Abstract
Recent spacecraft and lander missions to Mars have reinforced previous interpretations that Mars was a wet and warm planet in the geological past. The role of liquid water in shaping many of the surface features on Mars has long been recognized. Since the presence of liquid water is essential for survival of life, conditions on early Mars might have been more favourable for the emergence and evolution of life. Until a sample return mission to Mars, one of the ways of studying the past environmental conditions on Mars is through chemical and isotopic studies of Martian meteorites. Over 35 individual meteorite samples, believed to have originated on Mars, are now available for lab-based studies. Fe is a key element that is present in both primary and secondary minerals in the Martian meteorites. Fe-isotope ratios can be fractionated by low-temperature processes which includes biological activity. Experimental investigations of Fe reduction and oxidation by bacteria have produced large fractionation in Fe-isotope ratios. Hence, it is considered likely that if there is/were any form of life present on Mars then it might be possible to detect its signature by Fe-isotope studies of Martian meteorites. In the present study, we have analysed a number of Martian meteorites for their bulk-Fe-isotope composition. In addition, a set of terrestrial analogue material has also been analysed to compare the results and draw inferences. So far, our studies have not found any measurable Fe-isotopic fractionation in bulk Martian meteorites that can be ascribed to any low-temperature process operative on Mars.
Keywords: Mars, Martian meteorites, SNC, terrestrial analogues, iron isotopes, life
1. Introduction and background
The suggestion of McKay et al. (1996) that one of the Martian meteorites (ALH 84001) has preserved traces of Martian bacterial life led to a surge in scientific research on Martian meteorites. This has also resulted in the discovery and identification of more Martian meteorites from various places on the Earth. The total number of Martian meteorites known to date stands at over 35 (greater than 50 numbered individuals), almost a threefold increase in the total number of known Martian meteorites in the last decade. Collectively, they are called SNC meteorites after three major subdivisions based on texture and mineralogy. The largest group is called shergottites (a group named after the first meteorite of this type which fell in 1865 in a village in India called Shergahti). The next most common group of meteorites is nakhlites (named after the first meteorite fall of this type in El-Nakhla, Egypt in 1911), followed by chassignites (named after a meteorite fall in 1815 in Chassigny village, France). ALH 84001 is an orthopyroxenite, discovered in Antarctica in 1984, has a distinct mineralogy and petrology compared with the other three groups of Martian meteorites. All Martian meteorites recovered so far are basaltic igneous rocks that formed at elevated temperatures (greater than 1000°C) through volcanic activities. The crystallization ages of Martian meteorites vary over a large range; ALH 84001 being the oldest at ca 4.5 Ga and shergottites being the youngest (175–450 Ma) while nakhlites and chassigny are ca 1.3 Ga old (e.g. Nyquist et al. 2001). Almost all Martian meteorites also contain secondary minerals formed at low temperatures (less than 200°C) by interaction with ground water at the surface of Mars at some point in their geological past, thereby possessing the potential of preserving snapshots of hydrological conditions prevalent on Mars at that instant.
The tantalizing possibility that life might once have existed on the red planet has stimulated much of the scientific community for quite some time. The present-day climate on Mars is inhospitable for sustaining any life form. As we know, the presence of liquid water is a prerequisite for the survival of any life form. The past three decades of spacecraft missions have provided convincing evidence that during the early part of its geological history (greater than 3.5 Ga), Mars had an active hydrological cycle, similar to that of the Earth. However, during the later stages of its evolutionary history, Mars lost its surface water and atmosphere and became a cold and dry planet as we know it today. The important question as to whether there is extinct or extant life on Mars can be answered by dedicated sample return missions followed by human missions to Mars. Both these activities, although inevitable, are commercially and technologically challenging. However, until such return sample missions from Mars take place, one of the ways to look for past evidence of aqueous activity and life on Mars is through mineral, chemical and isotopic studies of Martian meteorites. In the present study, we have conducted Fe-isotope measurements on Martian meteorites to assess its suitability to seek evidence for any low-temperature aqueous process, including biological activity (if any) that might be preserved in these samples. In terrestrial systems, both these processes can induce significant fractionation in Fe-isotopic compositions. Excellent reviews of the current status of Fe-isotope studies of terrestrial and extraterrestrial systems can be found in Beard & Johnson (2004) and Johnson et al. (2004).
