Abstract
Large deposits of montmorillonite are present on the Earth today and it is believed to have been present at the time of the origin of life and has recently been detected on Mars. It is formed by aqueous weathering of volcanic ash. It catalyses the formation of oligomers of RNA that contain monomer units from 2 to 30–50. Oligomers of this length are formed because this catalyst controls the structure of the oligomers formed and does not generate all possible isomers. Evidence of sequence-, regio- and homochiral selectivity in these oligomers has been obtained. Postulates on the role of selective versus specific catalysts on the origins of life are discussed. An introduction to the origin of life is given with an emphasis on reaction conditions based on the recent data obtained from zircons 4.0–4.5 Ga.
Keywords: prebiotic, montmorillonite catalysis, ribonucleic acid, origin of life, sequence
1. Introduction
Indeed, if life originated on Earth, then little is known about the chemical processes that initiated the first life. It is possible that life originated elsewhere in the Universe and was delivered to the Earth, where it has prospered for the past 3.9 Ga. Since we do not know anything about other places where life may have originated and we know more about the reaction conditions on the primitive Earth, it will be assumed that life originated here.
Astronomers and geologists tell us that the first organic compounds that initiated the chemical processes leading to the origin of life resulted from the action of cosmic rays on the interstellar carbon formed by nuclear fusion of hydrogen and helium in stars. This process resulted in the formation of hydrocarbons, nitrogen- and oxygen-containing molecules. At the time of this writing, 126 organic molecules, radicals and ions have been identified in the interstellar dust clouds (www.cv.nrao.edu/~awootten/allmols.html). These clouds have lifetimes of about 108 years.
Shock waves from a supernova may initiate the formation of higher density loci in a dust cloud that served as the point where the dust cloud collapsed to form a Solar System. While the bulk of the mass in the dust cloud forms a protosun, there is sufficient material left over to form planets, asteroids and comets. It is believed that the latter are formed by the gravitational attraction of the planetesimals (small bodies) in the torus of dust around the protosun. The nuclear fusion processes of the Sun were initiated when the protosun became sufficiently massive and dense that nuclear fusion processes started.
Some of the organics in the original dust cloud underwent chemical changes in the course of the energetic processes that occurred during the formation of the Sun and planets. The structures of the organics closest to the Sun were changed by the strong radiation emanating from it, while the organics beyond Jupiter, which received much less radiation, changed little.
Comets, most of which formed beyond Jupiter, appear to contain more compounds that are comparable to those in interstellar dust clouds than the organics in meteorites (table 1). Meteorites, which are pieces of asteroids, are formed by the collisions of asteroids. The asteroids, which are mainly present in orbits between Mars and Jupiter, contain organics with a wide array of structures (table 2).
Table 1.
Some organics observed in the comas of comets (Ferris 2005).
| name | formula |
|---|---|
| methanol | CH3OH |
| formamide | HCONH2 |
| methane | CH4 |
| ethylene | C2H4 |
| methylacetylene | CH3C2H |
| formic acid | HCOOH |
| acetonitrile | CH3CN |
| methyl formate | HCOOCH3 |
| acetylene | C2H2 |
| ethane | C2H6 |
| hydrogen cyanide | HCN |
Table 2.
Soluble organic compounds detected in the Murchison Meteorite (++++>1000 p.p.m., +++>100 p.p.m., ++>10 p.p.m., +>1 ppm. Adapted from (Pizzarello 2004).).
| amino acids | ++ |
| carboxylic acids | +++ |
| dicarboxylic acids | ++ |
| hydroxy acids | ++ |
| sulfonic acids | ++++ |
| basic N-heterocycles | + |
| purines & pyrimidines | + |
| pyridine carboxylic acids | + |
| amides | ++ |
| amines | ++ |
| alcohols | ++ |
| aldehydes & ketones | ++ |
| aliphatic hydrocarbons | ++ |
| aromatic hydrocarbons | ++ |
| sugar alcohols & acids | + |
Both meteorites and comets are believed to have been sources of the organic compounds that initiated the first life on Earth.
In the past two years, important evidence about climatic conditions on the primitive Earth has been obtained. Plate tectonics erased the record of the rock formations 3.8 Ga ago, so that few clues remained, concerning the original conditions on the primitive Earth in the 4.5–4.0 Ga time period. Ancient zircons, refractory zirconium-containing pellets that were formed 4.0–4.5 Ga ago, do provide a source of information about the primitive Earth at that time (Amelin 2005). These pellets do not melt when subducted during plate tectonics; hence, the minerals trapped in the zircons were not changed. Consequently, these ancient zircons contain information about the reaction conditions on the primitive Earth. Comparison of the properties of the ancient zircons and those prepared in the laboratory made it possible to determine the conditions on the primitive Earth at the time, when the ancient zircons were formed. From these comparisons, it was possible to conclude that there was liquid water on the Earth 4.3 Ga ago (Watson & Harrison 2005) and that continents had already formed 4.3–4.4 Ga ago (Mojzsis et al. 2001; Wilde et al. 2001; Harrison et al. 2005). These conclusions are drastically different from the previous scenarios, where it was proposed that at that time there were magma oceans resulting from the impacts of large bodies on the Earth that generated the heat that melted and volatilized the Earth's crust (Righter & Drake 1999).
There is strong evidence for a late heavy bombardment on the Moon and presumably on the Earth, Mars and Venus 3.9 Ga ago (Gomes et al. 2005). One explanation for this bombardment is that Jupiter moved closer to the Sun at that time and the change in the gravitational field resulted in the ejection of many asteroids from their orbits and some of them collided with the inner planets and moons. While the conditions for the origin of life may have been present 4.3–4.5 Ga ago, this life may have been exterminated by the massive impacts 3.9 Ga ago. Alternatively, while much of the life was extinguished at that time, some survived in protected niches and it recolonized the Earth once the intensity of the impacts decreased. In another scenario, the impacts launched micro-organisms into the Earth's orbit and these microbes returned to the Earth several thousand years later, when it was more clement (Wells et al. 2003; Gladman et al. 2005).
2. Formation of more complex organics on the primitive Earth
The Earth appears to have had a habitable environment 4.3–4.5 Ga ago, which may have led to the origin of life. Compounds other than those brought to the Earth by meteorites and comets could have formed on the Earth. Stanley Miller, when a graduate student in the laboratory of Harold Urey, performed the first experiment designed to simulate the reaction conditions on the primitive Earth. He subjected a mixture of gases, which Urey felt modelled the atmospheric gases on the primitive Earth, to a spark discharge for a week and detected amino acids in the reaction products (Miller 1955). The atmosphere chosen, a mixture of methane, ammonia, hydrogen and water, was challenged by geologists since they believe that the atmosphere of the Early Earth was similar to that of the gases emanated from contemporary volcanoes. Volcanoes today give off varying amounts of gases, where the main emissions, in an approximate decreasing order of amounts, are H2O, CO2, SO2, CO, H2 and HCl (Symonds et al. 1994). These compounds are not likely to have generated the reduced compounds (hydrogen-containing) found in life, such as amino acids and nucleotides, and they do not generate reduced compounds, when a spark discharge is passed through the mixture. Since the oxidation level of the crust and mantle, which has been an essentially constant for the past 3.8 Ga, determines the oxidation state of the gases emitted by volcanoes, it is unlikely that the reduced gases used in the initial Miller experiment emanated from the volcanoes (Delano 2001).
