Abstract
Oxygen isotope data from ancient sedimentary rocks appear to suggest that the early Earth was significantly warmer than today, with estimates of surface temperatures between 45 and 85°C. We argue, following others, that this interpretation is incorrect—the same data can be explained via a change in isotopic composition of seawater with time. These changes in the isotopic composition could result from an increase in mean depth of the mid-ocean ridges caused by a decrease in geothermal heat flow with time. All this implies that the early Earth was warm, not hot.
A more temperate early Earth is also easier to reconcile with the long-term glacial record. However, what triggered these early glaciations is still under debate. The Paleoproterozoic glaciations at approximately 2.4 Ga were probably caused by the rise of atmospheric O2 and a concomitant decrease in greenhouse warming by CH4. Glaciation might have occurred in the Mid-Archaean as well, at approximately 2.9 Ga, perhaps as a consequence of anti-greenhouse cooling by hydrocarbon haze. Both glaciations are linked to decreases in the magnitude of mass-independent sulphur isotope fractionation in ancient rocks. Studying both the oxygen and sulphur isotopic records has thus proved useful in probing the composition of the early atmosphere.
Keywords: palaeoclimate, oxygen isotopes, sulphur isotopes, mass-independent fractionation, seawater composition
1. Introduction
Early atmospheric composition and climate play an important part in some origin of life theories. Theories that rely on in situ formation of organic molecules within the atmosphere–surface ocean system are extremely dependent on the redox state of the early atmosphere. The present wisdom is that highly reduced CH4–NH3 atmospheres are unlikely, while weakly reduced CO2–N2 atmospheres with small amounts of H2 (approx. 0.1%) are likely (Walker 1977; Kasting 1993; Kasting & Brown 1998). Recent work by Tian et al. (2005) suggests that atmospheric H2 concentrations might have been much higher (approx. 0.1 bar) as a consequence of slow hydrodynamic escape of hydrogen to space. Catling (2006) has questioned the new calculation, arguing that it is still over-simplified and the authors may have underestimated both the upper atmospheric temperatures and the hydrogen escape rates. Clearly, more work needs to be done to test whether the Tian et al. results are robust. Until then, it makes little sense to speculate further on what the correct answer may be. However, CH4–NH3 atmospheres remain unlikely.
The question of paleoclimates has received more recent attention. Kasting & Ono (2006) have just reviewed this topic in a recent issue of this same journal. Rather than repeating the discussion, we will summarize it briefly here, and discuss the changes that have occurred since the previous article was written. Detailed references can be found in the previous paper.
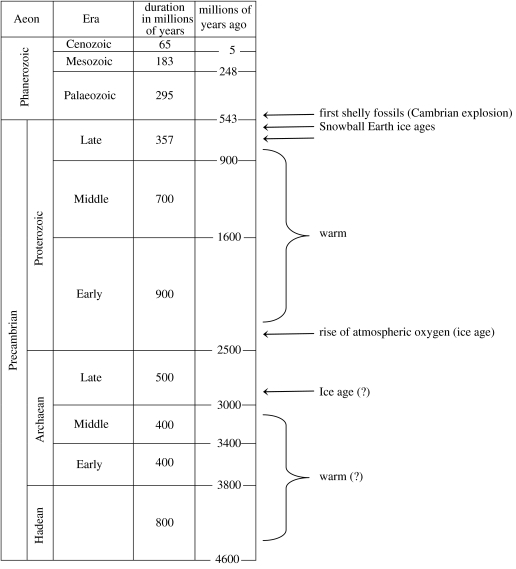
Figure 1, which is duplicated from Kasting & Ono (2006), shows the broad scope of what is known about climates during the Precambrian. The overall trend shows two long warm periods separated by brief glacial events. Much of the Precambrian, particularly the Middle Proterozoic, 2.2–0.8 Ga, appears to have been ice-free. This inference is more secure for the Proterozoic, where the rock record is reasonably good, than for the more sparsely represented Archaean. Major glaciations are known to have occurred in the Neoproterozoic at approximately 0.6 and 0.75 Ga and in the Paleoproterozoic at approximately 2.4 Ga. All three of these periods show evidence for continental-scale glaciation at low paleolatitudes and are thought by some authors, including us, to represent ‘Snowball Earth’ episodes. Though, for a variant on the standard Snowball Earth model, see the paper by Pollard & Kasting (2005).
Figure 1.
Geologic time-scale showing major climatic and evolutionary events during the Precambrian Era.
The Paleoproterozoic glaciation, which includes the Huronian sequence in southern Canada, appears to have coincided with the rise of atmospheric oxygen. The evidence for this comes both from conventional geological O2 indicators, such as detrital-reduced minerals and redbeds (e.g. Holland 1994), and from ‘unconventional’ mass-independently fractionated sulphur isotopes in rocks (Farquhar et al. 2000; Holland 2006; Kasting & Ono 2006). The coincidence in timing between the Paleoproterozoic glaciations and the rise of O2 is conveniently explained if the Late Archaean atmosphere was being kept warm by a combination of enhanced CO2 concentrations along with 1000 p.p.m.v. or more of biologically produced CH4. High greenhouse gas concentrations are required in order to maintain a mean surface temperature above freezing, despite the Sun being approximately 20% dimmer at this time. Methane concentrations of this magnitude should have accounted for approximately 30° of greenhouse warming; thus, the disappearance of CH4, as a result of increased O2 levels, could have easily triggered a major glaciation.
2. Was the Archaean climate hot? Evidence from oxygen isotopes in ancient sedimentary rocks
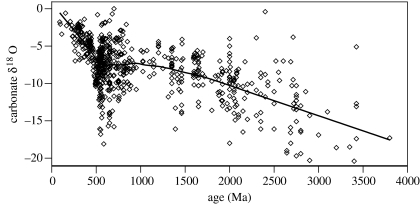
The evidence for glaciation in the Proterozoic, and possibly in the Archaean as well (see §4), is in apparent conflict with oxygen isotope data which suggest that the early Earth was hot. Indeed, any discussion on ancient climates eventually bumps into a long-standing question: why are virtually all Precambrian sedimentary rocks strongly depleted in 18O compared to their modern counterparts? This is an issue that has intrigued isotope geochemists ever since it was first pointed out by Perry (1967). Data from ancient carbonates have been compiled most recently by Veizer et al. (1999) and Shields & Veizer (2002) and are summarized in figure 2. Archaean, Proterozoic and early Phanerozoic carbonates are typically depleted in δ18O by 6–10‰. The same is true for cherts (Knauth & Epstein 1976; Knauth & Lowe 2003; Knauth 2005).
