Abstract
Over 130 molecules have been identified in the interstellar gas and circumstellar shells, the largest among them is a carbon chain with 13 atoms and molecular weight of 147 (twice that of the simplest amino acid glycine). The high reliability of astronomical identifications, as well as the fairly accurate quantitative analysis which can often be achieved, is emphasized. Glycine itself has been claimed, but a recent analysis indicates that few, if any, of the astronomical radio lines attributed to glycine are actually from that molecule. Polycyclic aromatic hydrocarbons (PAHs) have long been proposed as the source of the unidentified infrared bands between 3 and 16 μm, but no single PAH has been identified in space, partly because PAHs generally have weak or non-existent radio spectra. A remarkable exception is the non-planar corannulene molecule (C20H10) that has a strong radio spectrum; in the rich molecular cloud TMC-1, it is found that less than 10−5 of the carbon is contained in this molecule, suggesting that PAHs are not the dominant large molecules in the interstellar gas, as has been claimed. Owing to inherent spectroscopic limitations, determining the structures of the large molecules in space may require capture of the dust grains, which are continually entering the outer Solar System.
Keywords: prebiotic, interstellar molecules, radio astronomy
1. Introduction
When radio telescopes began to find many familiar organic molecules in space about 30 years ago, we formulated, not entirely seriously, the Fisher scientific principle: ‘if you cannot obtain a molecule from that well known chemical supply house, you will not find it in the interstellar gas’. This amounted to the statement that the molecules being found in space were stable, closed shell compounds of the kind familiar in any chemical stockroom. It was clear even then that this principle was suspect, strongly biased by observational selection, since we were largely limited to looking for molecules studied in the laboratory, for which radio frequencies could be found in the standard microwave compilations. We were simply following the footsteps of the laboratory spectroscopists, who since the beginning of microwave spectroscopy by Cleeton & Williams in the early 1930s had mainly studied stable molecules. With the discovery of strong astronomical lines which did not appear in the microwave tables, and the subsequent assignment of some of these on the basis of theoretical arguments and new laboratory work, it soon became clear that the Fisher scientific principle was indeed badly incomplete. Today we know that stable molecules that can be found in a chemical stockroom are a substantial component of the many molecules which can be observed in the interstellar gas and circumstellar shells, but only one component.
To set the astronomical stage for the discussion on the conditions on the early Earth, which led to the emergence of life, I want to simply summarize some of the salient properties of the molecules which have been identified recently. Since the discovery of ammonia and water by Townes, Welch and their collaborators nearly 40 years ago (Cheung et al. 1968, 1969), astronomical molecules have been found mainly in the radio band, but well before that, in the late 1930s, three free radicals, CH, CH+ and CN, were observed with the 100 in. Mt Wilson telescope in the near UV against nearby bright stars (cf. Herzberg 1963). More recently, half a dozen symmetrical non-polar molecules (e.g. C2, methane, acetylene) without radio spectra have been detected in the visual or infrared. As discussed later, the claim that polycyclic aromatic hydrocarbons (PAHs) are an important component of the interstellar gas is based on observations of the so-called unidentified infrared (UIR) emission bands in the middle infrared from 3 to 16 μm.
The total number of astronomical molecules is now over 130 (figure 1). Stable organic molecules continue to be an important component, but there are an even larger number of exotic molecules, some of which are so reactive and elusive in the laboratory that they were quite unknown until identified in space. These can be classified as: (i) carbon chains (linear molecules with alternating single and triple bonds, which on Earth tend to explosively polymerize), (ii) radicals with a single non-bonding electron (e.g. CCH and CCCH), (iii) carbenes with two non-bonding electrons (e.g. CH2, C3H2), molecular ions (e.g. HCO+, HNN+, ) and (iv) molecular isomers like HNC, which is 0.6 eV less stable than the familiar hydrogen cyanide (HCN). No gas phase negative molecular ions have been found, although they may exist in detectable amounts in interstellar regions well shielded by dust (Lepp & Dalgarno 1988).
Figure 1.
Molecules identified in the interstellar gas and circumstellar shells, ranked by the number of atoms. As the insert shows, the rate of discovery of about 5 per year has remained fairly steady for the past 35 years.
