Abstract
Selecting Yorkshire breeding stock for increased placental efficiency (PE; piglet weight divided by its placental weight) results in larger litters (i.e. ∼3 more piglets litter−1) and reduced placental sizes. Placental vessel density increases progressively after day 50 of gestation in the pig, and is positively correlated with PE and placental expression of vascular endothelial growth factor (VEGF), a potent angiogenic and permeability factor. To elicit its vascular effects, VEGF must bind to its receptors (R), VEGF-R1 and VEGF-R2. The objective of this study was to compare placental and endometrial blood vessel density and placental VEGF, VEGF-R1 and VEGF-R2 mRNA expression in day 70 and 90 conceptuses from Yorkshire females selected for high PE, low PE, and from unselected controls. A greater (P < 0.05) PE was observed for conceptuses in the high PE selection group when compared to the low PE selection group. Placental blood vessel density increased (P < 0.05) from day 70 to 90 (1.8 ± 0.1 versus 2.8 ± 0.2) in association with increases (P < 0.05) in placental VEGF mRNA expression. No selection group differences were observed in expression of VEGF, VEGF-R1, or VEGF-R2 on day 70. By day 90, however, placentae of conceptuses from the high PE group expressed greater (P < 0.05) amounts of VEGF and VEGF-R1 mRNA than the unselected controls and the low PE group. These data demonstrate that increased placental expression of the VEGF receptor system is associated with increased placental vascular density observed with the advancement of gestation in the pig. Although placental blood vessel density was not increased in the high PE selection group, elevated levels of the VEGF receptor system suggest an effect on increasing placental and endometrial blood vessel permeability and/or proximity in the high PE group.
Evidence suggests that uterine capacity becomes limiting to litter size after day 30 of gestation in the pig (Fenton et al. 1972; Monk & Erb, 1974; Webel & Dziuk, 1974). Superovulation and superinduction increase the number of conceptuses surviving to day 30, but these females do not farrow litters larger than controls (Dziuk, 1968; Fenton et al. 1970; Pope et al. 1972; Monk & Erb, 1974; Webel & Dziuk, 1974). Uterine capacity has been defined in terms of absolute uterine size (Christenson et al. 1987), but more recently, Ford et al. (2002) defined it in terms of the relative surface area of placental endometrial attachment required to support the nutrient requirements of an individual conceptus throughout gestation. Recent evidence suggests that placental efficiency (PE; fetal weight divided by the weight of its placenta) is an individual conceptus trait, and is highly variable within a litter (Wilson et al. 1999; Ford et al. 2002). Prolific Chinese Meishan gilts exhibit the same ovulation rate and uterine size as domestic Yorkshire gilts, while farrowing 3–4 more piglets per litter (Ford & Youngs, 1993). Meishan pigs overcome the limitation of uterine capacity by markedly increasing the ratio of the fetal size to placental size in association with a marked increase in placental vascularity when compared to domestic pig breeds (Biensen et al. 1998). These data are consistent with the concept that the Meishan pig, selected for thousands of years solely for the number of pigs born alive, with little regard for piglet birth weight or growth efficiency, may have dissociated fetal and placental growth (Peilieu, 1985).
In order to determine if placental size was heritable in our Yorkshire population, Wilson et al. (1999) developed a method of matching each piglet with its placenta at farrowing. As each piglet appeared at the vulva, its umbilical cord was double tagged and ligated, and the tagged cord allowed to retract into the birth canal. After farrowing and placental expulsion, each piglet's birth weight was matched with the weight of its placenta. Wilson et al. (1999) selected piglets with a birth weight of >1200 g and an above-average PE and placed them in the high PE selection group, and piglets of similar weight and below-average PE were placed in the low PE selection group. Interestingly, he noted that there was a four-fold variation in placental efficiency within an individual litter. When he bred boars and gilts of the high PE selection group together, placental weight was dramatically reduced and litter size was increased (∼3 live piglets litter−1) compared with the litters produced by the low PE boars and gilts (Wilson et al. 1999). These data are supported by Leenhouwers et al. (2001), who reported that piglets from females selected for high estimated piglet survival to weaning exhibited markedly greater PE than piglets from the low estimated piglet survival line.
We have also previously demonstrated that in the pig placental vessel density (or the number of placental vessels per unit area) increases markedly and progressively after day 50 of gestation and is positively correlated with PE as well as increases in placental expression of vascular endothelial growth factor (VEGF; Vonnahme et al. 2001). The potent angiogenic factor, VEGF, is a secreted 45-kDa dimeric ligand binding glycoprotein which increases endothelial cell proliferation, migration and capillary permeability when it binds to its receptors (R), VEGF-R1 and VEGF-R2 (Neufeld et al. 1999; Stacker & Achen, 1999).