Figure 2.
Average oxygen isotope ratios of marine carbonates deposited over the past 3.4 Ga, compiled by Jaffres (2005). Data are from Veizer et al. (1999) and Shields & Veizer (2002). The solid line represents a least-squares fit to the data.
On shorter time-scales, the oxygen isotopic composition of carbonates and cherts is routinely used as an indication of paleotemperatures. Low surface temperatures promote high fractionation of 18O between seawater and sediments (with the sediments being enriched in 18O); high surface temperatures promote more equal partitioning of 18O between seawater and sediments. Accumulation of low-δ18O water in the polar ice caps must also be taken into account. Isotopic analysis of deep-sea carbonate cores has provided detailed information about glacial–interglacial temperature variations over the past few millions of years.
The same technique can be applied on longer time-scales, provided that one allows for the fact that polar ice caps were absent prior to approximately 35 Ma, and for most of the Earth's history. However, the results have long been controversial. If the oxygen isotope data are taken at face value, seawater temperatures appear to have been as high as 70±15°C at 3.3 Ga (Knauth & Lowe 2003), and apparently remained significantly elevated (above 45°C) until as recently as 350 Ma.
The explanation offered most frequently for the high estimated paleotemperatures is that they reflect diagenetic alteration within sediments rather than ocean temperatures (Degens & Epstein 1962; Keith & Weber 1964). In this view, older sedimentary rocks are presumed to be more affected by this process than younger rocks because unaltered ancient sediments are less likely to have been preserved. However, careful analyses by many different workers (Burdett et al. 1990; Veizer et al. 1999; Kah 2000; Shields & Veizer 2002; Knauth & Lowe 2003) show that this explanation is unlikely to be valid. The demonstrably early nature of most marine calcite cements of the Precambrian carbonate record, probably related to a higher CaCO3 saturation state (Grotzinger 1990), implies that diagenetic alteration by later fluids would have been less significant at that time than during the Phanerozoic. The observed oxygen isotope trend in sedimentary rocks is almost certainly related to the properties of ancient seawater. The question is whether it reflects temperature or isotopic composition.
It is theoretically possible that the early Earth could have been hot. Climate calculations by Kasting & Ackerman (1986) showed that a dense greenhouse atmosphere containing 10 bars of CO2 could have produced a mean surface temperature of approximately 80°C at 4.5 Ga, despite a 30% lower solar luminosity at that time. Some models of the early Earth, e.g. Walker (1985), favour initial CO2 levels of this magnitude; other models (Sleep & Zahnle 2001) suggest that atmospheric CO2 levels were much lower. The only data that bear on surface temperatures during the very earliest part of Earth history are the oxygen isotope values of ancient zircons (Valley et al. 2002), and these tell us only that liquid water was present on parts of Earth's surface as early as 4.4 Ga. That is not a strong constraint on surface temperature, given that water can remain as liquid up to its critical temperature, i.e. 374°C for pure water, or approximately 400°C for water with modern ocean salinity. This is because the surface pressure exerted by a fully vaporized ocean (approx. 270 bar) is comparable to the critical pressure of seawater (285–300 bar; Bischoff & Rosenbauer 1984).
Once methanogens evolved, the Earth could have been warmed by CH4, as well as by CO2. Pavlov et al. (2000) calculated the climatic effects of modest amounts of both gases (CO2 partial pressures up to 0.1 bar, CH4 mixing ratios up to 0.01). Their calculations were performed for a time period near 2.8 Ga, when the Sun was approximately 80% as bright as today. Maximum surface temperatures in their calculations were about 320 K or 50°C. Since that time, the Pavlov et al. climate model has been updated. Specifically, the pressure dependence of the CH4 absorption coefficients in the near-infrared has been recalculated to more closely reproduce the data of Strong et al. (1993), as parameterized by Irwin et al. (1996). Details of the new model will be published elsewhere. The revised model allows more reliable simulations of dense CO2/CH4-rich atmospheres to be performed.
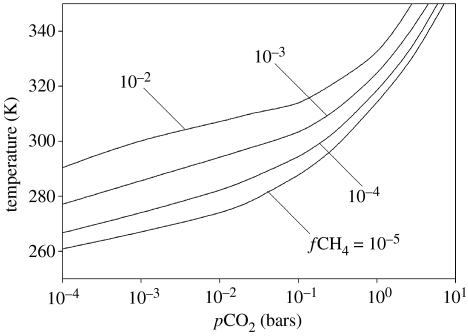
We used our new climate model to estimate what combination of CO2 and/or CH4 would have been necessary to produce a mean global surface temperature of 70°C at 3.3 Ga—the value suggested by the chert oxygen isotope data (Knauth & Lowe 2003). The solar luminosity at this time is estimated to have been approximately 77% of its present value (Gough 1981). The results are shown in figure 3. The calculations indicate that a temperature of 70°C could have been sustained by 2–6 bar of CO2 in combination with CH4 mixing ratios of 0–0.01. Higher CH4 mixing ratios correspond to lower CO2 partial pressures (pCO2). In these calculations, the atmospheric surface pressure has been calculated by adding the CO2 partial pressure to a background N2 partial pressure of 0.8 bar (the modern value). Other model assumptions are as in Pavlov et al. (2000).
Figure 3.
Surface temperature as a function of CO2 partial pressure and CH4 mixing ratio. The assumed solar constant was 77% of the present value, which is appropriate for 3.3 Ga. The climate model is from Pavlov et al. (2000), with modifications described in the text.
The CO2 and CH4 concentrations required to maintain a hot climate on the early Earth are not implausible by themselves. However, they may be inconsistent with other geologic evidence which suggests that the Early Archaean climate was more clement. Condie et al. (2001) computed the chemical index of alteration for Precambrian rocks of various ages and concluded that surface temperatures were moderate from approximately 3.5 until 3.0 Ga. Holland (1984) and Sleep & Hessler (2006) reached similar conclusions about the Early Archaean based on their own paleoweathering analyses. By comparison, the hot, CO2-rich atmospheres mentioned above should have resulted in extremely intense chemical weathering. The present atmosphere contains approximately 300 p.p.m.v., or 3×10−4 bar, of CO2. Dissolution of this CO2 in cloud droplets yields a pH of 5.7 for unpolluted rainwater. By comparison, a 3 bar CO2 atmosphere contains approximately 104 times this much CO2. The pH of rainwater drops one log unit for each factor of 100 increase in pCO2. Hence, if the hot early Earth model was correct, Archaean rainwater would have had a temperature of 70°C and a pH of 3.7. This should have produced incredibly intense weathering virtually everywhere; but that is not what is observed in the paleoweathering record.
