Abstract
The prebiotic synthesis of phosphorus-containing compounds—such as nucleotides and polynucleotides—would require both a geologically plausible source of the element and pathways for its incorporation into chemical systems on the primitive Earth. The mineral apatite, which is the only significant source of phosphate on Earth, has long been thought to be problematical in this respect due to its low solubility and reactivity. However, in the last decade or so, at least two pathways have been demonstrated which would circumvent these perceived problems. In addition, recent results would seem to suggest an additional, extraterrestrial source of reactive phosphorus. It appears that the ‘phosphorus problem’ is no longer the stumbling block which it was once thought to be.
Keywords: prebiotic phosphorylation, sources of phosphate, primitive Earth, reducing conditions, volcanic lightning
1. Introduction
We have all become quite accustomed to referring to the so-called biogenic elements—designated by the chemical symbols CHNOPS—without too much reflection as to why these elements play the role in biology that they do. However, a small group of notable scholars has addressed the question ‘why’. Lawrence Henderson (1913), for example, in a book entitled The fitness of the environment, pointed out that certain features of the environment were admirably suited to support life. Henderson (who is still well-worth reading by today's astrobiologists) pointed to (among other phenomena) the now famous example of the anomalous expansion of water at the freezing point. This property leads to the fact that the oceans do not freeze solid as a result of several cold winters, but rather freeze each winter from the top-down, and consequently, are able to melt again during the summer. Other characteristics of water considered by Henderson included other thermal properties such as its high specific heat, latent heat of melting and of evaporation, thermal conductivity and solvent properties. This approach was followed and expanded on by Blum (1951), in his book Time's arrow and evolution. Subsequently, George Wald (1958), in an introduction to the 1958 edition of Henderson's book, pointed out that H, O, N and C constitute 99% of all examples of living tissue and offered as a reason the fact that these four elements are (in that order) the smallest in the periodic table which reach stable electronic configurations by adding, respectively, 1, 2, 3 and 4 electrons. In other words, they are the simplest elements able to form bonds, and particularly (also as a result of their smallness), multiple bonds. In a later discussion of the same general topic, Wald (1962) expanded the argument to P and S:
I have tried to find a basis for the biological selection of S and P for group and energy transfer reactions in (1) the fact that they form more open and usually weaker bonds than their cogeners in the Second Period [of the periodic table], O and N; (2) their possession of 3d orbitals, permitting the expansion of their valences beyond four; and (3) their retention of the capacity to form multiple bonds, a property otherwise characteristic of C, N, and O. The capacity to form multiple bonds contributes principally to the thermodynamics of energy transfer.
Also of interest in this connection, are the remarks of Todd (1959):
This discussion of phosphate chemistry is by no means complete, but enough has been said to indicate in some degree why phosphate derivatives occur so widely as reactive intermediates in biological systems. Some of the reactions I have discussed can be simulated by derivatives of other acids, but the tribasic character of phosphoric acid gives it the tremendous flexibility in action which makes it almost ideal as a multi-purpose reagent in living processes.
Frank Westheimer (1987) has also added to the literature on this subject, and pointed to a number of properties of phosphate which fit this group for its role in both energy transfer and as a backbone feature of nucleic acids.
In light of the above arguments, we should not be surprised by the central role which phosphorus plays in biology, and presumably, must also have played in the origin of life. From the point of view of prebiotic chemistry, the next logical question is what was the exact sequence of events that started with inorganic materials, and led to the formation of the first important organic phosphate compounds. Since ATP is the central phosphorus-containing metabolite in biology and is involved in virtually all phosphorylation reactions, we could do worse than begin with the question of its synthesis and properties. A valuable observation in this regard was made by Jones & Lipmann (1960), who suggested that the role of ATP might have been preceded in prebiological chemistry by inorganic polyphosphates:
The inorganic polyphosphates which are found in a great variety of organisms seem more attractive as primary donors. Although now they give the impression of being metabolically fairly inert, they may represent an early means of energy distribution which was abandoned later on. Their availability from inorganic sources seems to be rather likely.