2. Water on Mars
The landscape visible on Mars through satellite images has long been used as a basis for arguing the involvement of running water on the Martian surface in shaping these features. Recent high-resolution images returned by European Space Agency's Mars Express spacecraft have indicated the possibility of dust-covered pack ice regions in the equatorial regions of Mars (Murray et al. 2005). The gamma ray spectrometer onboard NASA's Odyssey spacecraft has also detected abundant hydrogen within the 1 m subsurface regions beyond 60 °N and S of the equator (e.g. Feldman et al. 2002). The hydrogen atoms have been interpreted to be present as H2O constituting a large subsurface reservoir of water on Mars. The present-day conditions on Mars, however, are not favourable for the occurrence of liquid water on the surface. Based on surface chronological constraints, it has been proposed that during the early period of Mars' geological history (greater than 3.5 Ga), warm and wet conditions existed on the planet's surface (Carr 1987). It is not clear at what time in the geological past, liquid water ceased to occur on the surface of Mars leading to a much thinner atmosphere that exists today. At the landing site of the Mars Exploration Rover, Opportunity, there is ample evidence that water was involved in the formation of haematite concretions, found in abundance at this site. Various geological structures (e.g. cross-beddings, ripple marks) and elemental measurements in the rock formations at the Opportunity landing site have also supported the hypothesis that these deposits were formed from an evaporating water body (e.g. Squyres et al. 2004).
Other indirect evidence for action and effect of water on Mars come from Martian meteorite studies. Almost all Martian meteorites contain secondary minerals that are formed by the alteration of primary minerals in the presence of water. In particular, the nakhlite group of meteorites contains secondary minerals that have been interpreted to form a sequence of evaporitic mineral assemblage at low temperatures (Bridges & Grady 2000). The Martian meteorite, ALH 84001, contains carbonate rosettes that also formed in the presence of water. Thus, there is a great deal of evidence pointing towards a warm and wet Mars in the geological past, when conditions might have been suitable for the emergence of life.
3. Biosignatures in Martian samples
If life ever evolved on Mars, then there are various ways in which the biosignatures could be preserved in the Martian rock record. Fossilized remains of bacterial life forms were claimed to have been found in the carbonate rosettes of Martian meteorite, ALH 84001 (McKay et al. 1996). However, nearly 10 years after that report, still there is no consensus among scientists whether the morphological form preserved in ALH 84001 was biogenic or non-biogenic in origin. In a recent study, the same group of scientists have reported the occurrence of carbonaceous matter in veins inside the Nakhla Martian meteorite. The Nakhla meteorite was an observed fall and it is highly improbable that between its fall and passage to a curatorial facility, it was subjected to any significant terrestrial contamination. Furthermore, an interior portion of Nakhla was selected for this study, which virtually eliminates any possibility of terrestrial contamination. In addition, the carbon-isotopic composition of this material substantiates the claims made by the authors that the carbon is of extraterrestrial origin, likely to be indigenous to Mars (McKay et al. 2006).
4. Geochemical signatures of life
The preservation of organic compounds, the building blocks of life, in the rock record at geological time-scales can be extremely difficult, especially under the highly oxidizing conditions prevailing at the Martian surface at the present day. The same is true for the preservation of morphological features. However, the geochemical signatures of past biological activity may be preserved in a rock record in terms of specific elemental signatures or isotopic ratios. Many transition metal elements (e.g. Se, Zn and Fe) are used during metabolic activities in biological systems. Indirect evidence of life may potentially be identified through studies of these elements and isotope systems in relevant material.