Recent calculations of the gases, believed to be present in the atmosphere of the primitive Earth, suggest that the atmosphere had up to 30% H2 (Tian et al. 2005). This hypothesis was the result of the use of a lower value for the diffusive loss of hydrogen from the top of the Earth's atmosphere. The question remains whether the presence of hydrogen would result in the formation of reduced biomolecules, when a spark discharge is passed through a mixture of the oxidized gases emanating from a volcano that also contained a high level of hydrogen. Stanley Miller actually carried out this study in 1983 (Schlesinger & Miller 1983). Among the experiments preformed in this study was one, where a spark discharge was passed through a mixture of N2 (100 torr), H2 (300 torr), CO2 (100 torr) and 100 ml water, a model of an oxidizing atmosphere. Amino acid analysis showed the formation of a good yield of glycine, but much lower yields of the other amino acids than that observed in the Miller–Urey experiment. Thus, it appears that the yields of organics will be significantly lower, when hydrogen is the only reduced gas present in the electric discharge. If methane is present in the gas mixture, the yields of organics may be higher (Schlesinger & Miller 1983).
Hydrothermal systems were also a potential source of reduced compounds on the primitive Earth (Ferris 1992). These systems occur at spreading centres beneath the ocean, where magma is proximate to the ocean floor. The heat from the magma drives the circulation of ocean water down through the crust to the vicinity of the magma, where the oxidized compounds in the seawater, e.g. sulphate and oxidized metal ions, are reduced to sulphide and lower valent metal ions, respectively. These reduced substances are soluble at high temperatures (300–400°C) and pressures (250 bar) in the vent, but precipitate as metal sulphides, when they are ejected from the vent into the 2°C water present at the bottom of the ocean.
Laboratory studies carried out at high temperatures and pressures of a hydrothermal system yield reduced organic compounds. Examples include the reduction of molecular nitrogen and nitrogen oxides to ammonia with reduced iron oxides (Brandes et al. 1998). When iron sulphides were the reducing agents, 80% of nitrogen oxides ( and ) were reduced to ammonia in 15 min at 500°C. Another simulated hydrothermal process is the conversion of carbon dioxide to formate (HCOO−) with hydrogen generated by heating the mineral olivine to 300°C and 350 bar pressure (McCollom & Seewald 2001). Acetate and pyruvate have also been formed at high temperatures and pressures from similar starting materials (Huber & Wächtershäuser 1997; Cody et al. 2000).
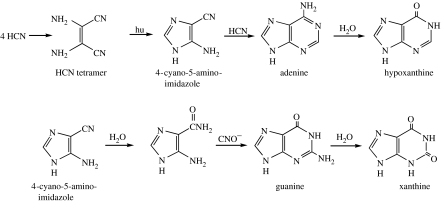
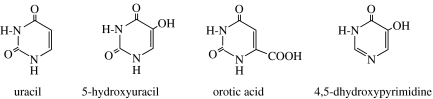
There have been numerous examples of the conversion of simpler organic compounds, delivered to or formed in the atmosphere and hydrothermal systems of the primitive Earth, to more complicated structures. For example, heating concentrated solutions of HCN yields adenine (Oro 1960). Adenine and other purines are also formed stepwise from 0.1 M aqueous solutions of HCN to give a tetramer (diaminomaleonitrile), which in turn yields adenine in subsequent photochemical and addition reactions (figure 1; Sanchez et al. 1967). Pyrimidines and amino acids have also been formed starting from 0.1 M HCN (figure 2; Ferris et al. 1978; Ferris & Hagan 1984; Voet & Schwartz 19823). An ammonium cyanide solution kept frozen at −78°C for 27 years yielded 2,6-diaminopurine, 5-aminouracil and 5-aminoorotic acid in addition to the compounds in figures 1 and 2 (Miyakawa et al. 2002).
Figure 1.
Synthetic pathways for the formation of purines from dilute HCN.
Figure 2.
Pyrimidines formed from 0.1 M HCN.
These studies suggest possible pathways for the formation of some purine and pyrimidine bases that may have reacted with ribose to form the nucleosides of RNA. A major problem to be solved is the polymerization of small molecules into the biopolymers essential for the first life. This will be discussed in the remainder of this paper.
3. Ribonucleic acid world
The formation of RNA is one of the first steps proposed for the origin of life with the assumption that the present DNA–protein world evolved from it (Gestland et al. 1999). The discovery that RNA catalysed reactions, in addition to storing genetic information, suggested that RNA was the most important biopolymer in the first life. RNA is also attractive because it would require the prebiotic synthesis of only one biopolymer (RNA) instead of two (DNA and protein). In addition, it has been determined that the RNA catalyses the formation of peptide bond in the ribosome, a key step in protein synthesis (Ban et al. 2000). This finding is consistent with the presence of an RNA world with the ribosome first being composed of only RNA, which was later decorated with proteins to make it more selective and effective in the formation of the peptide bond.
The direct formation of RNA by prebiotic processes is controversial. As will be outlined subsequently, there are no plausible prebiotic syntheses of RNA monomers so that many scientists in this field feel that the spontaneous formation of such complicated structures is very unlikely to have occurred on the primitive Earth.
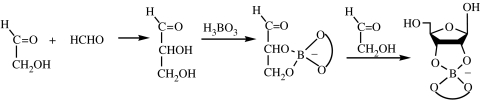
Earlier, it was also felt that the prebiotic synthesis of ribose, the basic structural element in RNA monomers, was unlikely, but a variety of prebiotic syntheses of ribose and ribose derivatives has since been reported using simple starting materials suggesting that prebiotic ribose synthesis may have occurred on the primitive Earth (Müller et al. 1990; Zubay 1998; Ricardo et al. 2004; Springsteen & Joyce 2004). For example, the reaction of glycolaldehyde with formaldehyde in the presence of borate generates borate complexes that stabilize the intermediates and the final products so that a complex mixture of products is not formed (figure 3). The four diastereomers of pentoses are formed as borate complexes.
Figure 3.
Ribose formation in the presence of borate.
Borate forms complexes where two organic ligands bind to one borate and the second ligand is shown by the curved line. There are four pentoses and all are formed in approximately equal amounts.
So far, there has been no reported efficient prebiotic conversion of ribose to the corresponding nucleosides by the reaction with purine or pyrimidine bases (Fuller et al. 1972). There are some promising leads for the phosphorylation of nucleosides (Osterberg & Orgel 1972; Osterberg et al. 1973), but the prebiotic conversion of the 5′-phosphorylated nucleotide to an activated nucleotide under prebiotic conditions has not been accomplished at the time of writing.