This observation provided a strong impetus for investigations into the properties of inorganic polyphosphates and their utility in phosphorylation reactions.
2. Phosphorylation reactions with polyphosphates or ammonium phosphates
In synthetic organic chemistry, reactive and usually unsaturated ‘condensing agents’ are commonly employed for phosphorylation reactions. A number of studies of possible ‘prebiotic’ phosphorylations have therefore utilized reactive compounds such as cyanamide and various related compounds (Steinman et al. 1964). Probably the most convincing models of prebiotic phosphorylations, however, have made use of inorganic polyphosphates or salts of phosphoric acid, which yield polyphosphates upon heating. A general summary of this approach is shown in figure 1.
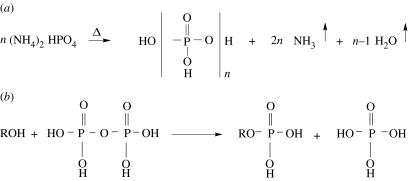
Figure 1.
(a) Balanced, idealized reaction equation for the production of condensed phosphates by the heating of ammonium phosphate at elevated temperatures (greater than 125°C). In an actual experiment, of course, the reaction may not be complete. (b) Reaction of an alcohol or nucleoside with pyrophosphoric acid (the simplest, but least reactive example of a condensed phosphate) to produce a phosphate monoester with elimination of phosphoric acid.
Figure 1 summarizes the reactions involved in the thermal phosphorylation of a nucleoside by heating in the presence of ammonium phosphate (Rabinowitz et al. 1968). The structure given as HO[PO3H]nH is the average composition of condensed phosphate of chain length n. At a moderate temperature such as 125°C, the primary products are pyrophosphate and tripolyphosphate. At elevated temperatures, long-chain polyphosphoric acid is formed. Solid-state phosphorylations such as these have been shown to be catalysed by urea (Lohrmann & Orgel 1971). Under these conditions, the escape of gaseous ammonia as well as water provides the driving force. Once formed, polyphosphates are effective phosphorylating agents, even in neutral aqueous solution (Schwartz & Ponnamperuma 1968). There is some question as to the availability of ammonia on the primitive Earth, due to its rapid photolytic conversion by ultraviolet photolysis to N2 (Ferris & Nicodem 1974). For this reason, the most reasonable scenario may be one involving the recycling of ammonia. For example, ammonia is generated by hydrolysis of many potentially important prebiotic reactants such as nitriles and amides. Thus, it is not difficult to imagine it being continually regenerated in solution, at least locally. The photochemical and geochemical cycling of ammonia is a complex problem, involving a number of processes. For a review and perspective on these questions, see Summers (1999).
As is often the case in prebiotic scenarios, a volcanic region would seem to be the sort of locale where polyphosphates as well as phosphorylation reactions could be driven by ammonium salts of phosphoric acid. While there is no shortage of models of prebiotic phosphorylation in the literature, we need properly to deal first with the question of the source material on the primitive Earth for all subsequent phosphorus chemistry. The phosphorylation methods summarized earlier are all subject to the criticism that they do not utilize a geologically plausible source of phosphate. In other words, where did the phosphate come from in the first place?
3. Known sources of inorganic phosphate
A summary of the most important naturally occurring phosphate minerals, both on the Earth and in meteorites, is shown in table 1 (the most important structural forms are shown; in actual fact, most occurrences involve a mixture of species).
Table 1.
Some naturally occurring phosphorus-containing minerals.