The astronomical molecules are mainly composed of the cosmically abundant biogenic elements H, C, N, O and S, but 11 molecules contain Si, and there is at least one molecule with F, Mg, Cl, Na, K, Al or P. A large number of molecular isotopic species have also been found, too many to discuss here in detail. Particularly conspicuous are species with 13C, 18O and D. On Earth, the 12C : 13C ratio is 89, but in the local interstellar gas it appears to be somewhat less, about 50, owing to the generation of elements in stars since the Sun was formed 4–5 billion years ago, and their dispersal into the interstellar gas by supernovae and stellar winds. As a result, when a carbon containing molecule is detected with a signal-to-noise ratio that greatly exceeds 50, it is usually possible to observe lines from its carbon-13 isotopic species. Since isotope shifts in molecules are large, typically many linewidths, the carbon-13 lines are readily separated from the stronger lines of the normal carbon-12 molecule and provide an important, sometimes crucial, test of the identification, further strengthening the qualitative analysis.
Owing to the high cosmic abundance of hydrogen, H2 is by orders of magnitude the most common molecule in space, both in the interstellar gas and circumstellar shells, and all the other molecules in figure 1 are only trace constituents. There has never been any serious doubt that this is true, but H2 is very difficult to observe in the dense, highly opaque gas where most of the astronomical molecules are found; hence, determining the exact amount of H2 relative to the other molecules has been difficult. The important CO : H2 ratio has been estimated in three ways, which give roughly the same result by: (i) assuming that the gas to dust ratio—the ratio of the absorption of starlight by the dust to the hydrogen density—is the same in molecular gas as in atomic gas, where the amount of H can be measured with the well-known radio 21 cm line, (ii) applying the virial theorem and assuming that the internal motion of molecular clouds is the result of self-gravity and (iii) appealing to gamma ray surveys of the Milky Way, which have been done by several space observatories, and assuming that the low-energy cosmic-ray primary protons responsible for the gamma rays penetrate equally well through both atomic and molecular gas—a standard assumption in cosmic-ray studies. A number of discussions on this technical issue have been published (Combes 1991; Solomon & Barrett 1991).
It is worth emphasizing the remarkably high quality of nearly all the astronomical identifications. Since interstellar molecular gas is typically cold, radio lines are often extremely sharp, even by laboratory standards, and in favourable cases it is possible to match astronomical lines to laboratory frequencies to a few parts in 107. At this accuracy, it does not take many lines to achieve a conclusive identification; a number of molecules in figure 1 have dozens of precisely measured astronomical lines, and as a result can be assigned with a confidence approaching certainty. When it is claimed, for example, that formaldehyde or methanol or a number of others among the many identified in space exist in a particular interstellar cloud, there is essentially no dissent from astronomers, chemists or physicists who examine the data. There is probably no area of astrophysics where the qualitative analysis is more secure.
The quantitative analysis also can be very good. The intensity of the molecular radio emission lines in space depends mainly on two factors, both of which can be well determined: the molecular electric dipole moment and the rotational partition function, which depends on the populations of the various levels. For stable molecules, the dipole moment can be measured from the Stark effect to about 1%, and even for some reactive molecules where the measurement is difficult, it can often be measured to almost the same value. In addition, for many reactive astronomical molecules, dipole moments can now be calculated with quantum numerical codes to a few per cent. Calculation of the partition function requires knowledge of the rotational excitation and the effective rotational temperature, but this can often be done in the order of 10%. The upshot is that determining the amount along the line of sight of a given molecule can usually be done to better than 50%, and often to 10–20%. When lines are optically thick, as often occurs, the determination is more complicated, but the quantitative analysis is then greatly facilitated by the measurements of optically thin rare isotopic species.
With modern radio telescopes and sensitive cryogenic receivers employing superconducting detectors near the temperature of liquid helium, it is remarkably easy to detect many of the simpler astronomical molecules. Rotational lines in a strong source can sometimes be observed in an observation of only a millisecond. However, the larger molecules are much harder to observe; detection of HC11N required, for example, some 30 h of observation per spectral line. However, most of the large differences in detectability are not the result of a precipitous decrease in abundance, but reflect simply the much more complicated rotational spectrum of large molecules, and the much larger rotational partition function. When this is taken into account, one is struck at how abundant the larger astronomical molecules can be relative to the smaller ones. There is no evidence of a cut-off with mass or size in the production of large molecules in space, one is simply encountering an inherent limitation in the spectroscopic techniques employed.