In an experimental study involving Fe reduction by dissimilatory bacteria, Beard et al. (1999) found significant Fe-isotope fractionation associated with this process. Subsequent experimental work using both reducing and oxidizing bacteria further confirmed large Fe-isotope fractionation associated with the metabolic processing of Fe by the bacteria (Beard et al. 2003; E. Hutchens, The Natural History Museum, London 2005, personal communication). Thus, in laboratory-based biological experiments, Fe isotopes have been found to be significantly fractionated by the bacterial action which provided the original premise for assessing the possibility of using fractionation of Fe isotopes in Martian rocks to identify any geochemical signature of extinct/extant life. In the present study, we have analysed bulk Martian meteorites and some terrestrial analogue material for their Fe-isotopic composition to identify any preserved geochemical signature of life.
5. Samples and methods
Iron is an important constituent of the rock-forming minerals that are produced at elevated temperatures experienced during magma genesis. Iron has four stable isotopes (54, 56, 57 and 58) and is an important constituent of the secondary minerals produced by alteration of the primary minerals, occurring in clay minerals and carbonates. Kinetic, equilibrium and nuclear processes fractionate the isotopes, in the same way as is commonly observed for other stable isotopes. At elevated temperatures, Fe isotopes display limited fractionation, detection and resolution of which requires a highly sensitive instrumentation, such as multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS). Stable Fe isotopes follow mass-dependent fractionation pattern. Standard delta notation is used to report Fe-isotope data in per mil (‰) and is defined as follows:
In the present work, we have measured bulk iron-isotope compositions of 12 Martian meteorites to ascertain variation in their δ56Fe and δ57Fe values. In addition, we have also analysed a set of terrestrial samples including Proterozoic basalts and granites from Southern India and sandstone samples from Antarctic dry valley (DV) regions, which may be considered Martian analogues (table 1). The Martian meteorite samples selected in our study represent all the three major groups described earlier. The terrestrial basalt and granite samples were selected from the Harker collection of the University of Cambridge, UK. The mineralogy and petrology of these samples have previously been studied extensively (Anand et al. 2003). The present-day climatic conditions in the DV regions of Antarctica represent an extreme end member of the range of conditions prevailing in the Earth today. This includes very cold temperatures and nearly no humidity, which presents a very harsh environment for the survival of life. Nevertheless, microbial communities, known as cryptoendoliths, have been found thriving within the sandstones of DV regions (Friedmann 1982). A selection of cryptoendolithic-bearing sandstones and cryptoendolith-free sandstones were obtained from the collection of British Geological Survey. Blackhurst et al. (2004) performed a detailed elemental study on bulk- and individual components of these samples and found some specific elemental fractionations associated with different portions of these samples. It was not clear if these elemental signatures were related to the maturity and diagenesis of the sandstone samples or if they could be ascribed to microbial activities. We chose three Antarctic sandstone samples from the study by Blackhurst et al. (2004) to conduct Fe-isotope measurements. Two cryptoendolith-free sandstones, VH59 and MM45, were analysed for their bulk Fe-isotope compositions. The third sample, BP8 was a cryptoendolith-bearing sample which had very low bulk FeO content. Only the top layer, termed ‘crust’ in the Blackhurst et al. (2004) study contained sufficient Fe to obtain Fe-isotopic composition.
Table 1.
List of samples analysed for Fe isotopes in this study. (Samples beginning with BM are from the Natural History Museum collections. NIPR stands for National Institute for Polar Research, Japan. Two of the Martian meteorites were donated to one of the co-authors (M.M.G.) for stable isotope studies by the NIPR. Samples beginning with numbers 153… are from Harker Collection of Cambridge University. The location and detailed descriptions of Antarctic samples are given by Blackhurst et al. (2004). The sample BP8 belongs to the same series of BP samples that have been described by Blackhurst et al. (2004).)
