Abstract
The theory of a chemoautotrophic origin of life in a volcanic iron–sulphur world postulates a pioneer organism at sites of reducing volcanic exhalations. The pioneer organism is characterized by a composite structure with an inorganic substructure and an organic superstructure. Within the surfaces of the inorganic substructure iron, cobalt, nickel and other transition metal centres with sulphido, carbonyl and other ligands were catalytically active and promoted the growth of the organic superstructure through carbon fixation, driven by the reducing potential of the volcanic exhalations. This pioneer metabolism was reproductive by an autocatalytic feedback mechanism. Some organic products served as ligands for activating catalytic metal centres whence they arose. The unitary structure–function relationship of the pioneer organism later gave rise to two major strands of evolution: cellularization and emergence of the genetic machinery. This early phase of evolution ended with segregation of the domains Bacteria, Archaea and Eukarya from a rapidly evolving population of pre-cells. Thus, life started with an initial, direct, deterministic chemical mechanism of evolution giving rise to a later, indirect, stochastic, genetic mechanism of evolution and the upward evolution of life by increase of complexity is grounded ultimately in the synthetic redox chemistry of the pioneer organism.
Keywords: iron–sulphur-world, surface metabolism, ancestral genome, nickel, carbon fixation, ligand feedback
1. Introduction
The molecules of extant life may be classified into four categories of different complexity: inorganic nutrients, low-molecular organic compounds, polymers and aggregates of polymers. The first category, the inorganic nutrients of life, consist of molecules of a few atoms, such as H2, N2, H2O, H2S, NH3, CH4, CO, CO2, HCN and P4O10. They are gaseous or volatile and escape from the interior of the Earth as magmatic (or volcanic) exhalations. Upon cooling of the flow of magmatic exhalations, gaseous H2O condenses to liquid water, the elixir of life.
The gaseous nutrients enter the so-called intermediary metabolism of the extant organisms and form a few hundred low-molecular bioorganic compounds, which constitute the second category of molecular complexity. This may be seen as the chemically creative part of biochemistry. The gaseous nutrients enter synthetic pathways with redox reactions and thoroughgoing transformations of the electron configurations.
Some of the low-molecular bioorganic compounds are bifunctional monomers (e.g. amino acids), capable of undergoing polycondensations to high-molecular polymers, such as proteins or nucleic acids. This constitutes the third category of molecular complexity. It is not so much a chemically creative stage as an organizational stage, in which the monomers are merely combined covalently and sequentially without undergoing redox changes or substantial changes of their electron configurations. The fourth category of complexity is made up of particles: aggregates of several polymer molecules as such or embedded in a lipid membrane.
For 100 years, after the pioneering work of von Nägeli (1884), most theories on the origin of life have located the emergence of the first reproducing organism of life at or above the high level of molecular complexity increase from monomers to macromolecules. As a consequence, the formation of the monomers is placed in an obscure ‘prebiotic broth’, wherein low-molecular compounds are believed to have accumulated over thousands or millions of years. These theories have been widely reviewed (de Duve 1995, 2002, 2005; Maynard Smith & Szathmary 1995; Zubay 1996; Brack 1998; Lahav 1999; Fry 2000; Wills & Bada 2000; Hazen 2005; Rauchfuß 2005; Thoms 2005). They are flawed in multiple ways. Here, it should suffice to note that they all postulate liquid water and that it is precisely the mass effect of liquid water, which tends to prevent the accumulation of polycondensation products (or polycondensation agents) due to hydrolysis.
2. The pioneer organism
The failures of the theories of an origin of life by polycondensation in a ‘prebiotic broth’ posed the challenge whether it would not be possible to develop a theory of an origin at the level of low-molecular organic compounds. As a solution to this problem, a theory of a chemoautotrophic origin from inorganic starting materials was developed (Wächtershäuser 1988b–d, 1990, 1992). This theory has been dubbed ‘iron–sulphur-world’ theory for reasons of the geochemical ubiquity of FeS and the catalytic, energetic and structural roles of iron and sulphur at the origin and during early evolution. I call the first organism of life ‘pioneer organism’. The term ‘organism’ stands for an organized being and the term ‘pioneer’ stands for its role as the starting point of evolution. It should become clear as the text proceeds that the pioneer organism is a chemically deterministic entity at the threshold between the abiotic world and the biotic world.
In the years following the first publication of the iron–sulphur-world theory, a number of experimental results have been obtained. They will be reported later. Suffice it to say here that these experiments have led to significant improvements of this developing theory. The theoretical–experimental research programme has, as its ultimate goal, to reconstitute the pioneer organism and watch it evolve.
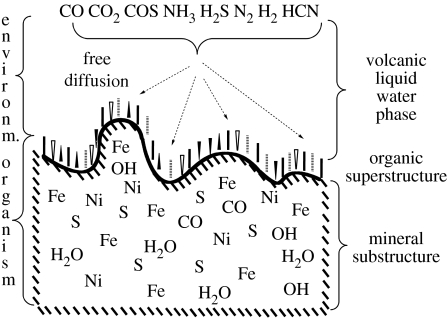
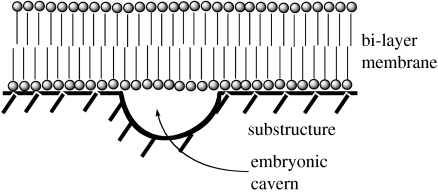
We turn first to a general characterization of the pioneer organism (figure 1). It has a minimal substructure–superstructure organization. The superstructure consists of low-molecular bioorganic compounds. They are bonded to the surfaces of an inorganic substructure, i.e. as an interphase between the inorganic substructure and the water phase. I use here the name pioneer organism for the totality of the organic molecules that are bonded at any given time to the inorganic surfaces plus the surface regions of the inorganic substructure. Extension and curvature of the surfaces of the substructure are indefinite. In the direction normal to the inorganic surfaces, the superstructure has an essentially monomolecular extension and a vectorial orientation. The water phase constitutes the environment and the source of nutrients.
Figure 1.
Cross-sectional representation of the minimal organization of the pioneer organism.
The inorganic substructure may form in a great variety of ways, which presently cannot be exhaustively defined. Three aspects of substructure formation deserve special mention and they are as follows:
Precipitation from solution may occur prior to or during the establishment of the pioneer organism. It has been experimentally ascertained earlier that fresh transition metal precipitates (Drobner et al. 1990; Huber & Wächtershäuser 1997, 1998, 2003; Huber et al. 2003) have a particularly high activity.
Cody et al. (2000) made the important observation that under high pressure and temperature CO mobilizes FeS to low-molecular carbonyl derivatives of unknown structure. These may be transported through volcanic flow channels to the site of the pioneer organism and become adsorbed onto or absorbed into the inorganic substructure.
It has been found experimentally that a number of synthetic reactions are dependent on alkaline conditions (Huber & Wächtershäuser 1998, 2003; Huber et al. 2003) and that the pH of the reaction medium may be stabilized by alkaline precipitates, like calcium hydroxide, magnesium hydroxide or basic magnesium carbonate. Therefore, such pH buffers may also be important in the substructure.
It has been stressed earlier that the inorganic substructure itself undergoes a chemical redox progression according to Ostwald's rule of steps from FeS to FeS2. This notion may now be expanded to heteroleptic (Fe,Ni)-precipitates progressing through carbonylations and sulphidizations. The surfaces of pyrite or other crystals may carry Fe–S-clusters, akin to those in Fe–S-enzymes (Wächtershäuser 1992), which may include other transition metals. This holds for all crystals in the progression, like mackinawite that has been detected in FeS precipitates after partial reaction with H2S to pyrite (Drobner et al. 1990). Amorphous FeS precipitates have been found to consist of nanocrystalline mackinawite (Michel et al. 2005). Greigite has been considered as an intermediate (Popper 1990) and as catalytic species (Russell et al. 2003, 2005).
The organic compounds of the superstructure arise by reductive carbon fixation, i.e. by redox reactions, for which liquid water is beneficial rather than detrimental. The redox reactions are catalysed by the transition metal centres in or on the surfaces of the inorganic substructure. The organic compounds become bonded to the substructure in statu nascendi, i.e. as ligands to the transition metal centres in the surface of the substructure. They subsequently undergo lateral transfer. In order to understand the mechanism of lateral transfer, we have to appreciate the omnipresence of water. The water molecules occupy all ‘free’ valences in the surface of the substructure and any lateral transfer of a ligand involves replacement of water molecules. For a closer understanding of the mechanism of lateral transfer, we have to introduce an important terminological distinction. The ligands that are directly bonded to the transition metal centres are termed ‘inner sphere’ ligands and those that are separated from the transition metal centres by a number of water molecules are termed ‘outer sphere’ ligands. Therefore, a lateral transfer of a ligand involves transfer from the inner sphere to an outer sphere of a first site, diffusion from the first site to a second site, and transfer from an outer sphere to the inner sphere of the second site. The constituents that detach by diffusing or flowing into the vast expanses of the open water (ocean) are irretrievably lost.
Such a pioneer organism may be seen as having a remarkable combination of three capabilities: for growth, reproduction and evolution. These central aspects of life coincide in the pioneer organism within one single type of process. This process may be briefly stated as follows. The thermodynamic driving force is provided by the chemical potential of the volcanic exhalations in the water phase. The kinetic reactivity is provided by the catalytic activity of the transition metal centres in or on the surfaces of the inorganic substructure. The combination of these thermodynamic and kinetic aspects has the following effects:
Synthetic carbon fixation reactions generate organic products that become bonded to the surfaces of the inorganic substructure in statu nascendi, which means growth.
Some of the synthetic organic products exhibit an autocatalytic positive feedback into the synthetic reactions whence they arise, which means reproduction.
The autocatalytic feedback effect exhibits variations, which is the basis for evolution.
Before we consider the pioneer organism in greater detail, we may look at the possible geochemical situation wherein this organism may arise and thrive.
3. The overall geochemical situation of the pioneer organism
The problem of the origin of life is a biological problem and its solution will be a biological theory in the sense that the explanandum consists of extant biological facts. This means that a theory of the origin and the early evolution of life will be a biomolecular theory. Its development should be guided by judging its explanatory power (Popper 1963), i.e. its power to explain many facts of extant biology with few theoretical assumptions. From a chemical point of view, the empirical merit of such a theory resides in its predictive power, i.e. its power to predict hitherto unknown chemical reactions that can be tested experimentally. Chemical thermodynamics, as explored by Amend & Shock (2001), serves to focus the experimental programme. The kinetic issues of the programme can only be resolved experimentally.
Turning now to the role of geology, it is unfortunate that we do not have any remains of the Hadean Earth with a direct biological signature. Therefore, the aim here can only be the establishment of compatibility between a theory of the early Earth and a theory of the origin and early evolution of life. Nisbet & Fowler (2004) have assigned to the geologists the role ‘to point out possible habitats where the first life could have been born’. Efforts in this direction promise the much needed constraints of the physical and chemical parameters for the experiments.
There are several reviews of recent theories on the early history of the Earth (Halliday 2004; Russell & Arndt 2005; Lunine 2006). It now appears from isotope studies of meteorites and Hadean zircon crystals that accretion, core–mantel differentiation and crust segregation proceeded rapidly and partly in parallel (Kleine et al. 2002; Yin et al. 2002; Jacobsen 2003; Harrison et al. 2005). These processes were followed as early as 4.4 Gyr, by oceans, continents and sedimentation (Mojzsis et al. 2001; Wilde et al. 2001). This new view extends the time frame for a possible origin of life deep into the Hadean. According to this emerging picture, a very hot magma and a relatively thin crust (de Wit 1998; Nisbet & Fowler 2004; Russell & Arndt 2005) would have been highly reducing and inflicted by heavy bombardment and by intense volcanism with highly reducing exhalations. It is exactly this range of conditions of the Hadean Earth that are crucial for the iron–sulphur-world theory of the origin of life.
In recent years, several remarkable geological habitats for the origin of life have been suggested:
Nisbet & Fowler (2004) suggest localized sites of origin in magnesium-rich komatiite lava deposits associated not only with iron but also with nickel sulphide.
Nisbet & Fowler (2004) further suggest alkaline hydrothermal systems or alkaline volcanism as the most likely setting, where ultramafic material (generating magnesium hydroxide) would be the source of alkali.
Cockell (2006) suggests that large impactors, long considered a source of heat-sterilization and as anathema for life, may actually have prepared the cradle of life by impact cratering. Thereby intense fracturing and deposition of dust and debris would have created a bottom bed with myriads of flow channels close to the magmatic source of the volcanic exhalations.
Nisbet & Fowler (2004) suggest with regard to cataclysmic large impacts that primitive life forms may have survived events of the magnitude of the Moon-forming impact by being contained in ejected rocks, which then orbited high in space and outside of the hot atmosphere for a sufficient time for later re-entry after the Earth had cooled down again.
These proposals are highly compatible with the theory of a chemoautotrophic origin of life as presented here, which assumes high pressure (Wächtershäuser 1988d, 1992), elevated temperatures (above or around 100°C), (Fe,Ni)-catalysis and the chemical potential of volcanic exhalations.
Let us now address two specific aspects of a possible cradle of life within the Hadean habitats that have been characterized earlier: hydrothermal systems and volcanic exhalations. These two aspects frequently occur side by side and are often confounded. The hydrothermal systems are cyclic. Ocean water cycles through the crust transporting heat and leached soluble salts from the crust into the ocean. For the origin of life they are of lesser importance. Volcanic exhalations are linear and hence primary, virgin magmatic gases (H2O, CO2, CO, H2S, etc.) escape as volcanic exhalations from the interior of the mantle. These, upon cooling, provide the chemical potential for the origin of life. Therefore, they form an indispensable part of the present theory, which means that the original homestead of life is situated in a volcanic flow system (Wächtershäuser 1988d). A Hadean volcanic flow system may be conceptually classified into three ideal types of flow regions:
A first upstream flow region (deep inside the Earth) with a temperature above the critical temperature of water may be likened to a flow reactor with a stationary solid structure, a mobile gas phase and perhaps entrained solid particles. It would be the source of the gaseous nutrients for the pioneer organism.
A second downstream flow region with a temperature below the critical temperature of water may be likened to a trickle-bed reactor with a stationary solid structure, a mobile gas phase and a mobile liquid water phase with dissolved volcanic exhalations. The water phase progressively condenses in gas flow direction beginning perhaps as a thin transient film that merely wets the solid surfaces and ultimately forming a liquid flow concurrent or countercurrent to the gas flow. Additional nutrients would have been formed here by reactions dependent on the presence of liquid water; and the formation of an organic superstructure may have already begun here.
A third flow region with a lower temperature may be a chromatographic reactor with a packed bed of solid debris or particles, in which a mobile liquid water phase passed through a myriad of flow channels that opened into the ocean. The third flow region may exhibit pH or sulphidization zoning and chromatographic characteristics for the organic products in the superstructure. It means that the constituents of the surface metabolism may have been selected by differential residence times, which means differential retention times in view of the state of volcanic flow. Reactive and chromatographic processes would have interacted along the flow path with best surface bonders being the slowest travellers.
