Abstract
One of the goals of the present Martian exploration is to search for evidence of extinct (or even extant) life. This could be redefined as a search for carbon. The carbon cycle (or, more properly, cycles) on Earth is a complex interaction among three reservoirs: the atmosphere; the hydrosphere; and the lithosphere. Superimposed on this is the biosphere, and its presence influences the fixing and release of carbon in these reservoirs over different time-scales. The overall carbon balance is kept at equilibrium on the surface by a combination of tectonic processes (which bury carbon), volcanism (which releases it) and biology (which mediates it). In contrast to Earth, Mars presently has no active tectonic system; neither does it possess a significant biosphere. However, these observations might not necessarily have held in the past. By looking at how Earth's carbon cycles have changed with time, as both the Earth's tectonic structure and a more sophisticated biology have evolved, and also by constructing a carbon cycle for Mars based on the carbon chemistry of Martian meteorites, we investigate whether or not there is evidence for a Martian biosphere.
Keywords: Earth, Mars, carbon, cycle, life
1. Introduction
The search for life beyond Earth is frequently (and not unrealistically) considered in terms of the presence, or otherwise, of liquid water. However, the importance of water is in its functions as a solvent and a transport medium, as well as its role in providing support to cell structure. Given appropriate ambient environmental parameters, such as temperature and pressure, other fluids could perform these functions, negating the requirement for liquid water as a necessity for life. Admittedly, water is the fluid with greatest versatility, in terms of the temperature range over which it is liquid, and to consider the origin of life in the absence of water is probably fantastic, but we know that life can survive in severe conditions on Earth, and remain viable in the absence of water, even if it originally arose when water was relatively plentiful. Thus, the presence (or absence) of water is not necessarily diagnostic for the occurrence (or lack) of life.
All life on Earth is based on carbon; the variety of organic and organo-metallic compounds formed cannot be paralleled by any other element. Thus, the presence of life (whether it be extinct, extant or dormant) must be evinced by the presence of carbon (the reverse is not true, because there are many non-biological sources of organic molecules). If that is the case, identification of how and where carbon occurs within an environment is an important signal to know whether the setting is, or is capable of, hosting life. On Earth today, there are three major carbon-bearing reservoirs that are interrelated: the atmosphere; the hydrosphere; and the lithosphere. Superimposed on these, and acting throughout, is the biosphere. There is also a net input of carbon to the Earth from extraterrestrial material, around 5×105 kg yr−1 (some 2×1015 kg over the Earth's lifetime). The presence of living organisms acts in subtle ways to upset the balance that exists between the other reservoirs (today, this effect is more noticeable than ever with the anthropogenic contribution to global CO2 levels). In the past, some of the carbon reservoirs either did not exist (e.g. there was no biosphere prior to ca 3.5–3.8 Gyr ago) or were not linked in the same way as they are today (e.g. no recycling at plate margins prior to the onset of plate tectonics). By examining the way the reservoirs interact, and tracking the pathways through which carbon moves from source to sink, interconnected carbon cycles are delineated for Earth. It is our aim to define similar pathways for carbon on Mars, and thus attempt to determine whether or not an equilibrium exists between the reservoirs, or whether there is an imbalance that might be ascribed to the influence of a living biology. For the purposes of this paper, the lithosphere is considered to be the igneous (and metamorphic) component of the crust and mantle, and the hydrosphere includes sedimentary rocks. The reason behind this, perhaps unusual, allocation of the sedimentary record to hydrosphere rather than lithosphere is because most sedimentary rocks require the active role of water in their production (limestone, clay, etc.). The reaction of CO2 from the atmosphere dissolving in water, eventually producing carbonate, is a reaction between atmosphere and hydrosphere. This applies to both Earth and Mars.
(a) Evolution of carbon-bearing reservoirs on Earth
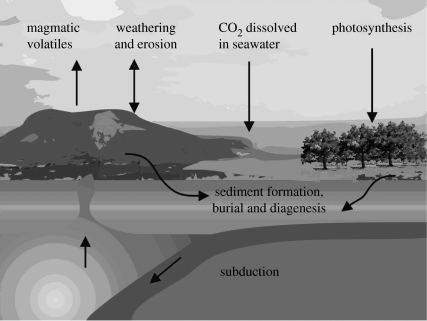
In order to progress with modelling a carbon cycle for Mars, it is instructive to examine Earth's carbon cycle (or, more properly, carbon cycles) and see what has changed as the Earth has evolved. Building a carbon cycle requires knowledge of the sinks and sources of carbon, and how they interact. Figure 1 is a schematic of the pathways through which carbon moves from one reservoir to another on Earth today.
Figure 1.
Schematic representation of the pathways through which the carbon cycles operate on Earth today.
Table 1 summarizes how the four reservoirs have evolved through the Earth's history. Thus, 4.5 Gyr ago, as the Earth first formed, it was subject to heavy and continuous bombardment by bodies not yet aggregated into planets. The result of this bombardment was to keep Earth's surface molten, to depths of many kilometres. The planet degassed any volatiles as rapidly as they were accreted, as well as losing volatiles from the original starting materials. Under these conditions, it is assumed that any atmosphere that formed was transient and rapidly removed by impact stripping (e.g. Maher & Stevenson 1988; Sleep et al. 1989). At the very start of Earth's history, there was neither hydrosphere nor biosphere. However, over a short time period, only a few million years (as indicated by the cratering record on the lunar surface; Ryder 2002), the bombardment slowed, Earth's surface cooled and solidified, and an atmosphere stabilized. The exact nature of the Earth's atmosphere is hotly debated. In the 1950s, following the laboratory work of Miller & Urey (1959), a highly reducing atmosphere was suggested. This idea fell out of popularity and the canonical view became that of an oxidizing atmosphere (approx. 80% CO2). However, the most recent models have revived the idea of a strongly reducing atmosphere (Schaefer & Fegley 2005). Water arrived from the late-stage accretion of planetesimals and dust—present models suggest ca 90% from asteroidal materials and ca 10% from comets (Morbidelli et al. 2000). Evidence for the earliest existence of water comes from the oxygen isotopic composition of the mineral zircon present in meta-sediments from the Jack Hills Formation of Western Australia. Most of these grains are around 4.2 Gyr old, but there are some detrital zircons with ages as ancient as 4.4 Gyr and their presence implies the existence of a fairly widespread ocean, as well as reworked crustal sediments (Mojzsis et al. 2001; Wilde et al. 2001). Therefore, by 4.4 Gyr ago, Earth already had an atmosphere, a lithosphere and a hydrosphere, and the reservoirs were actively connected. Tectonic activity occurred, where crust was subducted and recycled, but plate tectonics was not completely established (Watson & Harrison 2005). Although there is no evidence for a biosphere this early in Earth's history, it has been suggested that conditions could have been favourable for some micro-organisms to survive (Sleep et al. 1989), although not for any great length of time.
Table 1.