| sample name | source | description | |
|---|---|---|---|
| 1. | Shergotty | BM41021 | Martian basalt |
| 2. | Zagami | BM1993,M.10 | Martian basalt |
| 3. | Sah Al Uhaymir (SaU) 005 | BM2000,M40 | Martian basalt |
| 4. | Los Angeles (LA) 001 | BM2000,M12 | Martian basalt |
| 5. | Dar Al Gani (DaG) 476 | BM1999,M.73 | Martian basalt |
| 6. | Y980459 | NIPR | Martian basalt |
| 7. | Nakhla | BM1913,25 | Martian basalt |
| 8. | Governador Valadares (GV) | BM1975,M16 | Martian basalt |
| 9. | Lafayette | BM1959,755 | Martian basalt |
| 10. | MIL03346 | MWG | Martian basalt |
| 11. | Y000593 | NIPR | Martian basalt |
| 12. | Chassigny | BM19972 | Martian basalt |
| 13. | 98MA76 | 153271 | terrestrial basalt |
| 14. | 99MA40 | 153340 | terrestrial basalt |
| 15. | 98MA07 | 153208 | terrestrial basalt |
| 16. | 98MA79 | 153274 | terrestrial basalt |
| 17. | 99MA37 | 153338 | terrestrial basalt |
| 18. | 99MA74 | 153368 | terrestrial basalt |
| 19. | 98MA78 | 153273 | terrestrial basalt |
| 20. | 98MA22 | 153222 | terrestrial basalt |
| 21. | 99MA63a | 153362 | terrestrial granite |
| 22. | 98MA03a | 153203 | terrestrial granite |
| 23. | BP8 (crust) | Dry Valley region, Antarctica | cryptoendolith-bearing sandstone |
| 24. | MM45 | Dry Valley region, Antarctica | cryptoendolith-free sandstone |
| 25. | VH59 | Dry Valley region, Antarctica | cryptoendolith-free sandstone |
The initial step of sample preparation involved acid digestion of ca 200 mg powdered sample in HF–HClO4–HCl medium, followed by ion-exchange chromatography to separate Fe from other matrix elements. Further details of the procedure followed here can be found in Mullane et al. (2003). Subsequent to column chromatography, the Fe-isotope compositions were measured on a fixed resolution (m/Δm=500) MC-ICP-MS (IsoProbe, GV Instruments, UK) with respect to the Fe isotope standard IRMM-014, using the sample-standard bracketing method (Mullane et al. 2003). Blank subtraction is undertaken off-line. Correction for isobaric interference of 54Cr on 54Fe is made by monitoring the 52Cr signal and applying an on-line mathematical correction at mass 54. The ion-exchange chromatography strips out any Cr present in the sample. However, Cr can also be introduced during the analysis from the instrument components (e.g. cones) for which an online correction is usually applied as mentioned earlier. In typical cases, this correction is almost negligible (less than 0.0001% of the 54Fe signal) suggesting no significant isobaric interference of 54Cr on 54Fe. The other isobaric interference of 40Ar16OH background at mass 57 is reduced by analysing solutions at a concentration of approximately 4–10 p.p.m. and sample and standard solutions were concentration-matched to within ±5%. Both these procedures are routine in various labs conducting Fe-isotope measurements by sample standard bracketing technique (Mullane et al. 2003). The samples were run over a 15-month period and a number of industrial and natural standards were run to check the accuracy of the results. Fe solutions from other laboratories were also analysed for inter-laboratory comparisons. Each sample was run at least twice, usually, three to four times over a 15-month period. Each run consisted of six repeat measurements of 56/54Fe and 57/54Fe ratios with respect to international reference material, IRMM-014. The analytical set-up adopted in the present study was the same as that described by Mullane et al. (2003). Only those parameters were different that vary on the daily basis for the optimum running of the machine, such as the rate of gas flow in the nebulizer and hexapole. Table 2 lists the Fe-isotope compositions of some of the standard solutions that were run during this study. Typical external errors are less than 0.1‰ (2 sigma) in most cases.
Table 2.