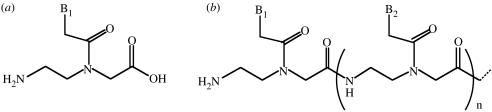
Those who express concerns about the prebiotic synthesis of activated nucleotides have proposed that there must have been a simpler preRNA, which in turn evolved into the RNA world. A variety of preRNA structures have been proposed including peptide nucleic acid (PNA) (Nielsen et al. 1991; figure 4), but to date no convincing prebiotic syntheses of the monomers or polymers of PNA have been described (Miller 1997).
Figure 4.
PNA, (a) the monomer and (b) the polymer.
4. Montmorillonite clay: a potential prebiotic catalyst
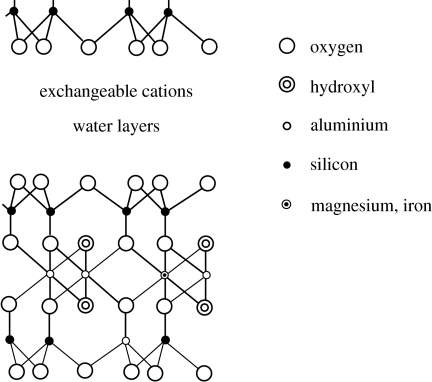
Clay minerals are found in abundance on the Earth today and one of them, montmorillonite, has been found to catalyse a number of organic reactions (Nikalje et al. 2000). This suggests that montmorillonite may also have catalysed reactions on the primitive Earth. Montmorillonite is formed by the weathering of volcanic ash and it is likely to have been present on the Early Earth, because it is believed that there were high levels of volcanic activity. Today, there are large deposits of montmorillonite on the Earth and it has recently been detected on Mars (Poulet et al. 2005). It is mined for use as a filter, binder, mild abrasive, absorbent (cat litter) as well as a catalyst. It is formed in platelets with three layers: the top and bottom layers, which are polymers of tetrahedral silicates, and these two layers are linked by octahedral aluminates (figure 5). Usually there are other elements substituted for silicon and aluminium depending on their abundance when the montmorillonite was formed. The usual isomorphous replacements elements are ferric and ferrous iron, and magnesium substituted for the octahedral aluminium and aluminium substituted in the tetrahedral silicate layer for silicon. The platelets have negative charges; hence, they have associated exchangeable cations, which are mainly Na+ and Ca+2, in the montmorillonite found on the Earth. These cations link the platelets together in stacked aggregates that are roughly similar to a deck of cards. Positively charged organics bind to the negatively charged platelets in the interlayers, while neutral and negatively charged organics may also be absorbed. The latter may bind by van der Waals interactions with the silicate surface and with each other.
Figure 5.
A unit of the montmorillonite structure. A portion of another montmorillonite sheet is at the top of the diagram.
Our studies, and those of others, show that nucleotides bind by van der Waals forces between the silicate layer of the montmorillonite and the purine and pyrimidine bases of the nucleotides, when the pH is near 7 (Lailach et al. 1968; Kawamura & Ferris 1999; Ertem & Ferris 2000). The strength of the binding of purine nucleotides is greater than that of the corresponding pyrimidine derivatives, an effect owing to the larger size of the planar purine ring.
When montmorillonite is used in the laboratory, it is usually converted to the homoionic form to be sure that the catalytic effect is owing to the clay and not to trace elements bound to it. This is done by the addition of a high concentration of cation salt like NaCl to replace the interlayer cations of the montmorillonite with Na+ (Lailach et al. 1968). Alternatively, the montmorillonite is converted to its acid form by treatment with cold, concentrated acid, e.g. HCl, which exchanges the metal cations for hydrogen ions and then exchanges the hydrogen ion with a metal cation by back titration to neutral pH with Na+ (Banin et al. 1985). This montmorillonite serves as an efficient catalyst for the formation of RNA oligomers, if the exchangeable cation is an alkali or an alkaline earth metal ion. Exceptions include ammonium ion, which also gives a catalytic montmorillonite while Mg2+ does not.
5. Montmorillonite catalysis of Ribonucleic acid oligomer formation
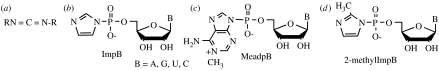
The ability of montmorillonite to catalyse a reaction was first observed in the studies on the cyclization of 3′-nucleotides to 2′,3′-cyclic nucleotides (Ferris et al. 1986). The yield of the cyclic product was twice as high, when the reaction was performed in the presence of Zn2+-montmorillonite. This finding prompted our investigation of the formation of a phosphodiester bond between 5′-nucleotides using a carbodiimide (figure 6a; Ferris et al. 1989) and then with the phosphorimidazolides of nucleosides using Na+-montmorillonite as the catalyst. (figure 6b; Kebbekus 1988; Ferris & Ertem 1993b). The latter reaction resulted in the formation of 6–14 mers, in an aqueous, pH 8 solution, with the oligomer length dependent on the base present in the nucleotide (Ferris & Ertem 1993a; Ding et al. 1996; Kawamura & Ferris 1999). Kinetic studies of the reaction of ImpA revealed that the montmorillonite enhanced the rate constant for oligomer formation by about 100–1000 times over that for the hydrolysis of the imidazole-activating group (Kawamura & Ferris 1994).
Figure 6.
(a) A carbodiimide condensing agent; (b) RNA monomer activated with imidazole; (c) a monomer activated with 1-methyladenine; (d) and a monomer activated with 2-methyl imidazole.
Changing the reaction conditions and the phosphate-activating group led to the formation of significantly longer oligomers. A ‘feeding reaction’, where the activated monomer is added daily to a 10 mer nucleic acid primer bound to montmorillonite, resulted in the addition of 30–40 mers to the primer in 12–14 days (figure 7; Ferris et al. 1996; Ferris 2002). Changing the phosphate-activating group from imidazole to 1-methyladenine (figure 6) resulted in the formation of 40–50 mers of A or U in 1–3 days without the need of a primer (Huang & Ferris 2003). These advances were important because they generated longer oligomers with the capability of storing more genetic information as well as having enhanced catalytic capability.
Figure 7.
Elongation of a primer by the daily addition of the activated monomer ImpA for 12–14 days.
6. Why the origin of life required catalysts
(a) Formation of biopolymers
The formation of biopolymers from monomers in aqueous solution required the presence of catalysts. One function of the catalyst is to selectively adsorb the reacting species from the aqueous solution. This is required to generate a localized higher concentration of the key reactants on the mineral surface. Organics bound to montmorillonite tend to be more stable than those in the aqueous phase (Williams et al. 2005). In addition, the potential presence of catalytic sites in the interlayers enhances the rate of polymerization. Catalysis is necessary to enhance the rate of oligomerization, since it is unlikely that an activated monomer will ever undergo spontaneous polymerization in aqueous solution, because these compounds hydrolyse rapidly in the presence of water. As noted above, the rate constant for the montmorillonite-catalysed oligomerization of 5′-phosphorimidazolide of adenosine is 100–1000 times greater than the rate of its hydrolysis. Hydrolysis is the main reaction product in the absence of montmorillonite along with a few percentage of dimers, where the rate constant of oligomer formation is only 10 times that of hydrolysis (Kawamura & Ferris 1994; Kanavarioti 1997).
