiological Society; Bethesda, MD: 1981. Functional organization of motoneuron pool and its inputs; pp. 423–507. [Google Scholar]
- Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr. Opin. Neurol. 2003;16:705–710. doi: 10.1097/01.wco.0000102630.16692.38. 10.1097/00019052-200312000-00010 [DOI] [PubMed] [Google Scholar]
- Ichiyama R.M, Gerasimenko Y.P, Zhong H, Roy R.R, Edgerton V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. 10.1016/j.neulet.2005.04.049 [DOI] [PubMed] [Google Scholar]
- Kuiken T.A, Dumanian G.A, Lipschutz R.D, Miller L.A, Stubblefield K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthetist Orthotist Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- Kuypers H.G. The motor system and the capacity to execute highly fractionated distal extremity movements. Electroencephalogr. Clin. Neurophysiol. 1978;34:429–431. [PubMed] [Google Scholar]
- Lacroix S, Havton L.A, McKay H, Yang H, Brant A, Roberts J, Tuszynski M.H. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J. Comp. Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. 10.1002/cne.20051 [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea D.A. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J. Neurophysiol. 2005;94:1120–1132. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lawrence D.G, Kuypers H.G. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lee Y.S, Lin C.Y, Robertson R.T, Hsiao I, Lin V.W. Motor recovery and anatomical evidence of axonal regrowth in spinal cord-repaired adult rats. J. Neuropathol. Exp. Neurol. 2004;63:233–245. doi: 10.1093/jnen/63.3.223-a. [DOI] [PubMed] [Google Scholar]
- Lemon R.N, Kirkwood P.A, Maier M.A, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog. Brain Res. 2004;143:263–279. doi: 10.1016/S0079-6123(03)43026-4. [DOI] [PubMed] [Google Scholar]
- Lovely R.G, Gregor R.J, Roy R.R, Edgerton V.R. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. 10.1016/0014-4886(86)90094-4 [DOI] [PubMed] [Google Scholar]
- Lovely R.G, Gregor R.J, Roy R.R, Edgerton V.R. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. 10.1016/0006-8993(90)91417-F [DOI] [PubMed] [Google Scholar]
- MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys. Ther. 2002;82:69–83. doi: 10.1093/ptj/82.1.69. [DOI] [PubMed] [Google Scholar]
- Orlovsky G.N, Feldman A.G. Neurophysiology. Plenum; New York, NY: 1972. Classification of lumboscaral neurons according to their discharge patterns during evoked locomotion. [Google Scholar]
- Orsal D, Barthe J.Y, Antri M, Feraboli-Lohnherr D, Yakovleff A, Gimenez y Ribotta M, Privat A, Provencher J, Rossignol S. Locomotor recovery in chronic spinal rat: long-term pharmacological treatment or transplantation of embryonic neurons? Prog. Brain Res. 2002;137:213–230. doi: 10.1016/s0079-6123(02)37018-3. [DOI] [PubMed] [Google Scholar]
- Pearson K.G. Generating the walking gait: role of sensory feedback. Prog. Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Petruska, J. C., Ichiyama, R. M., Crown, E., Kansey, K., Edgerton, V. R. & Mendell, L. M. 2004 Segmental and central inputs to motoneurons change following spinal cord transection and step training in rats Washington, DC: Society of Neuroscience Abstract Program No. 418.10.
- Raineteau O, Schwab M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001;2:263–273. doi: 10.1038/35067570. 10.1038/35067570 [DOI] [PubMed] [Google Scholar]
- Robinson G.A, Goldberger M.E. The development and recovery of motor function in spinal cats. I. The infant lesion effect. Exp. Brain Res. 1986a;62:373–386. doi: 10.1007/BF00238857. [DOI] [PubMed] [Google Scholar]
- Robinson G.A, Goldberger M.E. The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Exp. Brain Res. 1986b;62:387–400. doi: 10.1007/BF00238858. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Barbeau H. Pharmacology of locomotion: an account of studies in spinal cats and spinal cord injured subjects. J. Am. Paraplegia Soc. 1993;16:190–196. doi: 10.1080/01952307.1993.11735900. [DOI] [PubMed] [Google Scholar]
- Scott J.G, Mendell L.M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J. Neurophysiol. 1976;39:679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Shik M.L, Severin F.V, Orlovskii G.N. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Sjostrom A, Zangger P. Muscle spindle control during locomotor movements generated by the deafferented spinal cord. Acta Physiol. Scand. 1976;97:281–291. doi: 10.1111/j.1748-1716.1976.tb10265.x. [DOI] [PubMed] [Google Scholar]
- Sutton R.S, Barto A.G. MIT Press; Cambridge, MA: 1998. Reinforcement learning – an introduction. [Google Scholar]
- Thompson F.J, Parmer R, Reier P.J. Alteration in rate modulation of reflexes to lumbar motoneurons after midthoracic spinal cord injury in the rat. I. Contusion injury. J. Neurotrauma. 1998;15:495–508. doi: 10.1089/neu.1998.15.495. [DOI] [PubMed] [Google Scholar]
- Tillakaratne N.J, Mouria M, Ziv N.B, Roy R.R, Edgerton V.R, Tobin A.J. Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J. Neurosci. Res. 2000;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. 10.1002/(SICI)1097-4547(20000415)60:2%3C219::AID-JNR11%3E3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- Tillakaratne N.J, de Leon R.D, Hoang T.X, Roy R.R, Edgerton V.R, Tobin A.J. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler M, Danilov Y, Bach Y.R.P. Closing an open-loop control system: vestibular substitution through the tongue. J. Integr. Neurosci. 2003;2:159–164. doi: 10.1142/s0219635203000263. 10.1142/S0219635203000263 [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Fores J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J. Neurophysiol. 2004a;91:2838–2848. doi: 10.1152/jn.01177.2003. 10.1152/jn.01177.2003 [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Tsironis K, Skouras E, Navarro X, Neiss W.F. Peripheral and spinal motor reorganization after nerve injury and repair. J. Neurotrauma. 2004b;21:95–108. doi: 10.1089/089771504772695986. 10.1089/089771504772695986 [DOI] [PubMed] [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur. J. Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. 10.1111/j.1460-9568.1995.tb00686.x [DOI] [PubMed] [Google Scholar]
- Wirz M, Hornby R, Rupp R, Dietz V. Locomotor training with a driven gait orthosis in incomplete spinal cord injury. Gait Posture. 2005;21:S74. 10.1016/S0966-6362(05)80242-5 [Google Scholar]
- Zhou K, Doyle J.C, Glover K. Prentice Hall; Upper Saddle River, NJ: 1996. Robust and optimal control. [Google Scholar]