At 30 h per spectral line, it is clear why the larger molecules in figure 1 have so far been observed in only one or a few directions, covering a minute fraction (approx. 10−8) of the celestial sphere. On the other hand, there is good reason to believe that large molecules are much more widely distributed than the present observations directly demonstrate. Simple molecules like CO and HCN can be observed along the entire galactic plane; CO, the best overall tracer of molecular gas and the most widely studied and surveyed molecule here and in other galaxies, is readily observed over at least 10% of the celestial sphere and similarly widespread in other spiral galaxies (figure 2). The dust grains at the other end of the mass spectrum are at least as widely distributed as these simple molecules. Since large molecules tend to be more resistant to destructive processes like photodissociation and dissociative recombination than small ones, it would be astonishing if large molecules were not far more widely distributed than the present data reveal.
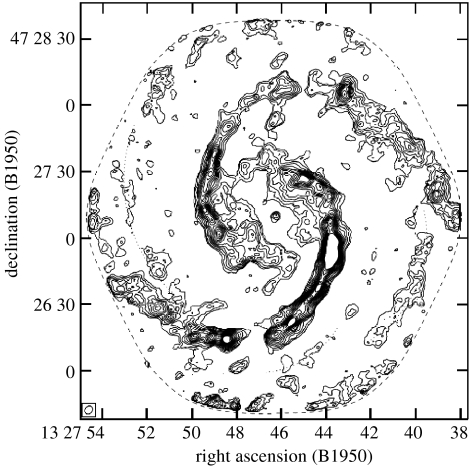
Figure 2.
CO line emission from the well-known large spiral galaxy M51, a map obtained with the CalTech millimeter-wave interferometer in Owen's Valley (Aalto et al. 1999). To astronomers, this map is particularly interesting because it shows how closely molecular clouds trace the spiral arms of this famous ‘grand design’ spiral system. The large concentrations of gas along the spiral arms are the so-called giant molecular clouds, each with a mass of a million solar masses or more (most of that H2, the dominant cosmic molecule).
The difference in mass between the largest molecules and the smallest interstellar dust grains is now so small—only about one-half an order of magnitude—that it is plausible to postulate a continuous transition from molecules to grains, and to suppose that these two constitute a single continuous population of chemically bonded structures. Within the observational uncertainties, the mass spectrum of this combined population is fairly flat, with approximately equal mass per decade of molecular weight, extending from the known interstellar molecules to the largest grains for which observational evidence exists: objects about 0.4 μm in size with the order of 1010 atoms. A crucial question then is how far the specific structures, which characterize all the known molecules, extend up the population of grains, objects whose structures are almost entirely unknown. The availability of free energy in the interstellar gas allows, in principle, the assembly of structures of arbitrary complexity; so, it is plausible that many of the interstellar grains have very specific structures, and are in fact large molecules. Determining just what those unknown structures are is a formidable challenge, because the refined spectroscopic techniques used to determine the structures of the known molecules are likely to fail at molecular weights greater than a few hundred. It may require the capture and laboratory analysis of the interstellar grains. This is not as utopian a requirement as it once seemed, because the Ulysses satellite showed that the larger grains are continually entering the outer Solar System in the direction expected from the solar motion through the interstellar gas (Grün & Landgraf 2001).