(b) Selectivity in the formation of oligomers
(i) Sequence selectivity
One of the early concerns in the studies of the prebiotic formation of biopolymers was that a random mixture of products would be formed (Kaplan 1971). This would lead to a mixture of all possible isomers that would generate only a trace amount of the desired catalyst for the replication and/or ligation of the oligomers. For example, the probability of the random formation of specific sequences of a protein containing 100 amino acids is 1 : 10130 (Kaplan 1971). The extent of this problem for RNA oligomer synthesis was quantified when it was calculated that a library of RNAs containing one copy of every possible 50 mer would consist of 1030 RNAs weighing 1010 g (Joyce & Orgel 1999). The formation of two identical copies of the same 50 mer would require the synthesis of 1054 RNAs weighing 1034 g. Two RNAs may be required to catalyse the synthesis of the other.
Catalysis provides a solution to this problem of RNA oligomers. Most catalysts lower the activation energy for a limited number of reaction pathways and this results in the formation of a limited number of structures rather than all possible isomers. This was found to be the case in the investigation of the montmorillonite-catalysed formation of 2–5 mers produced in the reaction between equal amounts of ImpA and ImpC (table 3; Miyakawa & Ferris 2003). Comparison of the selectivity in the oligomers formed clearly demonstrated that the number of sequences formed was much lower than predicted for a random process.
Table 3.
Montmorillonite-catalysed synthesis versus theoretical random synthesis in the ImpA–ImpC reaction (Miyakawa & Ferris 2003).
| mers | catalysed synthesis | random synthesis | ||
|---|---|---|---|---|
| isomers observed | proportions isomers of mer (%) | isomers predicted | yield of each isomer (%) | |
| 2 | 8 | 0.6–39 | 8 | 13 |
| 3 | 10 | 1.1–48 | 32 | 3.1 |
| 4 | 5 | 5.5–34 | 128 | 0.78 |
| 5 | 4 | 4.3–13 | 512 | 0.20 |
(ii) Phosphodiester bond formation
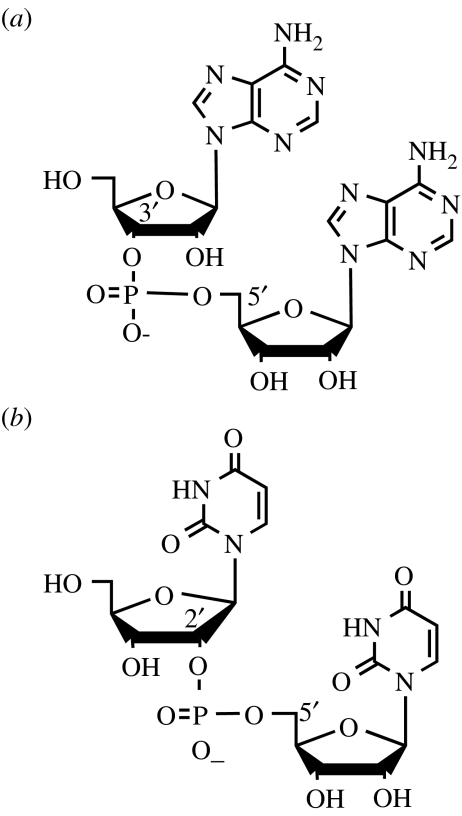
The oligomers produced by montmorillonite catalysis have 2′,5′- and 3′,5′- phosphodiester bonds (figure 8). When the reactions of either purine- or pyrimidine-activated nucleotides occur in the absence of montmorillonite in an aqueous solution, the ratio of 2′,5′- to 3′,5′-linked dimers is about 3 : 1 (Kanavarioti 1997; Kawamura & Ferris 1999). When the reaction is performed in the presence of montmorillonite, the ratios of ImpA to ImpI are 0.6 : 1 and 0.2 : 1 respectively, while those of ImpU to ImpC are 4 and 3, respectively (Ferris & Ertem 1993a; Ding et al. 1996; Ertem & Ferris 1997; Kawamura & Ferris 1999). Clearly, the montmorillonite changes the reaction pathway for the purine nucleotides, while the lowest energy reaction pathway for the pyrimidine nucleotides remains the same as it was in the absence of montmorillonite.
Figure 8.
Phosphodiester bonds, (a) 3′,5′-bond and (b) 2′,5′-bond.
(iii) Homochiral selectivity
Since the RNA monomers formed on the primitive Earth were likely to have been present as racemic mixtures, we initiated studies on the reaction products formed from d- and l-ImpA. It was observed that the linear dimers had a small excess (60 : 40) of homochiral dimers (dd & ll) over the corresponding heterochiral dimer (Joshi et al. 2000). Research is in progress where cyclic dimers, linear trimers and linear tetramers formed are being investigated.
7. Montmorillonite catalysis of vesicle formation
A surprising observation was that montmorillonite also catalyses the formation of vesicles (a spherical body encapsulating water inside a wall composed of linear, 10-carbon carboxylic acids; Hanczyc et al. 2003). In addition, some montmorillonite were incorporated into some of the vesicles. This suggests the possibility that the small activated monomers could diffuse through the wall of the vesicle and react on montmorillonite to form larger RNA oligomers that cannot pass out through the wall of the vesicle. This experiment has not been accomplished at this time, because the conditions required for RNA oligomer formation result in the destruction of the vesicle (Monnard et al. 2002).
8. Metal ions as prebiotic catalysts
(a) Catalysis of the reactions of 5′-phosphorimidazolides of nucleosides
A variety of metal ions also catalyse the oligomerization of activated monomers to form RNA oligomers. The best catalyst is the uranyl ion , which catalyses the formation of oligo(C)s, oligo(U)s and oligo(A)s from the corresponding ImpB (figure 6b) with maximum chain lengths of 10, 10 and 16, respectively (Sawai 1989, 1992; Sawai et al. 1989, 1992). catalysis differs from montmorillonite catalysis, in that most of the phosphodiester bonds are 2′,5′-linked. The overall yields of oligomers are usually greater than that of the montmorillonite catalysis because is not an effective catalyst for the hydrolysis of the imidazole-activating group. Pb2+, Zn2+ and lanthanide metal ions also catalyse oligomer formation with maximum chain lengths of 5, 4 and 3 mers, respectively (Sawai & Orgel 1975; Sawai 1976). Lu3+ gives the lowest yield of hydrolysis of the imidazole group (44%) and the highest yield of tetramers (0.9%) of the transition metal ions (Sawai & Yamamto 1996).
The catalytic effect of Pb2+ was much greater when the reaction was performed in the eutectic water phase at −18°C, where most of the water is present as ice (Kanavarioti et al. 2001; Monnard et al. 2003). Oligomers as long as 17 mers were formed with 80–90% yield. The high yields may be owing to the higher concentrations of activated monomers and the slower hydrolysis of the activated monomers at the low-reaction temperature.