Comparison of reservoirs on Earth and Mars at several different stages of planetary evolution. (The ages given are very approximate and are not boundary ages, but the time by which specific characteristics had become established. Thus, Earth's atmosphere did not become oxidizing 2.0 Gyr ago, but by 2.0 Gyr was oxidizing in composition.)
| age (Gyr) | Earth | Mars |
|---|---|---|
| 4.5 | Hadean | Noachian |
| cooling and outgassing; no plate tectonics; strongly reducing atmosphere; no biosphere; no carbon cycle | cooling and outgassing; no plate tectonics; atmosphere similar to early Earth?; no biosphere; no carbon cycle | |
| 3.5 | Archaean | Hesperian |
| plate tectonics established; abundant liquid water; reducing atmosphere; biosphere of simple organisms | plate tectonics ceased; hydrosphere/cryosphere of unknown extent; mildly reducing atmosphere, thicker than today (?) | |
| 2.0 | Proterozoic | Amazonian |
| abundant liquid water; oxidizing atmosphere; biosphere of simple organisms | hydrosphere/cryosphere of unknown extent; thicker atmosphere (?) | |
| present-day | Phanerozoic | |
| abundant liquid water; oxidizing atmosphere; biosphere of complex organisms | hydrosphere/cryosphere of unknown extent; thin, CO2-rich atmosphere |
At some point between the formation and 3.5 Gyr ago, Earth had stabilized to the extent that there were three dynamically interacting reservoirs: the atmosphere, the lithosphere and the hydrosphere; and plate tectonics was taking place with a mechanism of plate movement close to that which is in operation today (Sleep 2005). The atmosphere was mildly reducing in composition and composed of gases, including CO2 and CH4 (e.g. Kasting & Catling 2003). The presence of a biosphere is a matter of great debate: features within sediments that were interpreted as fossilized micro-organisms (e.g. Schopf 1993; Schopf et al. 2002) have, more recently, been reinterpreted as chemical traces (Brasier et al. 2002, 2004), although there have been more recent reports of microbial biomarkers in 3.5 Gyr-old volcanic pillow laves (Banerjee et al. 2006). Over the next billion years, the composition of Earth's atmosphere changed progressively; its gradual oxidation is shown by associated variations in the nature of the sediments laid down during this period, e.g. banded iron formations give way to sediments in which iron occurs as the oxidized species. The rise in atmospheric oxygen concentration is a combination of processes, including the spread of photosynthetic micro-organisms, a change in composition of volcanic gases and autooxidation of sulphides (e.g. Kasting & Catling 2003 and references therein). The mechanism of photosynthesis acts to remove CO2 from the atmosphere and replenishes it with oxygen. This reaction is balanced by respiration, which removes O2 from the atmosphere and replaces with CO2. As the biomass of photosynthesizing organisms increased, there was an increasing ‘fixing’ of carbon by burial, as the micro-organisms lived and died, gradually increasing the oxygen content of Earth's atmosphere. At around 2.3 Gyr ago, the switch from a reducing to an oxidizing atmosphere became permanent, and the atmosphere had evolved to a composition closer to that observed today (Holland 2002). The unambiguous establishment of life can be tracked through the fossil record, with the first appearance of macro-fossils ca 600 Myr ago (e.g. Conway Morris 1993). Through time, variations in global climate, impacts and tectonic processes have changed the surface of the Earth, and species have evolved and become extinct. However, overall, since the biosphere became established and the atmosphere oxidizing, there have been no gross changes to the way in which the terrestrial carbon cycles have operated.
(b) Evolution of carbon-bearing reservoirs on Mars
If the above sequence of events is a reasonable description of how Earth evolved in the first 2.5 billion years of its history, what sort of analogous description could be given for Mars? It is probable that 4.5 Gyr ago, Mars was very similar to Earth; it formed from the same starting materials in a similar environment. Once the planets cooled, however, the evolutionary pathways of Earth and Mars must have diverged almost immediately. Where Earth retained an atmosphere and acquired a hydrosphere, Mars very quickly lost the bulk of its atmosphere, and thus had no extensive surface hydrosphere. It is argued that water is a prerequisite for the operation of plate tectonics. Tectonic processes on Mars were modelled by Sleep (1994); evidence for a limited extent of plate tectonics came from magnetic measurements recorded by Mars Global Surveyor (Connerney et al. 1999). The most active phase of Mars' tectonic history, presumably including incipient plate tectonics, had ceased by ca 3.5 Gyr ago (Nimmo & Tanaka 2005; Solomon et al. 2005), precluding the formation of a dynamic carbon cycle similar to the one which had become established on Earth. Mars' evolution over the past 3 Gyr is chronicled through modification of its surface by fluid flow (either water or ice), which must also be associated with the change in thickness of the planet's atmosphere. One of the problems of tracking an evolutionary history for Mars is the lack of absolute ages for different lithological units. Crater counting over the landscape is the method by which relative ages are fixed, thus allowing dimension of Martian history into the various epochs (Noachian, Hesperian and Amazonian), but the absolute time at which one epoch merged into another is unknown. Comparison with the lunar cratering record (calibrated with dates measured on Apollo samples) allows broad timings to be fixed (Hartmann & Neukum 2001).
On the basis of the chronology outlined in table 1, and analogy with processes in operation on Earth, the most favourable epoch in which life might have become established on Mars was the Late Noachian/Early Hesperian. During this period, Mars was dynamically active and appeared still to possess an atmosphere; the main episodes of impact bombardment had ceased and the major volcanic provinces were emplaced (e.g. Phillips et al. 2001). It is rather regrettable that there are no Martian meteorites of this age (Nyquist et al. 2001). On Earth, the Archaean subdivision of the Precambrian is that which approximates most closely to the Hesperian on Mars. Unfortunately, the experience of characterizing terrestrial life forms of Archaean age is not straightforward; both the physical and chemical fossil records are confusing (Brasier et al. 2004). Since the likelihood of an automated robotic explorer uncovering unambiguous fossil evidence on Mars' surface is very slight, an alternative approach must be tried and hence this attempt to build a carbon cycle for Mars.
There have been many observations on Mars, both by remote sensing from orbit (e.g. most recently, Mars Global Surveyor, Mars Odyssey and Mars Express) and robotic landers (e.g. Pathfinder, Spirit and Opportunity). Today, carbon is known to occur on Mars in several reservoirs. As CO2, it comprises 95% of the thin (600 Pa) atmosphere. Both poles of Mars are capped by mixtures of water and CO2 ice. The present-day atmospheric pressure of CO2 is far too low to allow liquid water on the surface of Mars. And yet, from photogeological evidence it is clear that fluids existed on the surface in Mars' history. The conventional interpretation was that an early thick atmosphere (up to several bars) of CO2 allowed liquid water to flow on Mars (e.g. Pollack et al. 1987). The inference that carbon should occur as carbonates in the Martian crust and soils is confirmed to limited extent, and carbonates have indeed been identified by emission spectroscopy as present in the dust (Bandfield et al. 2003), although no massive carbonate deposits have been identified on the surface (Bibring et al. 2005). The presence of sulphur at the Martian surface (Baird et al. 1976), the subsequent identification of sulphates in situ (Squyres et al. 2004) and the evidence for the action of brines (Bridges et al. 2001) seem to suggest highly acidic conditions on early Mars (Hurowitz et al. 2006), in which case massive carbonate deposits might never have formed. Orbital imagery has shown features on Mars' surface that appear to have been carved by fluid (presumed to be water and/or ice), and secondary minerals produced by water have been identified in soils (Poulet et al. 2005), which suggests that Mars has had a hydrosphere. Features characterizing the Martian lithosphere have also been well documented by satellite imagery; the monumental volcanoes of the Tharsis region include Olympus Mons, the biggest volcano in the Solar System. Its multiple craters indicate that it has had a long and complex magmatic history. Most of the observations on the surface of Mars indicate that the dominant rock type is igneous; spectral measurements confirm that while basaltic material is the most common, other igneous rock types also occur (Christensen et al. 2005). Therefore, Mars has a lithosphere that appears to be comprised mostly of crystalline magmatic rocks.