As already mentioned, an equally difficult problem for the hot early Earth model is that it is difficult to reconcile with the glacial record discussed in §1 and 4. The Neoproterozoic, Paleoproterozoic and Mid-Archaean glaciations all occurred during the time that sedimentary rocks highly depleted in 18O were laid down. The glaciations themselves might have been relatively short-lived, and so they might not be well represented in the observed oxygen isotope record. However, one would still need to explain why the climate went from hot to cold and back to hot again multiple times in Earth's history. It is easier to believe that the surface temperature was moderate most of the time, and only a small push was needed in order for the climate to become glacial, or for it to recover.
3. Why the oxygen isotopic composition of seawater may have changed with time
If one accepts that the oxygen isotopes in ancient cherts and carbonates have not been uniformly reset, and if one rejects the idea that they record paleotemperatures, then one is forced to conclude that the oxygen isotopic composition of seawater must have changed with time. Various other authors, e.g. Perry et al. (1978), Walker & Lohmann (1989), Shields & Veizer (2002) and Wallmann (2004), have come to this same conclusion, and several of them have proposed mechanisms for causing such a change. To understand these mechanisms, it is necessary to briefly review the processes that control the oxygen isotopic composition of seawater.
It is by now well accepted that the major control on seawater oxygen isotopic composition involves exchange of 18O with basalt during circulation of seawater through the mid-ocean ridge hydrothermal systems (Muehlenbachs & Clayton 1976; Perry et al. 1978; Walker & Lohmann 1989; Muehlenbachs 1998; Wallmann 2001). Low temperature interactions that occur during weathering of continental surfaces exert a lesser effect (Wallmann 2001). Unaltered basalts are enriched in δ18O relative to seawater by approximately 5.7‰. High-temperature (above 350°C) interactions between seawater and rock that occur at depth within axial mid-ocean ridge circulation systems drive the isotopic composition of seawater towards that of the rock, i.e. towards increasing δ18O (Bowers & Taylor 1985). Low-temperature (below 350°C) interactions that occur at shallower depths and in off-axis vent systems, as well as in continental weathering, drive seawater isotopic composition towards low δ18O. If the nature of these interactions has not changed with time, then seawater isotopic composition should have remained constant (Muehlenbachs & Clayton 1976; Gregory & Taylor 1981; Muehlenbachs 1998).
However, there are numerous ways in which the nature of seawater–rock interactions could have changed over time. Perry et al. (1978) suggested that higher heat flow in the Archaean could have resulted in a higher percentage of magma being extruded as pillow basalts and pyroclastic material, leading to average water–rock interaction temperatures below 200°C. Walker & Lohmann (1989) suggested that the Archaean oceans might have been shallower and that the mid-ocean spreading ridges were mostly subaerial, also leading to cooler seawater–rock interactions. Wallmann (2004) suggested that blanketing of mid-ocean ridges by pelagic sediment during the last few hundred million years reduced the rate of cooler, off-axis hydrothermal alteration, thereby altering the balance in favour of high-temperature interactions.
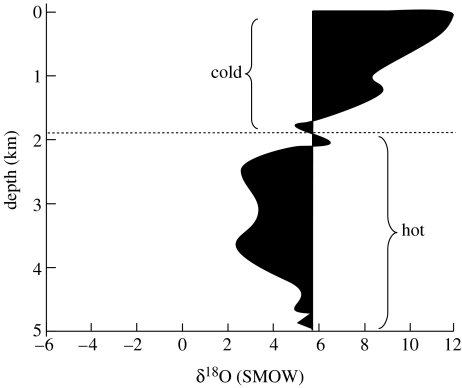
None of these mechanisms has been widely accepted, in part because none could account for the observed pattern of time variation of the oxygen isotope data (figure 2), and in part because it has been argued that they are directly contradicted by isotopic data from ophiolites (Holmden & Muehlenbachs 1993). Ophiolites are portions of ancient seafloor that have been obducted onto stable continental cratons, rather than subducted into the mantle, and thus have been preserved in the geologic record. Both modern ophiolites and at least one ancient one, the 2.0 Ga Purtiniq ophiolite from Canada, exhibit approximately the modern pattern (and magnitude) of δ18O depletion at large depths and enrichment at shallower depths. Figure 4 shows the pattern of oxygen isotope variation in the Cretaceous Samail ophiolite from Oman.
Figure 4.
δ18O values of the Cretaceous Samail ophiolite (shaded region). Unaltered seafloor basalt has a δ18O value of +5.7‰. Modified from Holmden & Muehlenbachs (1993).
We have recently proposed a new mechanism for causing changes in seawater isotopic composition. This idea was outlined in the talk that formed the basis for this paper, and it is presently being written up as a separate contribution (Kasting et al. submitted). It is thus not appropriate to go through all the details here. Briefly, though, our mechanism relies on a change in mean ridgecrest depth with time. Today, most mid-ocean ridges are submerged to a depth of at least 2.5 km (Bischoff 1980; Von Damm 1990). This puts their axial hydrothermal circulation systems within the ‘superconvective’ regime (Dunn & Hardee 1981), in which the efficiency of convective heat transfer is maximized. This allows deep hydrothermal penetration of seawater within the vent systems (Kasting & Holm 1992), which, in turn, allows for a large region in which high temperature water–rock interactions can occur. Such interactions transfer 18O from the basalt to the water, and so modern seawater is relatively rich in 18O.
The mid-ocean ridge environment could have been very different in the distant past because geothermal heat flow was much higher (e.g. Davies 1990). This has led several geophysicists to propose that newly created seafloor must be quite thick (Burke et al. 1976; Sleep & Windley 1982; Moores 1986, 1993, 2002; Sleep in press). In the model of Moores (2002), the seafloor was approximately 25 km thick in the Archaean, as compared to 7–8 km today. Seafloor basalts are more dense than continental crust, but less dense than the underlying mantle. Hence, the ocean floor should have risen isostatically, compared to today, thereby displacing seawater and flooding much of the continents. If the volume of the oceans has remained constant with time, then the mean ocean depth (and the mean ridgecrest depth) must have been less than today. Alternatively, ocean volume may have increased with time as a consequence of this same phenomenon (Kasting & Holm 1992), in which case ancient ridgecrest depths would have been even shallower. Shallow ridgecrest depths should have led to shallow hydrothermal penetration depths, greatly diminishing the region over which high temperature water–rock interactions could occur. The ancient oceans would have been less efficient at extracting 18O from basalts and would thus have had more negative δ18O values.