Fe isotopic composition of reference material analysed in this study.
| (reference material)IRMM-014 | δ56Fe measured (‰) | δ56Fe reported (‰) | δ57Fe measured (‰) | δ57Fe reported (‰) |
|---|---|---|---|---|
| Aristar (n=30) | −0.29±0.03 | — | −0.42±0.03 | — |
| Certipur (n=34) | 1.26±0.02 | — | 1.88±0.04 | — |
| Johnson Mattey (n=15) | 0.41±0.02 | 0.421±0.05a | 0.62±0.03 | — |
| Puratonic (n=15) | 0.09±0.04 | 0.082±0.035a | 0.15±0.04 | — |
| Hematite (n=11) | 0.52±0.02 | 0.500±0.091b | 0.80±0.03 | 0.745±0.142b |
R. Schoenberg, University of Hanover 2005 (personal communication).
6. Results and discussion
(a) Fe-isotope fractionation
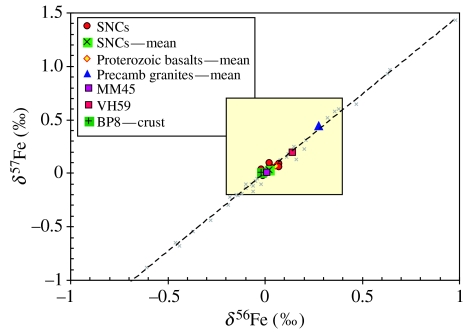
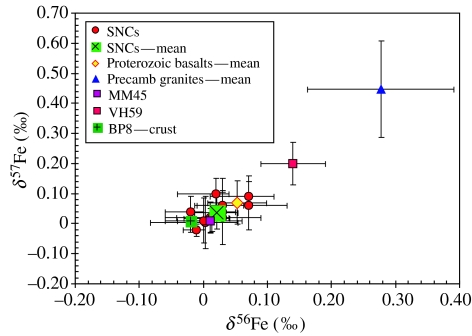
All the Martian meteorites analysed in the present study plot within a restricted range on a three-isotope plot (filled red circles in figure 1) and do not show any measurable fractionation that can be ascribed to low-temperature processes. These results are consistent with other studies that have reported Fe-isotopic compositions of some of the Martian meteorites investigated in the present study (Zhu et al. 2001; Poitrasson et al. 2004; Weyer et al. 2005). Figure 2 shows the spread in the measured δ56Fe and δ57Fe ratios in our samples with 2 sigma error bars. The grey crosses in figure 1 represent Fe-isotope data available on various planetary materials that have been compiled from various sources (Anand et al. 2006 and reference therein) and the dashed line is the best fit line to that data defining the mass fractionation line for Fe isotopes. The majority of Martian meteorites display a restricted range in Fe-isotopic composition from −0.02 to 0.07‰ for δ56Fe and from −0.02 to 0.10‰ for δ57Fe (figure 2), with a mean value (δ56Fe=0.02±0.03‰; δ57Fe=0.04±0.05‰) only slightly lighter than the mean value of eight Proterozoic basalts (δ56Fe=0.05±0.05‰; δ57Fe=0.07±0.07‰) from southern India analysed in this study. The two granite samples have the heaviest Fe-isotope values (table 3), similar to those reported by Poitrasson & Freydier (2005). The heavier Fe-isotopic values of granites have been interpreted to be owing to the exsolution of late magmatic aqueous fluids from the granitic melt that preferentially removed the lighter isotopes of iron, enriching the residual magma in the heavier isotopes (Poitrasson & Freydier 2005). The two ‘life-free’ Antarctic sandstones, MM45 and VH59, have contrasting Fe-isotopic signatures. The Fe-isotopic composition of MM45 is similar to the mean of Martian meteorite values, whereas VH59 has heavier isotopic values (figure 2; table 3). Blackhurst et al. (2004) remarked on the highly ‘immature’ nature of the VH59 sandstone. They also reported the higher modal abundance of feldspars and clay minerals in this sample, the latter being a direct result of low-temperature alteration process. It is, therefore, not entirely surprising that this sample shows a slightly fractionated Fe-isotope signature when compared with the more mature and less altered sandstone sample, MM45. In contrast, however, the ‘crust’ of the cryptoendolith-bearing sample, BP8, lacks any fractionated Fe-isotope signature and has Fe-isotope composition highly similar to that of bulk-Martian samples and terrestrial igneous rocks. This result is slightly unexpected and complicates our ability to use iron isotopes alone as possible bio-markers in the rock record. However, it may be worth noting that owing to analytical issues, we were only able to analyse one portion of the ‘life-bearing’ BP8 sample and that biogenic signature might still be present elsewhere in the sample. Another complexity is the occurrence of microbial communities in a layer just below the ‘crust’ which is essentially devoid of any Fe. Blackhurst et al. (2004) postulated that the microbial activity had probably leached the iron from the colonized layer into the layers above and below, namely in the ‘crust’ and the ‘substrate’, respectively, in which case, any biological fractionation in Fe isotopes should have been detected in the BP8 ‘crust’ sample. A more detailed study involving the measurement of Fe isotopes of individual layers of cryptoendolith-bearing sample is clearly necessary to further address this issue.