Three of the reservoirs through which carbon is cycled on Earth (atmosphere, hydrosphere and lithosphere) are present on Mars as well. Assuming the Martian reservoirs also contain carbon in one form or another, there is therefore a potential for carbon cycling similar to Earth. However, there is a gulf between defining the main reservoirs implicated in carbon cycling, and quantifying both the pathways of the interactions and the amounts of material involved. Previous attempts to detect carbon in Martian soil have met with limited success. The mass spectrometers on the Viking landers of 1976 measured carbon, but not its abundance or its isotopic composition, and the results were ambiguous in terms of evidence for life (Klein 1978). The Alpha Proton X-Ray spectrometer (APX) instrument on Mars Pathfinder found no carbon above the detection limit of the instrument (0.5 wt%; Bridges 2001). Since there have been no direct measurements of carbon abundance in rocks at Mars' surface, we cannot use remotely acquired data to define end-member compositions for the reservoirs. In order to do this, we rely on data acquired from analysis of Martian meteorites.
(c) Martian meteorites
There are presently about 60 rock fragments, derived from around 40 meteorites, that are recognized on Earth as pieces ejected from Mars during asteroidal impact. The Martian origin rests on the age, composition and noble gas inventory of the meteorites, and a full explanation for how it is known that the rocks are from Mars is given by Grady (2006). The advantage of studying these rocks is that their absolute ages can be measured; the disadvantage is their precise origin on Mars' surface is unknown. The most up-to-date listing of Martian meteorites is maintained at http://www2.jpl.nasa.gov/snc/; the most comprehensive bibliography is the Mars Meteorite Compendium at http://www-curator.jsc.nasa.gov/antmet/mmc/index.cfm (Meyer 2003).
Although the meteorites can be subdivided into four groups on the basis of agreed composition, all the specimens are igneous rocks—none is an example of Mars' sedimentary history. The different groups represent crystallization at fairly shallow depths (McSween 1994); on Earth, geologists would have labelled them as intrusives (lherzolite, pyroxenite and dunite) or extrusives (basalt). All the rocks were formed under fairly dry conditions, although some have been altered by fluids after formation. Most of the meteorites were shocked to pressures between 30 and 50 GPa during the impact event that ejected them from Mars' surface. The shergottites (after the type specimen Shergotty) are further subdivided into basaltic, lherzolitic and olivine-phyric types. Nakhlites (after Nakhla) are shallow cumulates that have been exposed to the Martian hydrosphere, and thus contain rich assemblages of carbonates, sulphates and halite. The chassignites (after Chassigny) are olivine-rich dunites. ALH 84001 is the sole orthopyroxenite and is rich in carbonates. Notwithstanding the fact that all the meteorites are igneous, it is possible to measure carbon in Martian meteorites, and on the basis of its speciation and isotopic composition, assign its provenance as primary (magmatic), secondary (alteration) or tertiary (trapped from shock) component.
2. Experimental methods
Two techniques were employed to garner carbon abundance data from the Martian meteorites. The first was that of high-resolution stepped combustion; the second acid dissolution. In each case, the carbon produced was in the form of carbon dioxide; this was introduced into a mass spectrometer for isotopic analysis. Stepped combustion gave data for primary magmatic carbon and carbon dioxide trapped from the Martian atmosphere, while acid dissolution gave data on the secondary carbonates produced by aqueous alteration on Mars' surface. A list of meteorites analysed, and their sources, is given in table 2. More detailed descriptions of the experimental procedures are given by Grady et al. (2004) and Wright et al. (1992) for stepped combustion and acid dissolution, respectively.
Table 2.
Samples analysed. (BM, Natural History Museum, London; MWG, Meteorite Working Group, USA; NIPR, National Institute of Polar Research, Japan. BS, basaltic shergottite; LS, lherzholitic shergottite; OS, olivine-phyric shergottite; C, chassignite; N, nakhlite; O, orthopyroxenite. SC, stepped combustion; AD, acid dissolution.)
| Sample | source | type | method | [C] (p.p.m.) | δ13C (‰) |
|---|---|---|---|---|---|
| Los Angeles | BM | BS | SC | 6.5 | −24.3 |
| QUE 94201 | MWG | BS | SC | 1.3 | −23.1 |
| Shergottya | BM | BS | SC | 4.3 | −19.4 |
| Zagamia | BM | BS | SC | 52.8 | −22.7 |
| DaG 476 | BM | OS | SC | 6.0 | −22.1 |
| SAU 005 | BM | OS | SC | 8.2 | −16.4 |
| ALHA 77005 | MWG | LS | SC | 1.7 | −21.4 |
| LEW 88516 | MWG | LS | SC | 1.8 | −20.2 |
| Chassignya | BM | C | SC | 2.1 | −20.8 |
| Governador Valadares | BM | N | AD | 27 | +11 |
| Lafayette | BM | N | AD | 10 | +1 |
| Nakhlaa | BM | N | AD | 26–55 | +13 to +49 |
| Y 000593 | NIPR | N | AD | 132–181 | +38 |
| ALH 84001 | MWG | O | AD | 280 | +41 |
Meteorites observed to fall.
(a) Stepped combustion
Approximately 100 mg sized chips of each meteorite were coarsely crushed to grains ca 100–200 μm in size. Powders from samples observed to fall were given no other pretreatment; meteorite finds were rinsed in 0.1 M HCl in order to remove any terrestrial weathering products. An aliquot of ca 10 mg was taken from the powdered reservoir, weighed and wrapped in high-purity platinum foil. The powder was introduced into the vacuum system, and, following evacuation to a pressure less than 10−5 mbar, was heated under a partial pressure of oxygen derived from the decomposition of Cu(II)O; heating was carried out in increments from room temperature to 1400°C. The combustion products (CO2, SO2 and H2O) were separated from each other using a variable temperature cryofinger immersed in liquid nitrogen. Following quantification in a calibrated volume, purified CO2 was introduced into the mass spectrometer for isotopic analysis. Carbon dioxide abundance was determined to be ±0.2 ng and δ13C to be±0.5‰.
(b) Acid dissolution
As for stepped combustion, material for analysis was drawn from a reservoir of coarsely crushed meteorite fragments. Aliquots of ca 30–50 mg powdered meteorite were reacted with 100% orthophosphoric acid (H3PO4) in two sequential steps at temperatures 25 and 75°C. This technique liberates CO2 from carbonates: calcite at the lower temperature; and Fe, Mg-rich carbonates at the higher temperature. Other dissolution products (H2O, H2S and SO2) were removed from the CO2 by passage of the gas mixture over a finger containing either lead ethanoate or silver wire. Again, following quantification in a calibrated volume, the cleaned and dried CO2 was introduced into the mass spectrometer for isotopic analysis. Carbon dioxide abundance was determined to be ±5 ng and δ13C to be ±1‰.
3. Results
(a) Primary carbon (the lithospheric reservoir)
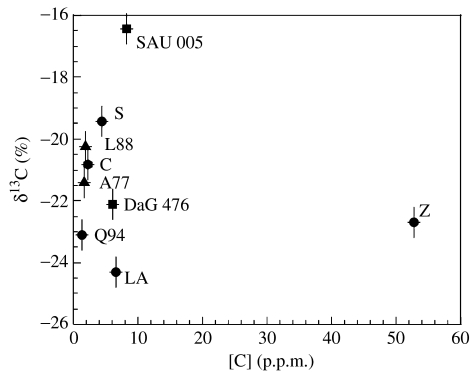
Material from the shergottite group of meteorites, plus chassignites, was selected for analysis, because previous work had shown that they had not been altered by secondary Martian fluids (Grady et al. 1997). Carbon isotopic data for primary carbon extracted from the meteorites are plotted in figure 2 and summarized in table 2. Abundance of carbon is variable, with a range from 1 to 50 p.p.m. (mean 9.4±16 p.p.m.). Only one meteorite (Zagami) has carbon abundance around 50 p.p.m.; the other samples have much lower carbon content, i.e. 1–10 p.p.m. It is probable that this carbon occurs as crystalline material along grain boundaries and dissolved in silicates. The reason for the higher apparent abundance of carbon in Zagami is unclear, but could be a sampling effect. Zagami is known to contain heterogeneously distributed pockets of igneous melt that are the final products of crystallization, and it is possible that the material taken from the powdered reservoir might have contained several of these inclusions. If data for Zagami are excluded, mean carbon abundance is more tightly constrained at 4.0±2.6 p.p.m. The isotopic composition of magmatic carbon has a spread of δ13C values from −24.3 to −16.4‰, with a mean of −21.2±2.3‰. A δ13C of −21.2‰ is very different from that which is assumed to represent bulk Earth (−5‰), a point which we will discuss in §4. Zagami exhibited an unusually high carbon abundance, but has a δ13C of −22.7‰, well within the error envelope of the mean. This implies that the high carbon content could indeed be from over-abundance of melt inclusions, and not owing to the presence of an additional component. In order to model carbon in Mars' magmatic reservoir, we take its abundance to be 4 p.p.m., with δ13C=−21.2‰.