| fluorapatite | Ca5(PO4)3F | terrestrial igneous minerals, meteorites |
| hydroxyapatite | Ca5(PO4)3(OH) | terrestrial sedimentary minerals |
| francolite | Ca5(PO4)2.5(CO3)0.5F | terrestrial sedimentary minerals |
| schreibersite | (Fe, Ni)3P | meteorites |
| whitlockite | Ca9(Mg, Fe)(PO4)6PO3OH | meteorites |
| chlorapatite | Ca5(PO4)3Cl | meteorites |
We are at once confronted by a problem in viewing this list. With the possible exception of meteoritic schreibersite (to be discussed later), all of the compounds in table 1 are only slightly soluble in water. While organisms can make use of the micromolar concentrations of phosphate which occur in natural waters, it is difficult to imagine how any useful synthetic (prebiotic) chemistry could take place. Owing to the high natural abundance of calcium, evaporation of seawater does not lead to an increase in phosphate concentration, but to precipitation of one or another form of apatite or francolite. Two possible exceptions to this general behaviour should be noted. Firstly, if the early oceans were slightly less alkaline than today's oceans, perhaps due to a higher partial CO2 pressure, then the precipitation of the acid calcium salt brushite (CaHPO4·2H2O) might have been possible (Gedulin & Arrhenius 1994). Secondly, under conditions of relatively high magnesium and ammonia concentrations, an additional precipitate becomes possible (Handschuh & Orgel 1973), the mineral struvite (MgNH4PO4·6H2O). Both of these latter minerals can generate condensed phosphates upon heating.
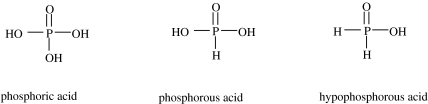
We have to go back to Addison Gulick (1955) to find the first explicit discussion of the problem of the low solubility of phosphate minerals, as well as a suggestion as to a possible solution. Gulick pointed out that the only significant source for phosphorus on Earth was the slightly soluble mineral apatite. He suggested that under the supposed ‘reducing conditions’ described by Oparin (1938) as well as Urey (1952), phosphate would be reduced to a form such as phosphite or hypophosphite (figure 2), which are considerably more soluble in the presence of calcium than phosphate.
Figure 2.
The three oxyacids of phosphorus. The salts are referred to as phosphates, phosphites and hypophosphites, respectively.
This suggestion was considered by Miller & Urey (1959), who noted that all the lower oxidation states of phosphorus were unstable under the pressures of hydrogen which these authors found to be probable for the primitive Earth. Although they remarked that stronger reducing agents than hydrogen might have been present and that such a possibility was deserving of further investigation, the possibility that reduction of phosphate could have occurred under unusual conditions actually remained unaddressed. Recent work has shown that such a process is indeed possible. This review will describe three scenarios by means of which phosphate could have been made available for prebiotic chemistry. The first is the possibility of phosphate reduction by a combination of reducing and high-energy conditions. The second is the discovery by Yamagata et al. that P4O10 can be volatilized from magma and is, in fact, being liberated from contemporary volcanoes. The third scenario is related to a recent demonstration that the meteoritic phosphide mineral schreibersite is surprisingly reactive.
4. Lightning in volcanic eruption clouds and phosphate
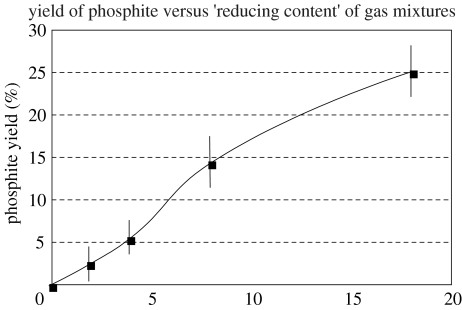
A phenomenon familiar to volcanologists is the occurrence of lightning discharges in eruption clouds. Gilbert & Lane (1994), for example, in a specialist report related to the topic of aviation safety, cite multiple cases of lightning in volcanic clouds, which were observed during eruptions at 11 different volcanoes, while Navarro-Gonzalez et al. (1996) list an additional six documented examples and yet more can be found on the Internet. Two features of these events make them interesting from the point of view of prebiotic chemistry: (i) the presence of reduced gases in volcanic eruptions and (ii) the presence of abundant dust in the clouds. It was pointed out by Russian workers in the 1970s that these conditions were suitable for prebiotic syntheses such as the Miller experiment and a number of kinds of organic compounds, including amino acids, were identified (chiefly by paper and thin-layer chromatography) in volcanic deposits (e.g. Markhinin & Podkletnov 1977). Today, we would require more definitive molecular characterization of compounds of potentially biological significance than were then applied, as well as evidence that they were not introduced by contamination. Nevertheless, conditions such as those described earlier also raise the possibility of reduction of phosphate to phosphite. In a simulation of the process, we have found that reduction is surprisingly effective, but strongly dependent on the reducing nature of the gas-mixture employed (figures 3 and 4).