The objective of this study was to compare placental and endometrial blood vessel density and placental VEGF, VEGF-R1, and VEGF-R2 mRNA expression from day 70 and day 90 conceptuses from females selected for high PE, low PE and from unselected controls after three additional generations of selection for PE beyond that reported by Wilson et al. (1999).
Methods
Tissue collection
Populations of Yorkshire pigs originally selected for high or low PE by Wilson et al. (1999) continued to be selected and bred within a group over four generations by the placental tagging technique. During generation 4, females from the high PE selection group (n= 4), low PE selection group (n= 4) and an unselected control group (n= 4) were bred to boars in their respective group at the onset of oestrus (day 0) and 12 h later. Females were killed by applying an electrical current across the brain (two per selection group) on day 70 and on day 90 of gestation at the Iowa State University Meat Laboratory, a USDA inspected facility, in accordance with procedures approved by the Institutional Animal Care & Use Committee and the FASS Guide (1999). Days 70 and 90 of gestation were chosen for sample collection as this is the initial period of rapid placental vascular proliferation in the Meishan pig (Biensen et al. 1998), and the interval when placental VEGF mRNA levels begin to increase in the Yorkshire pig (Vonnahme et al. 2001). The gravid uterus was immediately transported on ice to the laboratory (<5 min) for further processing. At the laboratory, each uterine horn was opened along the antimesometrial side. Noting its location in the horn, each fetus was removed, weighed, and a crown–rump length recorded. A 3 cm × 4 cm section of the uteroplacental interface located 2–3 cm from the umbilical attachment site of each fetus was collected, placed in a tissue cassette (Fisher Brand; Fisher Scientific, Pittsburgh, PA, USA) and fixed in 4 % paraformaldehyde. Next, a small portion (∼4 g) of placental tissue adjacent to the excised uteroplacental section was collected and snap frozen on liquid nitrogen for RNA extraction. The ends of each placenta were marked with dissection pins placed in the uterine wall, and placentae were peeled from the endometrium and weighed. The implantation site length for each conceptus was then measured using a cloth tape measure. In order to obtain a measure of PE, the weight of each fetus was divided by the weight of its placenta.
Histology and vascular density determination
After fixation, uteroplacental sections were dehydrated in a series of ethanol solutions and paraffin embedded using procedures previously established in our laboratory (Biensen et al. 1998). Vascular measurements were determined for utero-placental sections from 12 randomly selected conceptuses from litters in each selection group and the unselected control group on days 70 and 90. Paraffin-embedded placental/endometrial sections were sectioned at 5 μm, stained with periodic acid–Schiff reagent, counterstained with haematoxalin, and traced for quantification of vascular measurements as previously described (Vonnahme et al. 2001). For each placental: endometrial interface, images of two fields from each of four slides with stained tissues were projected on to a sheet of paper using a projecting microscope (XM150; Kramer Scientific, New York, NY, USA) and the areas occupied by placental and adjacent endometrial tissues were individually traced. In each of these areas, the blood vessels were traced so that the area and number of all vessels could be determined via image analysis (Bioquant; Nashville, TN, USA; Biensen et al. 1998), values were then averaged across the eight sections examined.
Placental VEGF, VEGF-R1, VEGF-R2 mRNA expression
To determine the levels of VEGF, VEGF-R1, and VEGF-R2 mRNA in the pig placenta, a pig-specific probe was needed for use in the RNase protection assay. Details for the porcine VEGF probe generation are previously published (Vonnahme et al. 2001). Primers for VEGF-R1 and VEGF-R2 were designed from the full-length porcine sequence established from ARS-USDA MARC (Clay Center, NE, USA; VEGF-R1 sense 5′-GCAGGATCCGACG-AGCAACAGCGGCTTCACG-3′ and antisense 5′-GTAG-AATTCAGCTGGAGTGGCAGAAAGT-3′ corresponding to base pairs 2039–2403; VEGF-R2 sense 5′-GCAGGAT-CCGGCTCTGGCCCAACAATCAGAG-3′ and antisense 5′ - GTAGAATTCCCTCGCACAAAGGGACACAT - 3′ corresponding to base pairs 230–544; primers designed with cloning restriction sites). While there has been no report of the soluble truncated form of VEGF-R1 in the pig, care was taken to design porcine VEGF-R1 primers homologous to regions within human and mouse sequences which do not share homology with the truncated form of VEGF-R1. Porcine placental RNA was reversed transcribed using Moloney murine leukaemia virus reverse transcriptase. Conditions were optimized, and the polymerase chain reaction (PCR) was performed. A 5-min extension time was added to the cycling programme to insure ample addition of dATP to the 3′ end of the PCR amplicon for ligation in to the pCR2.1 plasmid (Invitrogen, Carlsbad, CA, USA). The VEGF-R1 and VEGF-R2 inserts were then subcloned into Bluescript SK+-plasmid (Stratagene, La Jolla, CA, USA).