If the above argument sounds complicated, there may be an easier way to envision how this mechanism works. If Archaean mid-ocean ridgecrests were much shallower than today, seawater would have boiled well before it reached the critical temperature. Steam is much less dense than water, and so it has great difficulty in penetrating deeply into rock. Virtually, all water–rock interactions within the vent systems must therefore have occurred at temperatures below the 350°C ‘isotopic crossover point’, at which modern seawater begins to extract 18O from the rock. The ancient oceans should therefore have been isotopically lighter.
It remains to be shown that this mechanism is consistent with the ophiolite data. Recall from figure 4 that ancient ophiolites are depleted in 18O at depth, and enriched in 18O in shallower regions, just as mid-ocean ridge basalts are today. But this does not by itself imply that the oxygen isotopic composition of seawater has remained constant. That would be true only if the water–rock interaction temperature, and the water–rock ratio, was the same as today. To our knowledge, this has not been conclusively demonstrated. The Purtiniq ophiolite does indeed exhibit deep hydrothermal penetration, but this could have occurred in a cooler, off-axis vent system. Relatively cool, low-δ18O seawater interacting with basalt could have extracted the same amount of 18O from the rock as does hot, high-δ18O seawater today. Indeed, isotopic mass balance of the oceans requires that ancient mid-ocean ridge basalts should have been depleted in 18O at depth, and enriched in 18O in shallower regions, just as today. However, the different pressures and interaction temperatures resulted in steady-state seawater δ18O values that were approximately 10‰ lighter than modern values.
4. The Mid-Archaean glaciations: evidence from mass-independent sulphur isotope fractionation
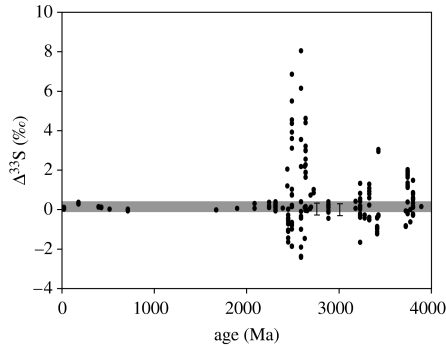
The latest development in the early Earth climate story concerns the (putative) glaciations that occurred during the Mid-Archaean, at approximately 2.9 Ga. Diamictites of possible glacial origin are found in the Pongola and Witwatersrand Supergroups in South Africa (Young et al. 1998; Crowell 1999) and in the Belingue greenstone belt in Zimbabwe (Nisbet et al. 1993). These possible glacial episodes have often been overlooked, as they lack corroborating indicators of glaciation, such as striations and dropstones. However, there is now good reason to reconsider them, as they appear to coincide with an anomaly in the mass-independent sulphur isotope record (Peters et al. 2005; Watanabe et al. 2005; Kasting & Ono 2006; Ono et al. submitted). Rock sequences that have been examined in this time period include the 2.76 Ga Hardey Formation and the 3.0 Ga Mosquito Creek Formation in Australia (Watanabe et al. 2005), the 2.9 Ga Pongola Basin (Ono et al. submitted), and the 2.71 Ga Kameeldoorns Formation of the Kaapvaal Craton in South Africa (Peters et al. 2005). Some, but not all, of these data are shown in figure 5. All these sequences exhibit Δ33S values of (0±0.5)‰. Here, Δ33S represents the deviation in parts per thousand of δ33S from the line defined by the more abundant isotopes, 32S and 34S. Henceforth, we will refer to such deviations as mass-independent fractionation, or MIF for short.
Figure 5.
Compilation of Δ33S versus time from rocks of all ages. Most of the data are from Ono et al. (in press) and references therein. The error bars at 2.8 and 3.0 Ga bracket the data from the Hardey and Mosquito Creek formations in Western Australia (Watanabe et al. 2005). Courtesy of Shuhei Ono.
Several different mechanisms have been suggested to explain the low MIF values observed between 2.8 and 3.2 Ga. To understand the significance of the low MIF values, we must first discuss how MIF signals are thought to be created. High MIF values are thought to be caused by photolysis of atmospheric SO2 at wavelengths less than 220 nm (Farquhar et al. 2000, 2001). In order for this signal to be preserved, sulphur must then leave the atmosphere in more than one chemical form and not be remixed in the ocean; otherwise, the MIF signal would be lost (Pavlov & Kasting 2002). Low MIF values are thought to result either from: (i) shielding of SO2 from short-wavelength solar UV radiation (Farquhar et al. 2000, 2001) or (ii) oxidation of all outgassed SO2 to sulphate prior to any incorporation of sulphur into sediments (Pavlov & Kasting 2002). In today's atmosphere, both these explanations apply; the high O2 and O3 concentrations simultaneously shield out short wavelength solar UV radiation and also ensure that SO2 is efficiently oxidized to sulphate. The rock record shows that MIF values are essentially zero for all sulphur samples younger than 2 Ga, indicating that one, or both, of these conditions has been satisfied since that time. Between 2.0 and 2.3 Ga, and between 2.8 and 3.2 Ga, the MIF values are measurably different from zero, but are within the range (0±0.5)‰. One explanation for these near-zero MIF values is that they reflect a combination of zero-MIF sulphur from the atmosphere with some reworked high-MIF sulphur from previously existing sediments (Farquhar & Wing 2003).
The low MIF values observed in rocks dated between 2.8 and 3.2 Ga have aroused considerable attention. These MIF signals are of the same magnitude as those seen between 2.0 and 2.3 Ga, suggesting that they also represent mixing between a zero-MIF atmospheric signal and reworked, high-MIF sulphur from older sediments. At least four different mechanisms have been invoked to explain the disappearance of sulphur MIF at this time.
Atmospheric O2 increased transiently at approximately 3.2 Ga as a consequence of the origin of oxygenic photosynthesis, then went back down again at 2.7–2.8 Ga for unspecified reasons (Ohmoto et al. 2005; Ono et al. submitted). The increase in O2 shut down sulphur MIF production and triggered glaciation by causing a drastic decrease in atmospheric CH4, as proposed for the later Paleoproterozoic glaciations.