Over 50 years ago in a well-known experiment, Stanley Miller and Harold Urey showed that by subjecting simple molecules thought to exist in the early terrestrial atmosphere to an electrical discharge, a large variety of more complicated biogenic molecules were produced, particularly amino acids (cf. Miller 1986). This experiment has been repeated and extended many times since, and it has been found that similar results are obtained with other dissociating and ionizing mechanisms, including UV radiation and X-rays. The significance of these experiments to the origin of life has been debated, with no firm conclusions emerging, except that the generation of biogenic molecules under astronomical conditions is not particularly difficult, and can be achieved in a variety of ways. Therefore, it is interesting that essentially all the starting gases of the various Miller–Urey experiments are now found in the interstellar gas, under conditions which are radically different from those in planetary atmospheres and oceans, with densities lower by at least 15 orders of magnitude. Some of the end products of the Miller–Urey experiment are found in space as well, e.g. formaldehyde and cyanoacetylene. One suspects that many more are currently lurking just below our current level of sensitivity, amino acids especially.
Amino acids in the interstellar gas have been sought by radio astronomers for a number of years, the search being mainly for the simplest amino acid, glycine. It is not easy to obtain amino acids in the vapour phase—when heated they tend to pyrolyse or dissociate—but enough glycine has now been produced as a vapour to measure its fairly complicated rotational spectrum to the required precision. The early searches for glycine were all negative, but two years ago Kuan et al. (2003) reported detection of a number of glycine lines, some 27 in several astronomical sources.
Unfortunately, this claim has not been confirmed. The amount of glycine claimed by Kuan et al. is in conflict with previously published upper limits (e.g. Combes et al. 1996; Ceccarelli et al. 2000), and glycine lines which should have appeared were not found. In a detailed analysis of the evidence, Snyder et al. (2005) recently concluded that few, if any, of the lines attributed by Kuan et al. to interstellar glycine were actually from that molecule. The spectroscopic data on which the claim of Kuan et al. was based have not been published or made available to other workers, and there is now a fairly wide consensus among radio astronomers and laboratory spectroscopists that glycine has not yet been found in space. But it is also clear that the spectroscopic and radio astronomical tools now exist to detect this fundamental amino acid and perhaps others, and that deeper searches with the new generation telescopes might well succeed and should be undertaken.
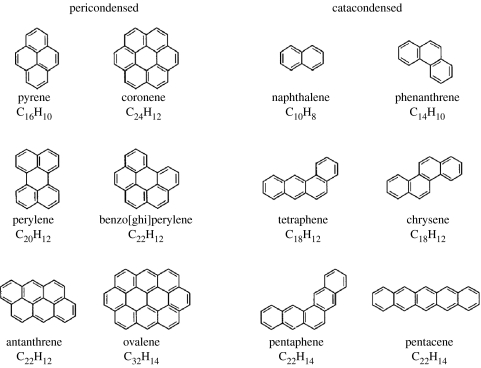
Finally, let me consider briefly the interesting case of PAHs, some of which are shown in figure 4. As stable organic molecules whose heavy atom backbone is graphitic in structure, it is entirely plausible that some PAHs exist in space, particularly in view of the great structural variety encountered in many molecules, which have been positively identified in the radio and infrared bands. But much stronger claims than that have been made over the past 20 years. It has been asserted that PAHs are the dominant large molecules in the interstellar gas and the most abundant organic molecules in the Universe, comprising as much as 20% of the interstellar carbon here and in other galaxies (cf. Tielens 2005). They have been proposed as the carriers of the mysterious diffuse interstellar bands (DIBs)—probably the outstanding unsolved problem in astronomical spectroscopy—as an important element in the ionization balance in dense clouds, as the sites of molecular production via catalytic surface reactions and as important contributors to the extinction of starlight. It is this dominant, extensive role of PAHs in the interstellar gas that is generally understood as the PAH hypothesis by astronomers and requires scrutiny.
Figure 4.
Typical PAHs. All are planar, and are either non-polar or only slightly so. Hydrogen atoms, bonded in the plane to the periphery of the carbon skeletons, are not shown.