Geochromatography has been previously invoked in conjunction with a prebiotic broth (Wing & Bada 1991, 2000) or with aquifers (Washington 2000). Corliss (1986) has analysed possible flow reactor conditions of a submarine hydrothermal vent. Subsequently, a number of other geologists have explored possible Hadean/Archaean hydrothermal vent scenarios for the origin of life (Russell et al. 1989, 1998, 2003, 2005; Holm 1992; Matsuno 1997; Russell & Hall 1997; Holm & Andersson 1998; Shock et al. 1998).
The involvement of volcanic exhalations in the origin of life has been invoked as early as 1974 (Mukhin 1974). Mukhin suggested volcanic carbon compounds as an additional source for the synthesis of organic compounds for the ‘prebiotic broth’. The present theory of a chemoautotrophic origin of life utilizes the volcanic flow system in a quite different manner. It is first of all the site at which the pioneer organism arises. In addition, it provides all the nutrients for this pioneer organism. This renders the chemo-autotrophic origin of life a local affair (Wächtershäuser 1988d) as opposed to the global affair of the prebiotic broth theory.
4. The basic chemical conditions of the pioneer organism
The geochemical setting of the Hadean Earth suggests a very general chemical framework for a volcanic origin of life. Regarding more specific chemical conditions of the pioneer organism, we have to rely on a different heuristic: biochemical retrodiction from extant biology. Four points considered here are as follows:
As to the source for carbon atoms, the extant biosphere is based mainly on CO2 fixation. Therefore, CO2 was originally considered as the least speculative candidate and CO was considered as a possible alternative (Wächtershäuser 1988d, 1990, 1992). In the meantime, based on experimental results, the emphasis has shifted from CO2 to CO, which makes good chemical, biological and geochemical sense. CO is highly reactive and in extant organisms, it has three central biological functions: as the carbon source for acetyl-CoA synthase; as the electron source for carbon monoxide dehydrogenase (CODH); and as the ligand in all the three hydrogenases (Pierik et al. 1999; Lemon & Peters 1999; Lyon et al. 2004). Some extant organisms use CO as sole carbon source (e.g. Rother & Metcalf 2004; Wu et al. 2005). This, however, seems to be a derived feature, since these organisms employ the usual CO2 reduction pathways fed by the CO2 that is produced by oxidation of CO. Finally, CO is today omnipresent in volcanic gases and the Hadean volcanic gases would have had a high ratio of CO: CO2. In addition to CO2 and CO, volcanic carbonyl sulphide (COS; Corazza 1986) has been considered as nutrient (Wächtershäuser 1992). Cyano ligands occurring at volcanic sites (Mukhin 1974) and in two of the three hydrogenases are also possible nutrients.
- As to the source of reducing power, a number of inorganic sources of electrons have been suggested that exist today and must have always existed under volcanic conditions:
- oxidative formation of pyrite (Wächtershäuser 1988b),
(4.1) - oxidation of ferrous compounds, like ferrous hydroxide (Huber & Wächtershäuser 2003),
(4.3)
The two half-reactions (4.1) and (4.2) with pyrite and CO2 as waste products are the two most crucial electron donors for the pioneer organism. Half-reaction (4.4) has been ‘excluded as the first source of electrons since its reducing potential is not sufficient for reducing CO2’ (Wächtershäuser 1988d), but not as a further source of electrons. The half reaction (4.1) has been demonstrated as viable source of electrons for the reduction of CO2 (Heinen & Lauwers 1996), which means CO2-fixation by oxidative pyrite formation. As a peculiar aspect of biomineralization, it has been discovered that the conversion of greigite to pyrite may be inhibited by aldehydes (Rickard et al. 2001). Pyrite chemistry, in conjunction with chemical origin of life reactions has been reviewed by Cody (2004). The surface bonding of pyrite has been studied, notably by Schoonen and co-workers, who found strong bonding of phosphate and phosphorylated compounds to pyrite (Bebie & Schoonen 1999).
As to the source of sulphur, volcanic H2S, the simplest of all sulphydryl compounds, has been considered as an essential nutrient and catalyst of the pioneer organism and for generating FeS, FeS2 and other transition metal sulphides in the substructure (Wächtershäuser 1988b).
Finally, as to the nature of the transition metal centres of the inorganic substructure, extant metallo-proteins suggest first and foremost Fe, which is the most abundant transition metal of the Solar System. Ni is a most important catalytic metal of Bacteria and Archaea and it is the second most abundant transition metal of the Solar System and a constant companion of Fe. Therefore, it has been suggested earlier (Wächtershäuser 1988d, 1990, 1992) and tested experimentally (Huber & Wächtershäuser 1997, 1998). The other biological transition metals, namely Mn, Co, Mo, W, Cu, Zn und V may also be considered. The suggestions for possible ligands are derived from the ligands of extant metallo-proteins, e.g. S, SH, S–S, H2O, OH, CO and CN. These possible metals and ligands combine to a rich set of possible inorganic structures within a precipitate (library) of polynuclear, polymodal and heteroleptic clusters on mineral surfaces (Wächtershäuser 2000a,b).
These reactants suggest a number of simple, testable reactions of volcanic nutrients in the formation and growth of a pioneer organism. These reactions may take place at the site of the pioneer organism for immediate infiltration into its surface metabolism or upstream thereof with subsequent transport of their products to the site of the pioneer organism. The following reactions have been experimentally demonstrated:
Formation of dihydrogen from FeS/H2S (Taylor et al. 1979; Drobner et al. 1990).
| (4.5) |
Nitrogen fixation with FeS/H2S under moderate conditions suggested theoretically (Wächtershäuser 1988d) and demonstrated with 15N–N2 (Dörr et al. 2003).
| (4.6) |
Formation of COS from CO and H2S by Ni-catalysis (Huber & Wächtershäuser 1997).
| (4.7) |
Formation of methylmercaptan from CO2 with FeS/H2S (Heinen & Lauwers 1996).
| (4.8) |
Formation of methylmercaptan from CO and H2S on NiS (Huber & Wächtershäuser 1997).
| (4.9) |
5. Synthetic reactions of the pioneer organism
We now turn to synthetic reactions that begin with simple volcanic nutrients and produce low-molecular organic compounds. We should bear in mind that the reactions are catalysed by metal centres in or on the surface of the inorganic substructure. We shall progress in the direction of increasing molecular complexity.
(a) Formation of C2-structures
The formation of activated thioacetic acid (CH3–CO–SH) from CH3SH and CO as evolutionary precursor of acetic acid thioesters was suggested theoretically (Wächtershäuser 1990). The formation of acetic acid and its thioester was demonstrated experimentally at 100°C (Huber & Wächtershäuser 1997):
| (5.1) |
The arithmetic is simple: C1+C1=C2. The reaction is catalysed by (Fe,Ni)S, NiS, Ni(OH)2 and CoS. It combines structure formation with energy conservation as group activation energy required for subsequent reactions. The reaction resembles the extant acetyl-CoA biosynthesis.
(b) Formation of C3-structures
The formation of pyruvate by CO-fixation was suggested theoretically (Huber & Wächtershäuser 1997) and demonstrated experimentally at 2000 bar and 250°C with FeS/RSH (Cody et al. 2000):
| (5.2) |
The arithmetic is C1+C1+C1=C3. In extant biochemistry, pyruvate is formed by reductive carboxylation of acetyl-CoA, seen here as the evolutionary successor reaction.
(c) Reductive amination
Reductive formation of α-amino acids from α-keto acids and NH3 was suggested (Wächtershäuser 1990) and demonstrated at 100°C (Hafenbradl et al. 1995; Huber & Wächtershäuser 2003):
| (5.3) |
The reaction proceeds with FeS and/or Fe(OH)2 as catalyst and electron source suggesting the oxidation of Fe2+ to Fe3+ as primary electron source, followed by the formation of FeS2 in the presence of H2S or of FeOOH, Fe2O3, Fe3O4, etc., in the absence of H2S. Glutamate synthase, one of the extant enzymes for reductive amination still depends on FeS-clusters for electron transport.
(d) Activation of amino acids and peptide cycle
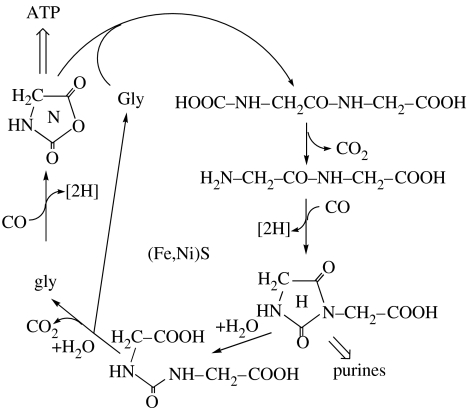
Amino acid activation and peptide formation were discovered when α-amino acids were added to the reaction system for forming the thioester of acetic acid. In the presence of (Fe,Ni)S, CO and H2S (or CH3SH) at 100°C, the reaction proceeds best under alkaline conditions with Mg(OH)2 as pH buffer. COS was seen as the activating intermediate, since its use instead of CO also produces peptides (Huber & Wächtershäuser 1998). Activation with COS in the absence of (Fe,Ni)S was studied at room temperature (Leman et al. 2004). Peptides formed with CO were shown to undergo N-terminal conversions, first to a hydantoin ring and then to a urea group, from which finally the N-terminal amino acid is cleaved hydrolytically (Huber et al. 2003). A hypothetical mechanism of the entire peptide cycle is shown in figure 2 for the simplest case of the dipeptide glycyl–glycine. In the first step, glycine (Gly) is activated to its aminoacyl N-carboxyanhydride (N) by reaction with COS obtained from CO and H2S. This means that the redox energy of reaction (4.3) is coupled to glycine activation via COS, which may well be the simplest pioneer energy coupling of life. Subsequently, the amino group of another molecule of glycine reacts as a nucleophile with the aminoacyl N-carboxyanhydride generating the dipeptide glycyl–glycine, which then reacts at its N-terminal end with COS (CO/H2S) generating hydantoin (H) as N-analogue of the N-carboxyanhydride (N).
Figure 2.
Dipeptide cycle of glycine. For possible alternatives see Huber & Wächtershäuser (1998) and Wächtershäuser (1998a).
Up to here, the peptide cycle is synthetic or anabolic. The subsequent hydrolysis to a urea derivative is still anabolic since the molecular weight increases. The final hydrolytic cleavage is catabolic and reminiscent of the extant Ni-enzyme urease. The combination of anabolic and catabolic cycle segments corresponds to the extant combination of ribosome-catalysed, anabolic protein synthesis with proteasome-catalysed, catabolic protein hydrolysis. In competition with N-terminal hydantoin formation, the free amino group of the dipeptide may react with another molecule of aminoacyl N-carboxyanhydride to form a tripeptide, which again may react competitively by N-terminal chain extension to a tetrapeptide or by N-terminal derivatization and degradation and so on. The peptide cycle may be seen as a catalytic cycle for the conversion of CO to CO2 akin to extant CODH. The peptide cycle will run as long as the redox energy supply of CO lasts. H2S is catalytic for the formation of COS. The hydrolytically sensitive COS is merely an intermediate and its accumulation is not required. With additional amino acids, peptides (hydantoins and ureas) with a variety of sequences are formed to coexist in mixture. This constitutes a dynamic library, in which the peptides (hydantoins and ureas) come and go with steady-state concentrations corresponding to the difference between the rates of formation and degradation. Importantly, this library of structures is also self-selecting, because a selective bonding of a certain structure as ligand to a metal centre of the substructure causes its stabilization against hydrolysis and thus an increase of its steady-state concentration. This means a self-selection of stable metallo-peptides.
(e) Phosphorylation
Volcanic gases contain volatile P4O10, which hydrolyses through intermediate polyphosphates and pyrophosphate (Yamagata et al. 1991) that function as phosphorylation agents, i.e. as inorganic substitutes for ATP (Baltscheffsky 1967). Pyrophosphate has been suggested as primordial phosphorylation agent (Baltscheffsky 1971). Extant organisms produce pyrophosphate by photo-phosphorylation (Baltscheffsky et al. 1966). Under standard temperature and pressure conditions, COS activates amino acids to subsequently react with AMP to aminoacyl-AMP and with phosphate to aminoacyl-phosphate, which subsequently reacts with further phosphate to pyrophosphate (Leman et al. 2006).
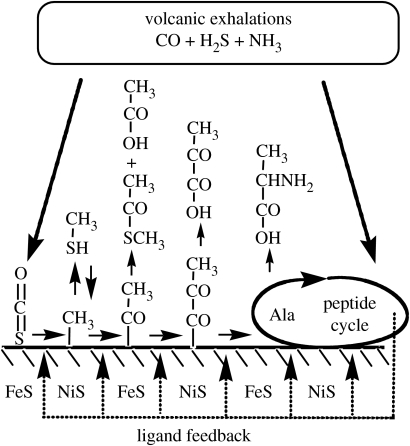
The aforementioned synthetic reactions may combine to autotrophic synthetic pathways. A pathway example progressing from CO to the peptide cycle is shown in figure 3. The reactions demonstrated so far may well be the tip of an iceberg of synthetic reactions for a possible pioneer organism waiting to be discovered experimentally.
Figure 3.
Integrated hypothetical reaction pathway with autocatalytic feedback (dotted arrows).
6. The pioneer metabolism, its reproduction and evolution
(a) Metabolism by ligand feedback
We next turn to mechanisms by which the synthetic reaction pathways in the superstructure of the pioneer organism may exhibit autocatalytic feedback, which would mean metabolic reproduction. We first note that growth of the superstructure refers to an accumulation of surface-bonding products. These products must be seen as ligands to the free metal valences in or on the surfaces of the inorganic substructure. At this instance, it is important to recognize that some of these catalyst ligands may increase the catalytic activity of the transition metal centres, to which they are bonded. This in turn would increase the rate of the pathway that produces the ligand (Wächtershäuser 1998a,c, 2000a,b; cf. Huber & Wächtershäuser 1998). Such ligand effects are quite idiosyncratic and theoretically not very predictable (Berrisford et al. 1995). Carefully chosen ligands may dramatically increase the activity of transition metal catalysts by factors of up to 103.
Let us now analyse which kinds of low-molecular organic products may become ligands for boosting catalytic transition metal centres. Typically, such ligands will have at least two functional groups (e.g. COOH and NH2) that can bond to a transition metal centre. Examples are hydroxy acids, e.g. glycolate, lactate, glycerate, malate, citrate, isocitrate and homocitrate, the latter being a ligand in extant nitrogenases (Kirn & Rees 1992; Rüttimann-Johnson et al. 2001); amino acids, e.g. glycine, alanine, serine, aspartate; and peptides or their hydantoin and urea derivatives. A possibility of such product feedback is indicated in figure 3.