This proposed mechanism suffers from two problems. (a) It is not clear why O2 levels would have gone back down for 300 million years after having increased for roughly this same length of time. Such a decrease would seem to be completely ad hoc. (b) The presence of abundant free O2 within this time-interval is inconsistent with the presence of detrital uraninite within the Witwatersrand sediment, although, to be fair, the question of whether these deposits are actually detrital in origin has been a long-standing topic of debate (Holland 1984, p. 315 ff.).
Atmospheric O2 increased more modestly at 3.2 Ga as a consequence of H2 drawdown by sulphate-reducing bacteria (Kasting & Ono 2006). In this model, the O2 was produced photochemically, rather than by photosynthesis, and its concentration remained low enough, below approximately 10−4 PAL (times the present atmospheric level), that it did not result in oxidation of detrital minerals. This mechanism is potentially viable, but has not been tested by detailed modelling.
The atmosphere became richer in H2 during this time-interval, 2.8–3.2 Ga, perhaps owing to decreasing solar ultraviolet fluxes and consequent slower hydrodynamic escape of hydrogen to space (A. Pavlov 2005, personal communication). The excess H2 converted virtually all the outgassed SO2 to H2S, eliminating the MIF signal through homogenization. This mechanism builds on the recent study by Tian et al. (2005), which indicates that hydrogen escape may have been slower than previously believed. This idea also remains to be tested by detailed modelling.
The MIF signal was reduced owing to the formation of organic haze from CH4 photolysis (Goldman & Kasting 2005; Peters et al. 2005). In this model, the Early Archaean Earth was warmed primarily by the greenhouse effect of CO2 and H2O. Then, at some point prior to 3.2 Ga, methanogens evolved and the atmospheric CH4/CO2 ratio began to increase. Once it reached a value of approximately 0.6 (Pavlov et al. 2001), organic haze began to form. The haze shielded SO2 from short wavelength photolysis, eliminating the sulphur MIF signal. It also blocked out enough visible sunlight to create an anti-greenhouse effect (McKay et al. 1991; Pavlov et al. 2000), which cooled the Earth sufficiently to cause glaciation.
This last hypothesis has now been tested by detailed modelling (Goldman et al. in preparation), and is found to be quantitatively self-consistent. It may also explain the apparently higher MIF signals observed in the Late Archaean, 2.4–2.7 Ga, compared to the Early Archaean, prior to 3.2 Ga (figure 5). In this model, the episode of glacial climates and low MIF values was brought to an end by the origin of oxygenic photosynthesis at approximately 2.8 Ga. Rather than causing an immediate increase in atmospheric O2, the origin of photosynthesis produced localized ‘oxygen oases’ in the surface ocean where primary productivity was high. At the same time, dissolved sulphate levels increased as a consequence of oxidation of sulphide by this dissolved O2. These factors combined to decrease the CH4 flux from shallow marine sediments, which otherwise ought to have been producing copious quantities of methane (Pavlov et al. 2003; Kharecha et al. 2005). The Late Archaean atmosphere, then, was still reduced in nature, with a tenuous organic haze that acted to maximize sulphur MIF production in the atmosphere by partially shielding SO2 from UV photolysis (Goldman et al. in preparation). The haze and the sulphur MIF signal went away for good when atmospheric O2 levels increased around 2.4 Ga.
Of these four mechanisms, we favour the last one because it has the highest degree of internal self-consistency, while at the same time it does the best job of explaining both the sulphur MIF data and the glaciations. All these hypotheses are extremely speculative, however, and they may all be proven wrong if subsequent analyses of 2.8–3.2 billion-year-old rocks turn up significant MIF signals. We hope that publishing them will encourage field geologists to measure sulphur MIF values in other rocks of this same age.
5. Conclusions
The topic of interest for this particular conference was supposed to be ‘Conditions surrounding the emergence of life’. We may not have directly addressed this topic in this paper, as the origin of life probably occurred prior to 3.5 Ga. However, we have attempted to show that by 3.3 Ga, at least, the climate was moderate. This suggests, but does not prove, that the climate was also moderate at the time when life arose. Hence, origin of life theories that require cold temperatures, e.g. Bada et al. (1994), should not be ruled out. High-temperature theories for the origin of life, e.g. Wächtershäuser (1998) remain viable as well, as hot environments can always be found at depth within the mid-ocean ridge systems or elsewhere in the solid Earth.
Conditions following the origin of life are also a topic of interest. If our proposed mechanisms for the origin of the Paleoproterozoic and Mid-Archaean glaciations are correct, then life evidently had a profound effect on the climate of the post-biotic Earth. James Lovelock's proposed ‘Gaia hypothesis’ for modulating climate (Lovelock 1979, 1988) might have been more evident on the Archaean Earth than it is today. We hope that others follow up our work and test this hypothesis in more detail.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
Discussion
C. N. Matthews (Department of Chemistry, University of Illinois at Chicago, USA). How did the recent results of the study of Titan's atmosphere obtained by the Huygens probe of the Cassini/Huygens mission affect your thinking about the atmosphere of the early Earth, since the haze of Titan was shown to be rich in large N-containing compounds formed by photochemical reactions in its N2–CH4 atmosphere?
J. F. Kasting. Our models of the photochemistry of organic haze formation draw heavily on models for haze formation in Titan's atmosphere. There are some key differences, however. Earth's early atmosphere would have been much warmer than Titan's atmosphere, and it would have contained appreciable concentrations of oxygen-containing species, e.g. CO2 and H2O. These species are frozen out of Titan's atmosphere. Hence, the haze in Earth's early atmosphere would likely have consisted of oxygenated hydrocarbons, ketones for example, in addition to hydrocarbons containing C, H and N. So, at this level, the comparison with Titan breaks down.
D. E. Canfield (Danish Center for Earth System Sciences and Institute of Biology, Odense, Denmark). Given the antiquity of methanogenesis based on 16sRNA, I'm surprised you have methanogenesis evolving as late as 3.2 billion years ago.
J. F. Kasting. This is a good point, and one that had bothered me as well. Our model, though, does not really require that methanogenesis evolve precisely at 3.2 Ga. It would also work if methanogenesis evolved prior to this time. The onset of organic haze formation, and eventually glaciation, could then have been triggered by a gradual drawdown of CO2 caused by growth and weathering of continents. In the model, haze formation begins when the atmospheric CH4/CO2 ratio exceeds approximately 0.6.