Figure 3.
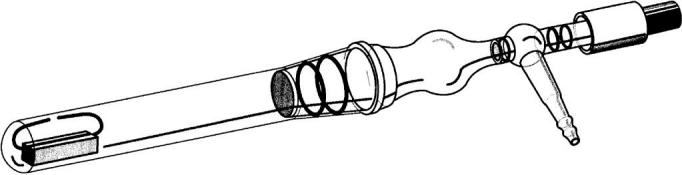
Apparatus used to study the effect of electric discharge on mineral samples. Samples of apatite, mixed with a clay mineral to provide improved adhesion, were deposited onto the ends of an open tungsten loop, which formed a gap of a few millimetres. Pyrex caps (not shown) were fitted over the ends of the loop to prevent direct interaction of the sample with the metal. The chamber was evacuated and back-filled with the gas mixture to be tested. A spark was induced between the ends of the loop by exposing the apparatus to a microwave field. (Illustration from Glindemann et al. 1999; reproduced with kind permission of Springer Science and Business Media.)
Figure 4.
The conversion of apatite to phosphite by spark discharge in a model atmosphere containing initially 60% CO2 and 40% N2, but with increasing additions of H2 and CO (the abscissa shows the sum of the contents of H2 and CO, which were present in equal concentrations). Data points (solid squares) are averages of three to five replicate analyses and the vertical lines show the average deviation of the mean of each set of measurements (De Graaf & Schwartz 2000; reproduced with kind permission of Springer Science and Business Media).
The interest of phosphite as a possible prebiotic phosphorus source is not only due to the higher solubility of its calcium salt compared to apatite (approx. 1000 times higher),1 but also to its greater reactivity as a phosphorylating agent. It has been established that ammonium phosphite, for example, readily reacts with nucleosides to yield nucleoside-phosphites (nucleoside H-phosphonates) under conditions in which ammonium phosphate is unreactive (figure 5).
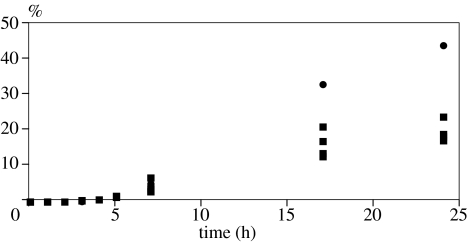
Figure 5.
Yield of uridine-5′-phosphite by reaction of uridine with ammonium phosphite (1 : 2 molar ratio) at 60°C. Solid squares, four sets of reactions giving the yields in the absence of urea; solid circles (upper series of data points), yields in the presence of urea (uridine : ammonium phosphate : urea=1 : 2 : 4). The initial time-lag observed for 5 h and less was probably due to the evaporation of water from the reaction. Under identical conditions as those shown here, ammonium phosphate produced no products (De Graaf & Schwartz 2005; reproduced with kind permission of Springer Science and Business Media).
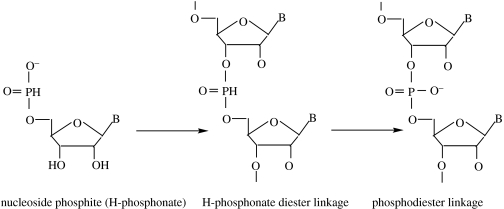
We have shown that nucleoside H-phosphonates are easily synthesized. Preliminary results also indicate that they are thermally condensed to form dimers linked by H-phosphonate diester bonds. While the monomers are resistant to oxidation, once incorporated into a dimer, the linkage is easily oxidized to a phosphodiester. It is, therefore, conceivable that they might act as intermediates in a pathway towards the formation of polynucleotides, as illustrated in figure 6.
Figure 6.