RNase protection assay
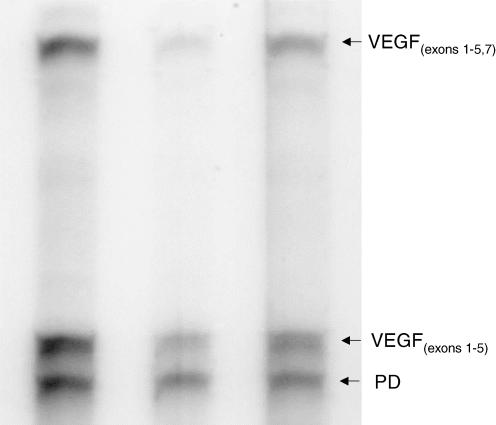
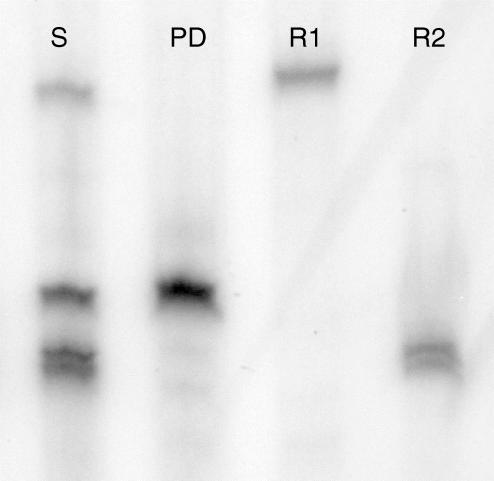
The in vitro transcription assay and RNase protection assay were performed as previously described (Wilson et al. 2000) in our laboratory with the following exceptions. Detection of placental VEGF mRNA and the housekeeping gene, pyruvate dehydrogenase (PD) was performed using the HybSpeed RNase Protection Assay kit as previously described (Vonnahme et al. 2001). Briefly, protected fragments resulted in two bands using the VEGF probe representing messages that coded for the VEGF165 fragment (exons 1–5 and 7) or the VEGF121, VEGF189 and VEGF206 fragments (exons 1–5; Fig. 1). To quantify VEGF mRNA levels for each placental sample, counts from both VEGF bands were summed and divided by the counts in the PD band to correct for slight differences in sample loading. Although the pattern of VEGF165 was positively correlated (r= 0.87; P < 0.0001) to the pattern of total VEGF mRNA expression, we cannot determine the exact patterns of isoforms 121, 189 and 206, and therefore we only report the total amount of VEGF mRNA expression. A second RNase protection assay was performed utilizing probes for VEGF-R1, VEGF-R2 and PD (Fig. 2). Plasmids containing either VEGF-R1 or VEGF-R2 were linearized with the Sac-1 restriction enzyme. Radiolabelled RNAs were synthesized from these linear DNA templates with 10 U of T7 RNA polymerase according to the manufacturer's protocol (Ambion, Austin, TX, USA). Preliminary RPAs were performed where a range of input sample RNA was added using a constant amount of high specific probe (i.e. 3 × 108 cpm μg−1). The intensity of the band representing each protected fragment should increase with increasing amounts of input RNA when there is excess probe present. When the signal remained the same with more input RNA, then there was not a molar excess of probe and the RPA would not be quantitative. The optimal amount of RNA added to each reaction was determined to be 20 μg. To quantify mRNA expression levels, counts from VEGF or VEGF-R1 or VEGF-R2 bands were divided by the counts in the PD band to correct for slight differences in sample loading. To correct for between-gel variation, a pool of placental RNA was included in each assay and therefore data are presented relative to the mRNA levels in the control sample.
Figure 1. RNase protection assay of placental RNA for VEGF and the housekeeping gene, pyruvate dehydrogenase (PD).