We may conceptualize ligand feedback from a different point of view. All products of the surface metabolism form a mixture (or library) of chemical compounds. Some may bond to the surface more or less strongly and others may not bond at all. Presently, from this library, the pioneer organism will automatically select those compounds, which are capable of bonding as ligands by selective residence or retention time; and optionally by protection from hydrolysis. In this sense, the products of the pioneer metabolism are self-selective. Increase in the size of the library of products increases the likelihood that it contains self-selecting ligands with autocatalytic feedback. Incidentally, from this point of view, racemates of the organic products of the pioneer metabolism mean a higher number of structures and thus a higher likelihood of positive feedback, which means that at this early stage of life lack of enantioselectivity of the synthetic reactions is an advantage rather than a disadvantage.
These considerations bring us to a most crucial aspect of the self-selecting library of ligands. Let us assume that one special reaction product of the pioneer metabolism (e.g. a certain peptide) binds as a ligand to a catalytic metal centre. Let us further assume that this ligand increases the catalytic activity for a rate-determining step in the reaction pathway to this special reaction product. This means that the overall rate of this pathway is increased. As a consequence, the steady-state concentration of the special reaction product is increased. This in turn means that the rate of this pathway is further increased, etc. This is how an autocatalytic feedback works. At this instance, however, we have to appreciate that the autocatalytic pathway generates not just the one special reaction product, but rather a whole set of reaction products, e.g. a set of peptides, hydantoin derivatives and urea derivatives. This means that the autocatalytic feedback effect of the one special product increases the steady-state concentration of each compound in the set of reaction products. This brings the steady-state concentrations of some other products up to levels, at which further feedback possibilities are actualized. In this manner, a single positive feedback effect will broaden the spectrum of products, which in turn will usher in further feedback effects. This means that the metabolism of the pioneer organism is self-expanding and undergoing a progressive increase of metabolic diversity and molecular complexity, thus exhibiting an ever-expanding avalanche of metabolic evolution.
Let us finally consider reproduction and evolution under the flow conditions of a chromatographic reaction system. Strong-bonding products, e.g. certain peptides, have a long retention time and migrate slowly in flow direction. This means that they tend to become concentrated in a relatively early flow zone, which may be termed the ‘ligand zone’. Local concentration of certain ligands in a ligand zone means a localization of the feedback effect of the ligands. This in turn increases the rate of reaction in the ligand zone and thus the production of more ligands and still stronger feedback in this zone. This means concentration and localization of the pioneer organism in spite of the volcanic flow situation.
(b) Evolution by double feedback
Another, not yet discussed, however, crucial aspect of evolution is based on a mechanism, which I have termed ‘double feedback’ (Wächtershäuser 1992, 1994). For understanding this mechanism, let us assume the pre-existence of a pioneer metabolism with a specific reaction pathway having a specific product that feeds back autocatalytically into the metabolism. We further assume that the self-expansion of the metabolism brings forth a new reaction pathway, which branches from the specific reaction pathway and thus weakens it, since it withdraws an intermediate. Now, if a product of the new branch pathway feeds back autocatalytically into this very same new branch pathway, the weakening of the pre-existent specific reaction pathway is amplified. Thus, a feedback which is positive (autocatalytic) for the branch pathway will be negative for the pre-existent specific reaction pathway and thereby for the whole metabolism. This is an egotistic, virus-like (‘virulytic’) feedback effect that tends to weaken or even kill the pioneer metabolism. A completely different situation arises, if the product of the new branch pathway exhibits a ‘double feedback’, an ‘egotistic feedback’ combined with an ‘altruistic feedback’. The egotistic feedback consists of the positive feedback into the new branch pathway. The altruistic feedback consists of a positive feedback into the pre-existent metabolism, thereby compensating for the egotistic feedback. The reaction products with such a double feedback effect are termed ‘vitalizers’ (Wächtershäuser 1992, 1994). There are numerous examples for such vitalizers in extant metabolism. The coenzymes catalyse many reactions including reactions within their own biosynthesis. The ribosomes catalyse the syntheses of all proteins including ribosomal proteins. The protein translocases catalyse the incorporation of numerous membrane proteins into membranes, including the proteins of the translocases themselves. The principle of double feedback holds at all levels of evolution from the pioneer organism through nucleic acid-implemented evolution to the evolution of the highest forms of human institutions (Wächtershäuser 1992, 1994).
(c) Metabolic inheritance
The chemical reactions are dependent on the (environmental) reaction conditions. By positive feedback (single or double) of the product of a reaction into this reaction itself, this dependence is lessened compared to the feedback-free de novo reaction, which means that the reaction becomes to a certain degree independent from the environment, i.e. autonomous. This means that an autocatalytic feedback reaction, once initiated de novo, will continue to run even under conditions, which no longer allow its de novo initiation. This is how history enters the picture. The existence of an autocatalytic feedback cycle signifies the historic fact of its previous de novo ignition. In this sense, each autocatalytic feedback effect constitutes an instance of inheritance or a memory effect and the whole process of evolution may be abstracted to a concatenation of such memory effects.
7. On the chemoautotrophic origin of the genetic machinery
So far, we have discussed the hypothetical pioneer organism as a chemical entity controlled by the universal laws of chemistry. We now investigate how this chemical entity could have been the starting point of the particular historic process of evolution, by which the chemoautotrophic pioneer organism evolved into organisms with a genetic machinery. This must have happened in coevolution with cellularization, which will be addressed later. It should be stressed at the outset that there is a huge body of literature on the problem of the origin of genetic machinery in the context of a cold prebiotic broth. Naturally, much of this work is not directly applicable to the context of a chemoautotrophic origin under hot, volcanic conditions. Therefore, we will restrict the present discussion to certain salient aspects of the new view of the problem that is forced upon us by the assumption of a chemoautotrophic pioneer organism.
All extant organisms have a multi-component genetic machinery consisting partly of proteins and partly of nucleic acids. The nucleic acids consist of structural units (nucleotide units) derived from phospho-ribose and heterocyclic bases. According to the theory of a chemo-autotrophic origin of life, these structural units must have originated by carbon fixation. Various more or less vague synthetic possibilities have been explored theoretically (Wächtershäuser 1988a,d, 1992). The peptide cycle shown in figure 2 now adds surprisingly direct experimental evidence. The hydantoin rings are formed at the N-terminal ends of peptides by carbon fixation. Such hydantoin rings have a close structural similarity to the imidazole portion of the purine bases in extant nucleic acids. The purines are to date biosynthesized by pathways involving carbon fixation. This suggests that in the extant biosynthesis of purines, the most ancient manner of synthesis by carbon fixation has been somehow preserved. Further, this experimental result brings the two ancient syntheses of peptides and purines into a close connection and it lends support to the possibility that among the earliest ‘nucleic acids’ may have been chemoautotrophically produced polypeptides with pendant hydantoin-derived bases (cf. Nielsen 1993). Such primitive ‘nucleic acids’ would have been followed later on by phospho-sugar-based nucleic acids.
The structural and functional properties of the general class of nucleic acids with a sugar-phosphate backbone have been intensely studied both theoretically and experimentally by Albert Eschenmoser and his school. It was found that α-threofuranosyl oligonucleotides with vicinally connected (3′→2′) phosphodiester bridges have similar base-pairing properties as RNA. Moreover, they form hybrids with RNA and DNA, which is a necessary condition for an intermediary evolutionary role. Most importantly, however, they show a much increased hydrolytic and thermal stabilities compared to RNA. Finally, the C4-sugar component could be the result of a C2+C2→C4 synthesis (Schöning et al. 2000). This makes it a good candidate for a nucleic acid originating in a hot chemoautotrophic system. In this context, the problem is further constrained by adding to the categories of structural and functional feasibility, the metabolic category of autotrophic synthetic accessibility. This is still a largely uncharted territory. One crucial problem within this territory, the problem of phosphorylation and of activation of nucleotides by phosphoanhydride formation, has been recently addressed experimentally (Leman et al. 2006). As explained earlier, this solution is quite compatible with the context of a chemoautotrophic origin of life and a potential solution of the problem of nucleic acid synthesis by nucleotide condensation.
Any solution of the problem in the synthesis of nucleic acids in a chemoautotrophic context will have to address not only the question, how the synthetic intermediates, like phosphorylated sugars, could have been formed by carbon fixation, but also the question how such intermediates could have operated as feedback components within an autocatalytic metabolic network. The earliest function of phosphorylated sugars and nucleotides—long before the advent of base pairing—must have been that of ligands for promoting transition metal catalysts. Sugars are excellent ligands. The heterocyclic bases in the early nucleotides would have acquired the additional function of acid–base catalysis. Finally, with the advent of base pairing, a third catalytic function was added: catalysis of peptide formation by anchimeric positioning of nucleic acids with terminal amino acid esters, to which we turn next.
Today almost all the peptides and proteins are synthesized by ribosomal catalysis. As a simple specific model en route to the ribosomal machinery, let us assume surface-bonded pro-tRNAs in the form of nucleic acid hairpin structures that became aminoacylated terminally by activated amino acids. Let us further assume that two of these surface-bonded pro-tRNAs became located side by side and attached by base pairing with their loops to a surface-bonded nucleic acid strand as pro-mRNA. The result of this base pair-assisted anchimeric positioning is a more efficient synthesis of dipeptides to be followed later by oligopeptides. This is the origin of the simplest ribosomal machinery consisting of two pro-tRNAs and one pro-mRNA. It embodies the chemical (as opposed to organizational) core of the ribosomal process of translation.
In the preceding paragraphs, we have addressed first the formation of nucleic acids by polycondensation and then the function of nucleic acids as catalysts for peptide formation. Let us now try to tie these two processes together. We assume that in the earliest phases of evolution, these two processes were essentially insensitive to or non-selective for particular nucleic acid sequences. Only later, with the appearance of more diversified amino acids, and longer nucleic acids and peptides, the question of a control of base sequences in nucleic acids and of amino acid sequences in peptides came into the picture. Sequence-controlled (template-directed) nucleic acid synthesis, whereby a sequence is copied from an old strand to a new strand of nucleic acid, is called ‘replication’ and sequence-controlled peptide synthesis, whereby a nucleic acid sequence is copied into a peptide sequence, is called ‘translation’.
We are now in a position to formulate a basic question for the origin of life: what was the temporal order in which the two processes of replication and translation must have emerged. Before we answer this question for the chemoautotrophic theory, let us briefly recall the answer as given in the context of the prebiotic broth theory (Bada 2004) in order to guard against confusion. It is assumed in the prebiotic broth theory that a large library of random sequences would have been generated and accumulated in the primitive ocean and that ‘by chance, some of these polymers acquired the ability to catalyse their own imperfect self-replication [and therefore] soon dominated’ … in the ‘overall pool of polymers’ Thus, replication is indispensable for converting the polymer chaos of the prebiotic broth into some sort of molecular order. In this evolutionary context, replication fidelity is seen as a value in itself. The process of translation would have appeared much later.
In the context of a chemoautotrophic origin of life, we come to a drastically different conclusion. Here, the two processes of sequence control, those of replication and translation, must have become established jointly, by coevolution (cf. Lahav 1999), whereby the emergence and evolution of replication slightly trailed the emergence and the evolution of translation. Each new increment of added potential sequence selectivity of translation would have served as the functional advantage for a subsequent increment of added sequence fidelity of template-directed nucleic acid synthesis. In the course of this coevolution of translation and replication, the primary, direct, chemical mechanism of evolution by variation of the synthesis of peptides with direct autocatalytic feedback became first supplemented and later increasingly substituted by a secondary, indirect, genetic mechanism of evolution, whereby variations of replicating nucleic acid sequences led indirectly to variations of peptide sequences. This transformation led to a step-by-step replacement of primitive metallo-peptides by more and more complex coded metallo-peptides. It was the basis for the admission of more diversified amino acids into the system and for the establishment and expansion of the genetic code. The conclusion seems to be inescapable: the evolution of nucleic acid replication and of nucleic acid-catalysed peptide synthesis must have been intrinsically linked. This conclusion is in sharpest contrast to all the notions of a world of RNA life with RNA replication and without translation (cf. Forterre 2005).
Turning now to the advent of protein folding, we note that in the primitive metallo-peptides metal centres served as catalysts and as electron transfer agents. With the advent of cysteine in the set of coded amino acids, the metal centres of the metallo-peptides acquired an additional function, namely that of folding determinants, which operate by a few strong covalent metal–sulphur bonds. This may be seen as the origin of proteins, which are distinguished from peptides by their ability to form folded structures. This means that the emergent folded metallo-proteins acquired, from the start, the hyperthermostability that is required by the high temperature of the volcanic environment of early life. A few strong covalent bonds were sufficient and the overall sequence fidelity was not critical. Only after a sufficient increase of replication fidelity and translation fidelity, proteins could arise that owed their folding stability, not to a few strong covalent bonds to transition metal centres, but rather to the cooperation of a multitude of weak non-covalent group interactions. Only then, the covalent metal–sulphur folding determinants could disappear opportunistically one by one in an irreversible evolution down the temperature scale (Wächtershäuser 1998c, 2001).
Finally, within the context of a chemoautotrophic origin of life, there is no compelling reason to assume a particularly late arrival of DNA and of the segregation of the processes of DNA synthesis by replication and RNA synthesis by transcription. It is suggested here that the early polymerases were not able to discriminate between ribonucleotides for RNA and deoxyribonucleotides for DNA. This means that after the advent of a gene for ribonucleotide reductase, the transition from replication to transcription would simply be controlled by turning on the production of this reductase.
Incidentally, within the context of a prebiotic broth, Carl Woese (1971) suggested the origin of translation as a gradual, continuous changeover from metal complexes with polypeptides of a non-translationally produced set to a similar set of translationally produced polypeptides with the initial benefit of an increased rate of production as opposed to the increased sequence fidelity. In a similar vein, it has been suggested that early on FeS reacted with non-translational peptides or proteins in a prebiotic broth to form catalytic Fe–S proteins (Eck & Dayhoff 1966; Hall et al. 1971, 1974a,b).
To sum up, the early, direct, deterministic, chemical mechanism of evolution by ligand feedback is the precondition for the later emergence of an indirect, stochastic, genetic mechanism of evolution by sequence variations. This conversion is truly an evolution of the mechanism of evolution. It must have occurred in multiple steps and in parallel with a protracted multi-stage process of cellularization (Wächtershäuser 1988d, 1992).
8. Cellularization
(a) Surface lipophilization
For explaining the first stage of cellularization, we recall the experimentally demonstrated formation of acetylthioester as evolutionary precursor of acetyl-CoA (Huber & Wächtershäuser 1997). In extant organisms, acetyl-CoA serves as the universal starting material for the biosynthesis of fatty acids and of isoprenoid lipids by the mevalonate pathway. In both pathways, the first step consists of a Claissen condensation of acetyl-CoA followed by further reiterative condensations. It has been suggested that thioester condensation was the first synthesis of lipids: long-chain fatty or isoprenoid acids (Wächtershäuser 1992). These functioned as primitive lipids in the age of the surface metabolism. They have a low solubility in water and therefore must have accumulated at the interface between the inorganic substructure and the water phase, becoming in fact part of the organic superstructure. This had the consequence of a progressive lipophilization of the surfaces of the inorganic substructure by a two-dimensional solvent of sorts. Lipophilization in turn has the effect of lowering the activities of H2O and H3O+ near the surfaces, which disfavours hydrolytic reactions, while it favours all the condensation reactions (altruistic feedback) and ushers in greatly expanded metabolic possibilities. Of course, the formation of fatty or isoprenoid acids is itself based on condensation reactions, which also benefit from the lipophilization (egotistic feedback). This form of double feedback is not based on the effect of single ligand molecules, but rather on the collective effect of the accumulation of fatty or isoprenoid acid molecules on the surface.