A hypothetical pathway starting with a nucleoside phosphite, leading to the formation of nucleoside-3′-5′ H-phosphonate linkages and, finally, to 3′-5′ phosphodiester linkages. While oxidation of a nucleoside H-phosphonate (monomer) is a difficult reaction, once incorporated into an H-phosphonate diester, oxidation proceeds readily with mild agents (De Graaf & Schwartz 2005). The final step of oxidation to a phosphodiester linkage could theoretically occur on the primitive Earth by reaction with Fe3+ or HOOH. Other linkage types (such as 5′-5′, 2′-5′ and so forth) are, of course, also possible.
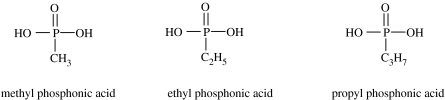
Phosphite, incidentally, also provides a possible link to the presence of phosphonic acids in carbonaceous chondrites. These compounds, which are the only phosphorus-containing organic compounds to be identified in meteorites, were reported by Cooper et al. (1992) in investigations of the Murchison meteorite (figure 7).
Figure 7.
Phosphonic acids identified in the Murchison meteorite. Note that they are all derivable from phosphorous acid in figure 2 by replacing the hydrogen atom attached to phosphorus with a methyl-, ethyl- or propyl-group.
An interesting possibility concerning the mechanism of synthesis of these compounds in meteorites is that they might derive from reactions of the PO3 radical di-anion and thus, ultimately, from a source such as phosphorous acid or phosphites. For example, recombination of ·PO3 with a methyl radical has been shown to produce methyl phosphonic acid, the most abundant phosphorus-containing compound in Murchison. Similarly, a suite of other phosphonic acids has been synthesized via ultraviolet irradiation of phosphite in the presence of formaldehyde or simple alcohols (De Graaf et al. 1995).
5. Volatilization of P4O10 and generation of polyphosphates in volcanic regions
Yamagata et al. (1991) reported the detection of condensed phosphates (primarily pyrophosphate and tripolyphosphate) in condensates from experiments in which apatite mixed with basalt had been subjected to elevated temperature, thus indirectly confirming an earlier prediction that P4O10 would be volatilized from apatite at high temperature (Griffith et al. 1977). More striking was the identification of the same products in condensates collected from a fumarole of the volcano Mount Usu. The concentrations reported for orthophosphate, pyrophosphate and tripolyphosphate were 1.13, 0.45 and 0.37 μM, respectively. These concentrations are very low and it is perhaps unlikely that they could have contributed directly to chemical evolution. More interesting is the question of the possible interaction of condensed phosphates with organic products on the surfaces (and perhaps even absorbed into the interior) of ash particles in eruption clouds. In the course of the experiments on phosphite production mentioned earlier, we were also able to detect ortho- pyro- and tripolyphosphate, essentially confirming the results of Yamagata et al. In our experiments, up to 50% of the phosphate exposed to the electric discharge was recovered in water extracts at the conclusion of the run, with roughly 10 and 2% of this total being in the form of pyro- and tripolyphosphate, respectively.
6. Schreibersite as a possible prebiotic source of condensed phosphates
Recently, an additional possibility and potential source of reactive phosphorus compounds has been suggested by Pasek & Lauretta (2005). As was noted in table 1, the phosphide mineral schreibersite is a well-known inclusion in iron meteorites and enstatite chondrites, generally in the form of discrete nodules or grains. These authors estimate that as much as 5% of total crustal P was at one time extraterrestrial schreibersite, added to the Earth by impacts during or at the end of the late heavy bombardment. However, the most interesting result of this new work is an investigation of the aqueous chemistry of the compound Fe3P, which was used to approximate schreibersite. Since the valance level of phosphorus in the mineral is 0 and it is known to consist of a solid solution of the element in the meteoritic Fe–Ni phase, it might have been expected to be rather unreactive and not produce much novel chemistry upon introduction into water. Quite surprisingly, however, reaction of synthetic Fe3P with pure water as well as with saline or other salt solutions was found to rapidly produce condensed phosphate as well as hypophosphate, a compound containing a P–P linkage. The chemistry of the reaction appears to be quite complex, but some of the products observed in a single experiment are listed in table 2.
Table 2.