Use of the VEGF probe resulted in two protected fragments, VEGF exons 1–5 and VEGF exons 1–5, 7. See text for details.
Figure 2. RNase protection assay of placental RNA for VEGF-R1, VEGF-R2 and the housekeeping gene, pyruvate dehydrogenase (PD).
Lanes represent the sum of all three probes (S), PD probe alone, VEGF-R1 (R1) probe alone and VEGF-R2 (R2) probe alone.
Statistics
Data were analysed using the general linear model procedure of SAS (SAS Institute Inc. Version 8.0; Cary, NC, USA). The experimental unit in this study was an individual conceptus, as it has been determined that PE is an individual conceptus trait (Wilson et al. 1999; Ford et al. 2002). In order to confirm that there was no effect of sow or selection scheme on PE, the percentage variation around mean PE was calculated. The effect of selection group and sow on percentage variation around the mean was analysed by the homogeneity of variance test using the Brown and Forsythe's variation of Levene's test. Percentage variation around the mean was calculated by subtracting mean PE from an individual's PE and dividing this by the individual's PE. This value was then put on a percentage basis. There was no effect of sow or selection group on the percentage of variation around the mean for PE (P= 0.98), indicating that an individual sow and our selection process did not impact the potential variation for PE within a litter. Day of gestation and selection group were included in the class statement. The effects of day of gestation and selection group were tested for the variables fetal weight, placental weight, PE, crown–rump length, implantation site length, relative number of placental blood vessels, relative number of endometrial blood vessels, placental blood vessel diameter, endometrial blood vessel diameter, and placental VEGF, VEGF-R1 and VEGF-R2 mRNA expression levels. Separation of means was accomplished using the least squared means procedure. P values of <0.05 were considered significant.
Results
Table 1 depicts the conceptus measurements (total conceptus number, fetal weight, crown–rump length, placental weight, PE, and implantation site length) taken from all conceptuses collected on day 70 and day 90 in the high PE selection group, an unselected control group as well as the low PE selection group. While there was no difference in fetal weight on day 70 among the three groups, the low PE selection group had significantly greater (P < 0.05) placental weights compared to the unselected control group and the high PE selection group conceptuses. Thus, the PE values of the conceptuses from the low PE selection group were significantly less (P < 0.05) than the PE in the unselected control group and the high PE selection group. The implantation site lengths of conceptuses in the high PE selection group were significantly shorter (P < 0.05) than those from the low PE selection group with the unselected control group being intermediate in this measurement. By day 90, fetal weight was greater (P < 0.05) in low PE selection group animals than in the high PE selection group, with the unselected control group being intermediate. Day 90 conceptuses from the high PE selection group had markedly lighter placentae (P < 0.05) than the unselected control group or the low PE selection group. Due to the markedly greater reduction in placental weight than fetal weight, the PE of the conceptuses in the high PE selection group on day 90 was significantly greater (P < 0.05) than the unselected control and the low PE selection groups. The implantation site lengths of conceptuses on day 90 from the high PE selection group were significantly reduced (P < 0.05) when compared to the implantation site length of conceptuses from both the unselected control and the low PE selection groups.
Table 1.
Conceptus measurements from selected placental efficiency groups on day 70 and day 90 of gestation
| Selection group1 | ||||
|---|---|---|---|---|
| High placental efficiency | Unselected controls | Low placental efficiency | ||
| Day 70 | Number of conceptuses | n= 27 | n= 24 | n= 20 |
| Fetal weight (g) | 218.44 ± 8.01a | 219.42 ± 7.61a | 255.80 ± 8.11a | |
| Crown–rump length (cm) | 17.85 ± 0.26a | 18.04 ± 0.18a | 19.08 ± 0.26b | |
| Placental weight (g) | 168.26 ± 9.82a | 170.75 ± 9.58a | 325.50 ± 18.66b | |
| Placental efficiency | 1.39 ± 0.08a | 1.34 ± 0.06a | 0.83 ± 0.04b | |
| Implantation site length (cm) | 16.85 ± 0.60a | 20.71 ± 0.90b | 24.45 ± 0.73c | |
| Day 90 | Number of conceptuses | n= 27 | n= 17 | n= 13 |
| Fetal weight (g) | 638.22 ± 37.48a | 664.35 ± 22.551b | 723.69 ± 44.88b | |
| Crown–rump length (cm) | 25.00 ± 0.49a | 26.03 ± 0.37b | 26.04 ± 0.37b | |
| Placental weight (g) | 190.00 ± 8.75a | 235.41 ± 20.43b | 242.92 ± 13.92b | |
| Placental efficiency | 3.35 ± 0.11a | 3.03 ± 0.17b | 3.02 ± 0.15b | |
| Implantation site length (cm) | 21.63 ± 0.65a | 26.41 ± 1.07b | 33.23 ± 2.71c | |
Average litter sizes were 13.8, 10.2, and 8.8 in the high placental efficiency selection group (n= 4), unselected controls (n= 4), and low placental efficiency selection group (n= 4), respectively.