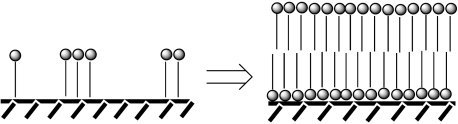
The promotion of lipid synthesis by lipid accumulation has the consequence of a formation of ever-longer molecules of the primitive lipids and ever more extensive lipid accumulation. At a certain surface concentration of the lipids, this leads to the self-organization of a multitude of lipid molecules in bi-layer membranes (figure 4). First, the membranes form discrete surface-supported patches. With further accumulation of the lipids, the membrane patches become larger until they coalesce into one coherent lipid membrane, which is still surface supported. In case of large mineral surfaces, the membrane may become similarly large. In case of a discrete mineral particle, the membrane may curve around the particle and ultimately surround it as an envelope (Wächtershäuser 1988d).
Figure 4.
Transition from surface lipophilization to a surface-supported bilayer membrane. The lipid molecule is shown as a single long hydrophobic rest bonded to a hydrophilic head group.
The process of accumulation of lipids is a developmental process, by which the surface situation slides inexorably from a fully hydrophilic situation to a hydrophobic situation with numerous and deep consequences for the metabolism. By the same token, the process of lipid accumulation is also an evolutionary process due to lipid feedback. This gives us an important general insight. At the beginning of life, there is no clear distinction between development and evolution. It is only after cellularization and after the emergence of the genetic machinery that a fast process of development becomes clearly distinguishable from a slow process of evolution.
(b) Semi-cellular structures
By further lipid accumulation, the surface-bonded membrane grew to cover larger surface areas of the substructure including eventually surface areas with tiny defects in the form of concave crevices or cavities, which naturally occur in the surfaces of inorganic material. For energetic reasons, a bi-layer lipid membrane would span such cavities rather than line them, thereby forming semi-cellular caverns (figure 5), in which the surface metabolism would continue. Further lipid accumulation would then enlarge the cavern. This mechanism created a ‘semi-cellular structure’, which is defined as an enclosed space, bounded partly by inorganic substructure and partly by membrane and filled with aqueous solution (cytosol). The remainder of the membrane would still have been anchored on the substructure.
Figure 5.
Embryonic semi-cellular cavern.
The semi-cellular structures constitute the beginning of individuation. However, the membranes of the semi-cellular structures are not very stable and subject to events of bursting and reconstitution. Still, the membranes of the semi-cellular structures are on average effective for isolating the interior cytosol from adverse conditions in the exterior water phase and for retaining and protecting products of the metabolism within the cytosol. The neutral, uncharged nutrients (e.g. CO, H2S and NH3) pass easily through the membrane so that the formation of the semi-cellular structure does not constitute a nutritional discontinuity. Within the semi-cellular structures, the inorganic surfaces are still available for catalysis. CO-fixation leads here to the formation of polar, ionic or zwitter-ionic organic compounds, which remain inside the semi-cellular structure. This increases the osmolality of the cytosol, which in turn leads to an osmotic pressure as driving force for further expansion of the semi-cellular structure. The cytosol is available for an unfolding cytosol metabolism with catabolic pathways beginning as internal salvage pathways by energy coupling. It is this stage that begins to accommodate a multi-component genetic machinery, which requires freedom of movement. Moreover, the membrane is now available for the step-wise evolution of membrane-related processes, such as chemiosmosis (Wächtershäuser 1997).
A main thrust of early evolution is concerned with the improvements of lipids in terms of the formation of membranes with greater stability. Since the membrane function of containment benefits from improvements of membrane stability, each improvement of the lipids with the effect of higher membrane stability is of immediate benefit for the semi-cellular organism. This leads eventually to the emergence of wedge-shaped lipids with two long-chain lipophilic rests attached to one small hydrophilic head group, e.g. to phosphatidyl lipids. With such wedge-shaped lipids, the lipid membranes become self-supporting cell membranes. This ushers in yet another stage of cellularization, the formation of self-supporting membranes. Before we turn to this important topic, we shall briefly touch upon a hypothesis for the origin of chemiosmosis.
(c) Origin of chemiosmosis
With the onset of semi-cellular structures having a stable, bi-layer lipid membrane separating an inner cytosol from the outer aqueous phase, chemical gradients develop automatically across the lipid membrane. A proton gradient arises as a necessary consequence of CO conversion into carbonic acid, formic acid, acetic acid, etc., which generates a pH difference between a relatively low internal pH and a relatively high external pH. The hydrophobic interior of a lipid membrane has a high resistance against the flow of ions, like H3O+ so that a high proton potential develops readily across the membrane.
The next step consists of a coupling of proton translocation across the membrane with membrane-bound redox reactions, which are thereby boosted from endergonic to exergonic energy profiles. Thereafter, some of the redox reactions become exergonic per se due to a change of the outer chemical conditions. These exergonic redox reactions can then drive proton translocation in the reverse direction against the proton gradient, thus increasing the proton potential. This constitutes a conversion of redox energy into proton gradient energy. Other redox reactions will remain endergonic. This means that the proton potential mediates an energy coupling between endergonic and exergonic redox reactions. An example of exergonic redox reactions would be the formation of pyrite by reaction (4.1), either at the outside of the membrane (Koch & Schmidt 1991) or at the inside of the membrane (Wächtershäuser 1997). Next, membrane-bound pyrophosphate formation (Baltscheffsky et al. 1966; Baltscheffsky 1967, 1971) would become established, now causing the proton potential to mediate energy coupling between redox energy and group activation energy. Finally, membrane-bound pyrophosphate formation would be first supplemented and later substituted by an emerging rotary ATPase.
This multi-step evolution of chemiosmosis, in which each step is facilitated by the previous steps, must have proceeded in coevolution with the establishment of the genetic machinery and in coevolution with cellularization. It may have taken place essentially completely within the semi-cellular phase of cellularization. Alternatively, it may have begun more or less deeply in the semi-cellular phase and found its completion in the subsequent pre-cell phase of cellularization, to which we turn next.
(d) Kandler's pre-cells
Carl Woese & George Fox (1977) introduced for the form of life at the deepest node in the tree of life the notion of a ‘progenote’, defined as ‘organism… in the throes of evolving the genotype–phenotype’. Subsequently, Woese specified: ‘the universal ancestor is not some unique entity, but rather, is a universal ancestor state’ with genetic mixing creating ‘a state of genetic communion’ and giving the appearance of a universal ancestor (Woese 1982), whose disjoint genes (likely RNA) ‘could have existed in high copy numbers’; and further: ‘the tempo of evolution at the time of the universal ancestor was very high’ (Woese 1987). This progenote concept was groundbreaking—the first coherent explanatory concept of life on the eve of speciation. It was the fruit of the discovery that all forms of cellular life classify into three distinct domains, Eubacteria (later Bacteria), Archaebacteria (later Archaea) and Eukaryotes (later Eukarya).
After the rooting of the tree of life (Gogarten et al. 1989; Iwabe et al. 1989) and its formal revision (Woese et al. 1990), Otto Kandler (1994a,b) proposed his pre-cell theory, which maintains some of the features of the progenote, while radically breaking with others. According to this theory, the organisms prior to speciation constitute a large coherent population of rapidly evolving ‘pre-cells’, defined as ‘metabolizing self-reproducing entities exhibiting most of the basic properties of a cell, but unable to limit the frequent mutual exchange of genetic information’. These pre-cells are seen as ‘multi-phenotypical’, having distinctly different metabolic phenotypes. Some sub-populations may be autotrophic, others heterotrophic; some anaerobic, others micro-aerophilic; some H2-consumers, others H2-producers, etc. In this way, the population of pre-cells, while unitary in terms of the genetic machinery, occupied a biosphere with diverse habitats. This brought the notion of a biosphere with ecosystems into the discussion, which meant that the pre-cells were sophisticated rather than primitive, the result of a long, Darwinian evolution over perhaps several hundred million years. A counterproposal of Koonin & Martin (2005) suggests that the entire process of evolution from first autocatalytic reactions to full-blown Bacteria and Archaea occurred at a single site on the ocean floor within a single growing FeS-mound, which would mean a period of several 10 000 years for the whole affair.
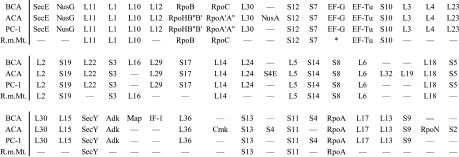
A characterization of the pre-cells, as they existed at the dawn of speciation, is in principle possible by comparison of bacterial and archaeal genomes. It was found (Wächtershäuser 1998b; cf. Lathe et al. 2000) that in all sequenced bacterial and archaeal genomes, a number of relatively short conserved gene clusters, mainly for transcription and translation, had different lengths from phyla to phyla and could be organized into overlapping gene cluster alignments, whereby astonishingly long segments of ancestral genome segments of the common ancestors of all Bacteria (BCA; 46 genes) and all Archaea (ACA; 53 genes) can be reconstructed (figure 6). Previously, only individual short operons (up to 11 genes) had been recognized as ancestral (Siefert et al. 1997).
Figure 6.
Reconstructed ancestral clusters of genes (designated as gene products) of the common ancestors of Bacteria (BCA) and Archaea (ACA), of the pre-cells (PC-1) at the eve of speciation and of the mitochondrion of R. americana (R.m.Mt.) (* four other genes instead of EF-G).
These two reconstructed genome segments are amazingly similar and even the few deviations are again constant throughout the domain Bacteria and throughout the domain Archaea, respectively. Lateral cross-phyla transfers of gene clusters cannot be invoked for explaining the extreme conformity within and between the domains of Bacteria and Archaea. The lateral transfer of such a large cluster of genes coding for plural components of vital and complex genetic machineries between distantly related groups of organisms would generate a high concentration of defunct hybrid machineries that cause havoc in the crucial processes of transcription and translation. They would be lethal. On the other hand, lateral transfers of individual genes of the genetic machinery that come to reside outside the reconstructed genome segments would be innocuous by causing only few hybrids and such transfers have indeed been demonstrated (Wächtershäuser 1998b). This means that the pre-cells (PC-1) on the eve of speciation already had a genome with a stable cluster of more than 40 canonical genes mainly for transcription and translation, which underwent little change of gene order over the billions of years that followed. Before the publication of the ancestral gene cluster, it was asserted that ‘gene order is not conserved in bacterial evolution’ (Mushegian & Koonin 1996).
The reconstructed gene cluster reveals much about the pre-cells on the eve of speciation (PC-1):
The presence of RNA polymerase genes means transcription from DNA to RNA.
The genome must have consisted of circular double-stranded DNA chromosomes as required for stability. Linear chromosomes as suggested by Woese (1998) for the progenote would have been unstable. The chromosomes may have existed each in redundant multiple copies (polyploidy) as suggested by Woese (1998); and they may have had the size of large extant plasmids.
The presence of the gene for the universal anti-termination factor NusG suggests that transcription may have continued over long DNA segments, perhaps around the entire circle of the chromosome, perhaps as a form of rolling circle transcription without promoters and perhaps without controlled termination, generating long polycistronic transcripts of varying lengths.
This suggests that pre-cell replication could have been a rolling circle process as well. A transition from rolling circle transcription to rolling circle replication could have happened without change of the polymerase, since the early polymerase would not have been selective enough for discriminating between the closely similar ribonucleotides and deoxyribonucleotides. The transition from transcription with ribonucleotides to replication with deoxyribonucleotides would have required nothing more than an activation of a ribonucleotide reductase for the conversion of ribonucleotides to deoxyribonucleotides. This would readily explain why RNA primers are needed in DNA replication to this day. Rolling circle replication would have involved all chromosomes. Initiating nicks would have occurred indiscriminately in both strands, which means roughly equal numbers of copies of both strands for subsequent annealing to double-stranded daughter chromosomes with sticky ends for ring formation. Relaxation by the nicks would have obviated helicase and topoisomerase functions.
The presence of genes for ribosomal proteins and elongation factors EF–Tu and EF–G means a highly advanced process of translation with a two-subunit ribosome (cf. Woese 1987, 1998).
The conserved amino acids in alignments of the sequences of the canonical proteins show that the process of translation in the pre-cells (PC-1) already involved a next to complete set of amino acids and by extension, a corresponding set of tRNAs and a corresponding set of aminoacyl-tRNA synthetases.
By extension, the pre-cells (PC-1) must have had a metabolism with scores of enzymes.
Upon fission of a polyploidal pre-cell (PC-1), the chromosomes, are partitioned (cf. Woese 1998) between the daughter pre-cells, as are all the other components.
- A far-reaching conclusion is drawn from the presence of the genes for SecE and SecY, the essential sub-units of protein translocase (van Wely et al. 2001). Since protein translocases operate in a lipid membrane environment, this means that the pre-cells (PC-1) had a stable lipid membrane. This suggests a mechanism for the frequent mutual exchange of the genetic information in accordance with the following set of postulates (Wächtershäuser 2003):
- The pre-cells had a bi-layer membrane and membrane stability required wedge-shaped phosphoglycerol lipids with a polar head group and two long-chain hydrophobic tails.
- The enzymes for the synthesis of the chiral phosphoglycerol lipids of the pre-cells were not enantio-specific and therefore generated racemic lipids.
- The lipid membrane permitted frequent fusions (and fissions) of the pre-cells with wholesale combination of the genomes and stepwise increase of polyploidy. Cell fusions are still found in extant Thermococcus coalescens (Kuwabara et al. 2005). Note that wholesale combination of genomes by fusions and fissions is not only far more intense than the horizontal transfer of individual genes from donor cell to acceptor cell. It is a qualitatively different process. It constitutes a highly promiscuous quasi-sexual process generating a huge gene pool undergoing a massively parallel, very rapid evolution of the pre-cell population, which fits the high tempo suggestion by Woese (1987). It involved large-scale reshuffling of the genetic endowments for uptake and utilization of nutrients and energy. These were differentially assorted under the selection pressures of different habitats, while the genetic machinery remained the same.
- The racemic lipid membranes of the pre-cells continuously underwent spontaneous symmetry breaking by spatial lipid segregation into an interdigitated micro-pattern of two homochiral lipid domains within each pre-cell membrane.
- By the frequent fusions and fissions, the pre-cells continuously segregated into two subsets with a predominance of one lipid enantiomer or the other. This occurred by strictly physicochemical forces.
- The racemic pre-cell membrane, while not as stable as a homochiral membrane, was stable enough for maintaining the integrity of hyperthermophilic pre-cells and for maintaining a definite organism–environment dichotomy over a long period of evolution, perhaps over hundreds of million years.