Products obtained by reaction of Fe3P with water. The reactions shown here were run in deionized water under argon as well as in the presence of air. Fe3P (1 g) was stirred in 25 ml H2O for 1 day at 20°C (selected data from Pasek & Lauretta 2005).
| product | μM concentration | |
|---|---|---|
| in argon | in air | |
| orthophosphate | 130 | 1100 |
| orthophosphite | 100 | 700 |
| hypophosphate | 18 | 280 |
| pyrophosphate | 23 | 150 |
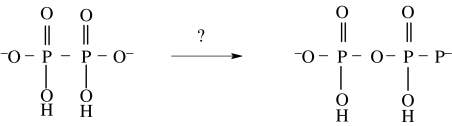
A mechanism which would explain the higher yields observed in the presence of oxygen has not yet been elucidated. Remarkably, however, pyrophosphate at a concentration of 23 μM was observed even under argon. The authors speculate that hypophosphate is the precursor of the pyrophosphate detected (figure 8).
Figure 8.
Possible formation of pyrophosphate from hypophosphate suggested by Pasek & Lauretta (2005). Since hypophosphate is known to be a relatively stable compound under most conditions and has been synthesized by UV irradiation of phosphite, this is a rather unexpected consequence of the reaction of Fe3P with water (Schwartz & van der Veen 1973).
Although this concentration seems low, it is at least an order of magnitude higher than the solubility of apatite as well as representing an activated form of phosphate which itself can, under certain conditions, produce phosphorylated products (Neuman et al. 1970). More data on this interesting reaction are clearly desirable, particularly if the results can be repeated with authentic schreibersite. A poster presented at this conference has, in fact, reported the substantiation of these results with meteoritic schreibersite (Terrence P. Kee 2006, personal communication).
7. Conclusion
A variety of pathways now are available as possible solutions for what this author used to refer to as the ‘phosphate problem’. The ‘problem’ seems to have evolved gradually into the less perplexing one of having to decide which of several possible mechanisms are most likely to have operated on the primitive Earth.
Endnote
There appear to be no literature citations for the solubility of CaHPO3. We have estimated this value in laboratory experiments as being greater than 0.001 M (Glindemann et al. 1999).
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
Discussion
J. F. Kasting (Department of Geosciences, Penn State University, PA, USA). I would just note that H2/CO rich atmospheres, which favour phosphite over phosphate, are considered to be plausible prebiotic atmospheres. H2 may have been high (about 0.1 bar) as a consequence of slow escape of hydrogen to space (Tian et al. 2005). CO could have been abundant (>0.1 bar) in the aftermath of large impacts (Kasting, Orig. of life, 1990). Thus, getting phosphate may have been relatively easy to do on the prebiotic Earth.
D. W. Deamer (Department of Chemistry and Biochemistry, University of California, Santa Cruz, USA). Question: Can you please comment on the roles of pH and CO2 in releasing phosphate from apatite in soluble form?
A. W. Schwartz. A pH lower than in the present ocean (about 8) would tend to increase the solubility of apatite. Calculations have shown that at a pH between 5 and 6 (in the range which could be produced by a high CO2 concentration), there would be about an order of magnitude more phosphate in solution. Although this is still a very low phosphate concentration for solution chemistry, the presence of simple organic acids, which chelate calcium, combined with evaporation, could strongly enhance the effect (Schwartz 1971).
R. J. P. Williams (Inorganic Chemistry Laboratory, University of Oxford, UK). I think it is not necessary to postulate reduced phosphorus compounds. The initial requirement of cells is to get energy in a useable form. The probability is that the first step is to make a vesicle with a gradient of protons associated with the membrane. This gradient will drive element uptake or rejection, explaining the observed gradients of ions and molecules essential before chemistry could occur (uphill in energy) in the prebiotic cell. We know that the proton gradient can drive the catalysed formation of pyrophosphate. Given pyrophosphate (ATP) all the rest of some redox chemistry—condensation—can be driven.
A. W. Schwartz. You may well be right and experiments may eventually substantiate this very reasonable conjecture. But this is theory rather than fact and represents a considerable extrapolation from biochemistry (i.e. requiring an enzyme) to possible prebiotic chemistry. Until we have a model system, which actually demonstrates that this can be achieved with plausible (simple) ingredients, it seems prudent to consider other possibilities.