(b) Catalysis of template-directed synthesis
Pb2+ and Zn2+ also catalyse the non-enzymatic template-directed synthesis of RNA oligomers (Sleeper et al. 1979; Lohrmann et al. 1980). Pb2+ catalyses the reaction of ImpG on a poly(C) template to form up to 40 mers of oligo(G)s that are mainly 2′,5′-linked. Pb2+ also catalyses the formation of up to 7 mers of 3′,5-linked oligo(A)s on a poly(U) template. Zn2+ catalyses the reaction of ImpG on a poly(C) template to form up to 30 mers that are mainly 3′,5′-linked (Lohrmann et al. 1980).
It is proposed that the metal ions serve as catalysts, because they bind to the activated monomer and growing polymer so that the phosphorimidazolide group of the activated monomer is proximal to the 2′ or 3′-hydroxyl on the 3′-end of the oligomer. The orientation of the two reactants facilitates the formation of the phosphodiester bond (Birdson & Orgel 1980). This proposal is supported by the observation that no metal ion is required to enhance the rate of reaction of the 2-methylphosphorimidazolide of guanosine (figure 6d) on a poly(C) template to give over 50 mers that are almost entirely 3′,5′-linked (Inoue & Orgel 1981). It is postulated that the 2-methyl group on the imidazole ring changes the orientation of the poly(C) helix so that the activated monomer is ideally positioned to react at the 3′-end of the growing oligonucleotide. This finding suggests that the role of the montmorillonite clay may also be that of the orientation of the activated monomers so that they are in a favourable orientation to react with each other.
9. Selectivity versus specificity
We have demonstrated that a limited number of sequences are formed in the montmorillonite-catalysed reaction of equal amounts of ImpA with ImpC (Miyakawa & Ferris 2003). The montmorillonite-catalysed reactions of phosphorimidazolides are regioselective in the formation of phosphodiester bonds with purine nucleotides bonded mainly by 3′,5′-phosphodiester bonds and with pyrimidines by 2′,5′-links (Ferris & Ertem 1993a; Ding et al. 1996; Ertem & Ferris 1997; Kawamura & Ferris 1999). Homochiral selectivity is observed in the reactions of with d,l-purine nucleotides (Joshi et al. 2000).
I propose that the selectivity observed with montmorillonite catalysis is more desirable than specificity. A specific catalyst will carry out mainly one reaction on a specific substrate. It will be difficult to initiate the first life with a requirement of a specific catalyst and a specific substrate for each step in the process. It is a more likely that selective catalysts acting on an assemblage of similar structures, a quasi-species (Eigen et al. 1988), initiated the origin of life. In addition, a selective catalyst might have catalysed more than one reaction as shown by both the montmorillonite catalysis of vesicle and phosphodiester bond formation.
10. Hypothetical stages in the origins of life
The progress in the formation of RNA monomers and the formation of RNA oligomers has been the emphasis of this discussion. The catalytic formation of RNA oligomers is an important first step in the origin of life since the availability of a catalyst and the activated monomers makes possible the continuous formation of the RNA oligomers that initiate the process. The presence of a subset of either ligase or replicase ribozymes of these oligomers will be important in the next step in the process. The ligase would generate longer oligomers that would have the potential to store more information than the 50 mers formed by montmorillonite catalysis. Alternatively a replicase would generate larger amounts of catalytic RNAs including those that are ligases, thus forming RNAs with the potential to store more genetic information. The presence of both replicase and ligase RNAs is a combination that has the potential to initiate the first life.
This first life could consist of an assemblage of these oligomers bound to a mineral or encapsulated inside a vesicle. Life on a mineral surface would not have to devise the process of cell division and would depend mainly on an external supply of activated monomers to survive. Life in a vesicle may require the evolution of metabolic processes as the source of the activated monomers.
While RNA oligomers have been used to describe the stages in the origins of life, this could also be the scenario for the formation of a preRNA that was a precursor to the RNA world. Similar stages, starting from genetic molecules very different from RNA nucleotides, could also have led to the first life.
Acknowledgments
My co-workers, listed in the references, are sincerely thanked for their thoughtful and skilful work on this project. Funding was provided by an NSF grant CHE-0413739 and NASA grant NAGS-12750 that supports the NY Centre for Studies on the Origins of Life.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
Discussion
D. W. Deamer (Department of chemistry and Biochemistry, University of California Santa Cruz, USA). Please comment on how activated nucleotides may be organized on the montmorillonite surface so that phosphodiester bond formation is promoted?
J. P. Ferris. It is not known how the activated nucleotides are organized on the montmorillonite. It is known that oligomers of G are formed on a poly(C) template as a result of the hydrogen bonding between the activated monomers and the C nucleotides in the template (Inoue & Orgel 1981). A similar reaction pathway may also occur on the montmorillonite, where the activated monomers bind next to each other in an orientation, where the 2′ or 3′-hydroxyl group of one activated monomer is oriented next to the activated phosphate group of a second monomer so that phosphodiester bond formation occurs.
Goodwin. Because of the importance of the possible formation of d-amino acid proteins instead of l-amino acids, proteins (as on all life formed on Earth), does the montmorillonite favour the catalytic formation of one particular chiral form of the RNA oligomer over the other form?
J. P. Ferris. Montmorillonite is not chiral; so it does not selectively catalyse the reaction of only one of the enantiomers of a d,l-mixture. We have found that in the reaction of a d,l-activated monomer of A that the homochiral (e.g. d,d and l,l-dimers) dimers, trimers and tetramers are formed preferentially over the heterochiral products (Joshi et al. in press). In the reaction of the d,l-activated monomers of U, the heterochiral products are favoured. We believe the difference in the selectivity between A and U is a function of the different orientations of the activated monomers when bound to the surface of the montmorillonite.
J. I. Lunine (Lunar and Planetary Sciences Department, University of Arizona, USA). (i) What types of treatments of the montmorillonite surface have other groups performed? (ii) Why is the activation treatment of the montmorillonite surface needed? (iii) Why is it that montmorillonite has the selective properties that you have found?
J. P. Ferris. (i) Two types of treatment are commonly used to form a homoionic montmorillonite (usually in the Na+ form of the clay). The various exchangeable metal ions bound to the clay are replaced by treating several times with excess of NaCl and then washing the clay until no more NaCl is detected in the wash. The second is to treat the montmorillonite briefly with cold (2°C) HCl and then wash it to remove the excess HCl and metal ions. Then, the acidic clay is titrated to pH 7 with Na+ (Banin et al. 1985). (ii) Homoionic clays are formed before use so that a montmorillonite with only one type of exchangeable metal ion is used. Then, one can be sure that the catalytic effects observed are due to the montmorillonite and not to one of the other metal ions bound to the montmorillonite. (iii) The selectivity is due to the catalyst lowering the activation energy of only a few reaction pathways. This is probably a function of the relative orientations of the activated monomers when bound to the montmorillonite.