Figure 1.
Plot of δ56Fe and δ57Fe values in a range of planetary samples. The grey crosses represent published Fe-isotopic composition on terrestrial and extraterrestrial samples. The compilation is not exhaustive but includes the largest fractionations in Fe-isotopic composition reported in literature. The thin dashed line is the best fit to the data points defined by grey crosses and define the mass fractionation line for Fe isotopes. The Fe-isotopic composition of samples analysed in the present study plot on the same mass-fractionation line. The Martian meteorite data show a restricted range in their Fe-isotopic composition. The terrestrial basalt and granite samples are from in and around the Proterozoic Cuddapah Basin of southern India (Anand et al. 2003). MM45 and VH59 are cryptoendolith-free sandstone samples from DV regions of Antarctica. BP8-crust is the top most portion of a cryptoendolith-bearing sandstone from DVs. The existence of bacterial colony has been reported beneath this crust (Blackhurst et al. 2004). A very low concentration of Fe in the colonized layer prevented the measurement of Fe-isotopic composition from this very horizon.
Figure 2.
Plot of δ56Fe and δ57Fe values of Martian meteorites and terrestrial samples analysed in the present study. This is an expanded version of the data presented in figure 1 which is bounded by the shaded rectangle. The error bars on each data point represent two standard deviations in Fe-isotopic measurements. Almost all samples analysed in the present study show limited fractionation in Fe isotopes. The terrestrial granites and a sandstone sample from Antarctica show slightly heavier Fe-isotopic signature but the values are well within the range of fractionation reported for terrestrial rocks from abiological systems.
Table 3.
Fe isotopic composition of samples analysed in this study.