References
- Blum H.F. Princeton University Press; Princeton, NJ: 1951. Time's arrow and evolution. [Google Scholar]
- Cooper G.W, Onwo W.M, Cronin J.R. Alkyl phosphonic acids and sulfonic acids in the Murchison meteorite. Geochim. Cosmochim. Acta. 1992;56:4109–4115. doi: 10.1016/0016-7037(92)90023-c. doi:10.1016/0016-7037(92)90023-C [DOI] [PubMed] [Google Scholar]
- De Graaf R.M, Schwartz A.W. Reduction and activation of phosphate on the primitive Earth. Orig. Life Evol. Biosph. 2000;30:405–410. doi: 10.1023/a:1006700512902. doi:10.1023/A:1006700512902 [DOI] [PubMed] [Google Scholar]
- De Graaf R.M, Schwartz A.W. Thermal synthesis of nucleoside H-phosphonates under mild conditions. Orig. Life Evol. Biosph. 2005;35:1–10. doi: 10.1007/s11084-005-0093-9. doi:10.1007/s11084-005-0093-9 [DOI] [PubMed] [Google Scholar]
- De Graaf R.M, Visscher J, Schwartz A.W. A plausibly prebiotic synthesis of phosphonic aicds. Nature. 1995;378:474–477. doi: 10.1038/378474a0. doi:10.1038/378474a0 [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Nicodem D.E. Ammonia: did it have a role in chemical evolution? In: Dose K, Fox S.W, Deborin G.A, Pavlovskaya T.E, editors. The origin of life and evolutionary biochemistry. Plenum Press; New York, NY: 1974. pp. 107–117. [Google Scholar]
- Gedulin B, Arrhenius G. Sources and geochemical evolution of RNA precursor molecules—the role of phosphate. In: Bengston S, editor. Early life on earth. Nobel symposium. vol. 84. Columbia University Press; New York, NY: 1994. pp. 91–110. [Google Scholar]
- Gilbert J.S, Lane S.J. Electrical phenomena in volcanic plumes. In: Casadevall T.J, editor. Volcanic ash and aviation safety. US geological survey bulletin. vol. 2047. US Government Printing Office; Washington, DC: 1994. pp. 31–38. [Google Scholar]
- Glindemann D, De Graaf R.M, Schwartz A.W. Chemical reduction of phosphate on the primitive Earth. Orig. Life Evol. Biosph. 1999;29:555–561. doi: 10.1023/a:1006622900660. doi:10.1023/A:1006622900660 [DOI] [PubMed] [Google Scholar]
- Griffith E.J, Ponnamperuma C, Gabel N. Phosphorus, a key to life on the primitive Earth. Orig. Life. 1977;8:71–85. doi: 10.1007/BF00927976. doi:10.1007/BF00927976 [DOI] [PubMed] [Google Scholar]
- Gulick A. Phosphorus as a factor in the origin of life. Am. Sci. 1955;43:479–489. [Google Scholar]
- Handschuh G.J, Orgel L.E. Struvite and prebiotic phosphorylation. Science. 1973;179:483–484. doi: 10.1126/science.179.4072.483. [DOI] [PubMed] [Google Scholar]
- Henderson L.J. Macmillan; Boston, MA: 1913. The fitness of the environment: an inquiry into the biological significance of the properties of matter. [Google Scholar]
- Jones M.E, Lipmann F. Chemical and enzymatic synthesis of carbamyl phosphate. Proc. Natl Acad. Sci. USA. 1960;46:1194–1205. doi: 10.1073/pnas.46.9.1194. doi:10.1073/pnas.46.9.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann L, Orgel L.E. Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science. 1971;171:490–494. doi: 10.1126/science.171.3970.490. [DOI] [PubMed] [Google Scholar]
- Markhinin E.K, Podkletnov N.E. The phenomenon of formation of prebiological compounds in volcanic processes. Orig. Life. 1977;8:225–235. doi: 10.1007/BF00930684. doi:10.1007/BF00930684 [DOI] [PubMed] [Google Scholar]
- Miller, Urey Organic compound synthesis on the primitive Earth. Science. 1959;130:245–251. doi: 10.1126/science.130.3370.245. [DOI] [PubMed] [Google Scholar]
- Navarro-Gonzalez, R., Basiuk, V. A. & Rosenbaum, M. 1996 Lightning associated to Archean volcanic ash–gas clouds. Chemical evolution: physics of the origin and evolution of life. Proc. 4th Trieste conference on chemical evolution, Trieste, Italy, 4–8 September 1995. pp. 123–142.