References
- Amelin Y. A tale of early Earth told in zircons. Science. 2005;310:1914–1915. doi: 10.1126/science.1121536. [DOI] [PubMed] [Google Scholar]
- Ban N, Nissen P, Hansen J, Moore P.B, Seitz T.A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. doi:10.1126/science.289.5481.905 [DOI] [PubMed] [Google Scholar]
- Banin A, Lawless J.G, Mazzurco J, Church F.M, Margulies L, Orenberg J.B. pH profile of the adsorption of nucleotides onto montmorillonite. Orig. Life Evol. Biosph. 1985;15:89–101. doi: 10.1007/BF01809491. doi:10.1007/BF01809491 [DOI] [PubMed] [Google Scholar]
- Birdson P.K, Orgel L.E. Catalysis of accurate poly(C)-directed synthesis of 3′-5′-linked oligoadenylates by Zn2+ J. Mol. Biol. 1980;144:567–577. doi: 10.1016/0022-2836(80)90337-x. doi:10.1016/0022-2836(80)90337-X [DOI] [PubMed] [Google Scholar]
- Brandes J.A, Boctor N.Z, Cody G.D, Cooper B.A. Abiotic nitrogen reduction on the early Earth. Nature. 1998;395:365–367. doi: 10.1038/26450. doi:10.1038/26450 [DOI] [PubMed] [Google Scholar]
- Cody G.D, Boctor N.Z, Filley T.R, Hazen R.M, Scott J.H, Sharma A, Yoder H.S., Jr Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science. 2000;289:1337–1340. doi: 10.1126/science.289.5483.1337. doi:10.1126/science.289.5483.1337 [DOI] [PubMed] [Google Scholar]
- Delano J. Redox History of the Earth's interior since ∼3900 Ma: implications for prebiotic molecules. Orig. Life Evol. Biosph. 2001;31:311–341. doi: 10.1023/a:1011895600380. doi:10.1023/A:1011895600380 [DOI] [PubMed] [Google Scholar]
- Ding Z.P, Kawamura K, Ferris J.P. Oligomerization of uridine phosphorimidazolides on montmorillonite: a model for the prebiotic synthesis of RNA on minerals. Orig. Life Evol. Biosph. 1996;26:151–171. doi: 10.1007/BF01809853. doi:10.1007/BF01809853 [DOI] [PubMed] [Google Scholar]
- Eigen M, McCaskill J, Schuster P. Molecular quasi-species. J. Phys. Chem. 1988;92:6881–6891. doi:10.1021/j100335a010 [Google Scholar]
- Ertem G, Ferris J.P. Template-directed synthesis using the heterogeneous templates produced by montmorillonite catalysis. A possible bridge between the prebiotic and RNA worlds. J. Am. Chem. Soc. 1997;119:7197–7201. doi: 10.1021/ja970422h. doi:10.1021/ja970422h [DOI] [PubMed] [Google Scholar]
- Ertem G, Ferris J.P. Sequence- and regio-selectivity in the montmorillonite-catalyzed synthesis of RNA. Orig. Life Evol. Biosph. 2000;30:411–422. doi: 10.1023/a:1006767019897. doi:10.1023/A:1006767019897 [DOI] [PubMed] [Google Scholar]
- Ferris J.P. Marine hydrothermal systems and the origin of life: chemical markers of prebiotic chemistry in hydrothermal systems. Orig. Life Evol. Biosph. 1992;22:109–134. doi: 10.1007/BF01808020. doi:10.1007/BF01808020 [DOI] [PubMed] [Google Scholar]
- Ferris J.P. Montmorillonite catalysis of 30–50 mer oligonucleotides: laboratory demonstration of potential steps in the origin of the RNA world. Orig. Life Evol. Biosph. 2002;32:311–332. doi: 10.1023/a:1020543312109. doi:10.1023/A:1020543312109 [DOI] [PubMed] [Google Scholar]
- Ferris J.P. Catalysis and prebiotic synthesis. In: Banfield J.F, Cervini J, Nealson K, editors. Molecular geomicrobiology. vol. 59. Mineralogical Society of America; Chantilly, VA: 2005. pp. 187–210. [Google Scholar]
- Ferris J.P, Ertem G. Montmorillonite catalysis of RNA oligomer formation in aqueous solution. A model for the prebiotic formation of RNA. J. Am. Chem. Soc. 1993a;115:12 270–12 275. doi: 10.1021/ja00079a006. doi:10.1021/ja00079a006 [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Ertem G. Oligomerization reactions of ribonucleotides: the reaction of the 5′-phosphorimidazolide of adenosine with diadenosine pyrophosphate on montmorillonite and other minerals. Orig. Life Evol. Biosph. 1993b;23:229–241. doi: 10.1007/BF01809373. doi:10.1007/BF01581901 [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Hagan W.J., Jr HCN and chemical evolution: the possible role of cyano compounds in prebiotic synthesis. Tetrahedron. 1984;40:1093–1120. doi: 10.1016/s0040-4020(01)99315-9. doi:10.1016/S0040-4020(01)99315-9 [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Joshi P.C, Edelson E.H, Lawless J.G. HCN: a plausible source of purines, pyrimidines, and amino acids on the primitive Earth. J. Mol. Evol. 1978;11:293–311. doi: 10.1007/BF01733839. doi:10.1007/BF01733839 [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Huang C.-H, Hagan W.J., Jr Clays as prototypical enzymes for the prebiological formation of phosphate esters. Orig. Life Evol. Biosph. 1986;17:173–174. doi: 10.1007/BF01808786. [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Ertem G, Agarwal V.K. Mineral catalysis of the formation of dimers of 5′-AMP in aqueous solution: the possible role of montmorillonite clays in the prebiotic synthesis of RNA. Orig. Life Evol. Biosph. 1989;19:165–178. doi: 10.1007/BF01808150. doi:10.1007/BF01808150 [DOI] [PubMed] [Google Scholar]
- Ferris J.P, Hill A.R, Jr, Liu R, Orgel L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature. 1996;381:59–61. doi: 10.1038/381059a0. doi:10.1038/381059a0 [DOI] [PubMed] [Google Scholar]
- Fuller W.D, Sanchez R.A, Orgel L.E. Studies in prebiotic synthesis IV. Synthesis of purine nucleosides. J. Mol. Biol. 1972;67:25–33. doi: 10.1016/0022-2836(72)90383-x. doi:10.1016/0022-2836(72)90383-X [DOI] [PubMed] [Google Scholar]
- Gestland T.R, Cech T.R, Atkins J.F. Cold Spring Harbor Press; New York, NY: 1999. The RNA world. [Google Scholar]
- Gladman B, Dones D, Levinson H.F, Burns J.A. Impact seeding and reseeding in the inner solar system. Astrobiology. 2005;5:483–496. doi: 10.1089/ast.2005.5.483. doi:10.1089/ast.2005.5.483 [DOI] [PubMed] [Google Scholar]
- Gomes R, Levison H.F, Tsiganis K, Morbidelli A. Origin of the cataclysmic late heavy bombardment period of the terrestrial planets. Nature. 2005;435:466–469. doi: 10.1038/nature03676. doi:10.1038/nature03676 [DOI] [PubMed] [Google Scholar]