It has been argued compellingly that the common ancestors of all Bacteria and of all Archaea and the ancestral organisms on the eve of speciation were hyperthermophiles (Di Giulio 2003; Schwartzman & Lineweaver 2004). We can now add that fusions and fissions are only possible among essentially isothermal pre-cells, which means that all pre-cells were hyperthermophiles.
The situation of the biosphere changes decisively with the segregation of the pre-cells into the two domains of prokaryotic life: Bacteria and Archaea. To this topic we turn next.
9. Divergence of the domains bacteria and archaea
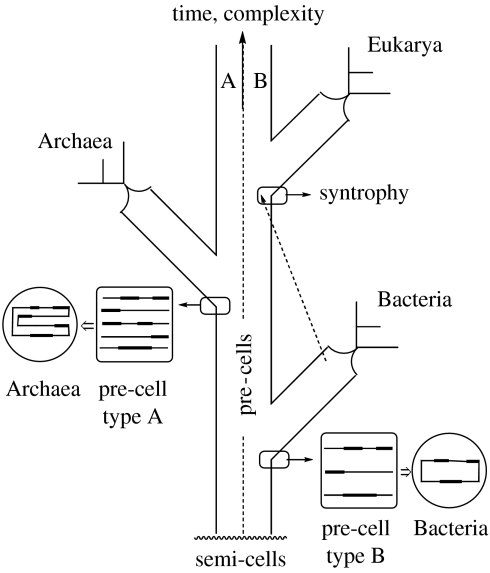
Kandler's (1994a,b, 1998) pre-cell theory marks a major departure from conventional phylogenetic thinking, a Gestalt switch. Conventionally, phylogenetic trees have been represented with bifurcations, whereby both branches are equivalent. Kandler breaks with this pattern regarding the two deepest nodes in Woese's tree of life (Woese 1987; Woese et al. 1990) and replaces it by a pattern of divergence from a continuing trunk evolution (figure 7). More specifically, Kandler suggests that founder populations of the domains Bacteria and Archaea diverged from a trunk of pre-cells. The Bacteria and Archaea are presented as diverging at different stages of the continuing, rapid trunk evolution of the population of pre-cells, the Bacteria branching off first at a less advanced stage and the Archaea branching off later at a more advanced stage.
Figure 7.
Divergence of the domains Bacteria and Archaea from a trunk evolution of pre-cells with two subpopulations A and B and origin of the domain Eukarya by endosymbiosis due to metabolic syntrophy between members of the domain Bacteria and members of the pre-cell subpopulation B with a predominance of Bacteria-type lipids (modified from Wächtershäuser 2003).
Kandler's new phylogeny is the basis for a mechanistic explanation of the origin of the domains of the Bacteria and Archaea. This new mechanistic explanation is based on lipid chirality (Wächtershäuser 2003). It assumes a changeover from racemic pre-cell lipids to the homochiral lipids of Bacteria and Archaea. For the divergence of the domains Bacteria and Archaea from the trunk evolution of pre-cells, the fortuitous emergence of new enzymes for enantio-specific lipid synthesis was decisive, notably the emergence of enantio-specific enzymes for reduction of dihydroxyacetone phosphate to glycerol-3-phosphate and glycerol-1-phosphate, respectively (Koga et al. 1998). Each of these two lipid enantiomers was selected as adaptation to the dominant lipid enantiomer in one (type B) or the other (type A) of the two placeholder subpopulations of pre-cells. A founder population of Bacteria with a homo-chiral lipid membrane diverged first from pre-cells with a relatively simple information processing machinery, while a founder population of Archaea with the antipodal homo-chiral lipid membrane diverged subsequently from more evolved pre-cells with more complex information processing (Wächtershäuser 2003). This means that two and only two domains could diverge. The divergence was physicochemically predetermined by the racemic nature of the ancestral pre-cell lipids. While some authors have adopted this lipid chirality mechanism (Pereto et al. 2004), other publications (Koonin & Martin 2005) are ignorant of the fact that the only strict difference between Archaea and Eukarya lies in their antipodal lipid chiralities (Koga et al. 1998). Moreover, the above proposal is in sharp contrast to the ‘genetic annealing theory’ by Carl Woese (1998, 2002).
After the divergence of the domains Bacteria and Archaea fusions and fissions would have continued, but more frequently within the domain populations than between the domain populations and the pre-cell population. This was the initiation of a subsequent relentless process, by which the unitary populations of Bacteria and Archaea embarked on their own distinct paths of evolution with a progressive alienation of the domains Bacteria and Archaea from each other. This must have occurred by progressive lipid incompatibility through head group modification and specialization of the lipophilic tails, mainly to fatty ester lipids in the bacterial domain and to ether lipids of isoprenoid alcohols in the archaeal domain (Wächtershäuser 2003).
The above proposal assumes that the divergence of the Bacteria and Archaea is due to extensive gains of novel (enantioselective) enzyme capabilities. It is astounding that some authors (Koonin & Martin 2005) counter-represented this mechanism as ‘differential loss’ from a ‘biochemically most-potent organism that ever lived’, which they peculiarly understand as ‘a form of life that existed in two dimensions’. The same authors assume in their own competing theory, a universal ancestor without lipids, believing, as they do, in ‘the unrelated isoprene ether versus fatty acid ester chemistries of the membrane lipids in Archaebacteria and Eubacteria, respectively’. They are factually wrong on four accounts according to well-established biochemistry: (i) the archaeal mevalonate lipid pathways and the bacterial fatty acid lipid pathways begin identically with the Claissen condensation of acetyl-CoA to β-keto butyric acid; (ii) both domains have phospho-glycerol lipids as their core lipids; (iii) the lipids of both the domains have overlapping sets of head groups and (iv) the membranes of both domains contain membrane reinforcer lipids of the squalene-type.
Returning again to the lipid-based theory of the origin of speciation (Wächtershäuser 2003), it is proposed that in the course of the bacterial evolution and the archaeal evolution, the genetic machinery changed independently, yet in an analogous manner. In the bacterial domain, the multiple plasmid-type chromosomes combined to one large circular bacterial chromosome. This facilitated linkage of bacterial replication with bacterial cell division. Analogous changes occurred independently in the archaeal domain. Some plasmids remained and their replication became synchronized with cell division. Others turned into rolling circle viruses. DNA-replication and transcription became separated. In both the Bacteria and Archaea, non-homologous enzyme complexes for DNA replication (Leippe et al. 1999) with coordinated leading strand and lagging strand synthesis departed from the much simpler system of rolling circle replication of the pre-cells, perhaps under the directing influence of the two different kinds of membrane lipids (cf. Pereto et al. 2004). In addition, the mechanisms of transcription and translation were refined independently in both the domains (Woese 1998). The Bacteria and Archaea continued to evolve as unitary populations until the emergence of fusion-prohibiting means such as rigid outer cell walls (Woese 1983; Kandler 1994a,b, 1998), which triggered the branching within the bacterial and archaeal phyla.
10. Divergence of the domain eukarya
With the above-explained divergence of the two mutually exclusive domains Bacteria and Archaea, the early evolution might be perceived as having come to a natural conclusion, if there were not this disturbing fact of a third domain, the nucleated Eukarya (formerly Eukaryotes). Numerous theories in the past have tried to reduce the emergence of the eukaryal cell to a combination of bacterial and archaeal cells. Specifically, these theories postulate an endosymbiosis between the cells of advanced species of the domains Archaea and Bacteria (reviewed in Doolittle 1998; Martin & Müller 1998; Moreira & Lopez-Garcia 1998; Martin 2005). All these theories have the unavoidable and unacceptable consequence that an endosymbiosis between the bacterial and archaeal cells would necessarily give rise to hybrid membranes with a mixture of bacterial lipids and archaeal lipids. As explained earlier, such a hybrid membrane would be less stable than the enantiomerically pure lipid membranes of the Bacteria and Archaea. This means that such an endosymbiosis between Bacteria and Archaea would be an evolutionary impossibility.
The theory advocated here (Wächtershäuser 2003) avoids this problem by postulating an endosymbiosis between bacterial cells and pre-cells (figure 7). The pre-cells engaging in this endosymbiosis are recruited from the subpopulation of pre-cells (type B) with membranes having a predominance of the bacterial-type lipid enantiomer. By such endosymbiosis, the emerging Eukarya acquire membranes consisting predominantly of bacterial-type lipids.
Two variants of endosymbiosis are imaginable. In the first variant, one wall-less bacterial cell would have spread around the pre-cell (cf. Martin & Müller 1998). In the second variant, several wall-less bacterial cells would have clustered around one pre-cell, subsequently merging with each other by fusion (cf. Moreira & Lopez-Garcia 1998). In both the variants, the pre-cell was preserved as a discrete entity. Moreover, it must be remembered that the pre-cells as well as the primitive wall-less Bacteria existed as huge populations. This means that the endosymbiosis could have been a population affair generating a large population of endosymbiotic entities. According to the present theory (Wächtershäuser 2003), the endosymbiosis between pre-cells and Bacteria would have been driven by a metabolic syntrophy between heterotrophic pre-cells and autotrophic Bacteria. The exchanged metabolic commodity could have been hydrogen (cf. Martin & Müller 1998; Moreira & Lopez-Garcia 1998).
The Eukarya differ fundamentally from the Bacteria and Archaea in terms of the organization of the genetic machinery with multiple chromosomes being housed within a nucleus bounded by a double membrane. This unique and complex organization must be explained by any theory on the origin of the Eukarya. The theory presented here delivers an explanation in addition to solving the lipid problem. It explains the eukaryal genetic machinery as an automatic, irreversible, almost deterministic consequence of the endosymbiosis, whereby a pre-cell was converted into the nucleus. The following 11 features that are shared by all extant Eukarya may be explained by the historic origin of the nucleus in an endosymbiotic pre-cell within a bacterial host.
The non-organellar genome is polychromosomal and the chromosomes are housed inside the nucleus. This can be explained by the hypothesis that a polychromosomal pre-cell turned into the nucleus, whereby the pre-cell chromosomes became trapped and separated from the outer cell membrane by the nuclear double membrane. Therefore, their replication could not become linked with the outer cell membrane during cell division. This means that there was no selective advantage in their unification into one large circular chromosome as was the case with the Bacteria and Archaea.
The nuclear membrane is endowed with pores and connected with an endoplasmic reticulum. This can be readily explained as an automatic consequence of the fusion of the outer bacterial cells in accordance with the above second variant, which is based on an explanatory principle due to Moreira & Lopez-Garcia (1998).
The non-organellar chromosomes are linear as opposed to the circular bacterial and archaeal chromosomes. This can be explained by their possible origin in the linear intermediates of the hypothesized rolling circle replication of the pre-cell chromosomes, perhaps with a multiplication in length.
Reproduction is based on a process of mitosis. The higher Eukarya, like plants and animals, have an open mitosis, while all others have a closed mitosis (Hausmann 1985). This finds a simple explanation in the hypothesis that the nucleus originated from a polychromosomal pre-cell, which means that closed mitosis is original and open mitosis is derived.
The nuclear chromosomes contain genes of bacterial origin. Approximately 75% of these bacterial genes in the nuclear chromosomes are clearly of non-organellar vintage (Esser et al. 2004). This can be explained with an evolutionary process, whereby the outer bacterial chromosomes were lost and their indispensable genes became relocated into the nuclear chromosomes.
The intermediary and energy metabolism is restricted to the outer cytoplasm. As a corollary protein synthesis by translation is also restricted to the outer cytoplasm, i.e. to exist in close quarters with the metabolism, where the enzymes are needed. This can be explained by the hypothesis that in the endosymbiosis, it would have been the bacterial host cell, which took charge of intermediary and energy metabolism, with the advantage that this metabolism moved closest to the influx of nutrients. Incidentally, some translation appears to have remained to this day inside the nucleus (Brogna 2001; Ibora et al. 2001). This is simply explainable as a leftover from pre-cell days.
Transcription occurs exclusively inside the nucleus and the mRNAs become short and capped. They are exported through the nuclear pores into the cytoplasm. This can be explained by the hypothesis that the expression of the genes became separated from their original location in the pre-cell turned nucleus and that the requirement of their transport through the nuclear pores did not permit preservation of long operons as they are active in extant Bacteria and Archaea.
The non-organellar ribosomes are synthesized in a peculiar manner. The ribosomal RNAs are synthesized inside the nucleus. The ribosomal proteins are synthesized outside the nucleus in the cytoplasm. Some of the ribosomal proteins are then imported into the nucleus for assembly with the ribosomal RNAs to form incomplete ribosomal sub-units. The incomplete ribosomal subunits are then exported through the pores of the nuclear membrane into the cytoplasm, where they are completed by the addition of further ribosomal proteins. The completed ribosomes are then used in the cytoplasm for translation. This roundabout pattern can be explained by the hypothesis that the incipient pre-cell endosymbiont inside the bacterial host would have maintained a pre-cellular process of translation for some time. The incomplete ribosomal subunits would simply reflect their origin in primitive pre-cellular ribosomes, thus giving us insights into the most ancient ribosomes and their early evolution.
Most genes in the nuclear chromosomes are infested with introns of unknown origin. This infestation with introns is not lethal or even particularly deleterious due to a peculiar processing (maturation) of the primary mRNAs inside the nucleus. The introns are spliced out and the remaining mRNA segments are interconnected to form a mature, functional mRNA. This can be explained by the hypothesis that a physical isolation of the intra-nuclear, pre-cell-derived space from the extra-nuclear Bacteria-derived cytoplasmic space would have ensued with endosymbiosis. This means that the intra-nuclear space would have become a safe compartment for intron splicing so that immature intron-laden mRNAs would have been prevented from entering the cytoplasm, thereby the metabolism would have been protected from being messed up with products of a translation of immature mRNAs.
The genes for the intermediary metabolism are most closely related to the Bacteria while the genetic machinery is most closely related to and actually more complex than the genetic machinery of the Archaea. This can now be explained with reference to the hypothetical phylogeny of figure 7. The Bacteria are shown as diverging first from relatively primitive pre-cells. The Archaea are shown as diverging later from evolutionarily more advanced pre-cells. The Eukarya are shown as diverging last (by endosymbiosis) from pre-cells that would have been still farther evolved than the pre-cells, from which the Archaea originated. This rendered the pre-cell-derived genetic machinery of the Eukarya similar to, but more evolved than the genetic machinery of the Archaea. The close relationship of the metabolic genes of the Eukarya to those of the Bacteria is simply due to their bacterial origin.
The genetic machinery inside the nucleus has somewhat ‘primitive’ features with numerous small RNAs being involved in replication, transcription and mRNA maturation. This can now be explained by the hypothesis that these ‘primitive’ features would date back as leftovers to the workings of the ‘primitive’ pre-cells that were involved in endosymbiosis.
With these 11 explanatory points, the presentation of my theory of the origin of the Eukarya by endosymbiosis of pre-cells and wall-less Bacteria is completed. The conventional assumption that a methanogen turned into the nucleus by symbiosis with a proteo-bacterium to form the incipient eukaryotic organism does not have a similar explanatory power. In closing, I would like to make a short, but significant digression into an older theory (Martin & Müller 1998). This older theory postulates that an endosymbiosis between a proteo-bacterial cell and a methanogen cell generated the first mitochondrion as the decisive initial event of the creation of the eukaryal cell, the nucleus appearing later by an unspecified series of endogenous events. Given all the facts of the Eukarya to be explained, the explanatory power of this older theory seems to be lacking. In addition, this older theory is at odds with the fact that the gene order of the mitochondrial genome of the protist Reclinomonas americana has a great similarity to the conserved gene cluster of the Bacteria (see figure 6).