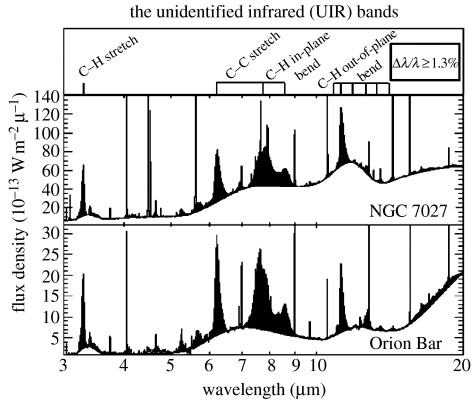
After these expansive claims, it comes as a surprise to find that no single PAH has been identified in the interstellar gas or in any other astronomical source. The observational foundation of the hypothesis is in fact fairly slender—the observation that several of the UIR bands between 3 and 16 μm in wavelength shown in figure 3 fall at about the wavelengths of the CH and CC vibrations of aromatic molecules. Since the UIR bands are broad, 1000 times or wider than the sharp radio lines of interstellar molecules, and vary somewhat in wavelength from source to source, the assignments are not free of ambiguity. It is also important to emphasize that they do not refer to any specific molecule. The PAH identification is a generic one, with a very large number of implicit free parameters, and this is one of the main reasons why the PAH hypothesis is so slippery, and hard to confirm or reject. The astronomical identification of PAHs is clearly far more ambiguous and less secure than the identifications of the radio and infrared molecules shown in figure 1.
Figure 3.
The UIR bands in two sources in our galaxy (Peeters et al. 2004). The Orion Bar is a central part of the famous Orion Nebula, produced by the intense UV radiation from several very hot embedded stars. NGC 7027 is a planetary nebula, an ionized shell of gas produced by a single very hot central star.
Since radio observations provide such secure identifications, it is natural to ask what light they shed on the PAH hypothesis. The answer until recently is discouraging, for two reasons: (i) PAHs tend to be non-polar or only slightly polar molecules, with weak rotational spectra or none at all and (ii) they have poor rotational partition functions, with the rotational intensity of those PAHs which are unsymmetrical and polar distributed over many transitions. Heterocyclic ring compounds like pyrrole and pyrimidine are quite polar and have strong spectra, but they are not hydrocarbons, and if the PAH hypothesis is to be stretched to include the huge class of heterocyclic aromatic molecules, it might also be extended to many more large organic molecules, including the carbon chains which are conspicuous in molecular clouds and circumstellar shells.
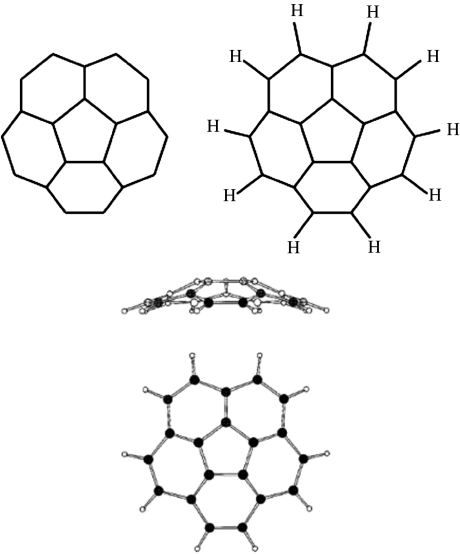
However, there is one remarkable PAH, the corannulene molecule (C20H10) shown in figure 5, whose microwave spectrum has recently been measured (Lovas et al. 2005), which is largely free of these objections, and allows an interesting test of the PAH hypothesis. The carbon backbone of this molecule is one-third of the C60 buckyball, a molecule so stable that in a well-adjusted carbon arc it can be produced with a yield approaching 50%. The central pentagon of corannulene curves the surface into the shallow cup shown in figure 5, and endows the molecule with a surprisingly large electric dipole moment along its symmetry axis (2.07±0.02 Debye, according to the recent Stark effect measurement by Lovas et al. 2005, versus 1.94 Debye for water). This is an order of magnitude larger than that of a typical polar PAH, producing a two orders of magnitude more intense rotational spectrum.
Figure 5.
The corannulene molecule (C20H10), a unique PAH with a strong radio spectrum. The carbon skeleton of this molecule is one-third of the very stable C60 buckyball. The central pentagon breaks the planar structure of normal PAHs, and bends the structure into a shallow cup as shown, endowing the molecule with a substantial dipole moment along the axis of the cup, and an intense radio spectrum.