- Hanczyc M.M, Fujikawa S.M, Szostak J.W. Experimental models of primitive cellular compartments: encapsulation, growth and division. Science. 2003;302:618–622. doi: 10.1126/science.1089904. doi:10.1126/science.1089904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T.M, Blichert-Toft J, Muller W, Albarede F, Holden P, Mojzsis S.J. Heterogeneous Hadean hafnium: evidence of continental crust at 4.4 to 4.5. Science. 2005;310:1947–1950. doi: 10.1126/science.1117926. doi:10.1126/science.1117926 [DOI] [PubMed] [Google Scholar]
- Huang W, Ferris J.P. Synthesis of 35–40 mers of RNA oligomers from unblocked monomers. A simple approach to the RNA world. Chem. Commun. 2003;12:1458–1459. doi: 10.1039/b303134a. doi:10.1039/b303134a [DOI] [PubMed] [Google Scholar]
- Huber C, Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science. 1997;2776:245–247. doi: 10.1126/science.276.5310.245. doi:10.1126/science.276.5310.245 [DOI] [PubMed] [Google Scholar]
- Inoue T, Orgel L.E. Substituent control of the poly(C)-directed oligomerization of guanosine 5′-phosphoroimidazolide. J. Am. Chem. Soc. 1981;103:7666–7667. doi:10.1021/ja00415a051 [Google Scholar]
- Joshi P.C, Pitsch S, Ferris J.P. Homochiral selection in the montmorillonite-catalyzed and uncatalyzed prebiotic synthesis of RNA. Chem. Commun. 2000:2497–2498. doi:10.1039/b007444f [Google Scholar]
- Joyce G.F, Orgel L.E. Prospects for understanding the origin of the RNA world. In: Gesteland R.F, Cech T.R, Atkins J.F, editors. The RNA world: the nature of modern RNA suggests a prebiotic RNA. Cold Spring Harbor Laboratory Press; New York, NY: 1999. pp. 49–77. [Google Scholar]
- Kanavarioti A. Dimerization in highly concentrated solutions of phosphorimidazolide activated mononucleotides. Orig. Life Evol. Biosph. 1997;27:357–376. doi: 10.1023/a:1006526002896. doi:10.1023/A:1006526002896 [DOI] [PubMed] [Google Scholar]
- Kanavarioti A, Monnard P.-A, Deamer D.W. Eutectic phases in ice facilitate nonenzymatic nucleic acid synthesis. Astrobiology. 2001;1:271–281. doi: 10.1089/15311070152757465. doi:10.1089/15311070152757465 [DOI] [PubMed] [Google Scholar]
- Kaplan R.W. The problem of chance in formation of protobionts by random aggregation of macromolecules. In: Buvet R, Ponnamperuma C, editors. Molecular evolution I. Chemical evolution and the origin of life. North-Holland Publishing Company; Amsterdam, The Netherlands; London, UK: 1971. pp. 319–329. [Google Scholar]
- Kawamura K, Ferris J.P. Kinetic and mechanistic analysis of dinucleotide and oligonucleotide formation from the 5′-phosphorimidazolide of adenosine on Na+-montmorillonite. J. Am. Chem. Soc. 1994;116:7564–7572. doi: 10.1021/ja00096a013. doi:10.1021/ja00096a013 [DOI] [PubMed] [Google Scholar]
- Kawamura K, Ferris J.P. Clay catalysis of oligonucleotide formation: kinetics of the reaction of the 5′-phosphorimidazolides of nucleotides with the non-basic heterocycles uracil and hypoxanthine. Orig. Life Evol. Biosph. 1999;29:563–591. doi: 10.1023/a:1006648524187. doi:10.1023/A:1006648524187 [DOI] [PubMed] [Google Scholar]
- Kebbekus P. Chemistry. Rensselaer Polytechnic Institute; Troy, NY: 1988. Formation of RNA oligomers on montmorillonite clay under possible prebiotic conditions; p. 23. [Google Scholar]
- Lailach G.E, Thompson T.D, Brindley G.W. Absorption of pyrimidines, purines, and nucleosides by Li-, Na-, Mg-, and Ca-montmorillonite (clay-organic studies XII) Clay. Clay Min. 1968;16:285–293. [Google Scholar]
- Lohrmann R, Bridson P.K, Orgel L.E. Efficient metal-ion catalyzed template-directed oligonucleotide synthesis. Science. 1980;208:1464–1465. doi: 10.1126/science.6247762. [DOI] [PubMed] [Google Scholar]
- McCollom T.M, Seewald J.S. A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim. Comochim. Acta. 2001;65:3769–3778. doi:10.1016/S0016-7037(01)00655-X [Google Scholar]
- Miller S.L. Production of some amino acids under possible primitive Earth conditions. J. Am. Chem. Soc. 1955;77:2351–2361. doi:10.1021/ja01614a001 [Google Scholar]
- Miller S.L. Peptide nucleic acids and prebiotic chemistry. Nat. Struct. Biol. 1997;4:167–169. doi: 10.1038/nsb0397-167. doi:10.1038/nsb0397-167 [DOI] [PubMed] [Google Scholar]
- Miyakawa S, Ferris J.P. Sequence- and regioselectivity in the montmorillonite-catalyzed synthesis of RNA. J. Am. Chem. Soc. 2003;125:8202–8208. doi: 10.1021/ja034328e. doi:10.1021/ja034328e [DOI] [PubMed] [Google Scholar]
- Miyakawa S, Cleaves H.J, Miller S.L. The cold origins of life: B. Implications based on pyrimidines and purines produced from frozen ammonium cyanide solutions. Orig. Life Evol. Biosph. 2002;32:209–218. doi: 10.1023/a:1019514022822. doi:10.1023/A:1019514022822 [DOI] [PubMed] [Google Scholar]
- Mojzsis S.J, Harrison T.M, Pidgeon R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth's surface 4,300 Myr ago. Nature. 2001;409:178–181. doi: 10.1038/35051557. doi:10.1038/35051557 [DOI] [PubMed] [Google Scholar]
- Monnard P.-A, Apel C.L, Kanavarioti A, Deamer D.W. Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology. 2002;2:139–152. doi: 10.1089/15311070260192237. doi:10.1089/15311070260192237 [DOI] [PubMed] [Google Scholar]
- Monnard P.-A, Kanavarioti A, Deamer D.W. Eutectic phase polymerization of activated ribonucleotide mixtures yields quasi-equimolar incorporation of purine and pyrimidine nucleobases. J. Am. Chem. Soc. 2003;125:13 734–13 740. doi: 10.1021/ja036465h. doi:10.1021/ja036465h [DOI] [PubMed] [Google Scholar]
- Müller D, Pitsch S, Kittaka A, Wagner E, Wintner C.E, Eschenmoser A. Chemie von a-Aminonitrilen. Aldomerisierung von Glycolaldehyd-phosphat zu racemischen Hexose-2,4,6-triphosphaten und (in Gegenwart von Formaldehyd) racemischen Pentose-2,4-diphosphaten: rac-Allose-2,4,6-triphosphat und rac-Ribose-2,4-diphosphat sind die Reaktionshauptprodukte. Helv. Chim. Acta. 1990;73:1410–1468. [Google Scholar]