Means ± s.e.m. within a row with different superscripts differ; P < 0.05.
Means ± s.e.m. within a row with different superscripts differ; P < 0.05.
Means ± s.e.m. within a row with different superscripts differ; P < 0.05.
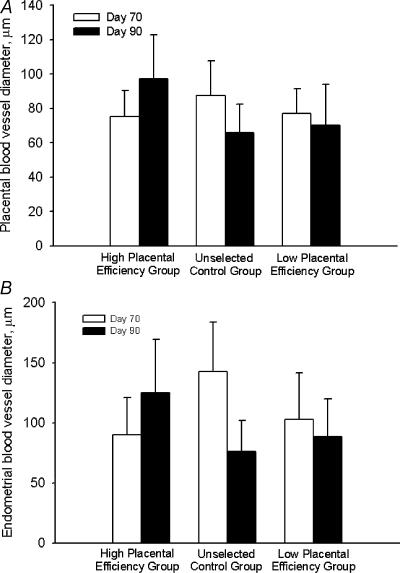
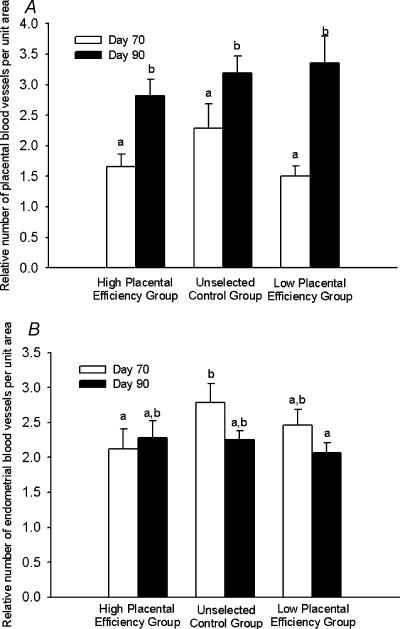
There was no effect of selection group or day of gestation on the diameters of blood vessels in either placental or endometrial tissue (Fig. 3). However, the relative number of placental blood vessels per unit area increased from day 70 to day 90 in all groups (Fig. 4A). In contrast, the relative number of endometrial blood vessels per unit area was relatively constant across day of gestation and selection group (Fig. 4B).
Figure 3. Placental (A) and endometrial (B) blood vessel diameters on day 70 and day 90 from the high placental efficiency, unselected control, and the low placental efficiency groups.
No differences were observed (P > 0.05).
Figure 4. Relative placental (A) and endometrial (B) blood vessel number per unit area tissue on day 70 and day 90 from the high placental efficiency, unselected control, and low placental efficiency groups.
a,b Means ± s.e.m. with different superscripts differ; P < 0.05.
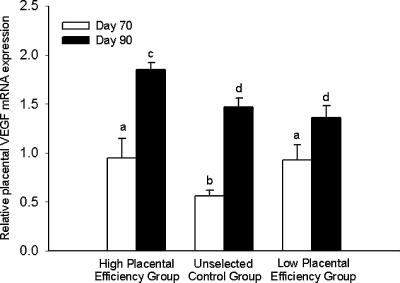
Placental VEGF mRNA expression increased (P < 0.05) from day 70 to day 90 in the placentae of conceptuses from all groups (Fig. 5). Placental VEGF mRNA expression did not differ between the conceptuses of the high PE and low PE selection groups on day 70 of gestation. The placentae from the unselected control conceptuses had a lower (P < 0.05) VEGF mRNA expression value than the two selected groups. On day 90, however, the placental VEGF mRNA expression levels attained in the high PE selection group were greater (P < 0.05) than those in the unselected control and low PE selection groups.
Figure 5. Relative placental VEGF mRNA expression levels on day 70 and day 90 of gestation.
a,b,c,d Means ± s.e.m. with different superscripts differ; P < 0.05.