Figure 2.
Abundance and isotopic composition of magmatic carbon in chassignites and shergottites. The component is defined as that liberated on combustion between 600 and 1000°C. The symbols represent the different shergottite subgroups: circles, basaltic; triangles, lherzholitic; squares, olivine-phyric. A77, Allan Hills 77005; C, Chassigny; DaG 476, Dar al Gani 476; L88, Lewis Cliff 88156; LA, Los Angeles; Q94, Queen Alexandra Range 94201; SAU 005, Sayh al Huaymir 005; S, Shergotty; Z, Zagami.
(b) Secondary carbon (the hydrosphere)
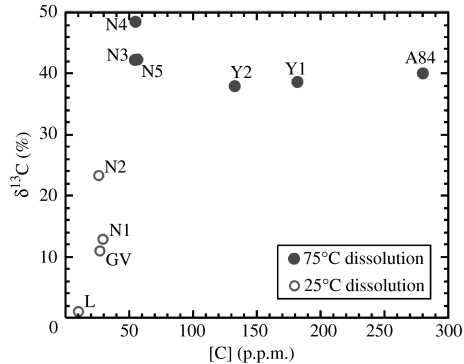
A different set of Martian meteorites from those subject to stepped combustion were used to determine the Martian secondary carbon reservoir. Previous work has shown that shergottites have not been altered significantly by water on Mars' surface, whereas the occurrence of secondary salts in the nakhlites and the orthopyroxenite ALH 84001 indicate that these specimens have experienced the effects of water (Wright et al. 1992; Bridges & Grady 2000). Data from acid dissolution of four nakhlites plus the orthopyroxenite ALH 84001 are given in table 2 and figure 3. Petrographic examination of these meteorites indicates that most of the carbonates occur as siderite or ankerite (i.e. iron-rich); therefore, the results from the higher temperature dissolution are probably more representative of the true abundance and δ13C of the carbonates. It is clear that the carbonates are 13C-enriched; this has been taken as evidence that the salts are produced by dissolution of Martian atmospheric CO2 in water at (or just below) the Mars' surface. As figure 3 indicates, the carbon present as carbonate within Martian meteorites is between 50 and 300 p.p.m., with elevated δ13C greater than +35‰.
Figure 3.
Abundance and isotopic composition of carbon in carbonates in the nakhlites and ALH 84001. The data were acquired using dissolution in 100% H3PO4 and are taken to represent carbon in the Martian hydrosphere. A84, Allan Hills 84001; GV, Governador Valadares; L, Lafayette; N, Nakhla; Y, Yamato 000593.
(c) Tertiary carbon (the atmosphere)
The partial pressure of CO2 in Mars' atmosphere is known from direct measurements of the planet (Nier et al. 1976); its isotopic composition has also been measured directly, albeit with very large errors (δ13C=0±50‰). A more precise value can be gained from analysing gas trapped in Martian meteorites. During stepped heating experiments, silicates start to soften and melt at temperatures above ca 900°C, releasing any gases trapped inside the lattice. It was the recognition of the presence of argon trapped during a shock process within the EET A79001 shergottite that first led to the recognition of Martian meteorites (Bogard & Johnson 1983); since this first analysis, atmospheric nitrogen, carbon dioxide, neon, krypton and xenon have been isolated from several Martian meteorites. There have been many discussions on how this trapped component is best deconvoluted, as it can be a composite of gases trapped during different shock events, together with species implanted by cosmic ray irradiation during Mars to Earth transit. However, most of the gas is assumed to have been trapped during the ejection event that removed the samples from Mars' surface to the orbit. Hence, the gas must have been trapped relatively recently, and is close to the modern day Martian atmosphere in composition. On the basis of gas released from Martian meteorites, a lower limit for the δ13C of Mars' atmosphere is +30‰ (Carr et al. 1985).
(d) Size of carbon-bearing reservoirs on Mars
(i) Magmatic carbon
As outlined previously, in order to model carbon in Mars' magmatic reservoir, we take its abundance to be 4 p.p.m., with δ13C=−21.2‰. Making a few simple assumptions, we can use these figures to calculate carbon abundance for Mars' lithosphere. The assumptions are the following: (i) the carbon is distributed homogeneously throughout the crust plus mantle, (ii) the thickness of the crust plus mantle in which this carbon is dispersed is 1750 km and (iii) the rocks are all basalt with a density of 3400 kg m−3. Using these three parameters, for a mean carbon abundance of 4 p.p.m. in Martian meteorites, the total amount of magmatic carbon in Mars' crust plus mantle is ca 2000×1015 kg (table 3). If we had used the carbon content of Zagami, the figure would have been 25 000×1015 kg. The first value is, to a good approximation, some two orders of magnitude lower than that of the Earth (324×1018 kg; table 3 and DesMarais 2001), which reflects the difference in carbon content of Martian meteorites (4 p.p.m.) and Earth's mantle (80 p.p.m.). If we normalize the mass of the Martian mantle (5×1023 kg) to that of Earth's mantle (4×1024 kg), then the amount of carbon on Mars would be equivalent to 16×1018 kg (or 200×1018 kg, assuming Zagami-type abundances).
Table 3.
Carbon on Earth and Mars.
| reservoir | abundance (1015 kg) | δ13C (‰) | ||
|---|---|---|---|---|
| Eartha | Mars | Eartha | Mars | |
| atmosphere (CO2) | 0.72 | 6.4 | −7 | >+40 |
| lithosphere (igneous rocks) | 324 000 | 2000 | −5 | −21.2 |
| hydrosphere (sedimentary rocks) | 84 000 | 25–150 | 0±2 | >+30 |
| biosphere (organics) | 1.56 | ? | −27 | ?? |
| input from extraterrestrial dust | 2 | 2b | ||
From DesMarais (2001).
Assuming that the higher flux of material to Mars is approximately balanced by its lower surface area.
In order to assess whether our estimate for the carbon content of the Martian mantle is realistic, it is useful to consider the more extensive data available for water. The Martian mantle has variously been considered as ‘dry’ (36 p.p.m. H2O; Wänke & Dreibus 1994), ‘relatively wet’ (up to 1.8 wt% H2O; McSween et al. 2001) or somewhere in between (several hundred parts per million of H2O; an inference from Lodders & Fegley 1997). This 500× variability in the estimate of water content makes it plain that there is likewise a considerable uncertainty in the abundance of carbon in the Martian mantle. The value used here, 4 p.p.m. C, is probably a lower limit, since CO2 would have degassed during the ascent from depth and subsequent emplacement. The problem is: what to set as the upper limit? Lodders & Fegley (1997) suggest a whole-Mars C content of 2960 p.p.m., while Morgan & Anders (1979) proposed 16.3 p.p.m.—an unknown proportion of these two figures could be owing to carbon sequestered in the Martian core. In light of these uncertainties, we continue with our figure of 4 p.p.m. C, but note that there is much work still to do in constraining this value.