- Nielsen P.E, Egholm M, Berg R.H, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Nikalje M.D, Puhukan P, Sudalai A. Recent advances in clay-catalyzed transformatiions. Org. Prep. Proced. 2000;32:1–40. [Google Scholar]
- Oro J. Synthersis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960;2:407–412. doi:10.1016/0006-291X(60)90138-8 [Google Scholar]
- Osterberg R, Orgel L.E. Polyphosphate and trimetaphosphate formation under potentially prebiotic conditions. J. Mol. Evol. 1972;1:241–248. doi: 10.1007/BF01660243. doi:10.1007/BF01660243 [DOI] [PubMed] [Google Scholar]
- Osterberg R, Orgel L.E, Lohrmann R. Further studies of urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973;2:231–234. doi: 10.1007/BF01654004. doi:10.1007/BF01654004 [DOI] [PubMed] [Google Scholar]
- Pizzarello S. Chemical evolution and meteorites, an update. Orig. Life Evol. Biosph. 2004;34:25–34. doi: 10.1023/b:orig.0000009826.76353.de. doi:10.1023/B:ORIG.0000009826.76353.de [DOI] [PubMed] [Google Scholar]
- Poulet F, Bibring J.-P, Mustard J.F, Gendrin A, Mangold N, Langevin Y, Arvidson R.E, Gondet B, Gomez C. Philosilicates on Mars and implications for early martian climate. Nature. 2005;438:623–627. doi: 10.1038/nature04274. doi:10.1038/nature04274 [DOI] [PubMed] [Google Scholar]
- Ricardo A, Carrigan M.A, Olcott A.N, Benner S. Borate minerals stabilize ribose. Science. 2004;303:196. doi: 10.1126/science.1092464. doi:10.1126/science.1092464 [DOI] [PubMed] [Google Scholar]
- Righter K, Drake M.J. Effect of water on metal-silicate partitioning of siderophile elements: a high pressure and temperature terrestrial magma ocean and core formation. Earth Planet. Sci. Lett. 1999;171:383–399. doi:10.1016/S0012-821X(99)00156-9 [Google Scholar]
- Sanchez R.A, Ferris J.P, Orgel L.E. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J. Mol. Biol. 1967;30:223. [PubMed] [Google Scholar]
- Sawai H. Catalysis of internucleotide bond formation by divalent ions. J. Am. Chem. Soc. 1976;98:7037–7039. doi: 10.1021/ja00438a050. doi:10.1021/ja00438a050 [DOI] [PubMed] [Google Scholar]
- Sawai H, Orgel L.E. Oligonucleotide synthesis catalyzed by the Zn2+ ion. J. Am. Chem. Soc. 1975;97:3532–3533. doi: 10.1021/ja00845a050. doi:10.1021/ja00845a050 [DOI] [PubMed] [Google Scholar]
- Sawai H, Yamamto K. Lanthanide ion as a catalyst for internucleotide bond formation. Bull. Chem. Soc. Jpn. 1996;69:1701–1704. doi:10.1246/bcsj.69.1701 [Google Scholar]
- Sawai H, Kuroda K, Hojo T. Uranyl ion as a highly effective catalyst for internucleotide bond formation. Bull. Chem. Soc. Jpn. 1989;62:2018–2023. doi:10.1246/bcsj.62.2018 [Google Scholar]
- Sawai H, Higa K, Kuroda K. Synthesis of cyclic and acyclic oligocytidylates by uranyl ion catalyst in aqueous solution. J. Chem. Soc. Perkin. 1992;I:505–508. doi:10.1039/p19920000505 [Google Scholar]
- Schlesinger G, Miller S.L. Prebiotic synthesis in atmospheres containing CH4, CO and CO2. J. Mol. Evol. 1983;19:376–382. doi: 10.1007/BF02101642. doi:10.1007/BF02101642 [DOI] [PubMed] [Google Scholar]
- Sleeper H.L, Lohrmann R, Orgel L.E. Template-directed synthesis of oligoadenylates catalyzed by Pb2+ ions. J. Mol. Evol. 1979;13:203–214. doi: 10.1007/BF01739480. doi:10.1007/BF01739480 [DOI] [PubMed] [Google Scholar]
- Springsteen G, Joyce G.F. Selective derivatization and sequestration of ribose from a prebiotic mix. J. Am. Chem. Soc. 2004;126:9578–9583. doi: 10.1021/ja0483692. doi:10.1021/ja0483692 [DOI] [PubMed] [Google Scholar]
- Symonds R.B, Rose W.I, Bluth G, Gerlach T.M. Volcanic gas studies: methods, results, and applications. In: Carroll M.R, Holloway J.R, editors. Volatiles in magmas. Reviews in Mineralogy. vol. 30. Mineralogical Society of America; Washington, DC: 1994. pp. 1–66. [Google Scholar]
- Tian F, Toon O.B, Pavlov A.A, De Sterck H. A hydrogen-rich early Earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. doi:10.1126/science.1106983 [DOI] [PubMed] [Google Scholar]
- Voet A.B, Schwartz A.W. Uracil synthesis via HCN oligomerization. Orig. Life. 1982;12:45–49. doi: 10.1007/BF00926910. doi:10.1007/BF00926910 [DOI] [PubMed] [Google Scholar]
- Watson E.B, Harrison T.M. Zircon thermometer reveals minimum melting conditions on earliest Earth. Science. 2005;308:841–844. doi: 10.1126/science.1110873. [DOI] [PubMed] [Google Scholar]
- Wells L.E, Armstrong J.C, Gonzalez G. Reseeding of the early Earth by impacts of returning ejecta during the late heavy bombardment. Icarus. 2003;162:38–46. doi:10.1016/S0019-1035(02)00077-5 [Google Scholar]
- Wilde S.A, Valley J.W, Peck W.H, Graham C.M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature. 2001;409:175–178. doi: 10.1038/35051550. doi:10.1038/35051550 [DOI] [PubMed] [Google Scholar]
- Williams L.B, Canfield B, Voglesonger K.M, Holloway J.R. Organic molecules formed in a “primordial womb”. Geology. 2005;33:913–916. doi:10.1130/G21751.1 [Google Scholar]
- Zubay G. Studies on the lead-catalyzed synthesis of aldopentoses. Orig. Life Evol. Biosph. 1998;28:13–26. doi: 10.1023/a:1006551410542. doi:10.1023/A:1006551410542 [DOI] [PubMed] [Google Scholar]
Additional references
- Banin A, Lawless J.G, Mazzurco J, Church F.M, Margulies L, Orenberg J.B. pH profile of the adsorption of nucleotides onto montmorillonite. Orig. Life Evol. Biosph. 1985;15:89–101. doi: 10.1007/BF01809491. [DOI] [PubMed] [Google Scholar]
- Inoue T, Orgel L.E. Substituent control of the poly(C)-directed oligomerization of guanosine 5′-phosphorimidazolide. J. Am. Chem. Soc. 1981;103:7666–7667. [Google Scholar]
- Joshi, P. C., Pitsch, S. & Ferris, J. P. In press Selectivity of montmorillonite catalyzed prebiotic reactions of d, l-nucleotides. Orig. Life Evol. Biosph. [DOI] [PubMed]