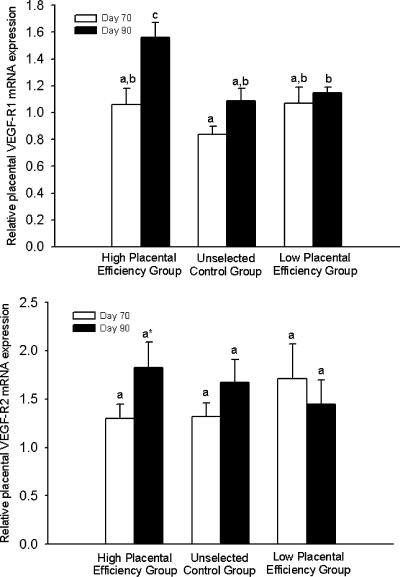
Placental VEGF-R1 mRNA expression levels did not differ by selection group on day 70 of gestation (Fig. 6A). While VEGF-R1 and VEGF-R2 mRNA expression did not increase from day 70 to day 90 in the placentae from unselected control and low PE selection groups, there was a significant increase (P < 0.05) in VEGF-R1 mRNA expression and a tendency (P= 0.08) for an increase in VEGF-R2 mRNA expression in placentae from the high PE selection group (Fig. 6A and B).
Figure 6. Relative placental VEGF-R1 (A) and VEGF-R2 (B) mRNA expression levels on day 70 and day 90 of gestation.
a,b,c Means ± s.e.m. with different superscripts differ; P < 0.05. * A tendency (P= 0.08) for an increase in VEGF-R2 on day 70 and day 90 in the high PE selection group.
Discussion
These data confirm our previous report (Vonnahme et al. 2001) that placental VEGF mRNA expression, placental vascular density and PE significantly increase from day 70to day 90 of gestation in the pig. More importantly, these data demonstrate for the first time that a multigenerational selection for increased PE results in a further elevation of placental mRNA expression of VEGF and VEGF-R1 and a tendency for increased VEGF-R2 by day 90 of gestation. Further, and in agreement with Wilson et al. (1999), breeding pairs which were selected for a higher PE produced conceptuses with smaller placentae and whose conceptuses occupied less uterine space during late gestation than conceptuses from breeding pairs selected for low PE. As the pig exhibits an appositional, non-invasive placental type, the surface area of endometrial: placental attachment as well as placental vascularity both play major roles in the limitations of uterine capacity. As previously mentioned, Meishan pigs overcome the limitation of uterine capacity by markedly reducing placental size, while at the same time increasing placental vascularity (Biensen et al. 1998). After day 70 of gestation, Meishan conceptuses fail to increase the size of their placenta, but rather, exhibit a marked and progressive increase in placental vascular density (Biensen et al. 1998). In contrast, Yorkshire conceptuses continue to exhibit marked increases in placental size after day 70, with only modest and variable increases in placental vascularity (Biensen et al. 1998; Vonnahme et al. 2001).
In association with the progressive increase in placental vascularity from day 50 to term, Meishan conceptuses exhibit progressive increases in the expression of VEGF, VEGF-R1 and VEGF-R2 (Vonnahme & Ford, 2002a). In contrast, while Yorkshire conceptuses also exhibit a progressive increase in placental VEGF mRNA expression during this period, placental VEGF-R1 mRNA and VEGF-R2 mRNA levels are highly variable. These data suggest a role for both VEGF receptors in mediating the progressive increase in the vascularity of the Meishan placenta. The biological implications of this differential pattern of vascular development in the placenta of the Meishan versus Yorkshire conceptuses was addressed by Vonnahme & Ford (2002b). They determined that Yorkshire, but not Meishan conceptuses, accelerate placental growth when adjacent litter mates perish as late as day 40 of gestation. This failure of surviving Meishan conceptuses to accelerate their placental growth may be a result of the breed's documented ability to increase placental vascularity to meet fetal demands. This strategy of a reduced placental growth and increased placental vascularity may also be present in piglets of domestic pig breeds, as selection of breeding stock with increased PE results in increased litter sizes and conceptuses with smaller, more vascular placentae (Wilson et al. 1999).