(ii) Sedimentary carbon
Abundance of the carbonates in the nakhlites is variable and linked to the occurrence of other secondary weathering products (clay minerals, gypsum, etc). Two possible explanations have been proposed to explain this variation: either the nakhlites represent a sequence of rocks that have suffered alteration as a pool of brine evaporates; or the samples that are represented by the nakhlites have come from different depths within a single lava flow that has been penetrated to varying degrees by percolating ground water. The data so far cannot distinguish between these two hypotheses. As detailed previously, there are between 50 and 300 p.p.m. carbon present as carbonate in the nakhlites. For argument's sake, we take this to be the reservoir of carbon representative of the hydrosphere; therefore, it can be used to deduce the size of Mars' sedimentary reservoir. Two main parameters required to determine the size of this reservoir are the areal extent of the deposits and their depth. These are completely unknown; there are no indications from either satellite imagery or thermal emission spectroscopy that there are vast carbonate deposits exposed on the surface or buried just below the surface (Bibring et al. 2005). Nonetheless, in order to gain some idea of Mars' carbon budget, we model the abundance of carbon present as carbonate on the basis of a global layer of surface materials 1 km thick, which contains 50–300 p.p.m. carbon as carbonate. Therefore, this calculates to an abundance of carbon of 25–150×1015 kg (table 3).
4. Discussion
(a) A carbon cycle for Mars?
Clearly, there is a carbon cycle on Mars, one that involves a seasonal movement of CO2 from polar cap to atmosphere. Furthermore, diurnal changes in temperature will cause CO2 to move in and out of the regolith on a regular basis. The carbon cycle we are trying to construct is more regional in scale and considers both transport of carbon from within the planet to surface layers and the prospect of cycling it back again. Rather than compare absolute abundances of carbon in the solid reservoirs of Earth and Mars, given the difference in size of the two planets, and the different crust/mantle/core ratios, it is perhaps more realistic to compare carbon concentrations in the reservoirs (it is not useful to compare atmospheric concentrations, given that Mars' atmosphere is 95% CO2 compared with 350 p.p.m. in the terrestrial atmosphere). Estimates of the carbon abundance of the terrestrial mantle vary, but fall in the range of 10–250 p.p.m. (e.g. Mathez et al. 1984; Trull et al. 1993), i.e. even the lowest estimates are higher than the value that we are inferring for the Martian mantle. Thus, Mars is either more completely degassed than Earth or its primordial carbon complement was lower than Earth's, or carbon is distributed in a different pattern from that on Earth. What implication does this have for a carbon cycle for Mars?
As figure 1 shows, carbon outgasses from the Earth largely in the form of CO2 from volcanoes. This typically has a δ13C of −5‰, as do the majority of diamonds, which are formed at depth and brought to the surface in kimberlite eruptions. The carbon that is added back into the mantle comprises an approximate 4 : 1 mix of carbonates (δ13C ca 0‰), with sediments derived from organic matter (δ13C ca −25‰); this balances out to give a mantle signature of δ13C ca −5‰ (e.g. DesMarais 2001). In order to make a similar calculation for Mars, we need to know the relative abundances of carbonates and any organic sediments (and, of course, we need to make the assumption that some sort of recycling mechanism exists). If there were a 1 : 1 mix of carbonates and sediments derived from organic matter, the sediments would have a δ13C ca −70‰. For a 10 : 1 carbonate to sediment mix, δ13C of the sediments would have to be less than −500‰; higher ratios would imply a sedimentary reservoir comprised of pure 12C. On Earth, isotopically light carbon is produced today by methanogenic micro-organisms; in Archaean times, such producers were the dominant species. One conclusion from this calculation is that on the basis of data from Martian meteorites, the Martian biosphere is fairly extensive (of the same order of magnitude in size as the carbonate reservoir) and 12C-enriched to slightly greater levels than those exhibited by terrestrial Archaean micro-organisms. We have measured isotopically light organic carbon in Martian meteorites, but could not determine whether it was indigenous to the samples or a contaminant (Wright et al. 1997). Studies by Jull et al. (2000), Sephton et al. (2002) and Gibson et al. (2006) also identified organic carbon in Martian meteorites with δ13C<−15‰. The first of these studies showed that the organic material was devoid of 14C; therefore, it could not be a modern terrestrial contaminant. The apparent similarity in δ13C between Martian magmatic and surface organic carbon allows the inference that surface materials simply represent the exsolution of magmatic carbon at higher levels during degassing. The lack of carbon isotopic fractionation would be consistent with an abiotic process acting to fix magmatic carbon on its one-way journey from depth towards the atmosphere. Such materials could then be recycled to depth with no impact on the overall isotopic balance, but this is not so with carbonate minerals with their elevated 13C-content, which tends to argue against large-scale recycling. The origin of the 13C-enrichment in the carbonates has been explained by atmospheric loss processes (Wright et al. 1990; Jakosky & Jones 1997). Perhaps we have to accept that irreversible loss of carbon from the Martian atmosphere explains not only the isotope systematics, but also the lack of widespread carbonates.
A more sober interpretation of the data is that if there is a series of carbon cycles in operation on Mars, then they are not driven by, or reliant on, subduction of sediments. The two main carbon-bearing reservoirs must be isolated from each other, and probably have been for more than 2 Gyr. In which case there is little evidence for any Martian biosphere.
(b) Unresolved issues
Although the pathways through which carbon cycles on Earth are well established and have been studied for many years, there are still fundamental details that are model-dependent. The most significant assumption made (and that has been assumed here) is that the carbon isotopic composition of Earth's mantle has a δ13C=−5‰. This value comes from analyses of diamonds, basalt glasses and exhaled volcanic volatiles (e.g. Deines 1980; Mattey et al. 1984, 1989; Mathez 1987). The value that we have inferred for Mars (δ13C=−21.2‰) is very different from that of Earth. It is unlikely that Earth and Mars, as large planetary bodies, would start off with widely differing carbon isotopic compositions. Given that Earth and Mars aggregated from the same materials some 4.6 Gyr ago, at approximately similar locations in the protoplanetary disc, there must be a reason why their primary carbon components appear to be so different in isotopic composition. Degassing of volatiles would drive the isotopic composition of residual material to higher values—therefore, if Mars is more completely degassed than Earth, then that would imply that the δ13C of its initial carbon complement should have been even more 12C-enriched than −21‰. The usual interpretation of terrestrial magmatic carbon is it may either come from a deep primordial reservoir or tap an intermediate level reservoir that has been contaminated by input of sediments from Earth's surface. If the magmatic reservoir on Earth has not been changed by subducted sediments (and the over-abundance of mantle versus crustal carbon would seem to imply that addition of sediments would not make too great a difference to the final isotopic composition), then has the terrestrial mantle signature remained constant through time? This is a difficult question to answer; although attempts have been made to investigate potential changes using the composition of inclusions in diamonds, it is still not certain whether differences are primary or have been imposed by subsequent events (e.g. Shirey et al. 2002).