11. Concluding remark
Our considerations from the vantage point of the pioneer organism are apt to change our view of the process of evolution. We recognize that we are faced in fact with two parallel, overlapping processes of evolution. With the pioneer organism, begins a primary and direct process of evolution. It is chemically determined and directionally fixed by the universal laws of carbon fixation on transition metal catalysts. Out of this primary, direct process of evolution arises later a secondary and indirect process of evolution by the genetic machinery. Accidental, non-directional DNA mutations form the material base for this secondary process of evolution. They affect the organism only indirectly by gene expression and by the fixation of beneficial DNA mutations in the population by selection. With the theory presented here, however, we see the necessity of interpreting this selection not merely as an adaptation to the environment, but first and foremost as an adaptation to the persisting chemically determined primary process of evolution; and chemically deterministic aspects of the process of evolution remain decisive even up to the level of the origin of the three domains of life.
Acknowledgments
The manuscript has been improved owing to much appreciated remarks by an unknown referee and due to corrections by the editor. Support by the Deutsche Forschungsgemeinschaft is gratefully acknowledged. I thank Dr Claudia Huber for helpful discussions and Dr Adelbert Bacher for generously supplying laboratory facilities and extraordinary material support. The paper would not exist without encouragement, help and correction (paragraph-by-paragraph and sentence-by-sentence) by Dorothy Wächtershäuser.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
Discussion
J. P. Ferris (Department of Chemistry and Chemical Biology, Rensslaer Polytechnic Institute, New York, USA). The first life will be very simple and may not have been enclosed in a membrane like a vesicle. The biomolecules for the first life may have been formed on a mineral surface and remained bound to the mineral. There could have been a steady state of molecules washed off from the surface and those formed on the surface from monomers in the environment. The processes of replication and ligation could have occurred on the mineral surface.
G. Wächtershäuser. If you replace the term ‘monomers in the environment’ by ‘inorganic nutrients’, this is the principle of my theory of a self-selecting chemoautotrophic surface metabolism (Wächtershäuser 1988d), which may extend to polypeptides or polynucleotides prior to or in coevolution with the extended cellularization process. There is self-selection because there is for each surface-bonded constituent, a certain rate of bleeding out that translates inversely into a residence or retention time. The self-selection leads automatically to stronger bonding constituents. A sufficient openness of the surface, i.e. an effective non-cellularity, is a precondition for this initial phase of self-selection. Salvage of detached (e.g. hydrolysed) monomers (in situ or downstream; and later inside the semi-cells or pre-cells) requires reactivation, which in turn requires conversion of hydrolytically stable redox energy into hydrolytically instable group activation energy.
C. N. Matthews (Department of Chemistry, University of Illinois at Chicago, USA). Many experiments in prebiotic chemistry have shown that amino acids, peptides, nitrogen heterocycles, etc., are readily formed, plausibly, without metal catalysts. Are transition metal catalysts, as in your model, essential for peptide synthesis, etc.?
G. Wächtershäuser. Our chemical experience tells us that transition metal catalysts, which can have different oxidation states, are essential for certain important metabolic redox reactions. Condensation reactions do not directly depend on the redox chemistry of transition metals, but only indirectly by way of the synthesis of the monomers and by way of energy coupling from redox energy to group activation energy. Polymers form the bulk material of extant life. Therefore, conventional theories have focused on proteins or nucleic acids and their components. Transition metals are ‘trace elements’ and therefore their essentiality for the origin has been underestimated in the conventional prebiotic broth theories. They may have been considered in conjunction with proteins, but not as the keys for a chemoautotrophic origin of life at the level of a formation of low-molecular organic compounds by redox chemistry.
D. W. Schwartzman (Department of Biology, Howard University, Washington DC, USA). Why is it necessary for emergence of Bacteria to have preceded archaeal emergence, if a temperature gradient likely existed at biogenesis? Thus, using the methodology of retrodiction archaeal protocells should have emerged at higher temperatures (present upper limit ca 120°C), bacterial protocells at lower temperatures (present upper limit ca 95°C) in the temperature/chemical gradient between the hydrothermal regime and the ambient seawater? (Note the likely correspondence of these temperature limits to distinct lipid stability of Archaea versus Bacteria.)
G. Wächtershäuser. The branching order proposal is a consequence of the tree topology and of the rooting of the tree, which both result from sequence comparisons. By genome comparison, the universal ancestor was already a highly advanced organism. I believe the conclusion is inescapable that it must have been a free cellular organism with a lipid membrane (a Kandler pre-cell), the result of perhaps several hundred million years of evolution. There are good reasons to believe that it was a hyperthermophile. From this point of departure, the Archaea and the Bacteria must have evolved irreversibly down the temperature scale. The particular thermophily of the particular extant bacterial and archaeal organisms that happen to be known to us may well reflect their individual degree of thermally downward evolution during the past several billion years. It does not mean that the particular thermophily of these particular extant organisms is identical to the thermophily at the origin of their domains. There certainly is room for the possibility that the last archaeal ancestor and the last bacterial ancestor may have had the same degree of hyperthermophily, which might even be more extreme than anything known to us today.
J. F. Allen (School of Biological Sciences, University of London, UK). I had a question, and some comments, to follow the fascinating lecture by Dr Wächtershäuser. I should like to present these now, since I believe that they are of general relevance to the topic of the meeting:
In this meeting, we have heard occasional reference to cells, but ‘cellularization’ has tended to be presented as a rather late stage, as if some acellular form of life came first, and evolution eventually hit upon cellular compartmentation as a useful architectural principle. Now, all living things that we know of either are cells, or they are composed of cells. I should therefore like to promote cells as ‘pre-conditions for the emergence of life on the early Earth’.
G. Wächtershäuser. According to my theory cellularization begins relatively early and occurs as an inevitable evolutionary process in several distinguishable stages: (stage 1) lipophilization of surface metabolists (beginning as the pioneer organism); (stage 2) semi-cellular organisms, bounded partly by the mineral substructure and partly by lipid membrane; (stage 3) pre-cells, surrounded by a closed lipid membrane, but subject to frequent fusions and fissions; and (stage 4) true cells with structures (either within the lipid membrane or as outer cell wall) that restrict cell fusion, first between domains and later within domains. This process is in my view pre-conditioned by the structure of the pioneer organism and it occurs as part of a coevolution.
J. F. Allen. If we consider the proposal presented by Dr Wächtershäuser, for catalysis of the first biochemical reactions by transition metals, I agree that redox chemistry is fundamental. However, I wish to add the word ‘vectorial’ to ‘redox chemistry’. This requires a definition of direction in space, which in turn requires a distinction between ‘inside’ and ‘outside’. If we allow this, then we require something resembling cells, and can consider the advantages of supposing that an autocatalytic, chemoautrophic mode of early metabolism occurred within compartments.
G. Wächtershäuser. I have always stressed that the surface metabolism is vectorial (Wächtershäuser 1988d, p. 457, left column, line 9). It establishes a below–above dichotomy, which not only gave way to, but also preconditioned the later cellular inside–outside dichotomy as placeholder. I have also always stressed the well-known fact, that from the point of view of statistical thermodynamics, the aqueous environment near a surface is fundamentally different from an aqueous environment far from the surface.
J. F. Allen. Firstly, in autocatalysis, the product of a series of reactions is also their substrate. Autocatalysis becomes more plausible if we imagine the autocatalytic cycle to be taking place in a confined space, so that the concentration of its components can build up. Surface catalysis alone cannot easily explain how a reactant becomes concentrated, since the volume of the solute is, for all practical purposes, infinite, being, one supposes, the volume of water in all the oceans of the World. On a flat surface, autocatalysis will tend to be prevented, or quenched, by dilution. There are other advantages of pre-formed inorganic cellular compartments, which I can enumerate.
Dr Wächtershäuser, please imagine the surface of a catalytic transition metal substrate, just as you propose, but fold it upon itself so that it becomes the inner surface of sphere. Do you not agree that we could then envisage the synthesis of simple organic molecules from inorganic precursors, in a self-reinforcing and self-sustaining cycle of reactions of water-soluble intermediates?
[There was a contribution from Dr Wächtershäuser at this point]
Now, in fact, such inorganic cellular compartments exist, and can also be produced, apparently, in the laboratory, as a result of mixing two solutions, which have solutes that react to form a ‘froth’ of insoluble precipitates. I have in mind the proposal of Russell that such structures, consisting of vesicles bounded by sulphides of iron and nickel, served as the first ‘incubators’ of life by permitting, containing, and sustaining chemoautotrophic synthesis (Martin & Russell 2003; Russell 2006).
A second advantage of supposing that life arose within pre-existing cellular compartments is that an internal environment is thus defined, and, as a result, homeostasis—the maintenance of a constant internal environment despite changes in the external environment—becomes possible. Homeostasis is surely another fundamental property of all living things.
A third advantage of ‘cells first’, at least in the cells envisaged in Russell's model (Russell & Hall 1997), is that a proton motive force can be understood to have been available right at the beginning, as a result of hydrothermal convection into mounds of these vesicles. This convection is thought to have brought relatively hot, alkaline and reducing solution into the vesicles, with a temperature, pH, and redox gradient across their boundaries becoming established owing to the cooler, more acidic, and more oxidizing external environment of the ocean. Mitchell's chemiosmotic theory (Mitchell 1961) appears to be universal for life, as we know it. There are few organisms that manage with only fermentation, and with substrate-level phosphorylation. The few that do manage depend on the products of chemiosmosis in other cells, and are therefore secondary, and derived. The first living cell was surely a chemiosmotic device, a compartmentalized system of energy transduction.
So, a proton motive force is another fundamental property of all living cells. Thus, hydrothermally produced electrochemical and pH gradient across pre-existing cell boundaries might explain, in principle, where the energy comes from for early biochemical synthesis. Geothermal convection is a source of energy that is continuous and indefinitely renewable. Given cells, convection could establish a ready-made chemiosmotic system of energy transduction, for coupling between oxidation–reduction, phosphoryl group transfer, and active transport.
[There was a contribution from Dr Wächtershäuser at this point]
Modern cells are bounded by lipid membranes with very low electrical conductivity, and very low permeability for hydrogen ions (protons). In complex cells such as those of eukaryotes, energy coupling membranes may constitute sub-compartments, or organelles, notably those of chloroplasts and mitochondria. However, there is no doubt that these originated, themselves, as separate cells, and that their coupling membranes were once cell membranes, facing the outside world.
It may argued that cellular compartments bounded by inorganic materials will be too leaky to protons and other ions to hold a proton motive force, in contrast to the more familiar compartments bounded by insulating lipid bilayers. My reply is that it must be recognized that an inorganic boundary needs to present only a kinetic barrier to free diffusion, since convection will continually replenish a reducing and alkaline internal, aqueous phase. An analogy might be that a sieve is not as good as a bucket for carrying water. However, if the mesh is fine, a sieve is better than nothing; it is a start. A sieve is also better than flat surface, from whose edges the water will rapidly spill away.
In summary: without cells, nothing can be described as ‘living’ in any sense, in which we might recognize the term. I submit that cells, pre-formed by geochemistry, not by biology, are a condition for the emergence of life on the early Earth.
G. Wächtershäuser. This whole proposal is based on an experimental artefact and on unsound geochemical assumptions and therefore it is without empirical merit. Let me briefly state the reasons. (i) The proposal of a precipitation of FeS-cells is chemically unknown and in need of experimental confirmation. Russell & Hall (1997) did publish a test result: a micrograph of a ‘freeze-dried section’ of a laboratory-generated iron sulphide mound comprising myriads of compartments (polygonal cavities about 10–20 μm in diameter with separating walls about 2–3 μm thick). The reported compartment structure is an artefact of sample preparation. Freeze drying of a hydrogel generates ice crystals, which upon evaporation leave behind pores. The size of the pores can be controlled by the rate of freezing. This is all very well known. (ii) We have no Hadean rocks that could show evidence for FeS-cells. (iii) The FeS-cell experiments were done with a FeCl2 solution of pH 1.9 (Russell et al. 1989). Acidity is crucial. Russell & Hall (1997) and Russell et al. (1998, 2003) calculated for the Hadean ocean pH 3.5, pH 4.5 (1998) or pH 5.5 (2003). This is anything but uncontroversial. Some geologists assume an alkaline Hadean ocean (Maisonneuve 1982; Kempe & Degens 1985, 1989; Grotzinger 1994; Sukumaran 2000). Others assume a nearly neutral Hadean ocean that developed from pH 5.6 at 100°C to pH 6.8 at 70°C (Morse & Mackenzie 1998). Therefore, a Hadean ocean with a combination of a low temperature (less than 20°C) and an acidic pH, as required (Russell et al. 2005) may well be a geochemical impossibility. (iv) Russell and co-workers used in their chemical garden experiments concentrations of FeCl2 of 3 wt% (1989) or 0.5 molar (1997), both by orders of magnitude too high.
As long as no valid empirical evidence is produced, a Hadean mound of FeS-cells must be considered unsubstantiated. In closing, it should be mentioned that any publication on the importance of porous structures for the origin of life should not ignore the seminal work by Hans Kuhn, who greatly elaborated on the importance of a pre-structured, porous rock environment for molecule-sorting processes in the origin of life (Kuhn 1972; Kuhn & Waser 1981, 1982; Kuhn & Kuhn 2003).