As mentioned previously, VEGF elicits its vasoactive actions via its receptors, VEGF-R1 and VEGF-R2. Although it was initially thought that the receptors for VEGF would be found exclusively on the endothelial cells, VEGF and its receptors have been localized to other tissues including the endometrium and placenta of the human (Charnock-Jones et al. 1994; Clark et al. 1996) and the pig (Winther et al. 1999; Charnock-Jones et al. 2001; Vonnahme et al. 2001). Vascular endothelial growth factor receptors seem to play different roles in the angiogenic process as VEGF-R2 deficient mouse conceptuses fail to develop endothelial cells or blood islands (Shalaby et al. 1995), whereas mice deficient in VEGF-R1 develop endothelial cells, but lack proper vascular lumen formation (Fong et al. 1995). While endothelial cell proliferation (Seetharam et al. 1995) and migration (Waltenberger et al. 1994) appear to be primarily a result of VEGF acting via VEGF-R2, vascular permeability and endothelial cell differentiation appear to be regulated by VEGF's actions via VEGF-R1 (Peters et al. 1993).
Vascular endothelial growth factor has been localized to the chorionic and uterine luminal epithelium of the pig (Winther et al. 1999; Vonnahme et al. 2001), and there is a progressive increase in immunostaining from mid- to late gestation (Vonnahme et al. 2001). In addition, we have localized VEGF-R1 and VEGF-R2 to the endothelium of adjacent placental and endometrial blood vessels during late gestation (S. P. Ford, unpublished data). These progressive increases in VEGF, VEGF-R1 and VEGF-R2 at the placental: endometrial interface may play a significant role in the reported decrease in intercapillary distance from 40 μm to 2 μm at the summit and lateral sides of the chorionic ridges from early to late gestation (Friess et al. 1980). This decrease in intercapillary distance results from epithelial cell thinning and from the protrusion of vessels into the chorionic and uterine luminal epithelial cells (Friess et al. 1980). Blood gases pass from maternal to fetal blood by simple diffusion, which is dependent on the surface area available for transfer, and the physical distance separating endometrial and placental capillaries (Villee, 1965). In contrast, Friess et al. (1980) presented morphological and histological evidence that the transport of less diffusible materials (i.e. glucose, amino acids, etc.) occurs in the chorionic troughs, where the chorionic and uterine luminal epithelium remain high columnar. Even in these areas, an increased density and/or permeability of fetal and endometrial capillaries would improve transfer mechanisms such as facilitated diffusion, active transport and pinocytosis (Sperhake, 1971).
While placental vascular density increased from day 70 to day 90 of gestation for all conceptuses in this study, we observed no preferential increase in placental vascular density in the high PE selection group to account for the greater fetal/placental weight ratio in this group on day 90 when compared to the low PE selection group or the unselected controls. It is conceivable that the elevated levels of VEGF-R1 and VEGF-R2 mRNA in the high PE selection group on day 90 may have resulted in an augmented capillary permeability and/or a reduced placental: endometrial intercapillary distance through endothelial cell proliferation and migration. Additional studies will be needed, however, to quantify selection group differences in intercapillary distances and the transport of blood gases and nutrients across the fetal: maternal interface to confirm this hypothesis.
References
- Biensen NJ, Wilson ME, Ford SP. The impact of either a Meishan or a Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J Anim Sci. 1998;76:2169–2176. doi: 10.2527/1998.7682169x. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Clark DE, Licence D, Day K, Wooding FB, Smith SK. Distribution of vascular endothelial growth factor (VEGF) and its binding sites at the maternal_fetal interface during gestation in pigs. Reproduction. 2001;122:753–760. [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Boocock CA, Ahmed A, Plevin R, Ferrara N, Smith SK. Vascular endothelial growth factor receptor localisation and activation in human trophoblast and choriocarcinoma cells. Biol Reprod. 1994;51:524–530. doi: 10.1095/biolreprod51.3.524. [DOI] [PubMed] [Google Scholar]
- Christenson RK, Leymaster KA, Young LD. Justification of unilateral hysterectomy-ovariectomy as a model to evaluate uterine capacity in swine. J Anim Sci. 1987;65:738–744. doi: 10.2527/jas1987.653738x. [DOI] [PubMed] [Google Scholar]
- Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum Reprod. 1996;11:1090–1098. doi: 10.1093/oxfordjournals.humrep.a019303. [DOI] [PubMed] [Google Scholar]