The high symmetry and the structural rigidity of corannulene, by causing many lines to coincide, yield a further increase in rotational intensity by a factor of about 30. The rotational spectrum of the molecule is that of a symmetric top, with the rotational levels segregated into a series of familiar K-stacks, distinguished by the value of the rotational angular momentum K along the molecular symmetry axis. Radiative transitions occur only within a given stack, with the usual selection rule for electric dipole transitions: ΔJ=0, ±1. However, collisions readily excite cross-stack transitions, and over 50 stacks will be collisionally excited in even a cold molecular cloud like TMC-1, even though the rotational temperature of a molecule there is typically only 10–20 K. The important point is that within each K-stack, the radiative transitions are essentially those of a linear molecule, with the same selection rule on J, the total angular momentum: ΔJ=0, ±1. In a strictly rigid symmetric top, the given transition J→J+1 has exactly the same frequency in each stack, but centrifugal distortion generally separates these into well-resolved lines. But—as Buckminister Fuller would have predicted—corannulene is very stiff, and cross-stack centrifugal distortion has so far proven too small to measure, with the important consequence that the lines of the various K-stacks will coincide in even a sharp line astronomical source. The result is to endow corannulene with a rotational partition function which in effect is that of a much smaller linear molecule—specifically, one which differs only slightly from that of a carbon chain like HC7N. Added to the gain from polarity, the upshot is that corannulene in space is expected to have radio lines 3–4 orders of magnitude stronger than those of a typical polar PAH of the same size and abundance.
By appealing to published survey data for the rich molecular cloud TMC-1 in the solar vicinity (Kaifu et al. 2004), William Klemperer and I have found from the upper limit, which can be set on lines of corannulene, that this PAH contains less than 1×10−5 of the carbon in this well-studied molecular cloud. This is so much less than the approximately 10% of the available carbon which has been claimed to reside in interstellar PAHs that one is inclined to question the whole PAH hypothesis. But in attempting to formulate a precise statement, one immediately encounters the ambiguity of a generic identification, and the problem of the many unspecified free parameters—the abundances of the individual PAHs. It may be, for example, that corannulene for some reason is not formed in the interstellar gas, perhaps owing to the slight steric strain which results from the curved carbon skeleton, or that the molecule is preferentially destroyed. Similar objections can probably be contrived for any other PAH which fails to appear. But at some point for the PAH hypothesis to remain credible, it seems clear that specific PAHs will have to be identified. Further observations with present instruments, particularly the new 100 m Green Bank telescope, might lower our limit here on corannulene by a further factor of 10, or, alternatively, provide evidence for this interesting molecule.
The following are some conclusions that are drawn from this brief survey of the molecules in space.
Only a very small fraction of the organic compounds in nature are found in planets or comets and other condensed objects. By far the larger quantity, more than 99.9% by mass, reside in the enormous molecular clouds in the interstellar gas here and other spiral galaxies.
The interstellar gas contains all the raw materials of the classic Miller–Urey experiments, and some of the end products as well; many others may be close to detection.
Familiar prebiotic organic compounds in space coexist with exotic organic compounds. Ion–molecule reactions, driven by cosmic-ray ionization, are important in interstellar chemical synthesis because they are very fast and typically proceed with no activation barrier, and so work at a temperature of only a few Kelvin.
The number of organic molecules identified with high confidence by spectroscopic techniques is already large (135), and more will certainly be found as radio and infrared telescopes are improved. However, fundamental spectroscopic limitations—especially the complexity of the spectra of large molecules—will probably limit the number to a few hundred.
The gap in mass between the largest interstellar molecules and the smallest grains is now small, implying that some of the grains are in fact large molecules, with specific but unknown structures.
Determining what these structures are will probably require the capture and analysis of the interstellar grains now known to enter the outer Solar System.
But there could be important surprises in store. The unassigned optical DIBs and the incompletely assigned UIR bands are almost certainly molecular in origin, and some quite plausibly are produced by large molecules.