References
- Amend J.P, Shock E.I. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 2001;25:175–243. doi: 10.1111/j.1574-6976.2001.tb00576.x. doi:10.1111/j.1574-6976.2001.tb00576.x [DOI] [PubMed] [Google Scholar]
- Bada J.L. How life began on Earth: a status report. Earth Planet. Sci. Lett. 2004;226:1–15. doi:10.1016/S0012-821X(04)00470-4 [Google Scholar]
- Baltscheffsky M. Inorganic pyrophosphate and ATP as energy donors in chromatophores from Rhodospirillum rubrum. Nature. 1967;216:241–243. doi: 10.1038/216241a0. doi:10.1038/216241a0 [DOI] [PubMed] [Google Scholar]
- Baltscheffsky H. Inorganic pyrophosphate and the origin and evolution of biological energy transformation. In: Buvet R, Ponnamperuma C, editors. Chemical evolution and the origin of life. North-Holland Biomedical Press; Amsterdam, The Netherlands: 1971. pp. 466–474. [Google Scholar]
- Baltscheffsky H, von Stedingk L.-V, Heldt H.-V, Klingenberg M. Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science. 1966;153:1120–1122. doi: 10.1126/science.153.3740.1120. [DOI] [PubMed] [Google Scholar]
- Bebie J, Schoonen A.A. Pyrite and phosphate in anoxia and an origin-of-life hypothesis. Earth Planet. Sci. Lett. 1999;171:11–15. doi:10.1016/S0012-821X(99)00134-X [Google Scholar]
- Berrisford D.J, Bolm C, Sharpless K.B. Ligand-accelerated catalysis. Angew. Chem. Int. Ed. 1995;34:1059–1070. doi:10.1002/anie.199510591 [Google Scholar]
- Brack A. Cambridge University Press; New York, NY: 1998. The molecular origins of life: assembling pieces of the puzzle. [Google Scholar]
- Brogna S. Pre-mRNA processing: insights from nonsense. Curr. Biol. 2001;11:R838–R841. doi: 10.1016/s0960-9822(01)00499-7. doi:10.1016/S0960-9822(01)00499-7 [DOI] [PubMed] [Google Scholar]
- Cockell C.S. The origin and emergence of life under impact bombardment. Phil. Trans. R. Soc. B. 2006;361 doi: 10.1098/rstb.2006.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody G.D. Transition metal sulfides and the origins of metabolism. Annu. Rev. Earth Planet. Sci. 2004;32:569–599. doi:10.1146/annurev.earth.32.101802.120225 [Google Scholar]
- Cody G.D, Boctor N.Z, Filley T.R, Hazen R.M, Scott J.H, Sharma A, H S, Yoder H.S., Jr Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science. 2000;289:1337–1340. doi: 10.1126/science.289.5483.1337. doi:10.1126/science.289.5483.1337 [DOI] [PubMed] [Google Scholar]
- Corazza R. Field workshop on volcanic gases, volcano (Italy), 1982, general report. Geothermics. 1986;15:197–200. doi:10.1016/0375-6505(86)90125-2 [Google Scholar]
- Corliss J.B. On the creation of living cells in submarine hot spring flow reactors: attractors and bifurcations in the natural hierarchy of dissipative systems. Orig. Life Evol. Biosph. 1986;19:381–382. doi:10.1007/BF02422084 [Google Scholar]
- de Duve C. Basic Books; New York, NY: 1995. Vital dust: life as a cosmic imperative. [Google Scholar]
- de Duve C. Oxford University Press; Oxford, UK: 2002. Live evolving: molecules, mind, and meaning. [Google Scholar]
- de Duve C. Cambridge University Press; New York, NY: 2005. Singularities: landmarks on the pathways to life. [Google Scholar]
- de Wit M.J. On Archaean granits, greenstones, cratons and tectonics: does the evidence demand a verdict? Precambrian Res. 1998;91:181–226. doi:10.1016/S0301-9268(98)00043-6 [Google Scholar]
- Di Giulio M. The universal ancestor and the ancestor of Bacteria were hyperthermophiles. J. Mol. Evol. 2003;57:721–730. doi: 10.1007/s00239-003-2522-6. doi:10.1007/s00239-003-2522-6 [DOI] [PubMed] [Google Scholar]
- Dörr M, et al. Eine mögliche präbiotische Bildung von Ammoniak aus molekularem Sickstoff auf Eisensulfidoberflächen. Angew. Chem. 2003;115:1579–1581. [Google Scholar]
- Doolittle W.F. A paradigm gets shifty. Nature. 1998;392:15–16. doi: 10.1038/32033. doi:10.1038/32033 [DOI] [PubMed] [Google Scholar]
- Drobner E, Huber H, Wächtershäuser G, Rose D, Stetter K.O. Pyrite formation linked with hydrogen evolution under anaerobic conditions. Nature. 1990;346:742–744. doi:10.1038/346742a0 [Google Scholar]
- Eck R.V, Dayhoff M.O. Evolution of the structure of ferredoxin based on living relics of primitive amino acid sequences. Science. 1966;152:363–366. doi: 10.1126/science.152.3720.363. [DOI] [PubMed] [Google Scholar]
- Esser C, et al. A genome phylogeny for mitochondria among α-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol. Biol. Evol. 2004;21:1643–1660. doi: 10.1093/molbev/msh160. doi:10.1093/molbev/msh160 [DOI] [PubMed] [Google Scholar]
- Forterre P. The two ages of the RNA world, and the transition to the DNA world: a story of viruses and cells. Biochimie. 2005;87:793–803. doi: 10.1016/j.biochi.2005.03.015. doi:10.1016/j.biochi.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Fry I. Rutgers University Press; New Brunswick, NJ: 2000. The emergence of life on Earth. [Google Scholar]
- Gogarten J.P, et al. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc. Natl Acad. Sci. USA. 1989;86:6661–6665. doi: 10.1073/pnas.86.17.6661. doi:10.1073/pnas.86.17.6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafenbradl D, Keller M, Wächtershäuser G, Stetter K.O. Primordial amino acids by reductive amination of α-oxo acids in conjunction with the oxidative formation of pyrite. Tetrahedron Lett. 1995;36:5179–5182. [Google Scholar]
- Hall D.O, Cammack R, Rao K.K. Role for ferredoxins in origin of life and biological evolution. Nature. 1971;233:136. doi: 10.1038/233136a0. doi:10.1038/233136a0 [DOI] [PubMed] [Google Scholar]
- Hall D.O, Cammack R, Rao K.K. The iron sulfur proteins: evolution of a ubiquitous protein from model systems to higher organisms. Orig. Life Evol. Biosph. 1974a;5:363–386. doi:10.1007/BF01207637 [PubMed] [Google Scholar]
- Hall D.O, Cammack R, Rao K.K. The iron–sulphur proteins: evolution of a ubiquitous protein from the origin of life to higher organisms. In: Dose K, Fox S.W, Deborin G.A, Pavlovskaya T.E, editors. The origin of life and evolutionary biochemistry. Plenum Press; New York, NY: 1974b. pp. 153–168. [Google Scholar]
- Halliday A.N. The origin and earliest history of the Earth. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry, vol. 1. Elsevier; Amsterdam, The Netherlands: 2004. pp. 509–557. [Google Scholar]
- Harrison T.M, Blichert-Toft J, Müller W, Albarede F, Holden P, Mojzsis S.J. Heterogeneous Hadean hafnium: Evidence of continental crust at 4.4 to 4.5 Ga. Science. 2005;310:1947–1950. doi: 10.1126/science.1117926. doi:10.1126/science.1117926 [DOI] [PubMed] [Google Scholar]
- Hausmann K. Georg Thieme Verlag; Stuttgart, Germany: 1985. Protozoologie. [Google Scholar]
- Hazen R.M. Joseph Henry Press; Washington, DC: 2005. Genesis, the scientific quest for life's origin. [Google Scholar]
- Heinen W, Lauwers A.M. Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. Orig. Life Evol. Biosph. 1996;26:131–150. doi: 10.1007/BF01809852. doi:10.1007/BF01809852 [DOI] [PubMed] [Google Scholar]
- Holm N.G. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1992. Marine hydrothermal systems and the origin of life. [Google Scholar]
- Holm N.G, Andersson E.M. Hydrothermal systems. In: Brack A, editor. The molecular origins of life. Cambridge University Press; New York, NY: 1998. pp. 86–99. [Google Scholar]
- Huber C, Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science. 1997;276:245–247. doi: 10.1126/science.276.5310.245. doi:10.1126/science.276.5310.245 [DOI] [PubMed] [Google Scholar]
- Huber C, Wächtershäuser G. G. Peptides by activation of amino acids with CO on (Fe,Ni)S surfaces and implications for the origin of life. Science. 1998;281:670–682. doi: 10.1126/science.281.5377.670. doi:10.1126/science.281.5377.670 [DOI] [PubMed] [Google Scholar]
- Huber C, Wächtershäuser G. Primordial reductive amination revisited. Tetrahedron Lett. 2003;44:1695–1697. doi:10.1016/S0040-4039(02)02863-0 [Google Scholar]
- Huber C, Eisenreich W, Hecht S, Wächtershäuser G. A possible primordial peptide cycle. Science. 2003;301:938–940. doi: 10.1126/science.1086501. doi:10.1126/science.1086501 [DOI] [PubMed] [Google Scholar]
- Ibora F.J, Jackson D.A, Cook P.R. Coupled transcription and translation within the nuclei of mammalian cells. Science. 2001;293:1139–1142. doi: 10.1126/science.1061216. doi:10.1126/science.1061216 [DOI] [PubMed] [Google Scholar]
- Iwabe N, Kuma K, Hasegawa M, Osawa S, Miyata T. Evolutionary relationship of Archaebacteria, Eubacteria and Eukaryotes inferred from phylogenetic trees of duplicated genes. Proc. Natl Acad. Sci. USA. 1989;86:9355–9359. doi: 10.1073/pnas.86.23.9355. doi:10.1073/pnas.86.23.9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen S.B. How old is planet Earth? Science. 2003;300:1513–1514. doi: 10.1126/science.1080682. doi:10.1126/science.1080682 [DOI] [PubMed] [Google Scholar]
- Kandler O. The early diversification of life. In: Bengtson S, editor. Early life on Earth: Nobel symposium no. 84. Columbia University Press; New York, NY: 1994a. pp. 152–160. [Google Scholar]
- Kandler O. Cell wall biochemistry in Archaea and its phylogenetic implications. J. Biol. Phys. 1994b;20:165–169. doi:10.1007/BF00700433 [Google Scholar]
- Kandler O. The early diversification of life and the origin of the three domains: a proposal. In: Wiegel J, Adams M.W.W, editors. Thermophiles: the keys to molecular evolution and the origin of life. Taylor & Francis; London, UK: 1998. pp. 19–31. [Google Scholar]
- Kirn J, Rees D.C. Crystallographic structure and functional implications of the nitrogenase molybdenum–iron protein from Azotobacter vinelandii. Nature. 1992;360:553–560. doi: 10.1038/360553a0. doi:10.1038/360553a0 [DOI] [PubMed] [Google Scholar]
- Kleine T, Münker C, Mezger K, Palme H. Rapid accretion and early core formation on asteroids and on terrestrial planets from Hf-W chronometry. Nature. 2002;418:952–955. doi: 10.1038/nature00982. doi:10.1038/nature00982 [DOI] [PubMed] [Google Scholar]
- Koch A.L, Schmidt T.M. The first cellular bioenergetic process: primitive generation of a proton-motive force. J. Mol. Evol. 1991;33:297–304. doi: 10.1007/BF02102860. doi:10.1007/BF02102860 [DOI] [PubMed] [Google Scholar]
- Koga Y, Kyuragi T, Nishihara M, Sone N. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipids with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J. Mol. Evol. 1998;46:54–63. doi: 10.1007/pl00006283. doi:10.1007/PL00006283 [DOI] [PubMed] [Google Scholar]
- Koonin E.V, Martin W. On the origin of genomes and cells within inorganic compartments. Trends Genet. 2005;21:647–654. doi: 10.1016/j.tig.2005.09.006. doi:10.1016/j.tig.2005.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, et al. Thermococcus coalescens sp. nov., a cell-fusing hyperthermophilic archaeon from Suiyo Seamount. Int. J. Syst. Evol. Microbiol. 2005;55:2507–2514. doi: 10.1099/ijs.0.63432-0. doi:10.1099/ijs.0.63432-0 [DOI] [PubMed] [Google Scholar]
- Lahav N. Oxford University Press; New York, NY: 1999. Biogenesis. [Google Scholar]
- Lathe W.C, Snel B, Bork P. Gene context conservation of a higher order of operons. Trends Biochem. Sci. 2000;25:474–479. doi: 10.1016/s0968-0004(00)01663-7. doi:10.1016/S0968-0004(00)01663-7 [DOI] [PubMed] [Google Scholar]
- Leippe D.D, Aravind L, Koonin E.V. Did DNA replication evolve twice independently? Nucleic Acids Res. 1999;27:3389–3401. doi: 10.1093/nar/27.17.3389. doi:10.1093/nar/27.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leman L, Orgel L, Ghadiri M.R. Carbonyl sulfide-mediated prebiotic formation of peptides. Science. 2004;306:283–286. doi: 10.1126/science.1102722. doi:10.1126/science.1102722 [DOI] [PubMed] [Google Scholar]
- Leman L.J, Orgel L.E, Ghadiri M.R. Amino acid dependent formation of phosphate anhydrides in water mediated by carbonyl sulfide. J. Am. Chem. Soc. 2006;128:20–21. doi: 10.1021/ja056036e. doi:10.1021/ja056036e [DOI] [PubMed] [Google Scholar]
- Lemon B.J, Peters J.W. Binding of exogeneously added carbon monoxide at the active site of the iron-only hydrogenase (CpI) from Clostridium pasteurianum. Biochemistry. 1999;38:12 969–12 973. doi: 10.1021/bi9913193. doi:10.1021/bi9913193 [DOI] [PubMed] [Google Scholar]
- Lunine J.I. Physical conditions on the early Earth. Phil. Trans. R. Soc. B. 2006;361:1721–1731. doi: 10.1098/rstb.2006.1900. doi:10.1098/rstb.2006.1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon E.J, Shima S, Boecher R, Thauer R.K, Grevels F.W, Bill E, Roseboom W, Albracht S.P. Carbon monoxide as an intrinsic ligand to iron in the active site of the iron–sulfur-cluster-free hydrogenase H2-forming methylenetetrahydromethanopterin dehydrogenase as revealed by infrared spectroscopy. J. Am. Chem. Soc. 2004;126:14 239–14 248. doi: 10.1021/ja046818s. doi:10.1021/ja046818s [DOI] [PubMed] [Google Scholar]
- Martin W. Archaebacteria (Archaea) and the origin of the eukaryotic nucleus. Curr. Opin. Microbiol. 2005;8:630–637. doi: 10.1016/j.mib.2005.10.004. doi:10.1016/j.mib.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Martin W, Müller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. doi:10.1038/32096 [DOI] [PubMed] [Google Scholar]
- Martin W, Russell M.J. On the origin of cells: an hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. R. Soc. B. 2003;358:59–85. doi: 10.1098/rstb.2002.1183. doi:10.1098/rstb.2002.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K. A design principle of a flow reactor simulating prebiotic evolution. Viva Origino. 1997;25:191–204. [Google Scholar]
- Maynard Smith J, Szathmary E. W.H. Freeman; Oxford, UK: 1995. The major transitions in evolution. [Google Scholar]
- Michel F.M, Antao S.M, Chupas P.J, Lee P.L, Parise J.B, Schoonen M.A.A. Short- to medium-range atomic order and crystallite size of the initial FeS precipitate from pair distribution function analysis. Chem. Mater. 2005;17:6246–6255. doi:10.1021/cm050886b [Google Scholar]