| sample | δ56Fe (‰) | errors (2 s.d.) | δ57Fe (‰) | errors (2 s.d.) |
|---|---|---|---|---|
| Los Angeles | −0.01 | 0.01 | −0.02 | 0.02 |
| Shergotty | 0.01 | 0.03 | 0.02 | 0.05 |
| Zagami | 0.03 | 0.03 | 0.06 | 0.04 |
| Sayh al Uhaymir | 0.00 | 0.05 | 0.00 | 0.09 |
| Nakhla | 0.01 | 0.03 | 0.02 | 0.04 |
| Chassigny | 0.00 | 0.03 | 0.01 | 0.06 |
| Governador Valadares | 0.02 | 0.02 | 0.10 | 0.06 |
| Lafayette | −0.02 | 0.02 | 0.04 | 0.04 |
| MIL 03346 | 0.07 | 0.04 | 0.09 | 0.06 |
| Y000593 | 0.03 | 0.02 | 0.04 | 0.02 |
| Y98045 | 0.03 | 0.06 | 0.02 | 0.09 |
| DaG 476 | 0.07 | 0.06 | 0.06 | 0.08 |
| Martian basalts (mean of 12 SNCs) | 0.02 | 0.03 | 0.04 | 0.05 |
| 1.9 Ga basalts (mean of eight samples) | 0.05 | 0.05 | 0.07 | 0.07 |
| Precambrian granites (mean of two samples) | 0.28 | 0.11 | 0.45 | 0.16 |
| Antarctic sandstone MM45 | 0.01 | 0.05 | 0.01 | 0.04 |
| Antarctic sandstone VH59 | 0.14 | 0.05 | 0.2 | 0.07 |
| Antarctic sandstone BP8 (crust) | −0.02 | 0.02 | 0.01 | 0.04 |
7. Conclusions and future direction
The Fe-isotopic composition of bulk-rock samples of Martian meteorites display a restricted range (δ56Fe: −0.02 to 0.07‰; δ57Fe: −0.02 to 0.10‰). All Fe-isotope data plot on the mass-dependent fractionation line defined for planetary materials. Terrestrial basalt samples have an Fe-isotopic composition nearly identical to that of Martian meteorites. Terrestrial granites, however, have slightly heavier Fe-isotope signatures, consistent with previous studies. We have not identified any Fe-isotope signatures in the bulk Martian samples and cryptoendolith-bearing sandstone samples from Antarctica that can be ascribed to a biologically mediated system. It is entirely possible that we have not yet analysed an appropriate sample from Mars that contains signatures of the past biological activities or we may need to explore other novel stable isotope tracers that may be more unambiguous biomarkers. The secondary minerals preserved in the Martian meteorites will be analysed in future work and they could potentially provide further information on Fe-isotope fractionation by low-temperature aqueous activities on the Martian surface. Culturing of bacteria on basaltic igneous rocks, similar to Martian meteorites, is also being carried out in the laboratory for Fe-isotopic studies, which is expected to shed some more light on the Fe-isotope fractionation owing to micro-organisms. Analysis of additional samples of microbe-rich communities from both cold and hot desert regions of the world may further aid in understanding the Fe-isotopic fractionations in biological systems.
Acknowledgments
We would like to thank Eta Mullane, Sarah James, Barry Coles, John Chapman and Dominik Weiss for their help in lab work and Fe-isotope measurements. Critical reviews from an anonymous reviewer helped improve the clarity of the paper. M.A. wishes to thank PPARC for a post-doctoral research assistantship.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
References
- Anand M, Gibson S.A, Subbarao K.V, Kelley S.P, Dickin A.P. Early Proterozoic melt generation processes beneath the intra-cratonic Cuddapah Basin, Southern India. J. Petrol. 2003;44:2139–2171. doi:10.1093/petrology/egg073 [Google Scholar]
- Anand, M., Russell, S. S., Blackhurst, R. & Grady, M. M. 2006 Fe isotopic composition of Martian meteorites and some terrestrial analogues. In XXXVII Lunar Planet. Sci. Conf, League City, TX, 13–17 March 2006 Abs#1824.