- Neuman M.W, Neuman W.F, Lane K. On the possible role of crystals in the origins of life IV. The phosphorylation of nucleosides. Curr. Modern Biol. 1970;3:277–283. doi: 10.1016/0303-2647(70)90010-9. [DOI] [PubMed] [Google Scholar]
- Oparin A.I. Macmillan; New York, NY: 1938. The origin of life. [Transl. by S. Morgulis.] [Google Scholar]
- Pasek M.A, Lauretta D.S. Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiolgy. 2005;5:515–535. doi: 10.1089/ast.2005.5.515. doi:10.1089/ast.2005.5.515 [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Chang S, Ponnamperuma C. Phosphorylation by way of inorganic phosphate as a potential prebiotic process. Nature. 1968;218:442–443. doi: 10.1038/218442a0. doi:10.1038/218442a0 [DOI] [PubMed] [Google Scholar]
- Schwartz A.W, Ponnamperuma C. Phosphorylation of adenosine with linear polyphosphate salts in aqueous solution. Nature. 1968;218:443. doi: 10.1038/218443a0. doi:10.1038/218443a0 [DOI] [PubMed] [Google Scholar]
- Schwartz A.W, van der Veen M. Synthesis of hypophosphate by ultraviolet irradiation of phosphite solutions. Inorg. Nucl. Chem. Lett. 1973;9:39–41. doi:10.1016/0020-1650(73)80083-2 [Google Scholar]
- Steinman G, Lemmon R.M, Calvin M. Cyanamide: a possible key compound in chemical evolution. Proc. Natl Acad. Sci. USA. 1964;52:27–30. doi: 10.1073/pnas.52.1.27. doi:10.1073/pnas.52.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D.P. Sources and sinks for ammonia and nitrite on the early Earth and the reaction of nitrite with ammonia. Orig. Life Evol. Biosph. 1999;29:33–46. doi: 10.1023/a:1006517823004. doi:10.1023/A:1006517823004 [DOI] [PubMed] [Google Scholar]
- Todd A. Some aspects of phosphate chemistry. Proc. Natl Acad. Sci. USA. 1959;45:1389–1397. doi: 10.1073/pnas.45.9.1389. doi:10.1073/pnas.45.9.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urey H.C. Yale University Press; New Haven, CT: 1952. The planets; p. 245. [Google Scholar]
- Wald G. Beacon Press; Boston, MA: 1958. Introduction to Henderson. op. cit. [Google Scholar]
- Wald G. Life in the second and third periods; or why phosphorus and sulfur for high-energy bonds? In: Kasha M, Pullman B, editors. Horizons in biochemistry. Academic Press; New York, NY: 1962. pp. 127–142. [Google Scholar]
- Westheimer F.H. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Watanabe H, Saitoh M, Namba T. Volcanic production of polyphosphates and its relevance to prebiotic chemistry. Nature. 1991;352:516–519. doi: 10.1038/352516a0. doi:10.1038/352516a0 [DOI] [PubMed] [Google Scholar]
Additional references
- Schwartz A.W. Phosphate: solubilization and activation on the primitive Earth. In: Buvet R, Ponnamperuma C, editors. Chemical evolution and the origin of life. North-Holland; Amsterdam, The Netherlands: 1971. pp. 100–108. [Google Scholar]
- Tian F, Toon O.B, Pavlov A.A, De Sterck H. A hydrogen-rich early Earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. doi:10.1126/science.1106983 [DOI] [PubMed] [Google Scholar]