D. E. Canfield. You have appealed to the rise in oxygen in two instances to drive CH4 oxidation and the glaciation. The first is at 2.7 Ga with the evolution of cyanobacteria and the second at around 2.4 Ga with the rise of O2 to higher levels. What is happening with methane between 2.7 and 2.4 Ga?
J. F. Kasting. Perhaps I went over this point too quickly in my talk. In order to fit the sulphur MIF data (figure 5 in our paper), we suggest that the atmospheric CH4 concentration (or, more precisely, the atmospheric CH4/CO2 ratio) was somewhat lower in the Late Archaean (2.4–2.7 Ga) than in the earlier Archaean. Our photochemical model then predicts a thinner organic haze, and this, in turn, results in a maximum in the sulphur MIF signal. These calculations will be published in a separate paper.
F. Westall (Centre de Biophysique Mole´culaire, CNRS, Orle´ans, France). The Early Archaean rocks document a basically ocean-covered Earth, supporting possibly shallow depths for mid-ocean ranges.
J. F. Kasting. Very interesting! This is consistent with our model for the oxygen isotopic evolution of seawater.
F. Westall. There is also strong evidence for extreme hydrothermal fluid alteration of the upper crust in the Early Archaean. How would this have affected the values of the coastal rocks (silicified volcanic, volcaniclastic, etc.) as opposed to the Early Archaean ocean water signal?
J. F. Kasting. We are just beginning to think about this question. We have included a brief discussion of Archaean greenstone belts in the EPSL paper (Kasting et al. submitted) referenced in the present paper. Aqueous alteration of greenstone basalts may have been important in controlling seawater oxygen isotopic composition during that time.
F. Westall. What is the influence of the CH4 haze on UV radiation penetration to the photic zone?
J. F. Kasting. The question of UV shielding by organic haze was addressed in the Pavlov et al. (2001) paper referenced in the present manuscript. Contrary to previous predictions by Sagan and Chyba, Pavlov et al. found that the ratio of the UV extinction optical depth of the haze to its visible optical depth was only a factor of approximately 3. The visible optical depth is constrained to be significantly less than 1; otherwise, the climate would have become extremely cold, and the methanogens that were producing CH4 would have died off. If this argument is correct, then the amount of UV shielding provided by the haze would have been minimal. The argument does depend strongly on the size distribution of the haze particles, so this question should perhaps be revisited with a more sophisticated model.
References
- Bada J.L, Bigham C, Miller S.L. Impact melting of frozen oceans on the early Earth: implications for the origin of life. Proc. Natl. Acad. Sci. USA. 1994;91:1248–1250. doi: 10.1073/pnas.91.4.1248. doi:10.1073/pnas.91.4.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff J.L. Geothermal system at 21°N, East Pacific Rise: physical limits on geothermal fluid and role of adiabatic expansion. Science. 1980;207:1465–1469. doi: 10.1126/science.207.4438.1465. [DOI] [PubMed] [Google Scholar]
- Bischoff J.L, Rosenbauer R.J. The critical point and two-phase boundary of seawater, 200–500 °C. Earth Planet. Sci. Lett. 1984;68:172–180. doi:10.1016/0012-821X(84)90149-3 [Google Scholar]
- Bowers T.S, Taylor H.P. An integrated chemical and stable isotope model of the origin of mid-ocean ridge hot spring systems. J. Geophys. Res. 1985;90:12 583–12 606. [Google Scholar]
- Burdett J.W, Grotzinger J.P, Arthur M.A. Did major changes in the stable-isotope composition of Proterozoic seawater occur? Geology. 1990;18:227–230. doi:10.1130/0091-7613(1990)018<0227:DMCITS>2.3.CO;2 [Google Scholar]
- Burke K, Dewey J.F, Kidd W.S.F. Dominance of horizontal movements, arc, and microcontinental collisions during the late permobile regime. In: Windley B.F, editor. The early history of the Earth. Wiley; New York, NY: 1976. pp. 113–130. [Google Scholar]
- Catling D.C. Comment on “A hydrogen-rich early Earth atmosphere”. Science. 2006;311:38a–38b. doi: 10.1126/science.1118412. doi:10.1126/science.1117827 [DOI] [PubMed] [Google Scholar]
- Condie K.C, DesMarais D.J, Abbott D. Precambrian superplumes and supercontinents: a record in black shales, carbon isotopes, and paleoclimates? Precambrian Res. 2001;106:239–260. doi:10.1016/S0301-9268(00)00097-8 [Google Scholar]
- Crowell J.C. GSA Memoir. 192 edn. Geological Society of America; Boulder, CO: 1999. Pre-Mesozoic ice ages: their bearing on understanding the climate system. [Google Scholar]
- Davies G.F. In: Heat and mass transport in the early Earth. Newsom H.E, Jones J.H, editors. Oxford University Press; New York, NY: 1990. pp. 175–194. [Google Scholar]
- Degens E.T, Epstein S. Relationship between O18/O16 ratios in coexisting carbonates, cherts and diatomites. Am. Assoc. Petrol. Geol. Bull. 1962;46:534–542. [Google Scholar]
- Dunn J.C, Hardee H.C. Superconvective geothermal zones. J. Volcanol. Geotherm. Res. 1981;11:189–201. doi:10.1016/0377-0273(81)90022-6 [Google Scholar]
- Farquhar J, Wing B.A. Multiple sulfur isotopes and the evolution of the atmosphere. Earth Planet. Sci. Lett. 2003;213:1–13. doi:10.1016/S0012-821X(03)00296-6 [Google Scholar]
- Farquhar J, Bao H, Thiemans M. Atmospheric influence of Earth's earliest sulfur cycle. Science. 2000;289:756–758. doi: 10.1126/science.289.5480.756. doi:10.1126/science.289.5480.756 [DOI] [PubMed] [Google Scholar]
- Farquhar J, Savarino J, Airieau S, Thiemens M.H. Observation of wavelength-sensitive mass-independent sulfur isotope effects during SO2 photolysis: application to the early atmosphere. J. Geophys. Res. 2001;106:1–11. doi:10.1029/2000JE001437 [Google Scholar]
- Goldman, S. & Kasting, J. F. 2005 What does the absence of mass-independent fractionation of sulfur isotopes at 2.8–3.2 Ga say about the early atmosphere? AGU Fall Meeting abstract (Abstract).
- Goldman, S., Kasting, J. F. & Farquhar, J. S. In preparation. Resolving the multiple sulfur isotope record: implications for climate and biological evolution.