By showing the vast domain of organic chemistry, the astronomical discoveries would seem to be important to the origin of life, but just how is quite unclear. The formation of a planet is a violent event, so the intricate chemical history of the gas from which the planet forms may be obliterated, requiring chemical evolution to begin de novo. On the other hand, enough chemically evolved material may survive planetary formation to leave a permanent impression on subsequent molecular evolution. With self-replication and amplification, only a small amount of material would suffice.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
References
- Aalto S, Hüttemeister S, Scoville N.Z, Thaddeus P. A new high-resolution map of the inner 2.5′ of M51. Astrophys. J. 1999;522:165–182. doi:10.1086/307610 [Google Scholar]
- Ceccarelli C, Loinard L, Castets A, Faure A, Lefloch B. Search for glycine in the solar type protostar IRAS 16293-2422. Astron. Astrophys. 2000;362:1122–1126. [Google Scholar]
- Cheung A.C, Rank D.M, Townes C.H, Thornton D.D, Welch W.J. Detection of NH3 molecules in the interstellar medium by their microwave emission. Phys. Rev. Lett. 1968;21:1701–1705. doi:10.1103/PhysRevLett.21.1701 [Google Scholar]
- Cheung A.C, Rank D.M, Townes C.H, Thornton D.D, Welch W.J. Detection of water in interstellar regions by its microwave radiation. Nature. 1969;221:626–628. doi:10.1038/221626a0 [Google Scholar]
- Combes F. Distribution of CO in the Milky Way. Annu. Rev. Astron. Astrophys. 1991;29:195–237. doi:10.1146/annurev.aa.29.090191.001211 [Google Scholar]
- Combes F, Nguyen-Q-Rieu, Wlodarczak G. Search for interstellar glycine. Astron. Astrophys. 1996;308:618–622. [Google Scholar]
- Grün E, Landgraf M. Fast dust in the heliosphere. Space Sci. Rev. 2001;99:151–164. [Google Scholar]
- Herzberg G. D. Van Nostrand Co., Inc.; Princeton, NJ: 1963. Molecular spectra and molecular structure. I. Spectra of diatomic molecules; pp. 496–497. [Google Scholar]
- Kaifu N, et al. A 8.8–50 GHz complete spectral line survey toward TMC-1 I. Survey data. Publ. Astron. Soc. Jpn. 2004;56:69–173. [Google Scholar]
- Kuan Y-J, Charnley S.B, Huang H.-C, Tseng W.-L, Kisiel Z. Interstellar glycine. Astrophys. J. 2003;593:848–867. doi:10.1086/375637 [Google Scholar]
- Lepp S, Dalgarno A. Heating of interstellar gas by large molecules or small grains. Astrophys. J. 1988;335:769–773. doi:10.1086/166965 [Google Scholar]
- Lovas F.J, McMahon R.J, Grabow J.-U, Schnell M, Mack J, Scott L.T, Kuczkowski R.L. Interstellar chemistry: a strategy for detecting polycyclic aromatic hydrocarbons in space. J. Am. Chem. Soc. 2005;127:4345–4349. doi: 10.1021/ja0426239. doi:10.1021/ja0426239 [DOI] [PubMed] [Google Scholar]
- Miller S.L. Current status of the prebiotic synthesis of small molecules. Chem. Scripta. 1986;26B:5–11. [PubMed] [Google Scholar]
- Peeters E, Allamandola L.J, Hudgins D.M, Hony S, Tielens A.G.G.M. The unidentified infrared features after ISO. In: Witt A.N, Clayton G.C, Draine B.T, editors. Astrophysics of dust. ASP conference series. vol. 309. ASP; San Francisco, CA: 2004. pp. 141–162. [Google Scholar]
- Snyder L.E, Lovas F.J, Hollis J.M, Friedel D.N, Jewell P.R, Remijan A, Ilyushin V.V, Alekseev A.E, Dyubko S.F. A rigorous attempt to verify interstellar glycine. Astrophys. J. 2005;619:914–930. doi:10.1086/426677 [Google Scholar]
- Solomon P.M, Barrett J.W. In: Dynamics of galaxies and their molecular cloud distributions (IAU symp. 146) Combes F, Casoli F, editors. Kluwer; Dordrecht, The Netherlands: 1991. pp. 235–241. [Google Scholar]
- Tielens A.G.G.M. Cambridge University Press; Cambridge, UK: 2005. The physics and chemistry of the interstellar medium. ch. 6, pp. 173–224. [Google Scholar]