Another issue that would affect carbon cycling is whether the atmosphere, hydrosphere and lithosphere are the sole non-biological carbon-bearing reservoirs. There is an additional reservoir of carbon on both Earth and Mars that has not been incorporated into our carbon cycle modelling. This is the cryosphere, an environment where carbon occurs as frozen species, either as carbon dioxide ice or trapped in water ice. On both planets, the extent of the cryosphere is unknown. On Earth, CO2 (and CH4) is locked in ice within permafrost as clathrates; carbon clathrates have also been proposed to occur at depth below the ocean floor, and it has been suggested that as much as 1016 kg methane might be trapped there (Buffett 2000). On Mars, the cryosphere is probably even more significant than on Earth; the cold surface temperature implies that a layer of permafrost is present across the whole globe; the depth of such a layer is unknown, but recent results from the MARSIS (Mars Advanced Radar for Subsurface and Ionosphere Sounding) experiment on Mars Express suggest that ice occurs to a depth of at least 1 km in certain parts of the surface (Picardi et al. 2005). The polar caps are, of course, the most apparent manifestation of Mars' cryosphere, and the annual ebbing and flowing in size of the caps indicate that there is a regular cycling of CO2 between the atmosphere and the cryosphere.
(c) Implications for a Martian biosphere
So far, we have not been able to construct a realistic regional scale carbon cycle for Mars. Working from the data that have already been acquired, there are still too many unknown or poorly constrained end-member compositions for the various different carbon reservoirs on Mars. On assuming that we had been able to derive an isotopic signature for putative Martian micro-organisms, what might that imply for a Martian biosphere, and its possible relationship to the terrestrial biosphere?
If living Martian micro-organisms had been found by any of the robotic rovers presently exploring Mars, then, de facto, there is no problem in inferring a biosphere, and it would only (!) be a matter of sending the correct analytical instrumentation to Mars to determine the molecular and isotopic characteristics of the organisms. Similarly, if dormant or extinct Martian organisms were found with a very different molecular or isotopic composition from that of terrestrial, there would be little problem in distinguishing between Martian and terrestrial biota. However, if micro-organisms with a similar molecular and carbon isotopic composition to terrestrial were found on Mars, then the question of their origins would have to be questioned very closely. Did the two biotas arise independently? Or did one planet ‘seed’ the other through exchange of materials? Such a process is possible (Gladman et al. 1996; Melosh 2003), and it is presumably only a matter of time before terrene meteorites are found on the Moon or Mars.
Presently, there is no evidence for life on Mars (reports of microfossils in the ALH 84001 Martian meteorite (McKay et al. 1996) have not been substantiated). The determination of carbon in situ in Martian rocks and soil is a technically challenging procedure. Despite all the missions that have observed the red planet, none has measured the carbon abundance of the surface. The best values that we have for the lithospheric reservoir are those derived from Martian rocks—and as this study has shown, those abundances are low. Any measurement of carbon at the Martian surface will require an extremely sensitive instrument. Analysis of primary magmatic carbon dissolved in silicate minerals requires temperatures up to 1000°C; a temperature of this magnitude is difficult to achieve on a space-borne instrument, as it requires high power. The Beagle 2 lander had, as part of its payload, a sophisticated instrument (the GAP (Gas Analysis Package)) that would have determined the content and stable isotopic composition of carbon at Mars' surface (Wright et al. 2000). Sadly, the loss of Beagle 2 meant that these parameters have still not been measured, and because none of the missions that are presently being planned is carrying the appropriate instrumentation, it is likely to be some time before we have absolute measurements for carbon at Mars' surface.
An alternative to robotic measurements in situ is to bring rocks back to Earth from Mars. The advantage of this is enormous; detailed highly specific analyses using the complete range of analytical instrumentation could be performed at the highest of resolutions. There are corresponding disadvantages, not least, that only limited samples would be available from a very restricted area of the planet. There is no simple solution to the problem—Mars seems to be a barren planet, hence any signs of life will be subtle and require careful analysis and verification. One solution is to send people to explore Mars. This can only happen after thorough exploration by robotic means; the authors do not anticipate that it will occur during their lifetimes.
5. Conclusions
It is clear that building a carbon cycle, or a set of inter-related carbon cycles, for Mars is difficult. Despite the intense investigation that Mars has experienced over the past decade, both through spacecraft missions and an increasing number and a variety of Martian meteorites, there are many free parameters with ill-defined values. From results derived from the chassignites and the shergottites, primary carbon is present in Martian meteorites as reduced, well-crystalline carbon. This is inferred to be present on grain surfaces as well as occluded within silicates and occurs in variable abundance (mean 4.0±2.6 p.p.m.). The δ13C of this component (−21.2±2.3‰) is much lighter than primary magmatic carbon from terrestrial basalts (−5‰). Secondary carbon is present in Martian meteorites (nakhlites) as Ca and Fe, Mg-rich carbonates. It also occurs in variable abundance (5–300 p.p.m.) and has a δ13C>+30‰, a value that is much higher than terrestrial carbonates (0±2‰). On the basis of their elevated δ13C, it is probable that the carbonates were produced on Mars' surface following dissolution of 13C-enriched Martian atmosphere in surface waters.
Working with end-members in δ13C of −21 and +30‰, it is difficult to see how a balance analogous to that between magmatic and sedimentary reservoirs on Earth could exist on Mars. The two main carbon-bearing reservoirs must be isolated from each other, and probably have been for more than 2 Gyr. On the basis of this simple-minded approach using data from Martian meteorites, there is little evidence for a biosphere on Mars.
Acknowledgments
The Royal Society is thanked for the opportunity to present this work at the Discussion Meeting on Life's Origins. The Natural History Museum, London, the Antarctic Meteorite Working Group, USA and the Meteorite Working Group, Japan are thanked for their generous provision of samples for analysis. Financial support from the PPARC is gratefully acknowledged.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
References
- Baird A.K, Toulmin P, Rose H.J, Christian R.P, Clark B.C, Keil K, Gooding J.L. Mineralogic and petrologic implications of Viking geochemical results from Mars—interim report. Science. 1976;194:1288–1293. doi: 10.1126/science.194.4271.1288. [DOI] [PubMed] [Google Scholar]
- Bandfield J.L, Glotch T.D, Christensen P.R. Spectroscopic identification of carbonate minerals in the Martian dust. Science. 2003;301:1084–1087. doi: 10.1126/science.1088054. doi:10.1126/science.1088054 [DOI] [PubMed] [Google Scholar]