- Gough D.O. Solar interior structure and luminosity variations. Solar Phys. 1981;74:21–34. doi:10.1007/BF00151270 [Google Scholar]
- Gregory R.T, Taylor H.P. An oxygen isotope profile in a section of Cretaceous oceanic crust, Samail ophiolite, Oman: evidence for δ18O buffering of the oceans by deep (>5 km) seawater-hydrothermal circulation at mid-ocean ridges. J. Geophys. Res. 1981;86:2737–2755. [Google Scholar]
- Grotzinger J.P. Geochemical model for Proterozoic stromatolite decline. Am. J. Sci. 1990;290:80–103. [Google Scholar]
- Holland H.D. Princeton University Press; Princeton, NJ: 1984. The chemical evolution of the atmosphere and oceans. [Google Scholar]
- Holland H.D. In: Early Proterozoic atmospheric change. Bengtson S, editor. Columbia University Press; New York, NY: 1994. pp. 237–244. [Google Scholar]
- Holland H.D. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B. 2006;361:903–915. doi: 10.1098/rstb.2006.1838. doi:10.1098/rstb.2006.1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmden C, Muehlenbachs K. The 18O/16O ratio of 2-billion-year-old seawater inferred from ancient oceanic crust. Science. 1993;259:1733–1736. doi: 10.1126/science.259.5102.1733. [DOI] [PubMed] [Google Scholar]
- Irwin P.G.J, Calcutt S.B, Taylor F.W, Weir A.L. Calculated k distribution coefficients for hydrogen- and self-broadened methane in the range 2000–9500 cm−1 from exponential sum fitting to band-modelled spectra. J. Geophys. Res. 1996;101:26 137–26 154. doi:10.1029/96JE02707 [Google Scholar]
- Jaffres, J. B. D. 2005 Development of a model to evaluate seawater oxygen isotope composition over the past 3.4 billion years. Bachelor of Science thesis, Dept. of Earth Sciences, James Cook University, North Queensland, Australia.
- Kah L.C. Depositional δ18O signatures in Proterozoic dolostones: constraints on seawater chemistry and early diagenesis. In: Grotzinger J.P, editor. Carbonate sedimentation and diagenesis in the evolving Precambrian World. 2000. SEPM Spec. Pub. 67, 345–360. [Google Scholar]
- Kasting J.F. Earth's early atmosphere. Science. 1993;259:920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- Kasting J.F, Ackerman T.P. Climatic consequences of very high CO2 levels in the earth's early atmosphere. Science. 1986;234:1383–1385. doi: 10.1126/science.11539665. [DOI] [PubMed] [Google Scholar]
- Kasting J.F, Brown L.L. In: Setting the stage: the early atmosphere as a source of biogenic compounds. Brack A, editor. Cambridge University Press; New York, NY: 1998. pp. 35–56. [Google Scholar]
- Kasting J.F, Holm N.G. What determines the volume of the oceans? Earth Planet. Sci. Lett. 1992;109:507–515. doi: 10.1016/0012-821x(92)90110-h. doi:10.1016/0012-821X(92)90110-H [DOI] [PubMed] [Google Scholar]
- Kasting J.F, Ono S. Paleoclimates: the first two billion years. Phil. Trans. R. Soc. B. 2006;361:917–929. doi: 10.1098/rstb.2006.1839. doi:10.1098.rstb.2006.1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasting, J. F., Howard, M.T., Wallmann, K., Veizer, J., Shields, G. & Jaffrés, J. Submitted. Paleoclimates, ocean depth, and the oxygen isotopic composition of seawater. Earth Planet. Sci. Lett
- Keith M.L, Weber J.N. Carbon and oxygen isotopic composition of selected limestones and fossils. Geochim. Cosmochim. Acta. 1964;28:1787–1816. doi:10.1016/0016-7037(64)90022-5 [Google Scholar]
- Kharecha P, Kasting J.F, Siefert J.L. A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology. 2005;3:53–76. doi:10.1111/j.1472-4669.2005.00049.x [Google Scholar]
- Knauth L.P. Temperature and salinity history of the Precambrian ocean: implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;219:53–69. doi:10.1016/j.palaeo.2004.10.014 [Google Scholar]
- Knauth L.P, Epstein S. Hydrogen and oxygen isotope ratios in nodular and bedded cherts. Geochim. Cosmochim. Acta. 1976;40:1095–1108. doi:10.1016/0016-7037(76)90051-X [Google Scholar]
- Knauth P, Lowe D.R. High Archean climatic temperature inferred from oxygen isotope geochemistry of cherts in the 3.5 Ga Swaziland Supergroup, South Africa. GSA Bull. 2003;115:566–580. [Google Scholar]
- Lovelock J.E. Oxford University Press; Oxford, UK: 1979. Gaia: a new look at life on Earth. [Google Scholar]
- Lovelock J. W.W. Norton; New York, NY: 1988. The Ages of Gaia. [Google Scholar]
- McKay C.P, Pollack J.B, Courtin R. The greenhouse and antigreenhouse effects on Titan. Science. 1991;253:1118–1121. doi: 10.1126/science.11538492. [DOI] [PubMed] [Google Scholar]
- Moores E.M. The Proterozoic ophiolite problem, continental emergence, and the Venus connection. Science. 1986;234:65–68. doi: 10.1126/science.234.4772.65. [DOI] [PubMed] [Google Scholar]
- Moores E.M. Neoproterozoic oceanic crustal thinning, emergence of continents, and origin of the Phanerozoic ecosystem; a model. Geology. 1993;21:5–8. doi:10.1130/0091-7613(1993)021<0005:NOCTEO>2.3.CO;2 [Google Scholar]
- Moores E.M. Pre-1 Ga (pre-Rodinian) ophiolites: their tectonic and environmental implications. GSA Bull. 2002;114:80–95. [Google Scholar]
- Muehlenbachs K. The oxygen isotopic composition of the oceans, sediments, and the seafloor. Chemical Geol. 1998;145:263–273. doi:10.1016/S0009-2541(97)00147-2 [Google Scholar]
- Muehlenbachs K, Clayton R.N. Oxygen isotope composition of the oceanic crust and its bearing on seawater. J. Geophys. Res. 1976;81:4365–4369. [Google Scholar]