- Mojzsis S.J, Harrison T.M, Pidgeon R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4.300 Myr ago. Nature. 2001;409:178–181. doi: 10.1038/35051557. doi:10.1038/35051557 [DOI] [PubMed] [Google Scholar]
- Moreira D, Lopez-Garcia P. Symbiosis between methanogenic Archaea and ∂-proteobacteria as the origin of eukaryotes: the synthrophy hypothesis. J. Mol. Evol. 1998;47:517–530. doi: 10.1007/pl00006408. doi:10.1007/PL00006408 [DOI] [PubMed] [Google Scholar]
- Mukhin L. Evolution of organic compounds in volcanic regions. Nature. 1974;251:50–51. doi:10.1038/251050a0 [Google Scholar]
- Mushegian A.R, Koonin E.V. Gene order is not conserved in bacterial evolution. Trends Genet. 1996;12:289–290. doi: 10.1016/0168-9525(96)20006-x. doi:10.1016/0168-9525(96)20006-X [DOI] [PubMed] [Google Scholar]
- Nielsen P.E. Peptide nucleic acid (PNA): a model structure for the primordial genetic material? Orig. Life Evol. Biosph. 1993;23:323–327. doi: 10.1007/BF01582083. doi:10.1007/BF01582083 [DOI] [PubMed] [Google Scholar]
- Nisbet E.G, Fowler C.M.R. The early history of life. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry, vol. 8. Elsevier; Amsterdam, The Netherlands: 2004. pp. 1–39. [Google Scholar]
- Pereto J, Lopez-Garcia P, Moreira D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 2004;29:469–477. doi: 10.1016/j.tibs.2004.07.002. doi:10.1016/j.tibs.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Pierik A.J, Roseboom W, Happe R.P, Bagley K.A, Albracht S.P.J. Carbon monoxide and cyanide as intrinsic ligands to iron in the active site of [NiFe]-hydrogenases. J. Biol. Chem. 1999;274:3331–3337. doi: 10.1074/jbc.274.6.3331. doi:10.1074/jbc.274.6.3331 [DOI] [PubMed] [Google Scholar]
- Popper C.R. Routledge; London, UK: 1963. Conjectures and refutations: the growth of scientific knowledge. [Google Scholar]
- Popper C.R. Pyrite and the origin of life. Nature. 1990;344:387. doi:10.1038/344387a0 [Google Scholar]
- Rauchfuß H. Springer; Berlin, Germany: 2005. Chemische Evolution und Ursprung des Lebens. [Google Scholar]
- Rickard D, Butler I.B, Olroyd A. A novel iron-sulfide switch and its implications for earth and planetary science. Earth Planet. Sci. Lett. 2001;189:85–91. doi:10.1016/S0012-821X(01)00352-1 [Google Scholar]
- Rother M, Metcalf W.W. Anaerobic growth of Methanosarcina acetivorans. Proc. Natl Acad. Sci. USA. 2004;101:16 929–16 934. doi: 10.1073/pnas.0407486101. doi:10.1073/pnas.0407486101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M.J, Arndt N.T. Geodynamic and metabolic cycles in the Hadean. Biogeosciences. 2005;2:97–111. [Google Scholar]
- Russell M.J, Hall A.J. The emergence of life from iron monosulfide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. Lond. 1997;154:377–402. doi: 10.1144/gsjgs.154.3.0377. [DOI] [PubMed] [Google Scholar]
- Russell M.J, Hall A.J, Turner D. In vitro growth of iron sulfide chimneys: possible culture chambers for origin-of-life experiments. Terra Nova. 1989;1:238–241. [Google Scholar]
- Russell M.J, Daia D.E, Hall A.J. The emergence of life from FeS bubbles at alkaline hot springs in an acid ocean. In: Wiegel J, Adams M.W.W, editors. Thermophiles: the keys to molecular evolution and the origin of life. Taylor & Francis; London, UK: 1998. pp. 77–125. [Google Scholar]
- Russell M.J, Hall A.J, Mellersh A.R. On the dissipation of thermal and chemical energies on the early Earth. In: Ikan R, editor. Natural and laboratory simulated thermal geochemical processes. Kluwer Academic Publishers; New York, NY: 2003. pp. 325–388. [Google Scholar]
- Russell M.J, Hall A.J, Boyce A.J, Fallick A.E. On hydrothermal convection systems and the emergence of life. Econ. Geol. 2005;100:419–438. doi:10.2113/100.3.419 [Google Scholar]
- Rüttimann-Johnson C, Rangaraj P, Shah V.K, Ludden P.W. Requirement of homocitrate for the transfer of a 49V-labelled precursor of the iron-vanadium cofactor from VnfX to nif-apodinitrogenase. J. Biol. Chem. 2001;276:4522–4526. doi: 10.1074/jbc.M007288200. doi:10.1074/jbc.M007288200 [DOI] [PubMed] [Google Scholar]
- Schöning K.-U, Scholz P, Guntha S, Wu X, Krishnamurthy R, Eschenmoser A. Chemical etiology of nucleic acid structure: the α-threofuranosyl-(3′→2′) oligonucleotide system. Science. 2000;290:1347–1351. doi: 10.1126/science.290.5495.1347. doi:10.1126/science.290.5495.1347 [DOI] [PubMed] [Google Scholar]
- Schwartzman D, Lineweaver C.H. The hyperthermophilic origin of life revisited. Biochem. Soc. Trans. 2004;32:168–171. doi: 10.1042/bst0320168. doi:10.1042/BST0320168 [DOI] [PubMed] [Google Scholar]
- Shock E.L, McCollom T, Schulte M.D. The emergence of metabolism from within hydrothermal systems. In: Wiegel J, Adams M.W.W, editors. Thermophiles: the keys to molecular evolution and the origin of life. Taylor & Francis; London, UK: 1998. pp. 59–76. [Google Scholar]
- Siefert J.L, Martin K.A, Abdi F, Widger W.R, Fox G.E. Conserved gene clusters in bacterial genomes provide further support for the primacy of RNA. J. Mol. Evol. 1997;45:467–472. doi: 10.1007/pl00006251. doi:10.1007/PL00006251 [DOI] [PubMed] [Google Scholar]
- Taylor P, Rummery T.E, Owen D.G. Reactions of iron monosulfide solids with aqueous hydrogen sulfide up to 160C. J. Inorg. Nucl. Chem. 1979;41:1683–1687. doi:10.1016/0022-1902(79)80106-2 [Google Scholar]
- Thoms S.P. S. Fischer Verlag; Frankfurt, Germany: 2005. Ursprung des Lebens. [Google Scholar]
- Nägeli C. Oldenbourg; München, Germany: 1884. Mechanisch-physiologische Theorie der Abstammungslehre. pp. 83–101. [Google Scholar]
- Wächtershäuser G. An all-purine precursor of nucleic acids. Proc. Natl Acad. Sci. USA. 1988a;85:1134–1135. doi: 10.1073/pnas.85.4.1134. doi:10.1073/pnas.85.4.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Pyrite formation, the first energy source for life: a hypothesis. Syst. Appl. Microbiol. 1988b;10:207–210. [Google Scholar]
- Wächtershäuser, G. 1988c German Patent Application P 38 12 158.1, filed April 4, 1988 and published November 3, 1988.
- Wächtershäuser G. Before enzymes and templates: theory of surface metabolism. Microbiol. Rev. 1988d;52:452–484. doi: 10.1128/mr.52.4.452-484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Evolution of the first metabolic cycles. Proc. Natl Acad. Sci. USA. 1990;87:200–204. doi: 10.1073/pnas.87.1.200. doi:10.1073/pnas.87.1.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Groundworks for an evolutionary biochemistry—the iron–sulfur world. Prog. Biophys. Mol. Biol. 1992;58:85–201. doi: 10.1016/0079-6107(92)90022-x. doi:10.1016/0079-6107(92)90022-X [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. Vitalysts and virulysts: a theory of self-expanding reproduction. In: Bengtson S, editor. Early life on Earth: Nobel symposium no. 84. Columbia University Press; New York, NY: 1994. pp. 124–132. [Google Scholar]
- Wächtershäuser G. The origin of life and its methodological challenge. J. Theor. Biol. 1997;187:483–494. doi: 10.1006/jtbi.1996.0383. [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. The origin of life as a transition from inorganic to organic polymers. In: Steinbüchel A, editor. Biochemical principles and mechanisms of biosynthesis and biodegradation of polymers. Wiley-VCH; Weinheim, Germany: 1998. pp. 1–8. [Google Scholar]
- Wächtershäuser G. Towards a reconstruction of ancestral genomes by gene cluster alignment. Syst. Appl. Microbiol. 1998b;21:473–477. [Google Scholar]
- Wächtershäuser G. The case for a hyperthermophilic, chemolithoautotrophic origin of life in an iron–sulfur world. In: Wiegel J, Adams M.W.W, editors. Thermophiles: the keys to molecular evolution and the origin of life. Taylor & Francis; London, UK: 1998c. pp. 47–57. [Google Scholar]
- Wächtershäuser G. Life as we don't know it. Science. 2000a;289:1307–1308. doi: 10.1126/science.289.5483.1307. doi:10.1126/science.289.5483.1307 [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. Die Entstehung des Lebens. In: Winnacker E.-L, Dichgans J, Erker G, Fritzsch H, Hölldobler B, Winterfeldt E, Donner W, editors. Unter jedem Stein liegt ein Diamant. S. Hirzel Verlag; Leipzig, Germany: 2000. pp. 15–23. [Google Scholar]
- Wächtershäuser G. Origin of life: RNA world versus autocatalytic anabolism. In: Dworkin M, editor. The prokaryotes, an evolving electronic resource for the microbial community. Springer; New York, NY: 2001. [Google Scholar]
- Wächtershäuser G. From pre-cells to Eukarya—a tale of two lipids. Mol. Microbiol. 2003;47:13–23. doi: 10.1046/j.1365-2958.2003.03267.x. doi:10.1046/j.1365-2958.2003.03267.x [DOI] [PubMed] [Google Scholar]
- Washington J. The possible role of volcanic aquifers in prebiologic genesis of organic compounds and RNA. Orig. Life Evol. Biosph. 2000;30:53–79. doi: 10.1023/a:1006692606492. doi:10.1023/A:1006692606492 [DOI] [PubMed] [Google Scholar]
- Wely, van K.H.M, Swaving J, Freudl R, Driessen A.J.M. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS Microbiol. Rev. 2001;25:437–454. doi: 10.1111/j.1574-6976.2001.tb00586.x. doi:10.1111/j.1574-6976.2001.tb00586.x [DOI] [PubMed] [Google Scholar]
- Wilde S.A, Valley J.W, Peck W.H, Graham C.M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature. 2001;409:175–178. doi: 10.1038/35051550. doi:10.1038/35051550 [DOI] [PubMed] [Google Scholar]
- Wills C, Bada J.L. Perseus Publishing; Cambridge, UK: 2000. The spark of life. [Google Scholar]
- Wing M.R, Bada J.L. Geochromatography on the parent body of the carbonaceous chondrite Ivuna. Geochim. Cosmochim. Acta. 1991;55:2937–2942. doi:10.1016/0016-7037(91)90458-H [Google Scholar]
- Woese C.R. Evolution of macromolecular complexity. J. Theor. Biol. 1971;33:29–34. doi: 10.1016/0022-5193(71)90214-1. doi:10.1016/0022-5193(71)90214-1 [DOI] [PubMed] [Google Scholar]
- Woese C.R. Archaebacteria and cellular origins: an overview. Zbl. Bakt. Hyg., I. Abt. Orig. C. 1982;3:1–17. [Google Scholar]
- Woese C.R. The primary lines of descent and the universal ancestor. In: Bendall D.S, editor. Evolution from molecules to men. Cambridge University Press; Cambridge, UK: 1983. pp. 209–233. [Google Scholar]
- Woese C.R. Bacterial evolution. Microbiol. Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C.R. The universal ancestor. Proc. Natl Acad. Sci. USA. 1998;95:6854–6859. doi: 10.1073/pnas.95.12.6854. doi:10.1073/pnas.95.12.6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C.R. On the evolution of cells. Proc. Natl Acad. Sci. USA. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. doi:10.1073/pnas.132266999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C.R, Fox G.E. The concept of cellular evolution. J. Mol. Evol. 1977;10:1–6. doi: 10.1007/BF01796132. doi:10.1007/BF01796132 [DOI] [PubMed] [Google Scholar]
- Woese C.R, Kandler O, Wheelis M.L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria and Eucarya. Proc. Natl Acad. Sci. USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. doi:10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, et al. Life in hot carbon monoxide: the complete genome sequence of Carboxydothermus hydrogenoformans Z-2901. PloS Genet. 2005;1:563–574. doi: 10.1371/journal.pgen.0010065. doi:10.1371/journal.pgen.0010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y, Watanabe H, Saitoh M, Namba T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature. 1991;352:516–519. doi: 10.1038/352516a0. doi:10.1038/352516a0 [DOI] [PubMed] [Google Scholar]
- Yin Q, Jacobsen S.B, Yamashita K, Blichert-Toft J, Télouk P, Albarède F. A short timescale for terrestrial planet formation from Hf-W chronometry of meteorites. Nature. 2002;418:949–952. doi: 10.1038/nature00995. doi:10.1038/nature00995 [DOI] [PubMed] [Google Scholar]
- Zubay G. Academic Press; San Diego, CA: 1996. Origins of life on the Earth and in the Cosmos. [Google Scholar]
Additional references
- Grotzinger J.P. Trends in Precambrian carbonate sediments and their implication for understanding evolution. In: Bengtson S, editor. Early life on Earth: Nobel symposium no. 84. Columbia University Press; New York, NY: 1994. pp. 245–258. [Google Scholar]
- Kempe S, Degens E.T. An early soda ocean? Chem. Geol. 1985;53:95–108. [Google Scholar]
- Kempe S, Kazmierczak J, Degens E.T. The soda ocean concept and its bearing on biotic evolution. In: Crick R.E, editor. Origin, evolution and modern aspects of biomineralization in plants and animals. Plenum Press; New York, NY: 1989. pp. 29–43. [Google Scholar]
- Kuhn H. Selbstorganisation molekularer Systeme und die Evolution des genetischen Apparats. Angew. Chem. 1972;84:838–862. [Google Scholar]
- Kuhn H, Kuhn C. Diversified world: drive to life's origin?! Angew. Chem. Int. Ed. 2003;42:262–266. doi: 10.1002/anie.200390098. [DOI] [PubMed] [Google Scholar]
- Kuhn H, Waser J. Molekulare Selbstorganisation und Ursprung des Lebens. Angew. Chem. 1981;93:495–515. [Google Scholar]
- Kuhn H, Waser J. Selbstorganisation der Materie und Evolution früher Formen des Lebens. In: Hoppe W, Lohmann W, Markl H, Ziegler H, editors. Biophysik. Springer; Berlin, Germany: 1982. pp. 860–907. [Google Scholar]
- Macleod G, McKeown C, Hall A.J, Russell M.J. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig. Life Evol. Biosph. 1994;24:19–41. doi: 10.1007/BF01582037. [DOI] [PubMed] [Google Scholar]
- Maisonneuve J. The composition of the Precambrian ocean waters. Sedimentary Geol. 1982;31:1–11. [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Morse J.W, Mackenzie F.T. Hadean ocean carbonate geochemistry. Aquatic geochem. 1998;4:301–319. [Google Scholar]
- Russell M.J. First life. Am. Sci. 2006;94:32–39. [Google Scholar]
- Sukumaran P.V. Evolution of the atmosphere and the oceans: evidence from the geological record. Resonance. 2000;5:4–12. [Google Scholar]