- Dziuk PJ. Effect of number of embryos and uterine space on embryo survival in the pig. J Anim Sci. 1968;27:673–676. doi: 10.2527/jas1968.273673x. [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies. FASS Guide For the Care and Use of Agricultural Animals in Agricultural Research and Teaching. 1999. p. 24. first revised edition. [Google Scholar]
- Fenton FR, Bazer FW, Robison OW, Ulberg LC. Effect of quantity of uterus on uterine capacity in gilts. J Anim Sci. 1970;31:104–106. doi: 10.2527/jas1970.311104x. [DOI] [PubMed] [Google Scholar]
- Fenton FR, Schwartz FL, Bazer FW, Robison OW, Ulberg LC. Stage of gestation when uterine capacity limits embryo survival in gilts. J Anim Sci. 1972;35:383–388. doi: 10.2527/jas1972.352383x. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breltman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Ford SP, Vonnahme KA, Wilson ME. Uterine capacity in the pig reflects a combination of uterine environment and conceptus genotype effects. J Anim Sci. 2002;80(1):E66–E73. [Google Scholar]
- Ford SP, Youngs CR. Early embryonic development in prolific Meishan pigs. J Reprod Fert. 1993;48:271–278. [PubMed] [Google Scholar]
- Friess AE, Sinowatz F, Skolek-Winnisch R, Trautner W. The placenta of the pig. I. Finestructural changes of the placental barrier during pregnancy. Anat Embryol. 1980;158:179–191. doi: 10.1007/BF00315905. [DOI] [PubMed] [Google Scholar]
- Leenhouwers JI, Knol EF, de Groot PN, Vos H, van der Lende T. Biologic Aspects of Genetic Differences in Piglet Survival, Doctoral Thesis. Wageningen, The Netherlands: 2001. Fetal development in the pig in relation to genetic merit for piglet survival. [Google Scholar]
- Monk EL, Erb RE. Effect of unilateral ovariectomy and hysterectomy on reproductive parameters in the gilt during early pregnancy. J Anim Sci. 1974;39:366–372. doi: 10.2527/jas1974.392366x. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Peilieu C. Livestock Breeds of China. Rome: FAO and China Academic Publishers; 1985. Pig breeds; pp. 159–194. [Google Scholar]
- Peters KG, De Vries C, Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci USA. 1993;90:8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CE, Christenson RK, Zimmerman-Pope VA, Day BN. Effect of number of embryos on embryonic survival in recipient gilts. J Anim Sci. 1972;35:805–808. doi: 10.2527/jas1972.354805x. [DOI] [PubMed] [Google Scholar]
- SAS (2003) SAS Systems for Windows. NC, USA: Cary; version 8.0. SAS Institute Inc. [Google Scholar]
- Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction form FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X-F, Breltman ML, Schuh AC. Failure of blood island formation and vasculogenesis in flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sperhake B. Inaug Diss Hannoever; Thesis. 1971. Zur Durchlassigkeit der placenta des schweines. Eine Literautrstudie. [Google Scholar]
- Stacker SA, Achen MG. The vascular endothelial growth factor family: signalling for vascular development. Growth Factors. 1999;17:1–11. doi: 10.3109/08977199909001058. [DOI] [PubMed] [Google Scholar]
- Villee CA. Placental transfer of drugs. Ann N Y Acad Sci. 1965;123:237–244. doi: 10.1111/j.1749-6632.1965.tb12262.x. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Ford SP. Proceedings for Perspectives in Pig Science. Sutton Bonington, UK: University of Nottingham; 2002a. Role of vascular endothelial growth factor (VEGF) in placental vascular development, placental efficiency and litter size in the pig. [Google Scholar]
- Vonnahme KA, Ford SP. Conceptus competition for uterine space: different strategies exhibited by the Meishan and Yorkshire pig. J Anim Sci. 2002b;80:1311–1316. doi: 10.2527/2002.8051311x. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Wilson ME, Ford SP. Relationship between placental vascular endothelial growth factor expression and placental/endometrial vascularity in the pig. Biol Reprod. 2001;64:1821–1825. doi: 10.1095/biolreprod64.6.1821. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt-1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Webel SK, Dziuk PJ. Effects of stage of gestation and uterine space on prenatal survival in the pig. J Anim Sci. 1974;38:960–967. doi: 10.2527/jas1974.385960x. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Biensen NJ, Ford SP. Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J Anim Sci. 1999;77:1654–1658. doi: 10.2527/1999.7771654x. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Sonstegard TS, Smith TPL, Fahrenkrug SC, Ford SP. Differential gene expression during elongation in the pig embryo. Genesis. 2000;26:9–14. doi: 10.1002/(sici)1526-968x(200001)26:1<9::aid-gene4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Winther H, Ahmed A, Dantzer V. Immunohistochemical localization of vascular endothelial growth factor (VEGF) and its two specific receptors, Flt-1 and KDR, in the porcine placenta and non-pregnant uterus. Placenta. 1999;20:35–43. doi: 10.1053/plac.1998.0350. [DOI] [PubMed] [Google Scholar]