- Nisbet E.G, Bickle M.J, Martin A, Orpen J.L. Sedimentology of the Brooklands Formation, Zimbabwe: development of an Archaean greenstone belt in a rifted graben. In: Bickle M.J, Nisbet E.G, editors. The Geology of the Belingwe greenstone belt, Zimbabwe. A.A. Balkema; Rotterdam, The Netherlands: 1993. pp. 87–120. [Google Scholar]
- Ohmoto H, Watanabe Y, Ikemi H. The absence of mass independent fractionation of sulfur isotopes in Archean sedimentary rocks: an insignificant phenomenon? Geochim. Cosmochim. Acta. 2005;69(Suppl. 5):A450. [Google Scholar]
- Ono, S., Beukes, N., Rumble, D. & Fogel, M. Submitted. Early evolution of atmospheric oxygen from multiple-sulfur and carbon isotope records of the 2.9 Ga Pongola Supergroup, Southern Africa. S Afr J Geol
- Pavlov A.A, Kasting J.F. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2:27–41. doi: 10.1089/153110702753621321. doi:10.1089/153110702753621321 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A, Kasting J.F, Brown L.L, Rages K.A, Freedman R. Greenhouse warming by CH4 in the atmosphere of early Earth. J. Geophys. Res. 2000;105:11 981–11 990. doi: 10.1029/1999je001134. doi:10.1029/1999JE001134 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A, Kasting J.F, Brown L.L. UV-shielding of NH3 and O2 by organic hazes in the Archean atmosphere. J. Geophys. Res. 2001;106:23 267–23 287. doi:10.1029/2000JE001448 [Google Scholar]
- Pavlov A.A, Hurtgen M.T, Kasting J.F, Arthur M.A. Methane-rich Proterozoic atmosphere? Geology. 2003;31:87–90. doi:10.1130/0091-7613(2003)031<0087:MRPA>2.0.CO;2 [Google Scholar]
- Perry E.C. The oxygen isotope chemistry of ancient cherts. Earth Planet. Sci. Lett. 1967;3:62–66. doi:10.1016/0012-821X(67)90012-X [Google Scholar]
- Perry E.C, Ahmad S.N, Swulius T.M. The oxygen isotope composition of 3800 m.y. old metamorphosed chert and iron formation from Isukasia, West Greenland. J. Geol. 1978;86:223–239. [Google Scholar]
- Peters, M., Farquhar, J. & Strauss, H. 2005 Mass Independent fractionation of sulphur isotopes in Precambrian sedimentary rocks: Indicator for changes in atmospheric composition and the operation of the global sulphur cycle. AGU Fall Meeting abstracts (Abstract).
- Pollard D, Kasting F. Snowball Earth: a thin-ice model with flowing sea glaciers. J. Geophys. Res. 2005;110:C07010. doi:10.1029/2004JC002525 [Google Scholar]
- Shields G, Veizer J. Precambrian marine carbon isotope database: version 1.1. Geol. Geochem. Geophys. 2002;3:6. [Google Scholar]
- Sleep, N.H. In press. Evolution of the Earth: plate tectonics through time (MS 143). In Treatise on geophysics, vol. 9 (ed. G. Schubert). Elsevier.
- Sleep N.H, Hessler A.M. Weathering of quartz as an Archean climate indicator. Earth Planet. Sci. Lett. 2006;241:594–602. doi:10.1016/j.epsl.2005.11.020 [Google Scholar]
- Sleep N.H, Windley B.F. Archean plate tectonics: constraints and inferences. J. Geol. 1982;90:363–380. [Google Scholar]
- Sleep N.H, Zahnle K. Carbon dioxide cycling and implications for climate on ancient Earth. J. Geophys. Res. 2001;106:1373–1399. doi:10.1029/2000JE001247 [Google Scholar]
- Strong K, Taylor F.W, Calcutt S.B, Remedios J.J, Ballard J. Spectral parameters of self- and hydrogen-broadened methane from to 2000 9500 cm−1 for remote sounding of the atmosphere of Jupiter. J. Quant. Spectrosc. Rad. Trans. 1993;50:363–429. doi:10.1016/0022-4073(93)90072-P [Google Scholar]
- Tian F, Toon O.B, Pavlov A.A, De Sterck H. A hydrogen rich early Earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. doi:10.1126/science.1106983 [DOI] [PubMed] [Google Scholar]
- Valley J.W, Peck W.H, King E.M, Wilde S.A. A cool early Earth. Geology. 2002;30:351–354. doi:10.1130/0091-7613(2002)030<0351:ACEE>2.0.CO;2 [Google Scholar]
- Veizer J, et al. 87Sr/86Sr,δ13C, and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999;161:59–88. doi:10.1016/S0009-2541(99)00081-9 [Google Scholar]
- Von Damm K.L. Seafloor hydrothermal activity: black smoker chemistry and chimneys. Annu. Rev. Earth Planet. Sci. 1990;18:173–204. doi:10.1146/annurev.ea.18.050190.001133 [Google Scholar]
- Wächtershäuser G. Origin of life in an iron–sulfur world. In: Brack A, editor. The molecular origins of life: assembling the pieces of the puzzle. Cambridge University Press; Cambridge, UK: 1998. pp. 206–218. [Google Scholar]
- Walker J.C.G. Macmillan; New York, NY: 1977. Evolution of the atmosphere. [Google Scholar]
- Walker J.C.G. Carbon dioxide on the early Earth. Orig. Life. 1985;16:117–127. doi: 10.1007/BF01809466. doi:10.1007/BF01809466 [DOI] [PubMed] [Google Scholar]
- Walker J.C.G, Lohmann K.C. Why the oxygen isotopic composition of seawater changes with time. Geophys. Res. Lett. 1989;16:323–326. [Google Scholar]
- Wallmann K. The geological water cycle and the evolution of marine δ18O values. Geochim. Cosmochim. Acta. 2001;65:2469–2485. doi:10.1016/S0016-7037(01)00603-2 [Google Scholar]
- Wallmann K. Impact of atmospheric CO2 and galactic cosmic radiation on Phanerozoic climate change and the marine delta 18-O record. Geol. Geochem. Geophys. 2004;5 [Google Scholar]
- Watanabe, Y., Ikemi, H. & Ohmoto, H. 2005 Implications of the absence of mass independent sulfur isotope fractionation in 2.76-Ga lacustrine and 3.0-Ga marine sediments. Abstracts of early Earth Symposium, Tokyo, May.
- Young G.M, von Brunn V, Gold D.J.C, Minter W.E.L. Earth's oldest reported glaciation; physical and chemical evidence from the Archean Mozaan Group (∼2.9 Ga) of South Africa. J. Geol. 1998;106:523–538. [Google Scholar]