- Banerjee N.R, Furnes H, Muehlenbachs K, Staudigel H, de Wit M. Preservation of ∼3.4–3.5 Ga microbial biomarkers in pillow lavas and hyaloclastites from the Barberton Greenstone Belt, South Africa. Earth Planet. Sci. Lett. 2006;241:707–722. doi:10.1016/j.epsl.2005.11.011 [Google Scholar]
- Bibring J.-P, et al. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science. 2005;307:1576–1581. doi: 10.1126/science.1108806. doi:10.1126/science.1108806 [DOI] [PubMed] [Google Scholar]
- Bogard D.D, Johnson P. Martian gases in an Antarctic meteorite? Science. 1983;221:651–654. doi: 10.1126/science.221.4611.651. [DOI] [PubMed] [Google Scholar]
- Brasier M.D, Green O.R, Jephcoat A.P, Kleppe A.K, Van Kranendonk M.J, Lindsay J.F, Steele A, Grassineau N.V. Questioning the evidence for Earth's oldest fossils. Nature. 2002;416:76–81. doi: 10.1038/416076a. doi:10.1038/416076a [DOI] [PubMed] [Google Scholar]
- Brasier M.D, Green O.R, Lindsay J.F, Steele A. Earth's oldest (∼3.5 Ga) fossils and the ‘Early Eden Hypothesis’: questioning the evidence. Orig. Life Evol. Biosph. 2004;34:257–269. doi: 10.1023/b:orig.0000009845.62244.d3. doi:10.1023/B:ORIG.0000009845.62244.d3 [DOI] [PubMed] [Google Scholar]
- Bridges N.T. Characteristics of the Pathfinder APXS sites: implications for the composition of martian rocks and soils. J. Geophys. Res. 2001;106:14 621–14 666. doi:10.1029/2000JE001393 [Google Scholar]
- Bridges J.C, Grady M.M. Evaporite mineral assemblages in the nakhlite (martian) meteorites. Earth Planet. Sci. Lett. 2000;176:267–279. doi:10.1016/S0012-821X(00)00019-4 [Google Scholar]
- Bridges J.C, Catling D.C, Saxton J.M, Swindle T.D, Lyon I.C, Grady M.M. Alteration assemblages in martian meteorites: implications for near-surface processes. Space Sci. Rev. 2001;96:365–392. doi:10.1023/A:1011965826553 [Google Scholar]
- Buffett B.A. Clathrate hydrates. Ann. Rev. Earth Planet. Sci. 2000;28:477–507. doi:10.1146/annurev.earth.28.1.477 [Google Scholar]
- Carr R.H, Grady M.M, Wright I.P, Pillinger C.T. Martian atmospheric carbon dioxide and weathering products in SNC meteorites. Nature. 1985;314:248–250. doi:10.1038/314248a0 [Google Scholar]
- Christensen P.R, et al. Evidence for magmatic evolution and diversity on Mars from infrared observations. Nature. 2005;436:504–509. doi: 10.1038/nature03639. doi:10.1038/nature04075 [DOI] [PubMed] [Google Scholar]
- Connerney J.E.P, et al. Magnetic lineations in the ancient crust of Mars. Science. 1999;284:794–798. doi: 10.1126/science.284.5415.794. doi:10.1126/science.284.5415.794 [DOI] [PubMed] [Google Scholar]
- Conway Morris S. The fossil record and the early evolution of the Metazoa. Nature. 1993;361:219–225. doi:10.1038/361219a0 [Google Scholar]
- Deines P. The carbon isotopic composition of diamonds: relationship to diamond shape, colour, occurrences and vapour composition. Geochim. Cosmochim. Acta. 1980;44:943–961. doi:10.1016/0016-7037(80)90284-7 [Google Scholar]
- Des Marais D.J. Isotopic evolution of the biogeochemical carbon cycle during the Precambrian. In: Valley J.W, Coles D.R, editors. Stable isotope geochemistry, vol. 43. Reviews in Mineralogy and Geochemistry. Mineralogical Society of America and Geochemical Society; Washington, DC: 2001. [Google Scholar]
- Gibson E.K, et al. Observation and analysis of in situ carbonaceous matter in Nakhla: part II. Lunar Planet. Sci. 2006;37:2039. [Google Scholar]
- Gladman B.J, Burns J.A, Duncan M, Lee P, Levison H.F. The exchange of impact ejecta between terrestrial planets. Science. 1996;271:1387–1392. [Google Scholar]
- Grady M.M. The history of research on meteorites from Mars. In: Mc Call G.J.H, Bowden A.J, Howarth R.J, editors. The history of meteorites and key meteorite collections: fireballs, falls and finds. Geological Society, Special Publication 256; London, UK: 2006. pp. 405–416. [Google Scholar]
- Grady M.M, Wright I.P, Pillinger C.T. A carbon and nitrogen isotope study of Zagami. J. Geophys. Res. Planet. 1997;E102:9165–9173. doi:10.1029/97JE00414 [Google Scholar]
- Grady M.M, Verchovsky A.B, Wright I.P. Magmatic carbon in Martian meteorites: attempts to constrain the carbon cycle on Mars. Int. J. Astrobiol. 2004;3:117–124. doi:10.1017/S1473550404002071 [Google Scholar]
- Hartmann W.K, Neukum G. Cratering chronology and the evolution of Mars. In: Kallenbach R, et al., editors. Chronology and evolution of Mars, vol. 96. Kluwer; Dordrecht, The Netherlands: 2001. pp. 165–194. [Google Scholar]
- Holland H. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim. Cosmochim. Acta. 2002;66:3811–3826. doi:10.1016/S0016-7037(02)00950-X [Google Scholar]
- Hurowitz J.A, McLennan S.M, Tosca N.J, Arvidson R.E, Michalski J.R, Ming D.W, Schröder C, Squyres S.W. In situ and experimental evidence for acidic weathering of rocks and soils on Mars. J. Geophys. Res. 2006;111:E02S19. doi:10.1029/2005JE002515 [Google Scholar]
- Jakosky B.M, Jones J.H. The history of Martian volatiles. Rev. Geophys. 1997;35:1–16. doi:10.1029/96RG02903 [Google Scholar]
- Jull A.J.T, Beck J.W, Burr G.S. Isotopic evidence for extraterrestrial organic material in the Martian meteorite, Nakhla. Geochim. Cosmochim. Acta. 2000;64:3763–3772. doi:10.1016/S0016-7037(00)00458-0 [Google Scholar]
- Kasting J.F, Catling D. Evolution of a habitable planet. Ann. Rev. Astron. Astrophys. 2003;41:429–463. doi:10.1146/annurev.astro.41.071601.170049 [Google Scholar]
- Klein H.P. The Viking biological experiments on Mars. Icarus. 1978;34:666–674. doi:10.1016/0019-1035(78)90053-2 [Google Scholar]
- Lodders K, Fegley B. An oxygen isotope model for the composition of Mars. Icarus. 1997;126:373–394. doi:10.1006/icar.1996.5653 [Google Scholar]
- McKay D.S, Gibson E.K, Thomas-Keprta K.L, Vali H, Romanek C.S, Clemett S.J, Chiller X.D.F, Maechling C.R, Zare R.N. Search for past life on Mars: possible relic biogenic activity in martian meteorite ALH84001. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- McSween H.Y. What we have learned about Mars from SNC meteorites. Meteoritics. 1994;29:757–779. [Google Scholar]
- McSween H.Y, Grove T.L, Lentz R.C, Dann J.C, Holzheid A.H, Riciputi L.R, Ryan J.G. Geochemical evidence for magmatic water from pyroxenes in the Shergotty meteorite. Nature. 2001;409:487–490. doi: 10.1038/35054011. doi:10.1038/35054011 [DOI] [PubMed] [Google Scholar]
- Maher K.A, Stevenson D.J. Impact frustration of the origin of life. Nature. 1988;331:612–614. doi: 10.1038/331612a0. doi:10.1038/331612a0 [DOI] [PubMed] [Google Scholar]
- Mathez E.A. Carbonaceous matter in mantle xenoliths: composition and relevance to the isotopes. Geochim. Cosmochim. Acta. 1987;51:2339–2347. doi:10.1016/0016-7037(87)90288-2 [Google Scholar]
- Mathez E.A, Dietrich V.J, Irving A.J. The geochemistry of carbon in mantle peridotites. Geochim. Cosmochim. Acta. 1984;48:1849–1859. doi:10.1016/0016-7037(84)90038-3 [Google Scholar]
- Mattey D.P, Carr R.H, Wright I.P, Pillinger C.T. Carbon isotopes in submarine basalts. Earth Planet. Sci. Lett. 1984;70:196–206. doi:10.1016/0012-821X(84)90005-0 [Google Scholar]
- Mattey D.P, Exley R.A, Pillinger C.T. Isotopic composition of CO2 and dissolved carbon species in basalt glass. Geochim. Cosmochim. Acta. 1989;53:2377–2386. doi:10.1016/0016-7037(89)90359-1 [Google Scholar]
- Melosh H.J. Exchange of meteorites (and life?) between stellar systems. Astrobiology. 2003;3:207–215. doi: 10.1089/153110703321632525. doi:10.1089/153110703321632525 [DOI] [PubMed] [Google Scholar]
- Meyer, C. 2003 Mars Meteorite Compendium. (http://www-curator.jsc.nasa.gov/antmet/mmc/index.cfm).