- Beard B.L, Johnson C.M. Fe isotope variations in the modern and ancient earth and other planetary bodies. Rev. Mineral. Geochem. 2004;55:319–357. [Google Scholar]
- Beard B.L, Johnson C.M, Cox L, Sun H, Nealson K.H, Aguilar C. Iron isotope biosignatures. Science. 1999;285:1889–1892. doi: 10.1126/science.285.5435.1889. doi:10.1126/science.285.5435.1889 [DOI] [PubMed] [Google Scholar]
- Beard B.L, Johnson C.M, Skulan J.L, Nealson K.H, Cox L, Sun H. Application of Fe isotopes to tracing the geochemical and biological cycling of Fe. Chem. Geol. 2003;195:87–117. doi:10.1016/S0009-2541(02)00390-X [Google Scholar]
- Blackhurst R.L, Jarvis K, Grady M.M. Biologically-induced elemental variations in Antarctic sandstones: a potential test for Martian microorganisms. Int. J. Astrobiol. 2004;3:97–106. doi:10.1017/S147355040400206X [Google Scholar]
- Bridges J.C, Grady M.M. Evaporite mineral assemblages in the nakhlite (Martian) meteorites. Earth Planet. Sci. Lett. 2000;176:267–279. doi:10.1016/S0012-821X(00)00019-4 [Google Scholar]
- Carr M.H. Water on Mars. Nature. 1987;326:30–35. doi:10.1038/326030a0 [Google Scholar]
- Feldman W.C, et al. Global distribution of neutrons from Mars: results from Mars Odyssey. Science. 2002;297:75–78. doi: 10.1126/science.1073541. doi:10.1126/science.1073541 [DOI] [PubMed] [Google Scholar]
- Friedmann E.I. Endolithic microorganisms in the Antarctic cold desert. Science. 1982;215:1045–1053. doi: 10.1126/science.215.4536.1045. [DOI] [PubMed] [Google Scholar]
- Johnson C.M, Beard B.L, Roden E.E, Newman D.K, Nealson K.H. Isotopic constraints on biogeochemical cycling of Fe. Rev. Mineral. Geochem. 2004;55:359–408. [Google Scholar]
- McKay D.S, Gibson E.K, Jr, Thomas-Keprta K.L, Vali H, Romanek C.S, Clemett S.J, Chillier X.D.F, Maechling C.R, Zare R.N. Search for past life on Mars: possible relic biogenic activity in Martian meteorite ALH84001. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- McKay, D. S. et al 2006 Observation and analysis of in-situ carbonaceous matter in Nakhla: part I. Lunar Planet. Sci. Conf. XXXVII, Abs#. 2251.
- Mullane E, Russell S.S, Gounelle M, Mason T, Din V, Weiss D, Coles B. Precise and accurate determination of iron isotopes by multi collector inductively coupled plasma mass spectrometry. In: Holland G, Tanner S.D, editors. Plasma source mass spectrometry: applications and emerging technologies. The Royal Society of Chemistry; Cambridge, UK: 2003. pp. 351–361. [Google Scholar]
- Murray J.B, et al. Evidence from the Mars express high resolution stereo camera for a frozen sea close to Mars' equator. Nature. 2005;434:352–356. doi: 10.1038/nature03379. doi:10.1038/nature03379 [DOI] [PubMed] [Google Scholar]
- Nyquist L.E, Bogard D.D, Shih C.-Y, Greshake A, Stoffler D, Eugster O. Chronology and evolution of Mars. vol. 96. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. Ages and geologic histories of Martian meteorites; pp. 105–164. [Google Scholar]
- Poitrasson F, Freydier R. Heavy iron isotope composition of granites determined by high resolution MC-ICP-MS. Chem. Geol. 2005;222:132–147. doi:10.1016/j.chemgeo.2005.07.005 [Google Scholar]
- Poitrasson F, Halliday A.N, Lee D-C, Levasseur S, Teutsch N. Iron isotope differences between Earth, Moon, Mars and Vesta as possible records of contrasted accretion mechanisms. Earth Planet. Sci. Lett. 2004;223:253–266. doi:10.1016/j.epsl.2004.04.032 [Google Scholar]
- Squyres S.W, et al. The opportunity Rover's Athena science investigation at Meridiani Planum. Mars. Science. 2004;306:1698–1703. doi: 10.1126/science.1106171. [DOI] [PubMed] [Google Scholar]
- Weyer S, Anbar A.D, Brey G.P, Münker C, Mezger K, Woodland A.B. Iron isotope fractionation during planetary differentiation. Earth Planet. Sci. Lett. 2005;240:251–264. doi:10.1016/j.epsl.2005.09.023 [Google Scholar]
- Zhu X.K, Guo Y, O'Nions R.K, Young E.D, Ash R.D. Isotopic homogeneity of iron in the early solar nebula. Nature. 2001;412:311–313. doi: 10.1038/35085525. doi:10.1038/35085525 [DOI] [PubMed] [Google Scholar]