- Miller S.L, Urey H.C. Organic compound synthesis on the primitive Earth. Science. 1959;130:245–251. doi: 10.1126/science.130.3370.245. [DOI] [PubMed] [Google Scholar]
- Mojzsis S.J, Harrison T.M, Pidgeon R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4,300 Myr ago. Nature. 2001;409:178–181. doi: 10.1038/35051557. doi:10.1038/35051557 [DOI] [PubMed] [Google Scholar]
- Morbidelli A, Chambers J, Lunine J.I, Petit J.M, Robert F, Valsecchi G.B, Cyr K.E. Source regions and timescales for the delivery of water to Earth. Meteorit. Planet. Sci. 2000;35:1309–1320. [Google Scholar]
- Morgan J.W, Anders E. Chemical composition of Mars. Geochim. Cosmochim. Acta. 1979;43:1601–1610. doi:10.1016/0016-7037(79)90180-7 [Google Scholar]
- Nier A.O, McElroy M.B, Yung Y.L. Isotopic composition of the martian atmosphere. Science. 1976;194:68–70. doi: 10.1126/science.194.4260.68. [DOI] [PubMed] [Google Scholar]
- Nimmo F, Tanaka K. Early crustal evolution of Mars. Ann. Rev. Earth Planet. Sci. 2005;33:133–161. doi:10.1146/annurev.earth.33.092203.122637 [Google Scholar]
- Nyquist L.E, Bogard D.D, Shih C.-Y, Greshake A, Stoffler D, Eugster O. Ages and geologic histories of martian meteorites. In: Kallenbach R, et al., editors. Chronology and evolution of Mars, vol. 96. Kluwer; Dordrecht, The Netherlands: 2001. pp. 105–164. [Google Scholar]
- Phillips R.J, et al. Ancient geodynamics and global-scale hydrology on Mars. Science. 2001;291:2587–2591. doi: 10.1126/science.1058701. doi:10.1126/science.1058701 [DOI] [PubMed] [Google Scholar]
- Picardi G, et al. Radar soundings of the subsurface of Mars. Science. 2005;310:1925–1928. doi: 10.1126/science.1122165. doi:10.1126/science.1122165 [DOI] [PubMed] [Google Scholar]
- Pollack J.B, Kasting J.F, Richardson S.M, Poliakoff K. The case for a wet, warm climate on early Mars. Icarus. 1987;71:203–224. doi: 10.1016/0019-1035(87)90147-3. doi:10.1016/0019-1035(87)90147-3 [DOI] [PubMed] [Google Scholar]
- Poulet F, Bibring J.-P, Mustard J.F, Gendrin A, Mangold N, Langevin Y, Arvidson R.E, Gondet B, Gomez C. Phyllosilicates on Mars and implications for early martian climate. Nature. 2005;438:623–627. doi: 10.1038/nature04274. doi:10.1038/nature04274 [DOI] [PubMed] [Google Scholar]
- Ryder G. Mass flux in the ancient Earth–Moon system and benign implications for the origin of life on Earth. J. Geophys. Res. (Planets) 2002;E107:5022. doi:10.1029/2001JE001583 [Google Scholar]
- Schaefer L, Fegley B. A reducing atmosphere from out-gassing of the early Earth (abstract) Div. Planet. Sci. 2005;37:676. [Google Scholar]
- Schopf J.W. Microfossils of the early Archean Apex chert: new evidence of the antiquity of life. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- Schopf J.W, Kudryavtsev A.B, Agresti D.G, Wdowiak T.J, Czaja A.D. Laser-Raman imagery of Earth's earliest fossils. Nature. 2002;416:73–76. doi: 10.1038/416073a. doi:10.1038/416073a [DOI] [PubMed] [Google Scholar]
- Sephton M.A, Wright I.P, Gilmour I, de Leeuw J.W, Grady M.M, Pillinger C.T. High molecular weight organic matter in martian meteorites. Planet. Space. Sci. 2002;50:711–716. doi:10.1016/S0032-0633(02)00053-3 [Google Scholar]
- Shirey S.B, Harris J.W, Richardson S.H, Fouch M.J, James D.E, Cartigny P, Deines P, Viljoen F. Diamond genesis, seismic structure, and evolution of the Kaapvaal–Zimbabwe Craton. Science. 2002;297:1683–1686. doi: 10.1126/science.1072384. doi:10.1126/science.1072384 [DOI] [PubMed] [Google Scholar]
- Sleep N.H. Martian plate tectonics. J. Geophys. Res. 1994;99:5639–5655. doi:10.1029/94JE00216 [Google Scholar]
- Sleep N.H. Evolution of the continental lithosphere. Ann. Rev. Earth Planet. Sci. 2005;33:369–393. doi:10.1146/annurev.earth.33.092203.122643 [Google Scholar]
- Sleep N.H, Zahnle K.J, Kasting J.F, Morowitz H.J. Annihilation of ecosystems by large asteroid impacts on the early Earth. Nature. 1989;342:139–142. doi: 10.1038/342139a0. doi:10.1038/342139a0 [DOI] [PubMed] [Google Scholar]
- Solomon S.C, et al. New perspectives on ancient Mars. Science. 2005;307:1214–1220. doi: 10.1126/science.1101812. doi:10.1126/science.1101812 [DOI] [PubMed] [Google Scholar]
- Squyres S.W, et al. The opportunity Rover's Athena science investigation at Meridiani Planum. Mars. Sci. 2004;306:1698–1703. doi: 10.1126/science.1106171. [DOI] [PubMed] [Google Scholar]
- Trull T, Nadeau S, Pineau F, Polve M, Javoy M. C–He systematics in hotspot xenoliths—implications for mantle carbon contents and carbon recycling. Earth Planet. Sci. Lett. 1993;118:43–64. doi:10.1016/0012-821X(93)90158-6 [Google Scholar]
- Wänke H, Dreibus G. Chemistry and accretion history of Mars. Phil. Trans. R. Soc. A. 1994;25:545–557. [Google Scholar]
- Watson E.B, Harrison T.M. Zircon thermometer reveals minimum melting conditions on earliest Earth. Science. 2005;308:841–844. doi: 10.1126/science.1110873. doi:10.1126/science.1110873 [DOI] [PubMed] [Google Scholar]
- Wilde S.A, Valley J.W, Peck W.H, Graham C.M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature. 2001;409:175–178. doi: 10.1038/35051550. doi:10.1038/35051550 [DOI] [PubMed] [Google Scholar]
- Wright I.P, Grady M.M, Pillinger C.T. The evolution of atmospheric CO2 on Mars—the perspective from carbon isotope measurements. J. Geophys. Res. 1990;95:14 789–14 794. [Google Scholar]
- Wright I.P, Grady M.M, Pillinger C.T. Chassigny and the nakhlites: carbon-bearing components and their relationship to martian environmental conditions. Geochim. Cosmochim. Acta. 1992;56:817–826. doi:10.1016/0016-7037(92)90100-W [Google Scholar]
- Wright I.P, Grady M.M, Pillinger C.T. Isotopically light carbon in ALH 84001—Martian metabolism or teflon contamination? Lunar Planet. Sci. 1997;28:1414. [Google Scholar]
- Wright I.P, Morgan G.H, Praine I.J, Morse A.D, Leigh D, Pillinger C.T. Beagle 2 and the search for organic compounds on Mars using GAP. Lunar Planet. Sci. 2000;31:1573. [Google Scholar]